Abstract
Neuromuscular impairment and reduced musculoskeletal integrity are hallmarks of spinal cord injury (SCI) that hinder locomotor recovery. These impairments are precipitated by the neurological insult and resulting disuse, which has stimulated interest in activity-based physical rehabilitation therapies (ABTs) that promote neuromuscular plasticity after SCI. However, ABT efficacy declines as SCI severity increases. Additionally, many men with SCI exhibit low testosterone, which may exacerbate neuromusculoskeletal impairment. Incorporating testosterone adjuvant to ABTs may improve musculoskeletal recovery and neuroplasticity because androgens attenuate muscle loss and the slow-to-fast muscle fiber-type transition after SCI, in a manner independent from mechanical strain, and promote motoneuron survival. These neuromusculoskeletal benefits are promising, although testosterone alone produces only limited functional improvement in rodent SCI models. In this review, we discuss the (1) molecular deficits underlying muscle loss after SCI; (2) independent influences of testosterone and locomotor training on neuromuscular function and musculoskeletal integrity post-SCI; (3) hormonal and molecular mechanisms underlying the therapeutic efficacy of these strategies; and (4) evidence supporting a multimodal strategy involving ABT with adjuvant testosterone, as a potential means to promote more comprehensive neuromusculoskeletal recovery than either strategy alone.
Keywords: neuroplasticity, bodyweight-supported treadmill training, estradiol, estrogen, muscle, PI3K, IGF-1, motor neuron, BDNF, FOXO, PGC-1 alpha, PGC-1 beta
1. Introduction
Spinal cord injury (SCI) results in profound sensorimotor impairment below the lesion site, which is precipitated by the insult to the spinal cord and exacerbated by a cascade of secondary cellular and molecular processes occurring in the acute and chronic post-injury phases [1,2,3]. Neuromuscular impairment and muscle loss are hallmarks of the SCI injury cascade that produce substantial impediments to physical therapy regimens intended to restore locomotion [3]. Activity-based physical rehabilitation therapies (ABTs) (e.g., bodyweight-supported treadmill training (BWSTT) or robotic assisted locomotor training with or without electric stimulation) have shown promise in promoting neuromuscular plasticity in humans and rodent models after motor-incomplete SCI [4,5]. However, ABTs are only partially effective in restoring function after SCI and their neuromuscular efficacy declines as injury severity increases [6], indicating the need for adjuvant therapeutic strategies to hasten functional recovery in this population.
Low testosterone (T) is also a secondary consequence of SCI, with hypogonadism being ~four times more prevalent in men after SCI than in non-injured populations [7]. Non-neurologically impaired men with low T exhibit low muscle mass, impaired muscle function [8], and worsened walking biomechanics [9], while T replacement therapy (TRT) improves muscle mass, neuromuscular function [10], and walking speed in older ambulatory hypogonadal men [11]. To-date, a causal relationship between low T and worsened neuromuscular function after SCI has not been elucidated, although TRT increases sublesional lean mass [12] and muscle cross-sectional area (CSA) in men with motor-complete SCI [13], in a manner completely independent from voluntary muscle contractility. Similarly, in male and female rodent SCI models, androgen treatment attenuates sublesional muscle loss and other phenotypic changes associated with impaired muscle function after SCI [14,15,16,17,18,19,20], and may promote slight improvement in locomotor recovery [15]. Herein, we will summarize the (1) molecular deficits underlying muscle loss after SCI; (2) independent influences of T and ABTs on neuromuscular function and musculoskeletal integrity subsequent to SCI; (3) hormonal and molecular mechanisms underlying the therapeutic efficacy of these strategies; and (4) evidence supporting a multimodal strategy involving BWSTT with adjuvant T, as a potential means to promote more comprehensive neuromusculoskeletal recovery after SCI.
2. Neuromuscular Adaptations after SCI
Motoneuron innervation of skeletal muscle fibers influences muscle mass and function, and fiber-type distribution [21]. After SCI, changes to the excitatory and inhibitory inputs to the motor unit (i.e., spinal motoneuron and the innervated fibers) exist [22]. For example, motor units that are dysfunctional after SCI result in a weaker and less fatigue resistant muscle [22,23], with rate of force development and motor axon conduction velocity decreasing [24,25]. These changes may exacerbate functional decline after SCI and can impair locomotor recovery, depending on the injury severity and degree of motor dysfunction [26]. The plethora of anatomical and functional consequences resulting from motor unit impairment after SCI have been detailed elsewhere [22]. Herein, we will focus on somatic motoneuron structural adaptations that influence impaired motor function after SCI [27] and how T administration [17,18] and locomotor training [28,29] may alter these adaptations and ultimately improve muscle function and/or locomotor recovery.
