Metabolic scaling means that reductions in habitat size that decrease predator body size will also reduce predator biomass that can be supported.
Abstract
Habitat reduction could drive biodiversity loss if the capacity of food webs to support predators is undermined by habitat-size constraints on predator body size. Assuming that (i) available space restricts predator body size, (ii) mass-specific energy needs of predators scale with their body size, and (iii) energy availability scales with prey biomass, we predicted that predator biomass per unit area would scale with habitat size (quarter-power exponent) and prey biomass (three-quarter–power exponent). We found that total predator biomass scaled with habitat size and prey resources as expected across 29 New Zealand rivers, such that a unit of habitat in a small ecosystem supported less predator biomass than an equivalent unit in a large ecosystem. The lower energetic costs of large body size likely mean that a unit of prey resource supports more biomass of large-bodied predators compared to small-bodied predators. Thus, contracting habitat size reduces the predator mass that can be supported because of constraints on predator body size, and this may be a powerful mechanism exacerbating reductions in biodiversity due to habitat loss.
INTRODUCTION
Habitat reduction is a key cause of biodiversity loss, but managing risks posed by ongoing habitat contraction and fragmentation is hindered by limited understanding of the effects on food webs (1, 2). Habitat size has many important influences (3, 4), but knowledge of the mechanistic drivers linking habitat size with characteristics of trophic structure, such as the capacity to support predators, is still surprisingly limited (1, 5).
The influence of habitat size on trophic structure via constraints on the body size of predators could be critically important in managing problems associated with contracting habitat size—the sizes of habitats required to support predator populations—and in understanding key features of the trophic structure of food webs. Many biological attributes scale with body size (6), but the dependence of both foraging area requirements and mass-specific metabolic rates on body size likely creates a habitat-size dependence, which is fundamentally important for the trophic structure of food webs. Body size is closely connected to habitat size because larger organisms usually require more space (7, 8). For example, home-range size typically has a power-scaling relationship with body size (9, 10). Moreover, because top predators tend to be among the largest organisms present, particularly in aquatic ecosystems (11), habitat size likely constrains the individual mass of organisms at higher trophic levels.
The capacity of food webs to support predators, as highlighted by Elton (12), also depends on the availability of energy at lower trophic levels and the loss of energy through metabolic processes (7, 11, 13). This connection between metabolism and trophic efficiency provides the second important link between habitat size and the body size of predators. Because metabolic rates generally have three-quarter–power scaling with body size (14), habitat size–related influences on predator body size will affect the metabolism and trophic efficiency of organisms at the top of food webs. Essentially, larger predators, because of the three-quarter–power scaling of metabolism with body size, are likely to be more efficient in their mass-specific use of energy compared to smaller predators.
On the basis of this dual dependence of both foraging area requirements and mass-specific metabolic rates on body size and using scaling relationships between prey biomass and productivity (15), we develop a simple theory predicting the shape of the relationship between habitat size and the capacity of an ecosystem to support predator biomass. Combining scaling of metabolic processes with body size, scaling of body size with habitat size, and scaling of prey productivity with prey biomass (described in the “Theoretical predictions” section), we predict that the total predator biomass per unit area should have a power-scaling relationship with habitat size with an exponent of 0.25 and a power-scaling relationship with prey biomass with an exponent of 0.75. We assessed empirical evidence for the predictions from the theory using measurements of prey biomass and predator biomass from 29 relatively unmodified grassland rivers in New Zealand spanning three orders of magnitude in habitat size.
RESULTS
Theoretical predictions
Our predictions are based on the general allometric scaling relationships, which have emerged from the metabolic theory of ecology (14), combined with the trophic control of food webs popularized by Elton (11) and recently expanded by Hatton et al. (15). Initially, we focus on the predator trophic level and connect the scaling relationships of body size with both habitat size and individual metabolism. The first of these connections is based on the inverse of the usual metabolic theory prediction of home-range allometry (that is, where body size is regarded as the dependent, not the independent, variable). Thus, body size of predators (M) is expected to scale with habitat size (H) according to an exponent b = 1
| (1) |
where M is individual mass, H is habitat size, and a1 is a constant of proportionality. This relationship is well supported across many types of organisms (9, 10, 14), including those that live in rivers (16, 17).
