Abstract
Endothermy, production and retention of heat by the body, appeared convergently in mammals, birds and four spiny-rayed teleost fish lineages. Of these, red-muscle endothermy over most or all of the body has only appeared in two groups: tunas and the opah (Lampris). Hitherto, tunas have been the only spiny-rayed fishes known to have bones containing embedded osteocyte cells; others have acellular bone. We examined bone histology in Lampris for the first time, demonstrating the presence of cellular bone very similar to that of tunas. This contrasts with the acellular condition of its ectothermic close relatives. The distribution of this character suggests that it co-evolved with red-muscle endothermy, hinting at a common physiological mechanism that would link bone histology to endothermy in these distantly related teleost lineages.
Keywords: bone histology, endothermy, osteocytes, Teleostei, Lampridiformes, Scombridae
1. Introduction
Endothermy, the capacity to produce and retain metabolic heat, is a well-known trait of modern mammals and birds. In a spectacular example of convergent evolution, endothermy also evolved independently in mackerel (Lamnidae) and thresher (Alopiidae) sharks and in four separate teleost lineages [1,2], allowing them to maintain active and efficient swimming in a broad range of water temperatures [3]. Teleost endothermy is little studied relative to that in tetrapods, but may provide key insights into the physiological requirements and evolution of endothermy.
Changes in bone microstructure are thought to correlate with tetrapod endothermy. This structure–function relationship has been a valuable tool for unravelling the metabolism and thermal biology of extinct taxa, including dinosaurs (e.g. [4,5]). However, the scarcity of comparative histological and microanatomical data on teleost bone hinders our understanding of the relationship between physiology and bone microstructure in endothermic teleosts.
All known endothermic teleosts belong to the mega-diverse predominantly marine clade Acanthomorpha, or spiny-rayed fishes [6]. These groups show two distinct endothermic strategies, different from those of birds and mammals: (i) billfishes (Xiphioidei) and the butterfly kingfish Gasterochisma melampus (Scombridae: Gasterochismatinae) independently developed heater organs consisting of modified extraocular muscles that locally warm the brain and eyes [1,2]; (ii) tunas (Scombridae: Thunnini) generate heat via the activity of red extraocular and swimming muscles and retain it with specialized counter-current blood vessels, warming not only the head but also the axial musculature [1,2]. The opah, Lampris sp. (Lampridiformes), has been recently shown to have both heat-generating extraocular and pectoral fin muscles, and a counter-exchange blood vessel network that heats cold oxygenated blood returning from the gills [7,8]. Thanks to this unique system, Lampris is the only known teleost to show endothermy over its entire body, allowing it to spend long periods of time hunting in the cold water between 50 and 400 m in depth [8].
Bone tissues in teleosts display a great variety of structures, ranging from: (i) cellular bone in which osteogenic cells (osteoblasts) are enclosed by the bone matrix and become trophic cells (osteocytes); to (ii) acellular bone that is entirely deprived of osteocytes, the osteoblasts withdrawing from the surface during deposition of the bone matrix [9–12]. Cellular bone is primitive to osteichthyans but is lost in the clade Neoteleostei, which includes acanthomorphs [11,13]. The only acanthomorphs with osteocytes reported thus far are tunas [9–11], in which bone tissues also show substantial remodelling [14]. The secondary reacquisition of osteocytes and the presence of conspicuous bone remodelling in tunas have been hypothesized to be linked with their active, endothermic metabolism [11,12,14]. Nevertheless, this assertion remains conjectural in the absence of comparative observations in lineages that independently evolved endothermy. In this context, Lampris provides an opportunity to test the hypothesized link between endothermy and cellular bone in teleosts. However, the bone microstructure of Lampris has thus far been unknown. Here we describe bone histology of this endothermic taxon and some of its ectothermic close relatives.
2. Material and methods
Histological sections were cut from bones of two extant lampridiforms (electronic supplementary material, table S1): Lampris sp. (MNHN-ZA-AC-A-7506, Muséum national d'Histoire naturelle, Paris, France) and Velifer hypselopterus (MNHN-ICOS-01117), along with six Late Cretaceous neoteleosts, including the stem-lampridiform [15] †‘Aipichthys’ velifer (MNHN.F.HAK1991). These fossils were included to document the trait in some of the earliest representatives of the neoteleost and acanthomorph clades (electronic supplementary material, table S1). Each sample was embedded in epoxy resin. The sections were sawn and ground to obtain 50–60 µm thickness, and examined with an Axiovert microscope in transmitted natural light.