The muscular adaptations to SCI have been detailed previously [30], which we summarize here in brevity. Individuals with SCI exhibit an 18–46% lower sublesional muscle cross-sectional area (CSA) than those without SCI, with the degree of atrophy depending on the injury severity [31] and muscle groups involved [32,33,34]. Muscle loss is worsened as injury severity progresses [31], a result of more impaired motor function and extended disuse in those with motor-complete SCI. For example, muscle atrophy occurs primarily in the initial six weeks after injury in those with motor-incomplete SCI [32], while whole muscle CSA and muscle fiber (f)CSA continually decline for at least 24 weeks after motor-complete SCI [33,35]. Atrophy of both type I (slow-oxidative) and type II (fast-glycolytic) fibers occurs in humans [35,36] and rodents in response to SCI [37], preceding intramuscular fat accumulation [32] and the well-characterized slow-to-fast fiber transition [38,39]. These structural and physiologic changes impair voluntary force generation and muscle endurance [40,41], factors that are associated with locomotor recovery after SCI [42,43]. However, skeletal muscle retains the ability to reverse the molecular cascade precipitating atrophy [44,45] and to improve contractility after SCI in response to sufficient stimuli [46]. In the sections below, we discuss evidence supporting the involvement of several molecular signaling pathways that likely influence muscle deficits after SCI.
2.1. Ubiquitin-Proteasome Signaling after SCI
Reduced phosphorylation of the forkhead box O (FOXO) proteins FOXO1 and FOXO3a promotes the transcription of muscle atrophy F-box (MAFbx or atrogin-1) and muscle ring finger-1 (MuRF1), muscle-specific E3 ubiquitin ligases that stimulate atrophy in response to disuse and other catabolic states [47]. The influence of these ubiquitin ligases on skeletal muscle atrophy has been demonstrated by the viral overexpression of MAFbx, which reduced the myotube diameter by ~85% in vitro, and by genetic elimination of MAFbx or MuRF1, which attenuated muscle atrophy in response to sciatic nerve transection [48]. Within several days of SCI, >50 protein ubiquination pathway genes are upregulated in sublesional muscle [49], an effect that likely influences the rapid muscle atrophy in response to injury. In particular, MAFbx and MuRF1 mRNA expressions are increased five- to >40-fold within two to eight days of injury [50,51,52,53,54], with protein expressions elevated >two-fold prior to the initiation of muscle atrophy [55]. Interestingly, the fold inductions of MAFbx and MuRF1 were associated with the rate of skeletal muscle atrophy in response to spinal cord transection [53], suggesting that a higher expression of these genes exacerbates muscle loss after SCI. In this regard, FOXO1 mRNA was 33% higher [54] and MAFbx and MuRF1 mRNA were >two-fold higher in rodents after spinal transection than in animals receiving sciatic nerve transection [53], potentially explaining the more rapid atrophy occurring after SCI than in other disuse conditions [53,56]. However, the increased expressions of these and other ubiquitin proteasome pathway genes do not persist after experimental SCI [49], with FOXO1, MAFbx, and MuRF1 gene expressions and pFOXO1 and pFOXO3 proteins reverting to the level of uninjured controls within two to 10 weeks of spinal cord transection [53,54,57]. Similarly, in humans with motor-complete SCI, MAFbx and MuRF1 expressions are high at one-month post-injury and decline by 50–75% at three- and 12-months, resulting from reductions in pFOXO1 and/or total FOXO3 proteins [58]. Indeed, in cohorts of men with chronic SCI, MAFbx and MuRF1 gene expression appear equal to [59] or less than uninjured controls, with FOXO1, FOXO3a, and MAFbx proteins being >35% lower than controls [60]. While our discussion focused on changes in the ubiquitin-proteasome pathway, it is important to note that other catabolic pathways likely influence muscle atrophy after SCI. For example, calpain 1 mRNA expression is increased as early as day 4 after spinal cord transection, an effect that persists for at least 15 days [50], which is important given the influence of calpain signaling on myofibrillar proteolysis [61].