Second, whole-organism metabolism scales with individual mass with a power exponent of three-quarters (14). This leads to the within-trophic level (that is, for predators) expected scaling between individual biomass (M) and abundance per unit area of habitat (N) (14)
| (2) |
The total standing biomass per unit area (B) is related to the abundance per unit area (N) and mean body mass (M) via
| (3) |
A habitat size–based expectation for predator biomass per unit area (Bpred) is derived by substitution from the above and ignoring organism-specific constants. Thus, we get
| (4) |
where c = a10.25 a2. These predictions only relate to the trophic level whose body size we expect to be limited by the habitat size, that is, predators. Yet, the collective biomass of predators in a habitat is also limited by the availability of energy at lower trophic levels, likely making Eq. 4 alone a weak predictor of predator biomass. Thus, prey availability must be incorporated to derive a scaling relationship that reliably predicts a habitat’s capacity to support predators. Incorporating prey availability into this equation is straightforward if one focuses on the trophic level immediately below, measured here as the standing stock biomass of primary consumers. This also yields a theory capable of predicting an important aspect of food web structure, that is, food web shape or predator/prey biomass ratios. If primary consumer productivity per unit area (Pprey) reflects energy available to predators, then the value of c in Eq. 4 should be proportional to Pprey, which scales with prey biomass to the power of 0.75 (15). Incorporating this scaling into our equation (that is, replacing c by c0Bprey0.75 in Eq. 4) gives
| (5) |
This predicts that predator biomass per unit area should have quarter- and three-quarter–power scaling relationships with habitat size and prey biomass, respectively. This relationship should have far-reaching consequences because it links both habitat size and prey availability with trophic structure.
Empirical results
We measured predators, including their body size and standing stock biomass, and the standing stock biomass of primary consumers in rivers spanning three orders of magnitude in habitat size measured by discharge. The size of predators was positively related to habitat size in our study rivers, having a power-scaling relationship very close to that expected in Eq. 1 {individual fish mass accounting for 50% of the cumulative biomass “P50” [in grams of dry mass (DM)] = 46.26H1.04, R2 = 0.50, F1,22 = 22.32, P < 0.001; 95% confidence interval (CI) for scaling exponent, 0.58 to 1.50; Fig. 1A}. In situations where downstream dispersal barriers (for example, waterfalls) prevented fish access and top predators were invertebrates, predator sizes were substantially smaller than those of fish (Fig. 1A). Available prey biomass was not related to P50 (R2 = 0.08, F1,25 = 2.07, P = 0.16; Fig. 1B).
Fig. 1. Biomass distributions among predator and prey trophic levels in 29 grassland rivers varying in both habitat size measured by discharge (in cubic meters per second) and the abundance of prey resources [primary consumers (in grams per square meter)].
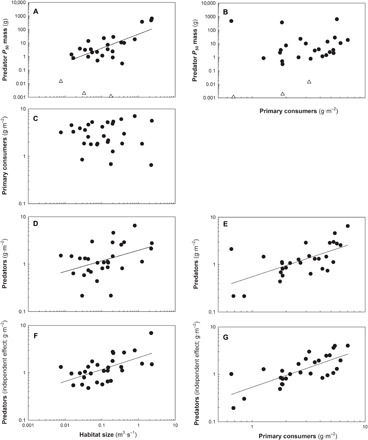
(A and B) Individual predator sizes accounting for 50% of the cumulative predator biomass [P50; DM] in rivers with fish (black circles) and in rivers lacking fish (open triangles). (C) Availability of prey energy at the base of the food web at each river measured by the biomass per unit area of primary consumers (in grams of DM per square meter). (D and E) Combined biomass of predators per unit area of stream (in grams of DM per square meter). (F) Relationship between habitat size and biomass per unit area of predators plotted with primary consumer biomass set at its mean to illustrate the independent effect of habitat size. (G) Relationship between habitat size and biomass per unit area of predators plotted with habitat size set at its mean to illustrate the independent effect of primary consumer biomass. The data for (F) and (G) were generated using the equation Bpred = 0.92H0.25 Bprey0.83, where Bpred is predator biomass per unit area, H is habitat size, and Bprey is primary consumer biomass per unit area. P50 predator mass was unavailable for two fishless rivers where predatory invertebrates were group-weighed.
The availability of prey at each river, measured by the biomass per unit area of primary consumers, was variable and unrelated to habitat size (R2 < 0.01, F1,27 = 0.14, P = 0.72; Fig 1C). Using a subsample of 15 rivers for which auxiliary data were available, further drivers of invertebrate secondary production, including mean annual temperature and mean individual primary consumer body mass, were also shown to be unrelated to habitat size (R2 = 0.13 and P = 0.18 and R2 = 0.05 and P = 0.41, respectively; fig. S1).
