Abstract
Luminal flow augments Na+ reabsorption in the thick ascending limb more than can be explained by increased ion delivery. This segment reabsorbs 30% of the filtered load of Na+, playing a key role in its homeostasis. Whether flow elevations enhance Na+-K+-2Cl− cotransporter (NKCC2) activity and the second messenger involved are unknown. We hypothesized that raising luminal flow augments NKCC2 activity by enhancing superoxide () production by NADPH oxidase 4 (NOX4). NKCC2 activity was measured in thick ascending limbs perfused at either 5 or 20 nl/min with and without inhibitors of production. Raising luminal flow from 5 to 20 nl/min enhanced NKCC2 activity from 4.8 ± 0.9 to 6.3 ± 1.2 arbitrary fluorescent units (AFU)/s. Maintaining flow at 5 nl/min did not alter NKCC2 activity. The superoxide dismutase mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin chloride blunted NKCC2 activity from 3.5 ± 0.4 to 2.5 ± 0.2 AFU/s when flow was 20 nl/min but not 5 nl/min. When flow was 20 nl/min, NKCC2 activity showed no change with time. The selective NOX1/4 inhibitor GKT-137831 blunted NKCC2 activity when thick ascending limbs were perfused at 20 nl/min from 7.2 ± 1.1 to 4.5 ± 0.8 AFU/s but not at 5 nl/min. The inhibitor also prevented luminal flow from elevating production. Allopurinol, a xanthine oxidase inhibitor, had no effect on NKCC2 activity when flow was 20 nl/min. Tetanus toxin prevents flow-induced stimulation of NKCC2 activity. We conclude that elevations in luminal flow enhance NaCl reabsorption in thick ascending limbs by stimulating NKCC2 via NOX4 activation and increased . NKCC2 activation is primarily the result of insertion of new transporters in the membrane.
Keywords: kidney, NADPH oxidase, sodium reabsorption, transport
INTRODUCTION
The thick ascending limb of the loop of Henle reabsorbs 30% of the filtered NaCl (7, 8) and contributes to the gradient that leads to urine concentration in the renal medulla, thus playing an important role in regulating blood pressure and volume homeostasis. Approximately 60% of NaCl reabsorption in the thick ascending limb is mediated by an electroneutral cotransporter that carries one Na+, one K+, and two Cl− (NKCC2); the rest is reabsorbed via Na+/H+ exchange and paracellular transport (28).
Luminal flow through the nephron is highly variable (21, 63). In the thick ascending limb it ranges from 20 (5, 42, 61) to 0 (21, 61, 63) nl/min. Acutely, it depends on tubuloglomerular feedback (29), glomerular filtration rate (41, 72), reabsorption of fluid by the proximal nephron (41, 73) and peristalsis of the pelvis (21, 61, 63). Chronically, urinary flow increases with high-salt (49) and high-protein (64) diets, diabetes (57), and hypertension (5, 14). Flow of the forming urine alters the reabsorption and secretion of a number of ions and compounds in nearly all nephron segments (17, 22, 31, 32, 55, 58, 69). Increasing luminal flow in thick ascending limbs elevates NaCl reabsorption, a process dependent on NKCC2, above what would be predicted simply on the basis of increased ion delivery (54, 68). The causes of this phenomenon are currently poorly understood. Furthermore, it is not known whether the effect is because of stimulation of NKCC2 activity rather than Na+-K+-ATPase.
Stretch of thick ascending limb cells caused by elevated luminal flow stimulates superoxide () production (24). Increases in NaCl delivery that augment NaCl reabsorption also enhance synthesis (1, 33). The thus produced may further increase Na+ reabsorption in this segment of the nephron. Tempol, a scavenger, decreases net NaCl reabsorption by ∼30% in isolated thick ascending limbs (52), a process dependent on NKCC2 activity. Exogenously added augments both NKCC2 (52) and Na+/H+ exchanger type 3 (NHE3) (36) activity. Furthermore, we have shown that flow-induced stimulates NHE activity in a protein kinase C (PKC)-dependent manner (32). However, it is not known whether flow-induced accounts for flow-enhanced NKCC2 activity.
Many enzymes are capable of producing (39, 40, 67, 71); however, the main sources of in thick ascending limbs are the NADPH oxidase isoforms 4 (NOX4) (34, 46) and 2 (NOX2) (74). NOX4 mediates ANG II-dependent production (12, 46) and has been related to pathological conditions such as diabetes (6, 27) and salt-sensitive hypertension (13, 74). More relevant to this report, we found that NOX4 is the source of stimulated by increases in luminal flow (34), and this NADPH oxidase isoform translocates in response to this stimulus (60). Although these studies suggest the importance of NOX4 as a source of in thick ascending limbs, currently there are no data showing that NOX4 plays a role in flow-augmented NaCl reabsorption or NKCC2 activity in thick ascending limbs. We hypothesized that flow stimulates production by NOX4, and , in turn, enhances NKCC2 activity.
MATERIALS AND METHODS
Chemicals and solutions.
Most reagents were purchased from Sigma Aldrich (St. Louis, MO). Sodium green tetraacetate and dihydroethidium were obtained from Invitrogen (Eugene, OR). 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) was from Cayman (Ann Arbor, MI). Manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) was from Calbiochem (Billerica, MA), and the NOX1/4 specific inhibitor GKT-137831 was from Selleckchem (Houston, TX). Tetanus toxin was purchased from List Biological Laboratories (Campbell, CA). The physiological saline used to perfuse and bathe thick ascending limbs contained (in mmol/l): 130 NaCl, 2.5 NaH2PO4, 4 KCl, 1.2 MgSO4, 6 l-alanine, 1 trisodium citrate, 5.5 glucose, 2 calcium dilactate, and 10 HEPES, pH 7.4 at 37°C. The osmolarity of this solution was 290 ± 3 mosmol/kgH2O.
Animals.
Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) were kept on a diet containing 0.29% Na+ and 1.1% K+ (Purina, Richmond, IN) for at least 5 days before the experiment. All protocols involving animals were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Isolation and perfusion of thick ascending limbs.