2.2. IGF-1 Signaling Pathway after SCI
IGF-1 mediated activation of the phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway promotes skeletal muscle anabolism [62]. In the immediate days after SCI, mRNA encoding IGF-1, the IGF-1 receptor (IGF-1R), and several IGF binding proteins (IGFBP) are upregulated in muscle [50,51,63], and muscle IGF-1 protein expression is increased [63]. Despite this, muscle pAkt is reduced within several days of SCI [51,55], while relatively normal total and phosphorylated 4E-BP1 and S6K1 expression persist in the first few weeks after SCI [50,51]. The inability of IGF-1 to increase pAkt and downstream targets acutely after SCI likely occurs because insulin receptor substrate-1 (IRS1) protein expression is rapidly reduced in response to SCI [51]. In this regard, IRS1 expression is required for IGF-1 mediated muscle hypertrophy, evidenced by the inability of genetic IGF-1 overexpression to promote skeletal muscle hypertrophy in IRS1 deficient animals [64]. Subsequently, the downregulation of IGF-1 signaling persists chronically after SCI, evidenced by (1) 25–40% lower circulating IGF-1 [65,66] and 50% lower IGF-1 protein expression in the muscle of individuals with chronic SCI compared with controls [60]; and (2) lower total Akt protein and lower total and phosphorylated mTOR and S6K1 in rodent muscle 10-weeks after spinal cord transection [57,67], accompanying 50% lower immunoprecipitated S6K1 activity [67]. Similarly, in humans with motor-complete SCI, total and phosphorylated mTOR and regulatory-associated protein of mTOR (RPTOR), an mTOR cofactor, were lower at three- and 12-months after injury, when compared with values obtained one-month post-SCI, likely influencing the lower p4E-BP1 and pS6 (subunit of S6K1) protein expression [58] that persists for upwards of 30 years after SCI [60]. In this regard, circulating IGF-1 has been shown to be highly correlated with increased thigh muscle CSA in men with chronic motor-complete SCI [68].
2.3. PGC-1α and PGC-1β Signaling after SCI
In skeletal muscle, peroxisome proliferator-activated receptor gamma co-activator (PGC)-1α regulates type I fiber expression and mitochondrial biogenesis [69], while PGC-1β regulates various aspects of mitochondrial structure and function [70]. As evidence, transgenic PGC-1α expression results in a whole body muscular phenotype that is high in type I oxidative fibers [71] and muscle-specific PGC-1α overexpression increases mitochondrial biogenesis [72]. In comparison, ablation of PGC-1β in skeletal muscle myofibers in adulthood reduced mitochondrial CSA and mitochondrial respiration without altering fiber-type distribution, resulting in increased oxidative stress and impaired exercise performance [70]. Baligand et al. used genome wide analysis and identified that genes associated with mitochondrial dysfunction and impaired oxidative phosphorylation were altered three to 14 days after SCI [49]. In particular, muscle PGC-1α mRNA expression was suppressed to a nearly undetectable level three days after spinal cord transection [54] and within two weeks, muscle PGC-1α protein was dramatically reduced, accompanying reductions in slow myosin heavy chain (MHC) protein expression [73] that is indicative of fewer oxidative fibers. Similarly, at eight-weeks post-transection, muscle PGC-1α gene expression and total and nuclear PGC-1α protein were >50% lower than controls, and slow MHC and slow troponin mRNA and protein were >60% lower [16]. However, at 17-weeks after spinal cord transection, muscle PGC-1α mRNA expression was no longer suppressed in rodents [74]. In this regard, the hallmark slow-to-fast fiber-type shift resulting from spinal cord transection is most pronounced in the first 100 days after injury, with relatively minor changes in type I MHC expression thereafter [38]. Regardless, muscle PGC-1α mRNA was 75% lower in men with chronic motor-complete SCI than in normally active men [75]. In comparison, relatively less is known about the changes in muscle PGC-1β after SCI. Interestingly, PGC-1β mRNA was reduced by 90% within three days of sciatic nerve transection [54], an effect that is mentioned because SCI typically results in relatively larger gene expression changes [76] and more rapid muscle loss than other disuse models [53,56]. Wu et al. also reported that muscle PGC-1β mRNA expression was 50% lower than controls at eight-weeks after spinal transection [74] and Kramer et al. reported 75% lower PGC-1β mRNA in men with chronic motor-complete SCI, when compared with ambulatory controls [75].
3. Androgenic Regulation of Muscle
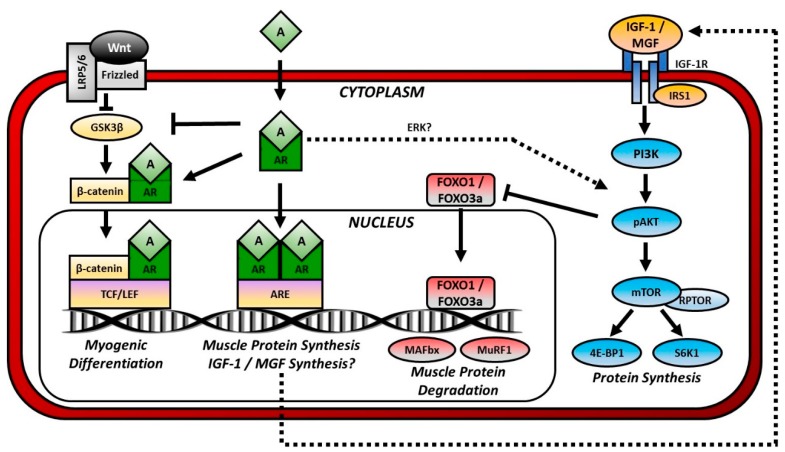
Maintenance of skeletal muscle mass requires relative balance between muscle protein synthesis (i.e., anabolic processes) and protein degradation (i.e., catabolic processes), with elevated catabolic signaling and/or reduced anabolic signaling influencing muscle loss. Readers are directed to the following in-depth review of the various signaling pathways that influence skeletal muscle anabolism and catabolism in response to disuse [77]. Herein, we will discuss how androgens influence muscle mass via classical AR-mediated signaling and through interactions with other intercellular anabolic and catabolic signaling pathways (Figure 1).