We observed an increase in the collective biomass per unit area of predators (Bpred), with habitat size (H) having a power-scaling relationship consistent with the 0.25 exponent predicted by Eq. 4 [Bpred (in grams of DM per square meter) = 1.98H0.23, F1,27 = 5.46, P = 0.027; exponent 95% CI, 0.027 to 0.42; Fig. 1D]. However, as expected, given the variability in energy availability to the base of the food web, the relationship was relatively weak (R2 = 0.17). Predator biomass per unit area was positively related to prey biomass per unit area with a scaling exponent of 0.79 (95% CI, 0.41 to 1.16), close to the expected 0.75 and with a reasonably strong relationship (R2 = 0.41, F1,27 = 18.97, P < 0.001; Fig. 1E).
When the observed three-quarter–power scaling of prey biomass per unit area was incorporated with quarter-power scaling of habitat size (Eq. 5), we observed stronger scaling patterns with predator biomass per unit area (Bpred = 0.92H0.25 Bprey0.83; model R2 = 0.62; F2,26 = 21.32; P < 0.001). Moreover, the scaling exponents for habitat size and prey biomass per unit area (0.25 and 0.83, respectively) were close to those expected (exponent 95% CIs, 0.12 to 0.39 and 0.52 to 1.13 for habitat size and prey biomass, respectively). Adjusted regression plots and partial regression coefficients also illustrated the strong and independent positive effects of both habitat size (partial R2 = 0.36) and prey biomass per unit area (partial R2 = 0.54) on predator biomass per unit area (Fig. 1, F and G). Akaike information criterion corrected for small sample size (AICc) scores indicated that the two-parameter model incorporating both habitat-size scaling and prey biomass per unit area scaling (AICc = 1.04) for predicting predator biomass was much better than single-parameter models with either habitat size (AICc = 21.14) or prey biomass per unit area (AICc = 11.05). Overall, these nonlinear relationships indicate that the amount of predator biomass that can be supported by a given unit of habitat in a river scales with both the available prey biomass and the habitat size of the river, as predicted by Eq. 5. Consequently, a unit of habitat in a small ecosystem supported less predator biomass than an equivalent unit of habitat in a large ecosystem with the same amount of prey biomass (Fig. 2).
Fig. 2. Overall effect of habitat-size constraints on trophic structure.
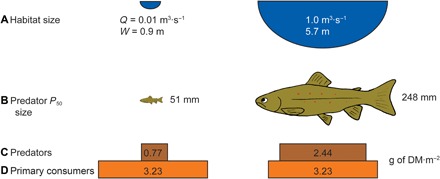
Habitat size (A), by constraining predator size (B), subsequently affects predator/prey biomass ratios (C and D) and capacity to support predator biomass (C) per unit of prey mass (D) based on our sampling in South Island, New Zealand rivers. For habitats sampled, a reduction in habitat size from 1.0 to 0.01 m3·s−1 [stream discharge (Q) associated with reduced width (W)] reduces predator size (for example, brown trout total length, from 248 to 51 mm) based on a back calculation of fish mass accounting for 50% of the cumulative biomass (P50) using Eq. 1 [P50 (in grams of DM) = 46.26H1.04]. Applying results for Eq. 5 (that is, Bpred = 0.92H0.25 Bprey0.83, where Bpred is predator biomass per unit area, H is habitat size, and Bprey is primary consumer biomass per unit area, with units indicated in the diagrams) and assuming similar resource availability across habitats [(D), 3.23 g of DM·per square meter], this reduction in predator size leads to a substantial reduction in the predator biomass (C) being supported per unit of prey biomass.
DISCUSSION
Habitat loss and fragmentation are some of the most pervasive causes of biodiversity loss especially among top predators (18, 19), thereby also compromising important ecosystem functions (2). The theory and empirical patterns that we describe, relating the size of habitats to their capacity to support predator biomass, provide important insights into the processes underlying these issues because they connect habitat size with trophic structure and add to our understanding of why habitat loss is such a pernicious cause of biodiversity loss. Given concordance between theory and observation in our study streams, the scaling of predator biomass with habitat size is likely to be a product of the laws of energy transformation embodied within metabolic scaling theory combined with the spatial constraints of habitat size on the body size of predators. Because larger organisms have, on average, slower metabolic rates (14), a unit of prey biomass can support a greater biomass of large-bodied predators compared to small-bodied predators. Thus, because larger habitats enable larger predators to persist (5, 9, 10, 20), all else being equal, the total predator biomass supported in those habitats should be greater. Moreover, because the allometry of foraging area requirements and mass-specific metabolic rates are well defined by metabolic theory, it was possible to derive a general theory following quarter-power scaling predicting the specific shape of these relationships. Essentially, this means that the effects of habitat size reduction on biodiversity loss are made worse because of reduced capacity to support predator populations. Thus, our findings indicate that predators are disproportionately vulnerable to habitat-size reductions or constraints, with associated far-reaching implications for management of both large predators and habitat loss.
