Abstract
Even though deficits in inhibitory control and conflict monitoring are well-known in ADHD, factors that further modulate these functions remain to be elucidated. One factor that may be of considerable importance is how inhibitory control is modulated by multisensory information processing. We examined the influence of concurrent auditory conflicting or redundant information on visually triggered response inhibition processes in adolescent ADHD patients and healthy controls. We combined high-density event-related potential (ERP) recordings with source localization to delineate the functional neuroanatomical basis of the involved neurophysiological processes. In comparison to controls, response inhibition (RI) processes in ADHD were compromised in conflicting conditions, but showed no differences to controls when redundant or no concurrent auditory information was presented. These effects were reflected by modulations at the response selection stage (P3 ERP) in the medial frontal gyrus (BA32), but not at the attentional selection (P1, N1 ERPs) or resource allocation level (P2 ERP). Conflicting information during RI exerts its influences in adolescent ADHD via response selection mechanisms, but not via attentional selection. It is not the mere presence of concurrent information, but the presence of conflicting information during RI that may destabilize goal shielding processes in medial frontal cortical regions, by means of increasing the automaticity of response tendencies. The occurring RI deficits might relate to the increased impulsivity in adolescent ADHD and a corresponding vulnerability to react to an increased automaticity of pre-potent response tendencies. ADHD patients show a bias to a specific content of information which can modulate inhibitory control.
Keywords: ADHD, Response inhibition, Automaticity of response tendencies, Goal-shielding processes, Action control
Highlights
-
•
Response inhibition (RI) performance in ADHD is modulated by multisensory information.
-
•
Only incongruent/conflicting concurrent information modulates RI performance.
-
•
RI deficits occur if this conflicting information braces the automaticity of response tendencies.
-
•
These deficits relate to a predisposition of ADHD to engage in impulsive behavior.
-
•
This may be due to deficient goal-shielding processes located in the MFG.
1. Introduction
Attention Deficit/Hyperactivity Disorder (ADHD) is one of the most prevalent neurodevelopmental disorders (Polanczyk et al., 2007). The diagnose of ADHD and the corresponding subtypes (inattentive, the hyperactive/impulsive or the combined ADHD subtype) is based on the expression of the three core symptoms inattention, hyperactivity and impulsivity (Ahmadi et al., 2014; Barkley, 1997; Randall et al., 2009). Besides these three core symptoms, deficits in executive functions, conflict monitoring and especially in inhibitory control are increasingly focused upon in current ADHD research (Albrecht et al., 2013; Bluschke et al., 2016b; Booth et al., 2005; Hart et al., 2013; Rubia et al., 2005; Wright et al., 2014). The deficits in inhibitory control are particularly important to consider because inhibitory deficits have been shown to be a major factor for the educational outcomes of adolescent ADHD (Berlin et al., 2003; Loe and Feldman, 2007). While the necessity to consider inhibitory deficits in adolescent ADHD is without question (Aron and Poldrack, 2005; Hart et al., 2014), it is unknown what factors, or boundary conditions modulate response inhibition processes in adolescent ADHD on a behavioral and neurofunctional level. This question is of major relevance for patients with adolescent ADHD, because employment opportunities are dependent on educational success. The exact identification of the nature of inhibitory deficits in adolescent ADHD may grant a possibility to create environmental settings, in which adolescents with ADHD are less prone to exhibit ADHD-specific behavioral problems and might thus help to improve educational outcome and opportunities in their future lives. On a neurofunctional level, the brain has been shown to undergo immense developmental processes during ongoing brain maturation between childhood and adolescence (Sowell et al., 2001, Sowell et al., 2003) and especially response inhibition functions are assumed to not fully mature until early adolescence (for review: Luna and Sweeney, 2004). In line with this observation, response inhibition deficits have been shown to be more pronounced in children than in adolescents with ADHD (Tillman et al., 2008). This suggests that examining children with ADHD might be challenging, as inhibitory deficits might be too pronounced to allow a reliable examination of response inhibition processes. This particularly relates to the examination of neurofunctional correlates of the modulation of response inhibition processes. Children with ADHD might be overstrained by the necessary number of trials, which would have to be presented in order to examine modulatory aspects of response inhibition with a sufficiently big signal-to-noise-ratio for the analysis of neurofunctional data. Concerning adults, response inhibition processes are not assumed to develop much further between adolescence and adulthood (Luna and Sweeney, 2004; Williams et al., 1999). Furthermore, the importance of educational settings for achievements in future life is continuously reduced in adulthood. Therefore, the examination of response inhibition processes seems particularly relevant in adolescent ADHD.
One factor that may be of considerable importance in the context of adolescent ADHD is how inhibitory control is modulated by multisensory information processing. In healthy controls it was shown that redundant auditory (concurrent) information facilitates response inhibition performance (Chmielewski et al., 2015), while conflicting auditory (concurrent) information compromises response inhibition performance (Chmielewski et al., 2015). The improvement of inhibitory control by means of presenting a redundant auditory NoGo stimulus alongside the primary visual NoGo information relates to a corresponding decrease in the automaticity of response tendencies, which is be beneficial for inhibitory control (Chmielewski et al., 2015, Chmielewski et al., 2016). Opposed to that, presenting a conflicting auditory Go stimulus alongside the primary visual NoGo information increases the automaticity of response tendencies and thus aggravates inhibitory control in healthy controls (Chmielewski et al., 2015, Chmielewski et al., 2016). In comparison to healthy controls, ADHD patients exhibit increased impulsivity (Bari and Robbins, 2013; Barkley, 1997; Douglas, 1999; Durston et al., 2009) and a predisposition to engage in automatic behavior (Clark et al., 2000). It is therefore possible that inhibitory control in adolescent ADHD patients might be more affected whenever the automaticity of response tendencies is increased (Dippel et al., 2015; Stock et al., 2015), i.e. when conflicting auditory Go information is presented alongside the primary visual NoGo information. Alternatively, a similar pattern of results might occur because conflict monitoring processes are dysfunctional in adolescent ADHD (Albrecht et al., 2008; Bluschke et al., 2016a; McLoughlin et al., 2009). More specifically, as overcoming conflicts is a prerequisite to successfully inhibit inappropriate responses in the conflicting NoGo condition in this paradigm, overstrained conflict monitoring functions might potentiate already existing deficits in response inhibition. If such response inhibition deficits (due to increased automaticity or due to deficient conflict monitoring processes) were only revealed in the conflicting condition in adolescent ADHD, this would suggest that the mere presence of additional sensory input does not necessarily compromise cognitive performance in adolescent ADHD. Rather, deficits in cognitive control and response inhibition strongly depend on the content of additional information that needs to be integrated. Importantly, this would suggest that there is an ADHD-inherent bias to a specific content of information, which modulates inhibitory control.
However, another pattern of results is also possible: Since ADHD patients show an increased vulnerability to distracting information (Mullane et al., 2009; Pelham et al., 2011), response inhibition processes in adolescent ADHD may be particularly vulnerable to effects of multisensory information. When only considering the increased distractibility, or the predisposition to allocate residual attentional capacity to irrelevant distractors in adolescent ADHD (Chen and Cave, 2016), response inhibition performance in adolescent ADHD should be compromised whenever additional information is presented, irrespective of the content of the information.
To examine what cognitive-neurophysiological subprocesses during the process of response inhibition are differentially modulated by the content of concurrent information in adolescent ADHD, we use a system neurophysiological approach using high-density EEG recordings and source localization techniques:
If response inhibition processes in adolescent ADHD are compromised by concurrent information due to an increased distractibility (i.e. irrespective of the content of information), we expect this to be reflected in the N1 and P1 amplitude likely reflecting perceptual gating and bottom-up attentional selection unrelated to stimulus content (Herrmann and Knight, 2001). The neural sources of visual P1 and N1 modulations should then be detected in extrastriate cortical areas (Di Russo et al., 2002; Gomez Gonzalez et al., 1994; Heinze et al., 1994; Herrmann and Knight, 2001). Alternatively, the effects might also be reflected in resource allocation processes (indicated by modulations in the P2 amplitude), which are deployed to process sensory input (Geisler and Murphy, 2000; Sugimoto and Katayama, 2013). If differences in resource allocation processes would contribute to potential response inhibition deficits, this will be associated with modulations in activity in parieto-occipital regions (Freunberger et al., 2007).
