Abstract
Aims/Introduction
A prolonged QT interval plays a causal role in life‐threatening arrhythmia, and becomes a risk factor for sudden cardiac death. Here, we assessed the association between microvascular complications and the QT interval in patients with type 2 diabetes.
Materials and Methods
Patients with type 2 diabetes (n = 219) admitted to Nippon Medical School Hospital (Tokyo, Japan) for glycemic control were enrolled. QT interval was measured manually in lead II on the electrocardiogram, and corrected for heart rate using Bazett's formula (QTc). Diabetic neuropathy, retinopathy and nephropathy were assessed by neuropathic symptoms or Achilles tendon reflex, ophthalmoscopy and urinary albumin excretion, respectively.
Results
In univariate analyses, female sex (P = 0.025), duration of type 2 diabetes (P = 0.041), body mass index (P = 0.0008), systolic blood pressure (P = 0.0011) and receiving insulin therapy (P < 0.0001) were positively associated with QTc. Patients with each of the three microvascular complications had longer QTc than those without: neuropathy (P = 0.0005), retinopathy (P = 0.0019) and nephropathy (P = 0.0001). As retinopathy or nephropathy progressed, QTc became longer (P < 0.001 and P < 0.001 for trend in retinopathy and nephropathy, respectively). Furthermore, QTc was prolonged with the multiplicity of the microvascular complications (P < 0.001 for trend). Multiple regression analyses showed that neuropathy, nephropathy and the multiplicity of the microvascular complications were independently associated with QTc.
Conclusions
Patients with type 2 diabetes with severe microvascular complications might be at high risk for life‐threatening arrhythmia associated with QT interval prolongation.
Keywords: Microvascular complications, QT interval, Type 2 diabetes mellitus
Introduction
Type 2 diabetes is associated with an increased risk of mortality, not only from cardiovascular diseases, but also from sudden cardiac death1, 2, 3. Sudden cardiac death is caused by abnormalities in coronary arteries, myocardium and electrical propagation in the heart4. In electrical propagation, QT interval, the time elapsed between the ventricular depolarization and repolarization, is a vulnerable period for the occurrence of arrhythmias. A prolonged QT interval plays a causal role in life‐threatening arrhythmia, such as torsades de pointes type ventricular tachycardia, and increases a risk of sudden cardiac death5, 6. Individuals with diabetes have been reported to have a longer QT interval than those without7, 8, 9, 10. Recent studies have reported that prolonged QT interval is an independent predictor of all‐cause and cardiovascular mortalities in type 2 diabetes11, 12. Thus, it is of great importance to clarify the patient characteristics that influence their QT interval for reducing mortality in type 2 diabetes.
Both macrovascular and microvascular complications are reported to be independent risk factors for sudden cardiac death in diabetes3, 13. Indeed, previous studies have shown that diabetes patients with macrovascular14, 15 or microvascular complications (neuropathy16, retinopathy16, 17, 18 and nephropathy17, 18) have longer QT intervals than those without these complications. Needless to say, there is a wide variety in the severity and multiplicity of complications among patients with diabetes. However, these previous studies have compared QT intervals only between the presence and absence of each complication; they have not taken the severity and multiplicity into account. Thus, the impact of the compound progression of the complications on QT interval has not been fully elucidated. In the present study, we investigated the relationships between the clinical characteristics, especially the severity and multiplicity of microvascular complications, and the QT interval in patients with type 2 diabetes.
Methods
Participants
Patients with type 2 diabetes who were admitted to Nippon Medical School Hospital (Tokyo, Japan) for glycemic control were enrolled (n = 219, 68 women and 151 men). Exclusion criteria included atrial fibrillation, major ventricular conduction defects (defined as long QRS duration >120 ms), uncontrolled endocrine disease, receiving steroid therapy and ketoacidosis. We also excluded the patients taking medications that can affect the QT interval, such as anti‐arrhythmic agents, antipsychotic agents, tricyclic antidepressants, tetracyclic antidepressants, α‐blockers, β‐blockers, probucol, digoxin, antifungal agents and antibacterial agents. The study protocol was approved by the institutional review board and conformed to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). All participants provided informed consent before enrollment.
