Abstract
Background
Modafinil, a nonamphetaminic wake-promoting compound, is prescribed as first line therapy in narcolepsy, an invalidating disorder characterized by excessive daytime sleepiness and cataplexy. Although its mode of action remains incompletely known, recent studies indicated that modafinil modulates astroglial connexin-based gap junctional communication as administration of a low dose of flecainide, an astroglial connexin inhibitor, enhanced the wake-promoting and procognitive activity of modafinil in rodents and healthy volunteers. The aim of this study is to investigate changes in glucose cerebral metabolism in rodents, induced by the combination of modafinil+flecainide low dose (called THN102).
Methods
The impact of THN102 on brain glucose metabolism was noninvasively investigated using 18F-2-fluoro-2-deoxy-D-glucose Positron Emission Tomography imaging in Sprague-Dawley male rats. Animals were injected with vehicle, flecainide, modafinil, or THN102 and further injected with 18F-2-fluoro-2-deoxy-D-glucose followed by 60-minute Positron Emission Tomography acquisition. 18F-2-fluoro-2-deoxy-D-glucose Positron Emission Tomography images were coregistered to a rat brain template and normalized from the total brain Positron Emission Tomography signal. Voxel-to-voxel analysis was performed using SPM8 software. Comparison of brain glucose metabolism between groups was then performed.
Results
THN102 significantly increased regional brain glucose metabolism as it resulted in large clusters of 18F-2-fluoro-2-deoxy-D-glucose uptake localized in the cortex, striatum, and amygdala compared with control or drugs administered alone. These regions, highly involved in the regulation of sleep-wake cycle, emotions, and cognitive functions were hence quantitatively modulated by THN102.
Conclusion
Data presented here provide the first evidence of a regional brain activation induced by THN102, currently being tested in a phase II clinical trial in narcoleptic patients.
Keywords: modafinil, astroglial connexin, FDG PET imaging, neuroglia, narcolepsy
Significance Statement
THN102 (modafinil/flecainide) is efficient to reduce excessive daytime sleepiness. The impact of THN102 on rat brain metabolism was investigated with FDG PET imaging. Cortex, striatum, and amygdala were more activated after THN102 compared with modafinil. This study provides insights on the mechanism of action of THN102 innovative product.
Introduction
Modafinil is a nonamphetaminic, wake-promoting compound used as first line treatment of excessive daytime sleepiness (EDS), associated with narcolepsy (Lavault et al., 2011; Thorpy and Dauvilliers, 2015; Barateau et al., 2016). It has also been proposed as a treatment to reduce EDS in Parkinson’s disease (Sheng et al., 2013; Rodrigues et al., 2016). Mechanisms involved in wake-promoting and procognitive actions of modafinil are complex, as it modulates multiple monoaminergic and GABAergic neuronal systems (Minzenberg and Carter, 2008).
Early studies using Fos as a marker of neuronal activity in cat (Lin et al., 1996) and rat (Engber et al., 1998) suggested the hypothalamus as main brain target of modafinil. Subsequent studies in rodents indicated that cortex and striatum were activated after wake enhancement by modafinil (Scammell et al., 2000; Willie et al., 2005). Imaging in rodents depicted higher metabolism in hippocampus, thalamus, and amygdala (Engber et al., 1998) and showed activating effects of modafinil in fronto-cortical areas (Gozzi et al., 2012); those areas are involved in both arousal (Duteil et al., 1990; Lin et al., 1992) and cognitive enhancement (Lynch et al.; Béracochéa et al., 2003). Using pharmacological magnetic resonance imaging, or surface electroencephalography, modafinil was found to increase brain activity notably in the hippocampus and frontal cortex in healthy volunteers (Joo et al., 2008a) and narcoleptic patients (Saletu et al., 2007; Joo et al., 2008b). Moreover, positron emission tomography (PET) using 18F-2-fluoro-2-deoxy-D-glucose (FDG) in narcoleptic patients unveiled that modafinil increased the glucose brain metabolism in the hippocampus (Kim et al., 2007).
More recently, modafinil was shown to modulate astrocyte functions by increasing cell-cell communication mediated by connexin channels (Liu et al., 2013), membrane proteins involved in cellular communication (Giaume et al., 2010), and sleep regulation (Franco-Pérez and Paz, 2009; Franco-Pérez et al., 2012; Clasadonte et al., 2017). It was further shown in rodents that modulating astrocyte connexin by flecainide impacted the pharmacological action of modafinil by enhancing its wake-promoting, procognitive, and, notably, antinarcoleptic effects (Duchêne et al., 2016; Lu and Chen, 2016). The mechanisms of action of the modafinil/flecainide combination (THN102) remain to be further investigated but would likely be based on a restoration of the functionality of astroglial connexins. Using FDG PET in the rat, the aim of the present study was to assess the CNS effects of THN102 at the functional level compared with modafinil or flecainide used alone, both at their effective and clinically relevant doses.
Materials and Methods
Animals
Male Sprague-Dawley rats (Elevage Janvier) were collectively housed with food and water ad libitum. They were kept in a temperature- and humidity-controlled facility with 12-hour-dark/-light cycles (lights on at 8:00 am). Experiments were performed during the light phase between 9:00 am and 1:00 pm. The study was conducted in accordance with the French legislation and European directives on the use of animals in research (EU Directive 2010/63/EU). The protocol has been approved by the local committee for animal use in research (APAFIS#7466-20 1611 04 1 7049220 v2).
Drugs and Chemicals
Drugs were administered by i.v. injection in the tail vein (1 mL/kg). Treatments included vehicle (VEH), modafinil 10 mg/kg (MOD; Orchid Pharma), flecainide 1 mg/kg (FLE; Sigma-Aldrich), or the combination of both (THN102). FDG for i.v. injection was purchased from Cyclopharma.
