Abstract
Aims/Introduction
The glucose‐lowering effects of the glucagon‐like peptide‐1 receptor agonist, liraglutide, have been shown to rely on remaining β‐cell function. However, the possible associations of remaining β‐cell function with the glucose‐lowering effects of liraglutide in combination with basal insulin remain unknown and warrant investigation.
Materials and Methods
This was a single‐center, retrospective, observational study carried out in a private hospital in Osaka, Japan. Type 2 diabetes patients who received a prescription change from insulin therapy, both multiple‐dose insulin and basal insulin‐supported oral therapy, to liraglutide and basal insulin combination and continued the therapy for 54 weeks without additional oral antidiabetic drugs or bolus insulin were retrospectively analyzed.
Results
Among the 72 participants who received a prescription change from multiple‐dose insulin and basal insulin‐supported oral therapy to liraglutide and basal insulin combination, 57 continued the therapy for 54 weeks. Of those who continued the therapy without receiving additional oral antidiabetic drugs or bolus insulin, seven participants achieved glycated hemoglobin < 7.0% at 54 weeks, but 30 participants did not. The participants who achieved glycated hemoglobin < 7.0% at 54 weeks had a significantly higher C‐peptide immunoreactivity index, a β‐cell function‐related index frequently used in Japanese clinical settings. The receiver operating curve analysis showed that the C‐peptide immunoreactivity index cut‐off value for the achievement of glycated hemoglobin <7.0% at 54 weeks is 1.103.
Conclusions
The current findings show that the glucose‐lowering effects of liraglutide rely on remaining β‐cell function, even when used with basal insulin; and suggest that liraglutide and basal insulin combination might require additional bolus insulin to fully compensate insulin insufficiency in individuals with reduced β‐cell function.
Keywords: β‐Cell function, Basal insulin, Liraglutide
Introduction
Type 2 diabetes is characterized by a progressive decline in β‐cell function and increased insulin resistance that contribute to chronic hyperglycemia and subsequent diabetic complications1. Therefore, antidiabetes strategies so far have mainly focused on improving β‐cell function and insulin resistance. Among them, glucagon‐like petide‐1 receptor (GLP‐1R) agonists, especially the so‐called long‐acting GLP‐1R agonists, such as liraglutide, have been gaining attention because they enhance insulin secretion glucose‐dependently and concurrently ameliorate insulin resistance through bodyweight reduction2, 3. Previous studies showed that the glucose‐lowering effects of the long‐acting GLP‐1R agonist, liraglutide, rely on remaining β‐cell function, as evaluated by postprandial levels of C‐peptide immunoreactivity (CPR) 4, the fasting C‐peptide index (CPI) or the CPR/plasma glucose ratio5, 6, 7, 8 and glucagon‐stimulated increments of CPR7. These findings suggest that the earlier initiation of long‐acting GLP‐1R agonists results in greater glucose‐lowering effects in patients with type 2 diabetes. A meta‐analysis of clinical trials on GLP‐1R agonists and basal insulin combination therapy showed that they improve glycated hemoglobin (HbA1c) similarly to basal–bolus insulin therapy with a reduced risk of hypoglycemia and bodyweight gain9. Therefore, it is conceivable that long‐acting GLP‐1R agonists are effective in type 2 diabetes patients with reduced remaining β‐cell function when used with basal insulin. However, this has not been tested in clinical trials or in clinical settings, and requires further investigation. The current retrospective study was designed to evaluate: (i) the HbA1c‐lowering effects of liraglutide and basal insulin combination therapy in type 2 diabetes patients who had previously received insulin therapy, both multiple‐dose insulin (MDI) and basal insulin‐supported oral therapy (BOT); and (ii) the possible association of remaining β‐cell function with the HbA1c‐lowering effects of the therapy.
Methods
Participants
A total of 72 type 2 diabetes patients who received a prescription change from insulin therapy, MDI or BOT to liraglutide and basal insulin combination therapy at Kansai Electric Power Hospital (Osaka, Japan) between September 2014 and February 2016 were retrospectively analyzed. Patients with type 1 diabetes, pregnancy, pancreatic disease, liver disease, renal disease, malignancy or receiving diabetogenic medication were excluded. Patients with severe renal failure (creatinine clearance rate < 30 mL/min) and/or those undergoing dialysis were also excluded, as serum CPR levels are modified in these conditions. Physical and laboratory data including HbA1c were acquired before and 12 ± 3, 24 ± 3, 36 ± 3 and 54 ± 3 weeks after the initiation of liraglutide and basal insulin combination therapy. A schematic diagram of the study participants is summarized in Figure 1. Of the 72 patients, 15 discontinued liraglutide as a result of hyperglycemia (1 patient), gastrointestinal side‐effects (4 patients) and other reasons (10 patients); 12 patients continued liraglutide therapy while receiving additional oral antidiabetic drugs (OAD) or insulin during 54 weeks, and the remaining 45 patients continued liraglutide therapy without additional OADs or bolus insulin. There were 37 patients whose HbA1c values at week 54 were available. No hypoglycemia requiring hospitalization occurred during the study period.
