Abstract
Cleaner shrimp and their reef fish clients are an interspecific mutualistic interaction that is thought to be mediated by signals, and a useful system for studying the dynamics of interspecific signalling. To demonstrate signalling, one must show that purported signals at minimum (a) result in a consistent state change in the receiver and (b) contain reliable information about the sender's intrinsic state or future behaviour. Additionally, signals must be perceptible by receivers. Here, we document fundamental attributes of the signalling system between the cleaner shrimp Ancylomenes pedersoni and its clients. First, we use sequential analysis of in situ behavioural interactions to show that cleaner antenna whipping reliably predicts subsequent cleaning. If shrimp do not signal via antenna whipping, clients triple their likelihood of being cleaned by adopting darker coloration over a matter of seconds, consistent with dark colour change signalling that clients want cleaning. Using experimental manipulations, we found that visual stimuli are sufficient to elicit antenna whipping, and that shrimp are more likely to ‘clean' dark than light visual stimuli. Lastly, we show that antenna whipping and colour change are perceptible when accounting for the intended receiver's visual acuity and spectral sensitivity, which differ markedly between cleaners and clients. Our results show that signalling by both cleaners and clients can initiate and mediate their mutualistic interaction.
Keywords: cleaning mutualism, shrimp vision, fish vision, visual ecology, interspecific signalling
1. Introduction
For a signalling system to arise and persist, the signal must, on average, increase the fitness of both the sender and the receiver, and be reliable [1–3]. A signal is reliable if some aspect of the signal (including presence/absence) correlates consistently with some attribute of the signaller (including future behaviour) [4]. Additionally, receiving the signal, or having information about the signaller's state, should result in a state change in the receiver that benefits the receiver [4,5]. In large part, work exploring signal reliability has focused on intraspecific signals, showing that signal reliability is maintained by genetic relatedness between signaller and receiver or by costs associated with signalling [4,6,7]. However, signals also mediate interspecific interactions, where genetic relatedness cannot explain signal reliability and where signallers and receivers may have vastly different sensory systems. Studying interspecific signalling systems offers an opportunity to test whether signal function and reliability arise in the absence of genetic relatedness, as well as to examine how senders and receivers with very different sensory capabilities may perceive and place selection pressure on signalling traits. Here, we use the interspecific mutualistic interactions between cleaner shrimp and their client fish to determine whether certain candidate signals reliably predict signaller intent.
The cleaner shrimp Ancylomenes pedersoni (Palaemonidae) and their reef fish clients are an example of a mutualistic system in which signalling probably occurs. Ancylomenes pedersoni live at ‘cleaning stations' on tropical coral reefs from which they provide cleaning services to reef fish clients, removing ectoparasites and dead skin from their mouths, gills and scales [8]. The cleaner receives a meal [8,9] and the client benefits from parasite removal [10]. Cleaning mutualisms provide a particularly promising system for studying interspecific signalling. First, signals that result in the mutualistic behaviour by definition have an average benefit for both sender and receiver [11]. Second, selection on signals that identify cleaners as beneficial partners rather than as food should be particularly intense, because misidentification by clients could result in the death of the cleaner [12]. Selection also may act on clients to identify themselves as seeking cleaning services, rather than a meal, because cleaners can choose not to clean clients.
Several behaviours observed in interactions between A. pedersoni and their clients have been proposed as signals. In particular, A. pedersoni have long white antennae that they often whip vigorously in the presence of clients. Numerous reports refer to this behaviour as a signal of intent to clean (e.g. [13–18]), but no studies have tested this interpretation. Many crustaceans also have chemosensory sensilla on the antennules (see [19]); so, alternatively, or in addition to being a signal, cleaners could whip their antenna to assess their olfactory environment. Additionally, client fish often rapidly change to darker coloration at cleaning stations [20–22]. Although some have suggested that colour change is a signal to cleaners (e.g. [23–25]), others have suggested it may be a physiological response to the act of being cleaned [26,27] or that it may serve to make parasites more visible to cleaners [23,28]. As with antenna whipping by cleaners, the signalling function of client colour change has not been rigorously investigated.
Here, we use several approaches to test whether antenna whipping and client colour change are reliable signals. First, we recorded in situ cleaner–client interactions and annotated the sequence of behaviours of both parties. We then used sequential analysis of the component behaviours to test whether the occurrence of one behaviour depends on a previous behaviour, as well as the probability of observing any particular sequence of behaviours [29]. We also used behavioural experiments in the laboratory to test whether shrimp whip their antennae in response to various visual stimuli in the absence of olfactory cues. We also observed whether shrimp that had antenna whipped then approached the visual stimulus, tapped it with their antennae, or climbed on it as if to clean.
Lastly, it is often implicitly assumed that a receiver can perceive a given signal. However, given the diversity of sensory capabilities across animals [30], it is important to account for the sensory abilities of a given receiver to test that the signal is perceptible. Here, we use information about colour vision and acuity in both cleaners and clients to modify still images and movies. These images show whether the spatial information available in the purported signals is perceptible by the relevant receiver visual system.
2. Material and methods
(a). Field recordings
To record interactions between cleaner shrimp and client fish, we deployed GoPro cameras (Hero3+, GoPro Corporation, San Mateo CA, USA) at cleaning stations. Two cameras were deployed at each station: one attached to a dive weight rested on the substrate 17–30 cm from the station, and a second attached to a PVC pole that provided a top-down view from between 37 and 65 cm away. All videos were recorded on the House Reef, CARMABI field station in Curaçao, the Netherlands Antilles (12°7′ N, 68°5′ W) during August 2015. Deployments occurred twice per day and lasted for 3–4 h, depending on camera battery life. The first deployment began at 07.00 and the other at 16.00, though A. pedersoni clean consistently throughout the day [17]. After deployments, divers left the area so that the cleaners and clients were undisturbed during filming, because diver presence can alter cleaner–client interactions [31]. In total, we recorded 133 h of footage at 10 cleaning stations (10.4 ± 1.8 h per station). Cleaning stations were between 5 and 8.5 m deep.
