Abstract
Spinal cord injury (SCI) resulting in tetraplegia is a devastating, life-changing insult causing paralysis and sensory impairment as well as distinct autonomic dysfunction that triggers compromised cardiovascular, bowel, bladder, and sexual activity. Life becomes a battle for independence as even routine bodily functions and the smallest activity of daily living become major challenges. Accordingly, there is a critical need for a chronic preclinical model of tetraplegia. This report addresses this critical need by comparing, for the first time, resting-, reflex-, and stress-induced cardiovascular, autonomic, and hormonal responses each week for 4 wk in 12 sham-operated intact rats and 12 rats with chronic, complete C6–7 spinal cord transection. Loss of supraspinal control to all sympathetic preganglionic neurons projecting to the heart and vasculature resulted in a profound bradycardia and hypotension, reduced cardiac sympathetic and parasympathetic tonus, reduced reflex- and stress-induced sympathetic responses, and reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Histological examination of the nucleus ambiguus and stellate ganglia supports the profound and distinct autonomic and cardiac deficits and reliance on angiotensin to maintain cardiovascular stability following chronic, complete cervical6–7 cord transection.
NEW & NOTEWORTHY For the first time, resting-, reflex-, and stress-induced cardiovascular, autonomic, and hormonal responses were studied in rats with chronic, complete C6–7 cord transection. Loss of supraspinal control of all sympathetic preganglionic neurons reduced cardiac sympathetic and parasympathetic tonus, reflex and stress-induced sympathetic responses, and sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure. Histological examination supports the distinct deficits associated with cervical cord injury.
Keywords: blood pressure regulation, parasympathetic nervous system, sympathetic nervous system, tetraplegia
INTRODUCTION
Spinal cord injury (SCI) resulting in tetraplegia is a devastating, life-changing insult causing paralysis and sensory impairment as well as autonomic dysfunction that triggers compromised cardiovascular, bowel, bladder, and sexual activity. With autonomic disruption, routine bodily functions, hygiene, and the smallest activity of daily living become major challenges. For example, autonomic loss and cardiovascular consequences negatively impact physical work capacity (19, 20). Accordingly, addressing autonomic losses would greatly improve the quality of life for individuals living with tetraplegia. In this context, individuals with SCI prioritize the recovery of autonomic functions such as cardiovascular, sexual, and bowel and bladder control above the ability to walk (1).
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality for individuals living with SCI (25). In fact, individuals living with SCI have threefold increased odds of developing CVD than their able-bodied counterparts (16). However, the magnitude of CVD hazard is heavily dependent on the level of SCI, whereby individuals with tetraplegia have a 16% greater risk of all-cause CVD than individuals with paraplegia (26). Thus, the neurological level of SCI has a profound impact on autonomic and cardiovascular outcomes (76).
Despite these profound autonomic and cardiac consequences associated with tetraplegia, virtually all preclinical investigations induce lesions in the thoracic region. Thoracic lesions are not applicable to cervical lesions when autonomic dysfunction and cardiovascular consequences are examined. Specifically, with complete cervical lesions, the heart and systemic blood vessels lose brain stem control to all sympathetic outflow and are affected by unregulated spinal reflexes. This leads to profoundly reduced sympathetic activity, bradycardia, hypotension, and a significantly reduced hemodynamic response to activities of daily living and reduced physical work capacity (29, 37, 59). As an example, the maximum heart rate for individuals living with tetraplegia is ∼110 and 130 beats/min (19, 28, 58). The loss of sympathetically induced veno- and vasoconstriction also causes peripheral pooling of blood (29, 30) and a reduced preload, stroke volume, cardiac output, and oxygen transport as well as a reduced ability to redistribute blood to working muscles during activities of daily living (58, 73). Furthermore, the reduced sympathetic activity markedly impairs sudomotor and piloerector function (61, 72), and the hemodynamic responses to activities of daily living are profoundly impacted by cardiac atrophy (36, 75). Thus, individuals with tetraplegia often experience hypotension, fatigue, dizziness, and even syncope during activities of daily living, which markedly reduces their physical work capacity (9, 37, 45). Taken together, cervical lesions result in the most dramatic autonomic outcomes and cardiovascular consequences. The magnitude of this difference between individuals with tetraplegia and paraplegia documents a critical need for a chronic preclinical model of tetraplegia.
This report begins to address this critical need by comparing resting-, reflex-, and stress-induced cardiovascular, autonomic, and hormonal responses each week for 4 wk in 12 sham-operated intact rats and 12 rats with chronic, complete C6–7 spinal cord transection. Histological examination of the stellate ganglia and nucleus ambiguus supports the profound and distinct autonomic and cardiac deficits and reliance on angiotensin to maintain cardiovascular stability following chronic, complete cervical6–7 cord transection.
MATERIALS AND METHODS
Animals and General Experimental Procedures
Experimental procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Michigan State University and complied with the National Institutes of Health’s Guide to the Care and Use of Laboratory Animals. The procedures were conducted in 24 male Sprague-Dawley rats (10–15 wk of age). Rats were instrumented with an intra-arterial telemetry device (PA-C40; Data Sciences International) for recording arterial pressure, heart rate, and locomotor activity as well as a catheter within the pericardial sac. After recovery, rats were subjected to complete, cervical6–7 spinal cord transection or sham transection and studied each week for 4 wk.
Surgical Procedures
Two survival surgeries were performed on each animal. Before each surgery, animals received atropine (0.05 mg/kg). In addition, multimodal preemptive analgesia was achieved using a local infiltration of bupivacaine (diluted to 0.25%, 5 mg/kg) and lidocaine (diluted to 0.5%, 5 mg/kg) around the incision site (sq) and the administration of a nonsteriodal anti-inflammatory agent (carprofen, 10 mg/kg ip). Animals were anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg ip), intubated, and prepared for aseptic surgery. Supplemental doses of Nembutal (10–20 mg/kg ip) were administered if the animals regained the blink reflex or responded during the surgical procedures. Carprofen and buprenorphine (0.1 mg/kg) were administered for 3 days postoperative. To avoid infections, the antibiotic cefazolin (10 mg/kg) was administered preoperative and for 3 days postoperative.
First Surgical Procedure
By using aseptic conditions, the hearts were approached via a left thoracotomy through the second intercostal space (49, 50), and a catheter was placed within the pericardial sac, as previously described (11, 12, 49, 50), for local, targeted administration of cholera toxin B subunit. Injecting tracers into the pericardial sac labeled a homogenous population of neurons projecting to the heart (49, 50). The opposite end of the intrapericardial catheter was tunneled subcutaneously and exteriorized on the dorsal aspect of the neck. Subsequently, a catheter from a telemetry device (PA-C40; Data Sciences International) was inserted into the left carotid artery and advanced into the descending aorta for recording arterial pressure. Finally, a catheter was placed in the intraperitoneal space for the infusion of fluids and drugs. The catheter was exteriorized on the dorsal aspect of the neck.
All animals remained on the feedback-based temperature control system and ventilator until they recovered from the anesthesia. Once the animals regained consciousness, they were placed in a “rodent recovery cage” (Thermocare Intensive Care Unit, Paso Robles, CA). Animals were returned to the housing room when fully recovered from the anesthesia and gained the ability to maintain an upright body position and body temperature. At least 10 days were allowed for recovery. During the recovery period, the rats were provided with supplemental enrichment treats (Bio-Serv, Flemington, NJ), handled, weighed, and acclimatized to the laboratory and investigators.
Second Surgical Procedure
Following the recovery period and after the animals returned to their presurgical weight, the second surgical procedure was performed. The animals were anesthetized as described earlier and subjected to a complete, cervical6–7 spinal cord transection (C6–7X) or sham spinal cord transection. Specifically, the spinous process and laminae of the sixth cervical vertebrae were removed, exposing the sixth to seventh cervical spinal cord segments. The spinal cord was subsequently transected between C6 and C7 using a microknife (Fine Science Tools 10316-14) and Vannas spring scissors (Fine Science Tools 15000-08). Spinal cord transection between the sixth and seventh cervical spinal cord segments eliminates supraspinal control of all sympathetic preganglionic neurons. The extensiveness of the spinal cord transection was established by visually inspecting the transection site. Undistinguishable procedures were followed for the sham-transected rats, except the spinal cord was not transected.