Rats weighing 115–170 g were anesthetized with xylazine and ketamine (20 and 100 mg/kg body wt, respectively). The abdominal cavity was opened, and the left kidney was bathed in ice-cold physiological saline before its removal. The inner stripe of the outer medulla was excised from coronal slices and placed in cold physiological saline to dissect medullary thick ascending limbs under a stereomicroscope at 4–10°C. Tubules ranging from 0.5 to 1.0 mm were transferred to a temperature-regulated chamber (37 ± 1°C) and retrograde perfused using concentric glass pipettes as described previously (23). Tubules were perfused at low (∼5 nl/min) or high (∼20 nl/min) flow rates within the physiological range. Flow rates were calibrated by measuring the rate of volume expansion of a drop of perfusion solution created at the end of the perfusion pipet under mineral oil in the absence of a tubule.
Measurement of NKCC2 activity.
NKCC2 activity was measured as previously reported (30, 53, 59). Briefly, isolated thick ascending limbs were perfused at 37°C with a solution designed to minimize NKCC2 activity containing (in mmol/l) 2.5 NaH2PO4, 1.2 MgSO4, 6 l-alanine, 1 Na3 citrate, 5.5 glucose, 2 Ca(lactate)2, 10 HEPES, 240 mannitol, and 10 µmol/l EIPA (0K+-0Cl−-4Na+ solution) to inhibit Na+/H+ exchange, and bathed in physiological saline. Tubules were loaded by bathing them with physiological solution containing 1.7 µmol/l cell-permeant sodium green tetraacetate for 10 min and then washing them for 12 min with physiological saline. Depending on the protocol, tubules were perfused during the equilibration period at either 5 ± 3 or 20 ± 3 nl/min to match the flow rate that was used during the first period as indicated in results. Sodium green was excited at 490 nm, and fluorescence emission was recorded with a Nikon DM510 filter to measure changes in intracellular Na+ () every 5 s. Data were acquired and processed using Metafluor (Molecular Devices, Sunnydale, CA). Next, the luminal solution was switched to physiological saline plus EIPA to stimulate NKCC2. After the measurements in the first period were complete (∼2 min), the perfusate was switched back to 0K+-0Cl−-4Na+ to inhibit NKCC2 activity. Later (10 min), the switch to physiological saline was made again after either changing luminal flow or adding drugs as indicated in results while was measured as in the first period. The first seven points after the luminal perfusion solution was switched to physiological saline were used to calculate the initial rate of fluorescence increase. NKCC2 activity was calculated from the initial rate of change normalized by the fluorescent baseline at the time of the solution switch.
Measurement of .
Tubules were mounted on pipets and perfused at ∼5 nl/min with physiological saline while loading with 5 µmol/l dihydroethidium for 15 min in the bath. Next, they were washed in dye-free solution for 30 min. Before the end of the wash period (15 min), flow was stopped. Oxyethidium and dihydroethidium were excited using 488 and 365 nm light, respectively. Emitted fluorescence intensities were measured between 520 to 600 nm (oxyethidium) and 400 to 450 (dihydroethidium) nm from regions of interest (ROI) with a custom-made dichroic mirror and emission filter (Chroma Technology) one time every 5 s. Data were acquired and processed using Metafluor. After 1 min of measurements in the absence of flow, luminal flow was increased to ∼20 nl/min while measurements were acquired for an additional 2 min. Regression analysis of the first 6–10 fluorescence ratio measurements was performed to obtain initial rates for the “no flow” and “flow” periods. Differences in these rates were assumed to represent flow-stimulated . In separate tubules, the NOX1/4 inhibitor was present in the bath during the wash period and throughout the experiment.
