Ibrutinib is the first approved therapy for the treatment of patients with Waldenström macroglobulinemia (WM). The approval was based on results of a study in WM patients that showed overall response rate (ORR) of 91% and median time to response of 4 weeks.1 Similar results were reported in another multicenter study in rituximab-refractory WM patients2 and in a study evaluating ibrutinib as primary therapy for WM patients.3 Temporary interruption of ibrutinib is sometimes needed to manage toxicities or perioperatively to minimize bleeding.4 We observed that some WM patients developed withdrawal symptoms while holding ibrutinib, which then resolved promptly after ibrutinib reinitiation. We performed a retrospective study that shows that withdrawal symptoms develop in 20% of WM patients who hold ibrutinib therapy. The rate of withdrawal symptoms was lower in patients who started ibrutinib at serum IgM levels ≥4,000 mg/dl and CXCR4 mutated patients, and higher in patients who had achieved a very good partial response (VGPR) on ibrutinib. Symptoms ensue within 2 days of ibrutinib hold and resolve rapidly following reinitiation of therapy. In two thirds of the patients who experience withdrawal, there is no evidence of disease progression during ibrutinib hold. In the patients who progress during the hold, response is regained within 3 months of ibrutinib reinitiation.
We identified patients seen at our institution between May 2012 and April 2017 who met diagnostic criteria for WM,5 met criteria to initiate therapy,6 and received ibrutinib at 420 mg PO once daily. The Institutional Review Board at the Dana-Farber Cancer Institute approved the study. Medical files were reviewed to identify WM patients who reported new-onset symptoms occurring during a temporary hold of ibrutinib therapy. Patients’ characteristics were compared using χ2, Fisher exact or rank sum tests as needed. The association of clinical factors with withdrawal symptoms was evaluated by fitting logistic regression models, and reported as odds ratio (OR) with 95% confidence interval (CI). All withdrawal symptoms were graded according to the Common Terminology Criteria for Adverse Events.7 P-values <0.05 were considered statistically significant. Calculations and graphs were obtained using STATA (StataCorp, College Station, TX, USA).
A total of 189 patients with WM who started ibrutinib were identified; 75 patients (40%) did not hold ibrutinib, and 114 (60%) held ibrutinib at some point during therapy. The median time on ibrutinib in patients who held ibrutinib was 22 months (95% CI 18-39 months) and 12 months (95% CI 9-15 months) for those who did not hold ibrutinib. There were no differences in characteristics between patients who held and did not hold ibrutinib (data not shown).
From patients who held ibrutinib, 92 (81%) did not experience withdrawal symptoms and 22 (19%) did. Of patients who experienced withdrawal symptoms, 17 (82%) were receiving ibrutinib in the relapsed and 4 (18%) in the frontline setting. All patients were holding ibrutinib for the first time since treatment initiation. The median drug hold length was 7 days (range 3-33 days). Reasons for holding ibrutinib included: procedure/surgery (n=14; 64%), toxicity (n=6; 27%), drug interaction (n=1; 5%), and patient choice (n=1; 5%). The median time to symptom development was 2 days (range 0-5 days) from the start of the drug hold.
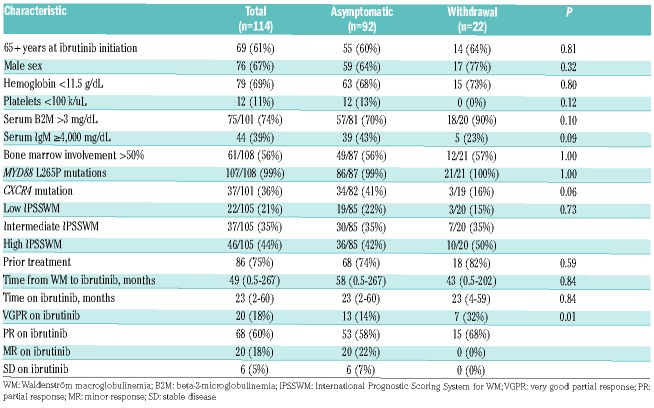
There was a trend towards lower odds of withdrawal symptoms in patients with serum IgM ≥4,000 mg/dl at the time of the hold than patients with serum IgM <4,000 mg/dl (23% vs. 77%; OR 0.39, 95% CI 0.13-1.15; P=0.09), and in CXCR4 mutated patients versus CXCR4 wild type patients (16% vs. 84%; OR 0.23, 95% CI 0.05-1.08; P=0.06), and a trend towards higher odds of withdrawal in patients who had achieved VGPR at the time of the hold than in patients who achieved PR (35 vs. 22%; OR 2.84, 95% CI 0.97-8.28; P=0.06). No withdrawal symptoms were reported in patients who achieved MR or did not respond to ibrutinib. There were no differences between patients who became symptomatic and remained asymptomatic during ibrutinib hold with regards to other clinical characteristics (Table 1).
Table 1.
Clinical characteristics of Waldenström macroglobulinemia patients who developed and did not develop ibrutinib withdrawal symptoms.