Injury reproducibility is an important characteristic of experimental models of SCI because it limits the variability in outcome measures. Although spinal cord contusion injuries may be the most clinically relevant model since most injuries in humans occur in a similar manner, this model has a greater variability in outcome measures and spared descending systems compared with the complete spinal cord transection model (4). Therefore, to enhance injury reproducibility, we used the complete spinal cord transection model (10, 13, 46–52, 62–64).
Acute postoperative care for spinal cord-injured animals.
Immediately following spinal cord transection, animals present with flaccid paralysis and loss of sensation below the level of the lesion. This poses a major challenge to the injured animal that requires 24-h assistance with basic needs that include eating, drinking, and micturition. This specialized care continues until the animal can eat and drink on its own as well as the return of reflexive micturition. Typically, this period lasts 7–10 days posttransection. Specifically, during the acute recovery period, animals received a continuous infusion (10 ml·100 g body wt−1·day−1) of warm Lactated Ringer’s Solution through the intraperitoneal catheter. The animals were also provided with novel, nutritionally complete gel diets (Bio-Serv) and fruit to encourage eating. In addition, using a syringe, the animals were hand-fed liquid diets such as BOOST nutritional drink or diluted gel diets (Bio-Serv) several times a day. Spinal transection also impairs micturition reflexes. Therefore, the urinary bladder was voided by manual compression every 4 h, 24 h/day, 7 day/wk. Once automatic voidance was observed, manual compression continued four times a day to empty completely any residual urine that may risk infections.
Body temperature regulation is a major challenge for animals with tetraplegia, and maintaining a thermoneutral environment was critical for recovery. Therefore, animals were housed in a Thermocare Intensive Care Unit, and temperature was maintained at 29°C.
Finally, injured animals were handled several times daily to passively move joints and massage muscles below the level of the lesion. These procedures prevented pressure sores and contractures. Sham-operated animals were also housed in a thermoneutral environment and handled daily and received nutritional supplements.
Experimental Procedures
Four experimental procedures were performed each week for 4 wk. The order of the experimental procedures was randomized to prevent an order effect. Following the completion of the experimental procedures, cholera toxin B subunit was injected into the pericardial sac. Five days following the injection of cholera toxin B subunit into the pericardial sac, the brains and stellate ganglia were harvested, as previously described (49, 50). Finally, magnetic resonance imaging studies were performed on a 7 Tesla high-field imaging system to document site of the injury.
For all experiments, conscious, unrestrained rats were studied in their home cages (∼13,350 cm3). The temperature within the cage was monitored and maintained near the thermoneutral zone for rats of ∼28–31°C (71) by use of a circulating water pad under the cage and a Pelonis fan-forced heater with thermostat.
Experimental procedure 1: resting sympathetic activity (cardiac sympathetic tonus) and resting sympathetic support of arterial blood pressure.
On the day of the experiment, rats were brought into the laboratory and allowed to adapt to the environment. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, the heart rate and arterial pressure responses to cardiac autonomic sympathetic and parasympathetic blockade (β1-adrenergic and muscarinic-cholinergic receptor blockade, respectively) were determined. Drug doses for the sympathetic and parasympathetic antagonists were calculated relative to the animal’s body weight on each experimental day. Cardiac muscarinic-cholinergic receptor blockade was achieved by infusion of the nonspecific muscarinic-cholinergic receptor antagonist atropine methyl bromide [methylatropine (MA); 3 mg/kg] through the intraperitoneal catheter. Because the heart rate response to MA reached its peak in 10–15 min, this time interval was standardized before the heart rate measurement. Cardiac β1-adrenergic receptor blockade was achieved by infusion of the specific β1-adrenergic receptor antagonist metoprolol (MT; 10 mg/kg) through the intraperitoneal catheter. MT was infused 15 min after MA, and again the heart rate response was measured after 15 min. Intrinsic HR (HRI) was the HR after complete cardiac autonomic blockade (muscarinic-cholinergic and β1-adrenergic receptor blockades). Sympathetic tonus was calculated as HRM − HRI, where HRM is heart rate after muscarinic-cholinergic receptor blockade (53). Finally, hexamethonium chloride [a nicotinic nACh (NN) receptor antagonist (20 mg/kg)] was given to abolish ganglionic transmission and calculate the sympathetic support of blood pressure (SSBP). SSBP was calculated as the reduction in blood pressure in response to ganglionic blockade. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 2: resting parasympathetic activity (cardiac parasympathetic tonus) and resting angiotensin support of blood pressure.
On an alternate day, animals were treated identically as described above, except that the order of cardiac autonomic blockade was reversed for the determination of parasympathetic tonus. Parasympathetic tonus was calculated as HRβ − HRI, where HRβ is HR after β1-adrenergic receptor blockade. Finally, captopril, an angiotensin-converting enzyme (ACE) inhibitor (1 mg/kg) was given to block the production of angiotensin and calculate the angiotensin support of blood pressure (ASBP). ASBP was calculated as the reduction in blood pressure in response to ACE inhibition. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 3: reflex-induced sympathetic activity.
On an alternate day, rats were brought into the laboratory and allowed to adapt to the environment. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, one dose of sodium nitroprusside was infused through the intraperitoneal catheter (0.05 mg/kg for the sham-transected intact rats and 0.03 mg/kg for the transected rats). The response to nitroprusside in the rats with tetraplegia was different from the response to nitroprusside in the intact rats. Specifically, the response to nitroprusside in the rats with tetraplegia was bimodal. Initially, blood pressure decreased, and heart rate increased slightly. However, the recovery blood pressure well overshot the control blood pressure and caused an increased blood pressure and decreased heart rate. In contrast, the blood pressure returned to control only for the intact rats. Accordingly, the mean peak reduction in mean arterial pressure and increase in heart rate in response to bolus injections of sodium nitroprusside (SNP) were measured for both groups of rats; however, the mean peak increase in mean arterial pressure and decrease in heart rate during the rebound in arterial pressure was measured for the rats with tetraplegia. At the end of the experiment, the animals were returned to their housing facilities.
Experimental procedure 4: stress-induced sympathetic activity.
On an alternate day, rats were brought in the laboratory and allowed to adapt to the environment. Beat-by-beat, steady-state hemodynamic variables were continuously recorded for ∼1 h to ensure stable hemodynamic conditions. Subsequently, each rat was placed within a standard rat restrainer. The steady-state arterial pressure and heart rate responses were continuously recorded for 20 min. At the end of the experiment, the animals were returned to their housing facilities.
Documentation of cervical6–7 spinal cord transection using magnetic resonance imaging.
Magnetic resonance imaging studies were performed on a 7 Tesla high-field imaging system housed in the Department of Physiology at Michigan State University (Bruker Biospin 70/30) equipped with a stereotactic animal holder complete with physiological monitoring (SA Instruments) for body temperature, respiration, and electrocardiogram. Animals were placed prone and their heads fixed inside the nosecone of the anesthesia system with the jaw positioned using a bite bar. Images were acquired using a transmitter/receiver quadrature volume coil at the proton frequency. T2-weighted images were acquired in the sagittal plane using a rapid acquisition and relaxation enhancement (Turbo-RARE) sequence provided by the manufacturer. Sequence parameters were as follows: repetition time (TR), 2,500 ms; echo time (TE), 22 ms; 15 slices (1 mm thick) contiguous, FOV 5.3 × 5.1 cm, 256 × 256 acquisition matrix, in-plane resolution 200 × 200 μm, and 16 signal averages (total acquisition time was ∼40 min).
Intrapericardial sac injections.