Clinical measurements
All participants underwent physical examinations including height, bodyweight and blood pressure on the first morning of hospitalization. Blood samples were taken after an overnight fast on the second day of admission. Fasting plasma glucose was measured by the glucose oxidase method (ADAMS Glucose GA‐1170; Arkray, Kyoto, Japan). Glycated hemoglobin was measured by high‐performance liquid chromatography (ADAMS A1c HA‐8160; Arkray), and is expressed as the percentage value of the National Glycohemoglobin Standardization Program according to the Japan Diabetes Society's guideline19. Serum total cholesterol, high‐density lipoprotein cholesterol and triacylglycerols were measured enzymatically (Sekisui Medical, Tokyo, Japan). Low‐density lipoprotein cholesterol was calculated using the Friedewald formula20. Serum calcium level was corrected for the concentration of albumin using the following formula if serum albumin level was <4.0 g/dL: corrected Ca (mg/dL) = Ca (mg/dL) + (4.0 – albumin [g/dL])21. Estimated glomerular filtration rate was calculated using the following formula, where Cr is creatinine: estimated glomerular filtration rate (mL/min/1.73 m2) = 194 × Cr−1.094 × age−0.287 (×0.742 if female)22.
QT interval
The 12‐lead electrocardiogram was recorded on the day of admission using an electrocardiograph (ECG‐1550; Nihon Kohden Co. Ltd, Tokyo, Japan). QT and RR intervals of three consecutive beats were measured manually on lead II. The QT interval was defined as the length from the beginning of QRS complex to the end of the downslope of the T wave. A corrected QT interval (QTc) was calculated according to Bazett's formula23: QTc = QT / (RR)1/2. The QTc >440 ms was considered prolonged14, 17, 24.
Assessment of microvascular complications
Neuropathy was screened with criteria for typical diabetic peripheral neuropathy proposed by the American Diabetes Association in 201025. The presence of typical diabetic peripheral neuropathic symptoms (decreased sensation, positive neuropathic sensory symptoms [e.g., numbness, prickling or stabbing, burning, or aching pain] in lower legs) or abnormal (decreased or absent) Achilles tendon reflex was diagnosed as neuropathy (including possible neuropathy). Retinopathy was diagnosed using ophthalmoscopy by an ophthalmologist. We categorized the severity of retinopathy as simple, preproliferative and proliferative retinopathy according to the Davis’ criteria26. Nephropathy was diagnosed by the presence of albuminuria (urinary albumin exertion ≥30 mg/g·Cr [spot]). Normo‐, micro‐ and macroalbuminuria were defined as urinary albumin excretion of <30, 30–300 and ≥300 mg/g·Cr (spot), respectively27. The multiplicity of microvascular complications was scored from 0 to 3 by the number of the complications (neuropathy, retinopathy and nephropathy) being diagnosed.
Statistical analysis
Continuous variables were described as the mean ± standard deviation or median and interquartile range. Categorical variables were expressed as the number and percentage. Differences in two independent groups were analyzed by Student's t‐test or the Mann–Whitney U‐test. Corrections for multiple comparisons were carried out using Dunnett's test. The Jonckheere–Terpstra test was carried out for the trend test. Correlations between QTc and other continuous variables were examined using Pearson's correlation test. Multivariate regression models were used to determine statistically significant predictive factors (neuropathy [model 1], retinopathy [model 2], nephropathy [model 3] and the number of microvascular complications [model 4]) for QTc. Each model was adjusted for age, sex, duration of type 2 diabetes, body mass index, systolic blood pressure and insulin therapy. A P‐value of <0.05 was considered significant. All analyses were carried out using JMP 11.2 software (SAS Institute Inc., Cary, North Carolina, USA) or SPSS 21.0 (IBM Co. Ltd., Armonk, New York, USA).
Results
Relationships between clinical characteristics and QTc
Table 1 shows the clinical characteristics of the patients, and the associations between the variables and QTc. The QTc of all participants was 430 ± 22 ms, and 35% of them (n = 76) had prolonged QTc. Significant differences were observed in QTc between women and men (436 ± 18 vs 427 ± 23 ms), and between the patients treated with insulin and those without (443 ± 21 vs 427 ± 21 ms). In addition, QTc was positively correlated with duration of type 2 diabetes, body mass index and systolic blood pressure. The patients with each microvascular complication had significantly longer QTc than those without (neuropathy [435 ± 20 vs 424 ± 22 ms], retinopathy [438 ± 21 vs 427 ± 21 ms] and nephropathy [436 ± 24 vs 425 ± 19 ms]).
