Abstract
OBJECTIVE
To determine the safety and pharmacokinetics of alpha-1 antitrypsin (AAT) in adults and children.
RESEARCH DESIGN AND METHODS
Short term AAT treatment restores euglycemia in the non-obese mouse model of type 1 diabetes. A phase I multicenter study in 16 subjects with new-onset type 1 diabetes studied the safety and pharmacokinetics of Aralast NP™ (AAT). This open-label, dose-escalation study enrolled eight adults ages 16–35 and eight children ages 8–15 within 100 days of diagnosis, to receive 12 infusions of AAT: a low dose of 45 mg/kg weekly for six weeks, followed by a higher dose of 90 mg/kg for six weeks.
RESULTS
C-peptide secretion during a mixed meal, HbA1c, and insulin usage remained relatively stable during the treatment period. At 72 hours after infusion of 90 mg/kg, mean levels of AAT fell below 2.0 g/L for 7 of 15 of the subjects. To identify a plasma level of AAT likely to be therapeutic, pharmacodynamic ex vivo assays were performed on fresh whole blood from adult subjects. PCR analyses were performed on inhibitor of IKBKE, NOD1, TLR1, and TRAD gene expression, which are important for activation of NF-κB and apoptosis pathways. AAT suppressed expression dose-dependently; 50% inhibition was achieved in the 2.5–5.0 mg/mL range.
CONCLUSIONS
AAT was well tolerated and safe in subjects with new-onset type 1 diabetes. Weekly doses of AAT greater than 90 mg/kg may be necessary for an optimal therapeutic effect.
Keywords: Diabetes Mellitus, Type 1, C-Peptide, Autoimmune Diseases, Pharmacokinetics, Serine Proteinase Inhibitors
INTRODUCTION
As maintenance of normal or near-normal glucose levels prevents the devastating complications of type 1 diabetes (1), a priority is to inhibit progression of the immune-mediated destruction of beta cells. While immunosuppression with cyclosporine may slow loss of beta cell function (2), drug-induced nephrotoxicity precludes clinical application. Other immune system interventions (3, 4), have slowed the tempo of beta cell destruction in subjects with recently diagnosed type 1 diabetes. These approaches have included monoclonal antibodies to CD3, a component of the T cell antigen receptor complex (5, 6), abatacept, an inhibitor of T cell costimulatory signals (7), and alefacept, a CD2-directed LFA-3/Fc fusion protein that targets memory and effector CD4 and CD8 cells (8). Although type 1 diabetes is widely believed to be caused by T cell-dependent autoimmunity, depletion of B cells using rituximab (9) also shows some efficacy.
Each of these interventions targeted some aspect of the adaptive immune system, and interactions between innate and adaptive immunity profoundly impact the adaptive immune response to antigen (10). Indeed, the texture of the innate immune system in the microenvironment in which T cells recognize antigen directs differentiation and maintenance of activated T cells into distinct effector or regulatory phenotypic subsets (11). The macrophage-rich invasive insulitis process is prominent during the pathogenesis of type 1 diabetes (12). These data suggest that blocking pro-inflammatory innate immune responses may be efficacious in treating type 1 diabetes.
Treatment with a tumor necrosis factor (TNF)-α receptor fusion protein, etanercept, in children with new-onset type 1 diabetes, may protect residual beta cell function (13). Likewise, treatment with anti-TNFα in non-obese diabetic (NOD) mice restored euglycemia and self-tolerance (14). In contrast, blockade of interleukin-1beta effects failed to halt progression of type 1 diabetes (15).
Alpha-1 antitrypsin (AAT), a member of the serpin superfamily, is an acute phase protein produced by the liver and is present in circulating blood (16). Inflammatory cytokines upregulate production of AAT by the liver and in a positive feed-back loop, AAT, a serine protease inhibitor, blunts expression of various pro-inflammatory cytokines and activation of the NF-κB pathway. Notably, AAT has been safely and effectively used therapeutically in humans with AAT deficiency for over 25 years (17). Treatment with human AAT prevents islet allograft rejection in mice with chemically induced diabetes (18) and reverses new-onset diabetes in NOD mice with restoration of self-tolerance (19). In this study, genome-wide arrays were analyzed by a systems biology approach. The results indicated that treatment blunted disease-associated expression of important pro-inflammatory molecular hubs, including TNF-α and NF-κB (19). Because of this remarkable result in NOD mice, and its safety record in AAT-deficient patients, the RETAIN trial was undertaken to determine the efficacy of AAT in subjects with new-onset type 1 diabetes. The purpose of this phase I study was twofold: 1) To establish the safety of weekly intravenous infusions of AAT at 45 mg/kg/wk for the first six weeks and 90 mg/kg/wk for the second six weeks in adult and pediatric participants with new-onset type 1 diabetes. 2) To determine the pharmacokinetics (PK) of AAT in adult and pediatric participants, and develop a pharmacokinetics/pharmacodynamics (PK/PD) model that may inform dosing regimens in future studies.
METHODS
Study design and participants
This was a phase I open-label, dose-escalation, safety, and PK study in which children and adults with new-onset type 1 diabetes received 12 intravenous infusions of AAT (Supplementary Figure 1). Enrollment was open between October 13, 2010 and July 20, 2011, and participants were enrolled from the ten participating clinical centers. Infusions one through six were administered at 45 mg/kg/wk, and infusions seven through 12 were administered at 90 mg/kg/wk. There were two cohorts of patients with new-onset type 1 diabetes: an adult cohort (eight participants age 16–35 years) and a pediatric cohort (eight pediatric patients age 8–15 years). Follow-up was completed on July 25, 2013. The complete protocol can be found in the supplementary material and on TrialShare (www.itntrialshare.org).