Twenty-eight to thirty days after the C6–7X or sham transection, the neuronal tracer cholera toxin B subunit (CTB) was injected into the pericardial space, as described recently (49, 50). Briefly, 10 μl of 1% CTB mixed with 1 μl of 3% Evans blue dye was injected into the pericardial space via the intrapericardial catheter. The Evans blue dye was used to visualize the injectate because CTB is colorless. Five to 7 days were allowed for CTB to be picked up at synaptic endings and transported in a retrograde fashion back to the cell bodies of neurons located within the nucleus ambiguus and stellate ganglia. Subsequently, the animals were deeply anesthetized and perfused transcardially. The stellate ganglia and brain stems were preserved as described previously (49, 51).
Tissue Processing, Analysis, and Immunohistochemistry
The right brain stem and the right and left stellate ganglia were embedded in tissue freezing medium and sliced sagittally (brain stem) and horizontally (stellate ganglia) at 30-μm thickness and placed into Netwell inserts. Tissue sections were washed in 10 mM Tris, 0.9% NaCl, and 0.05% thimerosal in 10 mM phosphate buffer, pH 7.4 (TPBS), containing 0.3% Triton X-100 (TPBS-Triton) for 3 × 10 min and then incubated in 10% heat-inactivated normal horse serum (NHS) in TPBS-Triton for ≥1 h. The sections were then incubated in goat anti-CTB antiserum (1:25,000; List Biologicals) in TPBS-Triton containing 10% NHS for 24 h to 3 days at room temperature. After rinsing (TPBS, 3 × 10 min each), sections were incubated with biotinylated donkey anti-goat immunoglobulin (1:500; Jackson Laboratories) in TPBS-Triton with 1% NHS overnight at room temperature. Sections were rinsed again (TPBS, 3 × 10 min each) and incubated for 4–6 h in 1:1,500 ExtrAvidin-HRP (catalog no. E-2886; Sigma) in TPBS-Triton. Immunoreactive neurons were revealed with the nickel-intensified diaminobenzidine reaction (43, 49, 50).
Structural Analysis
CTB-labeled nucleus ambiguus and stellate neurons were examined with an Olympus BH-2 microscope outfitted with a motorized stage, Neurolucida imaging software, and a high-resolution digital camera. Multiplanar photomicrographs were taken and the images stacked using MicroBright-field Neurolucida software. Selection criteria were similar to our earlier studies (49–51). Cell bodies and dendrites were reconstructed using the neuron-tracing feature on the Neurolucida system, and dendritic branching was assessed in the NeuroExplorer 3D visualization and morphometric analysis program included with the Neurolucida system.
Each neuron was analyzed using the Sholl analysis of dendritic branching (49–51, 67), which assumes that dendritic arborization is an indirect measurement of available postsynaptic space. A series of concentric rings calibrated at 10-μm intervals was superimposed on each neuron and centered on the cell body. Intersections between dendrites and each concentric ring were then counted. The location and number of intersections were plotted (49–51) and used for statistical comparisons. In each animal, nine to 10 sections were examined, and nine to 10 neurons per section were measured using the Neurolucida software. Only cells with clearly distinguishable perikarya and dendritic trees were assessed. The examined neurons were chosen randomly. Three additional morphological features were recorded: area of soma, overall length of all visible processes (maximum dendritic length), and number of intersections per animal. The morphological features were compared between C6–7X and sham-transected rats.
Data Analysis
All recordings were sampled at 2 kHz, and the data were expressed as means ± SE. A two-way ANOVA was used for each of the following comparisons between intact and C6–7X rats (group) over 4 wk (time): 1) resting arterial pressure and heart rate, 2) cardiac sympathetic and parasympathetic tonus, 3) intrinsic heart rate, 4) sympathetic and angiotensin support of blood pressure, 5) reflex-induced sympathetic activity, and 6) stress-induced sympathetic activity. In addition, the Sholl analysis was evaluated using a two-way ANOVA applied to the dendritic intersections found at each concentric ring. Group × branching order was applied to the numbers of dendrites according to their order of branching. Significant interactions allowed for further intergroup comparisons using a Holm-Sidak post hoc analysis.
Finally, Student’s unpaired t-test was used to compare soma area, maximum dendritic length, and number of intersections between intact and C6–7X rats. Significance was set at P < 0.05.
RESULTS
Figure 1 is a high-resolution sagittal image documenting the site of the cervical injury by fast-spin echo MRI. Images were acquired on a Bruker 7T 70/30 high-field MRI, using a rapid acquisition with relaxation enhancement (RARE) pulse sequence. Acquisition parameters were a 5 × 5 cm field of view, 200-μm in-plane resolution, 2.5-s TR, 22-ms TE, and 1-mm slice thickness. Total acquisition time was 40 min. The brain stem, cerebellum, vertebral bodies, and lesion are clearly visible.
Fig. 1.
High-resolution sagittal image documenting the site of the cervical injury by fast-spin echo MRI. The brain stem, cerebellum, vertebral bodies, and lesion are clearly visible.
Figure 2, A and B, presents resting mean arterial blood pressure and heart rate, respectively, each week for 4 wk in intact and C6–7X rats. Hemodynamic variables on the 4 experimental days for each week were averaged for both groups. The two-way analysis of variance revealed a group effect without a time effect or group × time interaction for arterial pressure. Thus, mean arterial pressure was significantly lower during the 4 wk in C6–7X rats and was not influenced by the duration of the injury. In support of this concept, mean arterial pressure on postinjury days 1, 2, and 3 averaged 87 ± 3, 88 ± 4, and 87 ± 3 mmHg, respectively, and was not different from mean arterial pressure averaged each week over weeks 1–4. Thus, the profound reduction in arterial pressure following cervical spinal cord injury begins as soon as 1 day after the injury. The two-way ANOVA revealed a group effect without a time effect but a significant group × time interaction for heart rate. Post hoc analysis documented that heart rate was significantly lower each week during the 4 wk in C6–7X rats. When an interaction effect is present, the impact of one factor depends on the level of the other factor. Thus, the magnitude of the effect of tetraplegia on heart rate was influenced by the duration of the injury. Importantly, the heart rate on postinjury days 1, 2, and 3 averaged 263 ± 11, 267 ± 9, and 280 ± 11, respectively, and was not significantly different from heart rate averaged each week over weeks 1 and 2. Thus, the profound bradycardia following cervical spinal cord injury begins as soon as 1 day after the injury and improves after the 2nd wk postinjury.
Fig. 2.
Resting mean arterial blood pressure (A) and heart rate (B) each week for 4 wk in chronic sham-transected (intact) and complete cervical6–7 spinal cord-transected (C6–7X) rats. Resting arterial blood pressure and heart rate were significantly lower in C6–7X rats compared with intact rats. *P < 0.05, intact vs. C6–7X, group effect; #P < 0.05, intact vs. C6–7X each week, group × time interaction.
Figure 3, A and B, presents resting cardiac sympathetic tonus and parasympathetic tonus, respectively, and Fig. 4 presents intrinsic heart rate each week for 4 wk in intact and C6–7X rats. The two-way analysis of variance revealed a group effect without a time effect or group × time interaction for cardiac sympathetic and parasympathetic tonus and intrinsic heart rate. Cardiac sympathetic and parasympathetic tonus were significantly lower during the 4 wk in C6–7X rats compared with intact rats. Furthermore, intrinsic heart rate, averaged over the 2 experimental days each week for 4 wk, was also significantly lower in C6–7X rats. The effects of tetraplegia on sympathetic and parasympathetic tonus and intrinsic heart rate were not influenced by the duration of the injury.
Fig. 3.
Cardiac sympathetic tonus (A) and cardiac parasympathetic tonus (B) each week for 4 wk in chronic sham-transected (intact) and complete cervical 6–7 spinal cord-transected (C6–7X) rats. Sympathetic and parasympathetic tonus were significantly lower in C6–7X rats compared with intact rats. *P < 0.05, intact vs. C6–7X, group effect.
Fig. 4.
