Risk-stratification and treatment intensification according to minimal residual disease (MRD) analysis has improved outcomes of patients with acute lymphoblastic leukemia (ALL).1,2 However, a significant proportion of patients with T-cell ALL (T-ALL) still experience early relapse or refractory disease. Robust prognostic markers able to identify high-risk patients at diagnosis have proved challenging, and risk-adapted management for TALL remains an unmet need. The work presented herein shows that the molecular marker, absence of biallelic deletion at the TRG locus, does not have a poor prognostic impact on the outcome of pediatric and adolescent patients with T-ALL treated on the risk-directed protocol of the Medical Research Council UK Acute Lymphoblastic Leukemia 2003 (MRC UKALL2003) trial.
Early T-cell Precursor (ETP) T-ALL, identified by immunophenotyping, was previously reported to confer a poor prognosis in pediatric, adolescent and adult patients.3,4 However, immunophenotyping was found to underestimate the number of patients with an ETP gene-expression signature,5 and inter-laboratory diagnosis for this immunophenotype is not standardized. Moreover, gene-expression profiling is not widely utilized for clinical use. Therefore, an alternative molecular method based on the V-J recombination status of the T-cell receptor γ (TRG) gene has been described.6 Given that TRG recombination occurs early during T-cell development,7 cells that have not undergone biallelic deletion at the TRG locus have been termed ‘ABD’ (Absence of Biallelic Deletion), with the majority of such ABD T-ALL cases having an ETP-ALL gene-expression signature.6 Consistent with the original reports of ETP-ALL,3 ABD was reported to be associated with inferior survival,6 although the patients studied were not treated on MRD risk-directed protocols. Thus we investigated whether ABD status adds further prognostic information for pediatric and adolescent patients with T-ALL treated on the MRC UKALL2003 trial, which used MRD risk to direct treatment intensity.1,8
Whole genome amplified (WGA) DNA was available from diagnostic samples of 152 of 393 (39%) T-ALL patients treated on this trial.9 Full details of the patient cohort, trial protocols, methods and statistical analyses are described in the Online Supplementary Information. The trial is registered at controlled-trials.com under ISRCTN number 07355119. Ethical approval for the trial was obtained previously from the Scottish Multi-Centre Research Ethics Committee on 25/02/2003, ref: 02/10/052, and samples were collected with informed consent according to the Declaration of Helsinki.
Baseline characteristics and survival outcomes of these 152 patients were not significantly different from the 241 patients not included in the study (Online Supplementary Table S1 and Table S2, and Online Supplementary Figure S1).
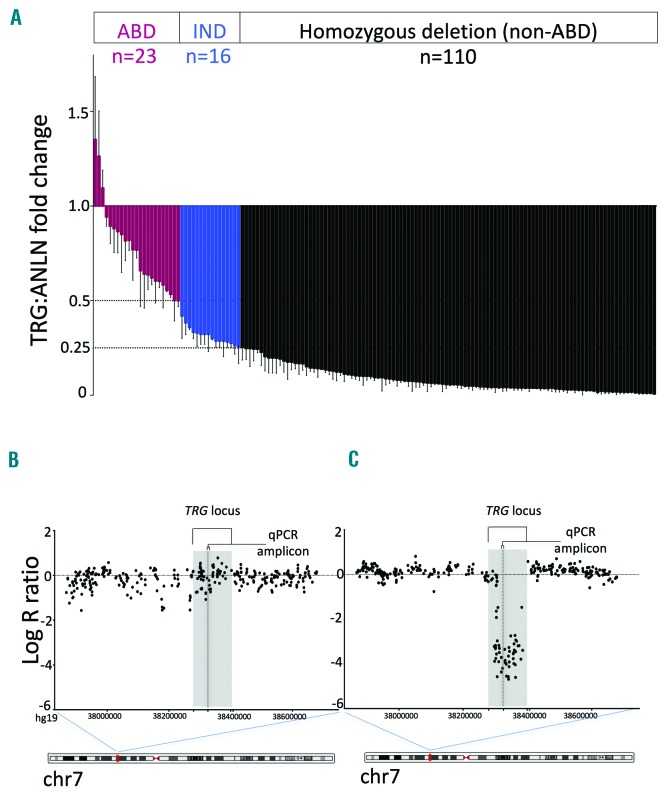
The TRG quantitative polymerase chain reaction (qPCR) assay determined fold change using the comparative DDCT method with ANLN as the reference gene (TRG:ANLN), as previously described.6 There was good agreement between results from WGA material and their corresponding non-WGA sample where available (R2=0.92) (Online Supplementary Figure S2A,B). Patients were assigned to the ABD group if the TRG:ANLN fold change was ≥0.5 and the diagnostic blast count ≥50% (to exclude lack of deletion due to contamination with non-leukemic cells), non-ABD if ≤0.25, and indeterminate if between these values (Figure 1A). Indeterminate results were confirmed using COA1 as an alternative reference gene (Online Supplementary Figure S2C). TRG locus deletions were also determined using data from Illumina CytoSNP-850K arrays.9
Figure 1.