Diabetic subjects that tested positive for islet autoantibodies, e.g., anti-insulin, anti-glutamate decarboxylase, anti-islet-cell antigen 512 (ICA-512), or anti-ZnT8, within 10 days of onset of insulin therapy were eligible for the study if their peak stimulated C-peptide after a mixed meal tolerance test (MMTT) exceeded 0.2 pmol/mL and they were within 100 days of diagnosis.
Exclusion criteria included: positive serology for hepatitis B, hepatitis C, HIV, or toxoplasmosis, or clinically active infection with Epstein-Barr virus, Cytomegalovirus, or tuberculosis; a history of serious cardiac, vascular, pulmonary, hepatic, or renal disease; a history of bleeding tendencies or thromboembolic events; leukopenia, thrombocytopenia, neutropenia, anemia, IgA deficiency, or impaired liver or kidney function; and known hypersensitivity to AAT or other serum products.
The protocol and consent documents were approved by independent institutional review boards. The participants or their parents provided written informed consent, and those under age 18 provided assent. An independent data safety monitoring board held regular safety reviews.
The safety sample includes all subjects who received any study treatment. Enrolled refers to any subject who enrolled at the screening visit. One pediatric subject enrolled but withdrew consent shortly thereafter and did not receive treatment. The characteristics of the patients are summarized in Table 1.
Table 1.
Baseline demographics and laboratory characteristics (All Treated)
| Characteristic Statistic or Category |
Adults (N=8) | Pediatrics (N=8) | Total (N=16) |
|---|---|---|---|
| Age (years) | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 20.6 (5.73) | 11.0 (2.07) | 15.8 (6.48) |
| Median (min, max) | 18.5 (16, 33) | 11.5 (8, 13) | 14.5 (8, 33) |
|
| |||
| Sex | |||
| Male | 5 (62.5) | 5 (62.5) | 10 (62.5) |
| Female | 3 (37.5) | 3 (37.5) | 6 (37.5) |
|
| |||
| Primary Race | |||
| White | 7 (87.5) | 8 (100) | 15 (93.8) |
| Black or African American | 1 (12.5) | 0 (0) | 1 (6.3) |
|
| |||
| Ethnicity | |||
| Not Hispanic or Latino | 7 (87.5) | 8 (100) | 15 (93.8) |
| Unknown | 1 (12.5) | 0 (0) | 1 (6.3) |
|
| |||
| Weight (kg) | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 69.6 (15.18) | 44.3 (8.28) | 57.0 (17.60) |
| Median (min, max) | 69.3 (48, 94) | 42.4 (36, 56) | 52.9 (36, 94) |
|
| |||
| Days Since Diagnosis | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 70.5 (30.11) | 64.4 (32.24) | 67.4 (30.30) |
| Median (min, max) | 75.5 (29, 102) | 67.5 (20, 99) | 69.0 (20, 102) |
|
| |||
| Baseline C-peptide Mean 2-hour AUC: pmol/mL [ng/mL] | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 0.71 (0.390) [2.14 (1.178)] | 0.72 (0.297) [2.17 (0.897)] | 0.71 (0.335) [2.16 (1.012)] |
| Median (min, max) | 0.60 (0.09, 1.38) [1.82 (0.28, 4.16)] | 0.72 (0.18, 1.14) [2.16 (0.56, 3.44)] | 0.65 (0.09, 1.38) [1.96 (0.28, 4.16)] |
|
| |||
| Baseline Peak C-peptide: pmol/mL [ng/mL] | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 0.98 (0.554) [2.97 (1.673)] | 0.94 (0.394) [2.85 (1.189)] | 0.96 (0.465) [2.91 (1.403)] |
| Median (min, max) | 0.92 (0.13, 1.95) [2.77 (0.40, 5.89)] | 0.91 (0.32, 1.64) [2.74 (0.97, 4.95)] | 0.91 (0.13, 1.95) [2.74 (0.40, 5.89)] |
|
| |||
| Baseline HbA1c: % [mmol/mol] | |||
| n | 7 | 8 | 15 |
| Mean (SD) | 7.93 (1.980) [63.16 (21.638)] | 8.05 (1.592) [64.49 (17.400)] | 7.99 (1.718) [63.87 (18.775)] |
| Median (min, max) | 7.20 (6.1, 11.6) [55.20 (43.2, 103.3)] | 7.70 (6.3, 10.8) [60.66 (45.4, 94.5)] | 7.60 (6.1, 11.6) [59.57 (43.2, 103.3)] |
|
| |||
| Baseline Insulin Use (units/day/kg) | |||
| n | 8 | 8 | 16 |
| Mean (SD) | 0.22 (0.170) | 0.30 (0.313) | 0.26 (0.247) |
| Median (min, max) | 0.16 (0.0, 0.5) | 0.23 (0.0, 0.8) | 0.17 (0.0, 0.8) |
AUC = area under the curve; max = maximum; min = minimum; SD = standard deviation
Note: Percentages are based on the total number of enrolled subjects.
Procedures
Patients were admitted to a clinical research center for continuous observation during the infusion period. The first infusion of AAT (Aralast NP™, Baxter AG) was given at a rate of 0.08 mL/kg/min for the entire infusion. For the second infusion the starting rate was 0.08 mL/kg/min, which was increased to 0.14 mL/kg/min, and then up to 0.2 mL/kg/min if tolerated.
