Abstract
Obesity or maternal overnutrition during pregnancy and lactation might have long-term consequences in offspring health. Fetal programming is characterized by adaptive responses to specific environmental conditions during early life stages. Programming alters gene expression through epigenetic modifications leading to a transgenerational effect of behavioral phenotypes in the offspring. Maternal intake of hypercaloric diets during fetal development programs aberrant behaviors resembling addiction in offspring. Programming by hypercaloric surplus sets a gene expression pattern modulating axonal pruning, synaptic signaling, and synaptic plasticity in selective regions of the reward system. Likewise, fetal programming can promote an inflammatory phenotype in peripheral and central sites through different cell types such as microglia and T and B cells, which contribute to disrupted energy sensing and behavioral pathways. The molecular mechanism that regulates the central and peripheral immune cross-talk during fetal programming and its relevance on offspring's addictive behavior susceptibility is still unclear. Here, we review the most relevant scientific reports about the impact of hypercaloric nutritional fetal programming on central and peripheral inflammation and its effects on addictive behavior of the offspring.
1. Introduction
According to the World Health Organization, nearly 39% of adults aged 18 years and over were overweight in 2016, and 13% were obese. Maternal obesity adversely impacts both maternal and offspring health, increasing the susceptibility to show metabolic abnormalities later in life such as obesity, dyslipidemia, type 2 diabetes mellitus, and hypertension as well as behavioral disorders related to schizophrenia, autism, and compulsive eating disorders [1, 2]. Maternal obesity or maternal overnutrition programs metabolic and hormonal nodes that modulate neuronal development during embryogenesis. For instance, neuronal maturation, including axonal pruning, synaptic plasticity, and stable tract formation between structures, is selectively programmed during pregnancy and lactation by consumption of high-sugar, high-fat, or high-sugar-high-fat diet formulas. Under this scenario, nutritional programming defines after-weaning selective behavioral phenotypes in offspring that might be exacerbated during adulthood, such as incentive-motivation behaviors leading to compulsive eating disorders.
Maternal obesity or overnutrition during fetal programming activates molecular and cellular mechanisms that command a new physiological state that might compromise basic metabolic and neuronal homeostasis. Metabolic imbalance during obesity can lead to overactivation of the immune system, triggering a process of chronic inflammation evidenced in animal models and humans. In fact, nutritional programming by hypercaloric diets promotes an inflammatory phenotype that contributes to disrupted energy sensing pathways in metabolic-relevant systems including adipose tissue, liver, pancreas, muscle, and the brain. Inflammation in the brain is associated with a cross-talk between peripheral and central cell types that potentially activates microglia in the brain. However, it is still unclear which immune cells infiltrate into the fetal brain leading to microglia activation and neuroinflammation during maternal nutritional programming. Moreover, it is unknown if maternal overnutrition during fetal programming originates central inflammation by microglial activation in the absence of peripheral immune cells infiltration. Finally, the role of neuroinflammation during maternal nutritional programming and its effects on defective behavior related to compulsive eating disorders in the offspring have only started to be dissected. In this review, we will discuss the role of maternal programming on peripheral and central immune cross-talk and its relevance in the development of incentive-motivation behavior such as addiction in the offspring.
Obesity is a metabolic condition showing positive energy balance driven by several factors including human genetics, life style, environment, body activity, and diet [6]. At first, obesity was conceived as a metabolic disorder showing an increase in white adipose tissue mass-modulated exclusively by the peripheral nervous system. However, recent evidence shows that the central nervous system (CNS) plays a major role in the modulation of adipose tissue mass and function. Also, the CNS is a major regulator of food intake and metabolism and seeks for rewards such as food; in a pathological state, CNS activation might lead to addiction-like behavior [7].
2. Obesity Is a Potential Deregulator of Energy-Satiety Integration in the CNS, Leading to Overfeeding
In nature, all living organisms require energy to sustain life and perform activities. Energy is mainly provided by food assigned into three main formulas such as proteins, carbohydrates, and fats. However, energy surplus such as overnutrition disrupts metabolic and hormonal homeostasis and has harmful consequences in health. On this matter, metabolic and hormonal signals from the periphery arrive to the CNS to give a message about the energy balance of the body. The CNS integrates these signals through evolutionary-conserved neuronal tracts connecting peripheral organs to selective brain structures. For instance, the brainstem receives information from the gut, while the hypothalamus integrates circulating/humoral signals. The brainstem and hypothalamus control satiety and integrate these signals by saying when and how much to eat [7]. However, under a pathological scenario satiety might be overestimated, leading to activation of the reward system, which modulates the incentive motivation to work and search for food despite an “apparent” satiety signal. The reward system integrates dopaminergic neurons located in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc) and also innervate several regions of the prefrontal cortex (PFC), central amygdala, basolateral amygdala (BLA), and hippocampus and dopamine neurons in the substantia nigra (SN) that project to the dorsal striatum, which has traditionally been associated with appetitive learning, performance, and motivation [8]. It has been hypothesized that incentive motivation for palatable food contributes to overfeeding during obesity [9, 10]. For instance, an evasion of satiety has been identified in obesity leading to positive energy balance and an increase of body mass index [11, 12]. Several researchers propose that, like drug-addiction, obesity sets an altered motivational behavior for seeking foods rich in fat and sugars (hypercaloric) that have high reward value (pleasant), which is potentially dependent on dopamine neurotransmission [10].
The proposal that overeating during obesity might be considered as food addiction in the Diagnostic and Statistical Manual of Mental Disorders (DSM) was initially based on phenotypic similarities between patterns of overeating in obese individuals and drug abuse in addicted persons. It has been shown in both animal models and humans that repeated exposure to high-fat or high-sugar diets disrupts the integration of energy-satiety peripheral signals into the CNS, leading to overfeeding which indeed shares behavioral similarities to addiction [10, 13]. For instance, obese humans might experience the following: (1) enhanced motivation over hypercaloric food intake including recurrent and excessive consumption, (2) an increased time spent seeking palatable food in contrast to habitual activities, and (3) frequent or permanent relapse to hypercaloric foods after dieting. Of note, obesity or palatable food overconsumption might be subject-specific and it is potentially modulated by environment-subject interaction. Based on this proposal, researchers have applied a selective Yale Food Addiction Scale (YFAS) to provide a standardized measure for the assessment and diagnosis of food addiction based mainly on substance dependence criteria [14, 15]. These evidences suggest that overfeeding during obesity might be related to incentive motivation for palatable food in human and animals. However, are humans born addicted or do they become addicted to food?