Statistics.
Results are expressed as means ± SE. Data were evaluated with Student's paired t-test. Flow-induced differences in the absence or presence of GKT-137831 were evaluated using a Student's t-test. P < 0.05 was considered significant.
RESULTS
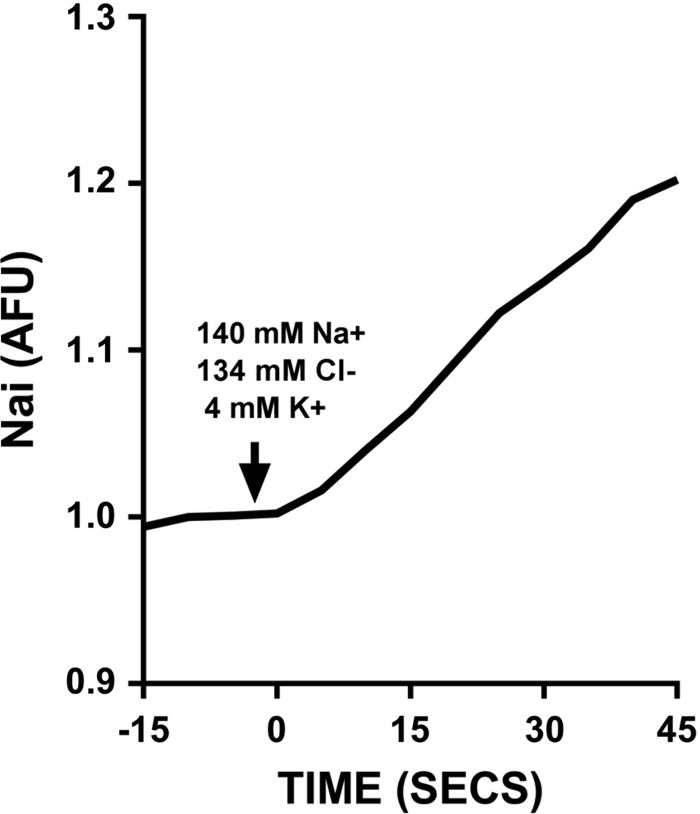
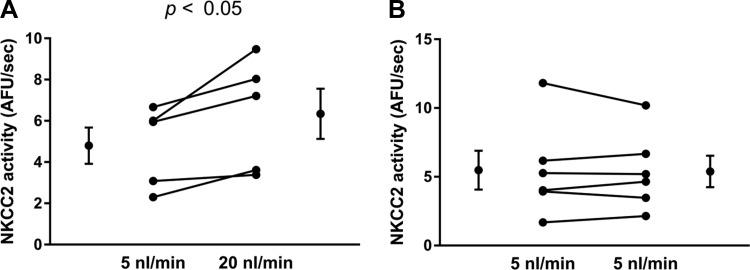
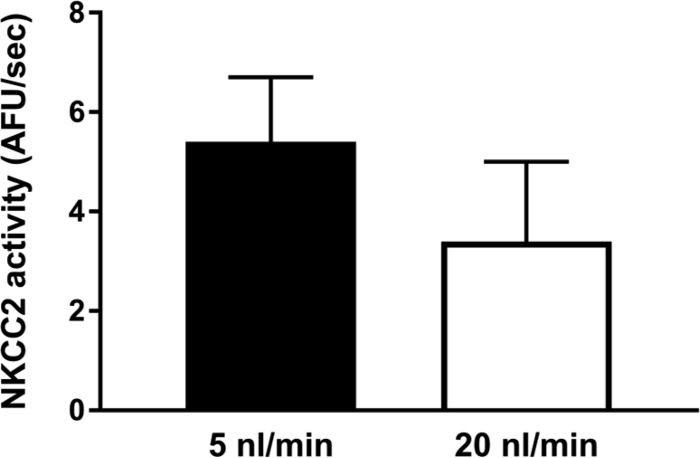
To begin to test our hypothesis, we first evaluated whether different flow rates yielded differences in NKCC2 activity. Figure 1 shows a representative experiment in which was measured over time with the fitted regression line used to calculate NKCC2 activity. was stable when the tubule was perfused with the EIPA-containing 0K+-0Cl−-4Na+ solution and abruptly increased when the luminal solution was switched to physiological saline. Over time the increase in reached a plateau. Figures 2–7, A and B, represent individual experiments and the means ± SE for all experiments. Figures 7C and 8 show means ± SE only. When isolated thick limbs were perfused at 5 nl/min, NKCC2 activity was 4.8 ± 0.9 arbitrary fluorescent units (AFU)/s. Increasing flow to 20 nl/min led to an increase in NKCC2 activity of 32% to 6.3 ± 1.2 AFU/s (n = 5, P < 0.05; Fig. 2A). To demonstrate that the increase in NKCC2 activity seen when luminal flow was increased was the result of the change in flow and not time, we performed control experiments in which luminal flow remained at 5 nl/min. During the initial period NKCC2 activity was 5.5 ± 1.4 AFU/s when tubules were perfused at 5 nl/min, and it was 5.4 ± 1.1 AFU/s, not significantly different, in the second period when flow remained the same (n = 6; Δ: 0.1 ± 0.3 AFU/s; Fig. 2B). These data demonstrate that basal NKCC2 activity is flow dependent.
Fig. 1.
Representative trace of an experiment measuring Na+-K+-2Cl− cotransporter (NKCC2) activity [expressed as increase in intracellular Na+ ()/s] in isolated perfused thick ascending limbs. AFU, arbitrary fluorescent units.
Fig. 2.
A: NKCC2 activity in tubules perfused at 5 and 20 nl/min (n = 5 experiments). B: effect of time on NKCC2 activity in tubules perfused at 5 nl/min (n = 6).
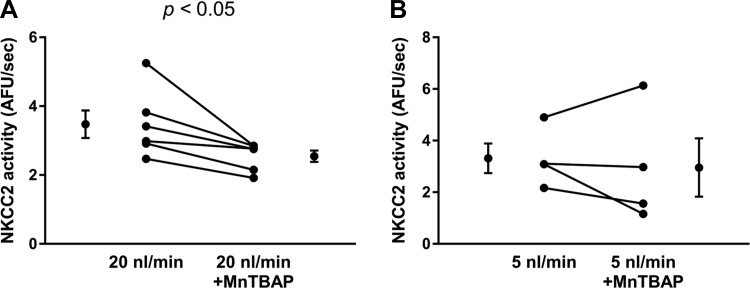
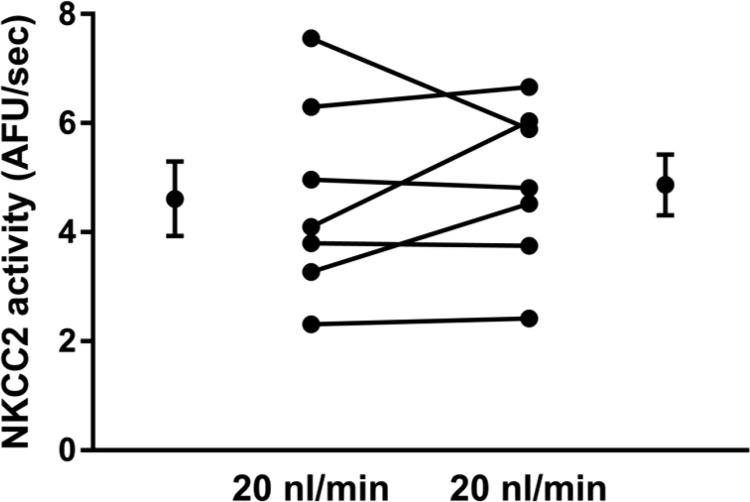
Next, we studied whether flow-induced plays a role in flow-enhanced NKCC2 activity by reducing with MnTBAP, a cell-permeable superoxide dismutase (SOD) mimetic and peroxynitrite scavenger (Fig. 3). When tubules were perfused at ∼20 nl/min basal NKCC2 activity was 3.5 ± 0.4 AFU/s. Treatment with the SOD mimetic MnTBAP (30 µmol/l) caused a 30% decrease in NKCC2 activity to 2.5 ± 0.2 AFU/s (n = 6, P < 0.05; Δ: 0.9 ± 0.3 AFU/s; Fig. 3A). We next studied the effect of scavenging with MnTBAP on NKCC2 activity when thick ascending limbs were perfused at ∼5 nl/min. In these experiments, basal NKCC2 activity was 3.3 ± 0.6 AFU/s during the first period. After a 10-min treatment with the mimetic MnTBAP it was 3.0 ± 1.1 AFU/s, not significantly different (n = 4; Δ: 0.4 ± 0.6 AFU/s; Fig. 3B). To demonstrate that the decrease in NKCC2 activity at high flow rates was because of MnTBAP and not simply a time effect, we performed experiments maintaining luminal flow at 20 nl/min in the absence of compound (Fig. 4). During the first period, NKCC2 activity was 4.6 ± 0.7 AFU/s, and during the second period it was 4.9 ± 0.6 AFU/s. Thus, no decrease in NKCC2 activity was seen because of time. Taken together, the data depicted in Figs. 3 and 4 suggest that luminal flow stimulates endogenous that, in turn, stimulates NKCC2 activity.