Figure 1.
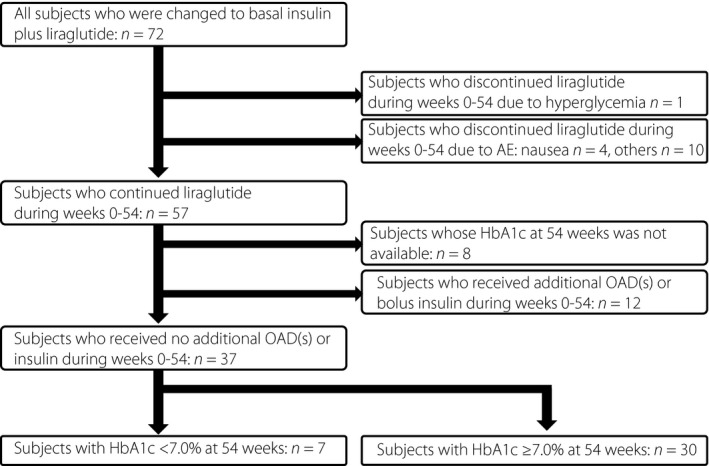
Schematic diagram of the study participants. HbA1c, glycated hemoglobin; OAD, oral antidiabetic drug.
Measurements
HbA1c was measured using high‐performance liquid chromatography with cation‐exchange resins that separate the stable form of β‐N1‐mono‐deoxyfructosyl Hb. The results are shown as National Glycohemoglobin Standardization Program values as recommended by the Japan Diabetes Society10. The CPI, which is closely correlated with other indices of β‐cell function, such as homeostatic model assessment of β‐cell function and insulinogenic index11, 12, 13, and has been widely used as a practical index to estimate β‐cell function in clinical settings14, was calculated as follows: 100 × fasting CPR / fasting plasma glucose. The patients continued pre‐existing insulin therapy up until the night before the measurement of fasting serum CPR in the morning to avoid hyperglycemia. CPR was measured using Lumipulse Presto C‐peptide (Fujirebio Inc., Tokyo, Japan). Other laboratory measurements including plasma glucose were measured by standard assays.
Statistical analysis
The patient characteristics and results are reported as the mean ± standard error of the mean (SEM). The statistical analysis was carried out using SPSS Statistics 22 software (IBM Corp., Armonk, NY, USA). Repeated measures were analyzed using a mixed‐effects model. Clinical parameters were compared between the two groups at single time‐points using the Mann–Whitney U‐test. P‐values < 0.05 were considered statistically significant. Receiver operating characteristic curves were constructed for the CPI. The sensitivity, specificity, cut‐off point and area under the receiver operating characteristic curve were also calculated.
Results
Of the 57 patients who initiated and continued liraglutide and basal insulin combination therapy for 54 weeks, we analyzed 37 patients who did not take additional OADs or bolus insulin (Figure 1). After the initiation of liraglutide and basal insulin combination therapy, HbA1c slightly improved (baseline 8.5 ± 0.3% and at 54 weeks 8.0 ± 0.2%; P = 0.138), with significant reductions in bodyweight (baseline 73.3 ± 2.8 kg and at 54 weeks 68.6 ± 2.4 kg; P < 0.01), insulin dose (baseline 25.9 ± 3.1 units/day and at 54 weeks 16.2 ± 1.4 units/day; P < 0.01) and insulin dose per bodyweight (baseline 0.34 ± 0.04 units/kg bodyweight/day and at 54 weeks 0.23 ± 0.02 units/kg bodyweight/day; P < 0.01; Figure 2a–d, black lines).
Figure 2.