3. Potential Role of Fetal Programming by Hypercaloric Food in the Development of Addictive Behaviors in the Offspring
Exposure to hypercaloric foods impacts the individual metabolic-hormonal settings of mothers and fathers and might also affect their offspring. Substantial scientific evidence has demonstrated the detrimental role of hypercaloric food intake during pregnancy and lactation leading to a failure in metabolic homeostasis, favoring the development of incentive-motivation behavior for food in the offspring. In this context, epidemiological data from human catastrophes such as the Dutch famine (1944), the siege of Leningrad (1942–1944), the great Chinese famine (1958–1961), and also the Överkalix study (1890-present) have shown that changes in diet intake regarding overfeeding or fasting are associated with disorders in the offspring such as diabetes mellitus type 2 and cardiovascular diseases [16–19]. In addition, there is evidence of behavioral alterations, including schizophrenia [20–22], affective disorders [23], and addiction [24, 25]. Based on this data, Barker (1998) proposed the “Barker hypothesis” suggesting that the transgenerational effect of diet exposure during pregnancy modulates metabolic and behavioral phenotypes in the offspring, a mechanism known as “fetal programming” [26]. In specific, this hypothesis claims that oversupply or absence of energy intake during embryonic-fetal development provides the fetus with physiological adaptations to a new milieu of metabolic-hormonal threshold to face a potential adverse postnatal environment similar to those whose parents were exposed to fetal programming. In fact, any stimulus or insult throughout embryonic-fetal development, including stress, infections, substance abuse, overnutrition, and behavioral alterations, might result in molecular adaptations that produce permanent structural, physiological, and metabolic changes in the fetus. Also, fetal programming might increase the risk of serious physiological problems in perinatal stages including miscarriage, fetal-congenital anomalies, thromboembolism, and gestational diabetes.
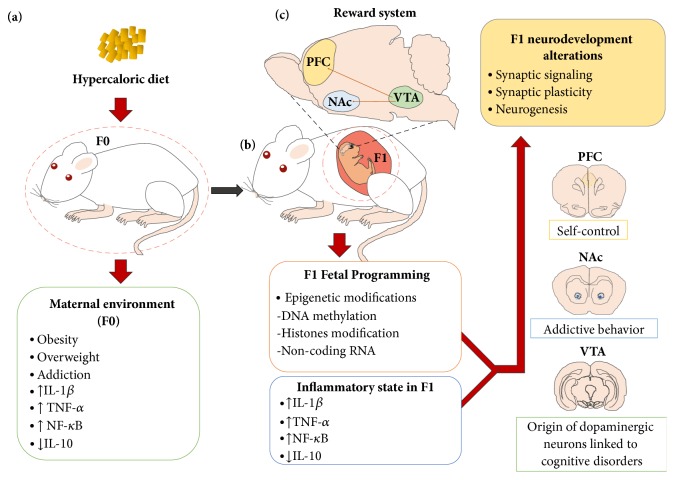
Initial reports demonstrated that energy-dense food disrupts the appetite-energy sensing and satiety systems, exacerbating the reward for food. Also, excessive consumption of palatable food can lead to a profound hyposensitivity to reward, leading to compulsive eating behavior similarly observed during drug seeking [27]. Nutritional programming in murine models induces alterations in behavior and synaptic plasticity, favoring higher consumption and sensitization to alcohol, methamphetamines, and cocaine [28, 29]. Maternal exposure to hypercaloric diets during pregnancy or lactation has shown to increase the long-term preference for junk food in the offspring [30], potentially associated with repeated, intermittent increases in extracellular dopamine (DA) in the NAc and the VTA [31–35]. Molecularly, these synaptic plasticity changes show greater expression of the ΔFosB gene [28] and both dopamine (e.g., DR2, DAT) and opioid pathway genes expression (e.g., the μ-opioid receptor) at early stages of development [35–39]. In fact, the opioid pathway regulates the rewarding effects of palatable food; an injection of μ-opioid agonists in the NAc increases preference for high-fat or sugar-rich foods [40, 41], and its antagonists decrease palatable food predilection, even at doses that show no effect on standard food intake [42, 43]. In addition, fetal programming by drugs such as nicotine exposure during pregnancy might also disrupt brain gene expression involved in neuronal glutamatergic (e.g., GluA1, GluA2, and CaMKIIα) and dopaminergic (e.g., DR2, DAT, and DR1) signaling plasticity in the hippocampus [44, 45], the laterodorsal tegmental nucleus [46], and the NAc [47–49]. In murine maternal overnutrition models, a transcription modulation of glutamatergic and dopaminergic systems in the NAc and prefrontal cortex that increases fat/sugar food preferences in the offspring has been identified [50–53] (Figure 1).
Figure 1.
Maternal programming by hypercaloric diets increases the development of addictive behavior in offspring. (a) Hypercaloric diet intake or obesity during pregnancy leads to activation of an inflammatory state of mothers (F0), favoring aversive intrauterine environment and selective increase in proinflammatory markers. (b) Positive inflammatory state in mothers correlates with the one in the offspring (F1) and also with activation of fetal epigenetic program including DNA methylation, histone modification (ip acetylation, methylation, sumoylation, and ubiquitination), and noncoding RNAs (ip miRNA, piRNA, and lncRNA), which are capable of altering gene expression profile in selective regions belonging to the reward system: prefrontal cortex (PFC), nucleus accumbens (NAc), and ventral tegmental area (VTA). (c) Epigenetic programming associates with neurodevelopment alterations in F1 including synaptic signaling, synaptic plasticity, and neurogenesis which contributes to addictive-like behavior.
In humans, it is still under investigation if obesity or maternal overnutrition during pregnancy leads to the development of food addiction in the offspring. Initial reports found that children from obese mothers or mothers with eating disorders presented more binge eating and night eating [54, 55] and consumed more carbohydrates compared to their normal weight counterparts [56]. Also, a positive correlation between addicted parents and food addiction in their offspring has been proven [57], although epidemiological studies are needed to support these findings.