If response inhibition deficits in adolescent ADHD would, however, only occur in the context of a specific content of multisensory information (due to an increased automaticity of response tendencies or due to conflict monitoring deficits in adolescent ADHD), we would expect this to reflected in the response selection stage. Such alterations in the response selection stage should relate to generation of response conflicts (Botvinick et al., 2001) and a corresponding engagement in goal-shielding processes at the response selection level, which are deployed to protect task goals (i.e. to successfully inhibit when responses would be inappropriate) from interference (Beste et al., 2017; Dreisbach and Haider, 2009; Gohil et al., 2017; Goschke and Bolte, 2014; Gruber and Goschke, 2004; Hofmann et al., 2012). During response inhibition it has repeatedly been shown that a frontal-midline NoGo-N2 event-related potential (ERP) component reflects pre-motor processes like conflict monitoring or updating of the response program, while a NoGo-P3 ERP-component reflects evaluative processing of the successful outcome of the inhibition or the inhibition process itself (Beste et al., 2010, Beste et al., 2011; Huster et al., 2013). These ERPs (NoGo-N2 and P3) at the response selection stage have already been shown to be reflected in alterations in the superior frontal gyrus (SFG), the supplementary motor area (SMA) and especially in the medial frontal gyrus (MFG) (Beste et al., 2010, Beste et al., 2011; Huster et al., 2013). More important, for the modulation of response inhibition processes by means of concurrent information, as intended in this study, especially the involvement of medial frontal areas has also been observed (Chmielewski et al., 2015, Chmielewski et al., 2016). This suggests that a potential modulation-related aggravation of response inhibition performance in adolescent ADHD should either be reflected in decreases in the NoGo-N2 or NoGo-P3 component and hence in a corresponding decreased activation in medial frontal structures during the response selection stage. This is particularly probable, because medial frontal and basal ganglia structures show changes in ADHD (Bos et al., 2017; Brieber et al., 2007; Hoogman et al., 2017) that are related to changes in GABA, glutamate and dopamine concentrations in this region, which also play a major role in inhibitory control (Ende et al., 2015; Umemoto et al., 2014; Villemonteix et al., 2015).
Taken together, we hypothesize two possible outcomes for the adolescent ADHD group. If there is an increased vulnerability to react to highly automatized response tendencies, or if, alternatively, deficits in processing conflicting information contribute to response inhibition deficits in the adolescent ADHD group, we expect response inhibition deficits to occur in response inhibition performance under conflicting information. This should be reflected in a decreased activation in the MFC and in alterations at the response selection level. More specifically, the vulnerability to react to highly automatized response tendencies should be reflected in a decreased P3 amplitude, while deficits in conflict monitoring processes should be reflected in a decreased N2 amplitude. If, however, the increased distractibility in adolescent ADHD is underlying for response inhibition deficits in adolescent ADHD, we would expect deficits to occur in response inhibition performance, whenever visual inhibitory information is accompanied by any concurrent auditory information (i.e. redundant or conflicting). This could either be reflected in perceptual gating and bottom-up attentional selection processes (P1 and N1), or at the resource allocation level (P2).
2. Materials and methods
2.1. Participants
N = 42 Caucasian participants (first language german) between 10 and 15 years of age were recruited. Adolescent ADHD patients were recruited by means of notices and direct addresses within the ADHD outpatient clinic within the University Hospital Carl Gustav Carus, while controls were recruited via notifications on newspapers and websites. ADHD diagnoses were determined according to standard clinical procedures (incl. Parent and child interview, teacher report, IQ testing, exclusion of potential underlying somatic disorders via EEG, EKG, audiometry and vision testing. ADHD patients (n = 21) with secured ICD-10 diagnoses (F90.0 - Hyperkinetic disorder: Disturbance of activity and attention, n = 16), (F90.1, − Hyperkinetic disorder: Hyperkinetic conduct disorder, n = 1) and F98.8, (Other specified behavioral and emotional disorders with onset usually occurring in childhood and adolescence: Attention deficit disorder without hyperactivity, n = 4) were included in the adolescent ADHD group (mean age: 13.16, S.D. = 1.40 years; 20 males; 4 left handed as assessed with the Edinburgh Handedness Inventory; 16 on medication using Medikinet retard (n = 6), Ritalin (n = 3), Concerta (n = 2), Strattera (n = 2), Elvanse (n = 2), Abilify (n = 1)). None of the controls (n = 21; mean age: 13.10, S.D. = 1.74 years; 11 males; 1 left handed as assessed with the Edinburgh Handedness Inventory) reported neurological or psychiatric disorders or use of medication. Vision and hearing were normal. There was no difference between the groups' IQ scores (ADHD: 105.4 ± 12.3; controls: 112 ± 14.5; p > .156), as obtained by the use of the Wechsler Intelligence Scale for Children (WISC-IV, Petermann and Petermann, 2011). Further, the Conners' Parent Rating Scale (Conners et al., 1998) supported the diagnosis of ADHD on all subscales (66.3 ± 1.48; cut-off > 60). The same is shown on the children's scale of Conners Comprehensive Behavior Rating Scales (61.38 ± 1.61; cut-off > 60). The DISPYPS-II (Diagnostik-System für psychische Störungen nach ICD −10) revealed scores above the cut-off for inattention (1.81 ± 0.16) and impulsivity (2.24 ± 0.32) (cut-off = 1) in adolescent ADHD. All adolescent ADHD participants scored at least once above the cut-off. All controls scored below cut-offs on all subscales in these tests. Written informed consent was obtained by the parents of the participating adolescents. The study was approved by the institutional review board of the Medical faculty of the TU Dresden and was conducted in accordance with the Declaration of Helsinki.
2.2. Task
A visual- auditory Go/NoGo task put forward by our group (Chmielewski et al., 2015) was chosen to examine response inhibition processes and their modulation by means of concurrent multisensory information. Go trials required the participants to press a response key, whenever the word “press” (German: “DRÜCK”) was presented on a computer screen. During NoGo trials, the word stop (German: STOPP) was presented on the screen and participants were asked to refrain from responding. While Go trials were never accompanied by auditory stimuli, additional auditory stimuli were presented in two-thirds of the NoGo trials: The auditory stimuli were spoken by the emotionally neutral female computer voice of “google translate”. The stimuli were adjusted in their time span (length of 400 ms) so that the visual and auditory stimuli had the same onset and offset. In 33% of the NoGo trials an auditory NoGo stimulus (spoken word “STOPP”) was presented, thus facilitating the withhold of the response (NoGocompatible) (Chmielewski et al., 2015, Chmielewski et al., 2016). In another 33% of the NoGo trials a concurrent auditory Go stimulus (spoken word “DRÜCK”) was presented. This creates a conflict between the concurrent auditory stimulus signaling participants to respond and the primary visual stimulus signaling participants to refrain from responding. This conflict should aggravate response inhibition performance (NoGoincompatible) (Chmielewski et al., 2015, Chmielewski et al., 2016). The remaining 33% of NoGo trials were not accompanied by an auditory stimulus (NoGowithout) and served as a baseline condition. A manipulation of Go trials by means of concurrent information was not applied, because a vast amount of research with Stroop and Flanker tasks has already shown that (Go trial) performance of children with ADHD decreases whenever incompatible information is displayed relative to conditions in which additional compatible information is displayed (Mullane et al., 2009). Another reason is that a rare occurrence of critical concurrent information promotes a mindless withdrawal of processing capacities in this task (Helton et al., 2005), meaning that participants get into a rather automatic rhythm of reacting, which makes them more prone to react in trials in which automaticity is increased by means of concurrent conflicting auditory (Go) information. Lastly, this design allows a comparison with results from other studies in adolescent neuropsychiatric disorders (Chmielewski et al., 2015, Chmielewski et al., 2016), which is desirable to support the creation of a new dimensional taxonomy of mental disorders (cf. Research Domain Criteria/RDoC initiative).
All participants received the instruction to respond only to visual stimuli and to ignore auditory stimuli. The task consisted of 70% (672) Go trials and 30% (288) NoGo trials. This ratio was chosen to increase the tendencies to erroneously press the key on NoGo trials. Trials were randomized and divided into six blocks with 160 trials. Inter-trial intervals varied between 1700 and 2100 ms. Each trial was terminated after the response, or after 1000 ms, if no response was obtained. Go trials were treated as correct, if the response was given within 1000 ms. NoGo trials were treated as correct, if no response was executed. Go trials were treated as misses, if no response was obtained, while NoGo trials were treated as false alarms (FA), if a response was obtained in the 1000 ms time window after stimulus presentation. A standardized instruction was given and a practice run of 60 trials was executed to familiarize participants with the task. In total, the task lasted ~32 min, which could hence result in boredom and disengagement from the task. In order to avoid disengagement from the task, participants were encouraged to make use of the breaks between the six experimental task blocks to be able to rest and then focus on the next block. Moreover, participants received monetary incentives (7.5€/h), which should increase the commitment to the task. Participants were also carefully monitored via a camera system to detect time periods in which participants were not focusing on the presented trials. Additionally, response activations were monitored to detect reappearing non-responses in Go trials, reappearing responses in NoGo trials or repetitive frustration-driven task-unrelated response button presses in short succession, which might be interpreted as signs of boredom/disengagement. Whenever this was the case (approximately for 20% of controls and patients with adolescent ADHD), participants were approached and politely asked to focus on the task. The experience that a lack of commitment was immediately noticed resulted in a reestablished commitment to the task and prevented the reoccurrence of signs of disengagement (i.e. wrong or non-responses). Additionally, the exact point in time, where this behavior occurred, was recorded and the respective trials were successively removed in the behavioral and EEG data to ensure only relevant data was included in the statistical analyses.