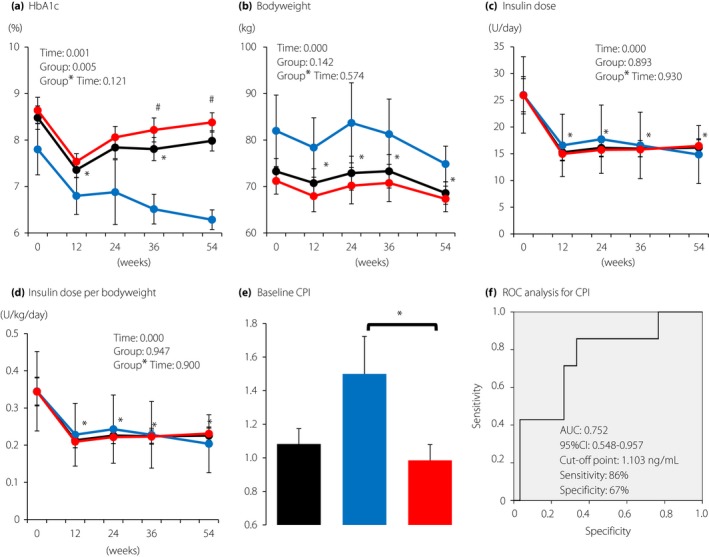
Changes during the 54‐week period in (a) glycated hemoglobin (HbA1c), (b) bodyweight, (c) insulin dose and (d) insulin dose per bodyweight. Black, all participants (n = 37); blue, patients with HbA1c < 7% at 54 weeks (n = 7); red, patients with HbA1c ≥ 7.0% at 54 weeks (n = 30). Each value represents the mean ± standard error of the mean. Time‐course curves were analyzed by mixed‐effects models including group, time, and the interaction of group and time, and the P‐values are shown. *P < 0.05 (vs 0 week) in all participants and # P < 0.05 (vs patients with HbA1c < 7.0% at 54 weeks) by the Mann–Whitney U‐test. (e) Comparison of C‐peptide index (CPI) in patients with HbA1c < 7.0% and those with HbA1c ≥ 7.0% at 54 weeks. Black bar, all participants (n = 37); blue bar, patients with HbA1c < 7.0% at 54 weeks (n = 7); red bar, patients with HbA1c ≥ 7.0% at 54 weeks (n = 30). Each value represents the mean ± standard error of the mean. *P < 0.05 by the Mann–Whitney U‐test. (f) Receiver operating characteristic (ROC) curves of CPI to predict the achievement of HbA1c < 7.0% at 54 weeks without adding additional antidiabetic drugs or bolus insulin. The area under the curve (AUC) is 0.752 (95% confidence interval 0.548–0.957). The estimated cut‐off point is 1.103 with 86% sensitivity and 67% specificity.
We then addressed whether remaining β‐cell function affects the achievement of HbA1c < 7.0% at 54 weeks after the initiation of liraglutide and basal insulin combination therapy. Among the 37 patients, the patients who achieved HbA1c < 7.0% showed higher HbA1c values and bodyweight throughout the observation period, although it did not reach statistical significance. These patients had a total insulin dose similar to patients who did not achieve HbA1c < 7.0%. Importantly, the baseline values of the β‐cell function index, CPI, were significantly higher in patients who achieved HbA1c < 7.0% than in those who did not achieve HbA1c < 7.0% (1.50 ± 0.22 vs 0.98 ± 0.10; P = 0.04; Figure 2e). The receiver operating characteristic analysis showed that the cut‐off CPI value for the achievement of HbA1c < 7.0% was 1.103 ng/mL (area under the curve 0.752; 95% confidence interval 0.548–0.957; sensitivity 86% and specificity 67%; Figure 2f).
We also carried out a subgroup analysis that showed that the changes in HbA1c from baseline to 54 weeks were affected only by baseline HbA1c, and did not differ by the baseline values of age, body mass index (BMI) and type 2 diabetes duration, the insulin dose or the type of pre‐existing insulin therapy (MDI vs BOT; Figure 3). Although the changes in bodyweight and insulin dose were significantly affected by baseline BMI, insulin dose and insulin therapy type, the bodyweight and insulin dose at 54 weeks were not affected by baseline insulin therapy type (Figure 3). Importantly, the dose of liraglutide did not differ by the baseline age (≥65 years, 0.86 ± 0.03 mg and <65 years, 0.81 ± 0.03 mg; P = 0.421), the medians of baseline HbA1c (≥8.2%, 0.82 ± 0.03 mg and <8.2%, 0.85 ± 0.03 mg; P = 0.538), baseline BMI (≥26.36 kg/m2, 0.87 ± 0.02 mg and <26.36 kg/m2, 0.81 ± 0.03 mg; P = 0.298), type 2 diabetes duration (≥16 years, 0.82 ± 0.03 mg and <16 years, 0.85 ± 0.03 mg; P = 0.620), baseline insulin dose (≥0.4 units/kg bodyweight/day, 0.85 ± 0.03 mg and <0.4 units/kg bodyweight/day, 0.82 ± 0.03 mg; P = 0.538) or the type of pre‐existing insulin therapy (MDI 0.85 ± 0.02 mg and BOT 0.79 ± 0.05 mg; P = 0.332). The ratio of basal insulin and liraglutide (units of basal insulin per mg of liraglutide) did not differ by baseline age (≥65 years, 20.74 ± 2.47 and <65 years, 18.13 ± 2.34; P = 0.354), the medians of baseline HbA1c (≥8.2%, 21.93 ± 2.75 and <8.2%, 17.16 ± 1.92; P = 0.159), type 2 diabetes duration (≥16 years, 21.88 ± 2.28 and <16 years, 17.22 ± 2.53; P = 0.258) or the type of pre‐existing insulin therapy (MDI 21.24 ± 2.06 and BOT, 15.76 ± 2.92; P = 0.204), but it differed by the medians of baseline BMI (≥26.36 kg/m2, 23.22 ± 2.70 and <26.36 kg/m2, 15.80 ± 1.75; P = 0.020) and the medians of baseline daily insulin dose per bodyweight (≥0.4 units/kg bodyweight/day, 23.98 ± 2.22 and <0.4 units/kg bodyweight/day, 15.00 ± 2.21; P = 0.014).