qPCR and SNP array analysis at the TRG locus. (A) ABD status by qPCR TRG:ANLN fold change of WGA diagnostic samples from 149 patients. Three patients with an indeterminate result are not shown as their blast counts were <50%. (B) Representative Log-R ratio plot at the TRG locus (GRCh37/hg19 chr7:37868112-38678273) from CytoSNP-850K arrays for an ABD and (C) a non-ABD patient. Location of the V-J region amplified in the qPCR assay is shown. ABD: Absence of Biallelic Deletion; qPCR: quantitative polymerase chain reaction; IND: indeterminate.
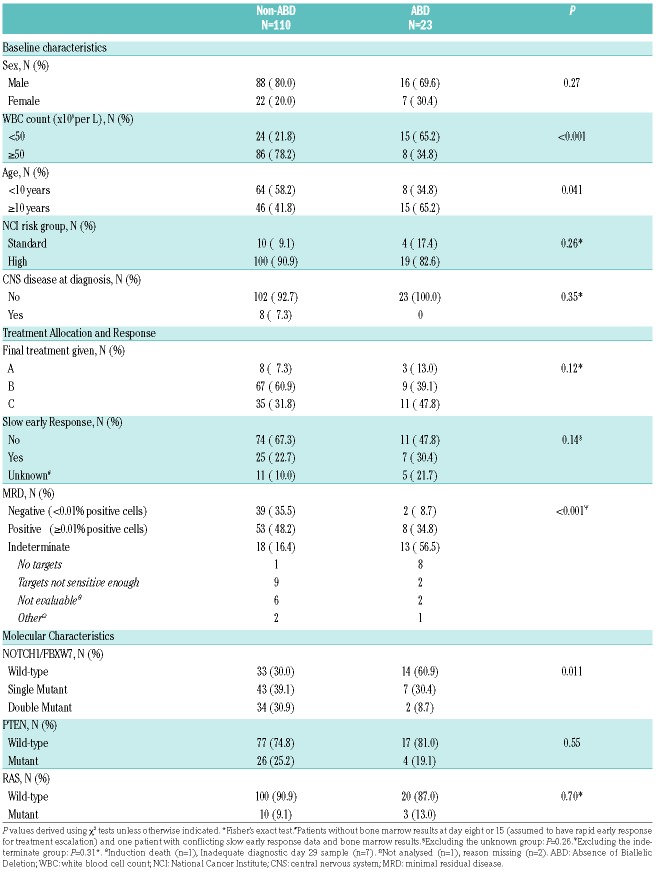
By qPCR, 23 of 152 samples (15%) were classified as ABD, 110 (72%) as non-ABD, and 19 (13%) were indeterminate (16 with fold change 0.26–0.49; three with fold change >0.5 but blast count <50%) (Figure 1A). Baseline characteristics and survival of the 19 patients with indeterminate status were similar to the other 133 patients (Online Supplementary Table S3 and Table S4, and Online Supplementary Figure S3). Baseline characteristics of the 133 patients according to ABD status are outlined in Table 1. Of these, 118 also had single nucleotide polymorphism (SNP) array results at the TRG locus that were concordant with the qPCR findings (Figure 1B,C), including 22 ABD patients; array data was uninterpretable or not available for the remaining 15 cases.
Table 1.
Baseline characteristics, treatment allocation, response and molecular characterization of the ABD and non-ABD groups.
