Abstract
Background and Purpose
Sperm from many species share the sperm‐specific Ca2+ channel CatSper that controls the intracellular Ca2+ concentration and, thereby, the swimming behaviour. A growing body of evidence suggests that the mechanisms controlling the activity of CatSper and its role during fertilization differ among species. A lack of suitable pharmacological tools has hampered the elucidation of the function of CatSper. Known inhibitors of CatSper exhibit considerable side effects and also inhibit Slo3, the principal K+ channel of mammalian sperm. The compound RU1968 was reported to suppress Ca2+ signaling in human sperm by an unknown mechanism. Here, we examined the action of RU1968 on CatSper in sperm from humans, mice, and sea urchins.
Experimental Approach
We resynthesized RU1968 and studied its action on sperm from humans, mice, and the sea urchin Arbacia punctulata by Ca2+ fluorimetry, single‐cell Ca2+ imaging, electrophysiology, opto‐chemistry, and motility analysis.
Key Results
RU1968 inhibited CatSper in sperm from invertebrates and mammals. The compound lacked toxic side effects in human sperm, did not affect mouse Slo3, and inhibited human Slo3 with about 15‐fold lower potency than CatSper. Moreover, in human sperm, RU1968 mimicked CatSper dysfunction and suppressed motility responses evoked by progesterone, an oviductal steroid known to activate CatSper. Finally, RU1968 abolished CatSper‐mediated chemotactic navigation in sea urchin sperm.
Conclusion and Implications
We propose RU1968 as a novel tool to elucidate the function of CatSper channels in sperm across species.
Abbreviations
- 2‐AG
2‐arachidonoylglycerol
- ABHD2
α/β hydrolase domain‐containing protein 2
- ASW
artificial sea water
- [Ca2+]i
intracellular Ca2+ concentration
- CASA
computer‐assisted sperm analysis
- HSA
human serum albumin
- HTF
human tubal fluid
- MES
2‐(N‐Morpholino)ethanesulfonic acid
- pHi
intracellular pH
- sEBSS
supplemented Earle's balanced salt solution
- TAPS
N‐[Tris(hydroxymethyl)methyl]‐3‐aminopropanesulfonic acid
- TYH
Toyoda, Yokoyama and Hosi's
- VAP
velocity average path
- Vm
membrane potential
Introduction
The intracellular Ca2+ concentration ([Ca2+]i) modulates the beat of the sperm flagellum and, thereby, the swimming behaviour (Publicover et al., 2008; Alvarez et al., 2014). In many but not all species, [Ca2+]i is controlled by the sperm‐specific Ca2+ channel http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=70 (cation channel of sperm) (Quill et al., 2001; Ren et al., 2001, Kirichok et al., 2006; Lishko et al., 2010; Loux et al., 2013; Seifert et al., 2015). CatSper appeared early in evolution, before the branching of eukaryotes into unikonts and bikonts (Cai and Clapham, 2008; Cai et al., 2014; Chung et al., 2017). So far, most of our knowledge about CatSper originates from physiological studies of native channels in mammalian and sea urchin sperm. In general, CatSper is activated by depolarization of the membrane potential (Vm) and by alkalization of the intracellular pH (pHi) (Kirichok et al., 2006; Lishko et al., 2010; Lishko et al., 2011; Strünker et al., 2011; Seifert et al., 2015). In sea urchin sperm, the egg's chemoattractant evokes rapid changes in Vm and pHi, stimulating Ca2+ influx via CatSper (Seifert et al., 2015, Espinal‐Enríquez et al., 2017), which controls chemotactic steering. Targeted ablation of genes encoding CatSper channel subunits provided insight into the function of CatSper in mouse sperm (Quill et al., 2001; Ren et al., 2001; Carlson et al., 2003; Carlson et al., 2005; Liu et al., 2007; Qi et al., 2007; Wang et al., 2009; Chung et al., 2011; Zeng et al., 2013; Chung et al., 2017). For instance, sperm from CatSper−/− mice suffer from impaired motility (Ren et al., 2001; Qi et al., 2007; Miki and Clapham, 2013), fail to traverse the oviduct (Ho et al., 2009; Miki and Clapham, 2013; Chung et al., 2014), and are unable to penetrate the egg coat (Ren et al., 2001), resulting in male infertility (Qi et al., 2007, Quill et al., 2001, Ren et al., 2001). CatSper is essential for fertilization also in humans. Mutations in CATSPER genes (Avenarius et al., 2009; Hildebrand et al., 2010) and CatSper dysfunction (Williams et al., 2015) are associated with male infertility. However, mouse and human CatSper channels have distinct properties, indicating that the channel might serve different functions (Kaupp and Strünker, 2016; Alvarez, 2017). For example, in human but not in mouse sperm, CatSper serves as a polymodal sensor that integrates a range of different chemical cues (Brenker et al., 2012; Schiffer et al., 2014; Brenker et al., 2018): human CatSper is activated by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2377 and prostaglandins (PGs) (Lishko et al., 2011; Strünker et al., 2011; Brenker et al., 2012), hormones present in the oviductal fluid (Schuetz and Dubin, 1981). The ensuing Ca2+ influx controls the swimming behaviour and promotes the penetration of the egg coat (Schaefer et al., 1998; Harper et al., 2003; Oren‐Benaroya et al., 2008; Publicover et al., 2008; Baldi et al., 2009; Kilic et al., 2009; Alasmari et al., 2013a; Schiffer et al., 2014; Tamburrino et al., 2014; Tamburrino et al., 2015). Moreover, progesterone facilitates the migration of human sperm in viscous medium encountered by the sperm during their voyage across the female genital tract (Alasmari et al., 2013b). However, in humans, neither the role of CatSper nor that of progesterone and PGs during fertilization has been fully established. The function of CatSper in species other than sea urchin, mouse, and human is largely unknown. To address these questions, we rely on pharmacological tools that allow manipulating the function of CatSper.
Several drugs have been identified that suppress CatSper activity, for example, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4269 (Lishko et al., 2011, Strünker et al., 2011), http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2522 (Strünker et al., 2011), MDL12330A (Brenker et al., 2012) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4212 (Carlson et al., 2009). In patch‐clamp experiments, NNC‐0396, mibefradil, and MDL12330A abolish CatSper currents (Lishko et al., 2011, Strünker et al., 2011, Brenker et al., 2012); HC‐056456 attenuates CatSper currents (Carlson et al., 2009), but it is unknown whether the drug inhibits the channel completely. Of note, none of these drugs is selective for CatSper. The drugs also inhibit the sperm‐specific K+ channel http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=69 (Navarro et al., 2007; Carlson et al., 2009; Brenker et al., 2014; Mansell et al., 2014), the principal K+ channel in mouse (Santi et al., 2010; Zeng et al., 2011) and human sperm (Brenker et al., 2014). Notably, each drug inhibits CatSper and Slo3 with similar potency. Moreover, NNC‐0396, mibefradil, and MDL12330A exhibit serious adverse actions in human sperm. At the high micromolar concentrations required to abolish Ca2+ influx via CatSper channels, NNC‐0396 and mibefradil evoke a sizeable and sustained increase of [Ca2+]i and pHi (Strünker et al., 2011; Brenker et al., 2012; Chávez et al., 2017) and stimulate acrosomal exocytosis (Chávez et al., 2017; Supporting Information Figure S3). Similarly, MDL12330A at high micromolar concentrations also evokes a sustained [Ca2+]i increase in human sperm (Brenker et al., 2012). Finally, the drugs affect the vitality and overall motility of sperm (Tamburrino et al., 2014) (Supporting Information Figure S3). HC‐056456 has not been further characterized in human sperm, because it is not commercially available. In conclusion, novel potent and selective CatSper inhibitors without toxic side effects are required.
Before the identification of CatSper channels, the steroidal http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=785 ligand RU1968 was reported to suppress progesterone‐ and PG‐induced Ca2+ signals in human sperm (Schaefer et al., 2000). The mechanism of RU1968 action in sperm has remained unclear, except that it did not involve the activation of sigma receptors (Schaefer et al., 2000). We wondered whether RU1968 might inhibit CatSper and re‐examined the actions of this compound in sperm.
Here, we show that RU1968 potently abolishs Ca2+ signals mediated by CatSper in mouse, human, and sea urchin sperm. Patch‐clamp recordings from mouse and human sperm confirmed that RU1968 inhibits CatSper. This compound does not affect mouse Slo3 and inhibits human Slo3 channels with about 15‐fold lower potency than human CatSper. When present during the capacitation process, RU1968 suppressed hyperactivation in human sperm and inhibited progesterone‐evoked motility responses, showing that these effects involve Ca2+ influx via CatSper. Finally, we demonstrate that RU1968 abolishs chemotaxis of sea urchin sperm. In summary, RU1968 is a potent cross‐species inhibitor of CatSper channels that is selective for CatSper over Slo3. This compound seems well‐suited to study CatSper channel function in sperm from invertebrates to mammals.
Methods
Sperm preparation
The studies involving human sperm were performed in agreement with the standards set by the Declaration of Helsinki. Samples of human semen were obtained from volunteers with their prior written consent. We obtained approval from the institutional ethics committees of the medical association Westfalen‐Lippe and the Medical Faculty of the University of Münster (4INie) and from the ethical committee of the University of Birmingham Life and Health Sciences (ERN12‐0570R).
For Ca2+ fluorimetry in sperm populations, single‐cell Ca2+ imaging, patch‐clamp recordings, single‐cell motility studies and assays for acrosomal exocytosis and viability, sperm were purified by the swim‐up procedure in human tubal fluid (HTF) medium containing (in mM): 93.8 NaCl, 4.69 KCl, 0.2 MgSO4, 0.37 KH2PO4, 2.04 CaCl2, 0.33 Na‐pyruvate, 21.4 lactic acid, 2.78 glucose, 21 HEPES and 4 NaHCO3, pH 7.35 (adjusted with NaOH). Sperm were washed and re‐suspended in HTF containing 3 mg·mL−1 human serum albumin (HSA, Irvine Scientific, Santa Ana, CA, USA). For Kremer test, sperm were purified by the swim‐up procedure in Supplemented Earle's balanced salt solution (sEBSS), containing (in mM): 98.5 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5.5 glucose, 25 NaHCO3, 2.5 Na‐pyruvate, 19 Na‐lactate, 0.81 MgSO4, 15 HEPES and 0.3% BSA, pH 7.4 (adjusted with NaOH). Before experiments, sperm were incubated for at least 300 min at 37°C in a 5% CO2 atmosphere.
For computer‐assisted sperm analysis (CASA), sperm were purified by the swim‐up procedure in HTF lacking NaHCO3 and HSA. Sperm were washed and re‐suspended in this medium (non‐capacitating conditions) or in HTF fortified with 25 mM NaHCO3 and 3 mg·mL−1 HSA (capacitating conditions). Before experiments, sperm were incubated for at least 300 min at 37°C and 5% CO2 atmosphere.
All animal care and experimental procedures complied with the Guidelines set by the Animal Center of Nanchang University (Approval: SYXK2010‐0002) and of the German Animal Welfare Act and the district veterinary office under approval by the LANUV (AZ.02.05.50.16.011 and AZ.84‐02.04.2012.A192). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). C57BL/6N wild‐type (from Janvier, Le Genest‐Saint‐Isle, France) and C57BL/6N CatSper1−/− mice [a kind gift from Prof. David Clapham (Howard Hughes Medical Institute, Janelia Research Campus, USA; Ren et al. 2011)] were kept under specific pathogen‐free conditions and in ventilated cages (Greenline, Tecniplast). Maximally five mice were housed per cage.
Mouse epididymides were obtained from at least 15‐week‐old male mice that were anaesthetized with CO2 or isoflurane (Abbvie Deutschland, Ludwigshafen, Germany) and killed by cervical dislocation. For patch‐clamp recordings, sperm were isolated from the cauda epididymis by swim‐out in HS solution containing (in mM): 135 NaCl, 5 KCl, 1 MgSO4, 2 CaCl2, 20 HEPES, 5 glucose, 10 lactic acid, 1 Na‐pyruvate, pH 7.4 (adjusted with NaOH). After 20 min swim‐out at 37°C and 10% CO2, the supernatant was collected. Sperm were washed twice and re‐suspended in HS solution. For Ca2+ fluorimetry, sperm were isolated by swim‐out in Toyoda, Yokoyama and Hosi (TYH) medium containing (in mM): 138 NaCl, 4.8 KCl, 2 CaCl2, 1.2 KH2PO4, 1 MgSO4, 5.6 glucose, 0.5 Na‐pyruvate, 10 Na‐lactate, 10 HEPES, pH 7.4 (adjusted with NaOH). After 15 min swim‐out at 37°C and 5% CO2, sperm were counted and capacitated in TYH‐medium supplemented with 25 mM NaHCO3 and 3 mg·mL−1 BSA.
Sperm from the sea urchin Arbacia punctulata were obtained by injecting 0.5 M KCl into the body cavity or electrical stimulation of the animal. The ejaculate (‘dry sperm’) was diluted in artificial sea water (ASW) containing (in mM): 423 NaCl, 9.27 CaCl2, 9 KCl, 22.94 MgCl2, 25.5 MgSO4, 0.1 EDTA, 10 HEPES, pH 7.8 (adjusted with NaOH).