FDG PET Imaging: Acquisition Protocol and Imaging Data Analysis
PET imaging study was performed using PET systems coupled with a computerized tomography scanner (Inveon microPET-CT; spatial resolution ~1.6 mm; Siemens) in anesthetized and fasted rats (1.5%–2.5% inhaled isoflurane, weight 250–350 g) (Bao et al., 2009). PET experiments were exclusively performed in the morning. Each day of experiment, 4 rats were randomly assigned to each group: animals were i.v. injected with VEH, MOD, FLE, or THN102 before being placed into the scanner for computerized tomography acquisition. Thirty minutes after the administration, 1 mL FDG (mean dose=42.2±26.3 MBq) was injected over 1 minute using a syringe pump. Dynamic PET acquisition begun immediately after the start of FDG infusion for 60 minutes. Blood glucose measurement was performed before 18F-FDG injection and at the end of PET acquisition using a portable glucometer (Accu-check Performa, Roche).
PET data were reconstructed using the FORE+OSEM2D algorithm including normalization, attenuation, scatter, and random corrections. To reduce noise and correct for partial volume effect, an iterative deconvolution using the point spread function of the scanner with a temporal based denoising was applied to each image. The method was previously reported and validated for use in small animal PET imaging (Wimberley et al., 2014; Reilhac et al., 2015).
Dynamic and summed (30–60 minutes) FDG PET images were spatially normalized to a standard rat brain FDG Schiffer’s template using Pmod software (version 3.6) (Schiffer et al., 2006). The brain kinetics of FDG may depend on its plasma kinetics (input function) and peripheral blood glucose level. Two normalization methods were thus performed to detect any regional change in FDG uptake by the brain. First, summed PET images were normalized by their respective whole-brain activity, thus highlighting the relative FDG uptake by the different brain regions. Then, the absolute metabolic rate of glucose (MRGlu) was estimated using pharmacokinetic modelling. To that end, a volume of interest was drawn on the vena cava to generate an imaged-derived input function of FDG, as previously described (Weber et al., 2002; Lanz et al., 2014). MRGlu Parametric PET images (PXMOD, Pmod software, version 3.6) were then generated for each animal from the dynamic brain PET images and corresponding imaged-derived input function (from 0 to 60 minutes) using the FDG Patlak model, considering blood glucose level measured immediately before PET (Patlak et al., 1983). The 0.71 lumped constant was used to take the difference between glucose and FDG metabolism into acount (Tokugawa et al., 2007).
Comparison of parametric images (FDG brain uptake and MRGlu) obtained in each group (n=5 animals/group) was performed using a statistical parametric mapping (SPM) and a voxel-to-voxel analysis (SPM8 softwared) as previously described (Schiffer et al., 2006; Soto-Montenegro et al., 2009). A brain mask was created from the FDG template and applied to all registered and normalized scans to include only cerebral voxels. Comparison was then performed using an ANOVA design to detect differences between groups. A significance level threshold of .05 (uncorrected for multiple comparisons) and a minimum cluster size of 200 voxels were selected. Only the clusters that were significant at P<.05 levels (corrected for multiple comparisons) were considered. The size of the clusters exceeding the threshold and their corrected significance were anatomically located using the Paxinos and Watson rat brain atlas (Paxinos and Watson, 2007).
Spontaneous Locomotor Activity Assessment
In an independent experiment, spontaneous locomotor activity was recorded in an open-field device (50x50x50 cm), during 2 hours, starting immediately after administration (VEH, MOD, FLE, and THN102). Cumulated traveled distance per 5-minute time bins was analyzed with ViewPoint. Experiments were performed with 8 drug-naïve animals in each group. The experimenter was blinded to treatment.
Determination of Modafinil Concentration in Serum and Brain
In parallel experiments, performed in another population of rats, brain and serum concentrations of modafinil were determined after MOD and THN102 administration in rats. Animals (n=8 rats/group) were anesthetized with isoflurane 30 minutes after i.v. administration and blood samples were collected, centrifuged (3000 g×15 min, 20°C), and stored at -80°C. Brains were collected immediately after blood sampling, freezed on dry ice, and stored at -80°C. Modafinil and flecainide concentrations in serum and brain lysate were determined using a reverse phase liquid chromatography with tandem mass spectrometry detection technique (Eurofins ADME Bioanalyses).
Statistical Analysis
Results of the locomotion and pharmacokinetic data are expressed as mean±SEM. Difference was considered significant at P<.05 levels. Statistical analysis was performed using Graphpad software (GraphPad Prism version 7). Cumulative traveled distance over time was compared using a 2-way repeated-measure ANOVA followed by Bonferroni’s posthoc test. Finally, serum and brain modafinil concentrations were compared using an unpaired t test.
Results
The goal of this study was to compare the brain metabolism after treatment with THN102, combination between modafinil and flecainide at low dose, to modafinil alone.
THN102 Locally Increases the Brain Glucose Metabolism
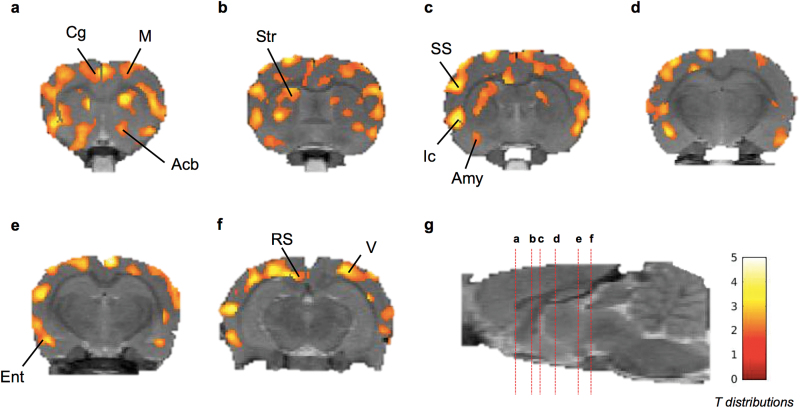
Normalization of FDG uptake by whole-brain activity was associated with a low variability. In the VEH group, the coefficient of variation (CV=SD/mean×100) of the relative uptake of FDG was CV=2.73% in the cortex. SPM analysis did not show any significant difference in the distribution of relative FDG brain uptake between FLE or MOD groups compared with the VEH group (P > .05). Significant increase in the relative brain uptake of FDG could be regionally observed in the THN102 group compared with the MOD group. The effect was observed bilaterally and was homogenously distributed in the cortex, amygdala and striatum (Table 1; Figure 1). Notably, a cluster of significant increase in FDG uptake could be observed in the nucleus accumbens. Similar distribution of increased relative FDG uptake could be observed in the THN102 group compared with the FLE or MOD alone (P<.05).