4. Obesity and Maternal Overfeeding Promote Peripheral and Central Inflammatory Response
An organism's first line of defense is the innate immune system and it includes physical barriers such as the skin, specific cell types such as macrophages, and complement proteins. The second line of defense is the inflammatory response which consists of an innate cellular system and humoral response that happens during injury to restore physiological homeostasis. The function of the immune system is not limited to fighting infections and repairing tissue damage, it also plays a key role in shaping neuronal tracts during CNS development. For instance, cells from the innate immune system, such as microglia, regulate neurogenesis, synaptic plasticity, and synaptic striping, and are the major antigen-presenting cells (APC) in the CNS [58]. Cross-talk between peripheral immune system and brain-resident immune cells is in part regulated by B and T lymphocytes, macrophages, and antibodies which migrate and penetrate the blood-brain barrier (BBB) [58, 59] and have also been reported in the cerebrospinal fluid [60]. Under an altered physiological scenario, peripheral immune cells located in the brain become proinflammatory entities and secrete cytokines promoting an exacerbated immune response by microglia. By doing this, peripheral immune cells integrate positive feedback with microglia that modulates neural growth and development [61]. Microglial cells are the brain-resident macrophages of the CNS; these oversee surveillance of the CNS integrity and respond to pathogens and injuries and also to very subtle alterations in their microenvironment [62]. In healthy brains, microglia remain in a ramified stated, and when activated, they enlarge their cell body, change to a phagocytic state, and execute similar functions to those of other tissue-resident macrophages such as proinflammatory cytokines secretion, antigen presentation, and ROS production and phagocytosis, leading to neuroinflammation [63]. Under this scenario, neuroinflammation is beneficial because it removes debris or dysfunctional neurons; under a pathological state, however, microglia activation is upregulated and can be damaging to neurons because of the excessive release of ROS and proinflammatory cytokines, leading to neuroinflammation [64], and might contribute to tissue damage and disease pathology. Although microglial activation could be harmful, microglia are necessary to provide essential trophic factors for the survival of neurons, like brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) [63].
An atypical form of inflammation, primarily induced by fatty acids accumulation in selective energy-dependent tissues including liver, adipose tissue, muscle, and brain occurs when there is a positive energy balance, as found in obesity or maternal overnutrition, [65]. Fatty acids promote a type of body inflammation termed “metainflammation” or “metabolic inflammation,” which involves several immune cells (T, B lymphocytes and microglia) and proinflammatory mediators (cytokines and chemokines) leading to metabolic and neurodegenerative disorders [66, 67]. Obesity or overnutrition during pregnancy leads to maternal immune activation (MIA), which favors changes in plasma and placental tissue-specific lipidomic profile, recruiting lipid species to membrane Toll-Like Receptor 4 (TLR4) and activating the nuclear factor-kappa B (NF-κB) pathway, through an increase of TLR2, TLR4, IL-6, IL-18, and TNF-α mRNA levels and macrophage markers cluster of differentiation including (CD)11b, CD14, and CD68 [68–70]. Maternal overnutrition in a sheep model of obesity demonstrated that inflammatory markers such as CD-68, TGF-β1, and TNF-α found in the mother are also identified in the offspring after birth [71]. These evidences show that imbalances in the peripheral and immune system cross-talk might compromise early stages of CNS development and differentiation potentially contributes to epilepsy, schizophrenia, cerebral palsy, Parkinson disease (PD), Alzheimer disease (AD), and ASD [72].
5. Central-Peripheral Immune System Cross-Talk Regulates Synaptic Plasticity, Neurogenesis, and Neurodegeneration
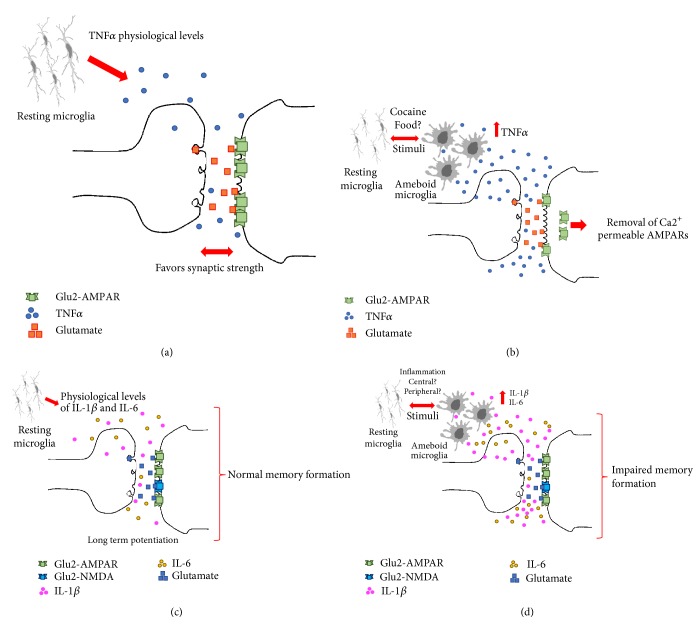
As mentioned before, neuroinflammation is how the innate immune system protects the CNS and controls initial stimulus, and although it is intended to be protective and beneficial, out-of-control inflammation can be fatal and contribute to tissue damage and disease pathology, including neurodegeneration [73]. Substantial scientific evidence has shown that neuroinflammation commands changes in cerebral plasticity and influences synaptic function and memory [72]. However, there are no conclusive data showing a cause-effect relationship in terms of the role of cytokines on synaptic plasticity modulation in the NAc, as a trigger to addictive behavior. Some data from neuroimmune interactions in humans in the behavioral response to drugs have shown small clues about their clinical relevance; however, these studies do not identify the molecular mechanisms of such interactions [74, 75]. Cause-effect relationship is even harder to identify because the CNS shows a selective cytokine profile when compared to peripheral cytokines during drug exposure. For instance, Calipari and colleagues did not find differences between the TNF-α expression in serum when mice were exposed to cocaine [76]. However, Lewitus et al. identified TNF-α release from microglia after cocaine consumption as a potential regulation of neuronal and behavioral plasticity during both the induction and expression of drug-induced behaviors [77]. Molecularly, initial reports have shown that glia releases TNF-α to fit surface expression of AMPARs, favoring the synaptic strength at excitatory synapses [78, 79]. In fact, TNF-α stimulation in brain slices reduced corticostriatal synaptic strength by removing of Ca2+ permeable AMPARs from the plasma membrane [80], suggesting that high levels of TNF-α affect synaptic plasticity, but physiological levels favor brain development and synaptic strength [79] (Figure 2). These evidences reinforce the previous hypothesis that TNF-α profile in the CNS modulates synaptic plasticity during addiction; however, it is still unknown whether central TNF-α accumulation might be a node of inflammatory activation during addiction or there is a cause-effect relationship involving a cross-talk between peripheral and central mechanism.
