Abstract
Research has increasingly highlighted the role that developmental plasticity—the ability of a particular genotype to produce variable phenotypes in response to different early environments—plays as an adaptive mechanism. One of the most widely studied genetic contributors to developmental plasticity in humans and rhesus macaques is a serotonin transporter gene-linked polymorphic region (5-HTTLPR), which determines transcriptional efficiency of the serotonin transporter gene in vitro and modifies the availability of synaptic serotonin in these species. A majority of studies to date have shown that carriers of a loss-of-function variant of the 5-HTTLPR, the short (s) allele, develop a stress-reactive phenotype in response to adverse early environments compared with long (l) allele homozygotes, leading to the prevalent conceptualization of the s-allele as a vulnerability allele. However, this framework fails to address the independent evolution of these loss-of-function mutations in both humans and macaques as well as the high population prevalence of s-alleles in both species. Here we show in free-ranging rhesus macaques that s-allele carriers benefit more from supportive early social environments than l-allele homozygotes, such that s-allele carriers which receive higher levels of maternal protection during infancy demonstrate greater social competence later in life. These findings provide, to our knowledge, the first empirical support for the assertion that the s-allele grants high undirected biological sensitivity to context in primates and suggest a mechanism through which the 5-HTTLPR s-allele is maintained in primate populations.
Keywords: 5-HTTLPR, G×E, maternal care, social play, rhesus macaque, developmental plasticity
1. Introduction
Natural selection can favour individuals that exhibit flexible responses to environmental contexts [1]. Such plasticity is necessary to respond to social and ecological heterogeneity, and at a species level, is often associated with widespread ecological adaptation [2–7]. Exemplars of the relationship between plasticity and ecological adaption include particular species of the macaque genus (Macaca) which are known for their widespread geographical distribution and ‘weed-like' persistence [4,8]. In these species, genetic variations which influence serotonergic signalling pathways have been hypothesized to contribute to the species' adaptability to a variety of environments by increasing an individual's sensitivity to context during development [9,10]. However, despite their potential adaptive value, interest in understanding the role serotonergic genetic variants play in promoting developmental plasticity has often been restricted to their association with psychiatric pathogenicity [11–16].
One such serotonergic variant, particularly well-studied in humans and rhesus macaques (Macaca mulatta), is the serotonin transporter gene (SLC6A4). Expression of SLC6A4 can be modulated by a polymorphic region (5-HTTLPR) located upstream of the SLC6A4 promotor region [17,18]. In vitro studies of the ‘long' (l) and ‘short' (s) alleles of the 5-HTTLPR, named after differences in their number of repetitive elements, have shown that the s-allele results in reduced expression of SLC6A4. This reduction in transcriptional activity associated with the s-allele of the 5-HTTLPR, in turn, results in decreased serotonin reuptake relative to the l-allele [19]. Phenotypically, rhesus macaque carriers of the s-allele show increased behavioural and endocrine stress-reactivity [20–23], social vigilance [24], receipt of social aggression [25], displays of submissive signals [26] and social isolation [27].
The s-allele of the 5-HTTLPR has also garnered attention for its environmentally dependent effects on behavioural phenotypes in rhesus monkeys. Across studies, developmental exposure to early life stressors (e.g. abusive parenting or maternal deprivation) magnifies the behavioural and neuroendocrine response to subsequent stressors, increases aggression and decreases social play of s-allele carrier, relative to l-allele homozygous, rhesus monkeys [19,20,22,28]. This observed gene-by-environment interaction (G×E), along with corresponding G×E interactions found in humans between our functionally analogous 5-HTTLPR and stress-mediated increases in psychiatric susceptibility, has led to the popular conceptualization of the l-allele as granting ‘protection' from, and the s-allele as granting ‘vulnerability' to, adverse early social environments [9,17,29–32].
The prevailing framework, which posits that the s-allele of the 5-HTTLPR is deleterious, has generated valuable insight into genetic contributors to psychiatric pathogenesis [30]. However, the framework does not address why such a universally deleterious mutation would have independently evolved in macaques and humans and have been maintained at high prevalence across populations in both primate species (reported allelic frequency ranges between 15–39% and 34–80% in rhesus macaque and human populations, respectively) [17,33,34]. Furthermore, in human populations with lower frequencies of the s-allele, we observe a loss-of-function single nucleotide polymorphism on the l-allele which lowers the transcriptional efficiency to that of the s-allele [35–38]. The high prevalence of the s-allele in primate species has led investigators to hypothesize that genetic variations which influence the transcriptional efficiency of SLC6A4 may confer an advantage in certain social contexts, at different developmental time points, or during different periods in life history [11–13,16,18,27,39–42].
One particular hypothesis, based on the observed environmentally dependent effects of the s-allele, argues that the s-allele has pleiotropic effects which ultimately maximize an s-carrier's developmental plasticity and that the 5-HTTLPR gene should, therefore, be re-conceptualized as a ‘plasticity' rather than a ‘vulnerability' gene [11,12,15,16]. This differential susceptibility hypothesis postulates that the s-allele of the 5-HTTLPR grants high biological sensitivity to context, and therefore, carriers experience heightened susceptibility to environmental input. Thus, not only should s-allele carriers exhibit stress-reactive phenotypes when exposed to adverse social environments during development, as has been observed, but s-allele carriers should also benefit the most from supportive social environments during development [5,11,12,14,43].
Although there is evidence supporting the differential susceptibility hypothesis for other proposed ‘plasticity genes' in humans, to our knowledge to date, no research has tested whether s-allele carriers of the 5-HTTLPR similarly display heightened developmental plasticity in response to high-investing social environments during development in non-human primates [44–46]. Here we address this important gap in scientific knowledge. Specifically, given the demonstrated value of high-quality maternal care to infant social development in primates [47–50], we used naturally occurring variation in maternal behaviour in a free-ranging population of rhesus macaques to test whether infants that are s-allele carriers of the 5-HTTLPR show increased sensitivity to high-investing social environments [49,51]. In accordance with the differential susceptibility hypothesis and based on documented G×E effects on social play in response to maternal deprivation [28], we predicted that social play in juvenile s-allele carriers (i.e. individuals of l/s or s/s genotypes) would be most influenced by indices of high-quality mother–infant attachment. We selected social play as our outcome measure for three key reasons: (i) it is an age-appropriate measure of social competence, requiring the proper display and interpretation of signals to announce non-aggressive intentions [52–54]; (ii) it positively predicts the formation of a primate's affiliative social networks [55–58]; and (iii) it is predictive of longitudinally stable social bonds which are important for cooperation, survival and reproductive success [59–62].