(b). Video annotation
Two observers who had been trained to identify cleaner and client behaviours, but were naive to our research question, watched and annotated all footage using the annotation program BORIS [32]. This resulted in an ethogram of nine focal behaviours (table 1). The observers also noted all interactions, defined as when a client was visible in the camera's field of view for more than five seconds, and which began when clients entered and ended when clients exited the field of view of the camera. A third observer then annotated all interaction footage at half speed to create a final dataset of behavioural interactions. Clients were identified to species during each interaction (see electronic supplementary material, table S1). Interactions were initially identified using the top-down footage, and final annotations were created using the side view footage, which provided a closer view and thus allowed for more detailed observation. These final annotations were checked against the order of behaviours noted by the first two annotators. Differences were resolved by majority rule, though in no instances did all three observers disagree about the identity or order of behaviours. Repeat instances of whipping, posing, dark colour change and jolting were often seen, and so were allowed in our sequences.
Table 1.
Focal client and cleaner behaviours. Icons correspond to those used in network figures.
| party | behaviour | description |
|---|---|---|
| client | interaction begin | client fish enters the camera's field of view |
| pose | client fish stops forward motion near a cleaning station, either resting on, or remaining still just above, the bottom; often accompanied by a flaring of the opercula and/or fins | |
colour change (dark) 
|
a rapid change to darker coloration, including barring/striping or full body colour change | |
colour change (light) 
|
a rapid change to lighter coloration | |
| jolt | a sudden twitching movement | |
| interaction end | client fish exits the camera's field of view | |
| cleaner | antenna whipping 
|
whipping of the antennae; usually accompanied by a body rocking motion |
| clean begin | begin physical contact between cleaner and client | |
| clean end | end physical contact between cleaner and client |
All cleaning stations where footage was annotated housed 3–5 individual cleaners. Although we annotated the behaviours of all shrimp, we analysed data from only the first shrimp to either antenna whip or clean (whichever came first) during each interaction. This allowed us to isolate the influence of single pairwise cleaner–client interactions, without the secondary influence of additional cleaners. Additionally, we restricted our analyses to the subset of client species in which we observed colour change or for which we found published evidence of colour change ability (electronic supplementary material, table S2).
(c). Sequential analysis
We analysed behavioural sequences from 199 interactions at eight cleaning stations (11–41 interactions per cleaning station); in total, we observed 18 individual shrimp and 10 species of client fish (electronic supplementary material, table S1). Sequences were analysed in two ways. First, we identified common strings of behaviours across the dataset and used the R [33] package TraMineR [34] to calculate the likelihood that a given series of behaviours occurred in a certain order. This allowed us to examine all behavioural sequences that lead to cleaning, as well as the probability that a shrimp that antenna whipped would clean later in the interaction, even if it was not the immediate next behaviour in a sequence.
Second, we examined sequences by focusing only on transitions between pairs of behaviours. We rearranged the data into a two-column format where the left column represented a behaviour and the right column represented the behaviour immediately following, reducing the behavioural sequences to their component transitions. Transitions were then summarized using an adjacency matrix [29], in which each row corresponds to an antecedent behaviour and each column to a consequent behaviour, with each cell representing the number of times each transition occurred across the entire dataset. We first created adjacency matrices for each cleaning station, to examine how the frequency of each behavioural transition varied across stations. Stations were highly consistent in which transition was the most common for each antecedent behaviour (see Results and electronic supplementary material, table S3); thus, data for all stations were combined for further analysis.
We analysed these transitions using the package igraph [35] in R. To test whether behaviours were followed by other behaviours more often than would be expected by chance, we created a permuted null distribution showing the frequency of each transition expected by chance and compared that distribution to our observed data (as in [36,37]). To do this, we permuted the second column of our two-column database of observed behavioural transitions 10 000 times, maintaining the relative frequency of each behaviour while randomizing the transitions between behaviours. For each of the 10 000 resampling iterations, we generated an adjacency matrix.
When permuting the transitions in our dataset, several temporally impossible transitions were created, including ‘clean begin → clean begin', ‘clean end → clean end’, transitions where any behaviour comes before ‘interaction begin' and transitions where any behaviour comes after ‘interaction end'. Additionally, we disallowed ‘colour change to light → colour change to light' because once an animal adopted light coloration, it was never observed to get even lighter immediately. By contrast, we did observe individuals transitioning to a darker mottled morph, and then later adopting fully dark coloration, meaning that ‘colour change to dark → colour change to dark’ was possible. This resulted in a set of 20 disallowed transitions. To account for this, after generating a resampled adjacency matrix, we replaced the disallowed transitions with 0 and divided the entire matrix by the total number of allowed transitions. Thus, each cell in the adjacency matrix represented the frequency of a given transition, normalized to the number of allowed transitions in the permuted dataset.
We then calculated a null distribution for each behavioural transition using the adjacency matrices from all resampling iterations; this represented a distribution of expected transition frequencies for each pair of behaviours if behavioural transitions occurred randomly, but with the relative frequency of each behaviour remaining constant. From the null distribution, we extracted the 99.92% quantile (the Bonferroni-corrected significance given that 61 allowed transitions were tested in parallel, although our results were qualitatively similar without the Bonferroni correction) for each transition and compared these quantiles to our observed values. Transitions in our observed dataset that occurred more frequently than their respective 99.92% null quantile were considered to have occurred significantly more often than expected by chance. We then used igraph to visualize the significant transitions as networks.