Figure 2.
Synaptic modulation by cytokine levels. (a) According to Bettie and cols. in 2002 and Stellwagen and cols. in 2006 results TNF-α was demonstrated to promote normal expression of AMPARs favoring synaptic strength at excitatory synapses. (b) In contrast, high levels of TNF-α can affect synaptic plasticity by removing of Ca2+-permeable AMPARs from plasma membrane according to Lewitus et al., 2014 (b). (c) Physiological levels of IL-1β and IL-6 are detrimental to maintain and modulate hippocampus activity [3, 4]. (d) Excess of IL-1β and IL-6 affect LTP and impair memory formation [3, 5].
Synaptic plasticity during addiction might also be modulated by chemokines. For instance, the CCL4 and CCL17 chemokines and their ligand CCL25 are upregulated in microglia following morphine treatment and might also induce local chemotaxis of axon terminals or dendritic spines. Blocking the synthesis of certain chemokines prevents the relapse of drug-seeking behavior following drug reexposure months later [81]. In addition, the modulation of the G-CSF chemokine concentration in the NAc and the prefrontal cortex is crucial to alter the motivation of cocaine consumption but not of food [76]. Together, chemokines have a very profound neuromodulatory effect on the regulation of neural circuits involved in addiction, but the molecular mechanisms on this scenario still need to be described in detail.
Central and peripheral immune cross-talk response might also modulate hippocampal neurogenesis. It has been reported that when CD4+ T lymphocytes are systemically depleted in mice, there are reduced hippocampal neurogenesis, learning impairment, and a decrease in BDNF secretion in the brain, whereas inoculation of CD4+ T lymphocytes promotes hippocampal neurogenesis and restores the deficits observed before [82, 83]. In models of autoimmune encephalitis in which inflammatory responses are upregulated, synaptic plasticity in the hippocampus and long-term potentiation (LTP) are affected because of the high levels of IL-1β. While peripheral inflammatory responses related to TNF-α and IL-6 have been identified to modulate hippocampus activity [84, 85], it has not been fully described whether central IL-1β comes from infiltrating lymphocytes or activated microglia within the CNS [86]. In any case, central physiological levels of IL-1β are detrimental, because excessive cytokines level or blockage of IL- 1 signaling through IL-1ra has been reported to cause memory impairment [87], potentially due to Ca2+ influx throughout NMDA receptors, Src kinases activation, and NR2A/B subunits phosphorylation [88].
As mentioned before for TNF-α and IL-1β, initial reports characterized that the IL-6 cytokine is required in normal physiological levels for the formation of LTP, but levels above normal affect memory formation and learning [89, 90]. Supporting those findings, IL-6 KO mice display memory impairment [84] and show reduced proliferating neuronal stem cells (NSCs) in the hippocampus [91]. Under physiological conditions, IL-6 is related to neural plasticity and NSCs proliferation through the JAK/STAT, MAPK/cAMP, Ras-MAPK, and PI3K pathways [92]. Lastly, the immune system is involved in regulation of hippocampal neurogenesis, through the kynurenine pathway [93], affecting directly the hippocampal neurogenesis and memory and learning processes [82] (Figure 2).
A positive inflammatory profile including the IL-1, IL-6, IL-1β, TNF-α, and IFN-γ has been evidenced in a wide range of neurodegenerative diseases. In particular, TNF-α production by active microglia is highly related to IFN-γ secreted by T cells into the CNS parenchyma [3]. Secreted IFN-γ and other inflammation mediators activate the MEK/ERK signaling pathway leading to TNF-α production and neuronal death [4]. Also, TNF-α and IFN-γ lead to microglial nitric oxide (NO) production, favoring oxidative stress, inhibiting neuronal respiration, and favoring neuronal apoptosis [94]. Of note, these responses seem to be independent, although there might be a node at different time-frame. For instance, the TNF-α targets its own receptor to activate the NF-κB-dependent proinflammatory pathway and the expression of inducible NO synthase (iNOS) that mediates NO production, which is regulated by NF-κB and STAT transcription factors [95].
In addition, IL-6 also might potentially favor neuronal death. By promoting the formation of the IL-6/IL-6R/gp130 complex, the proinflammatory IL-6 activates the JAK1, JAK2, and TYK2 pathway and promotes the phosphorylation of STAT1 and STAT3. In specific, IL-6 favors neuronal death by the STAT1 nuclear transcription factor, which associates with transcription of IFNs, including type I (IFN-α, IFN-β, and IFN-ω) and type II (IFN-γ), all of them involved in detrimental innate immune responses. However, IL-6 might also be anti-inflammatory and promote cell survival through STAT3-PI3K/Akt signaling activation by regulating cell cycle proteins (CDK2), antiapoptotic proteins (Bcl-2), and proapoptotic proteins (Bax, Bad, and caspase-9) [5, 96]. Thus, IL-6 secretion by activated microglia regulates inflammatory mediators and shows a dual role that prevents or promotes neuronal death.
In summary, because of their dual roles associated with threshold levels, cytokines in the brain may help to maintain cerebral homeostasis having a beneficial effect in normal physiological conditions; uncontrolled or chronic neuroinflammation, however, leads to neurodegeneration, disruption of cerebral plasticity, cognitive changes, and the appearance of aberrant behavior, also defined as “sickness behavior” by Cibelli and colleagues [74].