2.3. EEG recording and analysis
The EEG was recorded using 60 Ag/AgCl electrodes (sampling rate 500 Hz) connected to a “BrainAmp” amplifier (Brain Products Inc.). The electrode impedances were kept below 5 kΩ. Ag/AgCl-electrodes were mounted in an elastic cap and arranged in equidistant positions. After recording, data were down-sampled to 256 Hz and filtered (band-pass filter from 0.5 to 20 Hz, with a slope of 48 dB/oct each). It needs to be noted that the results reported below remained the same when a less steep slope of the filter (e.g. 16 db/oct) was used. Raw data were inspected manually by means of the ‘Raw Data Inspection’ function of the Brain Visions Analyzer (BVA; Brain Products Inc.) to reject non-ocular artifacts from the EEG (i.e. sharp offsets in the signal affecting ~0.9% ± 0.55% [SEM] of the data points in the experiment). Afterwards, an independent component analysis (“ICA” in the BVA; infomax algorithm) was conducted on the un-epoched data sets to manually remove recurring artifacts, such as horizontal and vertical eye movements, blinks and cardiac pulses. Then, the EEG data was segmented for Go trials, NoGo trials without concurrent information (NoGowithout), compatible NoGo trials (NoGocompatible), and incompatible NoGo (NoGoincompatible) trials. Go trials were only included when the correct response was executed in a time window until 1000 ms after target onset. NoGo trials were only included in the analysis when no response was executed at all. The segments were locked to the onset of the target stimulus (Go or NoGo stimulus) and ranged from −250 ms before to 1000 ms after this time point. An automated “Artifact Rejection” (BVA) procedure was conducted on all of the segments, using rejection criteria of amplitude differences >200 μV in a 100 ms period and activity of <0.5 μV in a 200 ms interval. By means of the artifact rejection procedure 0–3.5% of the segments were rejected, not differing between experimental conditions and groups (p > .5). Current source density (CSD) transformation (Nunez and Pilgreen, 1991) was conducted to eliminate reference potential from the data. The transformed values are given in μV/m2. An additional advantage of the CSD-transformation is that it serves as a spatial filter (Nunez and Pilgreen, 1991), which makes it possible to identify electrodes that best reflect activity related to cognitive processes (Mückschel et al., 2014). The baseline was corrected in the time interval from −200 to 0 ms (i.e. target onset). Then, individual averages were calculated for each condition and participant. As the segmentation process ensured that only correct Go and NoGo trials were included in the averages and because of raw data inspection and artifact rejection only a reduced number of all presented trials were included in the averages. For the ADHD group approximately 81.46% (596.29 ± 17.01[SEM] Trials) were included in the Go condition, 71.69% (68.82 ± 4.12) in the NoGowithout condition, 74.9% (71.9 ± 3.71) in the NoGocompatible condition and 54.45% (52.27 ± 4.35) of the trials in the NoGoincompatible condition were included in the analysis. For the control group in the Go condition approximately 92.72% (678.71 ± 15.28), in the NoGowithout 76.29% (68.82 ± 3.99), in the NoGocompatible 82.72% (71.9 ± 3.65), and in the NoGoincompatible condition 66.77% (52.27 ± 4.12) of the trials were included in averages and grand averages.
ERPs related to early attentional processes were quantified at electrodes P7 and P8 (P1: 80–140 ms, N1: 160–210 ms). Individual amplitude values for both electrodes were included in the statistical analysis for the P1 and N1 ERPs. To identify electrodes and time windows best reflecting relevant ERP components, all conditions of both groups (Go, NoGowithout, NoGocompatible and NoGoincompatible) were merged into a single grand average (supplemental fig. 3). P2, N2 and P3 peaks including topography plots were inspected. Based on this visual inspection and in accordance with the P2, N2 and P3 peaks and topography plots for each group and condition the electrode Cz was selected to quantify P2, N2 and P3 processes, as Cz was within the field of highest/lowest activation in these topography plots. The Cz was selected, as it is commonly considered best practice to only include those electrodes into analysis that show the largest amplitudes and/or are located in the topographic center of an ERP component (for more information on best practice, please refer to Keil et al., 2014). This choice was successively validated statistically by means of the following approach (Mückschel et al., 2014): Each electrode was compared against the average of all combined other electrodes using Bonferroni-correction for multiple comparisons (critical threshold p = .0007). We only chose the electrode(s) that showed significantly larger mean amplitudes (i.e., negative for N-potentials and positive for the P-potentials) than the remaining electrodes. Of note, this pattern of electrodes matched the electrodes found in the visual inspection of the data. Moreover, quantifying the P1 and N1 ERP-components at the P7 and P8 electrodes and quantifying the P2, N2 and P3 ERP-components at the Cz electrode is in accordance with previous studies employing this paradigm (Chmielewski et al., 2015, Chmielewski et al., 2016). While P2 and N2 ERP-components might also be quantified at other electrode sites in Go/Nogo paradigms not combining response inhibition with conflict monitoring processes, or not using multisensory (audio-visual) stimuli, the topography plots and a statistical validation in this and in the previous studies suggest to quantify Cz. Hence, P2, N2 and P3 were quantified at electrode Cz in a search window of ± 50 ms around the respective peak, which was obtained, when all conditions were merged into a single grand average (see supplemental Fig. 3). Following peaks were semi-automatically quantified in, or in the close proximity of, the following search windows: P2: 185 ± 50 ms, N2: 280 ± 50 ms and P3: 505 ± 50 ms, as indicated by the peaks and topography plots of the supplemental Fig. 3. Peak-to-baseline amplitudes and latencies were quantified for all ERP components at the single-subject level. Peak-to-baseline analyses were conducted, since peak-to-peak amplitudes should only be conducted, when the former peak is either not influenced by the experimental manipulation, or reflects the same underlying process as the component of interest (Handy, 2005). Within each of these search intervals (see above) the peak amplitude was semi-automatically extracted for the above-mentioned electrode positions. As search windows for the peak detection algorithm, especially for the response selection ERPs (P2, N2, P3) were rather small, peaks were manually re-located during the semi-automatic peak detection procedure if the search algorithm did not select the correct peak. This applied for ~15% of quantified peaks. Additionally, the P3 was quantified with larger time-windows of 140 and 200 ms to ensure that the identified maximal peaks for data quantification were not biased by the length of the search window used for peak detection.
2.4. Source localization
To examine sources related to amplitude modulations in ERPs, source localization was conducted using sLORETA (standardized low resolution brain electromagnetic tomography; Pascual-Marqui, 2002). sLORETA provides a single linear solution to the inverse problem without a localization bias (Pascual-Marqui, 2002; Sekihara et al., 2005). There is evidence of EEG/fMRI and EEG/TMS studies underlining the validity of the sources estimated using sLORETA (Dippel and Beste, 2015; Sekihara et al., 2005). For sLORETA, the intracerebral volume is partitioned into 6239 voxels at 5 mm spatial resolution. The standardized current density at each voxel is calculated in a realistic head model using the MNI152 template. For the statistics the sLORETA-built-in voxel-wise randomization tests with 2000 permutations, based on statistical nonparametric mapping (SnPM) were performed: Using sLORETA we only compared conditions showing differences in specific ERP-components between the adolescent ADHD and the control group. The logic of a randomization test using SnPM (Nichols and Holmes, 2002) is that if there is no experimental (i.e. group) effect, the labeling of the groups is arbitrary. Given the null hypothesis that the labelings are arbitrary, the significance of a statistic expressing the group effect can then be assessed by comparison with a distribution of values obtained when group-memberships are permuted (Nichols and Holmes, 2002). The randomization exchanges (permutates) the group memberships. Due to the non-parametric nature of the method, its validity need not rely on any assumption of Gaussianity. Voxels with significant differences (p < .01, corrected for multiple comparisons) between contrasted groups were located in the MNI-brain www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm. For the sLORETA procedure and the estimation of the sources underlying significant differences in amplitudes of ERP components between groups, only the time windows used for ERP amplitude quantification were used.
2.5. Statistics
Mixed effects ANOVAs (Greenhouse-Geisser-corrected, if necessary) were used to analyze the behavioral data (hits, misses and FA rates, as well as corresponding RTs). “Group” (adolescent ADHD vs. control) was used as between-subject factor and “condition” (NoGowithout/NoGocompatible/NoGoincompatible) as within-subject factor. For the ERP data, Go trials were additionally added to “condition”. For the P1 and N1 ERP-components “electrode” was included as additional within-subject factor in the mixed effects ANOVA. Post-hoc analyses using t-tests were Bonferroni-corrected. Post-hoc power values were calculated via the partial eta squared (ηp2) values and the number of participants (n) using the G*power software package (Faul et al., 2009). All variables included in the analyses were normal distributed according to Kolmogorov-Smirnov tests (all z < 0.78, p > .374). For the descriptive statistics the mean and standard error of the mean (S.E.M.) are given. To examine whether the factor “sex” affected the results, this factor was included as additional between-subject factor in separate ANOVAs. To control for possible effects of medication, the “medication type” in adolescent ADHD patients (i.e. Medikinet retard (n = 6), Ritalin (n = 3), Concerta (n = 2), Strattera (n = 2), Elvanse (n = 2), Abilify (n = 1), no medication (n = 5)) was included as a covariate in separate ANCOVAs.