Measurement of changes in intracellular Ca2+
In human sperm populations, changes in [Ca2+]i and pHi were measured with the fluorescent Ca2+ indicator Fluo4 and BCECF (Thermo Fisher, Waltham, MA, USA), respectively, in 384 multi‐well plates in a fluorescence plate reader (Fluostar Omega, BMG Labtech, Ortenberg, Germany) at 29°C, or in a rapid‐mixing device in the stopped‐flow mode (SFM400, Bio‐Logic, Grenoble, France) at 37°C. Sperm were loaded with Fluo4‐AM (10 μM) in the presence of Pluronic F‐127 (0.05% w/v) at 37°C for 45 min or with BCECF‐AM (10 μM) at 37°C for 15 min. After incubation, excess dye was removed by centrifugation at 700× g, for 10 min, room temperature. Sperm concentration was adjusted to 5 × 106 cells·mL−1 in HTF and equilibrated for 5 min at 29°C (Fluostar) or 37°C (stopped‐flow).
In plate‐reader experiments, wells were filled with 50 μL of the sperm suspension; the fluorescence was excited at 480 nm (Fluo‐4) or 440 and 480 nm (dual excitation, BCECF), and fluorescence emission was recorded at 520 nm. Fluorescence was monitored before and after injection of 25 μL (1:3 dilution) RU1968F1, followed after 5 min by injection of stimuli (1:10 dilution). The solutions were injected into the wells with an electronic multichannel pipette. Changes in Fluo‐4 fluorescence are depicted as ΔF/F (%), that is, the change in fluorescence (ΔF) relative to the mean basal fluorescence (F) before application of buffer or stimuli, to correct for intra‐ and inter‐experimental variations in basal fluorescence among individual wells. Changes in BCECF‐fluorescence ratio (R, 480/440 nm) are depicted as ΔR/R (%), that is, the change in ratio (ΔR) relative to the mean basal ratio (R) before application of buffer or stimuli, to correct for intra‐ and inter‐experimental variations in the basal fluorescence ratio among individual wells. In stopped‐flow experiments, the sperm suspension was rapidly mixed (1:1; flow rate = 1 mL·s−1) with HTF containing RU1968F1 and other stimuli, or with K8.6‐, KCl‐, or pHo8.6‐HTF containing RU1968F1. Fluorescence was excited with a SpectraX Light Engine modulated at 10 kHz (Lumencor, Beaverton, OR, USA) and passed through a 494/20 nm excitation filter (Semrock, Buffalo, NY, USA). Emission was passed through a 536/40 nm filter (Semrock) and recorded with a photomultiplier (H9656‐20; Hamamatsu Photonics, Hamamatsu, Japan). Signals were amplified with a lock‐in amplifier (7230 DSP, Signal Recovery, Oak Ridge, TN, USA) and recorded with a data acquisition pad (PCI‐6221; National Instruments, Germany) and BioKine software v. 4.49 (Bio‐Logic). K8.6‐HTF (in mM): 98.5 KCl, 0.2 MgSO4, 0.37 KH2PO4, 2.04 CaCl2, 0.33 Na‐pyruvate, 21.4 lactic acid, 2.78 glucose, 21 N‐[Tris(hydroxymethyl)methyl]‐3‐aminopropanesulfonic acid (TAPS) and 4 KHCO3, pH 8.6 (adjusted with KOH). KCl‐HTF (in mM): 98.5 KCl, 0.2 MgSO4, 0.37 KH2PO4, 2.04 CaCl2, 0.33 Na‐pyruvate, 21.4 lactic acid, 2.78 glucose, 21 HEPES and 4 KHCO3, pH 7.35 (adjusted with KOH). pHo8.6‐HTF (in mM): 93.8 NaCl, 4.69 KCl, 0.2 MgSO4, 0.37 KH2PO4, 2.04 CaCl2, 0.33 Na‐pyruvate, 21.4 lactic acid, 2.78 glucose, 21 TAPS and 4 NaHCO3, pH 8.6 (adjusted with NaOH). Changes in Fluo‐4 fluorescence are depicted as ΔF/F (%), that is, the change in fluorescence (ΔF) relative to the fluorescence (F) right after the mixing, to correct for intra‐ and inter‐experimental variations in basal fluorescence. For single‐cell Ca2+ imaging, sperm were incubated in the wells of PLL‐coated Greiner Cellview glass slides with Fluo‐4‐AM (5 μM) for 30 min at 37°C, followed by another 15 min at room temperature to allow settling of sperm on the glass surface. Afterwards, the buffer was replaced twice with 90 μL of ‘fresh’ HTF to remove excess extracellular dye. Progesterone and RU1968F1 were injected in a 1:10 dilution (10 μL) into the well, and the ensuing changes in [Ca2+]i were observed under an Olympus IX73 inverted microscope, equipped with a 20×/0.75 objective (U Plan S Apo, Olympus, Germany), coupled to an Andor Zyla 4.2 sCMOS camera (Andor Technology, Belfast, UK). Images were captured at 1 Hz. Changes in [Ca2+]i were determined from a region of interest around the head and neck of single sperm. Signals are displayed as (F‐F0)/(Fmax‐F0); F0 is the mean fluorescence of ≥5 images before injection of RU1968F1 or progesterone, whereas Fmax is the peak fluorescence signal evoked by a subsequent injection of ionomycin. This procedure corrects for intra‐ and inter‐experimental variations in resting [Ca2+]i and dye loading among individual sperm.
In mouse sperm populations, changes in [Ca2+]i were measured in sperm loaded with Cal520‐AM (5 μM) (ATT Bioquest, USA) in the presence of Pluronic F‐127 (0.02% w/v) for 45 min at 37°C in TYH buffer. After loading, excess dye was removed by three centrifugations (700× g, 7 min, room temperature). Recordings were performed using the stopped‐flow apparatus as described above, but with mixing at a flow rate of 0.5 mL·s−1. K8.6‐TYH (in mM): 4.8 NaCl, 138 KCl, 2 CaCl2, 1.2 KH2PO4, 1 MgSO4, 5.6 glucose, 0.5 Na‐pyruvate, 10 lactic acid, 10 TAPS, pH 8.6 (adjusted with KOH).
In sea urchin sperm populations, changes in [Ca2+]i were recorded in Fluo4‐loaded sperm. To this end, dry sperm (diluted 1:6 (v/v)) were loaded with Fluo4‐AM (10 μM) in the presence of Pluronic F‐127 (0.02% w/v) for 45 min at 18°C in ASW. After loading, sperm were diluted 1:20 (v/v) in ASW and allowed to equilibrate for 5 min. Recordings were performed using the stopped‐flow apparatus with a flow rate of 1 mL·s−1. Fluorescence was excited, recorded, and processed as described above.
KCl‐ASW (in mM): 216 KCl, 216 NaCl, 9.27 CaCl2, 22.94 MgCl2, 25.5 MgSO4, 0.1 EDTA, 10 HEPES, pH 7.8, (adjusted with NaOH).
Patch‐clamp recordings
Patch‐clamp recordings from human sperm were performed in the whole‐cell configuration, as previously described (Strünker et al., 2011). Seals between pipette and sperm were formed either at the cytoplasmic droplet or the neck region in standard extracellular solution (HS) containing (in mM): 135 NaCl, 5 KCl, 1 MgSO4, 2 CaCl2, 5 glucose, 1 Na‐pyruvate, 10 lactic acid and 20 HEPES, pH 7.4 (adjusted with NaOH). CatSper currents were recorded in divalent‐free solutions containing (in mM): 140 CsCl, 40 HEPES, 1 EGTA, pH 7.4 (adjusted with CsOH); the pipette solution contained (in mM): 130 Cs‐aspartate, 50 HEPES, 5 EGTA, 5 CsCl, pH 7.3 (adjusted with CsOH). Slo3 currents were recorded in HS with a pipette solution containing (in mM): 140 K‐aspartate, 50 HEPES, 10 NaCl, 5 KCl, 0.5 CaCl2, pH 7.3 (adjusted with KOH). Currents carried by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=124 were recorded in a bath and pipette solution containing (in mM): 120 NMDG, 100 2‐(N‐morpholino)ethanesulfonic acid (MES), 5 TEA‐Cl, 2 EGTA, pH 6 (adjusted with methanesulfonic acid). To depict mean changes in CatSper currents, CatSper current amplitudes were normalized to that of the monovalent currents in the absence of any progesterone, NH4Cl or RU1068F1. This procedure corrects for variations in amplitudes among individual sperm to ease and to improve the clarity of the graphical illustration. Patch‐clamp recordings from mouse sperm were performed in the whole‐cell configuration, as previously described (Kirichok et al., 2006; Zeng et al., 2011). Seals between pipette and sperm were formed at the cytoplasmic droplet. For Slo3 recordings, the extracellular solution contained (in mM): 160 KOH, 10 HEPES, 150 MES and 2 Ca(MES)2, adjusted to pH 7.4 with MES; the pipette solution contained (in mM): 155 KOH, 5 KCl, 10 BAPTA, 20 HEPES, 115 MES, pH 8.0 (adjusted with KOH). CatSper currents were recorded in divalent‐free solutions containing (in mM): 150 NaCl, 20 HEPES, 5 EDTA, pH 7.4 (adjusted with NaOH); and with a pipette solution containing (in mM) 135 Cs‐MES, 10 HEPES, 10 EGTA and 5 CsCl, pH 7.2 (adjusted with CsOH). Current amplitudes were normalized to that of the monovalent currents in the absence of NH4Cl or RU1968F1 (control) to correct for variations in amplitudes among individual sperm.
Human T‐type (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=536) and L‐type (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=529 + β2b + α2δ1) Ca2+ channels were studied in HEK293T cells (The European Collection of Cell Cultures, Porton Down, UK) that were cultured according to the supplier's protocol in the presence of penicillin G (100 U·mL−1) and streptomycin (10 mg·mL−1). Cells were transfected at 40% confluency with pcDNA3.1‐CACNA1C, pcDNA3.1‐CaVb2b and pIRES‐dsRed‐CaVa2d1 in a ratio of 2:1:1 μg, or with 2 μg of pCMV‐Entry‐CACNA1H, using the calcium‐phosphate precipitation method. Patch‐clamp recordings from HEK293T cells were performed in the whole‐cell configuration, using a HEKA EPC 10 amplifier with PatchMaster software (both HEKA Elektronik, Lambrecht, Germany). The extracellular solution contained (in mM): 125 TEA‐Cl, 15 glucose, 10 HEPES, 5 CaCl2, pH 7.4, (adjusted with CsOH); the pipette solution contained (in mM): 100 CsCl, 10 EGTA, 10 HEPES, 5 TEA‐Cl, 5 MgATP, 0.2 NaGTP, pH 7.4 (adjusted with CsOH). RU1968F1 was applied via a gravity‐driven perfusion system.
Analysis of sperm motility
To evaluate the acute action of RU1968F1 on motility parameters, sperm from a particular sample were incubated side‐by‐side in HTF lacking NaHCO3 and HSA (non‐capacitating medium) and in capacitating medium (25 mM NaHCO3/3 mg·mL−1 HSA). After 3 h of incubation at 37°C, sperm kinematic parameters were analysed by a CASA system (CEROS, Hamilton Thorn Research, Beverly, MA, USA) before and after application of RU1968F1. Sperm were bathed in RU1968F1 for 5 min prior to the experiment. To evaluate the long‐term action of RU1968F1 on sperm motility parameters, sperm were re‐suspended in capacitating medium (HTF containing 25 mM NaHCO3 and 3 mg·mL−1 HSA) with or without RU1968F1. After 3 h of incubation at 37°C, sperm kinematic parameters were analysed by CASA. To evaluate the action of RU1968F1 on progesterone‐induced hyperactivation, capacitated sperm were incubated for 5 min in the absence (control) and presence of progesterone, RU1968F1, and progesterone plus RU1968F1, and the motility was analysed by CASA. The following parameters were determined by CASA: curvilinear velocity (VCL, μm·s−1), amplitude of lateral head displacement (ALH, μm), linearity of progression (LIN, %), percentage of total, progressive and rapid motility as well as percentage of motile, hyperactivated sperm. The threshold values for hyperactivation were manually set (VCL >150 mm·s−1, ALH > 7 mm, LIN < 50% (Mortimer et al., 1998; Tamburrino et al., 2014). A minimum of 100 cells and five fields of view were analysed for each aliquot. The experiments were performed at 37°C.
Motility in human sperm evoked by uncaging of progesterone were studied in an observation chamber (100 μm depth) under an Olympus IX71 inverted microscope (Olympus, Tokyo, Japan), equipped with a 4× microscope objective (0.13 NA, UPLFLN‐PH, Olympus) under dark‐field illumination [red light‐emitting diode (LED), M660 L3‐C1, Thorlabs]. Movies were recorded at a total magnification of 6.4× with a high‐speed CMOS camera (Dimax HD, PCO, Kelheim, Germany) at 150 Hz. Photolysis of caged progesterone (1 μM) (Kilic et al., 2009) was achieved using a 200 ms light flash delivered by a 365 nm LED (M365 L2‐C, Thorlabs, Munich, Germany). Movies were processed and analysed using a customized CASA plugin for ImageJ. Changes in the velocity average path (VAP) are shown as VAP (%), that is, the change in VAP relative to the VAP right before the UV flash, to correct for intra‐ and inter‐experimental variations and for the different resting VAP in the absence and presence of the inhibitor. This procedure eases and improves the clarity of the graphical illustration.