6. Epigenetic Mechanisms during Fetal Programming and Transgenerational Inheritance of Addictive Phenotype
By now, we have shown evidences that metabolic inflammation during pregnancy leads to chronic inflammation in offspring that might disrupt synaptic plasticity and set a self-perpetuating cycle of addictive excessive consumption of natural and synthetic rewards [28, 97]. Despite the lack of epidemiological studies addressing the impact of human obesity or maternal overnutrition as a node to promote addiction-like phenotypes in offspring, recent evidence suggests that addiction in humans might have a common root from transgenerational inheritance, given that biomarkers that share identity in drug programming and addictive behavior have been previously described [98–101]. A transgenerational inheritance of addiction, eating disorders, or pathological phenotypes in offspring is modulated directly by epigenetics, which programs and adapts organisms in the early stages of development to face sudden changes in the environment, compared with evolutionary and natural selection processes, which tend to be slow [102]. Epigenetics refer to the mechanisms of long-term or stable regulation of gene expression that do not involve a change in gene sequences including DNA methylation and histone modification such as acetylation, sumoylation, ubiquitination, and methylation, as well as gene expression regulated by noncoding RNAs, miRNAs, piRNAs, and lncRNA [103]. The role of maternal programing by overnutrition during pregnancy and its effects on epigenetics linked to a proinflammatory profile in offspring have not been deeply analyzed yet. Initial work has shown that epigenetics modulate the immune system and potentially affects the CNS during pregnancy [103]. A recent paper identified that fat diet primes an epigenomic reprogramming of myeloid progenitor cells that led to an increased proliferation and enhanced innate immune responses [104], which the authors named as ‘‘innate immune memory” or ‘‘trained immunity,” able to mediate metabolic reprogramming for prolonged periods of time [105]. On this context, using a fetal-programing murine model with hypercaloric diet, Edlow et al. (2016) identified a significant number of deregulated miRNAs implicated in immune and proinflammatory processes, death cellular, adipogenesis and cellular stress in the males of offspring [106] (Figure 1). In humans, a positive correlation between body mass index (BMI) during prepregnancy with adiposity and inflammatory markers in offspring at birth has been demonstrated [107]. Also, Alexander and col. (2018) reported alterations in the methylation profile of genes involved in mitochondrial function, DNA repair, oxidative stress and inflammation in the placenta of children who were born from mothers with type 2 diabetes mellitus [108]. Of importance, a study in humans revealed that an adherence to Mediterranean diet for 5 years ended up in a significant increase in DNA methylation which positively correlated with a reduction in the concentrations of TNF-α, sICAM-1, and CRP [109].
These evidences suggest that gestational exposure to high-fat and high-sugar diets in murine models results in a disruption of the central reward system, which correlates with epigenetic biomarkers found in addicted-programed subjects. Programming sets the brain to potentiate changes in neuroplasticity [97, 110], neuronal death, neurotoxicity [111], etc., in postnatal life, which leads to the development of cognitive disorders such as addiction [112–115]. It is still under investigation if humans share the aberrant reward plasticity that has been described in murine models.
7. Conclusions
Maternal overnutrition leads to fetal adaptive responses which sets a neuronal gene expression program in offspring. Selective intake of diets rich in fats and sugars at critical stages recruit central and peripheral inflammatory cell type markers, such as microglia and T and B cells, which disrupt energy sensing and behavioral pathways increasing the susceptibility to show aberrant behaviors similar to addiction. In fact, an addictive phenotype might be transgenerational when inherited by selective epigenetic activation during fetal programming. These evidences propose the immune system activation by nutritional programming during gestation as a node to modulate changes in neuroplasticity related to incentive behavior phenotypes which must be addressed deeply for a better understanding.
Acknowledgments
The authors thank M.S. Alejandra Arreola-Triana for her support on editing this manuscript. This work was funded by National Council of Science and Technology in Mexico (CONACYT) (255317), 582196 CONACYT for Larisa Montalvo-Martínez and 573686 CONACYT for Roger Maldonado-Ruiz.
Disclosure
Alberto Camacho's present/permanent address is as follows: Department of Biochemistry, Faculty of Medicine, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, NL, México, and Center for Research and Development in Health Sciences, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, NL, México.
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Chavatte-Palmer P., Tarrade A., Rousseau-Ralliard D. Diet before and during pregnancy and offspring health: The importance of animal models and what can be learned from them. International Journal of Environmental Research and Public Health. 2016;13(6, article no. 586) doi: 10.3390/ijerph13060586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edlow A. G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenatal Diagnosis. 2017;37(1):95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin E., Ganz T., Lehrer R. I. Defensins and other endogenous peptide antibiotics of vertebrates. Journal of Leukocyte Biology. 1995;58(2):128–136. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff R. M., Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nature Reviews Immunology. 2012;12(9):623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 5.Lin W., Lin Y. Interferon-γ inhibits central nervous system myelination through both STAT1-dependent and STAT1-independent pathways. Journal of Neuroscience Research. 2010;88(12):2569–2577. doi: 10.1002/jnr.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Klaauw A., Farooqi I. The hunger genes: pathways to obesity. Cell. 2015;161(1):119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kim K., Seeley R. J., Sandoval D. A. Signalling from the periphery to the brain that regulates energy homeostasis. Nature Reviews Neuroscience. 2018;19(4):185–196. doi: 10.1038/nrn.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo S. J., Nestler E. J. The brain reward circuitry in mood disorders. 2013. [DOI] [PMC free article] [PubMed]
- 9.Yu Y.-H., Vasselli J. R., Zhang Y., Mechanick J. I., Korner J., Peterli R. Metabolic vs. hedonic obesity: A conceptual distinction and its clinical implications. Obesity Reviews. 2015;16(3):234–247. doi: 10.1111/obr.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volkow N. D., Wise R. A., Baler R. The dopamine motive system: Implications for drug and food addiction. Nature Reviews Neuroscience. 2017;18(12):741–752. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig D. S., Peterson K. E., Gortmaker S. L. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. The Lancet. 2001;357(9255):505–508. doi: 10.1016/s0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Maintaining a Healthy Weight On the Go: A Pocket Guide. NIH Public Access. 2010:1–24. [Google Scholar]