3. Results
3.1. Behavioral data
There were no significant differences between the adolescent ADHD patients (RTs: 486 ± 20 ms) and the control group (RT: 504 ± 21 ms) in Go trials RTs (t40 = 0.609; p = 0.273). Likewise, no differences between male (RTs: 505 ± 18 ms) and female participants (RTs: 467 ± 20 ms) were observed (t40 = 1.189; p = 0.241). Additionally, more Go misses were evident in adolescent ADHD patients (11.81 ± 0.3.29%) than in controls (4.76 ± 1.18%) (t40 = 2.01; p = .026). Again no differences, or respectively only differences on a trend level significance were evident between male (10.22 ± 0.2.33%) and female participants (2.81 ± 1.19%) were evident (t40 = 1.847; p = .072).
However, the rate of false alarms (FA) is the most important behavioral parameter in response inhibition tasks. Concerning the FA rates, a mixed effects ANOVA revealed a main effect of “condition” (F(2,80) = 62.28, p < .001; ηp2 = 0.609). Post-hoc paired t-tests showed FA rates to significantly increase from NoGocompatible (16.0 ± 2.1%) to the NoGowithout (21.7 ± 2.2%) to the NoGoincompatible (33.2 ± 2.8%) condition (all t ≥ 4.63; p < .001). Most importantly, an interaction “condition × group” was observed (F(2,80) = 3.75, p = 0.028; ηp2 = 0.086), which is shown in Fig. 1.
Fig. 1.
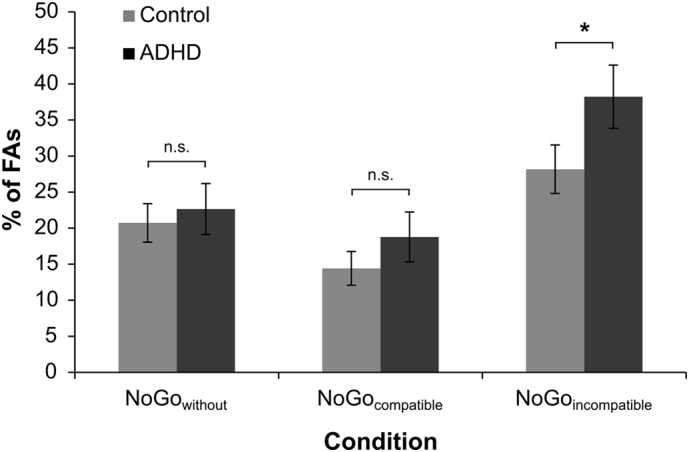
FA rates (with corresponding SEMs) for both groups in the NoGowithout, NoGocompatible and NoGoincompatible condition. Controls are depicted in black, individuals with ADHD in grey. The grey and black shaded bars depict the FA group differences in each NoGo condition.
Post-hoc paired t-tests revealed that significantly more FAs were committed by ADHD patients (38.2 ± 4.4) than healthy controls (28.2 ± 3.4%) in the NoGoincompatible condition (t40 = 1.82; p = 0.038). The groups did not differ in the NoGowithout (adolescent ADHD: 22.7 ± 3.5%; controls: 20.7 ± 2.7%) and NoGocompatible condition (adolescent ADHD: 18.8 ± 3.5%; controls: 14.4 ± 2.4%) (all t ≤ 1.04; p ≥ 0.152). A post-hoc power calculation revealed that the achieved power in this interaction was >95%. A main effect of “group” was not evident (F(1,40) = 1.52, p = .225; ηp2 = 0.037). In order to clarify whether sex differences contributed to the observed FA rates, the factor “sex” was added as an additional between subject factor to the model. The results of the model were the same and there were no main or interaction effects including the factor “sex” (all F < 1.47; p > 0.2). Similarly, when including the covariate “medication type”, this covariate did not change the model (all F ≤ .62; p ≥ .542). Concerning the RTs on FAs neither main effects nor any interactions could be observed (all F ≤ 1.56; p ≥ .217).
3.2. Neurophysiological data: Sensory and attentional processing
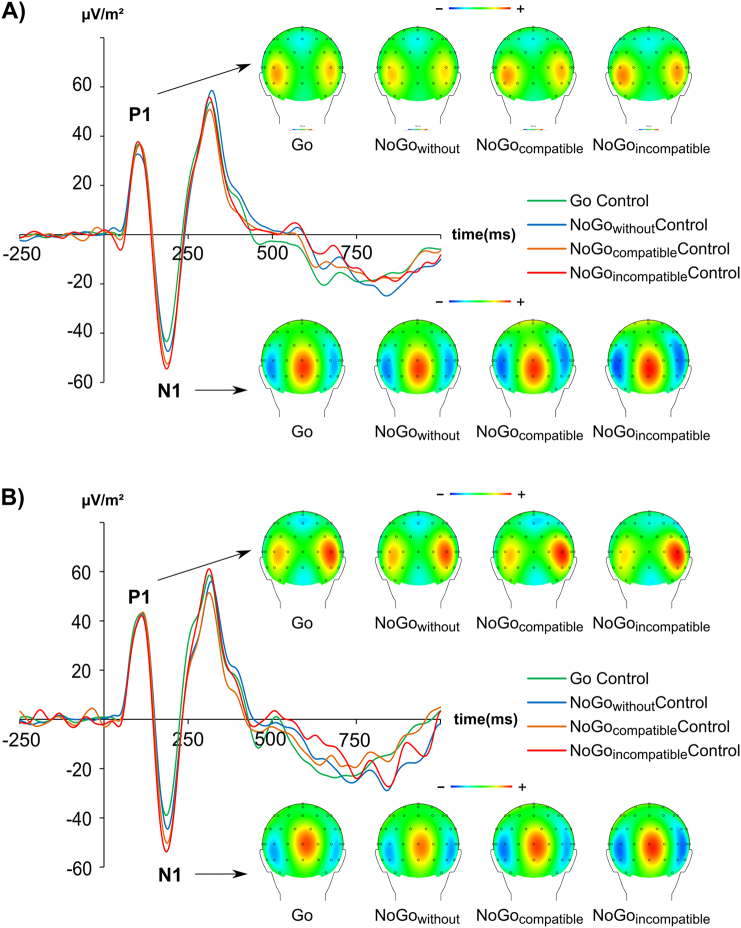
For all analyzed ERP components, there were no latency effects (all F < 1.33; p > 0.169). The neurophysiological data for the P1 and N1 is shown in Fig. 2.
Fig. 2.
Event-related potentials on Go and NoGo trials averaged across electrode P7 and P8 (only for creation of this figure). Time point zero denotes the time point of Go, or NoGo stimulus presentation. The different lines show the NoGowithout condition (blue lines), NoGocompatible condition (orange lines) and the NoGoincompatible condition (red lines) and the Go condition (green). The scalp topography plots show the distribution of the scalp electrical potential for the P1 (upper row), and N1 (lower row) on Go and NoGo trials. Figure part A shows the ERPs and scalp topographies for controls. Figure part B shows the ERPs and scalp topographies for ADHDs.
Concerning the P1 amplitude, in a repeated measures ANOVA with the within-group factors “electrode”, and “condition” and the between group factor “group” only a main effect of “electrode” was observed (F(1,40) = 17.94; p < .001; ηp2 = 0.310), showing the P1 amplitude to be significantly smaller (i.e. less positive) in the electrode P7 (43.3 ± 4.2 μV/m2) than in the electrode P8 (64.0 ± 4.9 μV/m2). None of the other factors (e.g. “condition”, “group”, “medication type”, “sex”) or interactive effects between them were significant (all F ≤ 2.94; p ≥ .094).
For the N1 amplitudes the mixed effects ANOVA revealed a main effect of “condition” (F(3,120) = 10.51; p < .001; ηp2 = 0.208), showing N1 amplitudes differences to occur between the Go (−56.1 ± 3.7 μV/m2), the NoGowithout (−59.2 ± 3.6 μV/m2), the NoGocompatible (−69.4 ± 4.4 μV/m2) and the NoGoincompatible (−70.9 ± 4.0 μV/m2) condition. Post-hoc paired t-tests, however, revealed that only conditions without concurrent information (Go and NoGowithout) differed from conditions with concurrent information (NoGocompatible and NoGoincompatible) (all t ≥ 2.79; p ≤ 0.008), while Go vs. NoGowithout and the NoGocompatible vs. NoGoincompatible condition did not significantly differ (all t ≤ 1.89; p ≥ 0.065). None of the other factors (e.g. “condition”, “group”, “medication type”, “sex”) or interactive effects between them were significant (all F ≤ 1.13; p ≥ 0.340).