3. Results
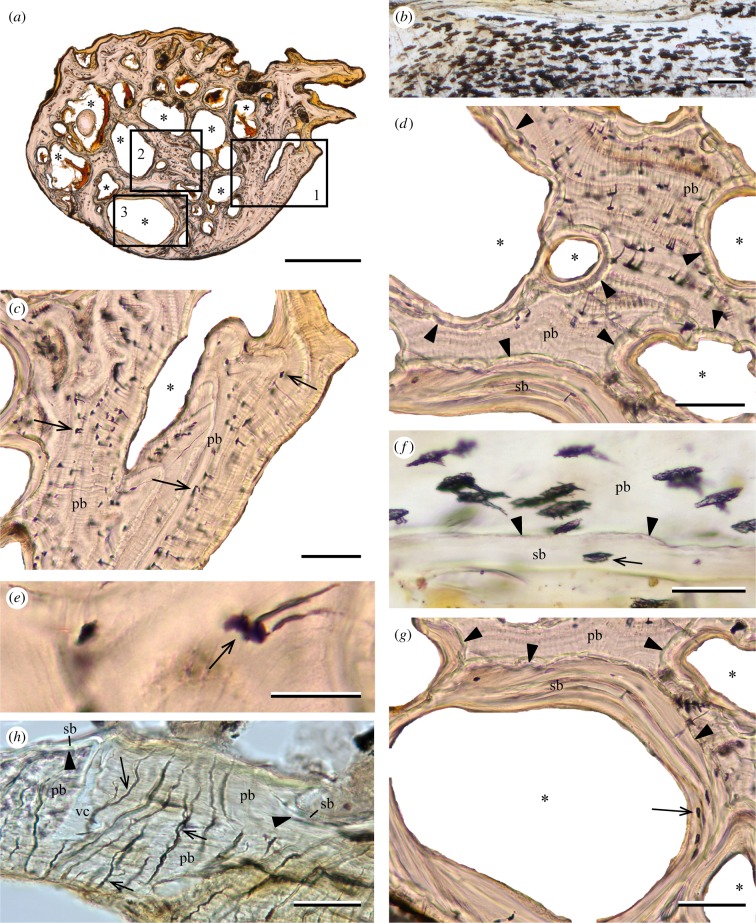
Primary bone in the rib of Lampris includes numerous elongate osteocyte lacunae (figure 1a,b), with their main axis perpendicular to the direction of growth. The canaliculi that housed the cytoplasmic extensions of the cells are fewer than in typical teleost bone [12]; they are relatively short, few are ramified (figure 1e), and they all point towards the periosteum (figure 1d). Primary bone shows a parallel-fibred structure, with layers of aligned osteocytes alternating with lines of arrested growth (figure 1c,d). The rib is spongy, with numerous cavities (figure 1a) surrounded by secondary bone limited by cementing lines (figure 1g). The secondary bone incorporates fewer osteocytes than areas of primary bone (figure 1f,g).
Figure 1.
(a–g) Lampris sp. (MNHN-ZA-AC-A-7506). Sections of the rib (natural transmitted light). (a) Entire cross section showing numerous vascular cavities (asterisks). (b) Longitudinal section showing numerous osteocyte lacunae. (c) Detail of inset 1 in (a), showing osteocyte lacunae (arrows) in primary bone. (d) Detail of inset 2 showing an area of primary bone with circularly-oriented osteocytes, and vascular cavities fringed by secondary bone. (e) Detail of an osteocyte (arrow) in primary bone with its radially-oriented canaliculi. (f) Longitudinal section showing osteocytes in primary bone. An osteocyte lacuna is seen in secondary bone (arrow). (g) Detail of inset 3 showing remodelled secondary bone including osteocytes without canaliculi (arrow). (h) Velifer hypselopterus (MNHN-ICOS-01117). Cross section of the scapula (transmitted natural light) showing the lack of osteocytes, but the presence of bifurcated (arrows) osteoblastic canaliculi inside the primary bony tissues. Some areas of secondary bone are visible. pb, primary bone; sb, secondary bone; vc, vascular cavity; *, marrow cavity. Arrowheads point to reversal lines between primary and secondary bone. Scale bars, (a) 500 µm, (b–d,g) 100 µm, (e–f,h) 50 µm.
The scapula of Velifer is composed of acellular bone: no osteocytes are incorporated in the bone tissue. However, it is perforated by numerous canals that are winding and bifurcated, indicating that they probably housed cytoplasmic extensions from surface osteoblasts (figure 1h). The rib of every Late Cretaceous taxon sampled (including the stem-lampridiform †‘Aipichthys’) also consists of acellular bone, without any identifiable features.