Kremer penetration assays were performed in sEBSS supplemented with methylcellulose (1% w/v) and 0.3% BSA, equilibrated overnight at 4°C (penetration medium). The penetration medium, with or without RU1968F1, was filled into flattened glass capillary tubes (dimensions: 1.2 × 4.8 × 50 mm, 400 μm depth; CM scientific, UK); one end of the tubes was sealed with CristaSeal wax (Hawksley, UK). The open ends of the tubes were submersed in a sperm suspension (3 × 106·mL−1) with or without stimuli and/or RU19868F1. Penetration was assessed after 1 h (37°C, 5.5% CO2) by counting sperm at 2 cm using a phase contrast microscope at a 200× magnification. Three fields of view were chosen, and in each field, three focal planes were counted, yielding nine fields altogether.
Human sperm viability and motility, as shown in Supporting Information Figure S3, was tested following incubation of sperm for 5 min at room temperature with RU1968F1, NNC‐55‐0396, mibefradil or the vehicle (DMSO). The fraction of immotile and dead sperm was assessed by counting and by an eosin vitality test, respectively, at 200× magnification under a phase‐contrast microscope (Axiostar, Carl Zeiss), in accordance with the WHO guidelines for semen analysis (WHO, 2010). For the eosin staining, 5 μL of the sperm suspension was mixed with 5 μL of eosin staining solution [0.5% (w/v) eosin Y dissolved in a 0.9% NaCl solution] on a microscope slide, covered with a 22 × 22 mm coverslip and incubated for 30 s at RT. Eosin‐positive (dead) versus eosin‐negative (live) sperm were counted. To determine the fraction of immotile and viable sperm, a total number of 400 sperm were assessed.
Sea urchin sperm chemotaxis was studied as described (Seifert et al., 2015). In brief, sperm (~108 cells·mL−1) were observed in a recording chamber (150 μm depth) under an IX71 microscope (Olympus), equipped with a 10× microscope objective (UPlanSApo; NA 0.4; Olympus), with stroboscopic (500 Hz) dark‐field illumination (white LED; K2 star; Luxeon). Movies were recorded with an EMCCD camera (DU‐897D; Andor) at 20 Hz through a bandpass filter (HQ520/40; Chroma). Photolysis of caged resact was achieved using a 200 ms pulse from a 365 nm LED (M365 L2‐C, Thorlabs). The relative dispersion was calculated as described before (Seifert et al., 2015).
Acrosomal exocytosis
Human sperm, capacitated for at least 300 min, were incubated with either 0.1% DMSO (vehicle control), RU1968F1 (10 μM), progesterone (10 μM) or a mixture of both (10 μM each) for 1 h at 37°C. Afterwards, sperm were washed by centrifugation and re‐suspended in 0.5 mL of hypo‐osmotic swelling medium (WHO, 2010). After 1 h at 37°C, sperm were washed again and fixed in 50 μL ice‐cold methanol. The sperm were layered on a slide, air‐dried, and stored at −20°C. For acrosome staining, sperm were incubated for 20 min in the dark with 1 mg·mL−1 FITC‐labelled Arachis hypogaea (peanut) lectin (PNA‐FITC, Sigma Aldrich) in PBS. Slides were analysed using an Axiolab A1 FL microscope (Carl Zeiss, Jena, Germany). For each condition, 200 curled‐tail (viable) cells were analysed for their acrosomal status, as previously described (Tamburrino et al., 2014).
Data analysis and statistical evaluation
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Experiments and data analysis were performed without randomization and blinding, except for the Eosin test and the manual counting of motile/immotile sperm. Otherwise, non‐treated and treated conditions were measured and analysed side‐by‐side by the same experimenter, using objective measures and analysis methods.
All data are presented as mean ± SD. Statistical analysis and fitting of dose–response relations were performed using GraphPad Prism 5 (Prism, La Jolla, USA). Half‐maximal inhibitory concentrations (IC50) were derived by nonlinear regression analysis, using a four parameter fit:
Y = signal amplitude; bottom and top = plateaus in the units of Y; x = log(concentration of inhibitor); IC50 = concentration of agonist that gives the response half way between bottom and top; n = Hill coefficient.
Most of the experiments were performed in a randomized block design, that is, for each experimental replicate, sperm prepared from one particular semen sample were subjected in parallel to all treatment conditions. If the experiment involved two conditions (control and treatment), we used the paired t‐test. If the experiment involved ≥3 conditions, we used one‐way randomized block ANOVA, assuming sphericity. When ANOVA's F‐test and the test for matching efficacy achieved P < 0.05, means were compared to the control's mean by Dunnett's multiple comparisons post hoc test, unless otherwise indicated. If experiments were not performed in a randomized block design, we used unpaired t‐test or one‐way ANOVA; when ANOVA's F‐test achieved P < 0.05 and Bartlett's test yielded no significant variance inhomogeneity, means were compared to the control's mean or to each other by Dunnett's or Bonferron's multiple comparisons post hoc test respectively. In Figures 6E, J, and 8I, for the ease of illustration and for clarity, we show data normalized to the control. We normalized the data only after the statistical analysis using one‐way ANOVA, because normalization makes any data set violate the ANOVA.
Figure 6.
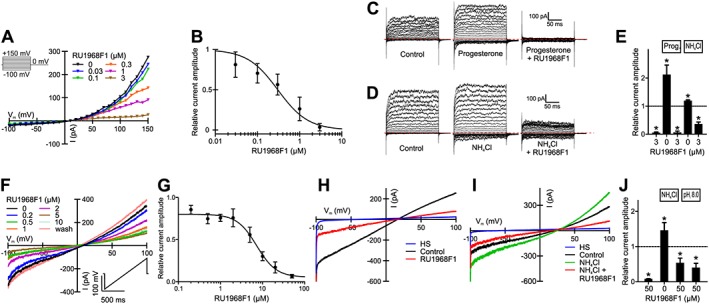
RU1968F1 inhibits monovalent cation currents carried by CatSper channels in human and mouse sperm. (A) Representative current–voltage relationship of CatSper currents recorded from a human sperm cell in divalent‐free extracellular and intracellular solution (pH 7.4) in the absence and presence of increasing RU1968F1 concentrations. Voltage was stepped from −100 to +150 mV in increments of 10 mV. Inset: Voltage protocol. (B) Dose–response relation for the inhibition of human CatSper currents by RU1968F1 at +100 mV (IC50 = 0.4 ± 0.3 μM; n = 5). (C) Representative monovalent CatSper currents recorded from a human sperm cell before (control) and after perfusion with progesterone (2 μM) and progesterone plus RU1968F1 (3 μM), evoked by the voltage protocol shown in (A). The dotted red line indicates the current at 0 mV. (D) Monovalent CatSper currents recorded from a human sperm cell before (control) and after perfusion with NH4Cl (10 mM) and NH4Cl plus RU1968F1 (3 μM), evoked by the voltage protocol shown in (A). The dotted red line indicates the current at 0 mV. (E) Mean amplitudes of monovalent currents at +100 mV recorded in the presence of RU1968F1, progesterone (2 μM), progesterone plus RU1968F1, NH4Cl (10 mM), and NH4Cl plus RU1968F1. Amplitudes were normalized to that evoked in the absence of any drug (control, dashed line). Data shown are means ± SD (n = 5). *P < 0.05, significantly different from control. Data were normalized only after performing the statistical analysis using one‐way ANOVA (see Methods for details and explanations). (F) Representative CatSper currents recorded from a mouse sperm cell in divalent‐free extracellular and intracellular solution (pH 7.2) in the absence and presence of increasing RU1968F1 concentrations. Voltage was ramped between −100 and +100 mV from a holding potential of 0 mV. Inset: Voltage protocol. (G) Dose–response relation for the inhibition of mouse CatSper currents at +100 mV (IC50 = 10 ± 1 μM, n = 3). Data shown are means ± SD. (H) Currents in the presence of extracellular divalent ions (HS) and monovalent currents in divalent‐free conditions (control) recorded from a mouse sperm cell evoked at pHi 8 before (control) and after perfusion with RU1968F1, using the voltage protocol shown in (F). (I) Currents recorded from a mouse sperm cell at pHi 7.2 before (control) and after perfusion with NH4Cl (30 mM) and NH4Cl plus RU1968F1, using the voltage protocol shown in (F). (J) Mean amplitudes of monovalent currents at +100 mV recorded from mouse sperm in the presence of RU1968F1, NH4Cl plus RU1968F1, and at pHi 8 in the presence of RU1968F1, using the voltage protocol shown in (F). Amplitudes were normalized to the monovalent currents evoked in the absence of any drug (control, dashed line). Data shown are means ± SD (n = 5). *P < 0.05, significantly different from control. Data were normalized only after performing the statistical analysis using one‐way ANOVA (see Methods for details and explanations).
Figure 8.
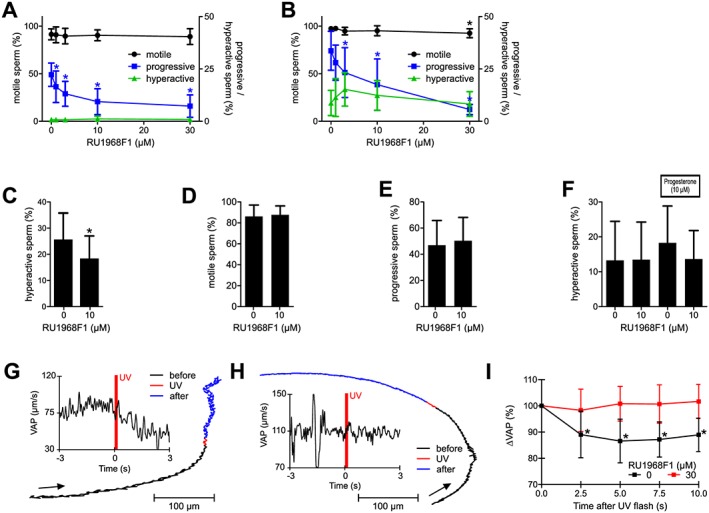
RU1968F1 interferes with hyperactivation and abolishes progesterone‐induced motility responses in human sperm. (A, B) Motility parameters of non‐capacitated (A) and capacitated (B) human sperm in the absence and presence of RU1968F1 (n = 8); sperm were bathed in the drug for 300 s. Data shown are means ± SD. *P < 0.05, significantly different from control (absence of RU1968F1). (C–E) Fraction of hyperactivated (C), motile (D), and progressively swimming (E) sperm after capacitation in the absence and presence of RU1968F1 (n = 11). Data shown are means ± SD. *P < 0.05, significantly different from control (absence of RU1968F1). (F) Hyperactivation evoked by bathing sperm for 300 s in RU1968F1, progesterone, or progesterone plus RU1968F (n = 11). Data shown are means ± SD. (G) Track of a single sperm cell recorded before (3 s), during (0.2 s) and after (2.8 s) uncaging of progesterone. The arrow indicates the direction of movement. Inset: time course of the VAP; the red bar indicates the uncaging of progesterone. (H) Track of a single sperm cell recorded before (3 s), during (0.2 s, flash), and after (2.8 s) uncaging of progesterone in the presence of RU1968F1 (30 μM). Inset: time course of VAP; the red bar indicates the uncaging of progesterone. (I) Mean relative changes in VAP averaged over 20–30 sperm in the field of view after uncaging of progesterone (n = 11). Data shown are means ± SD. *P < 0.05, significantly different from control (before flash, 0 s). Data were normalized only after performing the statistical analysis using one‐way ANOVA (see Methods for details and explanations).
Materials
The suppliers of the compounds used in these experiments are as follows: progesterone from Sigma and the caged form was prepared as described by Kilic et al., 2009; 8‐Br‐cAMP was from BioLog (Bremen, Germany); resact and its caged form were prepared as described by Böhmer et al., 2005 and Kaupp et al., 2003; mibefradil, MDL12330A, NNC‐0396 and PGE1 were supplied by Tocris (Wiesbaden‐Nordenstadt, Germany).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Synthesis of RU1968
We synthesized RU1968 from (±) estrone methyl ether (Figure 1A and Supporting Information Figure S1) that is readily accessible via the Torgov route (Ananchenko et al., 1962; Ananchenko and Torgov, 1963). Van Leusen reaction yielded the nitrile (1) (Van Leusen and Van Leusen, 2004), followed by addition of methyllithium to yield the ketone (2). Reductive amination with N,N‐dimethylethylenediamine established the aza side chain, yielding a mixture of four diastereomers (3) (at C‐17 and C‐20; Figures 1A compound 3). We separated two diastereomers (Figure 1A, compounds 3a and 3b), each a mixture of a cis and a trans isomer. Relative configurations [C‐18 (CH3) and C‐20 (CH)] were assigned by NMR spectroscopy (H,H‐COSY and NOE, Supporting Information Figures S1 and S2); the cis isomers were the dominant species (cis/trans ratio for compound 3a was 3:1; and for 3b, it was 4:1). Finally, cleavage of the phenolic methyl ether yielded a diastereomeric mixture of RU1968. The diastereomers eluted from a preparative HPLC in four fractions called RU1968F1‐4; RU1968F1 and 2 are derived from 3a cis and trans, respectively, whereas RU1968F3 and 4 are derived from 3b trans and cis, respectively (Figure 1C). Because the actions of RU1968F2‐4 in sperm were similar to that of RU1968F1 (see Figure 2 and Supporting Information Figures S3 and S6), we chose RU1968F1 to characterize its action in sperm. We first examined the action of RU1968F1 in populations of human sperm loaded with a fluorescent pHi or Ca2+ indicator (Supporting Information Figure S3A–D). The drug evoked negligible changes in pHi. At concentrations ≤7.5 μM, RU1968F1 caused a small, transient Ca2+ increase; [Ca2+]i peaked and returned to basal levels within about 250 s. At concentrations >7.5 μM, RU1968F1 evoked a slow decrease of [Ca2+]i. The mechanism(s) underlying the drug‐evoked changes in [Ca2+]i are unclear. In the absence of extracellular Ca2+, the drug did not change [Ca2+]i, indicating that Ca2+ release from internal stores is not involved (Supporting Information Figure S3C). Most importantly, even at high micromolar concentrations (30 μM), the drug did not affect overall human sperm motility and viability and did not evoke acrosomal exocytosis (Supporting Information Figure S3E–G). Thus, RU1968F1 lacks the toxic and adverse actions of NNC‐0396, mibefradil, and MDL12330A in human sperm.