3.3. Neurophysiological data: Response selection stage
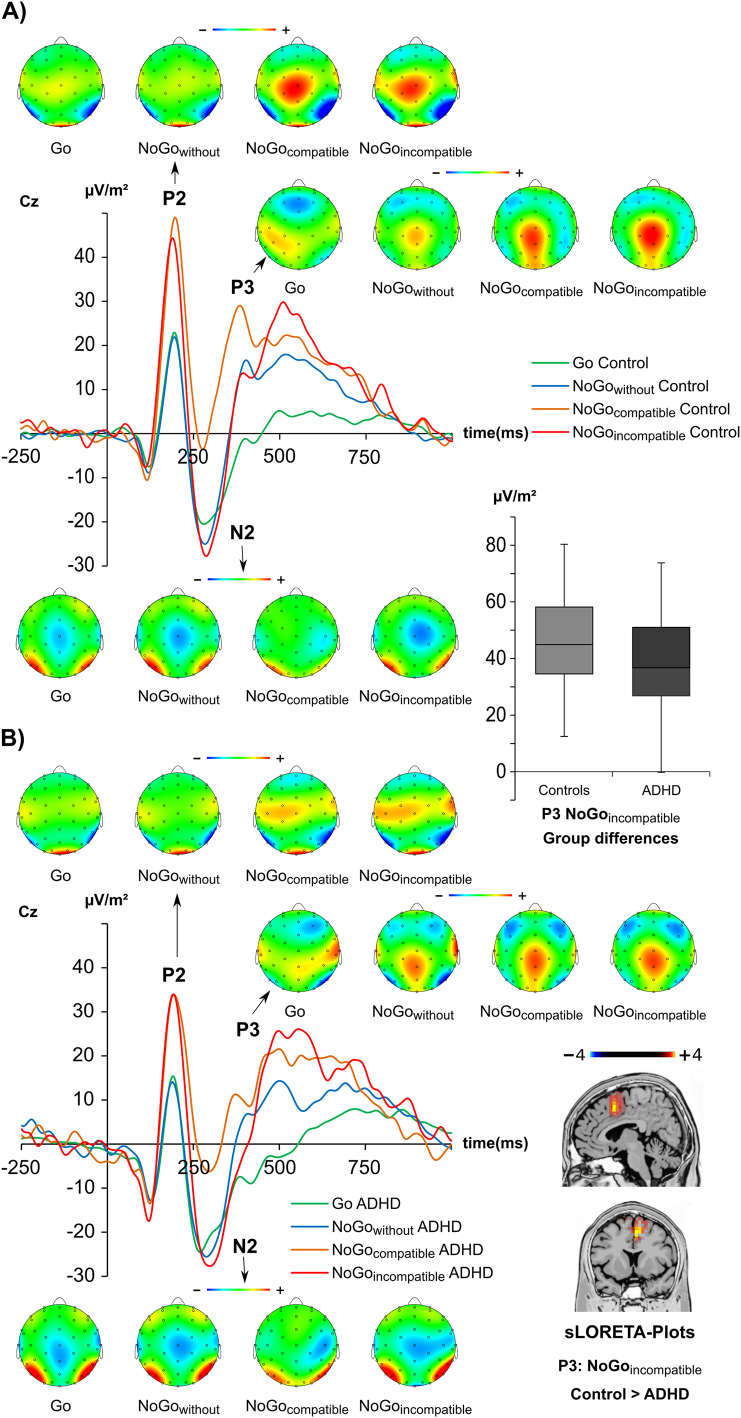
The P2, N2 and P3 ERPs are shown in Fig. 3.
Fig. 3.
Event-related potentials on Go and NoGo trials at electrode Cz. Time point zero denotes the time point of the Go, or NoGo stimulus presentation. The different lines show the NoGowithout condition (blue lines), NoGocompatible condition (orange lines), the NoGoincompatible condition (red lines) and the Go condition (green). The scalp topography plots show the distribution of the scalp electrical potential for the P2 (upper row), N2 (middle row) and P3 (lower row) on Go and NoGo trials. Figure part A shows the ERPs and scalp topographies for controls. Figure part B shows the ERPs and scalp topographies for ADHSs. Additionally the sLORETA source of the group differences in the NoGoincompatible condition is displayed in figure part B (corrected for multiple comparison, p < .01). The sLORETA colour scale denotes critical t-values. Additionally, a boxplot showing P3 differences between both groups is shown.
For the P2 amplitude (refer Fig. 3) a main effect of “condition” was evident (F(3,120) = 49.84; p < .001; ηp2 = 0.555) (Go: 25.3 ± 2.7 μV/m2; NoGowithout: 25.4 ± 3.0 μV/m2; NoGoincompatible: 49.0 ± 4.7 μV/m2; NoGocompatible: 51.5 ± 5.4 μV/m2). Post-hoc paired t-tests revealed that only conditions with concurrent information (NoGocompatible and NoGoincompatible) differed from conditions without concurrent information (Go and NoGowithout) (all t ≥ 7.69; p < .001). No differences were obtained for the other possible contrasts between conditions (all t ≤ 0.96; p ≥ .341). There was no main effect of “group”, or an interaction “condition × group” (all F ≤ 1.26; p ≥ .269). These results remained the same when controlling for “sex” and “medication type” (all F < 1.60; p > .194).
For the N2 amplitude (refer Fig. 3), a main effect of “condition” (F(3,120) = 18.70; p < .001; ηp2 = 0.319) was evident: N2 amplitudes differed between NoGocompatible (−15.2 ± 3.5 μV/m2), Go (−30.1 ± 2.6 μV/m2), NoGowithout (−32.6 ± 2.5 μV/m2) and NoGoincompatible trials (−36.3 ± 3.8 μV/m2). Post-hoc paired t-tests revealed that the NoGocompatible condition was smaller (i.e. less negative) than all other conditions (all t ≥ 5.17; p ≤ .001) and that the Go condition was smaller (i.e. less negative) than the NoGoincompatible condition (t41 = 2.14; p = 0.019). The NoGowithout condition did not differ from the Go, or the NoGoincompatible condition (all t ≤ 1.24; p ≥ 0.112). Moreover, neither a main effect of “group”, nor an interaction “condition × group” were evident (all F ≤ 0.426; p ≥ 0.551). These results remained the same when the factor “sex”, or the covariate “medication type” were included in the model (all F < 2.20; p > 0.109).
For the P3 amplitude (refer Fig. 3), a main effect of “condition” (F(3,120) = 76.47; p < .001; ηp2 = 0.657) was detected. P3 amplitude differences occurred between Go (15.3 ± 2.4 μV/m2), NoGowithout (27.6 ± 2.1 μV/m2), NoGocompatible (35.3 ± 2.6 μV/m2) and NoGoincompatible trials (42.0 ± 2.7 μV/m2). Post-hoc paired t-tests revealed that all conditions significantly differed from each other (all t ≥ 3.93; p ≤ .001). A main effect of “group” was not detected (F(1,40) = 0.28; p = .868; ηp2 = 0.001). Most importantly, an interaction “condition × group” was observed (F(3,120) = 4.86; p = .003; ηp2 = 0.108). Post-hoc paired t-tests revealed this interaction to be based on significant differences between both groups in the NoGoincompatible condition (adolescent ADHD: 37.4 ± 3.9 μV/m2; controls: 46.6 ± 3.9 μV/m2; t40 = 1.68; p = .049), while all other conditions did not significantly differ (all t ≤ 0.88; p ≥ .172). A post-hoc power calculation revealed that the achieved power in this interaction was >95%. The sLORETA analysis revealed that this group difference was related to decreased activation in the medial frontal gyrus (BA32) in adolescent ADHD (Fig. 3). These results remained the same when the factor “sex”, or the covariate “medication type” were also included in the model (all F < 1.51; p > 0.221). The results remained the same, when the larger time window of 140 to 200 ms was used during the peak quantification. For an analysis without CSD transformation refer to the supplemental material.
4. Discussion
We examined the influence of concurrent (conflicting or redundant) information on response inhibition processes in adolescent ADHD. We hypothesized that adolescent ADHD patients may only reveal deficits in conditions where multisensory conflicting stimuli are present and interfere with response inhibition processes. This would suggest that there is an ADHD-inherent bias to a specific content of information, which modulates inhibitory control. Underlining this hypothesis, the behavioral data showed that response inhibition deficits in adolescent ADHD were only present in the condition with conflicting concurrent information. In the response inhibition conditions with redundant or without concurrent auditory information no differences to healthy controls were observed. This shows that the concurrent auditory information is taken into account, but that it is not the mere presence of this concurrent information that compromises response inhibition performance in adolescent ADHD. Rather, it is the content of, or a conflict caused by the concurrent information that impacts inhibitory control in adolescent ADHD. These effects were robust as suggested by the power analysis of the data, revealing a power >95%. Additionally, the fact that false alarms in the NoGowithout condition were also at an intermediate level in adolescent ADHD further underlines that it is not the mere presence of auditory information in NoGo trials, but the content of information that matters. More important, the results show very specific effects between adolescent ADHD and controls that were only evident in the NoGoincompatible condition, but not in the NoGocompatible condition despite in both of these conditions the auditory information signals NoGo trials. If the auditory stimulus had prompted some response cancellation, this specificity would have been unlikely to occur. Interestingly, similar effects were not found for the false alarm RTs. This might, however, relate to the fact that outlier RTs might have differentially affected mean false alarm RTs in participants with very few false alarms vs. participants with a high number of false alarms.