4. Discussion
Prior to this study, lampridiform osteohistology was only reported from an unknown bone of Trachipterus [9] and from the dorsal-fin pterygiophores of Regalecus [16], two elongate-bodied lampridiforms that collectively are the sister-group of Lampris [15]. Both have acellular non-hyperostotic bones (hyperostotic bones occasionally contain a few embedded osteocytes in bones of species that otherwise have acellular bones [16]). Therefore, Lampris is the only known lampridiform with cellular bone in normal conditions (electronic supplementary material, table S2). Furthermore, the red muscles and counter-exchange vascular networks indicative of endothermy are not found in lampridiforms, suggesting the absence of endothermy in all lampridiforms other than Lampris [8] (figure 2).
Figure 2.
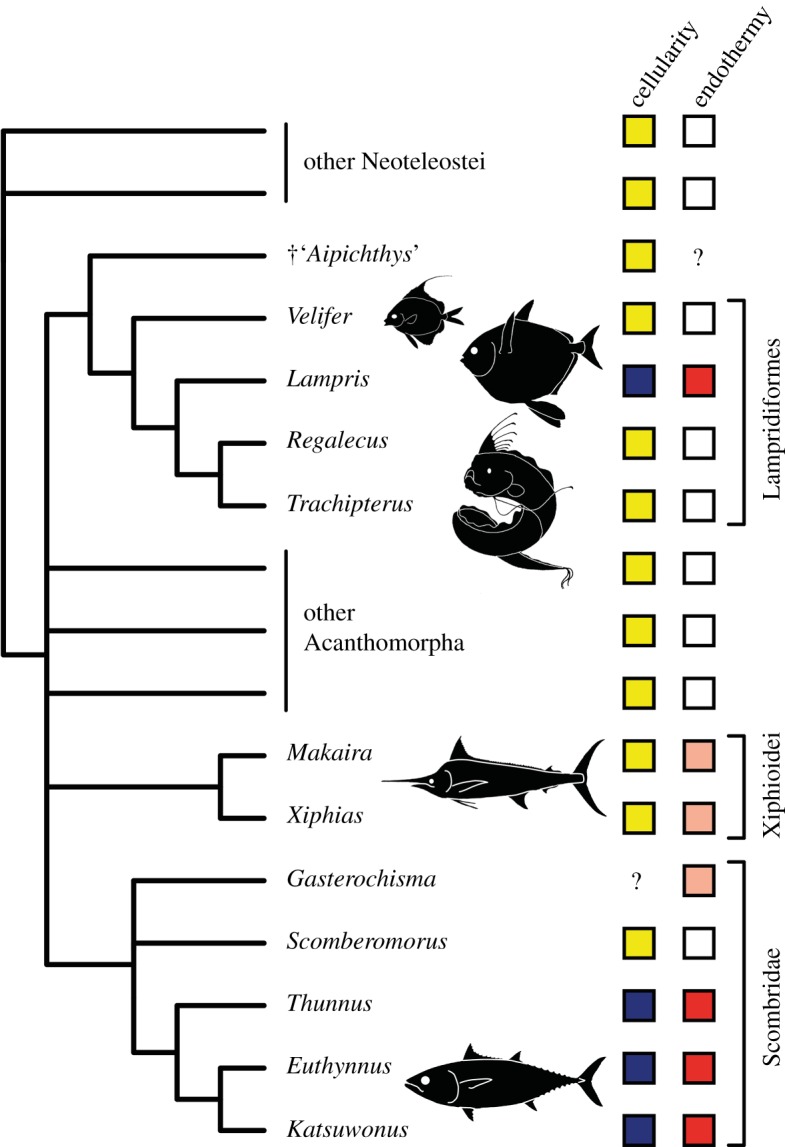
Phylogenetic distribution of the osteohistological and metabolic parameters considered in this study among Neoteleostei. Acellular bone is in yellow, cellular bone in dark blue. Ectothermic taxa are in white, taxa with brain heaters (cranial-only endothermy) in light pink, with red-muscle endothermy in red. Tree topology is adapted from [1,15,17]. (Online version in colour.)
In scombrids, cellular bone is described in Auxis, Euthynnus, Katsuwonus and Thunnus [9,11,12], i.e. ‘true’ tunas (Thunnini), which are all red-muscle endotherms [1,2]. Conversely, ectothermic scombrids such as the mackerels Scomber and Scomberomorus [1] have acellular bone [9,10] (figure 2; electronic supplementary material, table S2). Among the acanthomorphs that show cranial-only endothermy [1,2], billfishes also have acellular bone (electronic supplementary material, table S2) with histological features indicative of extensive remodelling, such as secondary osteons [18,19]. The absence of osteocytes in billfishes and ecologically comparable large-bodied fast-swimming pelagic predators (e.g. Coryphaena, Seriola) [9,10] excludes the possibility of a size-related distribution of osteocytes in acanthomorphs. Among teleosts virtually all taxa show either cellular or acellular bone consistently throughout all bones of the skeleton [10–12]. Therefore, we do not expect the presence of osteocytes to be affected by proximity with warmer regions of the body.