Intrinsic heart rate each week for 4 wk in chronic sham-transected (intact) and complete cervical 6–7 spinal cord-transected (C6–7X) rats. Intrinsic heart rate was determined as the heart rate response after complete cardiac autonomic blockade. C6–7X rats had a lower intrinsic heart rate compared with intact rats. *P < 0.05, intact vs. C6–7X, group effect.
Following the protocol to determine cardiac sympathetic tonus, ganglionic blockade was administered to determine sympathetic support of blood pressure. Similarly, following the protocol to determine cardiac parasympathetic tonus, an angiotensin-converting enzyme (ACE) inhibitor was administered to determine angiotensin support of blood pressure. Figure 5, A and B, presents sympathetic support of blood pressure and angiotensin support of blood pressure, respectively, each week for 4 wk in intact and C6–7X rats. The two-way analysis of variance revealed a group effect without a time effect or group × time interaction for both sympathetic and angiotensin support of blood pressure. Rats with tetraplegia had high angiotensin support of blood pressure with virtually no sympathetic support of blood pressure during the 4 wk. In sharp contrast, intact rats had high sympathetic support of blood pressure with virtually no angiotensin support of blood pressure during the 4 wk. The effects of tetraplegia on sympathetic and angiotensin support of blood pressure were not influenced by the duration of the injury.
Fig. 5.
Sympathetic (A) and angiotensin support (B) of blood pressure each week for 4 wk in chronic sham-transected (intact) and complete cervical 6–7 spinal cord-transected (C6–7X) rats. C6–7X rats had a reduced sympathetic support of blood pressure and an enhanced reliance on angiotensin to maintain arterial blood pressure compared with intact rats. *P < 0.05, intact vs. C6–7X, group effect.
Figure 6, A and B, presents the change in arterial pressure and heart rate, respectively, in response to 20 min of restrainer stress each week for 4 wk in intact and C6–7X rats. The two-way analysis of variance revealed a group effect, a time effect and a significant group × time interaction for the change in arterial pressure. There was a group effect and a group × time interaction for the change heart rate. C6–7X rats had virtually no arterial pressure or heart rate responses to restrainer stress each week for 4 wk. In contrast, intact rats had robust and significantly higher hemodynamic responses to restrainer stress. The arterial pressure and heart rate responses to restrainer stress were influenced by the duration of the study.
Fig. 6.
Change in arterial pressure (A) and heart rate (B) in response to 20 min of restrainer stress each week for 4 wk in chronic sham-transected (intact) and complete cervical 6–7 spinal cord-transected (C6–7X) rats. C6–7X rats had virtually no arterial pressure or heart rate responses to restrainer stress each week for 4 wk. *P < 0.05, intact vs. C6–7X, group effect; #P < 0.05, intact vs. C6–7X each week, group × time interaction.
Figure 7A presents reflex-induced sympathetic activity (arterial pressure and heart rate responses to a single dose of sodium nitroprusside) each week for 4 wk in intact and C6–7X rats. Figure 7B presents the rebound increase in arterial pressure above control and the decrease in heart rate for the C6–7X rats (intact rats did not have a rebound increase in arterial pressure). The two-way analysis of variance revealed no significant differences in the arterial pressure responses to SNP between the two groups. Although there were no differences in the arterial pressure responses, the reflex heart rate responses were significantly lower each week for 4 wk in the C6–7X rats compared with intact rats (group effect). Thus, the magnitude of the effect of tetraplegia on the heart rate response to SNP was not influenced by the duration of the injury. The rebound increase in arterial pressure above control induced a significant and robust reduction in heart rate in the C6–7X rats, documenting preservation of cardiovagal baroreflexes.
Fig. 7.
A: reflex-induced arterial pressure and heart rate responses to a single dose of sodium nitroprusside each week for 4 wk in chronic sham-transected (intact) and complete cervical 6–7 spinal cord-transected (C6–7X) rats. Although there were no differences in the arterial pressure responses, the reflex heart rate responses were significantly lower each week for 4 wk in the C6–7X rats compared with intact rats. B: rebound increase in arterial pressure above control and the decrease in heart rate for the C6–7X rats (intact rats did not have a rebound increase in arterial pressure above control). Rebound increase in arterial pressure above control induced a significant and robust reduction in heart rate in the C6–7X rats, documenting preservation of cardiovagal baroreflexes. *P < 0.05, intact vs. C6–7X, group effect.
Figure 8, A and B, presents photomicrographs of 30-μm sagittal sections through the right brain stem processed for CTB immunoreactivity from one sham-operated intact rat and one C6–7X rat, respectively. Cell bodies and dendrites were reconstructed, and dendritic branching was assessed. Each neuron was analyzed using the Sholl analysis of dendritic branching, which assumes that dendritic arborization is an indirect measure of available postsynaptic space. Figure 8C presents the mean number of intersections per CTB-labeled neurons between each ring in a series of concentric rings. The two-way analysis of variance revealed a significant group effect, ring effect, and group × ring interaction documenting that premotor cardioinhibitory vagal neuron dendritic arborization was markedly reduced in C6–7X rats (Fig. 8). In Fig. 9, premotor cardioinhibitory vagal neuron soma area (Fig. 9A), maximum dendritic length (Fig. 9B), number of intersections per animal (Fig. 9C), and total number of neurons (Fig. 9D) are presented. Although there was no difference in premotor cardioinhibitory vagal neuron soma area between intact and C6–7X rats, maximum dendritic length, number of interactions per animal, and the total number of labeled neurons were reduced in C6–7X rats.
Fig. 8.
Photomicrographs of 30-μm sagittal sections through the right brain stem processed for cholera toxin B subunit (CTB) immunoreactivity from 1 chronic sham-transected rat (intact; A) and 1 complete cervical 6–7 spinal cord-transected (C6–7X) rat (B). C: mean no. of intersections per CTB-labeled neurons between each ring in a series of concentric rings. Premotor cardioinhibitory vagal neuron dendritic arborization was reduced in C6–7X rats. *P < 0.05, intact vs. C6–7X, group effect.
Fig. 9.
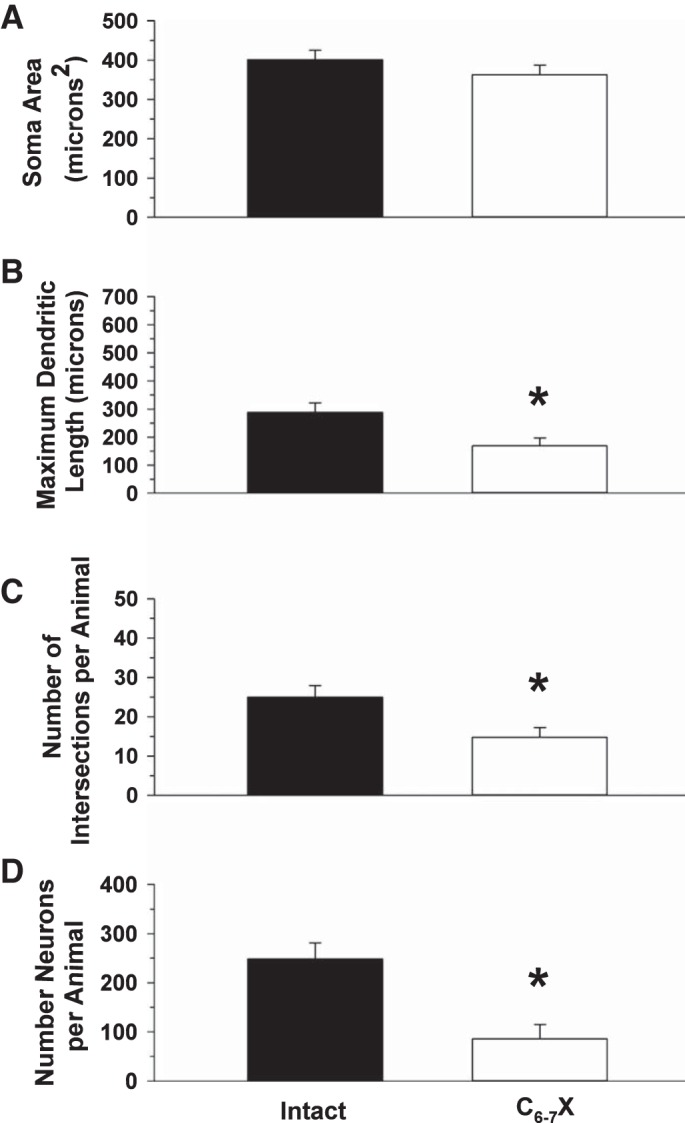
Soma area (A), maximum dendritic length (B), no. of intersections per animal (C), and total no. of neurons (D) of nucleus ambiguus neurons back-labeled from the heart from chronic sham-operated intact rats and complete cervical 6–7 spinal cord transected rats (C6–7X). Although there was no difference in premotor cardioinhibitory vagal neuron soma area between intact and C6–7X rats, maximum dendritic length, no. of interactions per animal, and the total no. of labeled neurons were reduced in C6–7X rats. *P < 0.05, intact vs. C6–7X, group effect.