Figure 1.
Androgen-mediated Anabolic and Anticatabolic Signaling Pathways in Muscle. Anabolic signaling: Androgens (A) pass through the plasma membrane and bind to cytosolic androgen receptors (AR). Dimerized and phosphorylated ARs pass through the nuclear membrane and bind to a region of the DNA termed the androgen response element (ARE), thereby initiating protein synthesis. Ligand-bound ARs may also enhance Wnt signaling as follows. Wnt binds to Frizzled and in turn disheveled (not shown). Disheveled inhibits the activity of glycogen synthase kinase-3β (GSK3β), which phosphorylates β-catenin and marks it for degradation. When GSK3β is inhibited, β-catenin accumulates and enters the nucleus where it binds to a region of the DNA termed the T-cell factor/lymphoid enhancer factor (TCF/LEF) that regulates genes involved in myogenic differentiation. Ligand-bound ARs enhance Wnt signaling by inhibiting GSK3β and attaching to β-catenin for nuclear shuttling. Androgens may also indirectly stimulate protein synthesis by activating the phosphatidylinositol-3 kinase (PI3K)/Akt signaling through actions of Erk or by promoting synthesis of insulin-like growth factor (IGF)-1 or mechano growth factor (MGF). IGF-1 and MGF bind cell-surface IGF-1 receptors (IGF-1R) and activate PI3K/Akt signaling. Anticatabolic signaling: Activation of PI3K/Akt signaling inhibits the transcription factor forkhead box O (FOXO). FOXO1 and FOXO3a activate muscle atrophy F-box (MAFbx or atrogin-1) and muscle ring finger-1 (MuRF1), and E3 ubiquitin ligases that prepare proteins for proteasome degradation.
3.1. Testosterone Synthesis and Metabolism
Testosterone is the most abundant bioactive androgen and is primarily synthesized by testicular Leydig cells in males [78] and by the ovaries in females [79], and to a lesser extent in other organs, such as the adrenal cortex [80]. The majority of T circulates are bound to protein transporters (i.e., sex-hormone binding globulin (SHBG) or albumin), with only a small fraction (~1–2%) circulating freely unbound to any protein transporter [81]. The fraction of free (i.e., unbound) T and albumin-bound T is bioavailable [82] because it can bind cell surface or cytosolic androgen receptors (ARs), in the case of free T, or cross the cell membrane and dissociate from its low affinity protein transporter, in the case of albumin, which allows subsequent cytosolic AR binding. In comparison, SHBG-bound T is biologically inactive because it cannot (1) bind cell-surface/plasma-membrane ARs; (2) cross cellular membranes and bind cytosolic ARs [83]; or (3) undergo enzymatic metabolism [84]. As such, elevated SHBG is associated with reduced androgenic action in target tissues expressing ARs, including skeletal muscle [78], bone [84], and spinal motoneurons [85,86,87], among others.
In addition to serving as a hormone, T is a substrate for the tissue-specific synthesis of two bioactive sex-steroid hormones, dihydrotestosterone (DHT) and estradiol (E2), via actions of the 5α-reductase and aromatase enzymes, respectively [78,84]. The localized conversion of T to DHT, via any of the three 5α-reductase isozymes [78], enhances tissue-specific androgen signaling given that DHT binds to ARs with ~three times the affinity of T [88] and that DHT maintains a longer presence in tissues because it is not aromatized to E2 nor converted to androstenedione (a weaker androgen), via 17β-hydroxysteroid dehydrogenase (17β-HSD) [78]. Similarly, the aromatization of T to E2 amplifies estrogen signaling in tissues expressing estrogen receptors (ERs) [89]. In males, this conversion primarily occurs in non-gonadal tissue, with >80% of circulating E2 derived from localized peripheral metabolism [90]. However, in females, the peripheral aromatization of T to E2 also amplifies estrogen signaling [79], an effect that is particularly evident after the menopausal transition or in other situations where ovarian estrogen synthesis declines [80].