Figure 3.
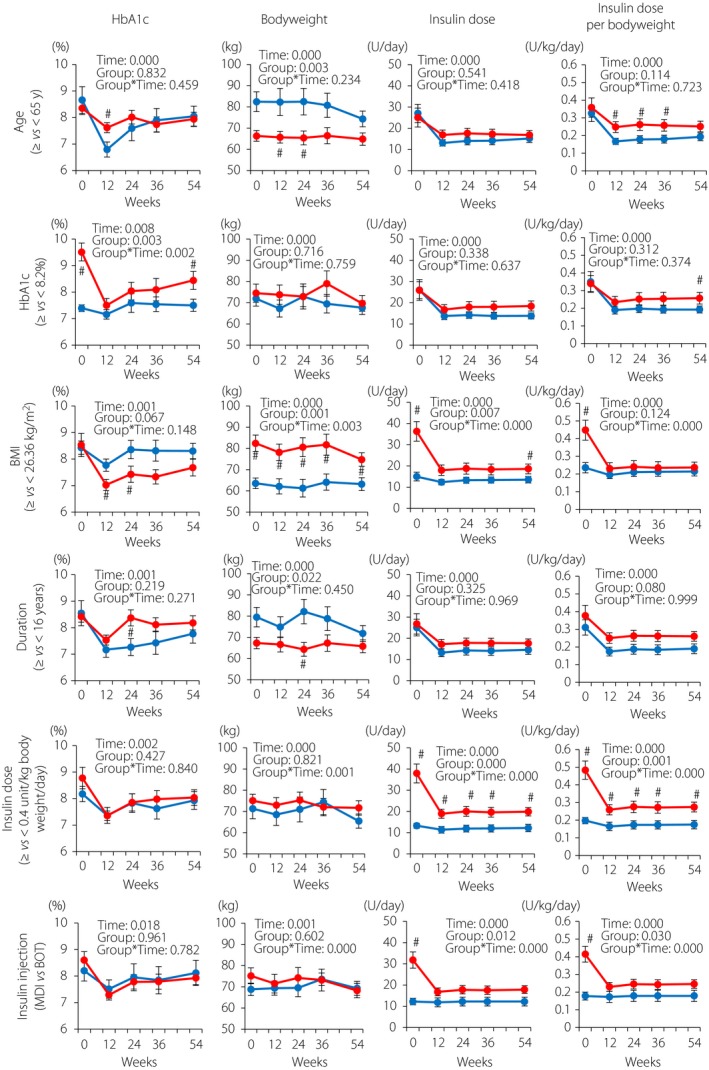
Subgroup analysis of glycated hemoglobin (HbA1c) and bodyweight changes by baseline HbA1c, body mass index (BMI), estimated duration of type 2 diabetes, insulin dose per bodyweight and insulin injection types. The patients who continued liraglutide and basal insulin combination therapy for 54 weeks without taking additional anti‐oral diabetic drugs or bolus insulin were stratified by age (<65 or ≥65 years), baseline HbA1c (median 8.2%), baseline BMI (median 26.36 kg/m2), estimated duration of diabetes (median 16.0 years), total insulin dose (median 0.4 units/kg bodyweight/day) and insulin injection types (multiple dose insulin [MDI] or basal insulin‐supported oral therapy [BOT]). Blue, patients with aged <65 years (n = 16) or below the medians (HbA1c, n = 18; BMI, n = 18; duration of type 2 diabetes, n = 18; and total insulin doses, n = 18) or those with BOT (n = 11); red, patients aged ≥65 years (n = 21) or above the medians (HbA1c, n = 19; BMI, n = 19; duration of type 2 diabetes, n = 19; and total insulin doses, n = 19) or those with MDI (n = 26). Each value represents the mean ± standard error of the mean. Time‐course curves were analyzed by mixed‐effects models including group, time and the interaction of group and time, and the P‐values are shown. # P < 0.05 (vs patients with values below the medians or patients with BOT) by the Mann–Whitney U‐test.