Figure 10, A and B, presents photomicrographs of 30-μm horizontal sections through the stellate ganglia processed for CTB immunoreactivity from one sham-operated intact rat and one C6–7X rat, respectively. Cell bodies and dendrites were reconstructed, and dendritic branching was assessed. Each neuron was analyzed using the Sholl analysis of dendritic branching. Figure 10C presents the mean number of intersections per CTB labeled neurons between each ring in a series of concentric rings. The two-way analysis of variance revealed a significant group effect, ring effect and group × ring interaction documenting that stellate ganglia neuron dendritic arborization was altered by the cervical spinal cord transection (Fig. 10). In Fig. 11, stellate ganglia neuron soma area (Fig. 11A), maximum dendritic length (Fig. 11B), number of intersection per animal (Fig. 11C), and total number of neurons (Fig. 11D) are presented. None of the neuron characteristics were altered by cervical spinal cord injury.
Fig. 10.
Photomicrographs of 30-μm horizontal sections through the stellate ganglia processed for CTB immunoreactivity from 1 chronic sham-transected rat (intact; A) and 1 complete cervical 6–7 spinal cord-transected rat (C6–7X; B). C: mean no. of intersections per CTB-labeled neurons between each ring in a series of concentric rings. Stellate ganglia neuron dendritic arborization was increased in C6–7X rats. *P < 0.05, intact vs. C6–7X, group effect.
Fig. 11.

Stellate ganglia neuron soma area (A), maximum dendritic length (B), no. of intersections/animal (C), and total no. of neurons (D). None of the neuron characteristics were altered by cervical spinal cord injury.
DISCUSSION
Based on published data from the National Spinal Cord Injury Statistical Center, the annual incidence of spinal cord injury in the USA alone is ∼17,000 each year (53a). Approximately 58.3% of these injuries result in tetraplegia (45% incomplete, 13.3% complete). Thus, there is a critical need for preclinical models of cervical SCI. Although there are significant efforts toward producing more clinically relevant preclinical models of cervical SCI, the models are difficult to produce and maintain and have dramatically high mortality rates. Accordingly, the models prepared to date are limited because the cervical injuries are unilateral (21, 41, 54, 60, 65, 68) or of limited degree of severity (2, 32, 33, 40, 56). The lack of a suitable preclinical model of cervical SCI has severely impacted the advancement of therapeutic strategies to reduce the devastating consequences of this injury. Furthermore, understanding the mechanisms driving autonomic dysfunction and cardiovascular consequences associated with tetraplegia is expected to provide mechanistic insights and therapeutic approaches for other autonomic neuropathies, including orthostatic hypotension, orthostatic intolerance, postural orthostatic tachycardia syndrome, syncope, and others. In addition, although the personal impact of SCI is unmeasurable, spinal cord injuries cost the nation at least $9.7 billion/yr for medical care, equipment, and disability support (5, 18). Thus, interventions designed to prevent complications have the potential to improve the quality of life for thousands of individuals and families with SCI as well as save our nation billions of dollars.
Cervical spinal cord injury is unique in that it completely disrupts all sympathetic outflow to the heart and blood vessels. Specifically, the cell bodies of sympathetic preganglionic neurons originate in the spinal gray matter of the thoracic (T1–T12) and upper lumbar (L1–L2) segments of the spinal cord. Axons of the sympathetic preganglionic neurons exit through the anterior roots of the spinal cord and synapse onto postganglionic sympathetic neurons in the sympathetic chain ganglia and prevertebral ganglia. Sympathetic postganglionic fibers are mainly adrenergic, releasing the neurotransmitter norepinephrine. The exception to this general rule involves the sympathetic fibers innervating sweat glands and piloerector muscles, which release acetylcholine. Sympathetic innervation of the heart and blood vessels of the upper limbs originates at segments T1–T4. Sympathetic innervation of the critical circulation to the splanchnic vasculature and lower limbs occurs at segments T6–L2. Thus, when the spinal cord is injured in the cervical region, the heart and systemic blood vessels lose brain stem control to all sympathetic outflow and are affected by unregulated spinal reflexes. Thus, cervical lesions result in the most dramatic autonomic outcomes and cardiovascular consequences. Furthermore, the loss of volitional motor control below the cervical level can lead to an extremely sedentary lifestyle with an increased incidence of secondary complications, including diabetes, cardiovascular disease, and an atherogenic lipid profile.
It must also be recognized and acknowledged that high-thoracic models (particularly with injury at thoracic 2 to thoracic 3) also decentralize practically all sympathetic preganglionic neurons, since these injuries spread (secondary cascade) at least one to two segments rostral within the cord. The remaining T1 segment is responsible for <10% of postganglionic cardiac innervation (70). Thus, high-thoracic models produce relevant cardiac and hemodynamic function that closely mimics that of cervical injuries, including left ventricular atrophy and reduced contractile function (17, 69).
Pathological neuroplasticity, following SCI, is associated with many lingering complications, such as chronic pain, spasticity, neurogenic bladder, and autonomic dysreflexia (3, 6, 7, 24, 27, 31, 38, 39, 42, 44, 55, 57, 66, 77). Pathological neuroplasticity is also associated with an increased susceptibility to life-threatening ventricular arrhythmias (46, 48, 51). These complications are exacerbated by the sedentary lifestyle of the typical individual with SCI. Thus, the management of autonomic and cardiovascular dysfunction associated with cervical SCI should focus on primary prevention programs to reduce or prevent associated complications, because prevention is the most cost-effective and innocuous strategy. This will require a detailed understanding of autonomic dysfunction and cardiovascular consequences associated with cervical lesions. This report addressed this critical need by comparing resting-, reflex-, and stress-induced cardiovascular, autonomic, and hormonal responses each week for 4 wk in sham-operated intact rats and rats with chronic, complete C6–7 spinal cord transection. It is important to note that only after years of attempts have we been successful in reliably and efficiently producing this model. During the first 7–10 days postinjury, the animals required intervention (bladder expression, feeding, movement) every 4 h. Maintaining body temperature was also critical. After 10 days, the rats no longer required feeding; however, manual bladder compression continued four times/day to completely empty any residual urine that may risk infections. With these efforts, we have achieved a <20% mortality rate. When problems occurred, it appeared to be due to our failure to maintain adequately the rat’s body temperature.
Although no behavioral testing was performed, all rats had complete paralysis of the forepaws and were unable to grasp objects. Although the rats could not grip objects, the rats could hold objects between their forepaws without closing the paw. The rats would support themselves on their shoulders and hold food between their forepaws to eat. The rats could also move their forelimb (elbow flexion) and had complete shoulder movement that allowed them to move modestly within the cage and groom their heads and faces.