Fig. 3.
A:) NKCC2 activity in tubules perfused at 20 nl/min with and without 30 µmol/l of the manganese superoxide () dismuatse mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP), n = 6. B: NKCC2 activity in tubules perfused at 5 nl/min with and without 30 µmol/l MnTBAP, n = 4.
Fig. 4.
Effect of time on NKCC2 activity in tubules perfused at 20 nl/min (n = 7).
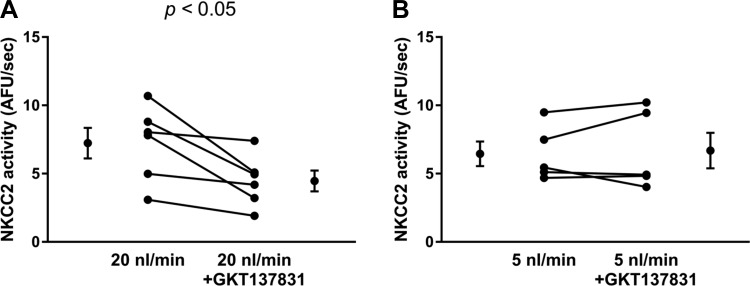
NOX4 is the enzyme that produces in response to increases in luminal flow in thick ascending limbs (34). Thus we tested whether a specific NOX1/4 inhibitor, GKT-137831, was able to blunt NKCC2 activity in tubules perfused at ∼20 nl/min (Fig. 5). When thick ascending limbs were perfused at ∼20 nl/min, NKCC2 activity during the initial basal period was 7.2 ± 1.1 AFU/s (Fig. 5A). After thick ascending limbs were treated with 5 µmol/l GKT-137831 (10 min), NKCC2 activity fell by 38% to 4.5 ± 0.8 AFU/s (n = 6, P < 0.05; Δ: 2.7 ± 0.8 AFU/s; Fig. 5A). In contrast, when thick ascending limbs were perfused at 5 nl/min, NKCC2 activity during the initial basal period was 6.5 ± 0.9 AFU/s. After thick ascending limbs were treated with GKT-137831 (10 min), NKCC2 activity remained unchanged at 6.7 ± 1.3 AFU/s (n = 5; Δ: −0.2 ± 0.5 AFU/s; Fig. 5B). These data suggest that NOX1/4 is the source of that augments NKCC2 activity in response to elevations of luminal flow.
Fig. 5.
A: NKCC2 activity in tubules perfused at 20 nl/min with and without 5 µmol/l NADPH oxidase (NOX) 1/4 specific inhibitor GKT-137831 (n = 6). B: NKCC2 activity in tubules perfused at 5 nl/min with and without 5 µmol/l NOX1/4 specific inhibitor GKT-137831 (n = 5).
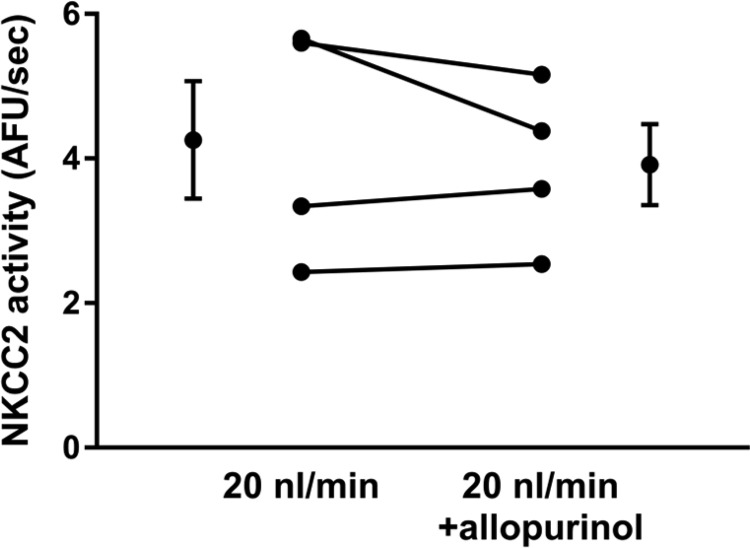
The other primary source of in thick ascending limbs is xanthine oxidase. Therefore, we investigated whether this enzyme contributes to flow-enhanced NKCC2 activity. To test the contribution of xanthine oxidase, we used 100 µmol/l allopurinol, a xanthine oxidase inhibitor (Fig. 6). When perfused at 20 nl/min, NKCC2 activity during the initial basal period was 4.3 ± 0.8 AFU/s. After thick ascending limbs were treated with allopurinol (10 min), NKCC2 activity was 3.9 ± 0.6 AFU/s, not significantly different (Δ: 0.3 ± 0.3 AFU/s; n = 4). These data suggest that xanthine oxidase is not the source of flow-stimulated that enhances NKCC2 activity.
Fig. 6.
NKCC2 activity in tubules perfused at 20 nl/min with and without 100 µmol/l xanthine oxidase inhibitor allopurinol (n = 4).
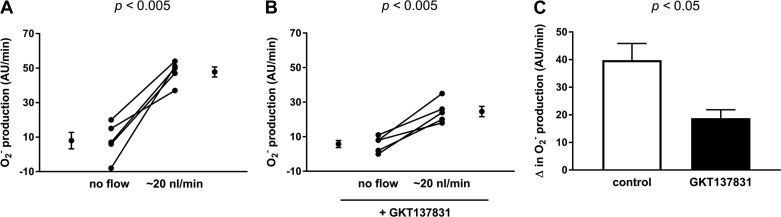
Finally, to demonstrate that the NOX1/4 inhibitor GKT-137831 did, in fact, blunt flow-induced , we measured the ability of 5 µmol/l GKT-137831 to prevent flow from elevating (Fig. 7). In the absence of luminal flow, production was 8.0 ± 4.8 AFU/min, and it increased to 47.8 ± 3.0 AFU/min when flow was increased to 20 nl/min in the absence of GKT-137831 (n = 5, P < 0.005; Δ: 39.8 ± 6.0 AFU/min; Fig. 7A). In contrast, in the presence of the NOX1/4 inhibitor, in the absence of luminal flow production was 5.8 ± 2.1 AFU/min, and it increased only to 24.6 ± 3.0 AFU/min when flow was increased to 20 nl/min (n = 5, P < 0.005; Δ: 18.8 ± 3.1 AFU/min), a 50% decrease in production (P < 0.05; Fig. 7C). These data show that GKT-137831 inhibits flow-induced production.