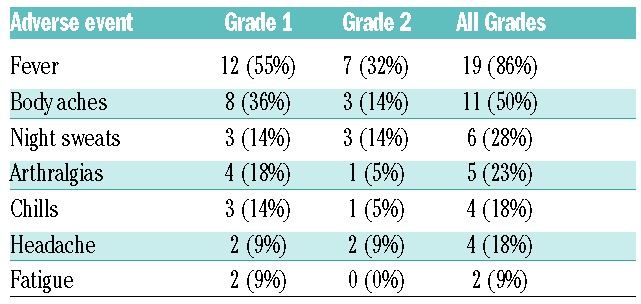
Patient-reported symptoms and grading are shown in Table 2. Twelve of the 22 patients (55%) who became symptomatic during ibrutinib hold had subsequent holds, and all reported recurrence of symptoms during the hold period. Four patients with fever were admitted to the hospital, and after exhaustive workup, no infectious etiology was identified. All patients reported resolution of symptoms the same day ibrutinib was restarted following the drug hold. Four patients known to develop these symptoms were treated with prednisone 10 mg PO twice daily during a subsequent hold and reported symptomatic relief.
Table 2.
Frequency and grade of adverse events reported during temporary ibrutinib hold.
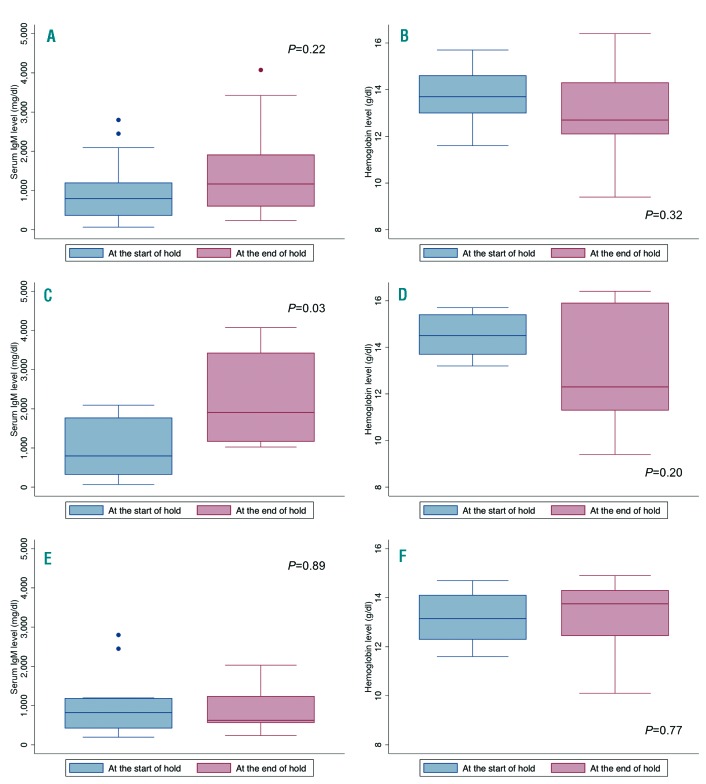
We then evaluated the possibility that the withdrawal symptoms experienced by WM patients during ibrutinib holds were due to progressive disease (PD) during the hold. We evaluated hemoglobin and serum IgM level before and after ibrutinib hold in patients who were symptomatic. The median serum IgM levels before and after ibrutinib hold were 796 mg/dl and 1167 mg/dl, respectively (P=0.22; Figure 1A). The median hemoglobin levels before and after ibrutinib hold were 13.7 g/dl and 12.7 g/dl, respectively (P=0.32; Figure 1B). During the hold, 7 of the symptomatic patients (35%) met criteria for PD. In these patients, median serum IgM levels before and after ibrutinib hold were 796 mg/dl and 1909 mg/dl, respectively (P=0.03; Figure 1C), and median hemoglobin levels were 14.1 g/dl and 12.2 g/dl, respectively (P=0.20; Figure 1D). In all cases, the serum IgM decreased upon restarting ibrutinib. The median time to response (at least 25% decrease in serum IgM compared to level at ibrutinib reinitiation) was 3 months (range 1-6 months) and the time until the serum IgM level went back to baseline (serum IgM level prior to ibrutinib hold) was 5 months (range 3-12 months). In the 15 patients who did not experience PD (65%), there were no differences in serum IgM (1061 mg/dl and 1202 mg/dl, respectively; P=0.89; Figure 1E) or hemoglobin levels (13.2 g/dl and 13.8 g/dl, respectively; P=0.77; Figure 1F) before and after ibrutinib hold.
Figure 1.
Box plots presenting the distribution of serum IgM and hemoglobin levels at the start and the end of ibrutinib hold, in patients with Waldenstrom macroglobulinemia who experienced withdrawal symptoms while holding ibrutinib. Figures 1A and 1B present the distribution in all patients, Figures 1C and 1D in patients who progressed during ibrutinib hold, and Figures 1E and 1F in patients who did not progress during ibrutinib hold.