Table 1.
Clinical characteristics and the association with corrected QT interval
| Variables | n = 219 | r | P‐value |
|---|---|---|---|
| Age (years) | 60 ± 13 | 0.0068 | 0.92 |
| Women, n (%) | 68 (31) | – | 0.025 |
| Duration of type 2 diabetes (years) | 6 (1–15) | 0.14 | 0.041 |
| BMI (kg/m2) | 25.0 ± 4.7 | 0.23 | 0.0008 |
| Systolic blood pressure (mmHg) | 126 ± 15 | 0.23 | 0.0011 |
| Diastolic blood pressure (mmHg) | 70 ± 11 | 0.080 | 0.24 |
| Fasting plasma glucose (mmol/L) | 9.79 ± 2.99 | 0.092 | 0.17 |
| HbA1c (%) | 9.5 ± 2.2 | 0.012 | 0.86 |
| Total cholesterol (mmol/L) | 5.09 ± 1.21 | 0.039 | 0.57 |
| HDL cholesterol (mmol/L) | 1.22 ± 0.32 | −0.083 | 0.22 |
| LDL cholesterol (mmol/L) | 3.08 ± 0.96 | 0.021 | 0.76 |
| Triacylglycerols (mmol/L) | 1.76 ± 1.30 | 0.11 | 0.10 |
| Serum sodium (mEq/L) | 139 ± 2 | 0.00070 | 0.99 |
| Serum potassium (mEq/L) | 4.1 ± 0.4 | −0.095 | 0.16 |
| Serum chlorine (mEq/L) | 104 ± 3 | −0.035 | 0.61 |
| Corrected serum calcium (mg/dL) | 9.2 ± 0.4 | −0.028 | 0.68 |
| eGFR (mL/min/1.73 m2) | 86.9 ± 26.5 | −0.043 | 0.52 |
| Microvascular complications | |||
| Neuropathy, n (%) | 108 (49) | – | 0.0005 |
| Retinopathy, n (%) | 50 (23) | – | 0.0019 |
| Nephropathy, n (%) | 86 (39) | – | 0.0001 |
| History of ischemic heart disease | 14 (6) | – | 0.088 |
| Insulin therapy, n (%) | 35 (16) | – | <0.0001 |
| QTc (ms) | 430 ± 22 | – | – |
Continuous variables are expressed as mean ± standard deviation or median (interquartile range). Correlations between continuous variables and corrected QT interval (QTc) were examined by Pearson's correlation test. Differences in two independent groups were analyzed by Student's t‐test or Mann–Whitney U‐test. BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low density lipoprotein; QTc, corrected QT interval.
Severity and multiplicity of microvascular complications and QTc
The QTc of the patients categorized by the severity of retinopathy and nephropathy are shown in Table 2. The patients with proliferative retinopathy had significantly longer QTc as compared with those without. In addition, as the severity of retinopathy progressed, QTc became longer. For nephropathy, the patients with microalbuminuria or macroalbuminuria had significantly longer QTc as compared with those with normoalbuminuria, and QTc was prolonged as the urinary albumin excretion increased. Furthermore, stepwise prolongation of QTc was observed with the multiplicity of the microvascular complications (Figure 1). Table 3 shows the series of multiple regression models of each microvascular complication and the multiplicity for predicting QTc. These analyses showed that neuropathy, nephropathy (the presence of albuminuria) and the multiplicity of microvascular complications were independently associated with QTc (models 1, 3 and 4).
Table 2.
Corrected QT interval according to the severity of retinopathy and nephropathy
| Microvascular complications | n | QTc (ms) | P‐value |
|---|---|---|---|
| Retinopathy | |||
| None | 169 | 427 ± 21 | ― |
| Simple retinopathy | 19 | 432 ± 22 | 0.78† |
| Preproliferative retinopathy | 14 | 441 ± 17 | 0.078† |
| Proliferative retinopathy | 17 | 443 ± 23 | 0.010† |
| Trend | <0.001‡ | ||
| Nephropathy | |||
| Normoalbuminuria | 133 | 425 ± 19 | – |
| Microalbuminuria | 60 | 435 ± 23 | 0.010§ |
| Macroalbuminuria | 26 | 442 ± 24 | 0.0005§ |
| Trend | <0.001‡ | ||
Corrected QT interval (QTc) values are expressed as mean ± standard deviation. † P‐values for Dunnett's test vs none. ‡ P‐values for the Jonckheere–Terpstra test. § P‐values for Dunnett's test vs normoalbuminuria.