Fig. 7.
production by thick ascending limbs without and with flow at 20 nl/min. A: without 5 µmol/l NOX1/4 inhibitor GKT-137831. B: with 5 µmol/l NOX1/4 inhibitor GKT-137831. C: GKT-137831 blunts flow-induced production by 50% (n = 5 for each group).
To investigate the mechanism by which flow activates NKCC2, we perfused tubules with tetanus toxin (30 nM). Tetanus toxin cleaves vesicle-associated membrane protein 2/3 thereby preventing insertion of new transporters in the membrane. After tetanus toxin treatment for 20 min, NKCC2 activity was 5.4 ± 1.3 AFU/s at a luminal flow of 5 nl/min. After increasing flow, activity was 3.4 ± 1.6 AFU/s, not significantly different (Fig. 8). Thus flow-stimulated NKCC2 activity was blocked by pretreatment with tetanus toxin.
Fig. 8.
NKCC2 activity in tubules pretreated with 30 nmol/l tetanus toxin perfused at 5 and 20 nl/min (n = 4).
DISCUSSION
The causes of flow-induced increases in Na+ reabsorption along the nephron in general and in the thick ascending limb specifically are poorly understood. Our hypothesis was that elevations in luminal flow stimulate NKCC2 activity via a process dependent on NOX4-generated . We found that: 1) increasing luminal flow in the physiological range stimulated NKCC2 activity in isolated perfused thick ascending limbs; 2) a SOD mimetic, MnTBAP, blunted NKCC2 activity when flow was 20 nl/min but not when it was 5 nl/min; 3) the xanthine oxidase inhibitor allopurinol had no effect on NKCC2 activity when luminal flow was 20 nl/min; 4) the NOX1/4 inhibitor GKT-137831 reduced NKCC2 activity when flow was 20 nl/min but not when it was 5 nl/min; 5) GKT-137831 prevented a flow-induced increase in production; and 6) flow-induced activation of NKCC2 was prevented by pretreatment with tetanus toxin.
Our finding that raising luminal flow within the physiological range stimulates NKCC2 activity is novel. The effect of flow specifically on this transporter had not been investigated previously. However, our results are supported by others in the literature. Raising luminal flow from 10 to 30 nl/min nearly doubled Na+ reabsorption by the loop of Henle in micropuncture experiments. Because the increase was eliminated by bumetanide, it was concluded that the effect was mostly beause of augmented Na+ transport by thick ascending limbs (68). This conclusion is supported by data showing that lumen to bath Na+ and Cl− reabsorption measured individually are both elevated by ∼50% in isolated perfused thick ascending limbs when the perfusion rate was raised from ∼5 to ∼16 nl/min (54). We have shown that increasing luminal flow in perfused thick ascending limbs enhances apical Na+/H+ exchange activity (32), which would be expected to augment bicarbonate reabsorption.
Although there are few reports concerning the effects of luminal flow on net NaCl reabsorption by thick ascending limbs or NKCC2 activity, the effects of flow have been more thoroughly studied in other nephron segments. Proximal tubule fluid and bicarbonate reabsorption both increase as luminal flow is elevated (15, 16, 18). As flow increased in steps from 5 to 30 nl/min, bicarbonate reabsorption was stimulated by >100% (17). It is important to note that the middle and upper ends of this range approximate physiological flows found in proximal tubules in vivo. In the cortical collecting duct, transport is also flow dependent. Raising luminal flow from ∼0.5–1 to ∼3–5 nl/min augmented net Na+ reabsorption at least fourfold or more in isolated perfused tubules (22, 48, 62). This stimulation appears to be the result of activation of the epithelial Na+ channel (ENaC) because amiloride-sensitive whole cell currents were enhanced by flow in oocytes expressing ENaC but not those that had been injected with water rather than RNA (62). Similarly, K+ secretion in the collecting duct is enhanced by elevations in luminal flow (62).
The ability of flow to stimulate NKCC2 activity is unlikely to be the result of just an increase in Na+, K+, and/or Cl− concentration. The F isoform of NKCC2 is most abundant in the inner stripe of the outer medulla. Its K1/2 values for Na+, K+, and Cl− in rodents are 21, 2, and 30 mmol/l, respectively (56). The tubules in this study were ∼0.5 mm in length. Assuming a net NaCl reabsorption of 100 pmol·mm−1·min−1, at an absolute flow rate of 5 nl/min, the Na+ and Cl− concentrations should only fall to ∼125 mmol/l by the end of the tubule. At a flow rate of 20 nl/min, the concentrations would be expected to fall to 132 mmol/l. Thus at both luminal flows the Na+ and Cl− concentrations at the end of the tubule are well above saturating concentrations. A similar case can be made for end-tubule K+ concentration. This argument indicates that something other than simple ion delivery is responsible for the measured increase in NKCC2 activity.
Raising luminal flow enhances production (33), and the latter stimulates NaCl reabsorption (52, 65), a process dependent on NKCC2. Consequently, we next tested the effects of reducing on NKCC2 activity at different flow rates. We found that the SOD mimetic MnTBAP diminished NKCC2 activity at 20 nl/min but had no effect when transporter activity was measured at 5 nl/min. The reduction in NKCC2 activity caused by MnTBAP at 20 nl/min was equal in magnitude to the increase caused by raising flow from 5 to 20 nl/min. The finding that MnTBAP had no effect on NKCC2 activity when the luminal flow was 5 nl/min is consistent with our previous data showing that production with a luminal flow of 5 nl/min is not significantly different from that in the absence of flow and that is augmented only at greater flows (1, 33). The data presented here indicate that flow-induced mediates most, if not all, of the effect of luminal flow on NKCC2 activity.