Adult cohort
The first cohort, comprised of eight participants whose ages ranged from 16–35 years, received AAT 45 mg/kg via IV infusion weekly for six weeks. One participant discontinued treatment before the completing the course of low-dose infusions but completed all follow-up visits. After completing the week-6 course of infusions, the remaining 7 participants underwent a 3-week washout period during which the protocol chair, the National Institute of Allergy and Infectious Diseases (NIAID) medical monitor, and ITN clinical trial physician reviewed safety data. Subsequently, each participant received 90 mg/kg/wk for the six weeks, for a total of 12 infusions. When all participants completed all of the infusions, a second safety review was performed.
Pediatric cohort
The pediatric cohort began enrolling after six participants in the adult cohort underwent the safety review and proceeded to the high dose. This cohort consisted of eight pediatric participants, age 8–15 years, who received AAT 45 mg/kg weekly for six weeks. One participant did not receive the 4th low-dose infusion because an elevated D-dimer level was detected, but the participant completed all other infusions. After completing the week-6 infusion, each participant underwent a minimum 3-week washout period. When at least six participants from the pediatric cohort had safely completed the low dose and at least six participants from the adult cohort had safely completed the high dose, dose escalation to 90 mg/kg/wk in the pediatric cohort proceeded for the next six weeks, for a total of 12 infusions. Safety reviews were performed as outlined for the adult cohort. All of the pediatric participants were cared for by pediatric endocrinologists and received a “team care” approach.
Clinical evaluations
Hemoglobin A1c
Hemoglobin A1c (HbA1c) levels were obtained at the screening visit, and before the 6th low-dose and 6th high-dose infusions. In addition, HbA1c levels were measured at 19 and 32 weeks after the first high-dose infusion, and 52, 65, 78, 91, and 104 weeks after the first low-dose infusion.
Insulin use
Insulin use was self-reported and recorded for five days leading up to each follow-up, screening, and infusion visit. In addition, usage was recorded at follow-up visits at 8, 19, and 32 weeks after the first high-dose infusion, and 52, 65, 78, 91, and 104 weeks after the first low-dose infusion.
C-peptide
Plasma C-peptide levels (ng/mL) were assessed during 2-hour or 4-hour MMTTs employing Boost High Protein Nutritional Energy Drink® (Mead-Johnson), and were performed after an overnight fast. Four-hour tests were done at the screening visit and at weeks 52 and 104. Two-hour tests were done eight and 19 weeks after the first high-dose infusion. For the 2-hour test, C-peptide levels were measured at −10, 0, 15, 30, 60, 90, and 120 minutes in relation to the baseline time of consumption of the mixed meal. The 2-hour mean area-under-the-curve (mAUC) was calculated by dividing the area under the curve (AUC) of plasma C-peptide levels from the 2-hour MMTT by 120 minutes. For this analysis, only the first 2 hours of a 4-hour test were used. The area under the curve (AUC) was computed using the trapezoidal rule. For this computation, the ‘time 0’ C-peptide values were taken as the average of C-peptide values measured at time points −10 and 0 minutes.
Biochemical assays
Biochemical autoantibody titers were assayed at Barbara Davis Center (Aurora, CO, USA) with radioimmunobinding assays. C-peptide concentrations were measured at the Northwest Lipid Research Laboratory (Seattle, WA, USA). Serum chemistry, hematology, viral load, and serology tests were done at a central laboratory (ICON Central Labs, Farmingdale, NY, USA).
Pharmacodynamic ex vivo assays
Peripheral blood mononuclear cells were obtained from subjects participating in studies under the auspices of Benaroya Research Institute-Juvenile Diabetes Research Foundation Center for Translational Research; these subjects were not participants in the RETAIN trial. Written informed consent was obtained from all subjects according to IRB-approved protocols at the Benaroya Seattle, WA, USA. Samples for all experiments were provided to the investigator by clinical core in a blinded manner. Subjects ranged in age from 23–39 (27.8 ± 4.9, mean ± SD), were 13–134 months post type 1 diabetes diagnosis (55.4 ± 45.5, mean ± SD), were 33% female, and all were Caucasian carrying type 1 diabetes-associated HLA-risk alleles. Fresh whole blood was incubated for three hours immediately after blood was drawn with increasing doses of AAT (0–6 mg/mL) or PBS to normalize volumes for serial dilutions of AAT; then Tempus reagent was added and samples were stored at −80°C prior to assay in batches. RNA was isolated and expression of select inflammatory and apoptotic genes thought to be relevant to the pathogenesis of type 1 diabetes were evaluated. The cDNA was synthesized using Invitrogen Superscript VILO RT. cDNA was then amplified with specific primers for signal transducer and activator of transcription 1 (STAT1), nucleotide binding oligomerization domain containing 1 (NOD1), nuclear factor kappa-B kinase subunit epsilon (IKBKE), toll like receptor 1 (TLR1), and TNFRSF1A associated via death domain (TRADD) using a robot-assisted 384-well plate format on an ABI 7900 in fast mode. Fold change was calculated using the ΔΔCt method (20) normalizing transcripts to the mean of the most stable housekeeping genes, RPL36AL or RPLPO. Assay-specific responses were normalized to no AAT treatment. Paired t-test was used to compare each AAT concentration to no AAT (blood incubated with PBS). For a subset of type 1 diabetes subjects, whole blood was stimulated with 100 ng/mL lipopolysaccharide (LPS) (Sigma L2887, Sigma-Aldrich, St. Louis, MO, USA) at 37°C in sterile round-bottom tubes in the presence of various concentrations of AAT. After 18 hours, cells were lysed with 0.5% Triton X-100, vortexed and stored at −80°C. Cytokines in supernatants were assayed by Luminex (BioRad, Hercules, CA, USA) in triplicate.