- 13.Kenny P. J. Common cellular and molecular mechanisms in obesity and drug addiction. Nature Reviews Neuroscience. 2011;12(11):638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 14.Gearhardt A. N., Corbin W. R., Brownell K. D. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52(2):430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Schulte E. M., Gearhardt A. N. Development of the Modified Yale Food Addiction Scale Version 2.0. European Eating Disorders Review. 2017;25(4):302–308. doi: 10.1002/erv.2515. [DOI] [PubMed] [Google Scholar]
- 16.Aiken C. E., Ozanne S. E. Transgenerational developmental programming. Human Reproduction Update. 2014;20(1):63–75. doi: 10.1093/humupd/dmt043.dmt043 [DOI] [PubMed] [Google Scholar]
- 17.Lalande M. Parental imprinting and human disease. Annual Review of Genetics. 1996;30:173–195. doi: 10.1146/annurev.genet.30.1.173. [DOI] [PubMed] [Google Scholar]
- 18.Vaiserman A. Early-life origin of adult disease: Evidence from natural experiments. Experimental Gerontology. 2011;46(2-3):189–192. doi: 10.1016/j.exger.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., He Y., Qi L., et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Susser E., Neugebauer R., Hoek H. W., et al. Schizophrenia after prenatal famine further evidence. Archives of General Psychiatry. 1996;53(1):25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 21.St Clair D., Xu M., Wang P., et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. Journal of the American Medical Association. 2005;294(5):557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 22.Xu M.-Q., Sun W.-S., Liu B.-X., et al. Prenatal malnutrition and adult Schizophrenia: Further evidence from the 1959-1961 chinese famine. Schizophrenia Bulletin. 2009;35(3):568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown A. S., Van Os J., Driessens C., Hoek H. W., Susser E. S. Further evidence of relation between prenatal famine and major affective disorder. The American Journal of Psychiatry. 2000;157(2):190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- 24.Franzek E. J., Sprangers N., Janssens A. C. J. W., Van Duijn C. M., Van De Wetering B. J. M. Prenatal exposure to the 1944-45 Dutch 'hunger winter' and addiction later in life. Addiction. 2008;103(3):433–438. doi: 10.1111/j.1360-0443.2007.02084.x. [DOI] [PubMed] [Google Scholar]
- 25.Painter R. C., Roseboom T. J., Bleker O. P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reproductive Toxicology. 2005;20(3):345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Barker D. J. P. In utero programming of chronic disease. Clinical Science. 1998;95(2):115–128. doi: 10.1042/CS19980019. [DOI] [PubMed] [Google Scholar]
- 27.Lenoir M., Serre F., Cantin L., Ahmed S. H. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2(8, article no. e698) doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peleg-Raibstein D., Sarker G., Litwan K., et al. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Translational Psychiatry. 2016;6(10, article no. e911) doi: 10.1038/tp.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocarsly M. E., Barson J. R., Hauca J. M., Hoebel B. G., Leibowitz S. F., Avena N. M. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiology & Behavior. 2012;107(4):568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayol S. A., Farrington S. J., Stickland N. C. A maternal “junk food” diet in pregnancy and lactation promotes an exacerbated taste for “junk food” and a greater propensity for obesity in rat offspring. British Journal of Nutrition. 2018;98(4):843–851. doi: 10.1017/S0007114507812037. [DOI] [PubMed] [Google Scholar]
- 31.Bassareo V., Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. The Journal of Neuroscience. 1997;17(2):851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajnal A., Smith G. P., Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2004;286(1):R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 33.Liang N.-C., Hajnal A., Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291(5):R1236–R1239. doi: 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- 34.Rada P., Avena N. M., Hoebel B. G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Naef L., Moquin L., Dal Bo G., Giros B., Gratton A., Walker C.-D. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Ong Z. Y., Muhlhausler B. S. Maternal "junk-food" feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. The FASEB Journal. 2011;25(7):2167–2179. doi: 10.1096/fj.10-178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong Z. Y., Wanasuria A. F., Lin M. Z. P., Hiscock J., Muhlhausler B. S. Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite. 2013;65:189–199. doi: 10.1016/j.appet.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Gugusheff J. R., Bae S. E., Rao A., et al. Sex and age-dependent effects of a maternal junk food diet on the mu-opioid receptor in rat offspring. Behavioural Brain Research. 2016;301:124–131. doi: 10.1016/j.bbr.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Fritz B. M., Muñoz B., Yin F., Bauchle C., Atwood B. K. A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience. 2018;372:1–15. doi: 10.1016/j.neuroscience.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M., Gosnell B. A., Kelley A. E. Intake of high-fat food is selectively enhanced by Mu opioid receptor stimulation within the nucleus accumbens. The Journal of Pharmacology and Experimental Therapeutics. 1998;285(2):908–914. [PubMed] [Google Scholar]
- 41.Zhang M., Kelley A. E. Enhanced intake of high-fat food following striatal mu-opioid stimulation: Microinjection mapping and Fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/S0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- 42.Kelley A. E., Bless E. P., Swanson C. J. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. The Journal of Pharmacology and Experimental Therapeutics. 1996;278(3):1499–1507. [PubMed] [Google Scholar]
- 43.Glass M. J., Billington C. J., Levine A. S. Opioids and food intake: Distributed functional neural pathways? Neuropeptides. 1999;33(5):360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Dávila-García M. I., Yarl W., Gondré-Lewis M. C. Gestational nicotine exposure regulates expression of AMPA and NMDA receptors and their signaling apparatus in developing and adult rat hippocampus. Neuroscience. 2011;188:168–181. doi: 10.1016/j.neuroscience.2011.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parameshwaran K., Buabeid M. A., Karuppagounder S. S., et al. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cellular and Molecular Life Sciences. 2012;69(5):829–841. doi: 10.1007/s00018-011-0805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNair L. F., Kohlmeier K. A. Prenatal nicotine is associated with reduced AMPA and NMDA receptor-mediated rises in calcium within the laterodorsal tegmentum: A pontine nucleus involved in addiction processes. Journal of Developmental Origins of Health and Disease. 2014;6(3):225–241. doi: 10.1017/S2040174414000439. [DOI] [PubMed] [Google Scholar]