In support of the differential susceptibility hypothesis, we found a G×E interaction whereby rhesus macaque s-allele carriers which experienced enhanced maternal protection during the first 12 weeks of life, exhibited the highest levels of social play at 2 years of age. L-allele homozygotes, by contrast, were comparatively insensitive to the effects of their early maternal environment. We also found a sex-specific G×E interaction whereby male rhesus macaque s-allele carriers which engaged in more safe-base exploration, an indicator of secure maternal attachment [63–66], similarly exhibited higher levels of social play at 2 years of age. To our knowledge, this is the first report in rhesus macaques to demonstrate that the s-allele, widely considered to be a deleterious genetic variant, instead confers developmental plasticity which responds to early nurturing social environments. Moreover, these findings provide the first observational evidence that increased developmental plasticity in s-carriers of the 5-HTTLPR produces a potentially adaptive response, and by extension, provide functional insight into robust maintenance of the s-allele in primate populations.
2. Methods
(a). Subjects and study site
This study was conducted on Cayo Santiago, a 15 ha island located 1 km off the eastern coast of Puerto Rico. The study site and monkey population are managed by the Caribbean Primate Research Centre (CPRC). During the study period, the population consisted of approximately 1200 free-ranging, daily-provisioned, rhesus macaques (Macaca mulatta) of Indian origin and robust genetic health [67,68]. For ease and reliability of identification, all monkeys are captured and tattooed with a unique alphanumeric identifier at 1 year of age. Subjects were the first n = 46 macaque infants (n = 24 males, n = 22 females) born to different mothers between August and September 2011 in two of the nine naturally formed and human-habituated social groups (groups R and S). This research protocol was approved by the Institutional Animal Care and Use Committees at the University of Puerto Rico, Stanford University and The University of Chicago. All procedures complied with both the National Institute of Health policies on the care and use of animals, and the legal requirements of Puerto Rico and the United States. On-site animal welfare standards and practices were ensured by the veterinarian and managerial staff at CPRC.
(b). Behavioural observations
Longitudinal behavioural data were collected from subjects during two different developmental stages. Observations were initiated at birth and continued throughout the first 12 weeks of life (August–December 2011). Subjects were again observed as juveniles for approximately 20 weeks when they were approximately 2 years of age (June–November 2013). All observations were collected between 07.00 h and 14.30 h, the order in which subjects were followed was randomized weekly, and observations were counterbalanced biweekly between morning and afternoon observation blocks in order to avoid circadian behavioural biases. Observers were at all times blind to the subject's genotype.
During the first 12 weeks of life, each infant was observed for two 30 min periods per week using continuous focal sampling methods [69]. Each observation period was recorded with a handheld video camera (Kodak PlaySport Z3) for subsequent coding. As previously described, the final infant behavioural dataset consisted of 542 h of recorded observation time [70]. Interobserver reliability greater than Cohen's κ = 0.9 for occurrences of maternal interactions was reached before coding of the videos was initiated. Mother–infant interactions were coded by all observers using automatic time-stamp functions in Microsoft Excel. Once imported, Microsoft Access was used to calculate frequencies of mother–infant interactions which were subsequently converted into hourly rates per subject. Coded behavioural interactions have been described in detail elsewhere [71–73]; a summary ethogram is included here (see the electronic supplementary material, S1).
Between the average age of 21.4 ± 0.6 (s.d.) and 26.6 ± 0.7 (s.d.) months, the same subjects (now juveniles) were observed and handheld devices (Psion Workabout™) loaded with ‘behaviour' software for event recording (Syscan International Inc., Montreal, Quebec) were used. Each subject was observed for one 30 min period per week using continuous focal sampling methods [69]. The final juvenile behavioural dataset consisted of 497 h of observational data [74]. For the present study, counts of social play were extracted as was the total time observed which ranged from 9 to 11.5 h per subject. Any instance of rough-and-tumble as well as non-aggressive (silent) wrestling and chasing, characterized by the use of a ‘play face', was recorded as social play [53,71].
In rhesus macaques, juvenile dominance rank is maternally inherited, and on Cayo Santiago these adult dominance ranks, based on results of agonistic encounters and displacements between individuals, are recorded in a database maintained by the CPRC staff. Additionally, during the first 12 weeks of life of our subjects, we collected ad libitum data on the outcome of dyadic agonistic interactions between mothers of our subjects to confirm dominance hierarchies [69]. As has been previously described, the dyadic interaction data were placed into a winner-loser matrix and used to equally distribute the mothers into high, middle or low ranking categories, separately for each of the two social groups [70,75]. Maternal rank was then used as a statistical blocking factor in our analysis to control for any effect of dominance status on social play.
(c). Genotyping
During the yearly ‘trapping season' (here, January–February 2012), infant subjects and their mothers were captured using standard CPRC protocols. Mothers and infants were separated and blood samples from the femoral vein of unanaesthetized infants were collected in tubes coated with ethylenediaminetetraacetic acid (EDTA), processed, stored in −80°C and shipped for genotyping. The genotyping protocol has been detailed elsewhere [17,19,22,76,77]. We were unable to genotype n = 4 subjects and their data were thus excluded from further analyses. Because of the paucity of rhesus macaques with homozygous s/s genotypes, and as is standard in similar behavioural genetic studies, we grouped individuals that carried at least one copy of the s-allele of the 5-HTTLPR [78,79].
(d). Statistical analyses
All data were analysed in JMP 13 Pro and SAS 9.4 for Windows (SAS Institute Inc, Cary, NC). In order to simplify the mother–infant interaction data for analysis, we first performed a principal components analysis (PCA) with varimax rotation. Following best practice for PCA [80,81], the raw data were normalized by using their correlation matrix for the PCA. The initial solution gave five principal components (PCs) with eigenvalues greater than 1 (PCs with eigenvalues less than 1 should be rejected), but the scree plot suggested that a three-PC solution might be optimal. We calculated both solutions. The three-PC solution yielded biologically meaningful PCs in line with previous literature [47,49,63,71–73,82], whereas the five-PC solution yielded similar results to the three-PC solution, but the additional PCs loaded only one or two behaviours (indicating poor data reduction). We, therefore, adopted the three-PC solution and calculated PC scores for each individual for further analysis.