Discussion
The current study showed that the achievement of HbA1c < 7.0% by liraglutide and basal insulin combination therapy was affected by remaining β‐cell function, and that the cut‐off value of the β‐cell function index, CPI, for this target was 1.103, suggesting that the therapy requires additional OAD or bolus insulin in patients with CPI < 1.103 to achieve HbA1c < 7.0% 1 year after initiation. The present study also suggested that liraglutide and basal insulin combination therapy improves HbA1c and bodyweight regardless of baseline characteristics, such as age, BMI, type 2 diabetes duration, insulin therapy type and the insulin dose.
Liraglutide exerts glucose‐lowering effects by enhancing basal and postprandial insulin secretion from pancreatic β‐cells2, 15. Therefore, it is conceivable that remaining β‐cell function is one of the critical determinants in the glucose‐lowering effects of liraglutide. Our groups and others previously reported that liraglutide exerts greater HbA1c‐lowering effects among non‐insulin‐treated type 2 diabetes patients with ample β‐cell function5, 7, 16. However, whether these HbA1c‐lowering effects depend on remaining β‐cell function in patients receiving basal insulin is unknown. As clearly shown in the current study, the HbA1c‐lowering effects depend on remaining β‐cell function, even in patients receiving liraglutide with basal insulin. Interestingly, the cut‐off CPI value for the achievement of HbA1c < 7.0% by liraglutide and basal insulin combination therapy (i.e., 1.103) is lower than that of liraglutide ± sulfonylurea therapy (i.e., 1.86) 7, suggesting that basal insulin can compensate for the β‐cell function required for liraglutide to enhance basal insulin secretion. However, as suggested by the current study, liraglutide and basal insulin combination therapy might require additional bolus insulin to fully compensate insulin insufficiency in individuals with reduced β‐cell function. Therefore, it would be interesting to evaluate the β‐cell function requirement for the achievement of HbA1c < 7.0% in individuals receiving both basal and bolus insulin. More than 70% of the 37 patients examined in the current study stopped receiving pre‐existing OADs and/or bolus insulin, and no washout periods were implemented before the initiation of the basal insulin and liraglutide combination therapy, making it difficult to evaluate patients’ responses based on changes in HbA1c from baseline to 54 weeks. In addition to the achievement of HbA1c < 7.0% at 54 weeks, we also evaluated CPI in HbA1c tertiles at 54 weeks, and found that the lowest HbA1c tertile had the highest CPI (Figure S2).
Insulin therapy, especially MDI, has been the most powerful tool for glycemic control, but the risk of hypoglycemia and increased bodyweight have been problems in the management of type 2 diabetes. GLP‐1R agonist and basal insulin combination therapy has been gaining attention, because it carries lower risks for hypoglycemia and bodyweight gain, and improves quality of life because of less frequent injections and exerts HbA1c‐lowering effects similarly to MDI according to randomized controlled trials17. Consistent with the results of randomized controlled trials, liraglutide and basal insulin combination therapy improved HbA1c, and decreased bodyweight and the insulin dose when switched from MDI or BOT in the current study (Figure 2) and in recent clinical trials in Japanese type 2 diabetes patients18, 19. Liraglutide and basal insulin combination therapy is effective regardless of baseline age, BMI, disease duration, insulin dose and the type of insulin injections (Figure 3). In addition, the ratio of basal insulin and liraglutide was not affected by baseline values of age, HbA1c, type 2 diabetes duration or the type of pre‐existing insulin therapy, suggesting a broad indication for a fixed‐ratio combination of liraglutide and basal insulin in Japanese type 2 diabetes patients similar to type 2 diabetes patients of other ethnicities20, 21. The ratio of basal insulin and liraglutide significantly differ between individuals with baseline BMI ≥ 26.36 kg/m2 and those < 26.36 kg/m2; and between individuals with baseline insulin dose ≥ 0.4 unit/kg/day and those with < 0.4 unit/kg/day, suggesting that the ratio should be determined in light of obesity and subsequent insulin resistance. However, it is important to note that liraglutide up to 0.9 mg q.d., unlike up to 1.8 mg q.d. in other countries, is currently approved in Japan and that the results need to be interpreted accordingly.