- 47.Carr K. D., Chau L. S., Cabeza de Vaca S., et al. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165(4):1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cornish J. L., Kalivas P. W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of Neuroscience. 2000;20(15):p. RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzschentke T. M., Schmidt W. J. Glutamatergic mechanisms in addiction. Molecular Psychiatry. 2003;8(4):373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 50.Pinheiro C. R., Moura E. G., Manhães A. C., et al. Concurrent maternal and pup postnatal tobacco smoke exposure in Wistar rats changes food preference and dopaminergic reward system parameters in the adult male offspring. Neuroscience. 2015;301:178–192. doi: 10.1016/j.neuroscience.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Pinheiro C. R., Moura E. G., Manhães A. C., et al. Maternal nicotine exposure during lactation alters food preference, anxiety-like behavior and the brain dopaminergic reward system in the adult rat offspring. Physiology & Behavior. 2015;149:131–141. doi: 10.1016/j.physbeh.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 52.Oginsky M. F., Goforth P. B., Nobile C. W., Lopez-Santiago L. F., Ferrario C. R. Eating 'Junk-Food' Produces Rapid and Long-Lasting Increases in NAc CP-AMPA Receptors: Implications for Enhanced Cue-Induced Motivation and Food Addiction. Neuropsychopharmacology. 2016;41(13):2977–2986. doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camacho A., Montalvo-Martinez L., Cardenas-Perez R. E., Fuentes-Mera L., Garza-Ocañas L. Obesogenic diet intake during pregnancy programs aberrant synaptic plasticity and addiction-like behavior to a palatable food in offspring. Behavioural Brain Research. 2017;330:46–55. doi: 10.1016/j.bbr.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Lamerz A., Kuepper-Nybelen J., Bruning N., et al. Prevalence of obesity, binge eating, and night eating in a cross-sectional field survey of 6-year-old children and their parents in a German urban population. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2005;46(4):385–393. doi: 10.1111/j.1469-7610.2004.00363.x. [DOI] [PubMed] [Google Scholar]
- 55.Lydecker J. A., Grilo C. M. Fathers and mothers with eating-disorder psychopathology: Associations with child eating-disorder behaviors. Journal of Psychosomatic Research. 2016;86:63–69. doi: 10.1016/j.jpsychores.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rising R., Lifshitz F. Relationship between maternal obesity and infant feeding-interactions. Nutrition Journal . 2005;4, article no. 17 doi: 10.1186/1475-2891-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burrows T., Skinner J., Joyner M. A., Palmieri J., Vaughan K., Gearhardt A. N. Food addiction in children: Associations with obesity, parental food addiction and feeding practices. Eating Behaviors. 2017;26:114–120. doi: 10.1016/j.eatbeh.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Maldonado-Ruiz R., Montalvo-Martínez L., Fuentes-Mera L., Camacho A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutrition & Diabetes. 2017;7(3):p. e254. doi: 10.1038/nutd.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buckman L. B., Hasty A. H., Flaherty D. K., et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain, Behavior, and Immunity. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strazielle N., Creidy R., Malcus C., Boucraut J., Ghersi-Egea J. F. T-Lymphocytes traffic into the brain across the blood-csf barrier: Evidence using a reconstituted choroid plexus epithelium. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150945.e0150945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buehler M. R. A proposed mechanism for autism: An aberrant neuroimmune response manifested as a psychiatric disorder. Medical Hypotheses. 2011;76(6):863–870. doi: 10.1016/j.mehy.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 62.Soulet D., Rivest S. Microglia. Current Biology. 2008;18(12):R506–R508. doi: 10.1016/j.cub.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 63.Salter M. W., Stevens B. Microglia emerge as central players in brain disease. Nature Medicine. 2017;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 64.von Bernhardi R., Eugenín-von Bernhardi L., Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Frontiers in Aging Neuroscience. 2015;7, article 124 doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carobbio S., Pellegrinelli V., Vidal-Puig A. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Advances in Experimental Medicine and Biology. 2017;960:161–196. doi: 10.1007/978-3-319-48382-5_7. [DOI] [PubMed] [Google Scholar]
- 66.Han C., Rice M., Cai D. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. American Journal of Physiology-Endocrinology and Metabolism. 2016;311(1):E32–E41. doi: 10.1152/ajpendo.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maldonado-Ruiz R., Fuentes-Mera L., Camacho A. Central Modulation of Neuroinflammation by Neuropeptides and Energy-Sensing Hormones during Obesity. BioMed Research International. 2017;2017 doi: 10.1155/2017/7949582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calder P. C. N-3 Fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proceedings of the Nutrition Society. 2013;72(3):326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 69.O'Neill L. A. J., Grahame Hardie D. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 70.Getz K. D., Anderka M. T., Werler M. M., Jick S. S. Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case–Control Study. Paediatric and Perinatal Epidemiology. 2016;30(5):479–487. doi: 10.1111/ppe.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghnenis A. B., Odhiambo J. F., McCormick R. J., Nathanielsz P. W., Ford S. P. Maternal obesity in the ewe increases cardiac ventricular expression of glucocorticoid receptors, proinflammatory cytokines and fibrosis in adult male offspring. PLoS ONE. 2017;12(12) doi: 10.1371/journal.pone.0189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estes M. L., McAllister A. K. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353(6301):772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ransohoff R. M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 74.Cibelli M., Fidalgo A. R., Terrando N., et al. Role of interleukin-1β in postoperative cognitive dysfunction. Annals of Neurology. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lacagnina M. J., Rivera P. D., Bilbo S. D. Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology. 2017;42(1):156–177. doi: 10.1038/npp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calipari E. S., Godino A., Peck E. G., et al. Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nature Communications. 2018;9(1) doi: 10.1038/s41467-017-01881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewitus G. M., Konefal S. C., Greenhalgh A. D., Pribiag H., Augereau K., Stellwagen D. Microglial TNF-α Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron. 2016;90(3):483–491. doi: 10.1016/j.neuron.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beattie E. C., Stellwagen D., Morishita W., et al. Control of synaptic strength by glial TNFα. Science. 2002;295(5563):2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 79.Stellwagen D., Malenka R. C. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 80.Lewitus G. M., Pribiag H., Duseja R., St-Hilaire M., Stellwagen D. An adaptive role of TNFα in the regulation of striatal synapses. The Journal of Neuroscience. 2014;34(18):6146–6155. doi: 10.1523/JNEUROSCI.3481-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwarz J. M., Smith S. H., Bilbo S. D. FACS analysis of neuronal-glial interactions in the nucleus accumbens following morphine administration. Psychopharmacology. 