The widespread distribution of acellular bone among lampridiforms and other acanthomorphs (including some of the stratigraphically oldest fossils) indicates that cellular bone evolved independently from acellular ancestors in the Lampris and tuna lineages (figure 2; electronic supplementary material, table S2). Our survey of endothermic acanthomorphs and their immediate ectothermic relatives then suggests that osteocytes are found only in red-muscle endotherms amidst the whole diversity of the group, this physiology correlating perfectly with the occurrence of secondarily cellular bone in the acanthomorph clade (figure 2).
This is reinforced by the observation that, despite their independent evolutionary origins, bone tissues of Lampris and tunas share peculiar histological features: conspicuous remodelled bone [12,14] and similar-looking osteocytes with an elongate cell body and very few cytoplasmic projections, differing from the ‘star-shaped’ lacunae of other teleosts that are typically more rounded with numerous cytoplasmic projections [12,20]. These similarities, and their difference from the primitive condition of cellular bone in non-acanthomorph teleosts, may reflect either functional similarity of osteocytes in Lampris and tuna, and/or constraints on their morphology resulting from evolutionary reacquisition. This remains uncertain.
Whether the correspondence documented here is due to a physiological advantage of reacquiring osteocytes in red-muscle endotherms, or to a passive consequence of elevated metabolism, it provides strong evidence for a structure–function correlation between bone microstructure and endothermy in acanthomorph teleosts. Unlike in tetrapods, histological indicators of metabolism have rarely been addressed by studies of teleost bone [21]. To overcome this limitation, more comparative structural studies incorporating physiological considerations, at the inter- and intraspecific scale, would be needed. Applying such findings to fossil taxa may permit breakthrough investigation into fish palaeophysiology (for instance, in potentially endothermic fossil acanthomorphs), a field that is currently poorly developed but is promising considering the advances in tetrapod palaeophysiology that were enabled by palaeohistology (e.g. [4,5]). This would allow a better understanding of bone biology and evolution in a group representing half of modern vertebrate diversity.
Supplementary Material
Acknowledgements
We thank P. Béarez and G. Clément (MNHN) for granting us permission to sample the new material studied here, M. Hautecoeur and S. Morel (MNHN) for preparing the thin sections studied and figured in the article and M. Paig-Tran (California State University, Fullerton, USA) for providing information on the histology of Regalecus. Comments by L. Legendre and N. Wegner substantially improved the manuscript.
Ethics
This research is based exclusively on specimens from natural history collections.
Data accessibility
Data are available as the electronic supplementary material.
Authors' contributions
D.D., F.J.M. and O.O. conceived the project. F.J.M. supervised specimen acquisition and preparation, and realized histological observations and photographs. D.D., F.J.M., M.F., R.B.J.B. and O.O. analysed and interpreted the data. D.D. and F.J.M. drafted the original manuscript and figures. All authors revised and edited the manuscript, approved the final version and agree to be accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
This research was supported by the Leverhulme Trust (grant no. RPG-2016-168) and by a Junior Research Fellowship at Wolfson College, University of Oxford (D.D.).