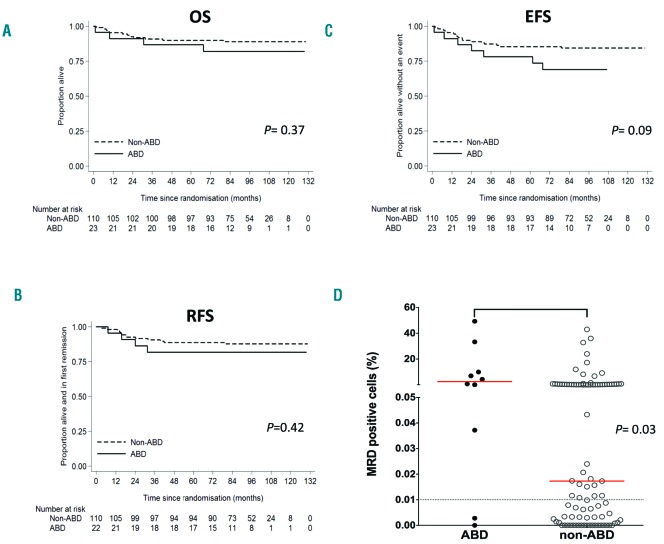
However, there was no statistically significant difference in overall survival (OS) between the ABD and non-ABD groups (5-year OS, 87% vs. 90%, respectively; hazard ratio (HR) 1.67, 95% confidence intervals 0.54–5.17; P=0.37) (Figure 2A; Online Supplementary Table S5). There was also no significant difference in the relapse-free survival (RFS) (82% vs. 89%; HR 1.58, 0.52–4.86; P=0.42) (Figure 2B; Online Supplementary Table S5). Although there was a trend towards an inferior event-free survival (EFS) in the ABD group, this did not reach statistical significance (77% vs. 85%; HR 2.12, 0.88–5.12; P=0.09) (Figure 2C; Online Supplementary Table S5), and was impacted by seven non-relapse events in the ABD group (one infection-related death at induction, four deaths from causes other than ALL and two second malignancies).
Figure 2.
Outcome and MRD-positivity in patients grouped according to their ABD status. Kaplan-Meier curves for (A) OS, (B) RFS and (C) EFS. (D) Scatter-plot showing percentage of MRD-positive cells at day 29. Patients with ≥0.01% positive cells were classified as MRD-positive. Red line: median level. OS: overall survival; EFS: event-free survival; RFS: relapse-free survival; ABD: Absence of Biallelic Deletion; MRD: minimal residual disease.
We have previously reported that patients treated on this trial with T-ALL and NOTCH1/FBXW7 double-mutant status had excellent OS (100%).10 However, the comparable survival of the ABD and non-ABD groups in our cohort could not be explained by this molecular subtype, since only two of the 23 ABD patients (9%) had NOTCH1/FBXW7 double-mutant status (Table 1).
There was, however, a significant association between MRD levels and ABD status. Only 9% of the ABD patients were MRD-negative at day 29 compared with 35% of the non-ABD patients (P=0.01, Fisher’s exact test). In addition, patients with ABD status and MRD results had higher median MRD levels compared with those with non-ABD status (2.519% vs. 0.0173% positive cells; P=0.03) (Figure 2D), suggesting that ABD status may highlight a high-risk group that is already identified by high MRD levels.
Moreover, more than three times as many ABD patients had an MRD indeterminate status compared to the non-ABD group (57% vs. 16%; P<0.001, Mantel-Haenszel test) (Table 1). This was mainly due to the fact that a higher proportion of ABD patients lacked MRD targets at diagnosis than the non-ABD patients (38% vs. 1%, respectively; P<0.001, Fisher’s exact test). MRD was evaluated by real-time qPCR analysis of the T-cell receptor gene rearrangement, and failure to detect a gene rearrangement target as an MRD marker in the ABD group is consistent with the same underlying biology as that of the ABD status, where developmental arrest occurs prior to V-J recombination. To address whether ABD status might be a useful alternative prognostic marker in this MRD indeterminate group, we analyzed outcome in this subgroup of patients. Our results show that in the MRD indeterminate cases who were eligible for RFS analysis, only three of 18 non-ABD (17%) and one of 12 ABD (8.3%) relapsed. Notably, MRD indeterminate status itself directed treatment intensity as these patients were not eligible on the trial for randomization to reduction of chemotherapy intensity. Thus, numbers are too small to make firm conclusions on the additional prognostic significance of ABD in this subgroup, which would need to be addressed in a larger prospective trial.