Implications for trophic structure
The habitat size-related influences on body size and the associated links with metabolic theory that we describe offer important insights into the control of food web trophic structure. First, a biomass pyramid where standing predator biomass is nearly as high as standing prey biomass, or even higher as in inverted biomass pyramids, is possible through a range of mechanisms (13). However, we show that these top-heavy trophic structures only occurred in large habitats. In those large habitats, the lower mass-specific metabolic costs associated with the larger size of the predators likely make the use of prey resources more efficient (Fig. 2). This outcome did not occur because larger habitats were more productive; none of the main drivers of primary consumer productivity (that is, abundance, nutrients, temperature, and body size) covaried with stream size (Fig. 1C and figs. S1 and S2). Thus, along with other mechanisms (13), habitat size likely plays an important role in determining precisely how top-heavy food webs can be within ecosystems.
Second, our findings also point to a mechanism that may be important in controlling the length of food chains. Combinations of three main mechanisms for controlling food chain length have typically been considered—(i) restrictions on energy supply, (ii) the size of ecosystems, and (iii) disturbances that affect the reliability of food supplies to upper trophic levels (21)—but the mechanism that might underpin any ecosystem size effect has been less clear (22). By accommodating larger predator body sizes, which are more energetically efficient, larger ecosystems may also allow more energy to be passed upward, thereby lengthening food chains. Thus, the combination of large predator body size, made possible by large habitat size, coupled with the metabolic advantages of large body size, could play a substantial role in the lengthening of food chains in large habitats.
Finally, because predators are key drivers of community structure and function (2, 23), effects of habitat-size alterations on important ecosystem functions could also be widespread and far-reaching. Disproportionate effects of habitat modification on predators (2, 18, 19), and theoretical and empirical work highlighting the spatial control of trophic interactions (24), also point to there being strong links between habitat size and food web structure. Our findings add to the weight of evidence suggesting that contractions in habitat size could destabilize predator-prey relationships and are also consistent with theory and observations suggesting that community stability decreases with habitat size (4, 23, 25).
Implications for the management of rivers
Given our expectation that capacity to support predator biomass will decline if habitat size is reduced, and will thus exacerbate the effects of habitat loss, it is not surprising that human-driven reduction of river flows has coincided with high rates of aquatic biodiversity loss and deleterious effects on aquatic biota, leading to a global crisis (26). Likewise, severe reductions in habitat size associated with drying in aquatic ecosystems have the most marked effects on large organisms at higher trophic levels (25, 27) and can be associated with thresholds that affect population persistence (28). Moreover, the nonlinear nature of the relationships that we describe also means that there are problems with those approaches, which predict the effects of alterations in habitat size from extrapolations of abundance to environment relationships as typically undertaken in physical habitat modeling (29). Thus, although it has been argued that river biota can be naturally resilient to variations in flow (30), our study highlights the strong vulnerability of river food webs to reductions in habitat size induced by flow reductions and issues with the way the effects of many habitat-size reductions in rivers are currently assessed.
Body size allometry of metabolism
The general applicability of the theory depends on the combination and occurrence of two fundamental biological scaling relationships: the body mass allometry of metabolism and the habitat-size dependence of predator mass. The quarter-power scaling of metabolism with body mass relationship, which underpins Eq. 2, is due to the well-known constraints on how animals operate, principally the fractal geometry of resource transport in animal bodies (31). Body size has far-reaching influences on multiple levels of organization (6), but the effect on the rate of energy conversion is likely to be one of the most important (14). Our predictions are based on the assumption, encapsulated in Eq. 2, that within-trophic–level mass-abundance scaling conforms to a three-quarter–power exponent. These relationships are two of the fundamental tenets of the metabolic theory of ecology (14). The within-trophic–level scaling of abundance and mass from metabolic theory (Eq. 2) applies broadly across biota (6), including freshwater fish, albeit with variable slope (32). The emergent effect is that the metabolic economies of scale provided by greater body mass mean that communities where larger predators predominate should be more efficient in their energy use and trophic transfer and that communities made up of larger predators should generally have more predator mass per unit of prey mass than those with smaller predator body sizes.
Because the scaling relationship in Eq. 1 depends on the body size of predators generally, not just on the body size of the largest predator in a habitat, how the body size of the predator biomass was assessed is important. We expect that the trophic efficiencies of lower metabolic rates per unit of predator mass will only accrue if the body size of the collective mass of predators present generally increases with habitat size, so measures such as P50 are most appropriate. Equation 5 does not account for variation in temperature across habitats of different size; however, this could be achieved through the incorporation of a Boltzman activation constant into Eqs. 1 to 5 (14). It might also be argued that real prey production may differ from the three-quarter–power scaling of standing stock biomass assumed in Eq. 5. However, if factors affecting productivity (for example, nutrient availability or temperature) are relatively consistent across the habitat size gradient or can be accounted for analytically, then the scaling of standing primary consumer biomass is a reliable measure of the energy from both autotrophic and heterotrophic energy pathways available to predators.