Figure 1.
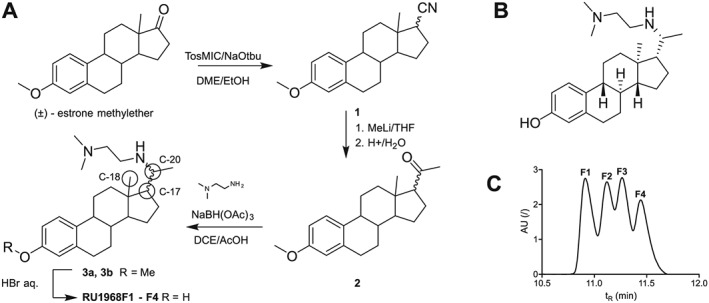
Synthesis of RU1968F1‐F4. (A) Synthesis of RU1968. Carbon atoms referred to in the text are marked with circles. (B) Structure of RU1968. (C) HPLC‐elution profile of the four diastereomers. Isomers are named according to their order of elution.
Figure 3.
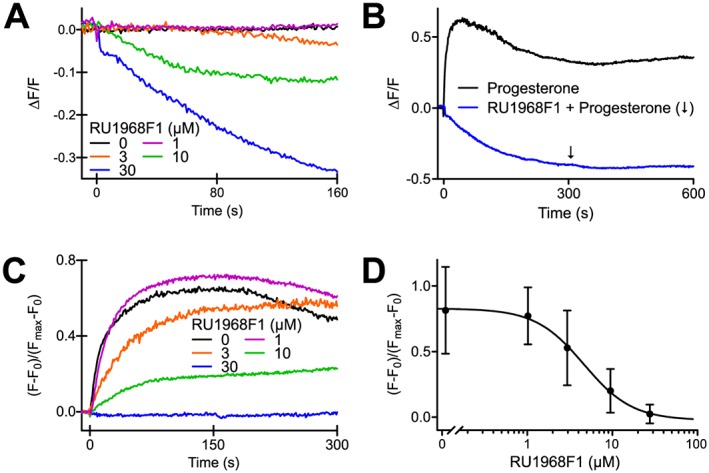
Action of RU1968F1 on progesterone‐evoked Ca2+ signals in single human sperm. (A) Changes in [Ca2+]i evoked by RU1968F1 in immobilized sperm. Sperm were challenged with RU1968F1 at t = 0. Traces represent averages of 122 (RU1968F1, 0 μM), 344 (1 μM), 42, (3 μM), 165 (10 μM) and 109 (30 μM) sperm from three donors. Signals are displayed as (F‐F0)/(Fmax‐F0); F0 is the mean fluorescence of ≥5 images before application of RU1968F1; Fmax is the peak fluorescence signal evoked by ionomycin (not shown) to gauge the maximal response amplitude. (B) Progesterone‐evoked Ca2+ responses (2 μM) in the absence and presence of RU1968F1 (30 μM); averages of 50 (control) and 109 (RU1968F1) sperm from three donors. Progesterone and RU1968F1 were applied at t = 0; following application of RU1968F1, progesterone was applied at the time point indicated by the arrow. (C) Amplitude of progesterone‐evoked Ca2+ responses in the absence and presence of different RU1968F1 concentrations; averages of 122 (RU1968F1, 0 μM), 344 (1 μM), 42 (3 μM), 165 (10 μM) and 109 (30 μM) sperm from three donors. (D) Mean amplitude of progesterone‐evoked Ca2+ signals (2 μM) in the presence of RU1968F1; number of sperm: 310 (RU1968F1, 0 μM), 552 (1 μM), 181 (3 μM), 302 (10 μM) and 222 (30 μM). Data shown are means ± SD. Fitting of a dose–response curve to the data yielded an IC50 of 4.8 ± 1.2 μM (standard error of the fit).
Figure 2.
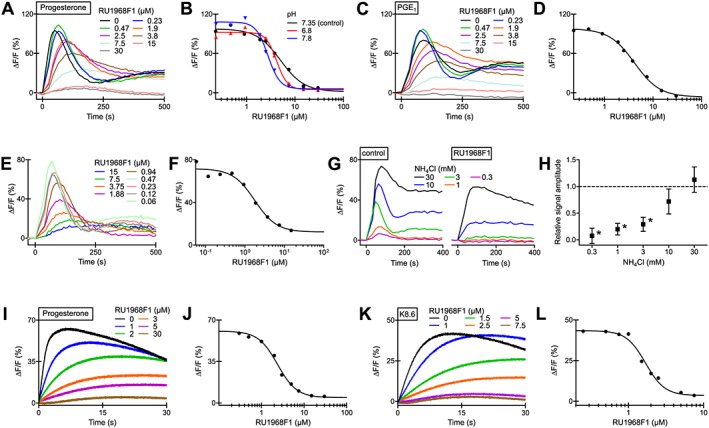
Action of RU1968F1 on CatSper‐mediated Ca2+ signals in human sperm populations. (A) Progesterone‐evoked Ca2+ signals in human sperm in the absence and presence of RU1968F1. ΔF/F (%) indicates the percentage change in fluorescence (ΔF) with respect to the mean basal fluorescence (F) before application of progesterone (500 nM). (B) Dose–response relation for the maximal signal amplitudes of the data from (A) (pH 7.35) (IC50 = 5.5 μM) and of progesterone responses studied at an extracellular pH of 6.8 (IC50 = 4.2 μM) or 7.8 (IC50 = 2.6 μM). (C) PGE1‐evoked Ca2+ signals in human sperm in the absence and presence of RU1968F1; PGE1 = 500 nM. (D) Dose–response relation for the maximal signal amplitudes of the data from (C) (IC50 = 3.1 μM). (E) NH4Cl‐evoked Ca2+ signals in the absence and presence of RU1968F1, NH4Cl = 3 mM. (F) Dose–response relation for the maximal signal amplitude of the data from (E) (IC50 = 1.8 μM) (G) Ca2+ signals evoked by various NH4Cl concentrations in the absence (control) and presence of RU1968F1 (30 μM). (H) Mean relative amplitude of Ca2+ signals evoked by various NH4Cl concentrations in the presence of RU1968F1 (30 μM) (n = 5); amplitude evoked in the absence of RU1968F1 = 1 (control). Data shown are means ± SD. *P < 0.05, significantly different from control. (I) Ca2+ signals evoked by simultaneous mixing of sperm with progesterone (500 nM) and RU1968F1 in a stopped‐flow apparatus. ΔF/F (%) indicates the percentage change in fluorescence (ΔF) with respect to the fluorescence (F) immediately after mixing. (J) Dose–response relation of the data from (I) (IC50 = 2.4 μM). (K) Ca2+ signals evoked by mixing of sperm with K8.6‐HTF and RU1968F1. The final K+ concentration and pH after mixing was 51.25 mM and 8.1 respectively. (L) Dose–response relation of the data from (K) (IC50 = 1.7 μM).
RU1968F1 is a potent cross‐species inhibitor of CatSper channels
To investigate whether RU1968F1 inhibits human CatSper channels, we studied progesterone‐ and PGE1‐evoked Ca2+ signals in human sperm in the presence of RU1968F1. RU1968F1 slowed down and completely suppressed the Ca2+ signals in a dose‐dependent fashion. The IC50 values of 4 ± 2 μM (progesterone) and 3.8 ± 0.5 μM (PGE1) (n = 7, mean ± SD) were similar to those reported previously (Schaefer et al., 2000) (Figure 2A–D). The compound was effective within a range of extracellular pH (pHo) values: at pHo 6.8 and 7.8, the IC50 value of the progesterone‐evoked Ca2+ signal was 4.4 ± 1.4 and 2.2 ± 0.6 μM, respectively (n = 6) (Figure 2B). The actions of RU1968F2‐4 alone and on progesterone‐evoked Ca2+ signals were similar to those of RU1968F1 (see Figure 2 and Supporting Information Figures S3 and S6).
Furthermore, we studied whether RU1968F1 also inhibits Ca2+ signals evoked by intracellular alkalization via the weak base NH4Cl (Figure 2E–H). The compound slowed down and almost completely suppressed Ca2+ signals evoked by NH4Cl ≤ 3 mM (Figure 2E, F); the IC50 value for 3 mM NH4Cl was 4.0 ± 2.8 μM (n = 5) (Figure 2F, G). Ca2+ signals evoked by 10 mM NH4Cl were only slightly attenuated, whereas for 30 mM NH4Cl, the signal was rather similar in the absence and presence of RU1968F1 (Figure 2G, H). We conclude that RU1968F1 inhibits also ΔpHi‐evoked Ca2+ responses. However, its potency seems to decrease with increasing ΔpHi. Alternatively, the presence of NH4 + or NH3 might impair binding of RU1968F1 to its blocking site. Arguing against that notion, the compound readily suppressed progesterone responses in sperm that were bathed for about 20 min in NH4Cl (30 mM) (Supporting Information Figure S5; IC50 = 3.5 ± 1.4 μM, n = 3); with time, the pHi slowly recovers from the NH4Cl‐evoked alkalization (Strünker et al., 2011).
Influx of Ca2+ via CatSper can also be evoked by simultaneous extracellular alkalization (which increases pHi) and depolarization by K+ (K8.6 buffer) (see Carlson et al., 2003). RU1968F1 suppressed Ca2+ signals evoked by simultaneous alkalization/depolarization (Figure 2K, L; IC50 = 1.2 ± 0.6 μM, n = 3) and by alkalization or depolarization alone (Supporting Information Figure S4). Finally, when sperm were mixed simultaneously with progesterone and RU1968F1 in a stopped‐flow apparatus, the inhibition of Ca2+ responses was similar to that under pre‐incubation conditions (Figure 2I, J) (IC50 = 3.0 ± 1.1 μM; n = 4). This result suggests that RU1968F1 rapidly reaches its blocking site.
Next, we studied the action of RU1968F1 in single human sperm by Ca2+ imaging. At concentrations ≥10 μM, RU1969F1 evoked a slow decrease of [Ca2+]i (Figure 3A). For low micromolar RU1969F1 concentrations, we did not observe Ca2+ transients, which might reflect differences in sensitivity of population versus single‐cell fluorimetry. In the presence of RU1968F1, progesterone‐induced Ca2+ responses were suppressed in a dose‐dependent fashion (Figure 3B–D) with an IC50 of 4.8 ± 1.2 μM (standard error of the fit). Altogether, the action of the RU1868F1 itself on [Ca2+]i and on progesterone‐evoked Ca2+ responses is similar when investigated in sperm populations and in single sperm.
We further tested whether the compound also inhibited Ca2+ influx via CatSper channels in mouse sperm. Mouse CatSper is insensitive to progesterone and PGs (Lishko et al., 2011). Therefore, we activated CatSper via simultaneous alkalization/depolarization or via 8‐Br‐cAMP, which activates mouse (Ren et al., 2001) and human (Brenker et al., 2012) CatSper at high concentrations. RU1968F1 suppressed Ca2+ responses evoked by alkalization/depolarization or 8‐Br‐cAMP with an IC50 of 0.83 ± 0.07 and 0.84 ± 0.03 μM respectively (n = 3) (Figure 4A–D).
Figure 4.
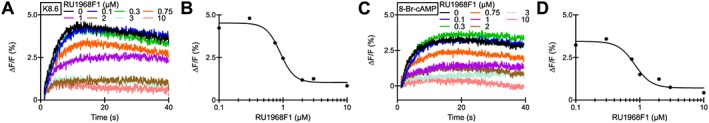
Action of RU1968F1 on CatSper‐mediated Ca2+ signals in mouse sperm populations. (A) Ca2+ signals evoked by simultaneous mixing of mouse sperm with K8.6‐TYH and RU1968F1 in a stopped‐flow apparatus. After mixing, the final K+ concentration and pH was 69 mM and 8.1 respectively. (B) Dose–response relation of the data from (A) (IC50 = 0.90 μM). (C) Ca2+ signals evoked by simultaneous mixing of mouse sperm with 8‐Br‐cAMP (20 mM) and RU1968F1. (D) Dose–response relation of the data from (C) (IC50 = 0.84 μM).