(d). Experiments with visual ‘synthetic clients’
To test whether antenna whipping may be an olfactory sensing behaviour, we examined whether shrimp antenna whip in the absence of client olfactory cues. We presented shrimp with purely visual ‘synthetic clients' in the laboratory. Shrimp were housed individually at Duke University (Durham, NC) on a 12 L : 12 D cycle, in artificial seawater (23–24°C; salinity 28–31ppt) made from Instant Ocean (United PetGroup, Blacksburg, VA, USA). Animals were fed one stick of Crab Cuisine (Hikari, CA, USA) on the day before a trial.
We used an iPad mini 2 (Apple, Inc., Cupertino, CA, USA), which rested against the glass outside of the tank, to display synthetic clients to cleaners (n = 9 individuals). At the beginning of the majority of trials, shrimp were resting on plastic perches 25 cm from the iPad. Synthetic clients were images that varied in shape (rectangles, circles or triangles), colour (black or white) and motion (‘moving' stimuli, which moved across without stopping, and ‘still' stimuli, which moved to the centre of the screen and stopped). Each stimulus covered the same total area, was present on the screen for 20 s, and was displayed on the same grey background. In between each 20 s stimulus presentation, 2 min of grey background served as a control. Stimuli were presented to the shrimp in random order, and over the course of the experiment, each shrimp saw each stimulus between four and ten times, for a total of 1210 trials.
To test whether cleaners were responding to the spatial aspects of the stimulus or the change in ambient light that occurred when stimuli entered the screen, we designed stimuli where the entire image was either lighter or darker grey. This eliminated the spatial stimulus while maintaining the same change in ambient light. We then performed a second set of behavioural trials (n = 5 individuals) where from the control grey screen, the screen would change to either the darker or the lighter shade for 20 s.
During trials, the tank was lit from overhead by an LED white light panel (Fotodiox Pro LED 312-DS, Fotodiox Inc., IL, USA) held at constant brightness and a correlated colour temperature of 5600 K. Trials were recorded using a GoPro camera that was placed at the side of the tank so that the observer was blind to stimulus identity. All footage was annotated using BORIS, and all instances of antenna whipping, moving towards the stimulus, tapping the stimulus with antennae or climbing on the stimulus were noted. To compare equal amounts of time for stimulus present and stimulus absent (control), we randomly extracted 20 s from each 2 min control clip for use in data analysis.
To statistically examine frequency of antenna whipping, we used the statistical software JMP Pro 13 (SAS Institute, Inc.). Shapiro–Wilk tests showed non-normality, and both Brown & Forsythe [38] and Levene [39] tests showed unequal variances, so we used an unequal variances (Welch's) t-test on ranked data to compare the mean tendencies of two groups [40], and a Welch's ANOVA to compare between more than two groups. Significance level was Bonferroni-corrected to 0.01 (n = 5 comparisons).
(e). Accounting for receiver vision
To examine whether spatial and colour information from signalling traits is available to the relevant receivers when accounting for receiver visual capabilities, we created still frames and videos of putative signals that were modified based on the receiver's acuity and colour vision. First, we used the R package AcuityView [41] to modify individual frames to display only the spatial information that is resolvable by a viewer, given the viewer's acuity and the distance to the object being viewed. We then modified the images based on receiver colour vision, where possible, and recompiled those frames into a video to examine whether signals are perceptible by signal receivers.
Ancylomenes pedersoni has low acuity (0.12 cycles degree−1) and monochromatic vision (λmax = 518 nm [42]). Therefore, we approximated A. pedersoni colour vision by displaying only the green channel from the image. For client vision, we selected clients with both the lowest and highest known acuities for reef-dwelling fish reported in a recent review of fish acuity [43]. Most reef fish, especially those in the shallow waters where cleaners are found, have either tri- or tetra-chromatic colour vision [30,44]; therefore, we display images as the colours appear to humans, under the assumption that many client fish could see at least all of the colour information available to humans.
3. Results
(a). Cleaning interactions are initiated by antenna whipping and/or colour change
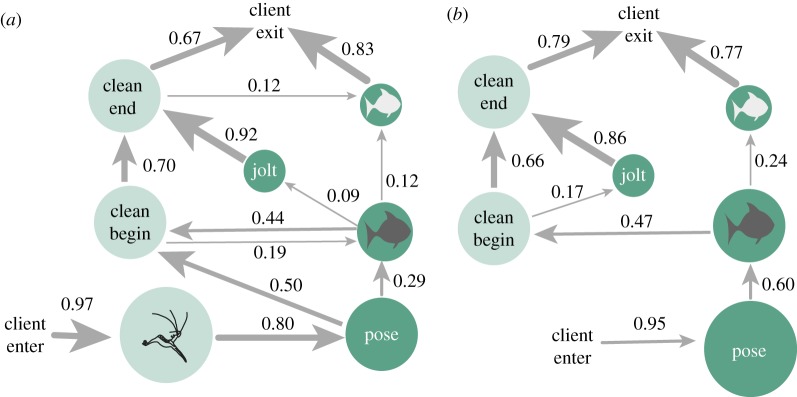
A network of our complete dataset showed that, following client arrival at a cleaning station, there were only two significant transitions away from client arrival. Thus, after a client arrived at a cleaning station, the immediate next behaviour was either antenna whipping by cleaners (probability = 0.70) or posing by clients (0.29) (figure 1; see electronic supplementary material, figure S1 for a complete network that includes transitions that were observed significantly less often than predicted by chance). Cleaning was observed 137 times, but only seven behavioural sequences lead to the initiation of cleaning (table 2). Of those, 95% of sequences pass through either antenna whipping, client colour change, or both.