We treat all consumers at trophic levels above primary consumers as one guild of “predators,” when, in most food webs, there are consumers that feed at multiple trophic levels above primary consumers (33). There are two reasons, connected to the jointly interacting bottom-up and top-down forces affecting food webs, for aggregating predators into one predator trophic level or guild. First, from a bottom-up perspective and assuming that allochthonous subsidies to organisms above primary consumers are minimal, the amount of energy available from primary consumers should be able to support all consumers at higher trophic levels (13). Second, and from a top-down perspective, it is quite possible that reduced predator size associated with habitat-size constraints will release lower-level predators from top-down control (see the Supplementary Materials and fig. S3). That will affect the distribution of biomass among predator sizes and trophic levels (inside the bottom-up constraint), so rather than making assumptions about which trophic level any top-down effect might influence, we grouped all predators into one guild.
Habitat-size dependence of predator body mass
The scaling of capacity to support predators with habitat size also hinges on the habitat-size dependence of predator body mass. The scaling of predator body mass with habitat size has been widely observed, usually with respect to maximum predator size and when habitat is measured as the home-range size of individuals (9, 10). For freshwater fishes, the main top predators in our study rivers, the measured exponents of the scaling relationships between predator size and habitat size (Eq. 1) follow the same general relationship, although the scaling exponent has sometimes tended to be slightly less than expected (b =1) (16), possibly due to variations in how the habitat size variable is defined (17). However, we observed scaling of predator mass with habitat size measured by river discharge with a relationship very close to that defined in Eq. 1, so how habitat size is defined may not be as important as measuring a variable, such as discharge in our case, that reflects the spatial dependence of the body size relationship.
Cross-ecosystem subsidies, physical habitat disturbance, and biogeographic constraints could also potentially influence predator body size (22). For example, Romero et al. (5) observed more top-heavy food webs in larger bromeliad phytotelmata habitats but found that this was more pronounced in stable climatic conditions. We specifically only considered relatively stable streams because disturbance can affect abundance of large predators regardless of habitat size (34). Similarly, cross-ecosystem prey additions from productive habitats (for example, forests) could facilitate larger predator sizes, so we only considered grassland streams. It is likely that large fishless streams, for example, would support comparatively small amounts of predator biomass per unit of prey mass because they lack the metabolic efficiencies of having large predators. Consistent with this, Petermann et al. (20) found that the strong effects of habitat size on trophic structure in bromeliad phytotelmata, leading to inverted biomass pyramids in large habitats, occurred more at sites where odonates were present compared to where they were not present. Thus, factors such as physical habitat disturbance and cross-ecosystem subsidies have the potential to explain additional variance in these scaling relationships (5) and so are worthy of investigation.
The scaling relationships driving predator body size likely occur because habitat size affects the availability of and access to spatially distributed resources that predators need (10). For example, experimentally halving the dimensions of streams with longitudinal fences without altering productivity per unit area reduced the body size of predatory fish present (34). Thus, if the area available for predators to forage efficiently is reduced, then their body size is likely to be constrained. Although rivers are open ecosystems due to longitudinal connectivity, for fish, rivers are also bounded ecosystems because the lateral limits of habitats they can forage in are constrained to the wetted channel. This is likely to be particularly important for predators with indeterminate growth such as fish, where environmental conditions have a large influence on eventual body size, and could explain why fish may not fit other predator-prey biomass scaling relationships (15), and for predators in other habitats (for example, African savanna) where habitat size constraints may place fewer restrictions on predator size.
Overall, our findings concur with the three-quarter–power scaling of predator biomass with prey biomass reported by Hatton et al. (15), supporting the fundamental primacy of bottom-up energetic constraints on predators. However, that habitat size explained substantial additional variance in predator biomass also indicates the importance of further factors in predator-prey biomass relationships. The disproportionate effects of habitat disruption on predator populations generally, compared to organisms at other trophic levels, (2, 18, 19), and the steeper slopes of species area curves at high trophic levels (35), agree with our findings and suggest that predators are generally affected by the constraints of habitat size.
MATERIALS AND METHODS
Study design
We assessed empirical support for our predictions using measurements assembled from 29 rivers in New Zealand using studies by our research group, where invertebrate and fish biomass per unit area had been measured during summer with Surber sampling and electrofishing, respectively (21, 36). Study reaches were chosen to reflect typical conditions on independent first- to fourth-order streams and rivers in the Waimakariri and Rakaia river catchments, South Island, New Zealand. To minimize potential for variations in either availability of allochthonous subsidies or habitat disturbance to influence our comparison, we only considered streams in grassland locations and those with single channels that had not been recently flooded, and we avoided flood-prone braided rivers (table S1 and Supplementary Materials).