AAT pharmacokinetics
Plasma samples for total AAT analysis were collected for the first and last low- and high-dose AAT infusions taken at 30 minutes prior to infusion and at the following times post infusion: 10 minutes, 6 hours, 24 hours, 48 hours, 72 hours, 120 hours, and 168 hours. Plasma AAT levels were determined by ELISA in the laboratory of Baxter Innovations GmbH (Vienna, Austria). The total plasma AAT concentrations measured included both the endogenous AAT and the infused AAT.
To adjust for exogenous levels of AAT, baseline values for each subject were subtracted from the concentration levels measured at each time point prior to analysis. The pre-dosing concentration prior to the first low-dose infusion was used as the baseline value.
Dose projections assumed a linear relationship between the change from baseline concentration levels and dose (dose proportionality). This assumption was based on historic results from the drug manufacturer and our current observations. Projections at higher doses were made using the last 90 mg/kg dose of AAT as the basis. Assuming dose proportionality, concentration levels at higher doses were calculated by multiplying the amount of increase per mg/kg by the amount of the higher dose. Geometric means and confidence limits were calculated on the sum of the resulting increase and the baseline value per subject.
RESULTS
Clinical outcomes
We screened 26 patients <100 days from diagnosis and enrolled 16 (Supplementary Figure 1). Infusions of AAT were given to eight adult and eight pediatric subjects, with the first infusion in February 2011 and the last in February 2012. Fourteen subjects completed all infusions, six infusions at the low dose (45 mg/kg/wk), and six infusions at the high dose (90 mg/kg/wk); one participant in the adult cohort discontinued drug administration after the 2nd low-dose infusion due to a suspected drug rash (grade 2), and one participant in the pediatric cohort did not receive the 4th low-dose infusion (elevated D-dimer) but completed all other infusions.
C-peptide responses
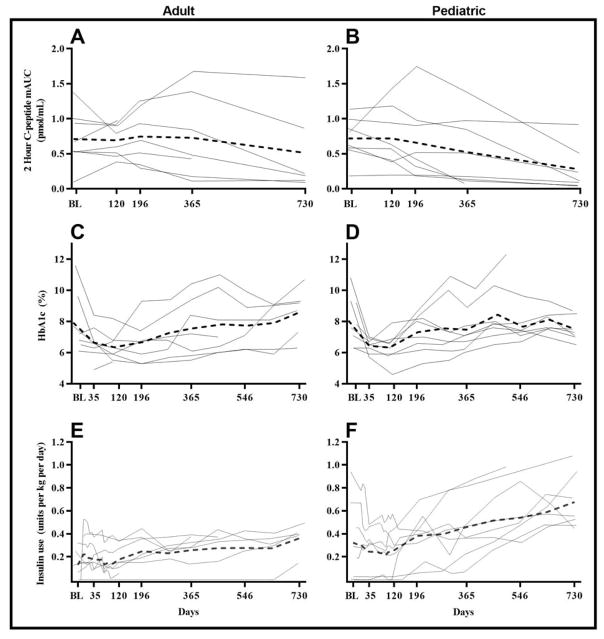
At week 52, for the adult cohort the mean (SD) for the C-peptide two-hour mAUC of the MMTT was 0.73 (0.60) pmol/mL [2.20 (1.82) ng/mL] (Figure 1, Panel A), which represented a mean (SD) rise from baseline of +0.013 (0.43) pmol/mL [+0.04 (1.30) ng/mL]. Although average C-peptide two-hour mAUC values were relatively stable over 52 weeks, those for individual subjects were variable.
Figure 1.
The means of C-peptide 2-hour AUC, HbA1c and insulin use (units per kg per day) over 730 days. A. C. & E. Adult cohort. B, D & F. Pediatric cohort. The means of the adult and pediatric cohorts are indicated by dotted lines, respectively. C-peptide mean 2-hour AUC is calculated as the area under the curve of C-peptide from 2-hour mixed meal tolerance test divided by the duration of test (120 minutes).
In contrast to the adult cohort, the average C-peptide two-hour mAUC over the first 52 weeks declined in the pediatric cohort (Figure 1, Panel B). In the pediatric cohort at week 52, the actual C-peptide two-hour mAUC was 0.53 (0.49) pmol/mL [1.60 (1.48) ng/mL], which was a mean (SD) change from baseline of −0.19 (0.41) pmol/mL [−0.58 (1.23) ng/mL].
HbA1c profiles
HbA1c values over time (in study days) are plotted by subject in Figure 1. Means of adult (Panel C) and pediatric (Panel D) cohorts at each visit are also plotted. Following stabilization of HbA1c levels during the early new-onset period, mean HbA1c levels for both the adult and pediatric cohorts remained stable during the infusion period and then gradually increased throughout the subsequent observation period. During the first 52 weeks, mean HbA1c levels generally remained in the range 6.5–7.5% (48–58 mmol/mol), but two subjects in the adult cohort and two subjects in the pediatric cohort, who were in the adolescence age range showed substantial increases in HbA1c levels after days 100–200, perhaps due to some combination of compliance and the insulin resistance of adolescence.