As the behavioural data were expressed as counts, social play was analysed as a restricted maximum-likelihood Poisson regression [83]. To control for differing observation times, total observation time was used as an offset in the model. Dispersion was estimated and corrected by the model. We first predicted social play, using a Poisson regression blocked for date of birth and maternal rank, and tested for sex (male or female), 5-HTTLPR genotype (l/l versus s/s or l/s), the sex-by-genotype interaction and their interactions with each of the three PCs. The resulting model showed evidence of over specification. Following best practice for linear models, we, therefore, simplified to a final model which only included statistically significant and hypothesis-driven interactions with maternal components (i.e. we retained genotype-by-PC interactions, but excluded non-significant genotype-by-sex-by-PC and sex-by-PC interactions) [80]. The final model showed no evidence of over specification. The model was tested with type III likelihood ratios. Post hoc contrasts and custom tests were performed, and Bonferroni corrected.
3. Results
(a). 5-HTTLPR genotyping
The distribution of 5-HTTLPR genotypes in our n = 42 subjects was as follows: males—l/l = 12, l/s = 9, s/s = 2 and females—l/l = 8, l/s = 11, s/s = 0 (see the electronic supplementary material, S2). In concurrence with previous reports [18,27,33,84], the frequency of the s-allele in our sample was 0.29. It did not deviate from Hardy–Weinberg equilibrium (χ2 = 1.17, p > .05).
(b). Principal components analysis data reduction
The PCA reduced maternal interaction measures to orthogonal dimensions that were consistent with previous research [47,49,71–73,82]. Rates of groom, retrieve, restrain, mother follow infant, contact initiate by the mother, and suckle positively loaded onto principal component 1 (PC1), which we labelled ‘maternal protectiveness', and explained 23% of the variance in the data. Rates of proximity initiate by the infant, contact initiate by the infant, proximity break by the infant, infant follow mother, proximity break by the mother, contact break by the mother and proximity initiate by the mother all positively loaded onto principal component 2 (PC2), which we labelled ‘safe-base exploration', and explained 19% of the variance in the data. Rates of abuse, both active and passive reject, and contact initiate by the infant positively loaded onto principal component 3 (PC3), along with negative loadings of proximity break by the mother, proximity initiate by the mother and suckle. We labelled PC3 ‘maternal rejection', and it explained 14% of the variance in the original data. The resulting loading is summarized in table 1.
Table 1.
Factor loadings of principal component analysis (PCA) for mother–infant interactions. (Italicized if |loading factor| > 0.3, per best practices in PCA [80,81].)
| behaviours | PC1 maternal protectiveness | PC2 safe-base exploration | PC3 maternal rejection |
|---|---|---|---|
| active reject | 0.012261 | 0.065555 | 0.847651 |
| passive reject | 0.034178 | 0.446231 | 0.693137 |
| restrain | 0.773081 | −0.067077 | −0.104231 |
| retrieve | 0.907778 | 0.100208 | −0.001903 |
| abuse | −0.164731 | 0.096278 | 0.574033 |
| groom | 0.658985 | −0.096778 | 0.012701 |
| contact-initiate, mother | 0.905545 | 0.092022 | −0.077655 |
| contact-break, mother | 0.09065 | 0.860042 | 0.214926 |
| proximity-initiate, mother | 0.128144 | 0.661496 | −0.398625 |
| proximity-break, mother | −0.108265 | 0.569013 | −0.568879 |
| mother follow infant | 0.814658 | 0.057434 | −0.044038 |
| suckle | 0.568331 | −0.156121 | −0.355401 |
| contact-initiate, infant | 0.030418 | 0.517165 | 0.318753 |
| contact-break, infant | 0.449584 | 0.142584 | 0.158238 |
| proximity-initiate, infant | −0.215658 | 0.601128 | 0.019315 |
| proximity-break, infant | 0.045577 | 0.440268 | 0.051163 |
| infant follow mother | 0.083051 | 0.822879 | 0.202861 |
(c). Gene-by-environment interaction
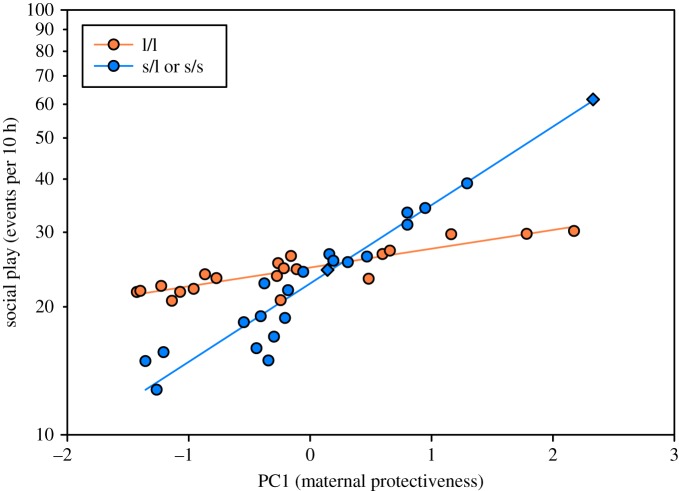
The interaction between 5-HTTLPR genotype and maternal protectiveness was significant (likelihood ratio (LR) χ2 = 4.46; p = 0.0347). Post hoc tests revealed that s-allele carriers which received higher levels of maternal protection during infancy exhibited greater social play at 2 years of age (LR χ2 = 12.88; p = 0.0003; figure 1). L-allele homozygote subjects showed no significant effect from maternal protection (LR χ2 = 1.08; p = 0.2997; figure 1).
Figure 1.
Maternal protectiveness influences juvenile social competence in a genotype-dependent manner. Infant genotype and levels of maternal protection experienced during the first 12 weeks of life interact to influence levels of social play exhibited at 2 years of age (LR χ2 = 4.46; p = 0.0347). Short-allele carriers of the 5-HTTLPR (i.e. l/s and s/s individuals; blue circles) which experienced higher levels of maternal protection during infancy exhibited greater social play as juveniles (LR χ2 = 12.88; p = 0.0003). L-allele homozygotes of the 5-HTTLPR (i.e. l/l individuals; orange circles) did not show a significant effect from maternal protection (LR χ2 = 1.08; p = 0.2997). The data points of the two s/s individuals are indicated with diamonds. The y-axis values (counts of social play per 10 h) are corrected for factors in the model equivalent to a least-squares line and are thus plotted as the expected value for each data point, plus the Pearson residual.