Figure 1.
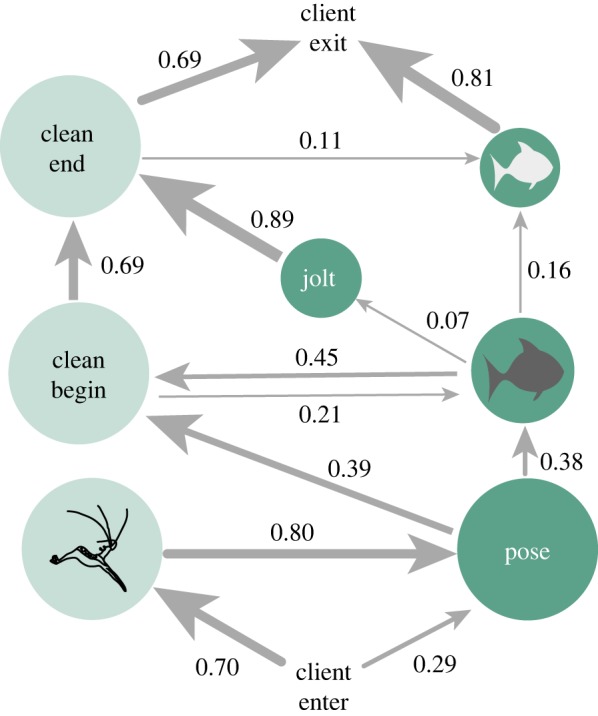
Network diagram of the significant behavioural transitions across 199 interactions between cleaner shrimp (light) and client fish (dark). Icons represent antennae whipping by shrimp, and colour change to dark and light by clients (table 1 caption). Network vertices (circles) represent behaviours and are scaled to represent the number of times that behaviour was observed. Transitional probabilities—the probability of progressing to a subsequent behaviour from a given current behaviour—between each behaviour are shown by numbers and the thickness of the network edges (arrows; thicker edges represent more common transitions). (Online version in colour.)
Table 2.
Behavioural sequences that led to cleaning (n= 137 interactions), and the probability of that sequence occurring. If a given behaviour occurred twice in immediate succession, it was collapsed to a single instance for clarity in the table.
| sequence | n | probability |
|---|---|---|
| antenna whip → pose → clean begin | 67 | 0.49 |
| antenna whip → pose → colour change dark → clean begin | 32 | 0.23 |
| pose → colour change dark → clean begin | 18 | 0.13 |
| antenna whip → clean begin | 9 | 0.07 |
| pose → clean begin | 7 | 0.05 |
| pose → antenna whip → clean begin | 3 | 0.02 |
| antenna whip → colour change dark → pose → clean begin | 1 | 0.01 |
(b). Antenna whipping signals cleaner intent to clean
(i). Sequential analysis
Visualizing the behavioural sequences where antennae whipping did occur (n = 144; figure 2a) and those where it did not (n = 55; figure 2b) showed that, when antennae whipping occurred, the only statistically significant transition from antenna whipping was to client posing. This indicates that antenna whipping is followed by a consistent state change by the receiver. When antenna whipping occurred, it was immediately followed by client posing in 80% (n = 115) of interactions. Additionally, in the 169 interactions where posing occurred, it was immediately preceded by antenna whipping in 73% (n = 119) of interactions. In an additional four interactions, antenna whipping preceded posing, but not immediately, as cleaning began before the fish had adopted a completely still pose. Examining each station individually showed that the most common behaviour after antenna whipping was posing at 6 of 8 stations; at the remaining two, antenna whipping was most often followed by further antenna whipping, and then by posing (electronic supplementary material, table S3).
Figure 2.
Network diagram of the significant transitions between behaviours across (a) 144 interactions in which antenna whipping by cleaner shrimp occurred and (b) 55 interactions in which antenna whipping by cleaner shrimp did not occur. Network parameters are described in figure 1. (Online version in colour.)
Antenna whipping also predicted cleaning behaviour by shrimp (i.e. the future behaviour of the signaller). In 82% of interactions (n = 112) where cleaning occurred, the previous behaviour by that same shrimp was antenna whipping. Additionally, of 144 interactions in which we observed antenna whipping, it was ultimately followed by cleaning in 80% of interactions (n = 115).
(ii). Synthetic clients
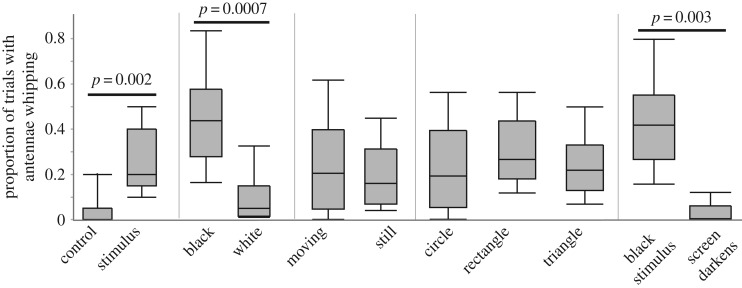
Experiments using synthetic clients showed that a visual stimulus is sufficient to elicit antenna whipping behaviour; shrimp antenna whipped in a significantly higher proportion of trials when stimuli were present than when stimuli were absent (t11.3 = 4.0, p = 0.002; figure 3). However, not all visual stimuli elicit antenna whipping. Shrimp antenna whipped significantly more frequently in response to black than white stimuli (t12 = 4.5, p = 0.0007), but equally to moving and still stimuli (t14 = 0.49, p = 0.63), as well as to circles, rectangles and triangles (F16=0.60, p = 0.56) (figure 3a). Antenna whipping towards dark stimuli was not explained simply by the change in ambient light associated with black stimuli: shrimp antenna whipped in response to black stimuli significantly more often than to the screen darkening (t9.7 = 5.6, p = 0.0003) (figure 3).