Table 1.
SPM Results Showing Clusters, Peak Coordinates, and Significant Levels of Brain Regions with an Increase in 18F-FDG Uptake When Comparing THN102 to MOD, FLE, or VEH
| Intergroup Comparisons | Significant Clusters | Significant Peak Coordinates within Clusters | Peak Regions | ||
|---|---|---|---|---|---|
| x | y | z | |||
| THN102 > VEH | Cortex Caudate and putamen (Striatum) Amygdala |
0.5 | 4.6 | 3.6 | R pre-limbic cortex |
| 6.7 | 4.6 | -5.6 | R primary auditory cortex | ||
| -5.7 | 3 | -8.8 | L temporal association cortex | ||
| -0.1 | 1.2 | -0.2 | L cingulate cortex area 1 | ||
| -5.5 | 8.2 | -0.6 | L layer 3 of cortex | ||
| THN102 > FLE | Cortex Caudate and putamen (Striatum) Amygdala |
6.5 | 4.6 | -5.8 | R primary auditory cortex |
| 4.3 | 2.8 | 2.0 | R primary somatosensory | ||
| -5.5 | 8.6 | -4.8 | L layer 3 of cortex | ||
| -2.1 | 3.6 | 3.8 | L forceps minor corpus callosum | ||
| -3.1 | 0.4 | -7.8 | L primary visual cortex monocular | ||
| THN102 > MOD | Cortex Caudate and putamen (Striatum) Amygdala |
5.7 | 7.2 | -1.6 | R dysgranular cortex |
| 2.1 | 1.4 | 4.4 | R secondary motor cortex | ||
| 2.5 | 0.8 | -5.2 | R secondary visual cortex | ||
| -0.7 | 0.4 | -4.2 | L retrosplenialdysgranular cortex | ||
| -3.7 | 6.8 | 3.0 | L layer 3 of cortex | ||
| -5.5 | 8.6 | -4.8 | L basolateral amygdaloïd nucleus, posterior | ||
Voxels comparison-based analysis was performed using SPM8 (n=5) rats per group using 1-way ANOVA followed by multiple comparison test. A significance level threshold of .05 and a minimum cluster size of 200 voxels were selected to identify significant clusters. Coordinates of significant peak (P<.05) were given according to the Paxinos and Watson rat brain atlas (Paxinos and Watson, 2007). FLE, flecainide 1 mg/kg; MOD, modafinil 10 mg/kg; THN102, modafinil 10 mg/kg+flecainide 1 mg/kg; VEH, vehicle.
Figure 1.
Coronal brain section showing statistical parametric map for increase in relative 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) uptake after Modafinil(MOD) +flecainide (THN102) treatment in comparison to MOD alone in rats. Following a cranio-caudal orientation centered on bregma (anterior is positive), coronal brain sections were at (a) 1.05, (b) -0.68, (c) -1.29, (d) -3.07, (e) -4.34, and (f) -5.76 millimeters from the bregma and (g) sagittal view of the brain with the marked coronal sections (red dotted lines) from a to f. Color scale represents all T distributions achieving statistical significance (see SPM statistical analysis). Acb, accumbens; Amy, amygdala; Cg, cingulate cortex; Ent, entorhinal cortex; Ic, insular cortex; M, motor cortex; RS, retrosplenial cortex; SS, somatosensory cortex; Str, striatum; V, visual cortex.
Within the significant clusters, further detailed analysis allowed to locate the relevant peak regions. Hence, THN102 enhanced the brain metabolism compared with MOD in the subsortical regions such as the dysgranular and retrosplenialdysgranular cortices, primary/secondary motor, dorsolateral enthorinal and visual cortices, the retrosplenialdysgranular area, as well as the layer 3 and basolateral amygdaloïd nuclei.
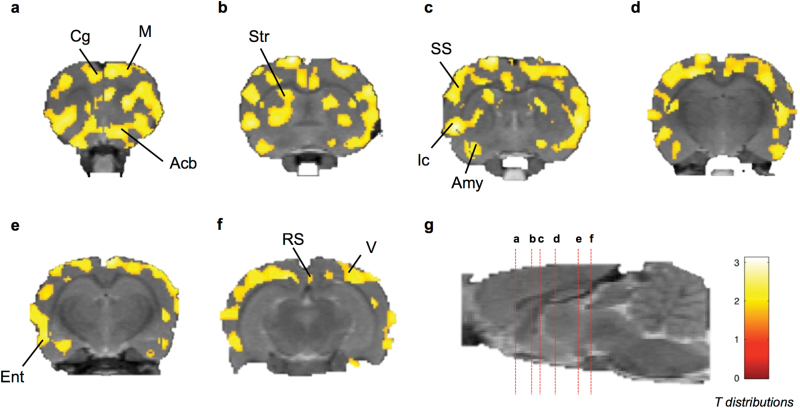
There was a significant increase in blood glucose levels measured before (85.8±4.5 mg/dL) and at the end (116±4.3 mg/dL) of PET acquisitions (P<.05). However, initial as well as final glucose concentrations were not different between the 4 treament groups, suggesting the absence of treatement-induced change in peripheral glucose metabolism. Compared with the relative FDG uptake, absolute quantification of the brain MRGlu was associated with a higher variability (CV=19.82% in the cortex of the VEH group). SPM analysis nonetheless highlighted patterns of significantly higher glucose consumption in the THN102 group compared with MOD alone (P<.05). The regional increase in MRGlu was consistent with the regional increase in the relative FDG uptake (Table 2; Figure 2). However, difference in MRGlu in the THN102 group compared with the VEH and FLE groups was not significant, which may be due to a higher variability of the MRGlu data.
Table 2.