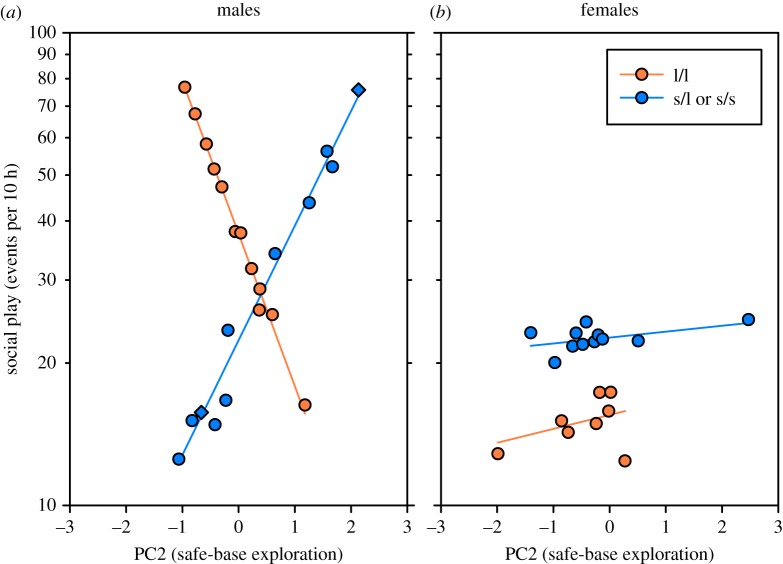
A three-way interaction between 5-HTTLPR genotype, safe-base exploration and sex was also highly significant (LR χ2 = 9.13; p = 0.0025; figure 2a). Post hoc tests demonstrated that male s-allele carriers which engaged in greater safe-base exploration during infancy exhibited greater social play at 2 years of age (LR χ2 = 20.41; p < 0.0001). Conversely, l-allele homozygote males which were observed conducting greater safe-base exploration during infancy exhibited decreased social play at 2 years of age (LR χ2 = 9.479; p = 0.0021). Females, regardless of genotype, showed no significant effect of safe-base exploration (l-allele homozygotes: LR χ2 = 0.0449; p = 0.8322) (s-allele carriers: LR χ2 = 0.0123; p = 0.9116) (figure 2b).
Figure 2.
Safe-base exploration influences juvenile social competence in a sex and genotype-dependent manner. Infant genotype, sex and levels of safe-base exploration experienced during the first 12 weeks of life interact to influence levels of social play exhibited at 2 years of age (LR χ2 = 9.13; p = 0.0025). (a) Male s-allele carriers of the 5-HTTLPR (i.e. l/s and s/s individuals; blue circles) which experienced higher levels of maternal protection during infancy exhibited greater social play as juveniles (LR χ2 = 9.13; p < 0.0001). Male l-allele homozygotes of the 5-HTTLPR (i.e. l/l individuals; orange circles) similarly displayed a relationship with safe-base exploration. However, it was in the opposite direction to male s-allele carriers whereby l-allele homozygote males which displayed higher levels of safe-base exploration during infancy exhibited decreased social play during juvenility (LR χ2 = 9.479; p = 0.0021). (b) No effect of genotype or infant safe-base exploration on social play during juvenility was discerned for females (s-allele carriers as blue circles: LR χ2 = 0.0123; p = 0.9116) (l-allele homozygotes as orange circles: LR χ2 = 0.0449; p = 0.8322). The data points of the two s/s individuals are indicated with diamonds. In (a,b), y-axis values (counts of social play per 10 h) are corrected for factors in the model equivalent to a least-squares line and are thus plotted as the expected value for each data point, plus the Pearson residual.
While there was no G×E interaction between 5-HTTLPR genotype and maternal rejection, we found a significant sex-by-environment interaction between infant sex and maternal rejection (LR χ2 = 4.33; p = 0.0375), whereby the relationship between early maternal rejection and juvenile social play differed significantly between male and female infants. However, in post hoc tests, neither sex showed a significant relationship on their own even before Bonferroni correction. The relationships between early maternal rejection and juvenile social play may have been particularly difficult to detect in this dataset: unlike for the other PCs, maternal rejection had a highly skewed distribution with only three individuals experiencing high levels of rejection.
4. Discussion
In this study, we found that rhesus macaque carriers of the risk-associated s-allele for the 5-HTTLPR gene engaged in social play as juveniles at higher frequencies if they had also experienced high levels of maternal protection as infants (figure 1). The fact that behavioural phenotypes of juveniles differ based on the quality of early maternal care in s-allele carriers, and that these carriers can show lower or higher levels of social play than l-allele homozygotes depending on early maternal care, provides evidence that the s-allele confers to its carriers a higher degree of developmental plasticity. This finding supports the interpretation provided by the differential susceptibility hypothesis whereby the s-allele increases the environmental sensitivity of the carrier during development and that under certain circumstances may actually confer benefits to its carriers. Safe-base exploration, an indicator of secure maternal attachment and which is suggested by fluid changes in mother–infant proximity and contact [63,64], provided an added opportunity for us to assess the interaction between differing quality of maternal environment and variants of the 5-HTTLPR gene. We found that male rhesus macaque carriers for the s-allele for the 5-HTTLPR which had demonstrated higher scores in safe-base exploration as infants also played at higher frequencies as juveniles (figure 2a).
While maladaptive G×E interactions in rhesus macaques have been widely reported between the s-allele and adverse rearing environments, to our knowledge this is the first report of G×E interactions between the s-allele and maternal care as an example of potentially adaptive developmental plasticity in rhesus macaques. Similar to the present study, prior interventional and observational studies in humans have reported G×E interactions that confer adaptive value for other genes traditionally thought to confer risk for psychiatric illness [45–47]. Our study expands this body of literature, which supports the differential susceptibility hypothesis for several widely interpreted ‘deleterious' alleles, to include the s-allele for the 5-HTTLPR in non-human primate populations [10–12,14–16,39,46,85].
Besides the differential susceptibility hypothesis, two other hypotheses address the evolution of the polymorphic 5-HTTLPR region. The balancing selection hypothesis posits that the stress-reactive phenotypes of s-allele carriers are in itself adaptive; the prevalence of the s-allele is justified by competitive social contexts in which the hypervigilant, observant and aggressive behavioural profile of s-carriers is adaptive [13,40,86]. Evidence supporting this hypothesis shows an association between polymorphic variation in serotonergic pathway genes and interspecific levels of social aggression across macaque species [9,42,87]. By contrast, the life-history modification hypothesis posits that the s- and l-alleles confer temporally shifted mating strategies. Thus, despite yielding proportionally similar reproductive output, genotypic variations in the 5-HTTLPR region are associated with both the age at which rhesus macaque males disperse from their natal groups and the age at which males are maximally reproductive [41,88]. Similarly, subordinate s-allele carrier rhesus macaque females—female members of the species do not disperse and instead form core matrilines within their natal group—experienced delayed sexual maturation relative to l-allele homozygous females [89]. Although genetic inquiries into the evolution of the SLC6A4 gene support the differential susceptibility hypothesis [18], the differential susceptibility, balancing selection and life-history modification hypotheses are not necessarily mutually exclusive and may represent three different simultaneous levels of stabilizing selection. For example, the hypervigilant and aggressive behavioural profile of the s-carriers, selected for in the balancing selection hypothesis, could conceivably result from predictive environmental programming during development and thus in reality be a form of informational adaptive developmental plasticity [10].