Figure 3.
The frequency with which the cleaner shrimp A. pedersoni antenna whips in response to visual stimuli on an iPad screen. Boxes are quantile plots, showing the median (line), 25th and 75th quantiles (box) and minimum and maximum (whiskers). Significant differences are shown by bars with p-values; note that the Bonferroni-corrected significance level for five comparisons was 0.01. Statistics are from a Welch's t-test (or ANOVA in the case of three groups) of unequal variances. Sample size for all comparisons is nine individuals, except for the black stimulus versus screen darkens, where the sample size was five individuals.
If antenna whipping was only a way to sample the olfactory environment and assess whether clients were present or not, one would expect no further behaviour after antenna whipping, because the lack of olfactory cues should indicate the stimulus is not a client fish. However, we found that after antenna whipping, shrimp interacted further with visual stimuli in more than half of trials. Moving towards the stimulus occurred in the highest proportion of trials, with a median (interquartile range) of 0.41 (0.33–0.47), followed by climbing on the stimulus as if to clean it (0.15, 0.05–0.30) and tapping on the stimulus with antennae (0.03, 0–0.05). Additionally, climbing on the stimulus was preceded by antenna whipping by the shrimp in a high proportion of trials, with a median (interquartile range) of 0.67 (0.63–0.74).
(iii). Accounting for reef fish vision
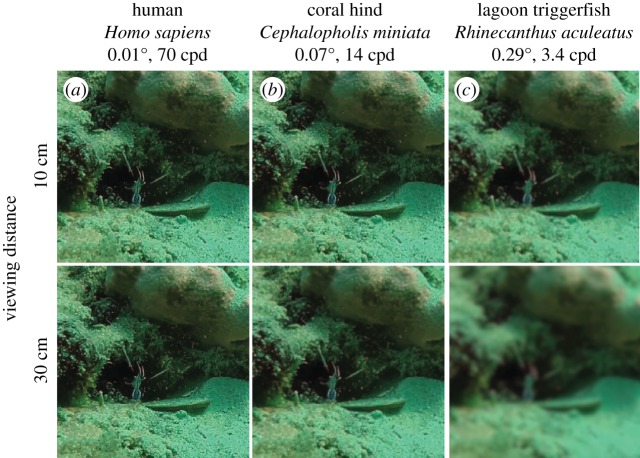
Both static images (figure 4) and videos (electronic supplementary material, videos S1–S3) suggest that antenna whipping is visible to clients with low acuity from 10 cm (a distance previously cited as a standard interaction distance [45,46], and which aligns with our observations of cleaner–client interactions), and to fish with high acuity from at least 30 cm.
Figure 4.
Images of A. pedersoni, for humans (a) and two reef fish at the high (b) and low (c) ends of known reef fish acuity. Based on the size of the shrimp, the image was assumed to be 5 cm across. Column labels show minimum resolvable angle in degrees and acuity in cycles per degree (cpd).
(b). Colour change may signal client desire to be cleaned in the absence of antenna whipping
Given that antenna whipping by cleaners appears to indicate intent to clean, we examined whether clients can still induce cleaning in the absence of antenna whipping. Examining a network for the 55 interactions in which antenna whipping did not occur (figure 2b) shows that once clients pose, the only statistically significant path to cleaning is via colour change to dark. In these interactions, change to dark was followed by cleaning in 47% of interactions (n = 25), and cleaning was preceded by a change to dark in 50% of interactions (n = 28). In the absence of antenna whipping, clients that posed but did not colour change to dark had a 15% chance of being cleaned. Thus, changing to a dark morph more than tripled the likelihood of cleaning. Further, in our experiments with synthetic clients we found that shrimp climbed onto dark stimuli in a significantly higher proportion of trials (median, interquartile range; 0.94, 0.67–0.95) than light stimuli (0.06, 0.00–0.33; t15.7 = 10.5, p < 0.0001).
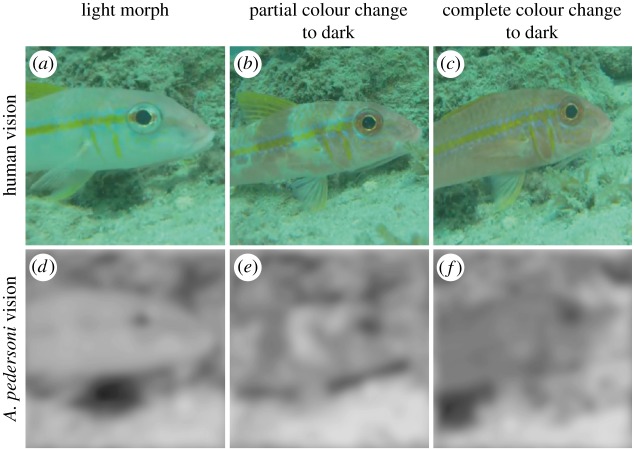
The order of colour change and cleaning that we observed suggests that colour change to a dark morph is not a response to the act of being cleaned. As can be seen in figures 1 and 2, and in electronic supplementary material, table S1, changing to a dark morph occurred before, or entirely without, physical contact by a cleaner shrimp in 74 of 110 cases (67%). Additionally, in 102 out of 137 cases, cleaning occurred without any colour change by the client, indicating that physical contact by cleaners is not sufficient to induce colour change. Thus, colour change to a dark morph does not appear to be an automatic response to presence at a cleaning station or to physical contact by shrimp. Lastly, despite having coarse, monochromatic vision, visual information about changes from light to dark is available to A. pedersoni viewers, especially given the short distances over which cleaners and clients interact (figure 5; electronic supplementary material, video S4).