Loss of supraspinal control to all sympathetic preganglionic neurons projecting to the heart and vasculature resulted in a profound bradycardia and hypotension (Fig. 2), reduced cardiac sympathetic and parasympathetic tonus (Fig. 3) and intrinsic heart rate (Fig. 4), reduced sympathetic support of blood pressure as well as enhanced reliance on angiotensin to maintain arterial blood pressure (Fig. 5), and reduced stress- (Fig. 6) and reflex-induced (Fig. 7A) sympathetic responses. In contrast, cardiovagal baroreflex responses were preserved in C6–7X rats (Fig. 7B), and, unexpectedly, parasympathetic tonus had a cardio-acceleration effect. The unpredicted cardio-acceleration effect suggests a complex and interacting influence of the autonomic nervous system on heart rate that is revealed (53) by a cervical injury. The profound bradycardia, altered cardiac autonomic tone, and enhanced reliance on angiotensin in rats with tetraplegia are expected to alter cardiac electrophysiology and increase the susceptibility to a variety of arrhythmias. Specifically, mammalian heart rate and cardiac electrophysiology are profoundly influenced by the sympathetic and parasympathetic divisions of the autonomic nervous system. The renin-angiotensin-aldosterone system also has a profound effect on cardiac electrophysiology by altering membrane ion channels (8, 35), suppression of the sarcoplasmic reticulum Ca2+-ATPase pump (34) or the ryanodine receptor (23, 74), and disruption of cell-to-cell coupling through modification of gap junctional conductance. Accordingly, the profound bradycardia and reliance on angiotensin in rats with tetraplegia is expected to alter cardiac electrophysiology and increase the susceptibility to a variety of arrhythmias. This expectation merits future consideration.
We previously reported that midthoracic spinal cord injury (T5X), which preserves supraspinal control of sympathetic preganglionic neurons projecting to the heart, increased cardiac sympathetic tonus (14, 46, 48, 62) and increased the susceptibility to life-threatening, sustained ventricular tachy-arrhythmias (14, 46, 48, 64). These functional changes were associated with structural sympathetic hyperinnervation of the heart (51). For example, using injections of cholera toxin B into the left and right stellate ganglia (which provides >90% of sympathetic supply to the heart) (51) or pericardial sac (49, 50), we documented a significant increase in cardiac sympathetic preganglionic (51) and postganglionic neuron (49) dendritic arborization following T5X. Furthermore, there was a significant increase in left ventricular sympathetic innervation density as measured through tyrosine hydroxylase immunohistochemistry following T5X (46, 51). Thus, midthoracic spinal cord injury resulted in cardiac sympathetic hyperinnervation and increased susceptibility to life-threatening arrhythmias.
We also reported that T5X reduced cardiac parasympathetic tonus (46) and caused structural plasticity in parasympathetic preganglionic neurons located within the nucleus ambiguus (50). Specifically, there was a significant increase in cardiac parasympathetic preganglionic neuron dendritic arborization, soma area, maximum dendritic length, and number of intersections/animal. This parasympathetic structural remodeling was associated with a profound cardiac remodeling. Specifically, T5X increased left ventricular (LV) chamber area, reduced LV wall thickness, and increased collagen content (50).
In sharp contrast to rats with T5X, rats with C6–7X lost supraspinal control to all sympathetic preganglionic neurons. Unexpectedly, C6–7X reduced dendritic branching of parasympathetic preganglionic neurons projecting to the heart. It is important to note that these neurons were back-labeled from the heart using injections of cholera toxin B subunit within the pericardial sac. Injecting tracers into the pericardial sac back-labels a homogenous population of neurons projecting to the heart.
The reduced dendritic branching of parasympathetic preganglionic neurons projecting to the heart may be the result of the low arterial pressure promoting activity-dependent neuroplasticity. Specifically, the low arterial pressure unloads arterial baroreceptors and reduces arterial baroreceptor activity. As a result, there is reduced input to nucleus ambiguus neurons resulting in low activity-dependent neuroplasticity. Importantly, these unexpected results merit further investigation and verification using an alternate procedure. Specifically, Golgi-Cox staining of the nucleus ambiguus should also be conducted because it is possible that C6–7X, by some unknown mechanism, reduced uptake of the cholera toxin B subunit.
C6–7X rats were associated with a complex structural remodeling of cardiac-projecting stellate ganglia neurons. Unexpectedly, C6–7X reduced proximal dendritic branching but increased distal dendritic branching of stellate postganglionic neurons projecting to the heart. The mechanisms mediating this complex structural neuroplasticity of cardiac-projecting stellate ganglia neurons is unknown but merits future investigation.
Conclusions
As noted, spinal cord injury resulting in tetraplegia is a devastating, life-changing insult causing paralysis and sensory impairment as well as autonomic dysfunction that triggers compromised cardiovascular, bowel, bladder, and sexual activity. Life becomes a battle for independence as even routine bodily functions and the smallest activity of daily living become a major challenge. This is also true for rodents. Accordingly, the reluctance to perform experiments on spinal cord-injured models, especially those with a cervical injury, is understandable. The challenges of animal care as well as the emotional concern for injured animals can be difficult to overcome. We are keenly aware of and sensitive to these issues. Accordingly, our spinal cord-injured animals are treated with the same care and respect we provide to humans with SCI (19, 20). This is a critical consideration, because addressing autonomic losses and cardiovascular consequences will greatly improve the quality of life for individuals living with tetraplegia.
A word of note regarding the study of rats is in order. Most of the procedures described in this paper cannot be performed in humans. Therefore, animal models are important to investigate therapeutic strategies and underlying mechanisms associated with tetraplegia. It is true, however, that the most effective interventions to the greatest number of individuals with spinal cord injury will require experiments using humans and nonhuman primates (15). However, most of the fundamental work on spinal cord injury can and should be performed in rodents and other animals (15). Once knowledge is gained from successful experiments in nonprimate species, experiments in nonhuman primates should be used to test invasive interventions. Because of testing in rodents, the novel procedures will have a higher potential for success in humans (15). Specifically, studies in rodents can identify potential benefits, determine mechanisms, and provide confidence in the efficacy and safety of an intervention (15).
Worldwide efforts are presently underway to develop strategies to reduce the catastrophic consequence of tetraplegia. In the US alone, the National Institutes of Health has invested several hundred million dollars in pursuit of therapeutic strategies. However, due largely to methodological limitations, this investment has had limited success. Among the major methodological limitations is the reliance on animal models that do not mimic the clinical or physiological situation. In this context, a conscious chronic model of tetraplegia has the potential to be of major importance for advancing the concepts and methods that drive the development of therapeutic strategies.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-122223.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.L.L. and S.E.D. conceived and designed research; H.L.L. and S.E.D. performed experiments, H.L.L. and S.E.D. analyzed data; H.L.L. and S.E.D. interpreted results of experiments; H.L.L., A.T., R.W.W., and S.E.D. prepared figures; H.L.L. and S.E.D. drafted manuscript, H.L.L., A.T., R.W.W., and S.E.D. edited and revised manuscript; H.L.L., A.T., R.W.W., and S.E.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rebecca A. Mulder for technical assistance.