Insulin usage
Analysis of insulin use was done using the average use over the five days leading up to the visit, divided by the weight in kilograms of the subject at the visit. Insulin use profiles over time (in study days) are plotted for each subject in Figure 1 (Panels E and F). Mean daily insulin use generally remained in the range 0.2–0.4 U/kg/day for both cohorts during the first 12 months, although the pediatric cohort tended to have a higher mean insulin use than the adult cohort, driven in part by two pediatric subjects whose insulin use drifted up to 0.8 U/kg/day at week 52. Beyond the 1-year period, there was a gradual increase in mean insulin use, which was modest in the adult cohort but greater and more variable in the pediatric cohort.
Safety
A total of 448 adverse events (AEs) were reported during the study. More AEs were reported in the pediatric cohort (319 events) than in the adult cohort (129 events). A smaller proportion of study drug-related AEs were reported in the pediatric cohort than in the adult cohort (17.2% [55 events] versus 41.1% [53 events]). Supplementary Table 1 contains a summary of these findings. At least one quarter of the safety sample reported the following AEs: hypoglycemia (93.8%), headache (37.5%), cough (31.3%), nasal congestion (31.3%), nausea (31.3%), contusion (25.0%), nasopharyngitis (25.0%), pyrexia (25.0%), rash (25.0%), upper respiratory tract infection (25.0%), and vomiting (25.0%) (Supplemental Table 2). Supplementary Table 1 notes there were 11 grade 3 events (nine hypoglycemias, one diabetic ketoacidosis, and one musculoskeletal pain event) and one grade 4 event (hypoglycemia). Of these, there were three reported serious adverse events (SAEs) during the post-treatment phase of the study (Supplemental Table 3). The adult subject with musculoskeletal pain was briefly hospitalized, as was the pediatric subject with diabetic ketoacidosis. The grade 4 hypoglycemic event in an adult subject was assessed as a significant medical event but did not require emergency intervention or hospitalization. None of these events were judged to be related to study therapy. One AE consisting of a rash was reported in the adult cohort and contributed to the investigator’s decision to discontinue study drug in that subject. No deaths were reported.
Ex vivo AAT pharmacodynamic assays
AAT inhibits neutrophil superoxide production (21), hepatocyte apoptosis (22), LPS-induced monocyte activation (23), and pro-inflammatory cytokine expression (24, 25). To further understand the mechanisms underlying the anti-inflammatory properties of AAT in the context of type 1 diabetes and the RETAIN trial, ex vivo PD assays were performed using fresh whole blood from adult type 1 diabetes subjects not enrolled in the RETAIN study. In the first set of experiments, arrays of 40 transcripts were assessed from three different subjects using a Fluidigm Biomark. Twenty-two genes from the test set were strongly affected by AAT (data not shown). From these preliminary analyses, IKBKE, NOD1, TLR1, and TRADD were selected for subsequent testing by quantitative real-time polymerase chain reaction (PCR) analysis, since these genes, which are involved in NF-κB activation and apoptosis pathways, were most robustly modulated by AAT. In the nine type 1 diabetes subjects tested, AAT suppressed expression of these four genes dose-dependently (Figure 2). Furthermore, ≥ 50% inhibition was achieved with AAT in the 2.5–5.0 mg/mL range. Results from each individual tested are shown in Supplemental Figure 2. As anticipated, AAT also inhibited secretion of IL-6 and IL-1β in fresh whole blood stimulated with bacterial LPS overnight (Supplemental Figure 3), confirming previous reports on the mechanism of action of AAT (23, 24).
Figure 2.
Dose response of AAT on fresh blood. Data are mean +/− SD from 9 Adult subjects with type 1 diabetes (normalized to HK genes + no AAT). Associations between No AAT and different concentrations of AAT are tested using Paired t-test and a p-value <0.05 determined statistical significance. *p<0.05, **p<0.005
AAT pharmacokinetics
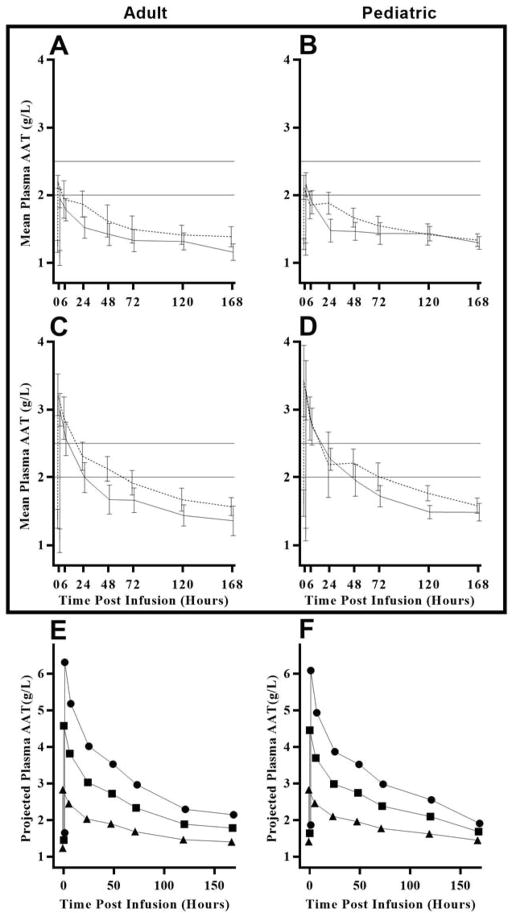
Subjects were given six weekly low-dose infusions of 45 mg/kg AAT and six weekly high-dose infusions of 90 mg/kg. There was a washout period of four weeks in adult subjects after the last low-dose AAT infusion prior to the first high dose infusion. In pediatric subjects, the washout period ranged from 4–8 weeks. Figure 3, Panels A, B, C, and D show mean plasma AAT concentrations over a period of one week (168 hours) for the first and last low and high dose infusions of the adult and pediatric cohorts. At the last high-dose infusion, the maximum concentration, at time 0, for all subjects was above 2.5 g/L, with means of 3.2 and 3.4 g/L for adult and pediatric cohorts, respectively. At 24 hours post-infusion, all seven adult subjects and five pediatric subjects had concentrations between approximately 2.0 and 2.5 g/L. Two pediatric subjects had concentrations of approximately 2.7 g/L, and one pediatric subject had a low concentration of 0.86 g/L at 24 hours post-infusion. At 72 hours post-infusion, concentration levels had dropped below 2.0 g/L for four of seven adults and for three of eight pediatric subjects. All but one subject had a concentration level below 2.0 g/L at 120 hours post-infusion. Note that the pre-infusion baseline AAT values rose after multiple weekly injections. Thus, before the last high dose injection in adults the AAT levels were 0.31 +/− 0.12 g/L higher than baseline, while for the pediatric group the increase was 0.42 +/− 0.22 g/L.