Figure 5.
As a representative example, the yellow goatfish Mulloidichthys martinicus in light (a,d), partial dark (b,e) or complete dark coloration (c,f), as seen by a human (a–c) or A. pedersoni (d–f). We assumed the client was 10 cm from the cleaner, a standard interaction distance [45,46], and that the portion of the fish being viewed is 7 cm long.
4. Discussion
Taken together, the data presented here support the hypothesis that signalling mediates cleaning interactions. This study (1) is the first study of signalling in cleaner shrimp to use in situ behavioural observations in the absence of the confounding presence of human observers, (2) suggests that antenna whipping by A. pedersoni is a signal and (3) is the first study of which we are aware to demonstrate a signalling role for client colour change.
(a). Antenna whipping is a reliable signal of a cleaner's intent to clean
Antenna whipping was consistently associated with the outcome of the interaction (i.e. subsequent cleaning behaviour by that shrimp), indicating that a receiver could extract reliable information about a cleaner's intent to clean from the signal. Additionally, antenna whipping is consistently followed by a state change (adopting a cleaning solicitation pose) by the receiver, a behaviour which allows cleaning to begin and thus benefits the receiver. Currently, the balance of evidence from two other empirical studies of signalling in cleaner shrimp [45,46] aligns with the claim that cleaner shrimp signal to identify themselves as willing to clean, rather than, for example, to attract clients from long distances to cleaning stations. However, it has been shown that clients will pose for anemones where A. pedersoni is not visible [13], and we viewed some interactions where clients posed without any signalling; thus, antenna whipping is not a necessary precursor to posing. Whether signalling modifies client posing behaviour, perhaps causing them to pose for longer or reinforcing that a given cleaning station is still active and thus resulting in repeated visits by the same client, has not yet been studied.
Several lines of evidence support the hypothesis that cleaner signalling traits have been evolutionarily modified for signalling. First, it has been noted in scientific and hobby literature that the antennae of A. pedersoni are longer and whiter than those of related non-cleaning species [14,15,47], suggesting some morphological modification of antennae in A. pedersoni for signalling. Second, studies of the congener A. longicarpus have shown that this species signals with slender white chelae [46], a signal similar in form to A. pedersoni, but using a different body part. Third, the cleaner shrimps A. longicarpus and Urocaridella sp. C both modify their signalling (chelae clapping and body rocking, respectively) based on hunger level [9,45,46], and when given a choice between a hungry and a satiated cleaner, client fish more often choose to be cleaned by the hungry cleaner [45], showing that clients respond to the modified signal.
(b). Colour change as a signal by client fish
Our results also suggest that client colour change to a darker morph may signal that a client wants to be cleaned. Clients who changed colour to a darker morph roughly tripled their chances of being cleaned when cleaners had not already signalled intent to clean, colour change is perceptible by cleaner shrimp, and in laboratory manipulations cleaners preferentially tried to clean dark stimuli over light stimuli. As further evidence, when we observed clients change back to a light morph (n = 41), it was followed either by departing the cleaning station (probability of 0.83) or by cleaning ending (0.17). Thus, clients may adopt a dark coloration as a signal before or during cleaning and switch back to light coloration only as cleaning ends. Ours is the first study, to our knowledge, to demonstrate any purported signalling function for client colour change by quantifying how it affects cleaner behaviour.
Our study focused on species of client fish with observed or previously recorded colour changing abilities. There are, however, client species that we never observed changing colour and for which we could find no evidence of colour change ability in the literature (electronic supplementary material, table S2), but these species also received cleaning services. Although it has been shown that cleaner fish prefer client species with higher average ectoparasite loads [48], the role that client signalling might play in whether some clients are preferred has not been examined.
Whether colour change has been modified in some way for a signalling function has not been studied. Numerous fishes colour change for mating, aggression or for camouflage [49], so a colour change signal may have been exapted from colour change in a different behavioural context. Lastly, behavioural sequences showed that colour change to dark is not a response to cleaning or posing, and that not all clients change colour in the presence of cleaners; this is in line with the hypothesis that colour change is a signal to solicit cleaning. The conditions under which individuals choose to colour change deserve further study.
5. Conclusion
Our study suggests that signalling, primarily by the cleaner but secondarily by the client, is important to initiate cleaning. In only 4 of 199 interactions did cleaning result when neither antenna whipping nor colour change occurred. These findings have implications for understanding how cleaning mutualisms are mediated and maintained. Cleaners and clients are an intriguing and accessible system for studying interspecific, communication-style exchanges of signals, in which reliability appears to have arisen as a result of shared interests between parties and not genetic relatedness between sender and receiver. Finally, mutualisms are a promising area for exploring the selection pressures that receivers can place on senders when the two parties have very different sensory capabilities. As shown here, differences in sensory systems between species can sometimes result in signals, such as antenna whipping, that the producer probably cannot resolve, but which are directed solely at partner species.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Stephen Nowicki and two anonymous reviewers for advice on earlier versions of this manuscript and Dr. Robert Fitak for statistical advice. We also thank the staff at the CARMABI field station for their assistance during fieldwork, and Helen Tan, Kelsey O'Donnell and Zoe Johnsen for assistance annotating videos.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4b4q7nq [50].