3.2. Hypogonadism and Testosterone Replacement after SCI
After SCI, 45–60% of men exhibit low T (hypogonadism, T < 300 ng/dL) [91,92,93] that results, in-part, from secondary testicular dysfunction [94] and impairments in the hypothalamic-pituitary axis [95,96]. Hypogonadism is ~four times more prevalent after SCI than in able-bodied men [7], with T concentrations remaining >25% below that of age-matched healthy men for an extended duration after SCI [91,97]. Additionally, a 50% greater decline in circulating T and >10-fold higher increase in SHBG occurs throughout the age-span in response to SCI [92], indicating that assessment of total T may underestimate hypogonadism incidence after SCI, a concept that we have demonstrated in other populations that exhibit elevated SHBG [81]. A causal relationship between low T and muscle loss after SCI has yet to be identified, although it is interesting to note that Finkelstein et al. reported that healthy men receiving pharmacologic treatment to maintain circulating T in the subphysiologic range (191 ± 78 ng/dL) exhibited a significant decline in whole body lean mass and thigh muscle area [98]. In this regard, the median T concentrations observed in several SCI cohort studies (i.e., 160–220 ng/dL) [93,99] were similar to that of Finkelstein et al., suggesting that the magnitude of hypogonadism after SCI is sufficient to induce muscle loss.
Our rodent severe contusion SCI model also exhibits 40–55% lower circulating T than controls for at least two months after injury [14,15,37,100] and androgen treatment attenuates muscle loss [14,15,16] and fCSA atrophy [17,18,19,20] in some, but not all, sublesional muscles in rodent SCI models [16,37,101]. In this regard, androgen-induced muscle preservation appears dependent upon muscle-specific AR expression, given that Phillips et al. reported muscle preservation in the sublesional levator ani/bulbocavernosus (LABC) muscle (high AR expression), but not soleus (low AR expression), in response to T treatment after SCI [37]. Importantly, neither E2 treatment [17] nor the conversion of T to DHT [14] influence muscle preservation in rodent SCI models, suggesting dispensability of aromatase and 5α-reductase activity in muscle. Indeed, Borst et al. [102] demonstrated that co-administration of T with finasteride (type II 5α-reductase inhibitor) to non-neurologically impaired men resulted in dramatically lower DHT, but did not prevent T-induced improvements in lean mass or muscle strength. Several small clinical trials have also assessed TRT in men with motor-complete SCI. For example, Cooper et al. observed ~40% lower urinary protein and creatinine excretion in SCI patients receiving TRT acutely after injury [103,104], indicating reduced whole body and muscle protein catabolism. Additionally, Bauman et al. reported that 12 months of TRT increased upper- and lower-extremity lean mass and resting energy expenditure in hypogonadal men with motor-complete SCI [12], with improvement persisting for at least 12-months after TRT discontinuation [105]. Lastly, a single case-study recently reported that TRT increased thigh muscle CSA in a man with motor-complete SCI [13].
3.3. Classical Androgen Signaling
Classical androgen-mediated genomic signaling is initiated by the binding of T or DHT to cytosolic ARs to form hormone-receptor complexes that translocate to the nucleus and bind androgen response elements (AREs) [106], resulting in the activation or repression of multiple gene pathways that regulate skeletal muscle [107]. Similarly, E2 stimulates classical estrogen-mediated genomic signaling that is characterized by binding cytoplasmic ERα or ERβ, which stimulates nuclear translocation of the hormone-receptor complex and binding to estrogen response elements (EREs). ER signaling is pronounced in bone [89], although the aromatization of T to E2 is not obligatory for skeletal muscle maintenance [98].
Androgens also exert actions that are independent of nuclear AREs [78]. For example, androgens [78] and estrogens [108] exert effects by binding non-traditional cell-surface sex-steroid hormone receptors (e.g., G-protein-coupled receptors (GPCR)) that rapidly alter several intracellular signaling pathways [106,109]. In mesenchymal multipotent C3H 10T1/2 cells, ligand-bound ARs also complex with cytosolic β-catenin [110]. This interaction results in the chaperoning and nuclear accumulation of the androgen-AR/β-catenin complex and stimulation of downstream T-cell factor (TCF)-4 genes, an effect that simultaneously promotes myogenic differentiation and suppresses adipogenic differentiation of mesenchymal pluripotent cells [110]. For further discussion on Wnt/β-catenin signaling, readers are directed to the following in-depth review [111]. Similarly, in C2C12 myotubes [112] and rodent skeletal muscle [113], androgens increase the phosphorylation of cytosolic glycogen synthase kinase-3β (GSK3β), which (1) inhibits the phosphorylation, ubiquitination, and degradation of β-catenin; and (2) promotes nuclear β-catenin accumulation and TCF activity. Ligand-bound ARs also interact with cytosolic β-catenin in motoneurons [114] and neuronal gonadotropin-releasing hormone cell lines [115] to promote nuclear shuttling of β-catenin and TCF-4 binding, which influences β-catenin-dependent transcriptional activity. To our knowledge, the functional significance of androgen-AR/β-catenin nuclear shuttling in motoneurons has not been determined. However, the loss of endogenous ARs accelerates motoneuron degeneration [116] and pharmacologically-induced stimulation of β-catenin protein expression in the spinal cord is associated with improved locomotor function after SCI [117,118,119], suggesting that nuclear β-catenin accumulation may promote motoneuron function.