SPM Results Showing Clusters, Peak Coordinates, and Significant Levels of Brain Regions with an Increase in MRGlu When Comparing THN102 to MOD in Rats
| Intergroup Comparisons | Significant Clusters | Significant Peak Coordinates within Clusters | Peak Regions | ||
|---|---|---|---|---|---|
| x | y | z | |||
| THN102 > MOD | Cortex Caudate and putamen (Striatum) Amygdala Accumbens |
4.1 | 0.8 | -5.2 | R primary visual cortex |
| 0.7 | 2.2 | 3.8 | R secondary motor cortex | ||
| 5.7 | 8.6 | -4.6 | R layer 3 of cortex | ||
| -1.5 | 0.6 | -0.4 | L primary motor cortex | ||
| -4.9 | 9.0 | 0.0 | L layer 3 of cortex | ||
| -6.5 | 6.4 | -8.8 | L dorsolateral enthorinal cortex | ||
Voxels comparison based analysis was performed using SPM8 (n=5) rats per group using 1-way ANOVA followed by multiple comparison test. A significance level threshold of .05 and a minimum cluster size of 200 voxels were selected to identify significant clusters. Coordinates of significant peak (P<.05) were given according to the Paxinos and Watson rat brain atlas (Paxinos and Watson, 2007). MOD, modafinil 10 mg/kg; MRGlu, metabolic rate of glucose; THN102, modafinil 10 mg/kg+flecainide 1 mg/kg.
Figure 2.
Coronal brain section showing statistical parametric map for increase in absolute metabolic rate of glucose (MRGlu) after Modafinil(MOD) +flecainide (THN102) treatment in comparison to MOD in rats. Following a cranio-caudal orientation centered on bregma (anterior is positive), coronal brain sections were at (a) 1.05, (b) -0.68, (c) -1.29, (d) -3.07, (e) -4.34, and (f) -5.76 millimeters from the bregma; and (g) sagittal view of the brain with the marked coronal sections (red dotted lines) from a to f. Color scale represents all T distributions achieving statistical significance (see SPM statistical analysis). Acb, accumbens; Amy, amygdala; Cg, cingulate cortex; Ent, entorhinal cortex; Ic, insular cortex; M, motor cortex; RS, retrosplenial cortex; SS, somatosensory cortex; Str, striatum; V, visual cortex.
THN102 Increases Spontaneous Locomotor Activity
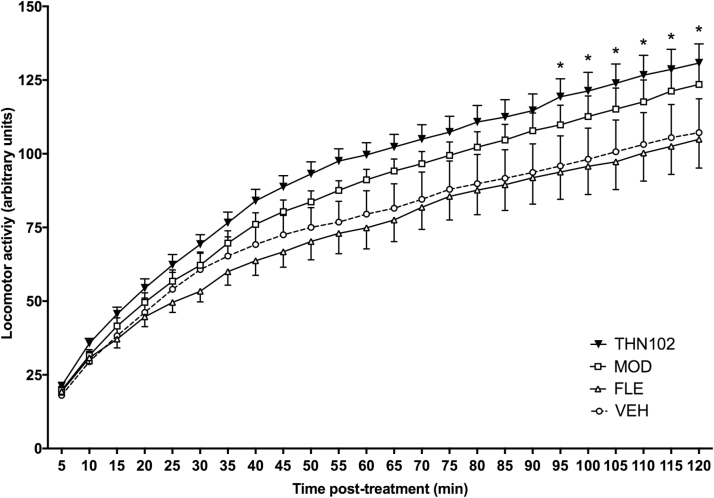
To assess whether doses of modafinil allowed to discriminate THN102 compared with modafinil alone, spontaneous motor activity in unrestrained awake rats was monitored for 2 hours after treatment, during the lights-on period (Figure 3). Locomotor activity in the MOD group tends to be increased compared with the VEH group without reaching significance (+15.2% at 120 minutes). THN102 administration induced a significant increase in cumulative locomotor activity compared with VEH-treated animals, beginning at 95 minutes (+22.0% at 120 minutes vs VEH, P=.0309). THN102 was more effective in increasing the locomotor activity compared with MOD; however, the difference was not statistically significant (+5.89% at 120 minutes). Moreover, no effect of FLE treatment was found at the 1-mg/kg dose when administered alone.
Figure 3.
Cumulative traveled distance over 2 hours after vehicle (VEH), flecainide 1 mg/kg (FLE), modafinil 10 mg/kg (MOD), or MOD 10 mg/kg+FLE 1 mg/kg (THN102) treatment in awake rats. Locomotor activity of rats was measured during 2 hours after treatment. Data are expressed as means±SEM (n=8 rats/group) and compared using 2-way ANOVA followed by Bonferroni’s multiple comparison test: *P<.05 THN102 vs VEH.
Brain and Serum Concentrations of Modafinil
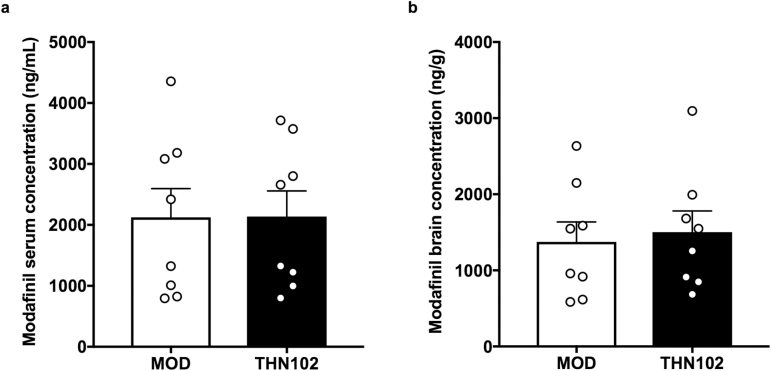
Serum and brain were collected 30 minutes after treatment with modafinil alone (MOD) or combined with flecainide (THN102; Figure 4). Quantification of modafinil concentration levels treated with MOD or THN102 demonstrated no significant difference between both groups in serum (2.12±0.472 ng/mL and 2.14±0.419 ng/mL, respectively) or in brain (1.37±0.262 ng/g and 1.50±0.277 ng/g, respectively). Therefore, there is no apparent pharmacokinetic interaction between MOD and FLE on MOD metabolism.
Figure 4.