Our data is in marked contrast to the results from another study where the ABD subgroup, identified using the same TRG qPCR assay, was associated with a dismal outcome (5-year OS: 25% vs. 72% in the ABD and non-ABD groups, respectively).6 However, these patients were not treated using MRD-directed therapy, suggesting that differences in treatment protocols may impact on the prognosis of this subgroup. Within the ABD group of our cohort, 11 patients (48%) received the more intensive chemotherapy arm, Regimen C, including all eight MRD-positive patients, although none of the ABD patients proceeded to an allogeneic stem cell transplant in first remission. Notably, there was no statistical difference in the RFS of ABD and non-ABD patients treated on Regimen C (P=0.21) (Online Supplementary Figure S4A, Online Supplementary Table S6). The RFS for ABD patients treated on Regimens A or B was 100%, although it should be noted that none of them were MRD-positive (Online Supplementary Figure S4B, Online Supplementary Table S6). There was a trend towards an increased risk of relapse in MRD-positive ABD patients when compared to MRD-positive non-ABD patients (HR 3.22, 0.83–12.52; P=0.07) (Online Supplementary Figure S4C), which might relate to higher median MRD levels.11
The comparable survival of the ABD and non-ABD groups treated on the UKALL2003 trial is consistent with the outcome reported for patients on this trial according to their ETP status by immunophenotyping.12 Moreover, the comparable survival between these two groups is also consistent with the outcome reported for ETP and non-ETP patients from other pediatric MRD risk-directed studies.13,14 In addition to this, our findings are similar to those recently reported for ABD status in adult T-ALL patients treated using response-based risk stratification and therapy intensification, including allogeneic stem cell transplantation.15
In conclusion, our data indicate that in pediatric/adolescent T-ALL, ABD status does not add further prognostic information nor justify treatment escalation beyond what can already be inferred by MRD analysis using a risk-adapted protocol.
Supplementary Material
Acknowledgements
The authors would like to thank Great Ormond Street Hospital Children’s Charity, Cancer Research UK and Bloodwise charities for funding and supporting this work.
Footnotes
Funding: this work was undertaken at UCL which receives a proportion of funding from the Department of Health’s NIHR Biomedical Research Centre’s funding scheme. Primary childhood leukemia samples used in the study were provided by the Bloodwise Childhood Leukaemia Cell Bank. The trial was funded by the UK MRC and Leukaemia and Lymphoma Research (now Bloodwise). The authors are grateful to all the hospital centers, clinical staff and patients who participated in the UKALL2003 trial.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. [DOI] [PubMed] [Google Scholar]
- 2.Goulden NJ, Knechtli CJC, Garland RJ, et al. Minimal residual disease analysis for the prediction of relapse in children with standard-risk acute lymphoblastic leukaemia. Br J Haematol. 1998;100(1):235–244. [DOI] [PubMed] [Google Scholar]
- 3.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain N, Lamb AV, O’Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127(15):1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuurbier L, Gutierrez A, Mullighan CG, et al. Immature MEF2C-dysregulated T-cell leukemia patients have an early T-cell precursor acute lymphoblastic leukemia gene signature and typically have non-rearranged T-cell receptors. Haematologica. 2014;99(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3816–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dik WA, Pike-Overzet K, Weerkamp F, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201(11):1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson S, Kirkwood AA, Goulden N, Vora A, Linch DC, Gale RE. Impact of PTEN abnormalities on outcome in pediatric patients with T-cell acute lymphoblastic leukemia treated on the MRC UKALL2003 trial. Leukemia. 2016;30(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkinson S, Koo K, Mansour MR, et al. Impact of NOTCH1/FBXW7 mutations on outcome in pediatric T-cell acute lymphoblastic leukemia patients treated on the MRC UKALL 2003 trial. Leukemia. 2013;27(1):41–47. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor D, Moorman AV, Wade R, et al. Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2017;35(6):660–667. [DOI] [PubMed] [Google Scholar]
- 12.Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166(3):421–424 [DOI] [PubMed] [Google Scholar]
- 13.Wood BL, Winter SS, Dunsmore KP, et al. T-lymphoblastic leukemia (T- ALL) shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype, and validation of the prognostic value of end-induction minimal residual disease (MRD) in Children’s Oncology Group (COG) Study AALL0434. Blood. 2014; 124(21):1.24993873 [Google Scholar]
- 14.Conter V, Valsecchi MG, Buldini B, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: a retrospective analysis. Lancet Haematol. 2016;3(2):e80–86. [DOI] [PubMed] [Google Scholar]
- 15.Bond J, Graux C, Lhermitte L, et al. Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a Group for Research on Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2017;35(23):2683–2691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.