3.4. Androgenic Crosstalk with IGF-1 and PI3K/Akt Signaling
Ligand-bound ARs activate PI3K/Akt signaling in muscle indirectly by stimulating the expression of (1) IGF-1 [113,120] and/or (2) other intracellular signaling molecules (e.g., Erk) that may activate or suppress components of this pathway independently of IGF-1 [121]. In support of this contention, the upstream promoter of the human IGF-1 gene exhibits two AREs that activate IGF-1 expression [122]. In rodents, androgen administration increases IGF-1 and mechano growth factor (MGF) mRNA expression by several fold in skeletal muscle within seven days of administration [113], an effect that persists for at least one month [120]. Similarly, within one month of initiating TRT, older men exhibit a >two-fold increase in IGF-1 protein expression in muscle [123]. In cultured rodent myoblasts, T treatment stimulates protein accretion and mammalian target of rapamycin (mTOR) mRNA expression, effects that are abrogated by co-incubation with bicalutamide (competitive AR antagonist), rapamycin (mTOR inhibitor), or LY294002 (PI3K inhibitor) [121,124], indicating that T-induced protein accretion is dependent upon both AR activation and PI3K signaling. Similarly, dose-dependent phosphorylation of Akt, mTOR, S6 (subunit of S6K1), and S6K1 occurs in cultured rodent and C2C12 myotubes in response to increasing concentrations of T [125,126]. Importantly, these effects are also abolished by co-incubation with LY294002, Akt inhibitor VIII, or rapamycin [126], demonstrating dependence upon the PI3K signaling pathway. Further, complete androgen withdrawal was sufficient to reduce muscle IGF-1 mRNA and suppress Akt, mTOR, S6K1, and 4E-BP1 phosphorylation (downstream PI3K targets) by >50% in rodents, with androgen (nandrolone or DHT) treatment increasing these factors two-fold above gonadally-intact animals [125,127]. Interestingly, Serra et al. reported that orchiectomy also increased RPTOR and reduced tuberous sclerosis complex protein 2 (TSC2) phosphorylation by >50%, changes associated with mTOR inhibition, while T treatment reversed these effects [128]. These findings indicate that androgens stimulate various IGF-1/PI3K/Akt signaling components in muscle and suggest that activation PI3K/Akt signaling influences androgen-mediated muscle hypertrophy. This contention is strengthened by the observation that MKR mice, which express a dominant negative IGF-1R in mature muscle fibers, exhibit attenuated hypertrophy of the highly androgen-sensitive LABC muscle in response to T treatment [128]. Regardless, IGF-1 and IGF-1R mRNA expression was not altered in animals receiving nandrolone for eight weeks after spinal cord transection, suggesting that androgens do not chronically stimulate IGF-1 expression in muscle in the absence of descending supraspinal input and/or mechanical strain [16].
3.5. Androgen Crosstalk with the Ubiquitin-Proteasome Pathway
Androgenic influence on FOXO expression and subsequent downstream signaling is evidenced by (1) increased total FOXO1 and FOXO3a protein and lower pFOXO3a in rodent muscle in response to orchiectomy (ORX) [125,128]; (2) 10- to 25-fold higher MAFbx and MuRF-1 mRNA expression in rodent skeletal muscle within seven days of ORX [128,129], an effect that persists for at least one month, albeit to a lesser magnitude over time [120,125]; and (3) the ability of androgen treatment to abolish these changes and prevent ORX-induced muscle atrophy [120,125,128,129]. Similarly, TRT suppresses ubiquitin-proteasome activity in the muscle of older men [130]. In C2C12 myoblasts and myotubes, androgen-induced suppression of MAFbx occurs indirectly via interactions among ligand-bound ARs and octamer binding transcription factor (Oct)-1 or Ankryn repeat domain protein 2 (Ankrd2), co-regulators of sex-steroid hormone induced transcriptional activity in target genes [131,132]. Interestingly, androgen treatment also attenuated muscle atrophy and the increases in FOXO1, MAFbx, and MuRF1 mRNA expression resulting from methylprednisolone administration after SCI [101], an effect that may be mediated by androgenic suppression of glucocorticoid receptor expression [120,133,134]. Alternatively, it is also possible that androgens indirectly influence FOXO signaling by stimulating PI3K/Akt signaling in muscle, given that FOXO1 and FOXO3a are downstream targets of pAkt signaling [62], although we are unaware of any study addressing this interesting possibility.