Figure 1.
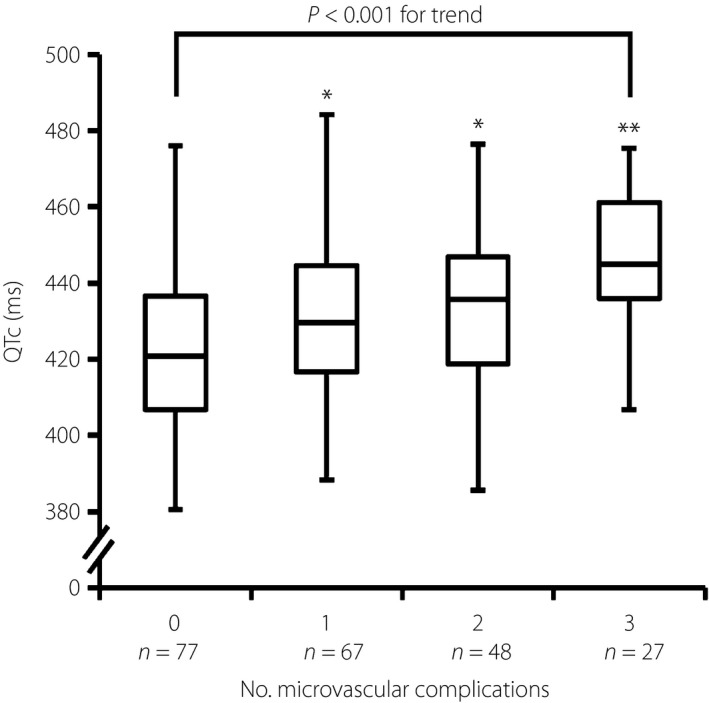
Multiplicity of microvascular complications and corrected QT interval (QTc). Distribution of the QTc according to the multiplicity of microvascular complications (diabetic neuropathy, retinopathy and nephropathy). The boxes indicate the central rectangular spans from the first quartile to the third quartile (interquartile range). A segment inside the rectangle shows the median. The whiskers display the lowest datum within 1.5 interquartile range of the lower quartile and the highest datum within 1.5 interquartile range of the upper quartile. *P < 0.05 and **P < 0.01 for Dunnett's test vs patients without microvascular complications (0).
Table 3.
Multiple linear regression models for corrected QT interval
| Model | Variables | β coefficient | t‐value | P‐value |
|---|---|---|---|---|
| 1 | Neuropathy | 3.06 | 2.14 | 0.034 |
| 2 | Retinopathy | 1.90 | 1.03 | 0.30 |
| 3 | Nephropathy | 4.63 | 3.16 | 0.0018 |
| 4 | Multiplicity of microvascular complications | 4.60 | 3.10 | 0.0022 |
Data are expressed as β coefficient (estimated partial regression coefficient), t and P values for the association of corrected QT interval with microvascular complications (neuropathy [model 1], retinopathy [model 2], nephropathy [model 3] and multiplicity of microvascular complications [model 4]) and clinical covariates (age, sex, duration of diabetes, body mass index, systolic blood pressure and insulin therapy).
Discussion
Considering that individuals with prolonged QT interval are at high risk of sudden cardiac death5, 6, we should pay more attention to the QT interval in patients with type 2 diabetes. To date, several reports have pointed out the associations between diabetic microvascular complications and QT interval prolongation16, 17, 18. However, the severity and multiplicity of microvascular complications have not been taken into account in these studies. In the present study, we showed that the severity of retinopathy and nephropathy was associated with QTc prolongation in patients with type 2 diabetes. Furthermore, the multiplicity of microvascular complications was also associated with the QTc prolongation. In multiple regression analyses, the presence of neuropathy, nephropathy and the multiplicity of the microvascular complications were independently associated with the QTc prolongation.