Figure 3.
Observed plasma AAT concentrations at each infusion visit and projected lower 95% confidence limits of plasma AAT blood concentrations at various dose levels by cohort. Panels A, B, C, and D show mean plasma AAT concentrations (solid line indicates first dose and dashed line indicates final dose). Note: Reference lines at 2.0 and 2.5 indicate the target range of concentration levels at 120 hours post infusion. There were six infusions at the low dose (45 mg/kg/wk) and six infusions at the high dose (90 mg/kg/wk). A. Adult cohort, low dose. B. Pediatric cohort, low dose. C. Adult cohort, high dose. D. Pediatric cohort, high dose. Panels E and F projections are based on the assumption of dose proportionality and a constant baseline plasma AAT concentration over time. Concentration levels after the last high dose infusion are used as bases for the projection. E. Adult cohort. F. Pediatric cohort. Projected dose (mg/kg) 90 (triangle), 180 (square), and 270 (circle).
To investigate the effect on plasma AAT concentrations for doses higher than 90 mg/kg, we assumed dose proportionality and that different doses of AAT decline at similar rates. Support for these assumptions is provided in Supplemental Figure 4. Dose projections are shown in Figure 3, which displays the lower limit of the 95% confidence interval of the projected concentrations at 90, 180, and 270 mg/kg AAT, separately for each cohort. In the adult cohort (Panel E), the lower confidence limit for the 180 mg/kg AAT dose is just below 2·5 g/L at 72 hours and just below 2.0 g/L at 120 hours. In the pediatric cohort (Panel F), the lower confidence limit for 180 mg/kg AAT is likewise just below 2.5 g/L at 72 hours, but is above 2.0 g/L at 120 hours. However, there are caveats. Projections for doses outside the range of doses on which we have data (45 and 90 mg/kg AAT) are inherently uncertain. In addition, these projections are made with assumptions of constant baseline values and dose proportionality. A violation of either of these assumptions would affect the plasma AAT concentration we have projected at higher doses.
Data sets are available for review at www.trialshare.com.
CONCLUSIONS
Various AAT preparations have been used therapeutically for over 20 years without notable side effects. In the present study AAT was given to adults and children with recently diagnosed type 1 diabetes to examine its safety and pharmacokinetics. Subjects were given weekly infusions of AAT, with six of these being 45 mg/kg and the next six being 90 mg/kg.
There was an impressive lack of grade 3 or higher adverse events and none of the three SAE could be related to the study drug. Of 448 adverse events (AE), the majority (38.8%) were instances of hypoglycemia, which are expected when type 1 diabetes is treated with insulin. Others have reported similar safety profiles in new-onset diabetes. In a report by Gottlieb et al (26), 12 recently diagnosed subjects were given eight consecutive weekly infusions of 80 mg/kg of AAT (Aralast, Baxter, Inc.), which were well tolerated and the only significant adverse events were related to hyperglycemia in seven of the 12 subjects. In another study by Rachmiel et al (27), 24 subjects with recent-onset type 1 diabetes received escalating doses 40, 60, or 80 mg/kg of AAT (Glassia, Kamada Ltd.) over a 28-wk period. The drug was well tolerated, although 41 adverse events were judged to be possibly or probably related to the drug, including headache, abdominal pain, and cough.
In the present study, the C-peptide AUC values during the 2-h MMTT remained relatively stable as a mean for the adult group for two years (Figure 2) although responses were quite heterogeneous. In the pediatric group, the decline of the mean was sharper, and fell to very low levels in four of the subjects at two years. Note that in both groups the mean C-peptide levels remained stable during the period that AAT was being administered. While comparisons to historical data should be interpreted with caution, an analysis of Diabetes TrialNet data found that shortly after diagnosis the 2-h C-peptide AUC levels declined at a rate of 0.0172 pmol/mL/month (0.206 per year) (28). In our study the C-peptide AUC had a mean increase of 0.013 for adults and a mean decrease of 0.191 for pediatric subjects over the first year, both lower than 0.206. It is not surprising that C-peptide secretion fell more rapidly in the pediatric group because function falls off more rapidly in younger subjects (28). Because our study had no placebo control it is not possible to conclude that AAT had any beneficial effect. The studies by Gottlieb et al (26) and Rachmiel et al (27) were also not controlled, so firm conclusions about efficacy cannot be made. The possibility that there were responders or non-responders to AAT can also not be evaluated without adequate controls.