3.6. Androgenic Influence on PGC-1α and PGC-1β Signaling after SCI
Testosterone stimulates muscle PGC-1α expression via direct AR-mediated and/or ER-mediated pathways following aromatization to E2. As evidence, (1) castration lowered muscle PGC-1α mRNA by 50% in male rodents and nandrolone treatment increased PGC-1α mRNA >two-fold [125]; (2) ovariectomy lowered muscle PGC-1α >70% in female rodents [135], while one week of E2 replacement increased PGC-1α mRNA and nuclear protein expression in postmenopausal women [136]; and (3) within eight days of initiating pharmacologic E2 therapy, muscle PGC-1α mRNA was increased by 30% in men [137]. As discussed above, PGC-1α [16,54] and -1β expressions [74] are suppressed in response to spinal cord transection, an effect that may influence the slow-to-fast fiber transition [30] and/or mitochondrial dysfunction after SCI [138]. In this regard, chronic androgen treatment increased PGC-1α mRNA >three-fold in the muscle of animals receiving spinal cord transection and prevented the SCI-induced reductions in total and nuclear PGC-1α protein, changes that were accompanied by higher slow troponin mRNA and protein expression and lower fast troponin mRNA [16]. Similarly, Gregory et al. reported that androgen treatment attenuated the slow-to-fast fiber transition after spinal cord transection [20]. In addition, Wu et al. observed >two-fold higher PGC-1β mRNA expression in the muscle of spinal cord transected animals receiving nandrolone for eight weeks, although this change did not reach the level of statistical significance [16]. Regardless, the role of aromatase in mediating the influence of androgens on muscle PGC-1α and/or PGC-1β expression remains to be determined after SCI.
4. Androgenic and Estrogenic Regulation of Motoneurons
The influence of SCI on somatic motoneuron structure depends largely upon injury characteristics and the muscle groups innervated (discussed in [27]). For example, Sengelaub et al. [17] and Byers et al. [18] reported no differences in quadriceps motoneuron counts or soma area four-weeks after mid-thoracic contusion SCI, although, dendritic length was decreased 40–50%. Similarly, four-weeks after mid-thoracic spinal transection, fewer dendrites (labeled via sciatic nerve) were present on spinal motoneuron, and dendritic arbor size and dendritic length were reduced in comparison to intact animals [28]. Reduced tibialis anterior and soleus motoneuron dendritic length was also observed four-months after mid-thoracic contusion SCI, with no difference in soma area [29]. Whereas, Bose et al. reported fewer motoneurons innervating the soleus at four-months after mid-thoracic contusion SCI, with residual motoneurons being relatively larger and having fewer total dendrites that exhibited longer dendritic lengths [27]. When taken together, these findings indicate that dendrite length is rapidly reduced after SCI, with the loss of relatively small motoneurons and short dendrites occurring thereafter.
ARs are expressed in the cytoplasm and nuclear regions of spinal motoneurons [86] in a roughly equal distribution among male and female rodents [85]. 5α-reductase type II is highly expressed in several gray- and white-matter areas of the spinal cord [139], including spinal motoneurons [140]. Spinal cord homogenates and cultured NSC34 cells (immortalized motoneurons) actively convert T to DHT [141] in a manner that is ~three-times greater than that occurring in other central nervous system (CNS) regions [142] or the seminal vesicles, an androgen-sensitive tissue that highly expresses 5α-reductase type II [143]. The neuronotrophic effects of androgens are evidenced by the ability of T and DHT to equally preserve motoneuron number and size in cultured rodent lumbosacral spinal cord segments [144]. Indeed, lumbosacral motoneurons innervating the quadriceps and other musculature are androgen responsive [145]. However, the androgen sensitivity of spinal motoneurons appears largely dependent upon the magnitude of AR expression in motoneurons and in the innervated skeletal muscle, given that (1) motoneurons innervating LABC (high AR expression) exhibit robust dendritic atrophy in response to ORX, which is completely prevented by T or DHT administration [146]; (2) motoneurons innervating quadriceps (relatively lower AR expression) do not exhibit dendritic atrophy in response to ORX [147]; and (3) transgenic AR overexpression in skeletal muscle increased somatic motoneuron androgenic responsiveness, evidenced by ORX-induced dendritic atrophy in normally non-androgen responsive motoneurons, which was completely prevented by T administration [147].