There are several potential mechanisms underlying the relationship between diabetic microvascular complications and QT interval prolongation. Several reports have suggested that microischemia, as a result of a small infarction in the myocardium, can prolong the QT interval28, 29. Indeed, several markers of early atherosclerosis (e.g., pulse wave velocity and carotid intima‐media thickness) have been reported to be positively associated with QT interval in patients with type 2 diabetes30, 31. Thus, it is highly possible that not only microvascular complications, but also QT interval prolongation, can be induced by the development of atherosclerosis. In the present study, however, no significant association was found between the history of ischemic heart disease and QTc in univariate analysis. This would be partly because of the small number of patients who had a history of ischemic heart disease (14 patients). Another possible reason for QT interval prolongation in patients with diabetes is the functional modulation of ion channels in myocardium. Hyperglycemia‐induced nitric oxide reduction can cause the inhibition of Ca2+‐adenosine triphosphatase and K+/Na+‐adenosine triphosphatase activities in cardiomyocytes, leading to an increase of cytosolic free calcium and subsequent prolongation of myocardial repolarization32. Such an impaired nitric oxide production, which can lead to endothelial dysfunction, oxidative stress and chronic inflammation in peripheral arteries, is also regarded as a cause of microvascular complications33. Overall, these findings strongly suggest that diabetic microvascular complications and QT interval prolongation share common etiologies (i.e., atherosclerosis and impaired nitric oxide production in type 2 diabetes).
In clinical practice, how to manage QT interval in patients with type 2 diabetes should be considered. Some medications, including specific anti‐arrhythmics, antipsychotics and antidepressants, are known to prolong QT interval34. Thus, we should monitor QT intervals carefully in patients who receive these medications. In contrast, therapeutic interventions for hypertension and obesity might have favorable effects on QT interval. Previous studies reported that both angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker shortened the QT interval in patients with hypertension35, 36. Another study reported an improved QT interval in obese individuals associated with weight reduction37. In addition, amelioration of glycemic control might decrease the QT interval. Although neither fasting plasma glucose nor glycated hemoglobin was associated with QTc in the present study, several previous reports showed that postprandial hyperglycemia17, 32 or acute hyperglycemia (induced by hyperglycemic clamp)38 prolonged the QT interval. In the present study, patients who received insulin therapy had a longer QTc than those who did not. A previous study also reported that QT interval was prolonged by insulin therapy in patients with type 2 diabetes39. Gastaldelli et al.40 suggested that insulin‐induced modifications in sympathetic activity and serum potassium concentration cause QT interval prolongation. Some reports also showed prolonged QT interval during insulin‐induced hypoglycemia41, 42.
The present study had several limitations. First, as this was a cross‐sectional study, we could not determine causal relationships between diabetic microvascular complications and QT interval prolongation. Second, the present study included only hospitalized patients with poor glycemic control. The results might therefore be not representative of all patients with type 2 diabetes. Third, atypical diabetic peripheral neuropathies, for example, painful and autonomic neuropathies, were not screened in the present study, because diagnostic criteria for these neuropathies have not been settled yet25. However, several reports have suggested that cardiac autonomic neuropathy affects the QT interval in patients with type 2 diabetes43, 44. As the heart rate variability test has been suggested to be useful for the assessment of cardiac autonomic neuropathy25, we also evaluated the coefficient of variation of R‐R intervals in 211 of 219 participants. The coefficient of variation of R‐R interval was actually associated with QTc negatively and independently in univariate analysis (r = −0.24, P = 0.0004) and multiple regression analysis (P = 0.0007), respectively. These results show that QTc is strongly associated with cardiac autonomic neuropathy, as suggested in the previous studies. Therefore, if atypical diabetic peripheral neuropathies were included in neuropathy, the association between neuropathy and QTc would become stronger.
In conclusion, the present study clearly showed the associations of the severity and multiplicity of diabetic microvascular complications with QT interval prolongation. Furthermore, neuropathy, nephropathy and the multiplicity of microvascular complications were independently associated with the QT interval. These results suggest that patients with type 2 diabetes with severe microvascular complications are at high risk for life‐threatening arrhythmia associated with prolonged QT interval.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
All the authors of this manuscript contributed significantly to this work.