Additionally, with safe-base exploration, we also report sex-dependent influences whereby infant females of either genotype were insensitive to differences in early life environment (figure 2b). One possible explanation for this sex-dependent interactions is that, as the species' dispersing sex, males may be more influenced by developmental differences in safe-base exploration which in itself incorporates behavioural indicators of independence [41]. Another additional explanation is that there are known sex-differences in the developmental trajectories of rates of social play in rhesus macaques, with males engaging in social play at much higher rates and at earlier ages than females [90,91]. It is conceivable that in female rhesus macaques social play may not be the most sensitive measure by which to test for the presence of developmental plasticity. In humans, s-allele carrier mothers self-report higher perceived attachment with their own infants if they self-report having themselves received better maternal care during childhood [92]. Given known female-bias in attraction toward infants in macaque species [93], future studies will need to address whether maternal care received during infancy by female rhesus monkey s-allele carriers influences their own maternal care as adults.
Finally, we report that male rhesus macaques of the 5-HTTLPR l/l genotype who demonstrated higher scores in safe-base exploration as infants, engaged in lower frequencies of social play as juveniles (figure 2a). This unexpected G×E interaction is contrary to the prevailing notion of the l-allele as resistant to environmental influences [9,17], and instead suggests that the l-allele may likewise confer individuals responsiveness to different environmental contexts and may do so in an opposing manner to that of the s-allele. Future studies are needed to investigate this possibility.
We note that our study is not without limitations. We have interpreted higher rates of social play as an adaptive response, but there are costs associated with it. For example, research on wild macaques has shown that while higher rates of social play do confer benefits such as better acquisition of motor skills, social play can impose energetic costs to the individual and result in decreased body growth [94]. Additionally, although early social play demonstrates a long-lasting relationship with the development of social skills and relationships critical to survival [52,53,55–57,59,60,62,95], the association between social play and later reproductive success has not been established in primates. Follow-up research is, therefore, necessary to determine the relationship between social play during primate juvenility and adult reproductive fitness [95], as has been shown in other mammalian species [61,96]. Finally, although we report a G×E interaction between the s-allele of 5-HTTLPR and maternal care in rhesus macaques which influences social play, follow-up studies need to determine the generalizability of this effect by investigating other cognitive and neuroendocrine measures during juvenility.
To conclude, this study demonstrates G×E interactions between early maternal care and 5-HTTLPR genotype of the infant which influences later social competence in rhesus macaques. These findings provide, to our knowledge, the first empirical evidence supporting the hypothesis that the s-allele of 5-HTTLPR grants developmental plasticity which can respond to high-investing early environments. Not only do these findings help dispel the popular conceptualization of the s-allele as invariantly deleterious, they provide a framework through which to further understand developmental plasticity in behaviour and its role in adapting to heterogeneous environmental conditions [97].
Supplementary Material
Acknowledgements
We thank Greg Ruber, Auberi Courchay, Clement Ludcher and Christine Fleener for assistance in collecting and coding behavioural data. We are indebted to John Addicott for creating our access database and to James Higham for providing access to it. We are grateful to the staff of CPRC for their support during this research project. We additionally thank Stephen Lindell for his work genotyping our subjects. Finally, we thank Alexander Georgiev, Constance Dubuc, Lauren Brent, Seth Dobson and James Curley for their thoughtful discussions.
Ethics
This research study was approved by the Institutional Animal Care and Use Committees at the University of Puerto Rico, Stanford University and The University of Chicago. All procedures complied with National Institute of Health policies on the care and use of animals.
Data accessibility
The corresponding data and SAS analysis code for this manuscript are available as an electronic supplementary material in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.t285760) [98].
Authors' contributions
J.E.M. designed the research study, conducted field behavioural data collection, created the study-specific database, generated the tables and drafted the manuscript. T.M.M. coordinated the parent longitudinal research project, conducted and coded field behavioural data collection, collected biological samples and assembled the parent longitudinal research project database. S.P.C. conducted field behavioural data collection and collected biological samples. J.P.G. and J.A.-D. conducted the statistical analyses, wrote the statistical analysis and results sections, and generated the figures. C.S.B. supervised genotyping in her laboratory. D.M. co-designed the parent longitudinal research project, secured funding for the parent project, and collected biological samples. K.J.P. co-designed the parent longitudinal research project, secured funding for the parent project, collected biological samples and supervised drafting of the manuscript. All authors reviewed, significantly revised and provided written final approval of this manuscript for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the National Institutes of Health (R01HD067175, P40OD012217 and 5T32MH020016) and a Stanford University Diversifying Academia, Recruiting Excellence (DARE) Doctoral Fellowship.