Finally, we investigated the action of RU1968F1 on CatSper in sperm of the sea urchin A. punctulata. To this end, we studied CatSper‐mediated Ca2+ responses evoked either by the chemoattractant resact, depolarization of Vm, or by NH4Cl. Irrespective of the stimulus, RU1986F1 suppressed the Ca2+ responses with IC50 values of 1.3 ± 0.1, 1.1 ± 0.4 and 4 ± 2 respectively (n = 3) (Figure 5A–F). Altogether, these results suggest that RU1968F1 is a potent cross‐species CatSper inhibitor.
Figure 5.
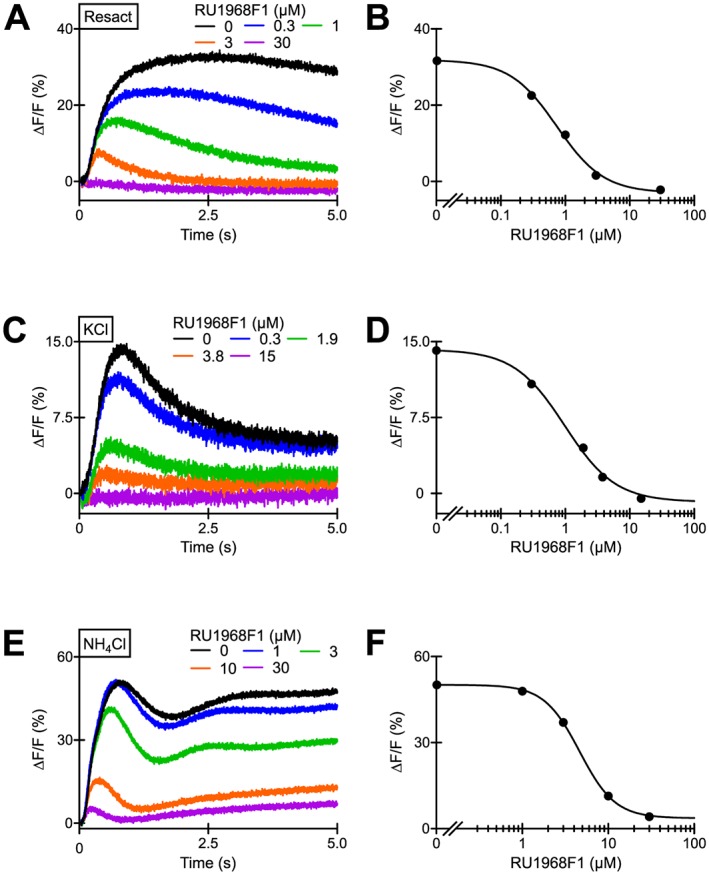
Action of RU1968F1 on Ca2+ responses mediated by CatSper channels in sea urchin sperm. (A) Resact‐induced Ca2+ signals in sea urchin sperm evoked by simultaneous mixing of sperm with resact (20 pM) and RU1968F1 in a stopped‐flow apparatus. (B) Dose–response relation of the data from (A) (IC50 = 0.7 μM). (C) Depolarization‐induced Ca2+ signals evoked by mixing of sperm with KCl‐ASW and RU1968F1. Final K+ concentration after mixing was 108 mM. (D) Dose–response relation of the data from (C) (IC50 = 1.0 μM). (E) Alkaline‐evoked Ca2+ signals in the presence of RU1968F1; the final NH4Cl concentration after mixing was 30 mM. (F) Dose–response relation of the data from (E) (IC50 = 4.6 μM).
To scrutinize this conclusion by an independent technique, we recorded CatSper currents in human and mouse sperm by whole‐cell patch clamping. In human sperm, monovalent CatSper currents were evoked by stepping the membrane voltage from −100 to +150 mV in increments of 10 mV from a holding potential of 0 mV. RU1968F1 completely suppressed these currents with an IC50 of 0.4 ± 0.3 μM (n = 5) (Figure 6A, B). Superfusion with progesterone or NH4Cl enhanced the current amplitudes (Figure 6C, D). The progesterone‐ and NH4Cl‐evoked currents were either completely suppressed or strongly attenuated by RU1968F1 (Figure 6C–E). In mouse sperm, monovalent CatSper currents were evoked by ramping the membrane voltage between −100 and +100 mV from a holding potential of 0 mV. Superfusion with RU1968F1 completely suppressed the currents with an IC50 of 10 ± 1 μM (Figure 6F, G). CatSper currents evoked at pHi 8 and by NH4Cl were strongly attenuated by the drug (Figure 6H–J). Thus, RU1968F1 inhibits human and mouse CatSper at rest and upon activation by ligands and ΔpHi. Similar to the results obtained by Ca2+ fluorimetry, the potency of the drug decreased with increasing pHi. We did not test whether higher RU1968F1 concentrations completely suppress the currents evoked by NH4Cl and at pHi 8.
RU1968F1 inhibits human but not mouse Slo3 channels
We studied the interaction of RU1968F1 with sperm ion channels other than CatSper. In mouse sperm, currents carried by the K+ channel Slo3 were similar in the absence and presence of RU1968F1 (Figure 7A, B). By contrast, in human sperm, the Slo3 current was inhibited with an IC50 of 7 ± 6 μM (n = 4) (Figure 7C, D). Thus, although not completely selective for CatSper, about 15‐fold higher RU1968F1 concentrations were required to block human Slo3. At concentrations up to 10 μM, the compound did not inhibit the voltage‐gated proton channel Hv1 and the ATP‐gated http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=77 (Supporting Information Figure S7), which are expressed in human and mouse sperm, respectively (Lishko et al., 2010; Navarro et al., 2011). We conclude that in mouse sperm, RU1968F1 acts rather selectively on CatSper. In human sperm, the compound also inhibited Slo3, but with about 15‐fold lower potency. Finally, RU1968F1 inhibited heterologously expressed L‐ and T‐type Ca2+ channels with IC50 values of about 20 and 10 μM, respectively (Supporting Information Figures S9 and S10), indicating that the compound also acts with lower potency on classic voltage‐gated Ca2+ channels of somatic cells.
Figure 7.
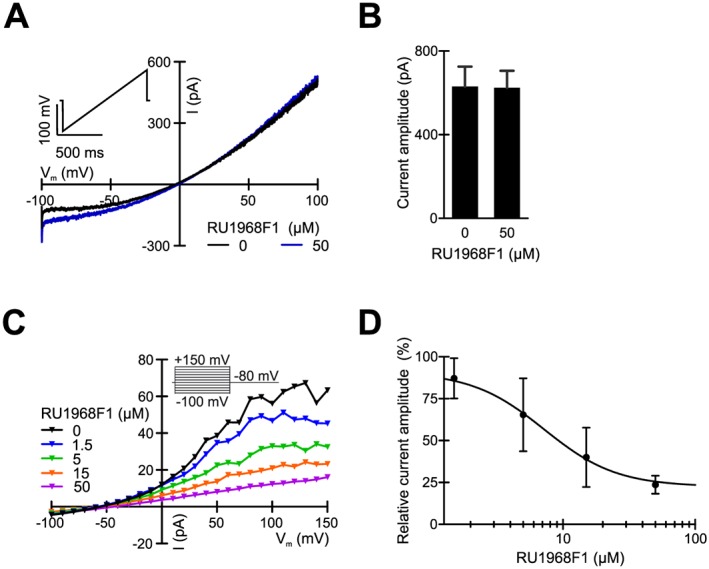
RU1968F1 inhibits human but not mouse Slo3 channels. (A) Representative Slo3 currents in a mouse sperm cell, recorded in the presence of extracellular divalent ions at symmetric intra‐ and extracellular K+ concentrations in the absence and presence of 50 μM RU1968F1. Inset: Voltage protocol. (B) Mean Slo3 currents in mouse sperm at +100 mV in the absence and presence of 50 μM RU1968F1 (n = 5). (C) Representative Slo3 currents recorded from a human sperm cell in the absence and presence of RU1968F1. Inset: Voltage protocol. (D) Dose–response relation for the inhibition of human Slo3 currents by RU1968F1 at +100 mV (IC50 = 7 ± 6 μM, n = 4). Data shown are means ± SD.
RU19681F1 suppresses progesterone‐evoked motility responses in human sperm
Next, we tested the action of RU1968F1 on the motility of human sperm using CASA. A brief incubation (5 min) of non‐capacitated or capacitated sperm with the compound did not impair overall motility (Figure 8A, B), whereas the fraction of progressively motile sperm decreased about twofold with increasing RU1968F1 concentrations (Figure 8A, B). Whether this is due to the inhibition of CatSper channels or represents an adverse action of the drug is unclear.
Furthermore, the penetration of the egg coat requires hyperactivated motility, which is characterized by an asymmetric flagellar beat, lower beating frequency, wiggly swimming trajectory and lower VAP (Suarez, 2008). In mouse sperm, CatSper channels are required for hyperactivation (Ren et al., 2001), whereas the control of hyperactivation by CatSper in human sperm is debated (Alasmari et al., 2013b; Tamburrino et al., 2014). We studied whether RU1968F1 affects hyperactivation in human sperm. A brief incubation (5 min) of capacitated sperm with RU1968F1 did not suppress spontaneous hyperactivated swimming. In fact, RU1968F1 concentrations <10 μM seem to slightly enhance hyperactivation, whereas higher concentrations had no effect (Figure 8B). Spontaneous hyperactivation develops during the capacitation process (compare Figure 8A, B). In sperm that were capacitated in the presence of RU1968F1 (10 μM), that is, incubated for some hours under capacitating conditions, the compound suppressed spontaneous hyperactivation (Figure 8C), while the fraction of progressively motile sperm or overall motility was not affected (Figure 8D, E). This result suggests that, in human sperm, CatSper channels are involved in the ability to undergo hyperactivation; though, the partial inhibition of Slo3 might contribute to this action of RU1968F1.
Finally, we studied the action of RU1968F1 on progesterone‐induced changes in swimming behaviour. Incubation of capacitated sperm with progesterone seemingly promoted hyperactivation, which was inhibited by RU1968F1 (Figure 8F); the effect of progesterone was, however, not statistically significant. Therefore, we studied the motility of human sperm before and after rapid activation of CatSper, using caged progesterone (Kilic et al., 2009). Uncaging of progesterone by a brief (200 ms) UV flash instantaneously evoked a wiggly swimming trajectory (Figure 8G) and a decrease of VAP (Figure 8G, I), resembling hyperactivated motility. The VAP reached its minimum ~5 s after uncaging of progesterone and did not recover within the recording time of 10 s. In the presence of RU1968F1, the swimming trajectory and the swimming pattern and VAP remained unchanged upon uncaging of progesterone (Figure 8H, I). We conclude that progesterone‐evoked hyperactivation requires Ca2+ influx via CatSper.
Furthermore, it is well established that progesterone facilitates the migration of human sperm into viscous medium (Alasmari et al., 2013b). In human sperm lacking functional CatSper channels, this facilitation was abolished (Williams et al., 2015). Using a modified Kremer's sperm‐mucus penetration test, we investigated whether inhibition of CatSper by RU1968F1 reproduced this phenotype. To this end, an open glass capillary, which contained medium fortified with methylcellulose, was immersed in a sperm suspension and the number of sperm at a penetration distance of 2 cm (Figure 9A–C) was determined. Data for shorter or longer penetration distances are presented in Supporting Information Figure S8. Consistent with previous results (Alasmari et al., 2013b), bathing sperm in progesterone enhanced the number of sperm penetrating the viscous medium (Figure 9A, C, and Supporting Information Figure S8A, C). The effects of progesterone were abolished by 1 μM RU1968F1 (Figure 9A and Supporting Information Figure S8A). Of note, at this concentration, the drug itself did not affect the number of penetrating cells (Figure 9B and Supporting Information Figure S8B). These effects of progesterone were also abolished when RU1968F1 was added to the capillary medium instead of to the sperm suspension (Figure 9C and Supporting Information Figure S8C). These results support the notion that progesterone acts via CatSper to promote swimming in high‐viscosity media and shows that RU1968F1 mimics the lack of functional CatSper channels. Of note, at concentrations >1 μM, RU1968F1 in a dose‐dependent fashion lowered the number of penetrating sperm both in the absence and presence of progesterone (Figure 9A, B, and Supporting Information Figure S8A, B). This probably reflects the compound‐related decrease of the fraction of progressively motile sperm.
Figure 9.