J Diabetes Investig 2018;9:946–951
References
- 1. Balkau B, Jouven X, Ducimetière P, et al Diabetes as a risk factor for sudden death. Lancet 1999; 354: 1968–1969. [DOI] [PubMed] [Google Scholar]
- 2. Curb JD, Rodriguez BL, Burchfiel CM, et al Sudden death, impaired glucose tolerance, and diabetes in Japanese American men. Circulation 1995; 91: 2591–2595. [DOI] [PubMed] [Google Scholar]
- 3. Jouven X, Lemaître RN, Rea TD, et al Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J 2005; 26: 2142–2147. [DOI] [PubMed] [Google Scholar]
- 4. Siscovick DS, Sotoodehnia N, Rea TD, et al Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord 2010; 11: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bednar MM, Harrigan EP, Anziano RJ, et al The QT interval. Prog Cardiovasc Dis 2001; 43: 1–45. [DOI] [PubMed] [Google Scholar]
- 7. Sivieri R, Veglio M, Chinaglia A, et al Prevalence of QT prolongation in a type 1 diabetic population and its association with autonomic neuropathy. The Neuropathy Study Group of the Italian Society for the Study of Diabetes. Diabet Med 1993; 10: 920–924. [DOI] [PubMed] [Google Scholar]
- 8. Pickham D, Helfenbein E, Shinn JA, et al High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med 2012; 40: 394–399. [DOI] [PubMed] [Google Scholar]
- 9. Festa A, D'Agostino R Jr, Rautaharju P, et al Relation of systemic blood pressure, left ventricular mass, insulin sensitivity, and coronary artery disease to QT interval duration in nondiabetic and type 2 diabetic subjects. Am J Cardiol 2000; 86: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 10. Sohaib SM, Papacosta O, Morris RW, et al Length of the QT interval: determinants and prognostic implications in a population‐based prospective study of older men. J Electrocardiol 2008; 41: 704–710. [DOI] [PubMed] [Google Scholar]
- 11. Okin PM, Devereux RB, Lee ET, et al Electrocardiographic repolarization complexity and abnormality predict all‐cause and cardiovascular mortality in diabetes: the Strong Heart Study. Diabetes 2004; 53: 434–440. [DOI] [PubMed] [Google Scholar]
- 12. Cox AJ, Azeem A, Yeboah J, et al Heart rate‐corrected QT interval is an independent predictor of all‐cause and cardiovascular mortality in individuals with type 2 diabetes: the Diabetes Heart Study. Diabetes Care 2014; 37: 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeung CY, Lam KS, Li SW, et al Sudden cardiac death after myocardial infarction in type 2 diabetic patients with no residual myocardial ischemia. Diabetes Care 2012; 35: 2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veglio M, Borra M, Stevens LK, et al The relation between QTc interval prolongation and diabetic complications. Diabetologia 1999; 42: 68–75. [DOI] [PubMed] [Google Scholar]
- 15. Veglio M, Bruno G, Borra M, et al Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population‐based cohort. J Intern Med 2002; 251: 317–324. [DOI] [PubMed] [Google Scholar]
- 16. Subbalakshmi NK, Adhikari PM, Sathyanarayana Rao KN, et al Influencing factors of QTc among the clinical characteristics in type 2 diabetes mellitus. Diabetes Res Clin Pract 2010; 88: 265–272. [DOI] [PubMed] [Google Scholar]
- 17. Li X, Ren H, Xu ZR, et al Prevalence and risk factors of prolonged QTc interval among Chinese patients with type 2 diabetes. Exp Diabetes Res 2012; 2012: 234084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christensen PK, Gall MA, Major‐Pedersen A, et al QTc interval length and QT dispersion as predictors of mortality in patients with non‐insulin‐dependent diabetes. Scand J Clin Lab Invest 2000; 60: 323–332. [DOI] [PubMed] [Google Scholar]
- 19. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 21. Payne RB, Little AJ, Williams RB, et al Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 1973; 4: 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 23. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920; 7: 353–370. [Google Scholar]