We did not explore in this study whether cellular stretch, pressure, shear stress, and/or ion delivery are responsible for flow-induced augmentation of . Previously we had shown that both stretch and ion delivery are the important parameters (24, 33). Given this, it is unlikely that cilia play an important role in flow-stimulated NKCC2 activity. To date, there have been no reports that cilia are important in either stretch or ion delivery-activated signaling cascades.
Our finding that mediates most flow-stimulated NKCC2 activity in thick ascending limbs is novel for thick ascending limbs. Although we have reported that elevated levels mediate flow-stimulated Na+/H+ exchange in this segment (31, 32), there have been no reports in other nephron segments that plays a role in flow-stimulated transport. It is probable that activation of PKC rather than changes in cAMP/protein kinase A (PKA) mediate flow-stimulated NKCC2 activity. We have reported that stimulates NKCC2 in thick ascending limbs via activation of PKC (65). Furthermore, we have also shown that PKC mediates flow-induced increases in (35).
The flow dependency of transport in the proximal tubule is because of mechanical stresses causing rearrangement of the cytoskeleton (19) and translocation of apical membrane transporters (20). The increases in fluid and bicarbonate reabsorption caused by elevations in luminal flow correlate with rises in intracellular Ca and can be blunted by reducing extracellular Ca (16). Thus, they may involve mechanosensitive transient receptor potential polycystin 2 or transient receptor potential vanilloid 4 channels similar to thick ascending limbs (9). In cortical collecting ducts, flow-induced activation of ENaC is the result of an increase in channel open probability that is not dependent on elevation of intracellular Ca (11, 48). In contrast, flow-stimulated K+ secretion mediated by the maxi-K+ channel (70) depends on an increase in intracellular Ca (43) and release of prostaglandins (22). These, in turn, likely affect two signaling cascades. First they reduce cAMP and PKA activity possibly via activation of phosphodiesterases. Second, they activate PKC (44).
The mechanism by which flow-induced increases NKCC2 activity has not been explored. Both increased insertion of transporters in the luminal membrane (4, 10, 51) and increased phosphorylation of NKCC2 have been reported as mechanisms of activation (3, 25, 26). We found that tetanus toxin, which prevents insertion of new transporters in the plasma membrane, prevented flow from stimulating NKCC2 activity. These data indicate that increased trafficking to the membrane is required for this phenomena and are in agreement with a previous study in thick ascending limbs that showed blocking trafficking with tetanus toxin inhibited Cl− absorption (51). However, our results do not rule out changes in membrane insertion mediated by NKCC2 phosphorylation (2) or activation of other transporters such as renal outer medullary K+ channel (ROMK), which is also affected by membrane trafficking (45). We did not directly test whether changes in phosphorylation are also involved. At present it is not possible to test this in native perfused thick ascending limbs because of technical limitations. Flow only increased activity by ∼30%. It is unlikely that attempting to measure changes of this magnitude in phosphorylation are possible in isolated perfused tubules.
Thick ascending limbs express several enzymes that can produce either as their primary product or as a byproduct. These include NOX4, xanthine oxidase, mitochondria respiratory enzymes, nitric oxide synthase, and cyclooxygenase to name a few. However, NOX4 and xanthine oxidase are the major sources under physiological conditions. Thus we tested their roles in flow-stimulated NKCC2 activity. We found that a selective NOX1/4 inhibitor but not a xanthine oxidase inhibitor reduced NKCC2 activity at a luminal flow of 20 nl/min but not at 5 nl/min. Furthermore, we found that the NOX1/4 inhibitor blunted flow-induced production. Given that NOX4 is expressed in far greater amounts than NOX1 (34), these data likely indicate that NOX4 is the source of that enhances NKCC2 activity in response to luminal flow.
Our finding that NOX4 is the source of the that is responsible for the stimulatory effect of luminal flow on NKCC2 activity is supported by other reports in the literature. NADH and NADPH oxidases are responsible for the majority of production in the outer medulla (75). from NADPH oxidases also blunts bioavailable NO in thick ascending limbs (47). Finally, we reported that NOX4 but not -1 or -2 mediates flow-induced production (34) and that NOX4 is the source of ANG II-stimulated production (46).
In contrast to our results, both H2O2 and were reported to be elevated by luminal flow in thick ascending limbs from Dahl salt-sensitive rats. H2O2 but not was reduced by knocking out NOX4. In contrast p67phox, a subunit of NOX2, blunted but did not eliminate and H2O2 production (74). The explanation for these results and ours is not clear. However, it has been reported that NOX4 produces both H2O2 and (37, 38, 46, 50, 66). Alternatively these results may only apply to Dahl salt-sensitive rats of the Milwaukee strain but not to outbred Sprague-Dawley rats. Given the importance of this issue to blood pressure regulation, further investigation to resolve these apparently contradictory data is warranted.
In summary, we have found that elevating luminal flow augments NKCC2 activity, and this likely explains the increases in net NaCl reabsorption reported by others under similar conditions. The increase in NKCC2 activity was mediated primarily by flow-induced production through NOX4 rather than xanthine oxidase. This flow-induced increase was largely the result of insertion of new transporters in the luminal membrane, although is it unclear whether NKCC2 phosphorylation or ROMK activity was also involved. Flow-stimulated NKCC2 activity and the resulting increase in net NaCl reabsorption have profound implications for Na+ homeostasis and water balance.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-070985 to J. L. Garvin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.S. and J.L.G. conceived and designed research; F.S. and N.J.H. performed experiments; F.S. and N.J.H. analyzed data; F.S., N.J.H., and J.L.G. interpreted results of experiments; F.S. prepared figures; F.S. and J.L.G. drafted manuscript; F.S., N.J.H., and J.L.G. edited and revised manuscript; F.S., N.J.H., and J.L.G. approved final version of manuscript.