Serum (a) and brain (b) concentrations of modafinil after modafinil 10 mg/kg+flecainide 1 mg/kg (THN102) and modafinil 10 mg/kg (MOD) treatment. Thirty minutes after the treatment injection, serum and brain levels of MOD were sampled and quantified by liquid chromatography with tandem mass spectrometry. Data are expressed as means with scattered plots±SEM (n=8 rats/group) and compared using an unpaired t test.
Discussion
In this study, we reported that THN102 significantly activates the cortico-amygdala-striata regions compared with MOD used alone.
Modafinil has been largely proposed in sleep medicine to treat EDS associated with narcolepsy (Lavault et al., 2011; Thorpy and Dauvilliers, 2015; Barateau et al., 2016), Parkinson’s disease (Sheng et al., 2013; Rodrigues et al., 2016), idiopathic hypersomnia (Lavault et al., 2011; Lopez et al., 2017), and obstructive sleep apnea/hypopnea syndrome (Black and Hirshkowitz, 2005). Numerous preclinical studies have generated a wealth of experimental data, which lead to many hypotheses regarding the mode of action of modafinil (Gerrard and Malcolm, 2007; Minzenberg and Carter, 2008). The central noradrenergic hypothesis has been supported by data showing that inhibition of catecholamine synthesis or antagonism of adrenergic receptors is able to attenuate the wake-promoting effects of modafinil (Duteil et al., 1991; Lin et al., 1992). Recent studies using mice lacking the noradrenaline synthesis or alpha1β-adrenoceptor (Stone et al., 2002a, 2002b; Hou et al., 2005) or brain imaging in humans (Minzenberg and Carter, 2008) also support the critical involvement of the locus cœruleus noradrenergic system in modafinil profile. The dopaminergic hypothesis has been very attractive and prevailing since the identification of an affinity of modafinil with dopamine transporter (Mignot et al., 1994; Wisor et al., 2001; Wisor and Eriksson, 2005; Korotkova et al., 2007; Qu et al., 2008). The brain disinhibitory hypothesis (Lin et al., 1996, 2000) has received less attention, yet it is supported by the fact that modafinil induces a significant decrease in GABA outflow (Ferraro et al., 1996) in many brains areas, notably those critically involved in sleep-wake cycle control.
Recent data indicated that not only neurons but also glial cells, in particular astrocytes, were modulated by modafinil, as it enhanced astrocyte coupling and the expression of one of their connexins, Cx30 (Liu et al., 2013). These data suggest that astroglial connexins might be involved in modafinil mode of action. Indeed, flecainide, an astroglial connexin inhibitor, was able to enhance modafinil procognitive and wake-promoting activities when coadministered to modafinil in rodents (Duchêne et al., 2016; Lu and Chen, 2016). THN102 is currently in phase II clinical trial on narcoleptic patients (NCT02821715). In this context, we compared the impact on brain functions between THN102 and modafinil in the rat by assessing their drug-induced changes in brain glucose metabolism.
The route of administration and dose of modafinil (10 mg/kg, i.v.) was based on a previous functional magnetic resonance imaging (fMRI) study in rats (Gozzi et al., 2012). Flecainide dose was chosen according to previous studies in rodents (Duchêne et al., 2016). Additionally, at selected doses, THN102 significantly increased locomotor activity, whereas modafinil alone showed only a tendency to increase it (nonsignificant), as partially described elsewhere (Simon et al., 1996; Edgar and Seidel, 1997; Zolkowska et al., 2009). As previously reported in mice (Duchêne et al., 2016), assessment of modafinil concentration in the serum and brain confirmed that flecainide did not increase the brain distribution of modafinil. Therefore, pharmacokinetic interaction between flecainide and modafinil can unlikely explain the brain effect of THN102 compared with vehicle and modafinil alone. Serum concentrations were close to modafinil levels found in subjects following dosing of clinically effective doses (McClellan and Spencer, 1998; Wong et al., 1999), suggesting potential relevance of the presented data to clinical conditions.
Several methods have been proposed to investigate the CNS effects of modafinil in human (Ellis et al., 1999; Spence et al., 2005; Hunter et al., 2006; Thomas and Kwong, 2006; Kim et al., 2007; Joo et al., 2008a; Minzenberg et al., 2008; Rasetti et al., 2010; Ghahremani et al., 2011; Minzenberg et al., 2011; Goudriaan et al., 2013; Schmaal et al., 2013; Funayama et al., 2014; Schmaal et al., 2014; Ikeda et al., 2017; Schmidt et al., 2017) and animals (Engber et al., 1998; van Vliet et al., 2008; Dawson et al., 2012; Gozzi et al., 2012), including fMRI, 2-deoxyglucose autoradiography, FDG PET, and cerebral blood flow assesement using single-photon emission computed tomography. These data tend towards a consensus over cortical and subcortical brain activations induced by modafinil including hypothalamic and thalamic regions (Thomas and Kwong, 2006; Gozzi et al., 2012), which are highly involved in the regulation of sleep-wake cycle (Szabadi, 2006; Lin et al., 2011). Other major structures are highlighted following modafinil administration as the caudate putamen (striatum), the amygdala, and the hippocampus (Engber et al., 1998; Ghahremani et al., 2011), known for their implication in driving emotions and cognitive functions (Joo et al., 2008a).
In our conditions, modafinil alone did not modulate glucose brain metabolism assessed by FDG PET compared with vehicle. Even though the dosage and the route were identical to a previous fMRI study (Gozzi et al., 2012), the observed cortical activation after treatment was not detected in our analysis. This difference highlights the discrepancies that may exist between the drug-induced response on blood oxygenation levels-dependent response and on energy consumption (Di et al., 2012; Cabrera et al., 2016). Another explanation for such discrepancies may be the presence of anesthesia. In the present study, animals were exposed to <2.5% isoflurane during drug administration and subsequent PET acquisition. It was reported that isoflurane may modulate the brain glucose uptake and may thus limit the sensitivity of the method to detect the CNS response to investigated compounds in vivo (Spangler-Bickell et al., 2016; Park et al., 2017). Working on awake rodents was shown feasible, although technically challenging (Spangler-Bickell et al., 2016; Park et al., 2017). In the present study, we chose to administer investigated treatments under isoflurane anesthesia to facilitate animal handling, i.v. administration, and avoid any stress due to the experimental procedure. Moreover, we showed that the locomotor activity was different between groups, which may nonspecifically impact FDG uptake by the brain in awake animals. Therefore, the whole procedure was performed under isoflurane anesthesia to highlight the intrinsic effect of each treatment on brain function and allow for dynamic PET acquisition for 60 minutes in immobile animals.