To the best of our knowledge, this is the first report of withdrawal symptoms experienced by WM patients temporarily holding ibrutinib. In a previous review paper, Woyach reported a case of a patient with chronic lymphocytic leukemia (CLL) who experienced similar symptoms to the ones reported here, such as fatigue and night sweats and without actual evidence of disease progression.8 Woyach goes on then to recommend minimizing temporary holds and using steroids in patients who experience withdrawal symptoms. However, there has not been a formal evaluation on this syndrome in CLL patients. At the time of this report, it is unknown if patients with mantle cell lymphoma have experienced similar ibrutinib withdrawal symptoms.
The mechanism by which ibrutinib withdrawal symptoms occur remains to be clarified. However, recent data demonstrate ibrutinib induces significant suppression of inflammatory cytokines (i.e., TNFα, IL2RA and CXCL13), as well as a downregulatory effect on T-cells and macrophages.9–12 It is possible that re-activation of both tumoral and microenvironmental cells during an ibrutinib hold could generate a cytokine release syndrome and cause the withdrawal symptoms reported here. Indeed, holding ibrutinib can result in the rapid reversal of its inhibitory effect, as evidenced by increase in serum IgM levels and regrowth of lymph nodes observed in WM and CLL patients with interrupted therapy. Moreover, our findings suggest withdrawal symptoms are more likely to occur in patients who achieve deep responses to ibrutinib therapy. This may in part be due to the deep suppression of CXCL13 on ibrutinib reported in WM patients who achieve major responses to therapy. Accordingly, lower rates of withdrawal symptoms were observed in patients with CXCR4 mutations, which are associated with superficial responses to ibrutinib and higher serum IgM levels in WM patients. Prospective evaluation of serum cytokine levels during the course of an ibrutinib hold would provide further evidence to elucidate this hypothesis.
Clinically, there appears to be two groups of patients who develop withdrawal symptoms. In one third of cases, withdrawal symptoms are associated with progressive disease characterized by increasing serum IgM levels, and in two thirds, symptoms occur in the absence of disease progression with no change in serum IgM or hemoglobin levels. However, minimizing ibrutinib holds, especially in patients who experience withdrawal symptoms, is of relevance in the light of recent findings reporting a shorter PFS in patients with <97% dose intensity on ibrutinib.13 Patients known to have ibrutinib withdrawal might benefit from a short course of prednisone 10 mg PO twice daily during the holding period.
We acknowledge the limitations of our study with regards to its retrospective nature and small sample size. In addition, the 20% incidence of ibrutinib withdrawal reported here could be an overestimation, since we might not know of patients not seen regularly at our clinic and were asymptomatic during ibrutinib holds. The time to response after ibrutinib reinitiation can also be an overestimation given that most patients had IgM levels measured at 3 months. However, we believe the ibrutinib withdrawal symptoms reported has clinical implications, and clinicians should be aware of this phenomenon.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015; 372(15):1430–1440. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Trotman J, Tedeschi A, et al. Ibrutinib for patients with rituximab-refractory Waldenstrom’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–250. [DOI] [PubMed] [Google Scholar]
- 3.Treon SP, Gustine J, Meid K, et al. Ibrutinib is highly active as first line therapy in symptomatic Waldenstrom’s Macroglobulinemia. Blood. 2017;130(Suppl 1):2767. [Google Scholar]
- 4.Castillo JJ, Palomba ML, Advani R, Treon SP. Ibrutinib in Waldenström macroglobulinemia: latest evidence and clinical experi ence. Ther Adv Hematol. 2016;7(4):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30(2):116–120. [DOI] [PubMed] [Google Scholar]
- 7.CTEP. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available at https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm-ctc_40 Accessed on December 17, 2017.
- 8.Woyach JA. How I manage ibrutinib-refractory chronic lymphocytic leukemia. Blood. 2017;129(10):1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemann CU, Herman SE, Maric I, et al. Disruption of in vivo Chronic Lymphocytic Leukemia Tumor-Microenvironment Interactions by Ibrutinib–Findings from an Investigator-Initiated Phase II Study. Clin Cancer Res. 2016;22(7):1572–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ping L, Ding N, Shi Y, et al. The Bruton’s tyrosine kinase inhibitor ibrutinib exerts immunomodulatory effects through regulation of tumor-infiltrating macrophages. Oncotarget. 2017;8(24):39218–39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren L, Campbell A, Fang H, et al. Analysis of the effects of the Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib on monocyte Fcgamma Receptor (FcgammaR) Function. J Biol Chem. 2016; 291(6):3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos JM, Tsakmaklis N, Patterson CJ, et al. CXCL13 levels are elevated in patients with Waldenstrom’s macroglobulinemia, and are predictive of major response to ibrutinib. Haematologica. 2017; 102(11):e452–e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustine J, Meid K, Dubeau T, et al. Impact of ibrutinib dose intensity on patient outcomes in previously treated Waldenstrom macroglobulinemia. Blood. 2017;130(Suppl 1):4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.