References
- 1.Pigliucci M. 2005. Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486. ( 10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 2.Thompson JD. 1991. Phenotypic plasticity as a component of evolutionary change. Trends Ecol. Evol. 6, 246–249. ( 10.1016/0169-5347(91)90070-E) [DOI] [PubMed] [Google Scholar]
- 3.Forsman A. 2014. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty S, Chakraborty D, Mukherjee O, Jain S, Ramakrishnan U, Sinha A. 2010. Genetic polymorphism in the serotonin transporter promoter region and ecological success in macaques. Behav. Genet. 40, 672–679. ( 10.1007/s10519-010-9360-2) [DOI] [PubMed] [Google Scholar]
- 5.Bjorklund DF, Ellis BJ. 2014. Children, childhood, and development in evolutionary perspective. Dev. Rev. 34, 225–264. ( 10.1016/j.dr.2014.05.005) [DOI] [Google Scholar]
- 6.Frankenhuis WE, Panchanathan K. 2011. Individual differences in developmental plasticity may result from stochastic sampling. Perspect. Psychol. Sci. 6, 336–347. ( 10.1177/1745691611412602) [DOI] [PubMed] [Google Scholar]
- 7.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 8.Maestripieri D. 2007. Macachiavellian intelligence. Chicago, IL: University of Chicago Press. [Google Scholar]
- 9.Suomi SJ. 2006. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann. NY Acad. Sci. 1094, 52–62. ( 10.1196/annals.1376.006) [DOI] [PubMed] [Google Scholar]
- 10.Nettle D, Bateson M. 2015. Adaptive developmental plasticity: what is it, how can we recognize it and when can it evolve? Proc. R. Soc. B 282, 20151005 ( 10.1098/rspb.2015.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchi I. 2011. The double edged sword of neural plasticity: increasing serotonin levels leads to both greater vulnerability to depression and improved capacity to recover. Psychoneuroendocrinology 36, 339–351. ( 10.1016/j.psyneuen.2010.08.011) [DOI] [PubMed] [Google Scholar]
- 12.Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. 2009. Vulnerability genes or plasticity genes? Mol. Psychiatry 14, 746–754. ( 10.1038/mp.2009.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homberg JR, Lesch KP. 2011. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 69, 513–519. ( 10.1016/j.biopsych.2010.09.024) [DOI] [PubMed] [Google Scholar]
- 14.Ellis BJ, Boyce WT. 2008. Biological sensitivity to context. Curr. Dir. Psychol. Sci. 17, 183–187. ( 10.1111/j.1467-8721.2008.00571.x) [DOI] [Google Scholar]
- 15.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. 2011. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Dev. Psychopathol. 23, 7–28. ( 10.1017/S0954579410000611) [DOI] [PubMed] [Google Scholar]
- 16.Belsky J, Pluess M. 2013. Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Dev. Psychopathol. 25, 1243–1261. ( 10.1017/S095457941300059X) [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, et al. 1997. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. J. Neural Transm. 104, 1259–1266. ( 10.1007/BF01294726) [DOI] [PubMed] [Google Scholar]
- 18.Shattuck MR, Satkoski-Trask J, Deinard A, Tito RY, Smith DG, Malhi RS. 2014. The evolutionary history of SLC6A4 and the role of plasticity in Macaca. Am. J. Phys. Anthropol. 153, 605–616. ( 10.1002/ajpa.22460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett AJ, et al. 2002. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry 7, 118–122. ( 10.1038/sj.mp.4000949) [DOI] [PubMed] [Google Scholar]
- 20.McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. 2009. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm. Behav. 55, 538–547. ( 10.1016/j.yhbeh.2009.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bethea CL, Streicher JM, Coleman K, Pau FKY, Moessner R, Cameron JL. 2004. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR). Behav. Genet. 34, 295–307. ( 10.1023/B:BEGE.0000017873.61607.be) [DOI] [PubMed] [Google Scholar]
- 22.Barr CS, et al. 2004. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic- pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry 55, 733–738. ( 10.1016/j.biopsych.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 23.Spinelli S, Schwandt ML, Lindell SG, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS. 2012. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev. Psychopathol. 24, 157–165. ( 10.1017/S0954579411000745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson KK, Ghodasra JH, Platt ML. 2009. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE 4, e4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwandt ML, Lindell SG, Sjöberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS. 2010. Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biol. Psychiatry 67, 323–330. ( 10.1016/j.biopsych.2009.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. 2008. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol. Behav. 93, 807–819. ( 10.1016/j.physbeh.2007.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brent L, Heilbronner S. 2013. Genetic origins of social networks in rhesus macaques. Sci. Rep. 3, 1042 ( 10.1038/srep01042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. 2003. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2, 336–340. ( 10.1046/j.1601-1848.2003.00051.x) [DOI] [PubMed] [Google Scholar]
- 29.Caspi A. 2003. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. ( 10.1126/science.1083968) [DOI] [PubMed] [Google Scholar]
- 30.Caspi A, Ahmad RH, Holmes A, Rudolf U, Terrie EM. 2010. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 167, 509–527. ( 10.1176/appi.ajp.2010.09101452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. 2002. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol. Psychiatry 7, 1058–1063. ( 10.1038/sj.mp.4001157) [DOI] [PubMed] [Google Scholar]
- 32.Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. 2004. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology 29, 2226–2234. ( 10.1038/sj.npp.1300552) [DOI] [PubMed] [Google Scholar]
- 33.Bennett AJ, Pierre PJ. 2010. Nonhuman primate research contributions to understanding genetic and environmental influences on phenotypic outcomes across development. In Handbook of developmental science, behavior, and genetics (eds Hood KE, Halpern CT, Greenberg G, Lerner RM), pp. 353–399. New York, NY: Wiley-Blackwell. [Google Scholar]
- 34.Chiao JY, Blizinsky KD. 2010. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc. R. Soc. B 277, 529–537. ( 10.1098/rspb.2009.1650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams RB, et al. 2017. Population differences in associations of serotonin transporter promoter polymorphism (5HTTLPR) di- and triallelic genotypes with blood pressure and hypertension prevalence. Am. Heart J. 185, 110–122. ( 10.1016/j.ahj.2016.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haberstick BC, et al. 2015. Population frequencies of the triallelic 5HTTLPR in six ethnicially diverse samples from North America, Southeast Asia, and Africa. Behav. Genet. 45, 255–261. ( 10.1007/s10519-014-9703-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iurescia S, Seripa D, Rinaldi M. 2016. Role of the 5-HTTLPR and SNP promoter polymorphisms on serotonin transporter gene expression: a closer look at genetic architecture and in vitro functional studies of common and uncommon allelic variants. Mol. Neurobiol. 53, 5510–5526. ( 10.1007/s12035-015-9409-6) [DOI] [PubMed] [Google Scholar]