How exactly the content of information affects response inhibition in adolescent ADHD is revealed by the neurophysiological data: As response inhibition deficits were not caused by the mere presence of concurrent information in adolescent ADHD, the behavioral data already suggest that perceptual and bottom-up attentional processes should not differ between adolescent ADHD patients and controls. This was corroborated by the ERP data showing no differences in the P1 and N1 amplitudes between both groups. The P1 and N1 are assumed to reflect perceptual gating and bottom-up attentional selection unrelated to stimulus content (Herrmann and Knight, 2001). Similarly, the P2 amplitude, which is assumed to reflect resource allocation processes (Geisler and Murphy, 2000; Sugimoto and Katayama, 2013), did not show differences between groups and experimental conditions. All these results suggest that modulations of response inhibition processes by the content of concurrent information should not occur until the response selection level.
At the response selection level, the content of information affected specific neurophysiological subprocesses and functional neuroanatomical structures: Even though no N2 amplitude differences were evident between groups, the NoGo-N2 amplitude increased with an increasing amount of conflict. This matches the assumption that the NoGo-N2 reflects pre-motor inhibition and/or conflict monitoring processes (Donkers and van Boxtel, 2004; Huster et al., 2013; Yeung et al., 2004). However, the finding that both groups show similar modulations of the N2 amplitude, when the amount of conflict was varied between experimental conditions, suggests that conflict monitoring deficits do not seem to play a role, despite their prevalence in adolescent ADHD (Albrecht et al., 2008; Bluschke et al., 2016a; McLoughlin et al., 2009; Mullane et al., 2009). This suggests that the content of information affects processes other than conflict monitoring in adolescent ADHD. Such a process is likely reflected in the NoGo-P3 amplitude, which paralleled the behavioral data and is assumed to reflect response inhibition or response overriding processes (Beste and Saft, 2013; Huster et al., 2013; Mückschel et al., 2015; Quetscher et al., 2014). Response inhibition deficits in the conflicting NoGo condition in adolescent ADHD were reflected by a selective decrease in the NoGo-P3 amplitude. This was associated with activation differences in the medial frontal cortex (BA32) in the source localization analysis. This finding seems reasonable, since the anterior cingulate cortex (BA32) has already been shown to exhibit a reduction of grey matter density as well as in GABA, glutamate and dopamine concentrations in adolescent ADHD compared to healthy subjects (Ende et al., 2015; Umemoto et al., 2014; Villemonteix et al., 2015). Importantly, and aside from inducing a conflict, it has been shown that presenting an auditory Go stimulus alongside the primary visual NoGo information increases the automaticity of response tendencies via neurophysiological mechanisms mediated by the anterior cingulate cortex (Chmielewski et al., 2015, Chmielewski et al., 2016). Studies in healthy controls have repeatedly shown that increasing the automaticity of response tendencies imposes higher demands on response inhibition processes (Dippel et al., 2015; Donkers and van Boxtel, 2004) that are reflected by the NoGo-P3 (Beste and Saft, 2013; Huster et al., 2013; Mückschel et al., 2015; Quetscher et al., 2014). Given that impulsivity is one of the core features in adolescent ADHD (Bari and Robbins, 2013; Barkley, 1997) and that adolescent ADHD is associated with a predisposition to engage in automatic behavior (Clark et al., 2000) and deficient response inhibition (Bluschke et al., 2016b; Dimoska et al., 2003; Rubia et al., 2005; Smith et al., 2004; Wright et al., 2014), it is likely that response inhibition processes in adolescent ADHD are more easily compromised by a high level of automaticity of response tendencies. It is therefore possible that adolescent ADHD patients show response inhibition deficits because a specific content of information fosters the execution of highly automated response tendencies in a given situation. Likely, this is reflected by decreased NoGo-P3 amplitudes in adolescent ADHD. It may be speculated that this difficulty relates to goal shielding processes, which are mechanisms employed to stabilize and protect task goals from interference (Beste et al., 2017; Dreisbach and Haider, 2009; Gohil et al., 2017; Goschke and Bolte, 2014; Gruber and Goschke, 2004; Hofmann et al., 2012). Since goal-shielding processes have been shown to depend on medial frontal brain regions (Beste et al., 2017; Dreisbach and Haider, 2009; Goschke and Bolte, 2014; Gruber and Goschke, 2004; Hofmann et al., 2012), the results from the source localization analysis support the interpretation that goal-shielding processes seem to be less robust in adolescent ADHD. Hence, the content of concurrent conflicting information seems to destabilize goal-shielding processes when the content of information (i.e. conflicting Go information) facilitates automated response tendencies. These facilitated automated response tendencies very likely compete against the desired goal (i.e. inhibitory control) and presumably tap into a predisposition of adolescent ADHD to engage in automatic, impulsive behavior. Moreover, the observation of an altered activity in the medial frontal regions also is in line with the assumption that the medial frontal cortex is necessary for the implementation of cognitive control in goal-directed behavior under decision uncertainty (Ridderinkhof et al., 2004), which relates to the conflicting condition, in which both information to react and to inhibit is present.
Interestingly, a similar pattern of behavioral results was observed, when this paradigm was utilized to examine adolescent autism spectrum disorder (aASD; Chmielewski et al., 2016). However, in aASD these response inhibition deficits in the conflicting NoGo condition did not relate to the increased automaticity of response tendencies and a corresponding destabilization of goal-shielding processes, as evident in the P3 and MFC. In aASD, this related to problems triggering conflict monitoring processes, as evident in the N2 amplitude and the superior frontal gyrus (Albrecht et al., 2008; Chmielewski et al., 2015; McLoughlin et al., 2009; Mullane et al., 2009), due to the presence of conflicting multisensory information. This underlines the importance of combining EEG and specific cognitive paradigms in different clinical populations in the light of the RDoC initiative.
In this study, there was no evidence for response inhibition deficits, when no concurrent information was present, which is usually reported (Wright et al., 2014). In the context of response inhibition deficits, it would, however, be important to consider that recent results suggests that especially the combined ADHD subtype is associated with inhibitory control deficits (Bluschke et al., 2016a). Moreover, it might be possible that different outcomes might occur for different subtypes. For example, as specifically the impulsive subtype should be susceptible to an increased automaticity of response tendencies as induced in the conflicting NoGo condition, we would expect this subtype's response inhibition deficits to be more pronounced. One might also speculate whether the inattentive subtype might be more affected by the mere presence of auditory information. The current sample was, however, not stratified for different ADHD subtypes, hence, future studies examining further differential effects depending on ADHD subtype might be promising. Similarly, it may be investigated in how far the medication profile of the patients had an effect and in how far medication might improve potential response inhibition deficits, as inhibitory control processes in adolescent ADHD patients have been shown to improve, when medication is administered (Broyd et al., 2005; Tamminga et al., 2016). Yet, since the behavioral and neurophysiological results did not significantly change when medication type was included as a covariate in the statistical analysis, this suggests the entire data can be considered to be unbiased by a possible effect of medication. Hence, possible effects in future studies focusing on medication effects may only be modest. Moreover, it needs to be noted that group effects on response inhibition were only seen in one specific experimental condition. Such a pattern is unlikely to emerge given that rather broad effects of medication treatment are usually reported for response inhibition performance in adolescent ADHD, which should hence be visible in all experimental conditions. If the medication had played a significant role (i.e. if medication was indeed able to ameliorate response inhibition deficits), no or only modest effects between both groups would have been obtained in this study. However, the effect sizes (partial eta squared) showed robust effects. Such a pattern is very unlikely, if not impossible, to emerge when medication would result in a general amelioration of response inhibition deficits. The finding that response inhibition deficits were only observed in a specific condition, despite adolescent ADHD patients being on medication, suggests that either medication is not sufficient to counteract response inhibition deficits when modulated by conflicting information, or that effects of multisensory conflicting information on response inhibition processes are mediated via neurobiological mechanisms unrelated to the dopaminergic or noradrenergic system. It might also be promising to integrate a task-unrelated word, such as “CHAIR” to the task in order to examine whether this affects adolescents with ADHD and healthy controls differently. As displaying additional Go and NoGo information mainly affected the automaticity of response tendencies, this might be an elegant solution to help to address the question whether a general distractibility as evident in adolescent ADHD might also interfere with response inhibition processes.