Authors' contributions
E.M.C. carried out fieldwork, annotated behavioural videos, performed sequential and network analysis and drafted the manuscript; P.A.G. assisted with fieldwork and the statistical aspects of sequential and network analysis, and gave feedback on the manuscript; S.J. participated in the design of the study and gave feedback on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding for this study was provided by The Crustacean Society grants in aid programme, a Duke University International Travel award and a Duke University Sigma Xi grant, all to E.M.C. P.A.G. and S.J. did not receive any funding specific to this project.
References
- 1.Kokko H. 1997. Evolutionarily stable strategies of age-dependent sexual advertisement. Behav. Ecol. Sociobiol. 41, 99–107. ( 10.1007/s002650050369) [DOI] [Google Scholar]
- 2.Otte D. 1974. Effects and functions in the evolution of signaling systems. Annu. Rev. Ecol. Syst. 5, 385–417. ( 10.1146/annurev.es.05.110174.002125) [DOI] [Google Scholar]
- 3.Johnstone RA, Grafen A. 1993. Dishonesty and the handicap principle. Anim. Behav. 46, 759–764. ( 10.1006/anbe.1993.1253) [DOI] [Google Scholar]
- 4.Searcy WA, Nowicki S. 2005. The evolution of animal communication. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Vehrencamp SL, Hall ML, Bohman ER, Depeine CD, Dalziell AH. 2007. Song matching, overlapping, and switching in the banded wren: the sender's perspective. Behav. Ecol. 18, 849–859. ( 10.1093/beheco/arm054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilford T, Dawkins MS. 1995. What are conventional signals? Anim. Behav. 49, 1689–1695. ( 10.1016/0003-3472(95)90090-X) [DOI] [Google Scholar]
- 7.Vehrencamp SL. 2000. Handicap, index, and conventional signal elements of bird song. In Animal signals: signalling and signal design in animal communication (eds Espmark Y, Amundsen T, Rosenqvist G), pp. 277–300. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 8.Becker JHA, Grutter AS. 2004. Cleaner shrimp do clean. Coral Reefs 23, 515–520. ( 10.1007/s00338-004-0429-3) [DOI] [Google Scholar]
- 9.Becker JHA, Grutter AS. 2005. Client fish ectoparasite loads and cleaner shrimp Urocaridella sp. C hunger levels affect cleaning behaviour. Anim. Behav. 70, 991–996. ( 10.1016/j.anbehav.2005.01.004) [DOI] [Google Scholar]
- 10.Bshary R, Oliveira RF, Oliveira TS, Canário AV. 2007. Do cleaning organisms reduce the stress response of client reef fish? Front. Zool. 4, 21 ( 10.1186/1742-9994-4-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher DH, James S, Keeler KH. 1982. The ecology of mutualism. Annu. Rev. Ecol. Syst. 13, 315–347. ( 10.1146/annurev.es.13.110182.001531) [DOI] [Google Scholar]
- 12.Hasson O. 1994. Cheating signals. J. Theor. Biol. 167, 223–238. ( 10.1006/jtbi.1994.1065) [DOI] [Google Scholar]
- 13.Huebner LK, Chadwick NE. 2012. Reef fishes use sea anemones as visual cues for cleaning interactions with shrimp. J. Exp. Mar. Bio. Ecol. 416–417, 237–242. ( 10.1016/j.jembe.2012.01.004) [DOI] [Google Scholar]
- 14.Limbaugh C, Pederson H, Chace FA. 1961. Shrimps that clean fishes. Bull. Mar. Sci. Gulf Caribb. 11, 237–257. [Google Scholar]
- 15.Ellis GR. 1985. Cleaner Shrimp. Freshw. Mar. Aquarium 8, 83–86. [Google Scholar]
- 16.Mahnken C. 1972. Observations on cleaner shrimps of the genus Periclimenes. Bull. Nat. Hist. Museum Los Angeles Cty. Sci. 14, 71–83. [Google Scholar]
- 17.Titus BM, Daly M, Exton DA. 2015. Temporal patterns of Pederson shrimp (Ancylomenes pedersoni Chace 1958) cleaning interactions on Caribbean coral reefs. Mar. Biol. 162, 1651–1664. ( 10.1007/s00227-015-2703-z) [DOI] [Google Scholar]
- 18.Johnasson M. 1987. Fish cleaning behaviour of shrimp. J. Zool. Lond. 213, 117–131. ( 10.1111/j.1469-7998.1987.tb03682.x) [DOI] [Google Scholar]
- 19.Mead K, Koehl M. 2000. Stomatopod antennule design: the asymmetry, sampling efficiency and ontogeny of olfactory flicking. J. Exp. Biol. 203, 3795–3808. [DOI] [PubMed] [Google Scholar]
- 20.Côté IM. 2000. Evolution and ecology of cleaning symbioses in the sea. Oceanogr. Mar. Biol. 38, 311–355. [Google Scholar]
- 21.Côté IM, Arnal C, Reynolds JD. 1998. Variation in posing behaviour among fish species visiting cleaning stations. J. Fish Biol. 53, 256–266. ( 10.1111/j.1095-8649.1998.tb01031.x) [DOI] [Google Scholar]
- 22.Losey GS. 1972. Behavioural ecology of the ‘cleaning fish’. Aust. Nat. Hist. 17, 232–238. [Google Scholar]
- 23.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 24.Feder HM. 1966. Cleaning symbiosis in the marine environment. In Symbiosis I: associations of microorganisms, plants, and other organisms (ed. Henry SM.), pp. 327–380. New York, NY: Academic Press. [Google Scholar]
- 25.Randall JE. 1962. Fish service stations. Sea Front. 8, 40–47. [Google Scholar]