- 38.Hu X-Z, et al. 2006. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 78, 815–826. ( 10.1086/503850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis BJ, Essex MJ, Boyce WT. 2005. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev. Psychopathol 17, 303–328. ( 10.1017/S0954579405050157) [DOI] [PubMed] [Google Scholar]
- 40.Dobson SD, Brent LJN. 2013. On the evolution of the serotonin transporter linked polymorphic region (5-HTTLPR) in primates. Front. Hum. Neurosci. 7, 588 ( 10.3389/fnhum.2013.00588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trefilov A, Berard J, Krawczak M, Schmidtke J. 2000. Natal dispersal in rhesus macaques is related to serotonin transporter gene promoter variation. Behav. Genet. 30, 295–301. ( 10.1023/A:1026597300525) [DOI] [PubMed] [Google Scholar]
- 42.Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ. 2006. Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behav. Genet. 36, 163–172. ( 10.1007/s10519-005-9017-8) [DOI] [PubMed] [Google Scholar]
- 43.Pluess M, Belsky J. 2010. Differential susceptibility to parenting and quality child care. Dev. Psychol. 46, 379–390. ( 10.1037/a0015203) [DOI] [PubMed] [Google Scholar]
- 44.Bakermans-Kranenburg MJ, Van IJzendoorn MH, Pijlman FT, Mesman J, Juffer F. 2008. Experimental evidence for differential susceptibility: dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers' externalizing behavior in a randomized controlled trial. Dev. Psychol. 44, 293–300. ( 10.1037/0012-1649.44.1.293) [DOI] [PubMed] [Google Scholar]
- 45.Dick DM, et al. 2011. CHRM2, parental monitoring, and adolescent externalizing behavior. Psychol. Sci. 22, 481–489. ( 10.1177/0956797611403318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky J, Pluess M, Widaman KF. 2013. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. J. Child Psychol. Psychiatry 54, 1135–1143. ( 10.1111/jcpp.12075) [DOI] [PubMed] [Google Scholar]
- 47.Maestripieri D. 2005. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc. R. Soc. B 272, 1243–1248. ( 10.1098/rspb.2005.3059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maestripieri D. 2012. Maternal influences on offspring growth, reproduction, and behavior in primates. In Maternal effects in mammals (eds Maestripieri D, Mateo J), pp. 256–291. Chicago, IL: University of Chicago Press. [Google Scholar]
- 49.Fairbanks LA. 1996. Individual differences in maternal style: causes and consequences for mothers and offspring. In Advances in the study of behavior (eds JS Rosenblatt, CT Snowdon), pp. 579–611. Cambridge, MA: Academic Press. [Google Scholar]
- 50.Berman CM. 1982. The ontogeny of social relationships with group companions among free-ranging infant rhesus monkeys I. Social networks and differentiation. Anim. Behav. 30, 149–162. [Google Scholar]
- 51.Fairbanks LA. 1993. What is a good mother? Adaptive variation in maternal behavior of primates. Curr. Dir. Psychol. Sci. 2, 179–183. ( 10.1111/1467-8721.ep10769793) [DOI] [Google Scholar]
- 52.Berman CM, Yanagi A. 2014. Functions of multiple play signals in free-ranging juvenile rhesus macaques (Macaca mulatta). Behaviour 151, 1983–2014. ( 10.1163/1568539X-00003227) [DOI] [Google Scholar]
- 53.Yanagi A, Berman CM. 2014. Body signals during social play in free-ranging rhesus macaques (Macaca mulatta): a systematic analysis. Am. J. Primatol. 76, 168–179. ( 10.1002/ajp.22219) [DOI] [PubMed] [Google Scholar]
- 54.Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall'Olio S, Fouts HN, Řeháková-Petrů M, Siviy SM, Pellis SM. 2016. Rough-and -tumble play as a window on animal communication. Biol. Rev. 91, 311–327. ( 10.1111/brv.12172) [DOI] [PubMed] [Google Scholar]
- 55.Sueur C, Pele M. 2016. Social network and decision-making in primates: a report on Franco-Japanese research collaborations. Primates 57, 327–332. ( 10.1007/s10329-015-0505-z) [DOI] [PubMed] [Google Scholar]
- 56.Shimada M, Sueur C. 2014. The importance of social play network for infant or juvenile wild chimpanzees at Mahale Mountains National Park, Tanzania. Am. J. Primatol. 76, 1025–1036. ( 10.1002/ajp.22289) [DOI] [PubMed] [Google Scholar]
- 57.Barale CL, Rubenstein DI, Beehner JC. 2015. Juvenile social relationships reflect adult patterns of behavior in wild geladas. Am. J. Primatol. 77, 1086–1096. ( 10.1002/ajp.22443) [DOI] [PubMed] [Google Scholar]
- 58.Shimada M, Sueur C. 2017. Social play among juvenile wild Japanese macaques (Macaca fuscata) strengthens their social bonds. Am. J. Primatol. 80, e22728. [DOI] [PubMed] [Google Scholar]
- 59.Weinstein T, Capitanio J. 2012. Longitudinal stability of friendships in rhesus monkeys (Macaca mulatta): individual-and relationship-level effects. J. Comp. Psychol. 126, 97–108. ( 10.1037/a0025607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brent LJN, Chang SWC, Gariépy J-F, Platt ML. 2014. The neuroethology of friendship. Ann. NY Acad. Sci. 1316, 1–17. ( 10.1111/nyas.12315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellis SM, Burghardt GM, Palagi E, Mangel M. 2015. Modeling play: distinguishing between origins and current functions. Adapt. Behav. 23, 331–339. ( 10.1177/1059712315596053) [DOI] [Google Scholar]
- 62.Durand S, Schank JC. 2015. The evolution of social play by learning to cooperate. Adapt. Behav. 23, 340–353. ( 10.1177/1059712315608243) [DOI] [Google Scholar]
- 63.Warfield JJ, Kondo-Ikemura K, Waters E. 2011. Measuring infant attachment security in rhesus macaques (Macaca mulatta): adaptation of the attachment Q-set. Am. J. Primatol. 73, 109–118. ( 10.1002/ajp.20883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suomi SJ. 2005. Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum. Dev. 48, 67–79. ( 10.1159/000083216) [DOI] [Google Scholar]
- 65.Kondo-Ikemura K, Waters E. 1995. Maternal behavior and infant security in Old World monkeys: conceptual issues and a methodological bridge between human and nonhuman primate research. Monogr. Soc. Res. Child Dev. 60, 97–110. ( 10.2307/1166173) [DOI] [Google Scholar]
- 66.McCormack K, Howell BR, Guzman D, Villongco C, Pears K, Kim H, Gunnar MR, Sanchez MM. 2015. The development of an instrument to measure global dimensions of maternal care in rhesus macaques (Macaca mulatta). Am. J. Primatol. 77, 20–33. ( 10.1002/ajp.22307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kessler MJ, Rawlins RG. 1986. The Cayo Santiago macaques: history, behavior, and biology. 1st edn. Albany, NY: State University of New York Press. [Google Scholar]