References
- 1.Block BA, Finnerty JR, Stewart AFR, Kidd J. 1993. Evolution of endothermy in fish: mapping physiological traits on a molecular phylogeny. Science 260, 210–214. ( 10.1126/science.8469974) [DOI] [PubMed] [Google Scholar]
- 2.Dickson KA, Graham JB. 2004. Evolution and consequences of endothermy in fishes. Physiol. Biochem. Zool. 77, 998–1018. ( 10.1086/423743) [DOI] [PubMed] [Google Scholar]
- 3.Watanabe YY, Goldman KJ, Caselle JE, Chapman DD, Papastamatiou YP. 2015. Comparative analyses of animal-tracking data reveal ecological significance of endothermy in fishes. Proc. Natl Acad. Sci. USA 112, 6104–6109. ( 10.1073/pnas.1500316112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padian K, de Ricqlès A, Horner JR. 2001. Dinosaurian growth rates and bird origins. Nature 412, 405–408. ( 10.1038/35086500) [DOI] [PubMed] [Google Scholar]
- 5.Legendre LJ, Guénard G, Botha-Brink J, Cubo J. 2016. Palaeohistological evidence for ancestral high metabolic rate in archosaurs. Syst. Biol. 65, 989–996. ( 10.1093/sysbio/syw033) [DOI] [PubMed] [Google Scholar]
- 6.Wainwright PC, Longo SJ. 2017. Functional innovations and the conquest of the oceans by acanthomorph fishes. Curr. Biol. 27, R550–R557. ( 10.1016/j.cub.2017.03.044) [DOI] [PubMed] [Google Scholar]
- 7.Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA. 2009. Evidence for cranial endothermy in the opah (Lampris guttatus). J. Exp. Biol. 212, 461–470. ( 10.1242/jeb.022814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegner NC, Snodgrass OE, Dewar H, Hyde JR. 2015. Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science 348, 786–790. ( 10.1126/science.aaa8902) [DOI] [PubMed] [Google Scholar]
- 9.Kölliker A. 1859. On the different types in the microscopic structure of the skeleton of osseous fishes. Proc. R. Soc. Lond. B 9, 656–668. ( 10.1098/rspl.1857.0132) [DOI] [Google Scholar]
- 10.Moss ML. 1961. Studies of the acellular bone of teleost fish. I. Morphological and systematic variations. Acta Anat. 46, 343–462. ( 10.1159/000141794) [DOI] [PubMed] [Google Scholar]
- 11.Meunier FJ. 1987. Os cellulaire, os acellulaire et tissus dérivés chez les Ostéichthyens: les phénomènes de l. acellularisation et de la perte de minéralisation [Cellular bone, acellular bone and derived tissues in osteichthyans: the phenomena of acellularisation and loss of mineralisation]. Ann. Biol. 26, 201–233 (in French). [Google Scholar]
- 12.Meunier FJ, Huysseune A. 1992. The concept of bone tissue in Osteichthyes. Netherlands J. Zool. 42, 445–458. ( 10.1163/156854291X00441) [DOI] [Google Scholar]
- 13.Parenti LR. 1986. The phylogenetic significance of bone types in euteleost fishes. Zool. J. Linn. Soc. 87, 37–51. ( 10.1111/j.1096-3642.1986.tb01329.x) [DOI] [Google Scholar]
- 14.de Ricqlès A, Meunier FJ, Castanet J, Francillon-Vieillot H. 1991. Comparative microstructure of bone. In Bone: a treatise, vol. III (ed Hall B. K.), pp. 1–78. Boca Raton, FL: CRC Press. [Google Scholar]
- 15.Davesne D, Gallut C, Barriel V, Janvier P, Lecointre G, Otero O. 2016. The phylogenetic intrarelationships of spiny-rayed fishes (Acanthomorpha, Teleostei, Actinopterygii): fossil taxa increase the congruence of morphology with molecular data. Front. Ecol. Evol. 4, 129 ( 10.3389/fevo.2016.00129) [DOI] [Google Scholar]
- 16.Paig-Tran EWM, Barrios AS, Ferry LA. 2016. Presence of repeating hyperostotic bones in dorsal pterygiophores of the oarfish, Regalecus russellii. J. Anat. 229, 560–567. ( 10.1111/joa.12503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miya M, et al. 2013. Evolutionary origin of the Scombridae (tunas and mackerels): members of a Paleogene adaptive radiation with 14 other pelagic fish families. PLoS ONE 8, e73535 ( 10.1371/journal.pone.0073535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poplin C, Poplin F, de Ricqlès A. 1976. Quelques particularités anatomiques et histologiques du rostre de l'espadon (Xiphias gladius L.) [A few anatomical and histological specificities of the swordfish (Xiphias gladius L.) rostrum]. Comptes Rendus Acad. Sci. Paris D 282, 1105–1108 (in French). [Google Scholar]
- 19.Atkins A, et al. 2014. Remodeling in bone without osteocytes: billfish challenge bone structure–function paradigms. Proc. Natl Acad. Sci. USA 111, 16 047–16 052. ( 10.1073/pnas.1412372111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stéphan P. 1900. Recherches histologiques sur la structure du tissu osseux des poissons [Histological research on the structure of fish bone tissue]. Bull. Sci. Fr. Belg. 33, 281–429 (in French). [Google Scholar]
- 21.Dean MN, Shahar R. 2012. The structure-mechanics relationship and the response to load of the acellular bone of neoteleost fish: a review. J. Appl. Ichthyol. 28, 320–329. ( 10.1111/j.1439-0426.2012.01991.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as the electronic supplementary material.