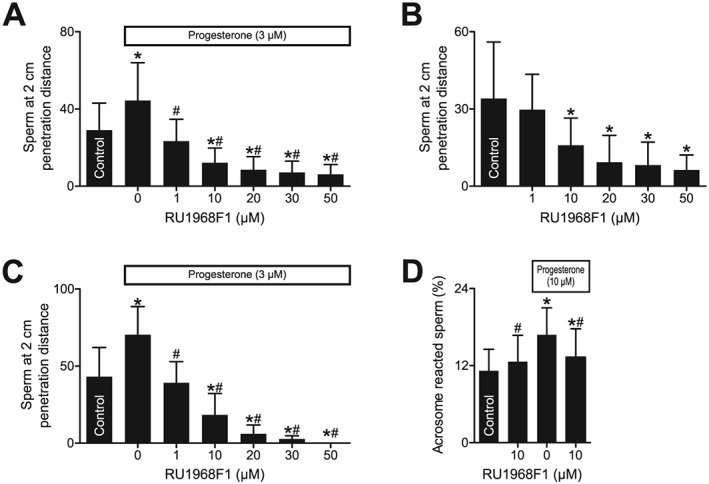
RU1968F1 suppresses penetration of sperm into viscous media. (A) Number of sperm at a penetration distance of 2 cm in a modified Kremer's sperm–mucus penetration test. The sperm were incubated in buffer (control), progesterone, or progesterone plus RU1968F1 (n = 21). Data shown are means ± SD. *P < 0.05, significantly different from control; # P < 0.05, significantly different from progesterone without RU1968F1. (B) Number of sperm after incubation in buffer (control) or RU1968F1 (n = 21). Data shown are means ± SD. *P < 0.05, significantly different from control (n = 21). (C) Number of sperm when the sperm were bathed in buffer (control) or progesterone, in the absence (0) or presence of RU1968F1 in the capillary (n = 6). Data shown are means ± SD. *P < 0.05, significantly different from control; #P < 0.05, significantly different from progesterone without RU1968F1. (D) Acrosome reaction evoked by RU1968F1, progesterone, or progesterone and RU1968F1 (n = 10). Data shown are means ± SD. *P < 0.05, significantly different from control, # P < 0.05, significantly different from progesterone.
Incubation of human sperm in high micromolar concentrations of progesterone evokes acrosomal exocytosis (Baldi et al., 2009) (Figure 9D), that is, the release of proteolytic enzymes from a secretory vesicle in the sperm head. In the presence of RU1968F1, this action of progesterone was attenuated (Figure 9D), whereas RU1968F1 itself did not evoke acrosomal exocytosis (Figure 9D and Supporting Information Figure S3). These results suggest that the progesterone‐induced acrosome reaction involves Ca2+ influx via CatSper and that RU1968F1 might allow the elucidation of the role of CatSper in this process in more detail. Altogether, we conclude that RU1968F1 can provide important insight on the role of progesterone action on CatSper to control sperm function.
RU19681F1 inhibits chemotaxis of sea urchin sperm
Finally, we tested whether RU1968F1 affected the CatSper‐mediated chemotactic steering of sea urchin sperm. In a shallow observation chamber under a dark‐field microscope, sperm were bathed in a caged derivative of the chemoattractant resact (Kaupp et al., 2003; Böhmer et al., 2005; Alvarez et al., 2012). A chemoattractant gradient was established by photolysis of caged resact via a UV flash in the centre of the recording chamber (Figure 10A). After the flash, sperm accumulated in the irradiated area, indicated by a decrease of sperm dispersion in the field of view (Figure 10B). Such accumulation was abolished by RU1968F1 (Figure 10), without affecting the overall motility of the sperm.
Figure 10.
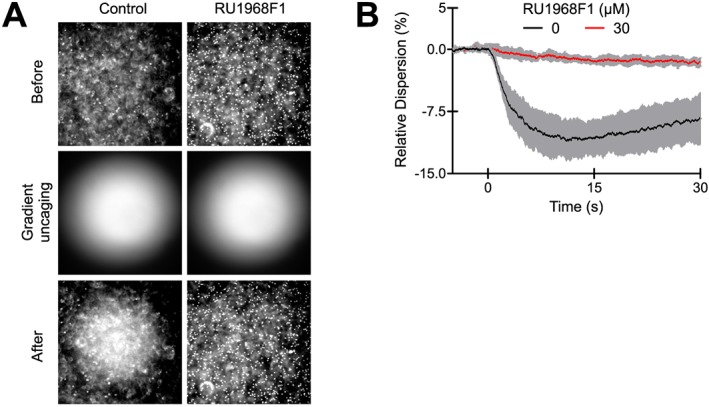
RU1968F1 abolishes chemotaxis of sea urchin sperm. (A) Dark‐field microscopy images of a sperm suspension before (top) and after (bottom) establishing a resact gradient by photolysis of caged resact (middle) in the absence (control, left panel) or presence of RU1968F1 (30 μM, right panel). RU1968F1 abolishes resact‐induced sperm accumulation. (B) Relative change of the sperm dispersion in the field of view evoked by uncaging of resact (t = 0, flash) in the absence (control) or presence of RU1968F1; a decrease of dispersion indicates sperm accumulation in the irradiated area (control, n = 5, RU1968F1, n = 6). Data shown are means ± SD.
Discussion
Here, we introduce RU1968F1 as a new pharmacological tool to elucidate the presence and role of CatSper channels in sperm. RU1968F1 is superior to previously known inhibitors: the compound is relatively selective for CatSper and lacks toxic side effects in human sperm. However, because the action of the compound is complex, we cannot exclude adverse actions in other sperm species. This cautious note has to be considered in futher studies of RU1968F1.
What is the molecular mechanism underlying inhibition of CatSper by RU1968F1? It has been proposed that progesterone acts via an endocannabinoid‐signalling pathway, involving the receptor α/β hydrolase domain‐containing protein 2 (ABHD2) (Miller et al., 2016). At rest, CatSper is inhibited by the endocannabinoid http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=729 in the flagellar membrane. Upon progesterone binding, ABHD2 degrades 2‐AG and, thereby, relieves CatSper from inhibition (Miller et al., 2016). Considering that RU1968F1 is a steroid, this compound might act as an antagonist at the steroid‐binding site on ABHD2. However, CatSper channel activation by PGs does not involve ABHD2 (Miller et al., 2016), and in mouse and sea urchin sperm, CatSper channels are not activated by progesterone or PGs (Lishko et al., 2011; Seifert et al., 2015). Moreover, activation of CatSper channels by alkaline pHi and depolarization does probably not involve a ligand‐binding site. Therefore, we suspect that RU1968F1 binds to residues in the pore region and, thereby, directly block ion flux. The inhibitory action of this compound on classical voltage‐gated Ca2+ channels also supports this conclusion. Of note, the potency of RU1968F1 to inhibit activation of CatSper channels by alkalization seems to decrease with increasing amplitude of ΔpHi. This might reflect a pH sensitivity of the blocking mechanism or pH‐dependent distribution of RU1968F1 across membranes. The latter is rather unlikely. Upon rapid mixing, the compound blocks Ca2+ influx through CatSper without a measurable latency, suggesting that the compound is more likely to act from the outside. However, the mechanism of CatSper channel inhibition will be difficult to elucidate rigorously by structure–function analysis or site‐directed mutagenesis, because CatSper channels resist functional expression.
What is the nature of the blocking site in Slo3 in human sperm? Human, but not mouse Slo3 channels, are inhibited by micromolar concentrations of progesterone (Brenker et al., 2014). This inhibition results from binding of progesterone either to a site on the channel itself or on its accessory subunit LRRC52 (Brenker et al., 2014). RU1968F1 might act via this steroid‐binding site on human Slo3. To improve the selectivity of RU1968F1, a structure–activity analysis is required to identify derivatives that do not act on human Slo3, but display a similar or even enhanced potency to inhibit CatSper. The fact that human Slo3 can be functionally expressed in cultured cells (Brenker et al., 2014) facilitates this endeavour.
Although the make‐up of Ca2+‐signalling pathways in sperm is quite diverse (Kaupp and Strünker, 2016; Alvarez, 2017), the CatSper channel is a common component in many, but not all species (Cai et al., 2014). Our finding that RU1968F1 inhibits CatSper across species opens the possibility to use the compound in a variety of experimental settings. First, teleost fish seem to lack CatSper genes (Cai and Clapham, 2008), although the swimming behaviour of zebrafish is controlled by Ca2+ (Fechner et al., 2015). However, the absence of CatSper channels in fish has been contested (Yanagimachi et al., 2017). RU1968F1 might help to solve this controversy. Second, the genome of many marine species, including the saprophytic fungus Allomyces macrogynus, the tunicate Ciona intestinalis and the seastar Asterias amurensis harbour CatSper genes (Cai and Clapham, 2008; Cai et al., 2014). Sperm from these species also undergo chemotaxis (Miller, 1975; Pommerville, 1978; Yoshida et al., 2002; Matsumoto et al., 2003). RU1968F1 might reveal whether chemotaxis involves CatSper. Third, the drug might help to define the diverse functions of CatSper among mammalian sperm. For example, mouse sperm undergo rotational motion that governs rheotaxis in gradients of flow velocities (Miki and Clapham, 2013). By contrast, CatSper−/− mouse sperm do not rotate and fail to undergo rheotaxis, suggesting that Ca2+ influx via CatSper channels is required. However, another study describes rheotaxis as a passive process that does not require Ca2+ influx (Zhang et al., 2016). CatSper recruit several proteins into Ca2+‐signalling domains that form a quadrilateral arrangement along the flagellar membrane (Chung et al., 2014; Chung et al., 2017). Targeted deletion of CatSper subunits disrupts these signalling domains (Chung et al., 2014, Chung et al., 2017). Therefore, the motility defects of CatSper−/− mouse sperm might be caused by the lack of Ca2+ influx via CatSper, by disruption of the supramolecular flagellar ultrastructure, or by a combination of both. Fourth, in human sperm, neither the role of oviductal CatSper ligands nor the role of CatSper during fertilization has been fully established. This is due to the demanding challenge to mimic the complex chemical, hydrodynamic and topographical environment of the oviduct in vitro (Xiao et al., 2017). We envision the use of RU1968F1 as a tool to study the role of CatSper and its ligands in human sperm, navigating across artificial or explanted oviducts.
Finally, mutations in CATSPER genes (Avenarius et al., 2009; Hildebrand et al., 2010) and the lack of functional CatSper channels (Williams et al., 2015) are associated with male infertility. In human sperm, at least in vitro, RU1968F1 mimics the lack of CatSper channels, indicating that inhibition of these channels in vivo might prevent fertilization. Thus, RU1968F1 could serve as a lead structure to develop new non‐hormonal contraceptives. Drugs that specifically target CatSper should exhibit no side effects, because the expression of the channel is confined to sperm.
Author contributions
A.R. and T.S. conceived and designed the study, coordinated the experiments and wrote the manuscript. A.R., C.S., C.B., D.F., T.E.N., Y.M.C., L.T., M.B., G.S., T.K.B., M.K., L.A., D.W., X.H.Z., E.B., S.P., U.B.K. and T.S. acquired, analysed and/or interpreted data and revised the manuscript critically for important intellectual content. All authors approved the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Relative stereochemistry at C18 and C20 in 3a. Right panel: H‐H COESY spectrum of 3a. C18‐CH3 groups were identified as singlets at δ = 0.73 ppm (blue, major isomer) and δ = 0.80 ppm (red, minor isomer). CH3 groups at C21 were identified as overlapping doublets (δ = 1.09 ppm major isomer and δ = 1.10 ppm minor isomer), each of which could be cross‐referenced to a multiplet at δ > 2.5 ppm, which consequently was assigned to the CH‐group at C20. (Major isomer, dashed blue lines, δ = 1.09 ppm → δ = 2.64 ppm; minor isomer, dashed red lines, δ = 1.10 ppm → δ = 2.76 ppm). Left panel: NOE experiments to assign relative stereochemistry between C18 and C20. Blue box: major isomer; irradiation of the C18‐CH3 singlet (δ = 0.73 ppm) results in a strong negative NOE resonance at δ = 2.64 ppm, indicating spatial proximity of C18‐ and C20‐protons. Cis configuration was assigned for this isomer. Red box: minor isomer; irradiation of the C18‐CH3 singlet (δ = 0.80 ppm) results in no observable NOE resonance at δ = 2.76 ppm, indicating no spatial proximity of C18‐ and C20‐protons. Trans configuration was assigned for this isomer.
Figure S2 Relative stereochemistry at C18 and C20 in 3b. Right panel: H‐H COESY spectrum of 3b. C18‐CH3 groups are easily identified as singlets at δ = 0.72 ppm (blue, major isomer) and δ = 0.85 ppm (red, minor isomer). CH3 groups at C21 are identified as doublets (δ = 1.16 ppm major isomer and δ = 1.05 ppm minor isomer), each of which could be cross referenced to a multiplet at δ > 2.5 ppm, which consequently was assigned to the CH‐group at C20. (Major isomer, dashed blue lines, δ = 1.16 ppm → δ = 2.55 ppm; minor isomer, dashed red lines, δ = 1.05 ppm → δ = 2.64 ppm). Left panel: NOE experiments to assign relative stereochemistry between C18 and C20. Blue box: major isomer; irradiation of the C18‐CH3 singlet (δ = 0.72 ppm) results in a strong negative NOE resonance at δ = 2.55 ppm, indicating spatial proximity of C18‐ and C20‐protons. Cis configuration was assigned for this isomer. Red box: minor isomer; irradiation of the C18‐CH3 singlet (δ = 0.85 ppm) results in no observable NOE resonance at δ = 2.64 ppm, indicating no spatial proximity of C18‐ and C20‐protons. Trans configuration was assigned for this isomer.