There were several limitations to the current study. First, the sample size of the current study was limited, as liraglutide and basal insulin combination therapy had only been approved in Japan for <3 years. Second, just 19% of the patients achieved HbA1c < 7.0% at 54 weeks, partly due to the new guideline of HbA1c targets for the elderly announced by the Japan Diabetes Society in May 2016 (e.g., HbA1c 6.5–7.5% for individuals aged ≥65‐years and individuals aged<75 years with normal cognitive function and activities of daily living, and with the use of insulin, sulfonylurea and glinide) 22. Third, because of the retrospective nature of the study, there is no pre‐fixed rule for dose adjustments of basal insulin by the physician‐in‐charge. Therefore, it is difficult to exclude the possibility that dose adjustments of basal insulin affected the overall results, including the CPI cut‐off value, although the basal insulin dose did not differ regardless of HbA1c < 7.0% achievement (Figure 2c,d). In addition, there is no pre‐fixed rule for prescription changes. The inclusion of the 12 patients originally excluded as a result of additional OADs or bolus insulin showed that baseline CPI was higher in the group achieving HbA1c < 7.0%, although it did not reach statistical significance (Figure S1). Five out of the 12 excluded patients had baseline CPI values (1.71 ± 0.13) that were higher than the mean CPI value of the group achieving HbA1c < 7.0% (Table 1). These five patients had higher baseline insulin dose per bodyweight (0.47 ± 0.08 units/kg bodyweight/day) and younger baseline age (52.2 ± 1.6 years), despite a similar baseline HbA1c (8.1 ± 0.4%) compared with the group achieving HbA1c < 7.0% (Table 1), suggesting that additional factors (e.g., overeating and insulin resistance) might also contribute to achievement of HbA1c < 7.0% by liraglutide and basal insulin combination therapy. Fourth, linear associations of CPI with β‐cell function at higher fasting glucose ranges were not investigated. However, fasting glucose levels in the current study participants were similar to or lower than those described in previous studies evaluating remaining β‐cell function to avoid insulin injections and those investigating associations of CPI with other indices of β‐cell function12, 13, 23. Despite these limitations, the current findings strongly suggest that liraglutide requires some remaining β‐cell function to exert glucose‐lowering effects, even when used with basal insulin, and suggest that liraglutide and basal insulin therapy requires additional OAD or bolus insulin to achieve ideal HbA1c target.
Table 1.
Baseline characteristics of the patients who received a prescription change from insulin therapy to liraglutide and basal insulin combination therapy
| All patients | Patients who achieved HbA1c < 7.0% at 54 weeks | Patients who did not achieve HbA1c < 7.0% at 54 weeks | P‐value | |
|---|---|---|---|---|
| n (%male) | 37 (59.5%) | 7 (71.4%) | 30 (56.7%) | |
| Age at baseline (years) | 63.0 ± 2.0 | 61.3 ± 5.1 | 63.4 ± 2.2 | 0.955 |
| Estimated duration of type 2 diabetes at baseline (years) | 15.5 ± 1.5 | 10.1 ± 3.8 | 16.8 ± 1.6 | 0.084 |
| BMI at baseline (kg/m2) | 27.9 ± 0.7 | 30.2 ± 2.1 | 27.4 ± 0.8 | 0.160 |
| HbA1c at baseline (%) | 8.5 ± 0.3 | 7.8 ± 0.5 | 8.6 ± 0.3 | 0.138 |
| HbA1c at 54 weeks (%) | 8.0 ± 0.2 | 6.3 ± 0.2 | 8.4 ± 0.2 | <0.001 |
| Patients with HbA1c < 7% at baseline (%) | 5.4 | 14.3 | 3.3 | |
| Fasting plasma glucose at baseline (mg/dL) | 120.9 ± 5.6 | 119.9 ± 7.3 | 121.2 ± 6.7 | 0.776 |
| Fasting C‐peptide at baseline (ng/mL) | 1.37 ± 0.15 | 1.79 ± 0.28 | 1.27 ± 0.18 | 0.077 |
| CPI at baseline | 1.08 ± 0.09 | 1.50 ± 0.22 | 0.98 ± 0.09 | 0.040 |
| Insulin injection type before liraglutide initiation, MDI/BOT (%) | 70/30 | 57/43 | 73/27 | |