A major challenge is determining the minimal concentration of AAT required to have a meaningful anti-inflammatory effect. In the study by Gottlieb et al (26), AAT treatment of recent-onset type 1 diabetes patients with 80 mg/kg led to a reduction of TLR4-induced cellular IL-1β in monocytes, and similar reductions were found with TLR7/8 and TLR3 agonists. Based on studies in rodents using injections of human AAT, we predicted that AAT levels increased from about 1 g/L to the range of 2–2.5 g/L would be sufficient to reduce pro-inflammatory responses and we predicted a dose of 90 mg/kg would accomplish this AAT concentration. Nonetheless, after infusion of 90 mg/kg, AAT levels remained above 2.0 g/L for only a few days.
To further evaluate the dose-related effects of AAT, pharmacodynamic assays were performed using fresh blood from adults with type 1 diabetes. Quantitative real-time PCR analysis revealed that AAT suppressed expression of genes involved in NF-κB activation (IKBKE, TLR1, and NOD1) and apoptosis pathways (TRADD) in all subjects tested. Importantly, ≥ 50% inhibition was achieved with AAT in the 2.5–5.0 mg/mL range. These novel findings offer new insight to the molecular mechanisms responsible for the early effects of AAT on human hematopoietic cells, and may help explain how direct effects of AAT on innate immunity could temper the adaptive response in type 1 diabetes and other inflammatory diseases (29–34). Inclusion of these PCR targets in future dosing studies may help determine dosing appropriate to reduce pro-inflammatory response beyond a few days.
In the NOD mouse model, both short-term treatment with TNF-α pathway blockade (14) and AAT (19) restored euglycemia and self-tolerance. The molecular correlates for this beneficial effect of AAT in the NOD model include inhibition of TNF-α and NF-κB expression as deduced by bioinformatics analysis of genome-wide arrays. Our study shows that AAT can exert similar effects on blood cells obtained from humans with new-onset type 1 diabetes. Unfortunately, drug levels required to achieve this inhibitory effect were not reached in the clinical study. The results shown in Figure 2 suggest that plasma AAT concentrations of more than 2.0 g/L are in a potentially therapeutic range. Because a dose of 90 mg/kg only maintains an AAT concentration over 2.0 g/L for a few days (Figure 3B and F), a higher dose may be required. To make projections on the doses required for improved outcomes, an analysis of proportionality was performed. As blood levels obtained with doses of 45 and 90 mg/kg fell at the same rate we reasoned that a higher dose of 180 mg/kg would have the same profile of decline. This allowed us to make projections of blood levels of AAT when injected at doses of 180 and 270 mg/kg. The projections predicted that the lower confidence limit for the 180 mg/kg AAT dose is just below 2.5 g/L at 72 hours and just below 2.0 g/L at 120 hours in adults, with similar levels projected in children (Figure 3D and H).
In conclusion, the intravenous administration of AAT to adults and children with new-onset type 1 diabetes was well tolerated and safe. However, our PK and PD studies suggest that weekly doses of more than 90 mg/kg of AAT may be required for optimal efficacy.
Supplementary Material
Acknowledgments
We thank all the members of the ITN RETAIN Study Team, who are listed in the Supplemental Materials. We particularly want to make note of the major contributions of Dr. Terry Strom, who died as the paper was being revised. He was an outstanding physician scientist and a major figure in the field of immunology.
Miscellaneous
This study is registered with ClinicalTrials.gov, number NCT01183468.
Contribution statement
GCW and TBS served as study chairs. GCW wrote the first draft of the manuscript. Other members of the writing group included MRE, KMH, SK, LJW, JGM, MK, and TBS. All authors were involved in the conduct of the trial, and the collection and review of the study data. The writing group had full access to all of the data and made the decision to publish the paper. The authors reviewed and commented on various versions of the paper, and the suggested revisions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the funding source
Research reported in this publication was performed as a project of the Immune Tolerance Network and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number UM1AI109565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Rho Federal Systems Division served as the Statistical and Data Coordinating Center under ITN SDCC contract: HHSN272200800029C, and DAIT-SACCC cooperative agreement: 1UM2-AI-11787. The Immune Tolerance Network was responsible for study design, data collection, analysis, and decision to submit the report for publication. Baxter AG provided the drug Aralast NP™ for the study, helped with the design and interpretation of pharmacokinetics study, and performed the ELISA measurements of plasma AAT. The writing team had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest statement
TBS & MK are funded for a study of AAT in the mouse model of type 1 diabetes, hold an issued patent on treatment of type 2 diabetes with AAT, and have applied for a patent on a recombinant AAT fusion protein. MRE reports grants from NIH during the conduct of the study. BJ and LW report a contract from NIH/NIAID DAIT ITN and grants during the conduct of the study from NIH/NIAID DAIT-SACCC.
ABBREVIATIONS
- AAT
Alpha-1 antitrypsin
- AUC
Area under the curve
- AEs
Adverse events
- ICA
Islet-cell antigen
- IKBKE
Nuclear factor kappa-B kinase subunit epsilon
- LPS
Lipopolysaccharide
- mAUC
Mean area-under-the-curve
- MMTT
Mixed Meal Tolerance Test
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institute of Health
- NOD
Non-obese diabetic
- NOD1
Nucleotide binding oligomerization domain containing 1
- PBMC
Peripheral blood mononuclear cell
- PCR
Polymerase chain reaction
- PD
Pharmacokdynamics
- PK
Pharmacokinetics
- SAE
Serious adverse events
- STAT1
Signal transducer and activator of transcription 1
- TLR1
Toll like receptor 1
- TNF
Tumor necrosis factor
- TRADD
TNFRSF1A associated via death domain
Contributor Information
Gordon C. Weir, Joslin Diabetes Center, Harvard Medical School, Boston, MA, 02215.