Some brain structures such as the locus coeruleus, thalamus, and hypothalamus, modulated by modafinil (Minzenberg et al., 2008; Gozzi et al., 2012; Schmaal et al., 2013) were not activated by THN102 compared with modafinil alone. More interestingly, this study reported that THN102 significantly increased the relative glucose brain uptake in the whole cortex, amygdala, and striatum compared with modafinil alone, hence suggesting a different activity on glucose metabolism between both treatment groups. Similar effects could be observed using pharmacokinetic modelling and estimation of the absolute MRGlu. Despite higher variability in this parameter, patterns of increased metabolic rate of glucose were detected in the cortex, striatum, amygdala, and accumbens of rats in the THN102 group compared with the MOD group. Such brain regions may be considered as substrates for the enhancement of modafinil effects by flecainide. They notably process attention states and motivation and code behavioral responses (Cardinal et al., 2002; Cho et al., 2013), functions that are altered in narcolepsy and that are portentially modulated by THN102 (Bayard et al., 2012).
The amygdala, cortex, and striatum are dopamine- and glutamine-rich brain areas (Darvas et al., 2011; Oikonomou et al., 2014). Data presented in this study point toward a putative role of THN102 on those dopaminergic and glutamatergic neurotransmission systems, potential targets of modafinil (Minzenberg and Carter, 2008). Furthermore, plasticity of astrocytes has also been demonstrated at least in the amygdala (Johnson et al., 2010) and cortex (Sims et al., 2015), providing potential evidence for the involvement of astrocyte networks in modafinil pharmacological profile (Duchêne et al., 2016).
Furthermore, our data confirm, for the first time, the greater effects of THN102 compared with modafinil in terms of brain activation, supporting the hypothesis that a modulation of connexins, and notably astroglial connexins Cx30 and Cx43 by flecainide, can impact modafinil mechanism of action. Recent PET studies in narcoleptic patients evidenced hypermetabolism in cortical regions compared with healthy controls, further pointing out the role of those areas in this disorder (Dauvilliers et al., 2010, 2017). Clinical FDG PET is feasible and may be useful to highlight the impact of THN102 on brain function in healthy volunteers and patients in the absence of anesthesia. Such studies might encompass dose-effect range and address the impact of flecainide on the neuropharmacology of modafinil in humans.
Taking together, we demonstrated here that THN102 enhanced both locomotor activity and glucose metabolism in cortical and subcortical areas involved in the regulation of sleep-wake cycle and behaviors. Our results further support the hypothesis that astrocyte connexins are involved in pharmacological responses of psychoactive drugs such as modafinil (Duchêne et al., 2016; Jeanson et al., 2016; Charvériat et al., 2017).
Funding
This work was supported by Theranexus Company and the Agence Nationale de la Recherche (grant number 14-CE16-0022).
Acknowledgments
We thank Emile Jaumain for helpful technical assistance.
Statement of Interest
F.M., M.C., and A.D. are full-time employees of Theranexus company. Y.D. served as consultant for Bioprojet Pharma, Flamel Technologies, Jazz Pharmaceuticals, Theranexus, Takeda, and UCB. The other authors declare no financial conflict of interest.
References
- Bao Q, Newport D, Chen M, Stout DB, Chatziioannou AF(2009)Performance evaluation of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med 50:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateau L, Lopez R, Dauvilliers Y(2016)Treatment options for narcolepsy. CNS Drugs 30:369–379. [DOI] [PubMed] [Google Scholar]
- Bayard S, Croisier Langenier M, Cochen De Cock V, Scholz S, Dauvilliers Y(2012)Executive control of attention in narcolepsy. Plos One 7:e33525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béracochéa D, Celerier A, Peres M, Pierard C(2003)Enhancement of learning processes following an acute modafinil injection in mice. Pharmacol Biochem Behav 76:473–479. [DOI] [PubMed] [Google Scholar]
- Black JE, Hirshkowitz M(2005)Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep 28:464–471. [DOI] [PubMed] [Google Scholar]
- Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ(2016)Neuroimaging the effectiveness of substance use disorder treatments. J Neuroimmune Pharmacol 11:408–433. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ(2002)Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352. [DOI] [PubMed] [Google Scholar]
- Charvériat M, Naus CC, Leybaert L, Sáez JC, Giaume C(2017)Connexin-dependent neuroglial networking as a new therapeutic target. Front Cell Neurosci 11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, Fudge JL(2013)Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci 33:14017–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Scemes E, Wang Z, Boison D, Haydon PG(2017)Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95:1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD(2011)Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem 18:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Comte F, Bayard S, Carlander B, Zanca M, Touchon J(2010)A brain PET study in patients with narcolepsy-cataplexy. J Neurol Neurosurg Psychiatry 81:344–348. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Evangelista E, de Verbizier D, Barateau L, Peigneux P(2017)[18F]fludeoxyglucose-positron emission tomography evidence for cerebral hypermetabolism in the awake state in narcolepsy and idiopathic hypersomnia. Front Neurol 8:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, Thompson RJ, McVie A, Thomson DM, Morris BJ, Pratt JA(2012)Modafinil reverses phencyclidine-induced deficits in cognitive flexibility, cerebral metabolism, and functional brain connectivity. Schizophr Bull 38:457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Biswal BB, Alzheimer’s Disease Neuroimaging Initiative (2012)Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fmri networks. Brain Connect 2:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne A, Perier M, Zhao Y, Liu X, Thomasson J, Chauveau F, Piérard C, Lagarde D, Picoli C, Jeanson T, Mouthon F, Dauvilliers Y, Giaume C, Lin JS, Charvériat M(2016)Impact of astroglial connexins on modafinil pharmacological properties. Sleep 39:1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duteil J, Rambert FA, Pessonnier J, Hermant JF, Gombert R, Assous E(1990)Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol 180:49–58. [DOI] [PubMed] [Google Scholar]