| Patients on OAD at baseline (%) | ||||
| Metformin | 46 | 57 | 43 | |
| DPP‐4 inhibitors | 49 | 43 | 50 | |
| Sulfonylureas | 11 | 0 | 13 | |
| SGLT2 inhibitors | 3 | 0 | 3 | |
| Glinides | 3 | 0 | 3 | |
| α‐Glucosidase inhibitors | 3 | 0 | 3 | |
| Thiazolidinedione | 0 | 0 | 0 | |
| Insulin dose before liraglutide initiation (unit/kg bodyweight/day) | 0.34 ± 0.04 | 0.34 ± 0.11 | 0.34 ± 0.04 | 0.458 |
| Insulin dose before liraglutide initiation (units/day) | 25.9 ± 3.1 | 26.0 ± 7.1 | 25.9 ± 3.5 | 0.955 |
| Insulin dose at 54 weeks (units/kg bodyweight/day) | 0.23 ± 0.20 | 0.20 ± 0.08 | 0.23 ± 0.18 | 0.391 |
| Insulin dose at 54 weeks (units/day) | 16.2 ± 1.4 | 14.9 ± 5.4 | 16.5 ± 1.3 | 0.556 |
| Liraglutide dose at 54 weeks (mg/day) | 0.84 ± 0.02 | 0.81 ± 0.06 | 0.84 ± 0.02 | 0.747 |
| A ratio of basal insulin to liraglutide at 54 weeks (units/mg) | 19.61 ± 1.72 | 17.78 ± 6.20 | 20.04 ± 1.63 | 0.531 |
Each value represents the mean ± standard error of the mean. CPI, 100 × (fasting serum C‐peptide [ng/mL]) / (fasting plasma glucose [mg/dL]). P‐values were calculated by the Mann–Whitney U‐test for patients who achieved glycated hemoglobin (HbA1c) < 7.0% at 54 weeks vs patients who did not achieve HbA1c < 7.0% at 54 weeks. BMI, body mass index; BOT, basal insulin‐supported oral therapy; CPI, C‐peptide index; DPP‐4, dipeptidyl peptidase‐4; MDI, multiple‐dose insulin; OAD, oral antidiabetic drugs; SGLT2, sodium‐glucose cotransporter 2.
In conclusion, the current study found that the patients who achieved HbA1c < 7.0% by liraglutide and basal insulin combination therapy had more remaining β‐cell function, and suggests that the earlier introduction of liraglutide allows better glycemic control outcomes, even when used with basal insulin.
Disclosure
Daisuke Yabe received consulting or speaker fees from MSD K.K., Novo Nordisk Pharma Ltd., Takeda Pharmaceutical Company Limited and Taisho Toyama Pharmaceutical Co. Ltd. Daisuke Yabe also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd., and Takeda Pharmaceutical Company Limited. Yoshiyuki Hamamoto received consulting or speaker fees from Novo Nordisk Pharma Ltd. Takeshi Kurose received consulting or speaker fees from Sanofi K.K. Takeshi Kurose also received clinically commissioned/joint research grants from the Japan Vascular Disease Research Foundation. Yutaka Seino received consulting or speaker fees from Eli Lilly Japan K.K., Sanofi K.K., Novo Nordisk Pharma Inc., Glaxo‐Smith‐Kline, Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Astellas Pharma Inc., BD, Nippon Boehringer Ingelheim Co., Ltd., Johnson & Johnson and Takeda Pharmaceutical Company Limited. Yutaka Seino also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly and Company, Taisho Toyama Pharmaceutical Co. Ltd., MSD K.K., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., and Arklay Co. Ltd. Ryota Usui, Yui Sakuramachi, Hitoshi Kuwata, Hisato Tatsuoka, Kenta Murotani and Yusuke Seino declare no conflict of interest.
Supporting information
Figure S1 ¦ Comparison of C‐peptide index (CPI) between patients with glycated hemoglobin <7.0% and those with glycated hemoglobin ≥7.0% at 54 weeks in 49 patients, including 12 who were originally excluded due to receiving additional oral anti‐diabetic drugs or bolus insulin.
Figure S2 ¦ Comparison of C‐peptide index (CPI) by glycated hemoglobin (HbA1c) at 54‐week tertiles.
Acknowledgments
The authors are grateful to H Abe of Kansai Electric Power Medical Research Institute and M Yamane of Kansai Electric Power Hospital for secretarial assistance. This study was funded by Grant‐in‐Aids for Scientific Research (C) from the Japan Society for Promotion of Science, Grants for Young Researchers from the Japan Association for Diabetes Education and Care, and a grant from the Japan Vascular Disease Research Foundation.