REFERENCES
- 1.Abe M, O’Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW Jr. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006. doi: 10.1152/ajprenal.00407.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ares GR, Caceres PS, Ortiz PA. Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 301: F1143–F1159, 2011. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ares GR, Haque MZ, Delpire E, Ortiz PA. Hyperphosphorylation of Na-K-2Cl cotransporter in thick ascending limbs of Dahl salt-sensitive rats. Hypertension 60: 1464–1470, 2012. doi: 10.1161/HYPERTENSIONAHA.112.202101. [DOI] [PubMed] [Google Scholar]
- 4.Ares GR, Ortiz PA. Constitutive endocytosis and recycling of NKCC2 in rat thick ascending limbs. Am J Physiol Renal Physiol 299: F1193–F1202, 2010. doi: 10.1152/ajprenal.00307.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer PG, Bianchi G, Liliana D. Renal micropuncture study of normotensive and Milan hypertensive rats before and after development of hypertension. Kidney Int 13: 452–466, 1978. doi: 10.1038/ki.1978.68. [DOI] [PubMed] [Google Scholar]
- 6.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burg M, Good D. Sodium chloride coupled transport in mammalian nephrons. Annu Rev Physiol 45: 533–547, 1983. doi: 10.1146/annurev.ph.45.030183.002533. [DOI] [PubMed] [Google Scholar]
- 8.Burg MB. Thick ascending limb of Henle’s loop. Kidney Int 22: 454–464, 1982. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- 9.Cabral PD, Capurro C, Garvin JL. TRPV4 mediates flow-induced increases in intracellular Ca in medullary thick ascending limbs. Acta Physiol (Oxf) 214: 319–328, 2015. doi: 10.1111/apha.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na(+)-K(+)-2Cl(-) cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009. doi: 10.1074/jbc.M109.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004. doi: 10.1074/jbc.M311783200. [DOI] [PubMed] [Google Scholar]
- 12.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013. doi: 10.1152/ajpheart.00974.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67: 440–450, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiBona GF, Rios LL. Mechanism of exaggerated diuresis in spontaneously hypertensive rats. Am J Physiol 235: 409–416, 1978. doi: 10.1152/ajprenal.1978.235.5.F409. [DOI] [PubMed] [Google Scholar]
- 15.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. II. Impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012. doi: 10.1152/ajprenal.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Z, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. III. Implication of cytosolic calcium in flow-dependent proximal tubule transport. Am J Physiol Renal Physiol 308: F839–F847, 2015. doi: 10.1152/ajprenal.00601.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006. doi: 10.1152/ajprenal.00255.2005. [DOI] [PubMed] [Google Scholar]
- 18.Du Z, Yan Q, Wan L, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport. Am J Physiol Renal Physiol 303: F386–F395, 2012. doi: 10.1152/ajprenal.00531.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci USA 105: 11418–11423, 2008. doi: 10.1073/pnas.0804954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010. doi: 10.1073/pnas.1015751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwyer TM, Schmidt-Nielsen B. The renal pelvis: machinery that concentrates urine in the papilla. News Physiol Sci 18: 1–6, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol 303: F632–F638, 2012. doi: 10.1152/ajprenal.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvin JL, Burg MB, Knepper MA. Active absorption by the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 255: F57–F65, 1988. doi: 10.1152/ajprenal.1988.255.1.F57. [DOI] [PubMed] [Google Scholar]
- 24.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008. doi: 10.1161/HYPERTENSIONAHA.107.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giménez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- 26.Giménez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003. doi: 10.1074/jbc.M303435200. [DOI] [PubMed] [Google Scholar]
- 27.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 28.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 29.Holstein-Rathlou NH. Dynamic aspects of the tubuloglomerular feedback mechanism. Dan Med Bull 39: 134–154, 1992. [PubMed] [Google Scholar]
- 30.Hong NJ, Garvin JL. Angiotensin II type 2 receptor-mediated inhibition of NaCl absorption is blunted in thick ascending limbs from Dahl salt-sensitive rats. Hypertension 60: 765–769, 2012. doi: 10.1161/HYPERTENSIONAHA.112.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong NJ, Garvin JL. Endogenous flow-induced nitric oxide reduces superoxide-stimulated Na/H exchange activity via PKG in thick ascending limbs. Am J Physiol Renal Physiol 308: F444–F449, 2015. doi: 10.1152/ajprenal.00583.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong NJ, Garvin JL. Endogenous flow-induced superoxide stimulates Na/H exchange activity via PKC in thick ascending limbs. Am J Physiol Renal Physiol 307: F800–F805, 2014. doi: 10.1152/ajprenal.00260.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong NJ, Garvin JL. Flow increases superoxide production by NADPH oxidase via activation of Na-K-2Cl cotransport and mechanical stress in thick ascending limbs. Am J Physiol Renal Physiol 292: F993–F998, 2007. doi: 10.1152/ajprenal.00383.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012. doi: 10.1152/ajprenal.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong NJ, Silva GB, Garvin JL. PKC-α mediates flow-stimulated superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 298: F885–F891, 2010. doi: 10.1152/ajprenal.00543.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006. doi: 10.1152/ajpregu.00447.2005. [DOI] [PubMed] [Google Scholar]
- 37.Kuroda J, Ago T, Nishimura A, Nakamura K, Matsuo R, Wakisaka Y, Kamouchi M, Kitazono T. Nox4 is a major source of superoxide production in human brain pericytes. J Vasc Res 51: 429–438, 2014. doi: 10.1159/000369930. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005. doi: 10.1111/j.1365-2443.2005.00907.X. [DOI] [PubMed] [Google Scholar]
- 39.Laakso J, Mervaala E, Himberg JJ, Teräväinen TL, Karppanen H, Vapaatalo H, Lapatto R. Increased kidney xanthine oxidoreductase activity in salt-induced experimental hypertension. Hypertension 32: 902–906, 1998. doi: 10.1161/01.HYP.32.5.902. [DOI] [PubMed] [Google Scholar]