Habitat size measurement
Habitat size was quantified by river discharge, measured using the velocity-area method at, or close to, the time of biological sampling. Habitat use by aquatic predators such as fish is highly dynamic and will likely alter in response to even short-term fluctuations in habitat size, so we wanted to know the actual sizes of habitat that predators were using at the time of sampling. Discharge does not incorporate the longitudinal dimensions of river habitats or directly measure the home range of the fish. However, because discharge integrates both the cross-sectional area of a channel and how the water moves through it, it is a more dynamic measure reflecting aspects of how organisms use the habitat. Thus, by capturing the spatial dimension most likely to constrain the size of predators within the sampled habitat, discharge measures habitat size in a way that reflects our predictions. Alternative variables such as catchment area would also not provide a reliable measure of habitat size for groundwater-fed springs and rivers because these tended to have higher discharge than predicted by catchment area (34).
Invertebrate and fish sampling
Invertebrates were sampled from five random locations in 25- to 35-m-long study reaches with Surber samplers (0.0625 m2, 250- or 300-μm mesh) and preserved in 70% ethanol. Preserved samples were sorted under a dissecting microscope, followed by enumeration and identification with appropriate keys (37). DM was calculated (not including cases and shells) either directly in bulk (36) or using length-weight regression based on representative samples of individuals (21).
Fish were sampled with three-pass electrofishing within stop-netted areas encompassing the dimensions of the study reach [see (34)]. The length of reaches sampled reflects areas that were logistically feasible to sample with electrofishing over the range of stream discharges considered and that were appropriate for sampling the range of predator types in these rivers. In four rivers where large fish [rainbow trout (>300 mm fork length)] were present, the fish were captured in a separate electrofishing operation within a larger reach size specifically targeting them; this larger sample area was used in the calculation of large-fish abundance. Fish DM was calculated using length-weight regression of fish captured (38) and summed to quantify fish biomass.
Statistical analysis
We classified predators as everything that was not a primary consumer using diet information (21). This meant that all invertebrates known to consume animal prey (predatory invertebrates) and all fish were classified as predators. All other invertebrates were classified as primary consumers (that is, taxa gaining most of their energy from algae, bacteria, fungi, and detritus). Predator size P50 was calculated as the size of the individual predator accounting for 50% of the cumulative predator biomass at each site, but other measures of predator size gave similar results (see the Supplementary Materials).
Although we lack the data necessary to produce secondary production estimates using standard cohort- or growth-based methods, we investigated whether the main drivers of production [that is, body size, temperature, and standing biomass (39, 40)] varied systematically with habitat size using data from a subset (n = 15) of our study streams for which appropriate temperature and body size data were available (fig. S1). Mean annual temperature was measured at half-hourly intervals between September 2004 and September 2006 at the 15 sites with WT-HR 1000 loggers (TruTrack Instruments) (21). Body size and standing biomass estimates were computed from the community data sets described above.
In our statistical analyses, to evaluate empirical support for Eqs. 1 to 5, we tested each prediction in turn and examined important assumptions associated with those predictions. Power-law scaling relationships were fitted using linear least-squares regression on log10-transformed data. We used AICc to evaluate one-parameter versus two-parameter models. All analyses were run in R (version 3.2.3).
Supplementary Material
Acknowledgments
We thank J. Cohen, J. Harding, A. Huryn, R. Law, J. O’Brien, D. Stouffer, R. Thompson, C. Townsend, M. Winterbourn, and several anonymous reviewers for discussions and comments that improved the manuscript; L. Morris, N. Etheridge, S. Howard, C. Ross, and K. McHugh for research assistance; and P. Nyström for use of data. We are grateful to the local farmers and the Department of Conservation for access to sites and to the University of Canterbury for use of the Cass Field Station. The research procedures were approved by the University of Canterbury Animal Ethics Committee. Funding: Funding was provided by the Marsden Fund of the Royal Society of New Zealand (08-UOC-023 and 08-UOC-034), the University of Canterbury, and the Brian Mason Scientific and Technical Trust. Author contributions: A.R.M. designed an initial empirical study from which the theory was subsequently developed between earthquakes by P.A.M., H.J.W., P.G.J., and A.R.M., with M.J.P. and H.S.G. subsequently improving the mathematical formulation and application to data, respectively. P.A.M., H.J.W., P.G.J., and H.S.G. were all involved in data collection. A.R.M. and H.J.W. performed the data analysis. A.R.M. wrote the first draft of the manuscript, and all authors contributed substantially to revisions. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The data will be available on Dryad at doi:10.5061/dryad.k580m37.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaap7523/DC1
Section S1. Drivers of secondary production
Section S2. Intermediate predators
Fig. S1. Variation in major drivers of secondary production.