A limitation of the study is the unequal distribution of male and female participants in the ADHD and control group did not allow including both “sex” and “group” to the analysis. Hence, this interesting question should be addressed in a new experiment with equally distributed male and female participants in both groups. It might additionally be argued that the “sex” of the patients may modulate the inhibitory control performance (Liu et al., 2013). Controlling for this factor did not change the pattern of results. The usage of EEG-source localization methods cannot yield as precise assessments of associated functional neuroanatomical structures as fMRI methods. However, given that there is evidence of EEG/fMRI and EEG/TMS studies underlining the validity of the sources estimated using sLORETA (Dippel and Beste, 2015; Sekihara et al., 2005) the applied source localization technique likely reflects reliable results.
In summary, the results show that concurrent information modulates inhibitory control processes in adolescent ADHD. Crucially, it is not the mere presence of concurrent information, but the content of information that is important to consider. The content of information does not exert its effect through modulations of perceptual gating and attentional selection processes, but through specific modulations at the response selection level. It seems that the content of concurrent conflicting information can destabilize goal-shielding processes in medial frontal (BA32) regions in adolescent ADHD when the conflicting information simultaneously increases the automaticity of response tendencies. If response tendencies are facilitated that compete against the desired goal (i.e. inhibitory control) and if the content of concurrent information presumably taps into a predisposition of adolescent ADHD to engage in impulsive behavior, response inhibition is compromised in adolescent ADHD. The shown ADHD-inherent bias to the content of information modulating inhibitory control may be important to consider in adolescent ADHD. Challenging the view that impulsivity is becoming worse under perceptual load and distraction in adolescent ADHD, the study shows that response inhibition is only modulated if the content of information taps into a predisposition of adolescent ADHD to engage in impulsive behavior, i.e. to react to pronounced distractors, which are triggering automatic behavior.
Acknowledgments
Acknowledgements
This work was supported by Grants from the Else-Kröner Fresenius Stiftung (2014_A46) and from the Deutsche Forschungsgemeinschaft (DFG) SFB 940 project B8 to C.B. and V.R. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the SLUB/TU Dresden.
Declaration of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.05.019.
Appendix A. Supplementary data
Supplementary material
References
- Ahmadi N., Mohammadi M.R., Araghi S.M., Zarafshan H. Neurocognitive profile of children with attention deficit hyperactivity disorders (ADHD): a comparison between subtypes. Iran. J. Psychiatry. 2014;9:197–202. [PMC free article] [PubMed] [Google Scholar]
- Albrecht B., Brandeis D., Uebel H., Heinrich H., Mueller U.C., Hasselhorn M., Steinhausen H.-C., Rothenberger A., Banaschewski T. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biol. Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B., Brandeis D., Uebel H., Valko L., Heinrich H., Drechsler R., Heise A., Müller U.C., Steinhausen H.-C., Rothenberger A., Banaschewski T. Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychol. Med. 2013;43:1997–2011. doi: 10.1017/S003329171200270X. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berlin L., Bohlin G., Rydell A.-M. Relations between inhibition, executive functioning, and ADHD symptoms: a longitudinal study from age 5 to 8(1/2) years. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2003;9:255–266. doi: 10.1076/chin.9.4.255.23519. [DOI] [PubMed] [Google Scholar]
- Beste C., Saft C. Action selection in a possible model of striatal medium spiny neuron dysfunction: behavioral and EEG data in a patient with benign hereditary chorea. Brain Struct. Funct. 2013:221–228. doi: 10.1007/s00429-013-0649-9. [DOI] [PubMed] [Google Scholar]
- Beste C., Willemssen R., Saft C., Falkenstein M. Response inhibition subprocesses and dopaminergic pathways: basal ganglia disease effects. Neuropsychologia. 2010;48:366–373. doi: 10.1016/j.neuropsychologia.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Beste C., Ness V., Falkenstein M., Saft C. On the role of fronto-striatal neural synchronization processes for response inhibition—evidence from ERP phase-synchronization analyses in pre-manifest Huntington's disease gene mutation carriers. Neuropsychologia. 2011;49:3484–3493. doi: 10.1016/j.neuropsychologia.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Beste C., Mückschel M., Rosales R., Domingo A., Lee L., Ng A., Klein C., Münchau A. The basal ganglia striosomes affect the modulation of conflicts by subliminal information-evidence from X-linked dystonia parkinsonism. Cereb. Cortex N. Y. N. 2017:1991 1–10. doi: 10.1093/cercor/bhx125. [DOI] [PubMed] [Google Scholar]
- Bluschke A., Chmielewski W.X., Roessner V., Beste C. Intact context-dependent modulation of conflict monitoring in childhood ADHD. J. Atten. Disord. 2016 doi: 10.1177/1087054716643388. [DOI] [PubMed] [Google Scholar]
- Bluschke A., Roessner V., Beste C. Specific cognitive-neurophysiological processes predict impulsivity in the childhood attention-deficit/hyperactivity disorder combined subtype. Psychol. Med. 2016;46:1277–1287. doi: 10.1017/S0033291715002822. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Li W., Parrish T.B., Gitelman D.R., Marsel Mesulam M. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J. Child Psychol. Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Bos D.J., Oranje B., Achterberg M., Vlaskamp C., Ambrosino S., de Reus M.A., van den Heuvel M.P., Rombouts S.A.R.B., Durston S. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry. 2017;58:810–818. doi: 10.1111/jcpp.12712. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brieber S., Neufang S., Bruning N., Kamp-Becker I., Remschmidt H., Herpertz-Dahlmann B., Fink G.R., Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Johnstone S.J., Barry R.J., Clarke A.R., McCarthy R., Selikowitz M., Lawrence C.A. The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2005;58:47–58. doi: 10.1016/j.ijpsycho.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Chen Z., Cave K.R. Zooming in on the cause of the perceptual load effect in the go/no-go paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2016;42:1072–1087. doi: 10.1037/xhp0000168. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Mückschel M., Dippel G., Beste C. Concurrent information affects response inhibition processes via the modulation of theta oscillations in cognitive control networks. Brain Struct. Funct. 2015:1–13. doi: 10.1007/s00429-015-1137-1. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Wolff N., Mückschel M., Roessner V., Beste C. Effects of multisensory integration processes on response inhibition in adolescent autism spectrum disorder. Psychol. Med. 2016;46:2705–2716. doi: 10.1017/S0033291716001008. [DOI] [PubMed] [Google Scholar]
- Clark C., Prior M., Kinsella G.J. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the six elements test and Hayling sentence completion test. J. Abnorm. Child Psychol. 2000;28:403–414. doi: 10.1023/a:1005176320912. [DOI] [PubMed] [Google Scholar]
- Conners C.K., Sitarenios G., Parker J.D.A., Epstein J.N. The revised Conners' parent rating scale (CPRS-R): factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Di Russo F., Martínez A., Sereno M.I., Pitzalis S., Hillyard S.A. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimoska A., Johnstone S.J., Barry R.J., Clarke A.R. Inhibitory motor control in children with attention-deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Biol. Psychiatry. 2003;54:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Dippel G., Beste C. A causal role of the right inferior frontal cortex in implementing strategies for multi-component behaviour. Nat. Commun. 2015;6(6587) doi: 10.1038/ncomms7587. [DOI] [PubMed] [Google Scholar]
- Dippel G., Chmielewski W., Mückschel M., Beste C. Response mode-dependent differences in neurofunctional networks during response inhibition: an EEG-beamforming study. Brain Struct. Funct. 2015:1–11. doi: 10.1007/s00429-015-1148-y. [DOI] [PubMed] [Google Scholar]
- Donkers F.C.L., van Boxtel G.J.M. Brain Cogn., Neurocognitive Mechanisms of Performance Monitoring and Inhibitory Control. Vol. 56. 2004. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition; pp. 165–176. [DOI] [PubMed] [Google Scholar]
- Douglas V.I. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay H.C., Hogan A.E., editors. Handbook of Disruptive Behavior Disorders. Kluwer Academic Publishers; Dordrecht, Netherlands: 1999. pp. 105–138. [Google Scholar]
- Dreisbach G., Haider H. How task representations guide attention: further evidence for the shielding function of task sets. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35:477–486. doi: 10.1037/a0014647. [DOI] [PubMed] [Google Scholar]
- Durston S., de Zeeuw P., Staal W.G. Imaging genetics in ADHD: a focus on cognitive control. Neurosci. Biobehav. Rev. 2009;33:674–689. doi: 10.1016/j.neubiorev.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Ende G., Cackowski S., VanEijk J., Sack M., Demirakca T., Kleindienst N., Bohus M., Sobanski E., Krause-Utz A., Schmahl C. Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015 doi: 10.1038/npp.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Freunberger R., Klimesch W., Doppelmayr M., Höller Y. Visual P2 component is related to theta phase-locking. Neurosci. Lett. 2007;426:181–186. doi: 10.1016/j.neulet.2007.08.062. [DOI] [PubMed] [Google Scholar]