REFERENCES
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD, Sharp KG, Steward O. Bilateral cervical contusion spinal cord injury in rats. Exp Neurol 220: 9–22, 2009. doi: 10.1016/j.expneurol.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: evidence for alpha-adrenoceptor hyper-responsiveness. Clin Auton Res 5: 267–270, 1995. doi: 10.1007/BF01818891. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139: 244–256, 1996. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 5.Berkowitz M, O’Leary PK, Kruse DL, Harvey C. Spinal Cord Injury: An Analysis of Medical and Social Costs. New York: Demos Medical Publishing, 1998. [Google Scholar]
- 6.Brock JA, Yeoh M, McLachlan EM. Enhanced neurally evoked responses and inhibition of norepinephrine reuptake in rat mesenteric arteries after spinal transection. Am J Physiol Heart Circ Physiol 290: H398–H405, 2006. doi: 10.1152/ajpheart.00712.2005. [DOI] [PubMed] [Google Scholar]
- 7.Brown A, Weaver LC. The dark side of neuroplasticity. Exp Neurol 235: 133–141, 2012. doi: 10.1016/j.expneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caballero R, Gómez R, Moreno I, Nuñez L, González T, Arias C, Guizy M, Valenzuela C, Tamargo J, Delpón E. Interaction of angiotensin II with the angiotensin type 2 receptor inhibits the cardiac transient outward potassium current. Cardiovasc Res 62: 86–95, 2004. doi: 10.1016/j.cardiores.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Claydon VE, Hol AT, Eng JJ, Krassioukov AV. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch Phys Med Rehabil 87: 1106–1114, 2006. doi: 10.1016/j.apmr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T(5) spinal rats. Am J Physiol Heart Circ Physiol 282: H1566–H1570, 2002. doi: 10.1152/ajpheart.00733.2001. [DOI] [PubMed] [Google Scholar]
- 11.Collins HL, DiCarlo SE. Attenuation of postexertional hypotension by cardiac afferent blockade. Am J Physiol Heart Circ Physiol 265: H1179–H1183, 1993. doi: 10.1152/ajpheart.1993.265.4.H1179. [DOI] [PubMed] [Google Scholar]
- 12.Collins HL, DiCarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J Appl Physiol (1985) 75: 114–120, 1993. doi: 10.1152/jappl.1993.75.1.114. [DOI] [PubMed] [Google Scholar]
- 13.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol 283: H1734–H1739, 2002. doi: 10.1152/ajpheart.00253.2002. [DOI] [PubMed] [Google Scholar]
- 14.Collins HL, Rodenbaugh DW, DiCarlo SE. Spinal cord injury alters cardiac electrophysiology and increases the susceptibility to ventricular arrhythmias. Prog Brain Res 152: 275–288, 2006. doi: 10.1016/S0079-6123(05)52018-1. [DOI] [PubMed] [Google Scholar]
- 15.Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med 13: 561–566, 2007. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology 81: 723–728, 2013. doi: 10.1212/WNL.0b013e3182a1aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeVeau KM, Martin EK, King NT, Shum-Siu A, Keller BB, West CR, Magnuson DSK. Challenging cardiac function post-spinal cord injury with dobutamine. Auton Neurosci 209: 19–24, 2018. doi: 10.1016/j.autneu.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVivo MJ. Causes and costs of spinal cord injury in the United States. Spinal Cord 35: 809–813, 1997. doi: 10.1038/sj.sc.3100501. [DOI] [PubMed] [Google Scholar]
- 19.DiCarlo SE. Improved cardiopulmonary status after a two-month program of graded arm exercise in a patient with C6 quadriplegia. A case report. Phys Ther 62: 456–459, 1982. doi: 10.1093/ptj/62.4.456. [DOI] [PubMed] [Google Scholar]
- 20.DiCarlo SE, Supp MD, Taylor HC. Effect of arm ergometry training on physical work capacity of individuals with spinal cord injuries. Phys Ther 63: 1104–1107, 1983. doi: 10.1093/ptj/63.7.1104. [DOI] [PubMed] [Google Scholar]
- 21.Dunham KA, Siriphorn A, Chompoopong S, Floyd CL. Characterization of a graded cervical hemicontusion spinal cord injury model in adult male rats. J Neurotrauma 27: 2091–2106, 2010. doi: 10.1089/neu.2010.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flesch M, Schiffer F, Zolk O, Pinto Y, Stasch JP, Knorr A, Ettelbrück S, Böhm M. Angiotensin receptor antagonism and angiotensin converting enzyme inhibition improve diastolic dysfunction and Ca(2+)-ATPase expression in the sarcoplasmic reticulum in hypertensive cardiomyopathy. J Hypertens 15: 1001–1009, 1997. doi: 10.1097/00004872-199715090-00011. [DOI] [PubMed] [Google Scholar]
- 24.Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11: 1766–1770, 2001. doi: 10.1016/S0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 25.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 43: 408–416, 2005. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 39: 310–317, 2001. doi: 10.1038/sj.sc.3101162. [DOI] [PubMed] [Google Scholar]
- 27.Hill CE, Beattie MS, Bresnahan JC. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171: 153–169, 2001. doi: 10.1006/exnr.2001.7734. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman MD. Cardiorespiratory fitness and training in quadriplegics and paraplegics. Sports Med 3: 312–330, 1986. doi: 10.2165/00007256-198603050-00002. [DOI] [PubMed] [Google Scholar]
- 29.Hopman MT, Dueck C, Monroe M, Philips WT, Skinner JS. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med 19: 98–103, 1998. doi: 10.1055/s-2007-971889. [DOI] [PubMed] [Google Scholar]
- 30.Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol (1985) 93: 1966–1972, 2002. doi: 10.1152/japplphysiol.00897.2001. [DOI] [PubMed] [Google Scholar]
- 31.Hou S, Duale H, Cameron AA, Abshire SM, Lyttle TS, Rabchevsky AG. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol 509: 382–399, 2008. doi: 10.1002/cne.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James ND, Shea J, Muir EM, Verhaagen J, Schneider BL, Bradbury EJ. Chondroitinase gene therapy improves upper limb function following cervical contusion injury. Exp Neurol 271: 131–135, 2015. doi: 10.1016/j.expneurol.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Y, Bouyer J, Haas C, Fischer I. Evaluation of the anatomical and functional consequences of repetitive mild cervical contusion using a model of spinal concussion. Exp Neurol 271: 175–188, 2015. doi: 10.1016/j.expneurol.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju H, Scammel-La Fleur T, Dixon IM. Altered mRNA abundance of calcium transport genes in cardiac myocytes induced by angiotensin II. J Mol Cell Cardiol 28: 1119–1128, 1996. doi: 10.1006/jmcc.1996.0103. [DOI] [PubMed] [Google Scholar]
- 35.Kaibara M, Mitarai S, Yano K, Kameyama M. Involvement of Na(+)-H+ antiporter in regulation of L-type Ca2+ channel current by angiotensin II in rabbit ventricular myocytes. Circ Res 75: 1121–1125, 1994. doi: 10.1161/01.RES.75.6.1121. [DOI] [PubMed] [Google Scholar]
- 36.Kessler KM, Pina I, Green B, Burnett B, Laighold M, Bilsker M, Palomo AR, Myerburg RJ. Cardiovascular findings in quadriplegic and paraplegic patients and in normal subjects. Am J Cardiol 58: 525–530, 1986. doi: 10.1016/0002-9149(86)90027-5. [DOI] [PubMed] [Google Scholar]
- 37.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med 22: 39–45, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Krassioukov AV, Weaver LC. Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin Exp Hypertens 17: 361–373, 1995. doi: 10.3109/10641969509087077. [DOI] [PubMed] [Google Scholar]
- 39.Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci 19: 7405–7414, 1999. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane MA, Lee KZ, Salazar K, O’Steen BE, Bloom DC, Fuller DD, Reier PJ. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol 235: 197–210, 2012. doi: 10.1016/j.expneurol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Streijger F, Tigchelaar S, Maloon M, Liu J, Tetzlaff W, Kwon BK. A contusive model of unilateral cervical spinal cord injury using the infinite horizon impactor. J Vis Exp 65: pii:3313, 2012. doi: 10.3791/3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llewellyn-Smith IJ, Martin CL, Fenwick NM, DiCarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol 503: 741–767, 2007. doi: 10.1002/cne.21414. [DOI] [PubMed] [Google Scholar]