Fig. S2. Variation in stream water–specific conductivity in relation to discharge from 15 rivers in the Waimakariri and Rakaia river catchments, New Zealand.
Fig. S3. Patterns in abundance of predatory invertebrates.
Table S1. Location (New Zealand map grid) and habitat size (measured in terms of discharge and stream order) of sites sampled from the Waimakariri and Rakaia river catchments of the South Island, New Zealand to provide information on predator and prey abundance, together with the source of the data.
REFERENCES AND NOTES
- 1.Ryall K. L., Fahrig L., Response of predators to loss and fragmentation of prey habitat: A review of theory. Ecology 87, 1086–1093 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Dobson A., Lodge D., Alder J., Cumming G. S., Keymer J., McGlade J., Mooney H., Rusak J. A., Sala O., Wolters V., Wall D., Winfree R., Xenopoulos M. A., Habitat loss, trophic collapse, and the decline of ecosystem services. Ecology 87, 1915–1924 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Sabo J. L., Finlay J. C., Kennedy T., Post D. M., The role of discharge variation in scaling of drainage area and food chain length in rivers. Science 330, 965–967 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Mellin C., Huchery C., Caley M. J., Meekan M. G., Bradshaw C. J. A., Reef size and isolation determine the temporal stability of coral reef fish populations. Ecology 91, 3138–3145 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Romero G. Q., Piccoli G. C. O., de Omena P. M., Gonçalves-Souza T., Food web structure shaped by habitat size and climate across a latitudinal gradient. Ecology 97, 2705–2715 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Woodward G., Ebenman B., Emmerson M., Montoya J. M., Olesen J. M., Valido A., Warren P. H., Body size in ecological networks. Trends Ecol. Evol. 20, 402–409 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Arim M., Abades S. R., Laufer G., Loureiro M., Marquet P. A., Food web structure and body size: Trophic position and resource acquisition. Oikos 119, 147–153 (2010). [Google Scholar]
- 8.Marquet P. A., Taper M. L., On size and area: Patterns of mammalian body size extremes across landmasses. Evol. Ecol. 12, 127–139 (1998). [Google Scholar]
- 9.Harestad A. S., Bunnell F. L., Home range and body-weight—A reevaluation. Ecology 60, 389–402 (1979). [Google Scholar]
- 10.Jetz W., Carbone C., Fulford J., Brown J. H., The scaling of animal space use. Science 306, 266–268 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Riede J. O., Brose U., Ebenman B., Jacob U., Thompson R., Townsend C. R., Jonsson T., Stepping in Elton’s footprints: A general scaling model for body masses and trophic levels across ecosystems. Ecol. Lett. 14, 169–178 (2011). [DOI] [PubMed] [Google Scholar]
- 12.C. Elton, Animal Ecology (Sidgwick and Jackson, 1927). [Google Scholar]
- 13.McCauley D. J., Gellner G., Martinez N. D., Williams R. J., Sandin S. A., Micheli F., Mumby P. J., McCann K. S., On the prevalence and dynamics of inverted trophic pyramids and otherwise top-heavy communities. Ecol. Lett. 21, 439–454 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- 15.Hatton I. A., McCann K. S., Fryxell J. M., Davies T. J., Smerlak M., Sinclair A. R. E., Loreau M., The predator-prey power law: Biomass scaling across terrestrial and aquatic biomes. Science 349, aac6284 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Minns C. K., Allometry of home range size in lake and river fishes. Can. J. Fish. Aquat. Sci. 52, 1499–1508 (1995). [Google Scholar]
- 17.Woolnough D. A., Downing J. A., Newton T. J., Fish movement and habitat use depends on water body size and shape. Ecol. Freshw. Fish 18, 83–91 (2009). [Google Scholar]
- 18.Petchey O. L., Downing A. L., Mittelbach G. G., Persson L., Steiner C. F., Warren P. H., Woodward G., Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 104, 467–478 (2004). [Google Scholar]
- 19.Duffy J. E., Biodiversity loss, trophic skew and ecosystem functioning. Ecol. Lett. 6, 680–687 (2003). [Google Scholar]
- 20.Petermann J. S., Farjalla V. F., Jocque M., Kratina P., MacDonald A. A. M., Marino N. A. C., de Omena P. M., Piccoli G. C. O., Richardson B. A., Richardson M. J., Romero G. Q., Videla M., Srivastava D. S., Dominant predators mediate the impact of habitat size on trophic structure in bromeliad invertebrate communities. Ecology 96, 428–439 (2015). [DOI] [PubMed] [Google Scholar]