- Duteil J, Rambert FA, Pointeau AM, Mangiameli P, Assous E(1991)Flerobuterol: a potential antidepressant drug related to beta-adrenergic agonists. Experimental profile in mice. Fundam Clin Pharmacol 5:695–708. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF(1997)Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther 283:757–769. [PubMed] [Google Scholar]
- Ellis CM, Monk C, Simmons A, Lemmens G, Williams SC, Brammer M, Bullmore E, Parkes JD(1999)Functional magnetic resonance imaging neuroactivation studies in normal subjects and subjects with the narcoleptic syndrome. Actions of modafinil. J Sleep Res 8:85–93. [DOI] [PubMed] [Google Scholar]
- Engber TM, Dennis SA, Jones BE, Miller MS, Contreras PC(1998)Brain regional substrates for the actions of the novel wake-promoting agent modafinil in the rat: comparison with amphetamine. Neuroscience 87:905–911. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tanganelli S, O’Connor WT, Antonelli T, Rambert F, Fuxe K(1996)The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett 220:5–8. [DOI] [PubMed] [Google Scholar]
- Franco-Pérez J, Ballesteros-Zebadúa P, Fernández-Figueroa EA, Ruiz-Olmedo I, Reyes-Grajeda P, Paz C(2012)Sleep deprivation and sleep recovery modifies connexin36 and connexin43 protein levels in rat brain. Neuroreport 23:103–107. [DOI] [PubMed] [Google Scholar]
- Franco-Pérez J, Paz C(2009)Quinine, a selective gap junction blocker, decreases REM sleep in rats. Pharmacol Biochem Behav 94:250–254. [DOI] [PubMed] [Google Scholar]
- Funayama T, Ikeda Y, Tateno A, Takahashi H, Okubo Y, Fukayama H, Suzuki H(2014)Modafinil augments brain activation associated with reward anticipation in the nucleus accumbens. Psychopharmacology (Berl) 231:3217–3228. [DOI] [PubMed] [Google Scholar]
- Gerrard P, Malcolm R(2007)Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat 3:349–364. [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED(2011)Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology 36:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N(2010)Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:87–99. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L(2013)Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fmri. Addict Behav 38:1509–1517. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S, Bifone A, Zucconi GG, Bentivoglio M(2012)Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology 37:822–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou RH, Freeman C, Langley RW, Szabadi E, Bradshaw CM(2005)Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology (Berl) 181:537–549. [DOI] [PubMed] [Google Scholar]
- Hunter MD, Ganesan V, Wilkinson ID, Spence SA(2006)Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry 163:2184–2186. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Funayama T, Tateno A, Fukayama H, Okubo Y, Suzuki H(2017)Modafinil enhances alerting-related brain activity in attention networks. Psychopharmacology (Berl) 234:2077–2089. [DOI] [PubMed] [Google Scholar]
- Jeanson T, Duchêne A, Richard D, Bourgoin S, Picoli C, Ezan P, Mouthon F, Giaume C, Hamon M, Charvériat M(2016)Potentiation of amitriptyline anti-hyperalgesic-like action by astroglial connexin 43 inhibition in neuropathic rats. Sci Rep 6:38766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL(2010)Astrocytes in the amygdala. Vitam Horm 82:23–45. [DOI] [PubMed] [Google Scholar]
- Joo EY, Tae WS, Jung KY, Hong SB (2008a) Cerebral blood flow changes in man by wake-promoting drug, modafinil: a randomized double blind study. J Sleep Res 17:82–88. [DOI] [PubMed] [Google Scholar]
- Joo EY, Seo DW, Tae WS, Hong SB (2008b) Effect of modafinil on cerebral blood flow in narcolepsy patients. Sleep 31:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Yoon IY, Shin YK, Cho SS, Kim SE(2007)Modafinil-induced hippocampal activation in narcolepsy. Neurosci Lett 422:91–96. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Klyuch BP, Ponomarenko AA, Lin JS, Haas HL, Sergeeva OA(2007)Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology 52:626–633. [DOI] [PubMed] [Google Scholar]
- Lanz B, Poitry-Yamate C, Gruetter R(2014)Image-derived input function from the vena cava for 18F-FDG PET studies in rats and mice. J Nucl Med 55:1380–1388. [DOI] [PubMed] [Google Scholar]
- Lavault S, Dauvilliers Y, Drouot X, Leu-Semenescu S, Golmard JL, Lecendreux M, Franco P, Arnulf I(2011)Benefit and risk of modafinil in idiopathic hypersomnia vs Narcolepsy with cataplexy. Sleep Med 12:550–556. [DOI] [PubMed] [Google Scholar]
- Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M(1992)Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 591:319–326. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M(1996)Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A 93:14128–14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Gervasoni D, Hou Y, Vanni-Mercier G, Rambert F, Frydman A, Jouvet M(2000)Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J Sleep Res 9:89–96. [DOI] [PubMed] [Google Scholar]
- Lin JS, Anaclet C, Sergeeva OA, Haas HL(2011)The waking brain: an update. Cell Mol Life Sci 68:2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Petit JM, Ezan P, Gyger J, Magistretti P, Giaume C(2013)The psychostimulant modafinil enhances gap junctional communication in cortical astrocytes. Neuropharmacology 75:533–538. [DOI] [PubMed] [Google Scholar]
- Lopez R, Arnulf I, Drouot X, Lecendreux M, Dauvilliers Y(2017)French consensus. Management of patients with hypersomnia: which strategy?Rev Neurol (Paris) 173:8–18. [DOI] [PubMed] [Google Scholar]
- Lu J, Chen M(2016)Glial gap junctions boost modafinil action on arousal. Sleep 39:1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Palmer LC, Gall CM(2011)The likelihood of cognitive enhancement. Pharmacol Biochem Behav 99:116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KJ, Spencer CM(1998)Modafinil: a review of its pharmacology and clinical efficacy in the management of narcolepsy. CNS Drugs 9:311–324. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC(1994)Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep 17:436–437. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS(2008)Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33:1477–1502. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS(2008)Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science 322:1700–1702. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Yoon JH, Carter CS(2011)Modafinil modulation of the default mode network. Psychopharmacology (Berl) 215:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Singh MB, Sterjanaj EV, Antic SD(2014)Spiny neurons of amygdala, striatum, and cortex use dendritic plateau potentials to detect network UP states. Front Cell Neurosci 8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TY, Nishida KS, Wilson CM, Jaiswal S, Scott J, Hoy AR, Selwyn RG, Dardzinski BJ, Choi KH(2017)Effects of isoflurane anesthesia and intravenous morphine self-administration on regional glucose metabolism ([18F]FDG-PET) of male sprague-dawley rats. Eur J Neurosci 45:922–931. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD(1983)Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3:1–7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C(2007)The rat brain in stereotaxic coordinates, 6th ed. [DOI] [PubMed] [Google Scholar]
- Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y(2008)Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 28:8462–8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, Arrillaga-Romany IC, Goldberg TE, Callicott JH, Apud JA, Weinberger DR(2010)Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology 35:2101–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilhac A, Charil A, Wimberley C, Angelis G, Hamze H, Callaghan P, Garcia MP, Boisson F, Ryder W, Meikle SR, Gregoire MC(2015)4D PET iterative deconvolution with spatiotemporal regularization for quantitative dynamic PET imaging. Neuroimage 118:484–493. [DOI] [PubMed] [Google Scholar]
- Rodrigues TM, Castro Caldas A, Ferreira JJ(2016)Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: systematic review and meta-analysis. Parkinsonism Relat Disord 27:25–34. [DOI] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Semlitsch HV, Saletu-Zyhlarz GM, Mandl M, Zeitlhofer J, Saletu B(2007)Low-resolution brain electromagnetic tomography (LORETA) identifies brain regions linked to psychometric performance under modafinil in narcolepsy. Psychiatry Res 154:69–84. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS, Saper CB(2000)Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 20:8620–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer WK, Mirrione MM, Biegon A, Alexoff DL, Patel V, Dewey SL(2006)Serial micropet measures of the metabolic reaction to a microdialysis probe implant. J Neurosci Methods 155:272–284. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE(2013)Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry 73:211–218. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, Joos L, Dom G, Pattij T, van den Brink W, Veltman DJ(2014)Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychol Med 44:2787–2798. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Muller F, Dolder PC, Schmid Y, Zanchi D, Liechti ME, Borgwardt S(2017)Comparative effects of methylphenidate, modafinil and MDMA on response inhibition neural networks in healthy subjects. Int J Neuropsychopharmacol 20:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng P, Hou L, Wang X, Wang X, Huang C, Yu M, Han X, Dong Y(2013)Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. Plos One 8:e81802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Hémet C, Costentin J(1996)Analysis of stimulant locomotor effects of modafinil in various strains of mice and rats. Fundam Clin Pharmacol 10:431–435. [DOI] [PubMed] [Google Scholar]
- Sims RE, Butcher JB, Parri HR, Glazewski S(2015)Astrocyte and neuronal plasticity in the somatosensory system. Neural Plast 2015:732014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Montenegro ML, Vaquero JJ, Pascau J, Gispert JD, García-Barreno P, Desco M(2009)Detection of visual activation in the rat brain using 2-deoxy-2-[(18)F]fluoro-D: -glucose and statistical parametric mapping (SPM). Mol Imaging Biol 11:94–99. [DOI] [PubMed] [Google Scholar]
- Spangler-Bickell MG, de Laat B, Fulton R, Bormans G, Nuyts J(2016)The effect of isoflurane on18f-FDG uptake in the rat brain: a fully conscious dynamic PET study using motion compensation. EJNMMI Res 6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SA, Green RD, Wilkinson ID, Hunter MD(2005)Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry 187:55–61. [DOI] [PubMed] [Google Scholar]
- Stone EA, Cotecchia S, Lin Y, Quartermain D (2002a) Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse 46:269–270. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Suckow RF, Quartermain D (2002b) Stress-induced subsensitivity to modafinil and its prevention by corticosteroids. Pharmacol Biochem Behav 73:971–978. [DOI] [PubMed] [Google Scholar]
- Szabadi E.(2006)Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol 61:761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ, Kwong K(2006)Modafinil activates cortical and subcortical sites in the sleep-deprived state. Sleep 29:1471–1481. [DOI] [PubMed] [Google Scholar]
- Thorpy MJ, Dauvilliers Y(2015)Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med 16:9–18. [DOI] [PubMed] [Google Scholar]
- Tokugawa J, Ravasi L, Nakayama T, Schmidt KC, Sokoloff L(2007)Operational lumped constant for FDG in normal adult male rats. J Nucl Med 48:94–99. [PubMed] [Google Scholar]
- van Vliet SA, Blezer EL, Jongsma MJ, Vanwersch RA, Olivier B, Philippens IH(2008)Exploring the neuroprotective effects of modafinil in a marmoset parkinson model with immunohistochemistry, magnetic resonance imaging and spectroscopy. Brain Res 1189:219–228. [DOI] [PubMed] [Google Scholar]
- Weber B, Burger C, Biro P, Buck A(2002)A femoral arteriovenous shunt facilitates arterial whole blood sampling in animals. Eur J Nucl Med Mol Imaging 29:319–323. [DOI] [PubMed] [Google Scholar]
- Willie JT, Renthal W, Chemelli RM, Miller MS, Scammell TE, Yanagisawa M, Sinton CM(2005)Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience 130:983–995. [DOI] [PubMed] [Google Scholar]
- Wimberley C, Angelis G, Boisson F, Callaghan P, Fischer K, Pichler BJ, Meikle SR, Grégoire MC, Reilhac A(2014)Simulation-based optimisation of the PET data processing for partial saturation approach protocols. Neuroimage 97:29–40. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM(2001)Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS(2005)Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience 132:1027–1034. [DOI] [PubMed] [Google Scholar]
- Wong YN, Simcoe D, Hartman LN, Laughton WB, King SP, McCormick GC, Grebow PE(1999)A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. J Clin Pharmacol 39:30–40. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH(2009)Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]