- 43.Llewellyn-Smith IJ, DiCarlo SE, Collins HL, Keast JR. Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera. J Comp Neurol 488: 278–289, 2005. doi: 10.1002/cne.20552. [DOI] [PubMed] [Google Scholar]
- 44.Llewellyn-Smith IJ, Weaver LC. Changes in synaptic inputs to sympathetic preganglionic neurons after spinal cord injury. J Comp Neurol 435: 226–240, 2001. doi: 10.1002/cne.1204. [DOI] [PubMed] [Google Scholar]
- 45.Low DA, da Nóbrega AC, Mathias CJ. Exercise-induced hypotension in autonomic disorders. Auton Neurosci 171: 66–78, 2012. doi: 10.1016/j.autneu.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Lujan HL, Chen Y, DiCarlo SE. Paraplegia increased cardiac NGF content, sympathetic tonus, and the susceptibility to ischemia-induced ventricular tachycardia in conscious rats. Am J Physiol Heart Circ Physiol 296: H1364–H1372, 2009. doi: 10.1152/ajpheart.01286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lujan HL, DiCarlo SE. Fundamental hemodynamic mechanisms mediating the response to myocardial ischemia in conscious paraplegic mice: cardiac output versus peripheral resistance. Physiol Rep 5: e13214, 2017. doi: 10.14814/phy2.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol 293: H3333–H3339, 2007. doi: 10.1152/ajpheart.01019.2007. [DOI] [PubMed] [Google Scholar]
- 49.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol (1985) 113: 1332–1341, 2012. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lujan HL, Janbaih H, DiCarlo SE. Structural remodeling of the heart and its premotor cardioinhibitory vagal neurons following T(5) spinal cord transection. J Appl Physiol (1985) 116: 1148–1155, 2014. doi: 10.1152/japplphysiol.01285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol 299: R985–R995, 2010. doi: 10.1152/ajpregu.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lujan HL, Palani G, Peduzzi JD, DiCarlo SE. Targeted ablation of mesenteric projecting sympathetic neurons reduces the hemodynamic response to pain in conscious, spinal cord-transected rats. Am J Physiol Regul Integr Comp Physiol 298: R1358–R1365, 2010. doi: 10.1152/ajpregu.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lujan HL, Rivers JP, DiCarlo SE. Complex and interacting influences of the autonomic nervous system on cardiac electrophysiology in conscious mice. Auton Neurosci 201: 24–31, 2016. doi: 10.1016/j.autneu.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.National Spinal Cord Injury Statistical Center Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham, 2016. [Google Scholar]
- 54.Nicaise C, Frank DM, Hala TJ, Authelet M, Pochet R, Adriaens D, Brion JP, Wright MC, Lepore AC. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J Neurotrauma 30: 1092–1099, 2013. doi: 10.1089/neu.2012.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol 184: 373–380, 2003. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Onifer SM, Nunn CD, Decker JA, Payne BN, Wagoner MR, Puckett AH, Massey JM, Armstrong J, Kaddumi EG, Fentress KG, Wells MJ, West RM, Calloway CC, Schnell JT, Whitaker CM, Burke DA, Hubscher CH. Loss and spontaneous recovery of forelimb evoked potentials in both the adult rat cuneate nucleus and somatosensory cortex following contusive cervical spinal cord injury. Exp Neurol 207: 238–247, 2007. doi: 10.1016/j.expneurol.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan B, Zahner MR, Kulikowicz E, Schramm LP. Effects of corticospinal tract stimulation on renal sympathetic nerve activity in rats with intact and chronically lesioned spinal cords. Am J Physiol Regul Integr Comp Physiol 293: R178–R184, 2007. doi: 10.1152/ajpregu.00044.2007. [DOI] [PubMed] [Google Scholar]
- 58.Patil RD, Karve SV, DiCarlo SE. Integrated cardiovascular physiology: a laboratory exercise. Am J Physiol 265: S20–S31, 1993. doi: 10.1152/advances.1993.265.6.S20. [DOI] [PubMed] [Google Scholar]
- 59.Phillips WT, Kiratli BJ, Sarkarati M, Weraarchakul G, Myers J, Franklin BA, Parkash I, Froelicher V. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol 23: 641–716, 1998. doi: 10.1016/S0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- 60.Popovich PG, Lemeshow S, Gensel JC, Tovar CA. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp Neurol 233: 615–622, 2012. doi: 10.1016/j.expneurol.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price MJ, Campbell IG. Effects of spinal cord lesion level upon thermoregulation during exercise in the heat. Med Sci Sports Exerc 35: 1100–1107, 2003. doi: 10.1249/01.MSS.0000074655.76321.D7. [DOI] [PubMed] [Google Scholar]
- 62.Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens 25: 349–358, 2003. doi: 10.1081/CEH-120023544. [DOI] [PubMed] [Google Scholar]
- 63.Rodenbaugh DW, Collins HL, DiCarlo SE. Paraplegia differentially increases arterial blood pressure related cardiovascular disease risk factors in normotensive and hypertensive rats. Brain Res 980: 242–248, 2003. doi: 10.1016/S0006-8993(03)02982-2. [DOI] [PubMed] [Google Scholar]
- 64.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol 285: H2605–H2613, 2003. doi: 10.1152/ajpheart.00319.2003. [DOI] [PubMed] [Google Scholar]
- 65.Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol 210: 489–500, 2008. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N. Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168: 2269–2274, 2002. doi: 10.1016/S0022-5347(05)64369-8. [DOI] [PubMed] [Google Scholar]
- 67.Sholl D. The quantification of neuronal connectivity. In: Organization of the Cerebral Cortex (edited by Da S.). New York: Wiley, 1956. [Google Scholar]
- 68.Singh A, Krisa L, Frederick KL, Sandrow-Feinberg H, Balasubramanian S, Stackhouse SK, Murray M, Shumsky JS. Forelimb locomotor rating scale for behavioral assessment of recovery after unilateral cervical spinal cord injury in rats. J Neurosci Methods 226: 124–131, 2014. doi: 10.1016/j.jneumeth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Squair JW, Liu J, Tetzlaff W, Krassioukov AV, West CR. Spinal cord injury-induced cardiomyocyte atrophy and impaired cardiac function are severity dependent. Exp Physiol 103: 179–189, 2018. doi: 10.1113/EP086549. [DOI] [PubMed] [Google Scholar]
- 70.Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res 455: 187–191, 1988. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 71.Swoap SJ, Overton JM, Garber G. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287: R391–R396, 2004. doi: 10.1152/ajpregu.00731.2003. [DOI] [PubMed] [Google Scholar]
- 72.Theisen D. Cardiovascular determinants of exercise capacity in the Paralympic athlete with spinal cord injury. Exp Physiol 97: 319–324, 2012. doi: 10.1113/expphysiol.2011.063016. [DOI] [PubMed] [Google Scholar]
- 73.Thijssen DH, Steendijk S, Hopman MT. Blood redistribution during exercise in subjects with spinal cord injury and controls. Med Sci Sports Exerc 41: 1249–1254, 2009. doi: 10.1249/MSS.0b013e318196c902. [DOI] [PubMed] [Google Scholar]
- 74.Tokuhisa T, Yano M, Obayashi M, Noma T, Mochizuki M, Oda T, Okuda S, Doi M, Liu J, Ikeda Y, Yamamoto T, Ohkusa T, Matsuzaki M. AT1 receptor antagonist restores cardiac ryanodine receptor function, rendering isoproterenol-induced failing heart less susceptible to Ca2+ -leak induced by oxidative stress. Circ J 70: 777–786, 2006. doi: 10.1253/circj.70.777. [DOI] [PubMed] [Google Scholar]
- 75.West CR, Campbell IG, Shave RE, Romer LM. Resting cardiopulmonary function in Paralympic athletes with cervical spinal cord injury. Med Sci Sports Exerc 44: 323–329, 2012. doi: 10.1249/MSS.0b013e31822b7441. [DOI] [PubMed] [Google Scholar]
- 76.West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 50: 484–492, 2012. doi: 10.1038/sc.2012.17. [DOI] [PubMed] [Google Scholar]
- 77.Yeoh M, McLachlan EM, Brock JA. Chronic decentralization potentiates neurovascular transmission in the isolated rat tail artery, mimicking the effects of spinal transection. J Physiol 561: 583–596, 2004. doi: 10.1113/jphysiol.2004.074948. [DOI] [PMC free article] [PubMed] [Google Scholar]