- 26.Hobson ES. 1965. Diurnal-nocturnal activity of some inshore fishes in the Gulf of California. Copeia 3, 291–302. ( 10.2307/1440790) [DOI] [Google Scholar]
- 27.Hobson ES. 1971. Cleaning symbiosis among California inshore fishes. Fish. Bull. 69, 491–523. [Google Scholar]
- 28.Vaughan DB, Grutter AS, Costello MJ, Hutson KS. 2017. Cleaner fishes and shrimp diversity and a re-evaluation of cleaning symbioses. Fish Fish. 18, 698–716. ( 10.1111/faf.12198) [DOI] [Google Scholar]
- 29.Castellan NJ. 1979. The analysis of behavior sequences. In The analysis of social interactions: methods, issues, and illustrations (ed. Cairns RB.), pp. 81–118. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 30.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.Titus BM, Daly M, Exton DA. 2015. Do reef fish habituate to diver presence? Evidence from two reef sites with contrasting historical levels of SCUBA intensity in the Bay Islands, Honduras. PLoS ONE 10, e0119645 ( 10.1371/journal.pone.0119645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. ( 10.1111/2041-210X.12584) [DOI] [Google Scholar]
- 33.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 34.Gabadinho A, Ritschard G, Müller NS, Studer M. 2011. Analyzing and visualizing state sequences in R with TraMineR. J. Stat. Softw. 40, 1–37. ( 10.18637/jss.v040.i04) [DOI] [Google Scholar]
- 35.Csardi G, Nepusz T.. 2006. The igraph software package for complex network research. InterJournal Complex Syst. 1695. [Google Scholar]
- 36.Green PA, Patek SN. 2018. Mutual assessment during ritualized fighting in mantis shrimp (Stomatopoda). Proc. R. Soc. B 285, 20172542 ( 10.1098/rspb.2017.2542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakeman R, Robinson BF, Quera V. 1996. Testing sequential association: estimating exact p values using sampled permutations. Psychol. Methods 1, 4–15. ( 10.1037/1082-989X.1.1.4) [DOI] [Google Scholar]
- 38.Brown MB, Forsythe AB. 1974. Robust tests for the equality of variances. J. Am. Stat. Assoc. 69, 364–367. ( 10.1080/01621459.1974.10482955) [DOI] [Google Scholar]
- 39.Levene H. 1960. Robust tests for the equality of variances. In Contributions to probability and statistics (ed. Olkin I.), pp. 278–292. Palo Alto, CA: Stanford University Press. [Google Scholar]
- 40.Ruxton GD. 2006. The unequal variance t-test is an underused alternative to Student's t-test and the Mann-Whitney U test. Behav. Ecol. 17, 688–690. ( 10.1093/beheco/ark016) [DOI] [Google Scholar]
- 41.Caves EM, Johnsen S. 2017. AcuityView: an R package for portraying the effects of visual acuity on scenes observed by an animal. Methods Ecol. Evol. 9, 793–797. ( 10.1111/2041-210X.12911) [DOI] [Google Scholar]
- 42.Caves EM, Frank TM, Johnsen S. 2016. Spectral sensitivity, spatial resolution, and temporal resolution and their implications for conspecific signalling in cleaner shrimp. J. Exp. Biol. 219, 597–608. ( 10.1242/jeb.122275) [DOI] [PubMed] [Google Scholar]
- 43.Caves EM, Sutton TT, Johnsen S. 2017. Visual acuity in ray-finned fishes correlates with eye size and habitat. J. Exp. Biol. 220, 1586–1596. ( 10.1242/jeb.151183) [DOI] [PubMed] [Google Scholar]
- 44.Marshall J, Carleton KL, Cronin T. 2015. Colour vision in marine organisms. Curr. Opin. Neurobiol. 34, 86–94. ( 10.1016/j.conb.2015.02.002) [DOI] [PubMed] [Google Scholar]
- 45.Becker JHA, Curtis LM, Grutter AS. 2005. Cleaner shrimp use a rocking dance to advertise cleaning service to clients. Curr. Biol. 15, 760–764. ( 10.1016/j.cub.2005.02.067) [DOI] [PubMed] [Google Scholar]
- 46.Chapuis L, Bshary R. 2010. Signalling by the cleaner shrimp Periclimenes longicarpus. Anim. Behav. 79, 645–647. ( 10.1016/j.anbehav.2009.12.012) [DOI] [Google Scholar]
- 47.Wicksten MK. 1995. Within-species variation in Periclimenes yucatanicus (Ives), with taxonomic remarks on P. pedersoni Chace (Crustacea: Decapoda: Caridea: Palaemonidae). Proc. Biol. Soc. Washingt. 108, 458–464. [Google Scholar]
- 48.Arnal C, Côté IM, Sasal P, Morand S. 2000. Cleaner-client interactions on a Caribbean reef: influence of correlates of parasitism. Behav. Ecol. Sociobiol. 47, 353–358. ( 10.1007/s002650050676) [DOI] [Google Scholar]
- 49.Townsend C. 1929. Records of changes in color among fishes. Zoologica IX, 321–378. [Google Scholar]
- 50.Caves EM, Green PA, Johnsen S. 2018. Data from: Mutual visual signalling between the cleaner shrimp Ancylomenes pedersoni and its client fish Dryad Digital Repository. ( 10.5061/dryad.4b4q7nq) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Caves EM, Green PA, Johnsen S. 2018. Data from: Mutual visual signalling between the cleaner shrimp Ancylomenes pedersoni and its client fish Dryad Digital Repository. ( 10.5061/dryad.4b4q7nq) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.4b4q7nq [50].