- 21.McHugh P. A., McIntosh A. R., Jellyman P. G., Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol. Lett. 13, 881–890 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Post D. M., The long and short of food-chain length. Trends Ecol. Evol. 17, 269–277 (2002). [Google Scholar]
- 23.McCann K. S., Rasmussen J. B., Umbanhowar J., The dynamics of spatially coupled food webs. Ecol. Lett. 8, 513–523 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Martinson H. M., Fagan W. F., Denno R. F., Critical patch sizes for food-web modules. Ecology 93, 1779–1786 (2012). [DOI] [PubMed] [Google Scholar]
- 25.McHugh P. A., Thompson R. M., Greig H. S., Warburton H. J., McIntosh A. R., Habitat size influences food web structure in drying streams. Ecography 38, 700–712 (2015). [Google Scholar]
- 26.Vörösmarty C. J., McIntyre P. B., Gessner M. O., Dudgeon D., Prusevich A., Green P., Glidden S., Bunn S. E., Sullivan C. A., Liermann C. R., Davies P. M., Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Ledger M. E., Brown L. E., Edwards F. K., Milner A. M., Woodward G., Drought alters the structure and functioning of complex food webs. Nat. Clim. Chang. 3, 223–227 (2013). [Google Scholar]
- 28.White R. S. A., McHugh P. A., McIntosh A. R., Drought survival is a threshold function of habitat size and population density in a fish metapopulation. Glob. Chang. Biol. 22, 3341–3348 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Lancaster J., Downes B. J., Ecohydraulics needs to embrace ecology and sound science, and to avoid mathematical artefacts. River Res. Appl. 26, 921–929 (2010). [Google Scholar]
- 30.Jowett I. G., Biggs B. J. F., Application of the ‘natural flow paradigm’ in a New Zealand context. River Res. Appl. 25, 1126–1135 (2009). [Google Scholar]
- 31.West G. B., Brown J. H., Enquist B. J., A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Keeley E. R., An experimental analysis of self-thinning in juvenile steelhead trout. Oikos 102, 543–550 (2003). [Google Scholar]
- 33.Trebilcol R., Baum J. K., Salomon A. K., Dulvy N. K., Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol. Evol. 28, 423–431 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Jellyman P. G., McHugh P. A., McIntosh A. R., Increases in disturbance and reductions in habitat size interact to suppress predator body size. Glob. Chang. Biol. 20, 1550–1558 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Roslin T., Várkonyi G., Koponen M., Vikberg V., Nieminen M., Species-area relationships across four trophic levels—Decreasing island size truncates food chains. Ecography 37, 443–453 (2014). [Google Scholar]
- 36.Nyström P., McIntosh A. R., Winterbourn M. J., Top-down and bottom-up processes in grassland and forested streams. Oecologia 136, 596–608 (2003). [DOI] [PubMed] [Google Scholar]
- 37.M. J. Winterbourn, K. L. D. Gregson, C. H. Dolphin, Guide to the Aquatic Insects of New Zealand (Entomological Society of New Zealand, 2006). [Google Scholar]
- 38.Jellyman P. G., Booker D. J., Crow S. K., Bonnett M. L., Jellyman D. J., Does one size fit all? An evaluation of length–weight relationships for New Zealand’s freshwater fish species. N. Z. J. Mar. Freshw. Res. 47, 450–468 (2013). [Google Scholar]
- 39.Morin A., Empirical models predicting population abundance and productivity in lotic systems. J. North Am. Benthol. Soc. 16, 319–337 (1997). [Google Scholar]
- 40.A. D. Huryn, A. C. Benke, Relationship between biomass turnover and body size for stream communities, in Body Size: The Structure and Function of Aquatc Ecosystems, A. G. Hildrew, D. G. Raffaelli, R. Edmonds-Brown, Eds. (Cambridge Univ. Press, 2007), pp. 55–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaap7523/DC1
Section S1. Drivers of secondary production
Section S2. Intermediate predators
Fig. S1. Variation in major drivers of secondary production.
Fig. S2. Variation in stream water–specific conductivity in relation to discharge from 15 rivers in the Waimakariri and Rakaia river catchments, New Zealand.
Fig. S3. Patterns in abundance of predatory invertebrates.
Table S1. Location (New Zealand map grid) and habitat size (measured in terms of discharge and stream order) of sites sampled from the Waimakariri and Rakaia river catchments of the South Island, New Zealand to provide information on predator and prey abundance, together with the source of the data.