Figure S3 The action of RU1968F1 itself in human sperm. (A) RU1968F1‐induced Ca2+ signals in human sperm. (B) Dose–response relation of the RU1968F1‐evoked signal amplitudes (n = 3). Error bars indicate SD. (C) RU1968F1‐induced Ca2+ signals in human sperm at 10 nM extracellular Ca2+. (D) Changes in pHi evoked by RU1968F1 or NH4Cl in sperm loaded with the pH indicator BCECF. The signals were recorded in the FluoStar. (E) Fraction of immotile sperm in the absence (n = 9) and presence of the RU1968F1 (30 μM), MDL12330A (100 μM), Mibefradil (60 μM), or NNC‐55‐0396 (30 μM) (n = 5). Error bars indicate SD. *P < 0.05 versus control. (F) Fraction of vital cells in the absence and presence of the inhibitors (n = 5). Vitality was evaluated by the Eosin test, performed according to the WHO manual. Error bars indicate SD. *P < 0.05 versus control (G) Fold increase in acrosome‐reacted sperm after treatment with RU1968F1 (30 μM), Mibefradil (40 μM), or NNC‐55‐0396 (20 μM) (n = 10). Error bars indicate SD. *P < 0.05 versus control.
Figure S4 RU1968F1 inhibits depolarization‐ and alkaline‐evoked Ca2+ signals in human sperm. (A) Ca2+ signals evoked by mixing of sperm with pH 8.6‐HTF and RU1968F1 in a stopped‐flow apparatus. The final pH after mixing was 8.1. (B) Ca2+ signals evoked by mixing of sperm with K+‐HTF and RU1968F1 in a stopped‐flow apparatus. The final K+ concentration after mixing was 51.25 mM.
Figure S5 RU1968F1 inhibits progesterone responses in sperm bathed in NH4Cl. (A) Progesterone‐induced Ca2+ signals (500 nM) in human sperm bathed for 20 min in 30 mM NH4Cl. (B) Dose–response relation of signals from (A). Mean IC50: 3.1 ± 1.2 (n = 5).
Figure S6 Action of RU1968F2‐F4 on their own and on CatSper‐mediated Ca2+ signals in human sperm populations. (A) RU1968F2‐induced Ca2+ signals in human sperm. (B) Progesterone (500 nM)‐induced Ca2+ signals in human sperm in the presence of RU1968F2; (C) Dose–response relation of the signal amplitudes from (B) (IC50 = 1.6 μM). (D) RU1968F3‐induced Ca2+ signals in human sperm. (E) Progesterone‐induced Ca2+ signals in the presence of RU1968F3. (F) Dose–response relation of the signal amplitudes from (E) (IC50 = 2.7 μM). (G) RU1968F4‐induced Ca2+ signals in human sperm. (H) Progesterone‐induced Ca2+ signals in human sperm in the presence of RU1968F4. (I) Dose–response relation of the signal amplitudes from (H) (IC50 = 5.8 μM).
Figure S7 Action of RU1968F1 on Hv1 currents in human sperm and ATP‐evoked Ca2+ responses in mouse sperm. (A) Voltage‐gated proton currents recorded from a human sperm cell before (blue) and after superfusion with RU1968F1 (red). (B) Mean outward current before and after application of RU1968F1 (n = 3). Arrow in panel (A) indicates the time point at which outward currents were determined. Error bars indicate SD. (C) ATP‐evoked Ca2+ signals in CatSper1−/− mouse sperm in the absence and presence of RU1968F1. The Ca2+ responses were recorded in the FluoStar. ATP was injected at t = 0 s.
Figure S8 RU1968F1 suppresses penetration of sperm into viscous media. (A) Number of sperm at penetration distances of 0–4 cm in a modified Kremer's sperm‐mucus penetration test. Sperm were incubated in buffer (0) or RU1968F1 (n = 21). Error bars indicate SD. *P < 0.05 versus control (0, without RU1968F1). #P < 0.05 versus progesterone without RU1968F1 (B) Number of sperm after incubation in buffer (0), progesterone, or progesterone plus RU1968F1 (n = 21). Error bars indicate SD. *P < 0.05 versus control, #P < 0.05 versus progesterone without RU1968F1 (0, without progesterone). (C) Number of sperm bathed in buffer (control) or progesterone when the capillary included buffer (0) or RU1968F1 (n = 6). Error bars indicate SD. *P < 0.05 versus control (0, without progesterone), #P < 0.05 versus progesterone without RU1968F1 (0, without progesterone).
Figure S9 RU1968F1 inhibits CaV1.2 channels. Representative whole‐cell current recorded from a HEK293T cell expressing human CaV1.2 + β2b + α2δ1 before (A) and after perfusion of the cell with 50 μM RU1968F1 (B). (C) Plotting the mean peak current versus voltage demonstrates the incomplete block of CaV1.2 by RU1968F1 (n = 5). (D) Peak currents at +20 mV recorded every 2 s while the cell was perfused with external solution or solution containing 50 μM RU1968F1. The reduction and recovery of the current during the wash‐in (red) and wash‐out (blue) of RU1968F1, respectively, was fitted with an exponential function. The time constants were used to calculate an approximate affinity (k D) of 31 ± 9 μM (n = 4).
Figure S10 RU1968F1 inhibits CaV3.2 channels. Representative whole‐cell current recorded from a HEK293T cell expressing CaV3.2 before (A) and after perfusion of the cell with RU1968F1 (B). (C) Plotting the mean peak current versus voltage demonstrates the incomplete block of CaV3.2 by RU1968F1 (n = 5). (D) Peak currents at +20 mV recorded every 2 s while the cell was perfused with external solution or solution containing 50 μM RU1968F1. The reduction and recovery of the current during the wash‐in (red) and wash‐out (blue) of RU1968F1, respectively, was fitted with an exponential function. The time constants were used to calculate an approximate affinity (k D) of 16 ± 3 μM (n = 4).
Acknowledgements
This work was supported by the German Research Foundation (SFB645 to T.S., U.B.K. and D.W.; SFB1089 to U.B.K.; SPP1926, 1726 and TRR83 to D.W.; and CRU326 to T.S.); the Cells‐in‐Motion (CiM) Cluster of Excellence, Münster (FF‐2016‐17 to T.S.); the ImmunoSensation Cluster of Excellence, Bonn (to U.B.K. and D.W.); the Fritz‐Thyssen‐Foundation and the Boehringer Ingelheim Foundation (to D.W.), the fund Innovative Medical Research (IMF) of the University of Münster Medical School, Münster, (I‐BR121507 to C.B.); and the CAPES Foundation (Ministry of Education, Brazil, to E.T.N.). We thank David Clapham (Janelia Research Campus, USA) for kindly providing the CatSper1‐KO mice.
Rennhack, A. , Schiffer, C. , Brenker, C. , Fridman, D. , Nitao, E. T. , Cheng, Y.‐M. , Tamburrino, L. , Balbach, M. , Stölting, G. , Berger, T. K. , Kierzek, M. , Alvarez, L. , Wachten, D. , Zeng, X.‐H. , Baldi, E. , Publicover, S. J. , Benjamin Kaupp, U. , and Strünker, T. (2018) A novel cross‐species inhibitor to study the function of CatSper Ca2+ channels in sperm. British Journal of Pharmacology, 175: 3144–3161. https://doi.org/10.1111/bph.14355.
References
- Alasmari W, Barratt CLR, Publicover SJ, Whalley KM, Foster E, Kay V et al (2013a). The clinical significance of calcium‐signalling pathways mediating human sperm hyperactivation. Hum Reprod 28: 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L et al (2013b). Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J Biol Chem 288: 6248–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174 (Suppl 1): S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez L (2017). The tailored sperm cell. J Plant Res 130: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez L, Dai L, Friedrich BM, Kashikar ND, Gregor I, Pascal R et al (2012). The rate of change in Ca2+ concentration controls sperm chemotaxis. J Cell Biol 196: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez L, Friedrich BM, Gompper G, Kaupp UB (2014). The computational sperm cell. Trends Cell Biol 24: 198–207. [DOI] [PubMed] [Google Scholar]
- Ananchenko SN, Limanov VY, Leonov VN, Rzheznikov VN, Torgov IV (1962). Syntheses of derivates of oestrane and 19‐norsteroids from 6‐methoxytetralone and 6‐hydroxytetralone. Tetrahedron 18: 1355–1367. [Google Scholar]
- Ananchenko SN, Torgov IV (1963). New syntheses of estrone, d,1‐8‐iso‐oestrone and d,1‐19‐norestosterone. Tetrahedron Lett 4: 1553–1558. [Google Scholar]
- Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LLH, Kahrizi K et al (2009). Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet 84: 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Muratori M, Marchiani S, Tamburrino L, Forti G (2009). Nongenomic activation of spermatozoa by steroid hormones: facts and fiction. Mol Cell Endocrinol 308: 39–46. [DOI] [PubMed] [Google Scholar]
- Böhmer M, Van Q, Weyand I, Hagen V, Beyermann M, Matsomoto M et al (2005). Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J 24: 2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krähling M et al (2012). The CatSper channel: a polymodal chemosensor in human sperm. EMBO J 31: 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Zhou Y, Müller A, Echeverry FA, Trötschel C, Poetsch A et al (2014). The Ca2+−activated K+ current of human sperm is mediated by Slo3. Elife 3: e01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Schiffer C, Wagner IV, Tüttelmann F, Röpke A, Rennhack A et al (2018). Action of steroids and plant triterpenoids on CatSper Ca2+ channels in human sperm. Proc Natl Acad Sci U S A 115: E344–E346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Clapham DE (2008). Evolutionary genomics reveals lineage‐specific gene loss and rapid evolution of a sperm‐specific ion channel complex: CatSpers and CasSperβ. PLos One 3: e3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang X, Clapham DE (2014). Early evolution of the eukaryotic Ca2+ singaling machinery: conversation of the CatSper channel complex. Mol Biol Evol 31: 2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Burnett LA, del Camino D, Quill TA, Hille B, Chong JA et al (2009). Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PloS One 4: e6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF (2005). Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem 280: 32238–32244. [DOI] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill TA, Ren D, Clapham DE, Hille B et al (2003). CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci U S A 100: 14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez JC, De la Vega‐Beltrán JL, José O, Torres P, Nishigaki T, Treviño CL et al (2017). Acrosomal alkalization triggers Ca2+ release and acrosome reaction in mammalian spermatozoa. J Cell Physiol . https://doi.org/10.1002/jcp.26262. [DOI] [PubMed] [Google Scholar]
- Chung JJ, Miki M, Kim D, Shim SH, Shi HF, Hwang JY et al (2017). CatSperζ regulates the structural continuity of sperm Ca2+ signaling domains and is required for normal fertility. Elife 6: e23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE (2011). A novel gene required for male fertility and functional CatSper channel formation in spermatozoa. Nat Commun 2: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE (2014). Structurally distinct Ca2+ signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 157: 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Brit J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal‐Enríquez J, Priego‐Espinosa DA, Darszon A, Beltrán C, Martínez‐Mekler G (2017). Network model predicts that CatSper is the main Ca2+ channel in the regulation of sea urchin sperm motility. Sci Rep 7: 4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner S, Alvarez L, Bönigk W, Müller A, Berger TK, Pascal R et al (2015). A K+‐selective CNG channel orchestrates Ca2+ signaling in zebrafish sperm. Elife 4: e07624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CV, Kirkman‐Brown JC, Barratt CLR, Publicover SJ (2003). Encoding of progesterone stimulus intensity by intracellular [Ca2+] ([Ca2+]i) in human spermatozoa. Biochem J 372: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, Auer J et al (2010). Genetic male infertility and mutation of CatSper ion channels. Eur J Hum Genet 18: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez SS (2009). CatSper‐null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod Feril Dev 21: 345–350. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Solzin J, Hildebrand E, Brown JE, Helbig A, Hagen V et al (2003). The signal flow and motor response controlling chemotaxis of sea urchin sperm. Nat Cell Biol 5: 109–117. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Strünker T (2016). Signaling in sperm: more different than similar. Trends Cell Biol 27: 101–109. [DOI] [PubMed] [Google Scholar]
- Kilic F, Kashikar ND, Schmidt R, Alvarez L, Dai L, Weyand I et al (2009). Caged progesterone: a new tool for studying rapid nongenomic actions of progesterone. J Am Chem Soc 131: 4027–4030. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE (2006). Whole‐cell patch‐clamp measurements of spermatozoa reveal an alkaline‐activated Ca2+ channel. Nature 439: 737–740. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Federenko A, Kirichok Y (2010). Acid extrusion from human spermatozoa is mediated by flagellar voltage‐gated proton channel. Cell 140: 327–337. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y (2011). Progesterone activates the principal Ca2+ channel of human sperm. Nature 471: 387–391. [DOI] [PubMed] [Google Scholar]
- Liu J, Xia J, Cho KH, Clapham DE, Ren D (2007). CatSperβ, a novel transmembrane protein in the CatSper channel complex. J Biol Chem 282: 18945–18952. [DOI] [PubMed] [Google Scholar]
- Loux SC, Crawford KR, Ing NH, Gonzales‐Fernandez L, Macias‐Garcia B, Love C et al (2013). CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol Reprod 89: 123. [DOI] [PubMed] [Google Scholar]