- 24. Maebuchi D, Arima H, Doi Y, et al QT interval prolongation and the risks of stroke and coronary heart disease in a general Japanese population: the Hisayama study. Hypertens Res 2010; 33: 916–921. [DOI] [PubMed] [Google Scholar]
- 25. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis MD, Kern TS, Rand LI. Diabetic retinopathy In: Alberti KGMM, Zimmet P, DeFronzo RA, Keen H. (eds). International Textbook of Diabetes Mellitus, 2nd edn Chichester, UK: Wiley, 1997; 1413–1446. [Google Scholar]
- 27. Inomat S, Haneda M, Moriya T, et al Revised criteria for the early diagnosis of diabetic nephropathy. Nihon Jinzo Gakkai Shi 2005; 47: 767–769 (Japanese). [PubMed] [Google Scholar]
- 28. Rana BS, Band MM, Ogston S, et al Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am J Cardiol 2002; 90: 483–487. [DOI] [PubMed] [Google Scholar]
- 29. Tomassoni G, Pisanó E, Gardner L, et al QT prolongation and dispersion in myocardial ischemia and infarction. J Electrocardiol 1998; 30(Suppl): 187–190. [DOI] [PubMed] [Google Scholar]
- 30. Takebayashi K, Aso Y, Matsutomo R, et al Association between the corrected QT intervals and combined intimal‐medial thickness of the carotid artery in patients with type 2 diabetes. Metabolism 2004; 53: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto Y, Tanaka M, Senmaru T, et al The QT interval could be a marker of subclinical atherosclerosis in patients with type 2 diabetes. J Diabetes Metab 2013; 4: 302. [Google Scholar]
- 32. Fiorentini A, Perciaccante A, Valente R, et al The correlation among QTc interval, hyperglycaemia and the impaired autonomic activity. Auton Neurosci 2010; 154: 94–98. [DOI] [PubMed] [Google Scholar]
- 33. Brownlee M. The pathobiology of diabetic complications. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 34. Al‐Khatib SM, LaPointe NM, Kramer JM, et al What clinicians should know about the QT interval. JAMA 2003; 289: 2120–2127. [DOI] [PubMed] [Google Scholar]
- 35. González‐Juanatey JR, García‐Acuña JM, Pose A, et al Reduction of QT and QTc dispersion during long‐term treatment of systemic hypertension with enalapril. Am J Cardiol 1998; 81: 170–174. [DOI] [PubMed] [Google Scholar]
- 36. Porthan K, Viitasalo M, Hiltunen TP, et al Short‐term electrophysiological effects of losartan, bisoprolol, amlodipine, and hydrochlorothiazide in hypertensive men. Ann Med 2009; 41: 29–37. [DOI] [PubMed] [Google Scholar]
- 37. Omran J, Firwana B, Koerber S, et al Effect of obesity and weight loss on ventricular repolarization: a systematic review and meta‐analysis. Obes Rev 2016; 17: 520–530. [DOI] [PubMed] [Google Scholar]
- 38. Santini V, Ciampittiello G, Gigli F, et al QTc and autonomic neuropathy in diabetes: effects of acute hyperglycaemia and n‐3 PUFA. Nutr Metab Cardiovasc Dis 2007; 17: 712–718. [DOI] [PubMed] [Google Scholar]
- 39. Takebayashi K, Naruse R, Morita K, et al The effect of insulin therapy and plasma glucose levels on corrected QT intervals in patients with type 2 diabetes. J Clin Med Res 2012; 4: 1–5.22383920 [Google Scholar]
- 40. Gastaldelli A, Emdin M, Conforti F, et al Insulin prolongs the QTc interval in humans. Am J Physiol Regul Integr Comp Physiol 2000; 279: 2022–2025. [DOI] [PubMed] [Google Scholar]
- 41. Chow E, Bernjak A, Williams S, et al Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014; 63: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 42. Kacheva S, Karges B, Göller K, et al QT prolongation caused by insulin‐induced hypoglycaemia ‐ An interventional study in 119 individuals. Diabetes Res Clin Pract 2017; 123: 165–172. [DOI] [PubMed] [Google Scholar]
- 43. Kahn JK, Sisson JC, Vinik AI. QT interval prolongation and sudden cardiac death in diabetic autonomic neuropathy. J Clin Endocrinol Metab 1987; 64: 751–754. [DOI] [PubMed] [Google Scholar]
- 44. Pappachan JM, Sebastian J, Bino BC, et al Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J 2008; 84: 205–210. [DOI] [PubMed] [Google Scholar]