Mario R. Ehlers, Immune Tolerance Network, Clinical Trials Group, San Francisco, CA, 94107.
Kristina M. Harris, Immune Tolerance Network, Biomarker & Discovery Research, Bethesda, MD, 20814.
Sai Kanaparthi, Immune Tolerance Network, Biomarker & Discovery Research, Bethesda, MD, 20814.
Alice Long, Benaroya Research Institute at Virginia Mason, Translational Research Program, Seattle, WA, 98101.
Deborah Phippard, Immune Tolerance Network, Biomarker & Discovery Research, Bethesda, MD, 20814.
Lia J. Weiner, Rho Federal Systems Division, Chapel Hill, NC, 27517.
Brett Jepson, Rho Federal Systems Division, Chapel Hill, NC, 27517.
James G. McNamara, National Institute of Allergy and Infectious Diseases, Bethesda, MD, 20892.
Maria Koulmanda, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, 02215.
Terry B. Strom, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, 02215.
References
- 1.The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128(7):517–23. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Stiller CR, Dupre J, Gent M, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223:1362–7. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- 3.Rigby MR, Ehlers MR. Targeted immune interventions for type 1 diabetes: not as easy as it looks! Curr Opin Endocrinol Diabetes Obes. 2014;21(4):271–8. doi: 10.1097/MED.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher MP, Goland RS, Greenbaum CJ. Making progress: preserving beta cells in type 1 diabetes. Ann N Y Acad Sci. 2011;1243:119–34. doi: 10.1111/j.1749-6632.2011.06321.x. [DOI] [PubMed] [Google Scholar]
- 5.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54(6):1763–9. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(25):2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 7.Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–9. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1(4):284–94. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11(3):221–30. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, et al. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia. 2007;50(3):596–601. doi: 10.1007/s00125-006-0569-9. [DOI] [PubMed] [Google Scholar]
- 13.Mastrandrea L, Yu J, Behrens T, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32(7):1244–9. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koulmanda M, Bhasin M, Awdeh Z, et al. The role of TNF-alpha in mice with type 1- and 2- diabetes. PLoS One. 2012;7(5):e33254. doi: 10.1371/journal.pone.0033254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran A, Bundy B, Becker DJ, et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381(9881):1905–15. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102(12):4751–804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers MR. Immune-modulating effects of alpha-1 antitrypsin. Biol Chem. 2014;395(10):1187–93. doi: 10.1515/hsz-2014-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A. 2005;102(34):12153–8. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koulmanda M, Bhasin M, Hoffman L, et al. Curative and beta cell regenerative effects of alpha1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc Natl Acad Sci U S A. 2008;105(42):16242–7. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittgen TD. Real-time quantitative PCR. Methods. 2001;25(4):383–5. doi: 10.1006/meth.2001.1260. [DOI] [PubMed] [Google Scholar]
- 21.Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS letters. 1992;300(1):21–4. doi: 10.1016/0014-5793(92)80156-b. [DOI] [PubMed] [Google Scholar]
- 22.Van Molle W, Libert C, Fiers W, Brouckaert P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J Immunol. 1997;159(7):3555–64. [PubMed] [Google Scholar]
- 23.Janciauskiene S, Larsson S, Larsson P, Virtala R, Jansson L, Stevens T. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by alpha1-antitrypsin. Biochem Biophys Res Commun. 2004;321(3):592–600. doi: 10.1016/j.bbrc.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 24.Pott GB, Chan ED, Dinarello CA, Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85(5):886–95. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koulmanda M, Bhasin M, Fan Z, et al. Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci U S A. 2012;109(38):15443–8. doi: 10.1073/pnas.1018366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb PA, Alkanani AK, Michels AW, et al. alpha1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1beta responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(8):E1418–26. doi: 10.1210/jc.2013-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachmiel M, Strauss P, Dror N, et al. Alpha-1 antitrypsin therapy is safe and well tolerated in children and adolescents with recent onset type 1 diabetes mellitus. Pediatr Diabetes. 2015 doi: 10.1111/pedi.12283. [DOI] [PubMed] [Google Scholar]
- 28.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61(8):2066–73. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallois D, Grimm CH, Avner P, Boitard C, Rogner UC. The type 1 diabetes locus Idd6 controls TLR1 expression. J Immunol. 2007;179(6):3896–903. doi: 10.4049/jimmunol.179.6.3896. [DOI] [PubMed] [Google Scholar]
- 30.Moreno L, Gatheral T. Therapeutic targeting of NOD1 receptors. Br J Pharmacol. 2013;170(3):475–85. doi: 10.1111/bph.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieto P, Vallejo-Cremades MT, Benito G, et al. NOD1 receptor is up-regulated in diabetic human and murine myocardium. Clin Sci (Lond) 2014;127(12):665–77. doi: 10.1042/CS20140180. [DOI] [PubMed] [Google Scholar]
- 32.Cuda C, Badawi A, Karmali M, El-Sohemy A. Effects of polymorphisms in nucleotide-binding oligomerization domains 1 and 2 on biomarkers of the metabolic syndrome and type II diabetes. Genes Nutr. 2012;7(3):427–35. doi: 10.1007/s12263-012-0287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schertzer JD, Tamrakar AK, Magalhaes JG, et al. NOD1 activators link innate immunity to insulin resistance. Diabetes. 2011;60(9):2206–15. doi: 10.2337/db11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.