- Mansell SA, Publicover SJ, Barratt CLR, Wilson SM (2014). Patch clamp studies of human sperm under physiological ionic conditions reveal three functionally and pharmacologically distinct cation channels. Mol Hum Reprod 20: 392–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Solzin J, Helbig A, Hagen V, Ueno SI, Kawase O et al (2003). A sperm‐activating peptide controls a cGMP‐signaling pathway in starfish sperm. Dev Biol 260: 314–324. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki M, Clapham DE (2013). Rheotaxis guides mammalian sperm. Curr Biol 23: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Mannowetz N, Iavarone AT, Safavi R, Gracheva EO, Smith JF et al (2016). Unconventional endocannabinoid singaling governs sperm activation via the sex hormone progesterone. Science 352: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL (1975). Chemotaxis of the spermatozoa of Ciona intestinalis . Nature 254: 244–245. [DOI] [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D (1998). Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod 13: 2139–2146. [DOI] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE (2007). KSper, a pH‐sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A 104: 7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Miki K, Clapham DE (2011). ATP‐activated P2X2 current in mouse spermatozoa. Proc Natl Acad Sci U S A 108: 14342–14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren‐Benaroya R, Orvieto R, Gakamsky A, Pinchasov M, Eisenbach M (2008). The sperm chemoattractant secreted from human cumulus cells is progesterone. Hum Repod 23: 2339–2345. [DOI] [PubMed] [Google Scholar]
- Pommerville J (1978). Analysis of gamete and zygote motility in allomyces . Exp Cell Res 113: 161–172. [DOI] [PubMed] [Google Scholar]
- Publicover SJ, Giojalas LC, Teves ME, Mendes Machado de Oliveira GS, Morales Garcia AA, Barratt CLR et al (2008). Ca2+ signaling in the control of motility and guidance in mammalian sperm. Front Biosci 13: 5623–5637. [DOI] [PubMed] [Google Scholar]
- Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, Krapivinsky L et al (2007). All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A 104: 1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill TA, Ren D, Clapham DE, Garbers DL (2001). A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q et al (2001). A sperm ion channel required for sperm motility and male fertility. Nature 413: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Martinez‐Lopez P, De la Vega‐beltran JL, Butler A, Alisio A, Darszon A et al (2010). The Slo3 sperm‐specific potassium channel plays a vital role in male fertility. FEBS Lett 584: 1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Habenicht UF, Bräutigam M, Gudermann T (2000). Steroidal sigma receptor ligands affect signaling pathways in human spermatozoa. Biol Reprod 63: 57–63. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hofmann T, Schultz G, Gudermann T (1998). A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc Natl Acad Sci U S A 95: 3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C, Müller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A et al (2014). Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep 15: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz AW, Dubin NH (1981). Progesterone and prostaglandin secretion by ovulated rat cumulus cell‐oocyte complexes. Endocrinology 108: 457–463. [DOI] [PubMed] [Google Scholar]
- Seifert R, Flick M, Bönigk W, Alvarez L, Trötschel C, Poetsch A et al (2015). The CatSper channel controls chemosensation in sea urchin sperm. EMBO J 34: 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R et al (2011). The CatSper channel mediates progesterone induced Ca2+ influx in human sperm. Nature 471: 382–386. [DOI] [PubMed] [Google Scholar]
- Suarez SS (2008). Control of hyperactivation in sperm. Hum Reprod Update 14: 647–657. [DOI] [PubMed] [Google Scholar]
- Tamburrino L, Marchiani S, Minetti F, Forti G, Muratori M, Baldi E (2014). The CatSper calcium channel in human sperm: relation with motility and involvement in progesterone‐induced acrosome reaction. Hum Reprod 29: 418–428. [DOI] [PubMed] [Google Scholar]
- Tamburrino L, Marchiani S, Vicini E, Muciaccia B, Cambi M, Pellegrini S et al (2015). Quantification of CatSper1 expression in human spermatozoa and relation to functional parameters. Hum Reprod 30: 1532–1544. [DOI] [PubMed] [Google Scholar]
- Van Leusen D, Van Leusen AM (2004). Synthetic uses of tosylmethyl isocyanide (TosMic). Organic Reactions 57: 417–666. [Google Scholar]
- Wang H, Liu J, Cho KH, Ren D (2009). A novel, single, transmembrane protein CatSperγ is associated with CatSper1 channel protein. Biol Reprod 81: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HL, Mansell S, Alasmari W, Brown SG, Wilson SM, Sutton KA et al (2015). Specific loss of CatSper function is sufficient to compromise fertilization capacity of human spermatozoa. Hum Reprod 30: 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010). World Health Organization Department of Reproductive Health and Research. WHO laboratory manual for the examination and processing of human semen, fifth edition.
- Yanagimachi R, Harumi T, Matsubara H, Yan W, Yuan S, Hirohashi N et al (2017). Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol Reprod 96: 780–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Copetta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA et al (2017). A microfluidic culture model of the human reproductive tract and 28‐day menstrual cycle. Nat Commun 8: 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Murata M, Inaba K, Morisawa M (2002). A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc Natl Acad Sci U S A 99: 14831–14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu J, Meriano J, Ru C, Xie S, Luo J et al (2016). Human sperm rheotaxis: a passive physical process. Sci Rep 6: 23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Navarro B, Xia XM, Clapham DE, Lingle CJ (2013). Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization‐ and voltage‐activated currents in mouse spermatozoa. J Gen Physiol 142: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM (2011). Deletion of the Slo3 gene abolishes alkalization‐activated K+ current in mouse spermatozoa. Proc Natl Acad Sci U S A 108: 5879–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Relative stereochemistry at C18 and C20 in 3a. Right panel: H‐H COESY spectrum of 3a. C18‐CH3 groups were identified as singlets at δ = 0.73 ppm (blue, major isomer) and δ = 0.80 ppm (red, minor isomer). CH3 groups at C21 were identified as overlapping doublets (δ = 1.09 ppm major isomer and δ = 1.10 ppm minor isomer), each of which could be cross‐referenced to a multiplet at δ > 2.5 ppm, which consequently was assigned to the CH‐group at C20. (Major isomer, dashed blue lines, δ = 1.09 ppm → δ = 2.64 ppm; minor isomer, dashed red lines, δ = 1.10 ppm → δ = 2.76 ppm). Left panel: NOE experiments to assign relative stereochemistry between C18 and C20. Blue box: major isomer; irradiation of the C18‐CH3 singlet (δ = 0.73 ppm) results in a strong negative NOE resonance at δ = 2.64 ppm, indicating spatial proximity of C18‐ and C20‐protons. Cis configuration was assigned for this isomer. Red box: minor isomer; irradiation of the C18‐CH3 singlet (δ = 0.80 ppm) results in no observable NOE resonance at δ = 2.76 ppm, indicating no spatial proximity of C18‐ and C20‐protons. Trans configuration was assigned for this isomer.
Figure S2 Relative stereochemistry at C18 and C20 in 3b. Right panel: H‐H COESY spectrum of 3b. C18‐CH3 groups are easily identified as singlets at δ = 0.72 ppm (blue, major isomer) and δ = 0.85 ppm (red, minor isomer). CH3 groups at C21 are identified as doublets (δ = 1.16 ppm major isomer and δ = 1.05 ppm minor isomer), each of which could be cross referenced to a multiplet at δ > 2.5 ppm, which consequently was assigned to the CH‐group at C20. (Major isomer, dashed blue lines, δ = 1.16 ppm → δ = 2.55 ppm; minor isomer, dashed red lines, δ = 1.05 ppm → δ = 2.64 ppm). Left panel: NOE experiments to assign relative stereochemistry between C18 and C20. Blue box: major isomer; irradiation of the C18‐CH3 singlet (δ = 0.72 ppm) results in a strong negative NOE resonance at δ = 2.55 ppm, indicating spatial proximity of C18‐ and C20‐protons. Cis configuration was assigned for this isomer. Red box: minor isomer; irradiation of the C18‐CH3 singlet (δ = 0.85 ppm) results in no observable NOE resonance at δ = 2.64 ppm, indicating no spatial proximity of C18‐ and C20‐protons. Trans configuration was assigned for this isomer.
Figure S3 The action of RU1968F1 itself in human sperm. (A) RU1968F1‐induced Ca2+ signals in human sperm. (B) Dose–response relation of the RU1968F1‐evoked signal amplitudes (n = 3). Error bars indicate SD. (C) RU1968F1‐induced Ca2+ signals in human sperm at 10 nM extracellular Ca2+. (D) Changes in pHi evoked by RU1968F1 or NH4Cl in sperm loaded with the pH indicator BCECF. The signals were recorded in the FluoStar. (E) Fraction of immotile sperm in the absence (n = 9) and presence of the RU1968F1 (30 μM), MDL12330A (100 μM), Mibefradil (60 μM), or NNC‐55‐0396 (30 μM) (n = 5). Error bars indicate SD. *P < 0.05 versus control. (F) Fraction of vital cells in the absence and presence of the inhibitors (n = 5). Vitality was evaluated by the Eosin test, performed according to the WHO manual. Error bars indicate SD. *P < 0.05 versus control (G) Fold increase in acrosome‐reacted sperm after treatment with RU1968F1 (30 μM), Mibefradil (40 μM), or NNC‐55‐0396 (20 μM) (n = 10). Error bars indicate SD. *P < 0.05 versus control.
Figure S4 RU1968F1 inhibits depolarization‐ and alkaline‐evoked Ca2+ signals in human sperm. (A) Ca2+ signals evoked by mixing of sperm with pH 8.6‐HTF and RU1968F1 in a stopped‐flow apparatus. The final pH after mixing was 8.1. (B) Ca2+ signals evoked by mixing of sperm with K+‐HTF and RU1968F1 in a stopped‐flow apparatus. The final K+ concentration after mixing was 51.25 mM.
Figure S5 RU1968F1 inhibits progesterone responses in sperm bathed in NH4Cl. (A) Progesterone‐induced Ca2+ signals (500 nM) in human sperm bathed for 20 min in 30 mM NH4Cl. (B) Dose–response relation of signals from (A). Mean IC50: 3.1 ± 1.2 (n = 5).
Figure S6 Action of RU1968F2‐F4 on their own and on CatSper‐mediated Ca2+ signals in human sperm populations. (A) RU1968F2‐induced Ca2+ signals in human sperm. (B) Progesterone (500 nM)‐induced Ca2+ signals in human sperm in the presence of RU1968F2; (C) Dose–response relation of the signal amplitudes from (B) (IC50 = 1.6 μM). (D) RU1968F3‐induced Ca2+ signals in human sperm. (E) Progesterone‐induced Ca2+ signals in the presence of RU1968F3. (F) Dose–response relation of the signal amplitudes from (E) (IC50 = 2.7 μM). (G) RU1968F4‐induced Ca2+ signals in human sperm. (H) Progesterone‐induced Ca2+ signals in human sperm in the presence of RU1968F4. (I) Dose–response relation of the signal amplitudes from (H) (IC50 = 5.8 μM).
Figure S7 Action of RU1968F1 on Hv1 currents in human sperm and ATP‐evoked Ca2+ responses in mouse sperm. (A) Voltage‐gated proton currents recorded from a human sperm cell before (blue) and after superfusion with RU1968F1 (red). (B) Mean outward current before and after application of RU1968F1 (n = 3). Arrow in panel (A) indicates the time point at which outward currents were determined. Error bars indicate SD. (C) ATP‐evoked Ca2+ signals in CatSper1−/− mouse sperm in the absence and presence of RU1968F1. The Ca2+ responses were recorded in the FluoStar. ATP was injected at t = 0 s.
Figure S8 RU1968F1 suppresses penetration of sperm into viscous media. (A) Number of sperm at penetration distances of 0–4 cm in a modified Kremer's sperm‐mucus penetration test. Sperm were incubated in buffer (0) or RU1968F1 (n = 21). Error bars indicate SD. *P < 0.05 versus control (0, without RU1968F1). #P < 0.05 versus progesterone without RU1968F1 (B) Number of sperm after incubation in buffer (0), progesterone, or progesterone plus RU1968F1 (n = 21). Error bars indicate SD. *P < 0.05 versus control, #P < 0.05 versus progesterone without RU1968F1 (0, without progesterone). (C) Number of sperm bathed in buffer (control) or progesterone when the capillary included buffer (0) or RU1968F1 (n = 6). Error bars indicate SD. *P < 0.05 versus control (0, without progesterone), #P < 0.05 versus progesterone without RU1968F1 (0, without progesterone).
Figure S9 RU1968F1 inhibits CaV1.2 channels. Representative whole‐cell current recorded from a HEK293T cell expressing human CaV1.2 + β2b + α2δ1 before (A) and after perfusion of the cell with 50 μM RU1968F1 (B). (C) Plotting the mean peak current versus voltage demonstrates the incomplete block of CaV1.2 by RU1968F1 (n = 5). (D) Peak currents at +20 mV recorded every 2 s while the cell was perfused with external solution or solution containing 50 μM RU1968F1. The reduction and recovery of the current during the wash‐in (red) and wash‐out (blue) of RU1968F1, respectively, was fitted with an exponential function. The time constants were used to calculate an approximate affinity (k D) of 31 ± 9 μM (n = 4).
Figure S10 RU1968F1 inhibits CaV3.2 channels. Representative whole‐cell current recorded from a HEK293T cell expressing CaV3.2 before (A) and after perfusion of the cell with RU1968F1 (B). (C) Plotting the mean peak current versus voltage demonstrates the incomplete block of CaV3.2 by RU1968F1 (n = 5). (D) Peak currents at +20 mV recorded every 2 s while the cell was perfused with external solution or solution containing 50 μM RU1968F1. The reduction and recovery of the current during the wash‐in (red) and wash‐out (blue) of RU1968F1, respectively, was fitted with an exponential function. The time constants were used to calculate an approximate affinity (k D) of 16 ± 3 μM (n = 4).