- 68.Widdig A, Muniz L, Minkner M, Barth Y, Bley S, Ruiz-Lambides A, Junge O, Mundry R, Kulik L. 2017. Low incidence of inbreeding in a long-lived primate population isolated for 75 years. Behav. Ecol. Sociobiol. 71, 18 ( 10.1007/s00265-016-2236-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–266. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 70.Mandalaywala TM, Parker KJ, Maestripieri D. 2014. Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychol. Sci. 25, 1893–1902. ( 10.1177/0956797614544175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. 2006. Influence of parenting style on the offspring's behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav. Brain Res. 175, 90–95. ( 10.1016/j.bbr.2006.08.002) [DOI] [PubMed] [Google Scholar]
- 72.Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. 2006. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behav. Neurosci. 120, 1017–1024. ( 10.1037/0735-7044.120.5.1017) [DOI] [PubMed] [Google Scholar]
- 73.Maestripieri D. 1998. Parenting styles of abusive mothers in group-living rhesus macaques. Anim. Behav. 55, 1–11. ( 10.1006/anbe.1997.0578) [DOI] [PubMed] [Google Scholar]
- 74.Coyne SP, Lindell SG, Clemente J, Barr CS, Parker KJ, Maestripieri D. 2015. Dopamine D4 receptor genotype variation in free-ranging rhesus macaques and its association with juvenile behavior. Behav. Brain Res. 292, 50–55. ( 10.1016/j.bbr.2015.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mandalaywala TM, Higham JP, Heistermann M, Parker KJ, Maestripieri D. 2014. Physiological and behavioural responses to weaning conflict in free-ranging primate infants. Anim. Behav. 97, 241–247. ( 10.1016/j.anbehav.2014.09.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindell SG, Yuan Q, Zhou Z, Goldman D, Thompson RC, Lopez JF, Suomi SJ, Higley JD, Barr CS. 2012. The serotonin transporter gene is a substrate for age and stress dependent epigenetic regulation in rhesus macaque brain: potential roles in genetic selection and gene×environment interactions. Dev. Psychopathol. 24, 1391–1400. ( 10.1017/S0954579412000788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. 2003. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol. Clin. Exp. Res. 27, 812–817. ( 10.1097/01.ALC.0000067976.62827.ED) [DOI] [PubMed] [Google Scholar]
- 78.Watson KK, Li D, Brent LJN, Horvath JE, Gonzalez-Martinez J, Ruíz-Lambides AV, Robinson AG, Skene JHP, Platt ML. 2015. Genetic influences on social attention in free-ranging rhesus macaques. Anim. Behav. 103, 267–275. ( 10.1016/j.anbehav.2015.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uher R, McGuffin P. 2008. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol. Psychiatry 13, 131–146. ( 10.1038/sj.mp.4002067) [DOI] [PubMed] [Google Scholar]
- 80.Howitt D, Cramer D. 2008. Introduction to statistics in psychology, 4th edn. Harlow, UK: Pearson Education Limited. [Google Scholar]
- 81.Lehman A, O'Rourke N, Hatcher L, Stepanski EJ. 2013. JMP for basic univariate and multivariate statistics: methods for researchers and social scientists, 2nd edn. Cary, NC: SAS Institute Inc. [Google Scholar]
- 82.Schino G, D'Amato FR, Troisi A. 1995. Mother-infant relationships in Japanese macaques: sources of inter-individual variation. Anim. Behav. 49, 151–158. ( 10.1016/0003-3472(95)80162-6) [DOI] [Google Scholar]
- 83.Littell RC, Stroup WW, Freund RJ. 2002. SAS for linear models, 4th ed. Cary, NC: SAS Institute. [Google Scholar]
- 84.Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. et al. 2010. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 9, 575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellis BJ, Bjorklund DF. 2012. Beyond mental health: an evolutionary analysis of development under risky and supportive environmental conditions: an introduction to the special section. Dev. Psychol. 48, 591–597. ( 10.1037/a0027651) [DOI] [PubMed] [Google Scholar]
- 86.Jedema HP, et al. 2010. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry 15, 512–522, 446 ( 10.1038/mp.2009.90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thierry B. 2007. Unity in diversity: lessons from macaque societies. Evol. Anthropol. 16, 224–238. ( 10.1002/evan.20147) [DOI] [Google Scholar]
- 88.Krawczak M. 2005. Male reproductive timing in rhesus macaques is influenced by the 5HTTLPR promoter polymorphism of the serotonin transporter gene. Biol. Reprod. 72, 1109–1113. ( 10.1095/biolreprod.104.038059) [DOI] [PubMed] [Google Scholar]
- 89.Wilson ME, Kinkead B. 2008. Gene-environment interactions, not neonatal growth hormone deficiency, time puberty in female rhesus monkeys. Biol. Reprod. 78, 736–743. ( 10.1095/biolreprod.107.065953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tartabini A. 1991. Social play-behavior in young rhesus-monkeys Macaca mulatta at 3 different ages - from the 3rd To the 6th month of life. Behav. Processes 24, 185–192. ( 10.1016/0376-6357(91)90074-A) [DOI] [PubMed] [Google Scholar]
- 91.Kulik L, Amici F, Langos D, Widdig A. 2015. Sex differences in the development of social relationships in rhesus macaques (Macaca mulatta). Int. J. Primatol. 36, 353–376. ( 10.1007/s10764-015-9826-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Meaney MJ, Sokolowski MB, Fleming AS. 2011. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 10, 325–333. ( 10.1111/j.1601-183X.2010.00671.x) [DOI] [PubMed] [Google Scholar]
- 93.Silk JB. 1999. Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Anim. Behav. 57, 1021–1032. ( 10.1006/anbe.1998.1065) [DOI] [PubMed] [Google Scholar]
- 94.Berghänel A, Schülke O, Ostner J. 2015. Locomotor play drives motor skill acquisition at the expense of growth: a life history trade-off. Sci. Adv. 1, e1500451 ( 10.1126/sciadv.1500451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pellis SM, Pellis VC, Himmler BT. 2014. How play makes for a more adaptable brain: a comparative and neural perspective. Am. J. Play 7, 73–98. [Google Scholar]
- 96.Graham KL, Burghardt GM. 2010. Current perspectives on the biological study of play: signs of progress. Q. Rev. Biol. 85, 393–418. ( 10.1086/656903) [DOI] [PubMed] [Google Scholar]
- 97.Sih A. 2011. Effects of early stress on behavioral syndromes: an integrated adaptive perspective. Neurosci. Biobehav. Rev. 35, 1452–1465. ( 10.1016/j.neubiorev.2011.03.015) [DOI] [PubMed] [Google Scholar]
- 98.Madrid JE, Mandalaywala TM, Coyne SP, Ahloy-Dallaire J, Garner JP, Barr CS, Maestripieri D, Parker KJ. 2018. Data from: Adaptive developmental plasticity in rhesus macaques: the serotonin transporter gene interacts with maternal care to affect juvenile social behaviour Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.t285760) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Madrid JE, Mandalaywala TM, Coyne SP, Ahloy-Dallaire J, Garner JP, Barr CS, Maestripieri D, Parker KJ. 2018. Data from: Adaptive developmental plasticity in rhesus macaques: the serotonin transporter gene interacts with maternal care to affect juvenile social behaviour Dryad Digital Repository. (http://dx.doi.org/10.5061/dryad.t285760) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The corresponding data and SAS analysis code for this manuscript are available as an electronic supplementary material in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.t285760) [98].