- Geisler M.W., Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2000;37:309–315. doi: 10.1016/s0167-8760(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Gohil K., Bluschke A., Roessner V., Stock A.-K., Beste C. ADHD patients fail to maintain task goals in face of subliminally and consciously induced cognitive conflicts. Psychol. Med. 2017:1–13. doi: 10.1017/S0033291717000216. [DOI] [PubMed] [Google Scholar]
- Gomez Gonzalez C.M., Clark V.P., Fan S., Luck S.J., Hillyard S.A. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994;7:41–51. doi: 10.1007/BF01184836. [DOI] [PubMed] [Google Scholar]
- Goschke T., Bolte A. Emotional modulation of control dilemmas: the role of positive affect, reward, and dopamine in cognitive stability and flexibility. Neuropsychologia. 2014;62:403–423. doi: 10.1016/j.neuropsychologia.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Gruber O., Goschke T. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes, executive control of human action. Acta Psychol. (Amst.) 2004;115:105–121. doi: 10.1016/j.actpsy.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Handy T.C. Event-Related Potentials: A Methods Handbook. MIT Press; 2005. Basic principles of ERP quantification; p. 40. [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hart H., Chantiluke K., Cubillo A.I., Smith A.B., Simmons A., Brammer M.J., Marquand A.F., Rubia K. Pattern classification of response inhibition in ADHD: toward the development of neurobiological markers for ADHD. Hum. Brain Mapp. 2014;35:3083–3094. doi: 10.1002/hbm.22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze H.J., Mangun G.R., Burchert W., Hinrichs H., Scholz M., Münte T.F., Gös A., Scherg M., Johannes S., Hundeshagen H. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Helton W.S., Hollander T.D., Warm J.S., Matthews G., Dember W.N., Wallaart M., Beauchamp G., Parasuraman R., Hancock P.A. Signal regularity and the mindlessness model of vigilance. Br. J. Psychol. Lond. Engl. 2005;1953(96):249–261. doi: 10.1348/000712605X38369. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Knight R.T. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Schmeichel B.J., Baddeley A.D. Executive functions and self-regulation. Trends Cogn. Sci. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hoogman M., Bralten J., Hibar D.P., Mennes M., Zwiers M.P., Schweren L.S.J., van Hulzen K.J.E., Medland S.E., Shumskaya E., Jahanshad N., de Zeeuw P., Szekely E., Sudre G., Wolfers T., Onnink A.M.H., Dammers J.T., Mostert J.C., Vives-Gilabert Y., Kohls G., Oberwelland E., Seitz J., Schulte-Ruther M., Ambrosino S., Doyle A.E., H?vik M.F., Dramsdahl M., Tamm L., van Erp T.G.M., Dale A., Schork A., Conzelmann A., Zierhut K., Baur R., McCarthy H., Yoncheva Y.N., Cubillo A., Chantiluke K., Mehta M.A., Paloyelis Y., Hohmann S., Baumeister S., Bramati I., Mattos P., Tovar-Moll F., Douglas P., Banaschewski T., Brandeis D., Kuntsi J., Asherson P., Rubia K., Kelly C., Martino A.D., Milham M.P., Castellanos F.X., Frodl T., Zentis M., Lesch K.-P., Reif A., Pauli P., Jernigan T.L., Haavik J., Plessen K.J., Lundervold A.J., Hugdahl K., Seidman L.J., Biederman J., Rommelse N., Heslenfeld D.J., Hartman C.A., Hoekstra P.J., Oosterlaan J., von Polier G., Konrad K., Vilarroya O., Ramos-Quiroga J.A., Soliva J.C., Durston S., Buitelaar J.K., Faraone S.V., Shaw P., Thompson P.M., Franke B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017;4:310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster R.J., Enriquez-Geppert S., Lavallee C.F., Falkenstein M., Herrmann C.S. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2013;87:217–233. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Keil A., Debener S., Gratton G., Junghöfer M., Kappenman E.S., Luck S.J., Luu P., Miller G.A., Yee C.M. Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51:1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Liu T., Xiao T., Shi J. Response inhibition, preattentive processing, and sex difference in young children: an event-related potential study. Neuroreport. 2013;24:126–130. doi: 10.1097/WNR.0b013e32835d846b. [DOI] [PubMed] [Google Scholar]
- Loe I.M., Feldman H.M. Academic and educational outcomes of children with ADHD. J. Pediatr. Psychol. 2007;32:643–654. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann. N. Y. Acad. Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- McLoughlin G., Albrecht B., Banaschewski T., Rothenberger A., Brandeis D., Asherson P., Kuntsi J. Performance monitoring is altered in adult ADHD: a familial event-related potential investigation. Neuropsychologia. 2009;47:3134–3142. doi: 10.1016/j.neuropsychologia.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mückschel M., Stock A.-K., Beste C. Psychophysiological mechanisms of Interindividual differences in goal activation modes during action cascading. Cereb. Cortex. 2014;24:2120–2129. doi: 10.1093/cercor/bht066. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Smitka M., Hermann A., von der Hagen M., Beste C. Deep brain stimulation in the globus pallidus compensates response inhibition deficits: evidence from pantothenate kinase-associated neurodegeneration. Brain Struct. Funct. 2015:1–7. doi: 10.1007/s00429-015-1041-8. [DOI] [PubMed] [Google Scholar]
- Mullane J.C., Corkum P.V., Klein R.M., McLaughlin E. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2009;15:321–342. doi: 10.1080/09297040802348028. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Pilgreen K.L. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1991;8:397–413. [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pelham W.E., Waschbusch D.A., Hoza B., Gnagy E.M., Greiner A.R., Sams S.E., Vallano G., Majumdar A., Carter R.L. Music and video as distractors for boys with ADHD in the classroom: comparison with controls, individual differences, and medication effects. J. Abnorm. Child Psychol. 2011;39:1085–1098. doi: 10.1007/s10802-011-9529-z. [DOI] [PubMed] [Google Scholar]
- Petermann F., Petermann U. Fourth Edition. Pearson Assessment; Frankfurt/Main: 2011. Wechsler Intelligence Scale for Children. [Google Scholar]
- Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Quetscher C., Yildiz A., Dharmadhikari S., Glaubitz B., Schmidt-Wilcke T., Dydak U., Beste C. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct. Funct. 2014:3555–3564. doi: 10.1007/s00429-014-0873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall K.D., Brocki K.C., Kerns K.A. Cognitive control in children with ADHD-C: how efficient are they? Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2009;15:163–178. doi: 10.1080/09297040802464148. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Toone B., Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am. J. Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Sahani M., Nagarajan S.S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. NeuroImage. 2005;25:1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Johnstone S.J., Barry R.J. Inhibitory processing during the Go/NoGo task: an ERP analysis of children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004;115:1320–1331. doi: 10.1016/j.clinph.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stock A.-K., Riegler L., Chmielewski W.X., Beste C. Paradox effects of binge drinking on response inhibition processes depending on mental workload. Arch. Toxicol. 2015 doi: 10.1007/s00204-015-1565-y. [DOI] [PubMed] [Google Scholar]
- Sugimoto F., Katayama J. Somatosensory P2 reflects resource allocation in a game task: assessment with an irrelevant probe technique using electrical probe stimuli to shoulders. Int. J. Psychophysiol. 2013;87:200–204. doi: 10.1016/j.ijpsycho.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Tamminga H.G.H., Reneman L., Huizenga H.M., Geurts H.M. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: a meta-regression analysis. Psychol. Med. 2016;46:1791–1807. doi: 10.1017/S0033291716000350. [DOI] [PubMed] [Google Scholar]
- Tillman C.M., Thorell L.B., Brocki K.C., Bohlin G. Motor response inhibition and execution in the stop-signal task: development and relation to ADHD behaviors. Child Neuropsychol. J. Norm. Abnorm. Dev. Child. Adolesc. 2008;14:42–59. doi: 10.1080/09297040701249020. [DOI] [PubMed] [Google Scholar]
- Umemoto A., Lukie C.N., Kerns K.A., Müller U., Holroyd C.B. Impaired reward processing by anterior cingulate cortex in children with attention deficit hyperactivity disorder. Cogn. Affect. Behav. Neurosci. 2014;14:698–714. doi: 10.3758/s13415-014-0298-3. [DOI] [PubMed] [Google Scholar]
- Villemonteix T., De Brito S.A., Slama H., Kavec M., Balériaux D., Metens T., Baijot S., Mary A., Peigneux P., Massat I. Grey matter volume differences associated with gender in children with attention-deficit/hyperactivity disorder: a voxel-based morphometry study. Dev. Cogn. Neurosci. 2015;14:32–37. doi: 10.1016/j.dcn.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.R., Ponesse J.S., Schachar R.J., Logan G.D., Tannock R. Development of inhibitory control across the life span. Dev. Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wright L., Lipszyc J., Dupuis A., Thayapararajah S.W., Schachar R. Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. J. Abnorm. Psychol. 2014;123:429–439. doi: 10.1037/a0036295. [DOI] [PubMed] [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material