2013;230(4):525–535. doi: 10.1007/s00213-013-3180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf S. A., Steiner B., Akpinarli A., et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. The Journal of Immunology. 2009;182(7):3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- 83.Leiter O., Kempermann G., Walker T. L. A Common Language: How neuroimmunological cross talk regulates adult hippocampal neurogenesis. Stem Cells International. 2016;2016 doi: 10.1155/2016/1681590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baier P. C., May U., Scheller J., Rose-John S., Schiffelholz T. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behavioural Brain Research. 2009;200(1):192–196. doi: 10.1016/j.bbr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 85.Tan H., Cao J., Zhang J., Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. Journal of Neuroinflammation. 2014;11, article no. 93 doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nisticò R., Mori F., Feligioni M., Nicoletti F., Centonze D. Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1633) doi: 10.1098/rstb.2013.0162.20130162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goshen I., Kreisel T., Ounallah-Saad H., et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8-10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Viviani B., Bartesaghi S., Gardoni F., et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. The Journal of Neuroscience. 2003;23(25):8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei H., Chadman K. K., McCloskey D. P., et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822(6):831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Balschun D., Wetzel W., Del Rey A., et al. Interleukin-6: a cytokine to forget. The FASEB Journal. 2004;18(14):1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- 91.Bowen K. K., Dempsey R. J., Vemuganti R. Adult interleukin-6 knockout mice show compromisedneurogenesis. NeuroReport. 2011;22(3):126–130. doi: 10.1097/WNR.0b013e3283430a44. [DOI] [PubMed] [Google Scholar]
- 92.Kang M. K., Kang S. K. Interleukin-6 induces proliferation in adult spinal cord-derived neural progenitors via the JAK2/STAT3 pathway with EGF-induced MAPK phosphorylation. Cell Proliferation. 2008;41(3):377–392. doi: 10.1111/j.1365-2184.2008.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zunszain P. A., Anacker C., Cattaneo A., et al. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bassani T. B., Vital M. A. B. F., Rauh L. K. Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arquivos de Neuro-Psiquiatria. 2015;73(7):616–623. doi: 10.1590/0004-282X20150057. [DOI] [PubMed] [Google Scholar]
- 95.Mass E., Ballesteros I., Farlik M., et al. Specification of tissue-resident macrophages during organogenesis. The New York Academy of Sciences. 2016;353(6304) doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arulampalam V., Kolosenko I., Hjortsberg L., Björklund A.-C., Grandér D., Tamm K. P. Activation of STAT1 is required for interferon-alpha-mediated cell death. Experimental Cell Research. 2011;317(1):9–19. doi: 10.1016/j.yexcr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Gutiérrez-Martos M., Girard B., Mendonça-Netto S., et al. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addiction Biology. 2017 doi: 10.1111/adb.12541. [DOI] [PubMed] [Google Scholar]
- 98.Donkin I., Versteyhe S., Ingerslev L. R., et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metabolism. 2016;23(2):369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Wu K. L. H., Wu C.-W., Tain Y.-L., et al. Environmental stimulation rescues maternal high fructose intake-impaired learning and memory in female offspring: Its correlation with redistribution of histone deacetylase 4. Neurobiology of Learning and Memory. 2016;130:105–117. doi: 10.1016/j.nlm.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Holland M. L., Lowe R., Caton P. W., et al. Early-life nutrition modulates the epigenetic state of specific rDNA genetic variants in mice. Science. 2016;353(6298):495–498. doi: 10.1126/science.aaf7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rijlaarsdam J., Cecil C. A. M., Walton E., et al. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2017;58(1):19–27. doi: 10.1111/jcpp.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bohacek J., Mansuy I. M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nature Reviews Genetics. 2015;16(11):641–652. doi: 10.1038/nrg3964. [DOI] [PubMed] [Google Scholar]
- 103.Álvarez-Errico D., Vento-Tormo R., Sieweke M., Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nature Reviews Immunology. 2015;15(1):7–17. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 104.Christ A., Günther P., Lauterbach M. A., et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172(1-2):162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Netea M. G., Quintin J., van der Meer J. W. M. Trained immunity: a memory for innate host defense. Cell Host & Microbe. 2011;9(5):355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Edlow A. G., Guedj F., Pennings J. L. A., Sverdlov D., Neri C., Bianchi D. W. Males are from Mars, and females are from Venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. American Journal of Obstetrics & Gynecology. 2016;214(5):623–623e10. doi: 10.1016/j.ajog.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCloskey K., Ponsonby A.-L., Collier F., et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatric Obesity. 2018;13(1):46–53. doi: 10.1111/ijpo.12187. [DOI] [PubMed] [Google Scholar]
- 108.Alexander J., Teague A. M., Chen J., et al. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PLoS ONE. 2018;13(2):p. e0190698. doi: 10.1371/journal.pone.0190698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arpón A., Riezu-Boj J. I., Milagro F. I., et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. Journal of Physiology and Biochemistry. 2016;73(3):445–455. doi: 10.1007/s13105-017-0552-6. [DOI] [PubMed] [Google Scholar]
- 110.Hao S., Dey A., Yu X., Stranahan A. M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain, Behavior, and Immunity. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kessing C. F., Tyor W. R. Interferon-α induces neurotoxicity through activation of the type i receptor and the GluN2A subunit of the NMDA receptor. Journal of Interferon & Cytokine Research. 2015;35(4):317–324. doi: 10.1089/jir.2014.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crews F., Zou J., Qin L. NIH public access. Brain, Behavior, and Immunity. 2013;25:1–20. [Google Scholar]
- 113.Crews F. T., Walter T. J., Coleman L. G., Vetreno R. P. Toll-like receptor signaling and stages of addiction. Psychopharmacology. 2017;234(9-10):1483–1498. doi: 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harricharan R., Abboussi O., Daniels W. M. U. Addiction: A dysregulation of satiety and inflammatory processes. Progress in Brain Research. 2017;235:65–91. doi: 10.1016/bs.pbr.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 115.Wakida N., Kiguchi N., Saika F., Nishiue H., Kobayashi Y., Kishioka S. CC-chemokine ligand 2 facilitates conditioned place preference to methamphetamine through the activation of dopamine systems. Journal of Pharmacological Sciences. 2014;125(1):68–73. doi: 10.1254/jphs.14032FP. [DOI] [PubMed] [Google Scholar]