J Diabetes Investig 2018;9:822–830
References
- 1. U.K. prospective diabetes study 16 . Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 2. Yabe D, Seino Y. Defining the role of GLP‐1 receptor agonists for individualized treatment of type 2 diabetes. Expert Rev Endocrinol Metab 2014; 9: 659–670. [DOI] [PubMed] [Google Scholar]
- 3. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 4. Iwao T, Sakai K, Sata M. Postprandial serum C‐peptide is a useful parameter in the prediction of successful switching to liraglutide monotherapy from complex insulin therapy in Japanese patients with type 2 diabetes. J Diabetes Complications 2013; 27: 87–91. [DOI] [PubMed] [Google Scholar]
- 5. Kozawa J, Inoue K, Iwamoto R, et al Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin‐secreting capacity. J Diabetes Investig 2012; 3: 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Usui R, Yabe D, Kuwata H, et al Retrospective analysis of safety and efficacy of insulin‐to‐liraglutide switch in Japanese type 2 diabetes: a caution against inappropriate use in patients with reduced beta‐cell function. J Diabetes Investig. 2013; 4: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Usui R, Yabe D, Kuwata H, et al Retrospective analysis of safety and efficacy of liraglutide monotherapy and sulfonylurea‐combination therapy in Japanese type 2 diabetes: association of remaining beta‐cell function and achievement of HbA1c target one year after initiation. J Diabetes Complications 2015; 29: 1203–1210. [DOI] [PubMed] [Google Scholar]
- 8. Meier JJ, Vilsbøll T, Donsmark M, et al Greater glycaemic control is achieved with the once‐daily human GLP‐1 analogue liraglutide vs comparators across the continuum of estimated beta cell mass. Diabetologia 2011; 1: S322. [Google Scholar]
- 9. Eng C, Kramer CK, Zinman B, et al Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet 2014; 384: 2228–2234. [DOI] [PubMed] [Google Scholar]
- 10. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabet Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okuno Y, Komada H, Sakaguchi K, et al Postprandial serum C‐peptide to plasma glucose concentration ratio correlates with oral glucose tolerance test‐ and glucose clamp‐based disposition indexes. Metabolism 2013; 62: 1470–1476. [DOI] [PubMed] [Google Scholar]
- 12. Okuno Y, Sakaguchi K, Komada H, et al Correlation of serum CPR to plasma glucose ratio with various indices of insulin secretion and diseases duration in type 2 diabetes. Kobe J Med Sci 2013; 59: E44–E53. [PubMed] [Google Scholar]
- 13. Iwata M, Matsushita Y, Fukuda K, et al Secretory units of islets in transplantation index is a useful predictor of insulin requirement in Japanese type 2 diabetic patients. J Diabetes Investig 2014; 5: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saisho Y. Postprandial C‐peptide to glucose ratio as a marker of beta cell function: implication for the management of Type 2 diabetes. Int J Mol Sci 2016; 17: 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yabe D, Eto T, Shiramoto M, et al Effects of DPP‐4 inhibitor linagliptin and GLP‐1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open‐label, 2‐arm parallel comparative, exploratory trial. Diabetes Obes Metab 2017; 19: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondo Y, Satoh S, Nagakura J, et al Defining criteria for the introduction of liraglutide using the glucagon stimulation test in patients with type 2 diabetes. J Diabetes Investig 2013; 4: 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maiorino MI, Chiodini P, Bellastella G, et al Insulin and glucagon‐like peptide 1 receptor agonist combination therapy in Type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Care 2017; 40: 614–624. [DOI] [PubMed] [Google Scholar]
- 18. Seino Y, Kaneko S, Fukuda S, et al Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig. 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seino Y, Min KW, Niemoeller E, et al Investigators EG‐LAS. randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gough SC, Bode BW, Woo VC, et al One‐year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26‐week extension to a 26‐week main trial. Diabetes Obes Metab 2015; 17: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gough SC, Bode B, Woo V, et al Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014; 2: 885–893. [DOI] [PubMed] [Google Scholar]
- 22. Report Committee . Glycemic targets for elderly patients with diabetes: Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. J Diabetes Investig. 2017; 8: 126–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Funakoshi S, Fujimoto S, Hamasaki A, et al Utility of indices using C‐peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patientes with type 2 diaebtes. J Diabetes Investig 2011; 2: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Comparison of C‐peptide index (CPI) between patients with glycated hemoglobin <7.0% and those with glycated hemoglobin ≥7.0% at 54 weeks in 49 patients, including 12 who were originally excluded due to receiving additional oral anti‐diabetic drugs or bolus insulin.
Figure S2 ¦ Comparison of C‐peptide index (CPI) by glycated hemoglobin (HbA1c) at 54‐week tertiles.