- 40.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leyssac PP, Karlsen FM, Holstein-Rathlou NH, Skøtt O. On determinants of glomerular filtration rate after inhibition of proximal tubular reabsorption. Am J Physiol Regul Integr Comp Physiol 266: R1544–R1550, 1994. doi: 10.1152/ajpregu.1994.266.5.R1544. [DOI] [PubMed] [Google Scholar]
- 42.Liang M, Berndt TJ, Knox FG. Mechanism underlying diuretic effect of L-NAME at a subpressor dose. Am J Physiol Renal Physiol 281: F414–F419, 2001. doi: 10.1152/ajprenal.2001.281.3.F414. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007. doi: 10.1152/ajprenal.00057.2007. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Wei Y, Sun P, Wang WH, Kleyman TR, Satlin LM. Mechanoregulation of BK channel activity in the mammalian cortical collecting duct: role of protein kinases A and C. Am J Physiol Renal Physiol 297: F904–F915, 2009. doi: 10.1152/ajprenal.90685.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackie TD, Kim BY, Subramanya AR, Bain DJ, O’Donnell AF, Welling PA, Brodsky JL. The endosomal trafficking factors CORVET and ESCRT suppress plasma membrane residence of the renal outer medullary potassium channel (ROMK). J Biol Chem 293: 3201–3217, 2018. doi: 10.1074/jbc.M117.819086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol 303: C781–C789, 2012. doi: 10.1152/ajpcell.00457.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mori T, Cowley AW Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- 48.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006. doi: 10.1152/ajprenal.00514.2005. [DOI] [PubMed] [Google Scholar]
- 49.Mozaffari MS, Jirakulsomchok S, Shao ZH, Wyss JM. High-NaCl diets increase natriuretic and diuretic responses in salt-resistant but not salt-sensitive SHR. Am J Physiol Renal Fluid Electrolyte Physiol 260: F890–F897, 1991. doi: 10.1152/ajprenal.1991.260.6.F890. [DOI] [PubMed] [Google Scholar]
- 50.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006. doi: 10.1152/ajprenal.00248.2005. [DOI] [PubMed] [Google Scholar]
- 52.Ortiz PA, Garvin JL. Superoxide stimulates NaCl absorption by the thick ascending limb. Am J Physiol Renal Physiol 283: F957–F962, 2002. doi: 10.1152/ajprenal.00102.2002. [DOI] [PubMed] [Google Scholar]
- 53.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. doi: 10.1152/ajprenal.0075.2001. [DOI] [PubMed] [Google Scholar]
- 54.Osono E, Nishimura H. Control of sodium and chloride transport in the thick ascending limb in the avian nephron. Am J Physiol Regul Integr Comp Physiol 267: R455–R462, 1994. doi: 10.1152/ajpregu.1994.267.2.R455. [DOI] [PubMed] [Google Scholar]
- 55.Pandit MM, Strait KA, Matsuda T, Kohan DE. Na delivery and ENaC mediate flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 302: F1325–F1330, 2012. doi: 10.1152/ajprenal.00034.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl- cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002. doi: 10.1074/jbc.M110442200. [DOI] [PubMed] [Google Scholar]
- 57.Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 260: F946–F952, 1991. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- 58.Preisig PA. Luminal flow rate regulates proximal tubule H- transporters. Am J Physiol Renal Physiol 262: F47–F54, 1992. doi: 10.1152/ajprenal.1992.262.1.F47. [DOI] [PubMed] [Google Scholar]
- 59.Ramseyer VD, Ortiz PA, Carretero OA, Garvin JL. Angiotensin II-mediated hypertension impairs nitric oxide-induced NKCC2 inhibition in thick ascending limbs. Am J Physiol Renal Physiol 310: F748–F754, 2016. doi: 10.1152/ajprenal.00473.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saez F, Hong NJ, Garvin JL. Luminal flow induces NADPH oxidase 4 translocation to the nuclei of thick ascending limbs. Physiol Rep 4: e12724, 2016. doi: 10.14814/phy2.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakai T, Craig DA, Wexler AS, Marsh DJ. Fluid waves in renal tubules. Biophys J 50: 805–813, 1986. doi: 10.1016/S0006-3495(86)83521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt-Nielsen B. August Krogh Lecture. The renal concentrating mechanism in insects and mammals: a new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995. doi: 10.1152/ajpregu.1995.268.5.R1087. [DOI] [PubMed] [Google Scholar]
- 64.Seney FD Jr, Persson EG, Wright FS. Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol Renal Fluid Electrolyte Physiol 252: F83–F90, 1987. doi: 10.1152/ajprenal.1987.252.1.F83. [DOI] [PubMed] [Google Scholar]
- 65.Silva GB, Ortiz PA, Hong NJ, Garvin JL. Superoxide stimulates NaCl absorption in the thick ascending limb via activation of protein kinase C. Hypertension 48: 467–472, 2006. doi: 10.1161/01.HYP.0000236646.83354.51. [DOI] [PubMed] [Google Scholar]
- 66.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 68.Unwin RJ, Walter SJ, Shirley DG. Lithium reabsorption in perfused loops of Henle: effects of perfusion rate and bumetanide. Am J Physiol Renal Physiol 266: F806–F812, 1994. doi: 10.1152/ajprenal.1994.266.5.F806. [DOI] [PubMed] [Google Scholar]
- 69.Wang T. Flow-activated transport events along the nephron. Curr Opin Nephrol Hypertens 15: 530–536, 2006. doi: 10.1097/01.mnh.0000242180.46362.c4. [DOI] [PubMed] [Google Scholar]
- 70.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 71.Wu R, Lamontagne D, de Champlain J. Antioxidative properties of acetylsalicylic Acid on vascular tissues from normotensive and spontaneously hypertensive rats. Circulation 105: 387–392, 2002. doi: 10.1161/hc0302.102609. [DOI] [PubMed] [Google Scholar]
- 72.Yip KP, Holstein-Rathlou NH, Marsh DJ. Mechanisms of temporal variation in single-nephron blood flow in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F427–F434, 1993. doi: 10.1152/ajprenal.1993.264.3.F427. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Mircheff AK, Hensley CB, Magyar CE, Warnock DG, Chambrey R, Yip KP, Marsh DJ, Holstein-Rathlou NH, McDonough AA. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1004–F1014, 1996. doi: 10.1152/ajprenal.1996.270.6.F1004. [DOI] [PubMed] [Google Scholar]
- 74.Zheleznova NN, Yang C, Cowley AW Jr. Role of Nox4 and p67phox subunit of Nox2 in ROS production in response to increased tubular flow in the mTAL of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F450–F458, 2016. doi: 10.1152/ajprenal.00187.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. doi: 10.1161/01.HYP.37.2.547. [DOI] [PubMed] [Google Scholar]