ERα and ERβ are also expressed in cytoplasmic and nuclear regions of spinal motoneurons [148,149] and cultured motoneurons actively synthesize E2 in culture [149], owing to aromatase expression [148,149]. However, cultured rodent embryonic and adult spinal cord homogenates do not appear to synthesize E2 when incubated with androstenedione [142] or T [141]. Regardless, E2-mediated preservation of motoneuron dendritic growth [150] is as robust as that resulting from T or DHT treatment [151] and is highly dependent upon motoneuronal ERα expression, at least during development [152]. In culture, E2 treatment also improves spinal motoneuron cell survival during glutamate-induced toxicity, an effect that was not inhibited by ICI 182,780 (competitive ERα/ERβ agonist) [153], indicating that estrogen-mediated improvement in spinal motoneuron viability occurs independent of nuclear ER-signaling. In this regard, GPCR-30 (or G protein-coupled estrogen receptor-30) has been identified as a cell-surface/transmembrane ER that rapidly stimulates intracellular signaling [108] and E2 has been shown to improve motoneuron viability during glucose-oxygen deprivation, an effect that was blocked by co-treatment with G15 (GPCR-30 inhibitor) [154].
4.1. Influence of Androgens and Estrogens on Motoneuron Structure after SCI
As discussed above, altered dendritic length is a hallmark of SCI that has been observed in motoneurons innervating sublesional vastus lateralis [17,18], tibialis anterior [29], and soleus muscle [27]. In adult female rats, T treatment completely prevented the reduction in dendritic length of quadricep motoneurons resulting from SCI, without altering motoneuron counts, soma volume, or other motoneuron structural variables [18]. Whereas, DHT or E2 treatment each attenuated the reduction in quadriceps dendritic length by 50–60% [17]. The functional consequences of preserved dendritic length after SCI remains to be determined. However, it is interesting to note that our laboratory has reported that T treatment slightly increased hindlimb locomotor recovery in male rodents subsequent to moderate-severe contusion SCI, with the largest benefit occurring in response to supraphysiologic T [15], while E2 treatment is known to produce comparatively larger locomotor improvements in both male and female rodents after SCI [155].
4.2. Molecular Pathways Underlying Androgen-Mediated Motoneuron Protection
Androgenic regulation of brain-derived neurotrophic factor (BDNF) and tyrosine receptor kinase (Trk)B (BDNF receptor) in spinal motoneurons appears particularly important for motoneuron anatomical preservation [156,157]. As evidence, both somatic motoneurons and the spinal nucleus of the bulbocavernosus (SNB) express BDNF and TrkB mRNA and protein [146,158], with >80% of motoneuron dendrites identified as TrkB-positive [159]. In male rodents, ORX reduced BDNF mRNA and protein expressions in SNB [159] and in motoneurons innervating the quadriceps [158], while T treatment prevented these effects. BDNF regulates soma size independently of T, as evidenced by the ability of exogenous BDNF to maintain SNB motoneuron soma size in ORX male rats after bilateral axotomy [160]. In comparison, both BDNF and T appear necessary for the maintenance of SNB dendritic length, given that neither exogenous BDNF nor T independently prevented the reduction in SNB motoneuron dendritic length resulting from bilateral axotomy in ORX males, while co-treatment with BDNF and T increased dendritic length [161]. In females, T treatment also increased SNB motoneuron count by 50%, an effect that was prevented by the co-administration of TrkB-IgG [162]. These findings are particularly striking and suggest that androgen treatment may represent a means of stimulating BDNF/TrkB signaling, given the prevalence and severity of hypogonadism after SCI [91,92,93] and that the stimulation of motoneuron BDNF/TrkB signaling enhanced functional recovery in rodent SCI models [163].
In addition, estrogenic activation of GPCR-30 [108] or ERα [164] stimulates intercellular PI3K/Akt signaling, which promotes motoneuron survival [155]. As evidence, treatment of cultured spinal motoneurons with LY294002 (PI3K inhibitor) blocked the anti-apoptotic effects of E2 treatment during glucose-oxygen deprivation, resulting in a 50% increase in apoptotic motoneurons [154]. Similarly, PC12 cell viability was reduced ~50% upon treatment with rapamycin (mTOR inhibitor), demonstrating the necessity of downstream PI3K/Akt/mTOR signaling for cell survival [165]. Interestingly, LY294002 co-administration suppressed E2-mediated voluntary locomotor recovery in male rodents after SCI [154], indicating that PI3K/Akt signaling influences locomotor recovery after SCI. Estrogens may also exert neuroprotection via other pathways that have been reviewed by others [155].
5. Activity-Based Physical Rehabilitation after SCI
After SCI, increasing focus has been placed on improving activity-dependent neuromuscular plasticity through the use of BWSTT, a therapy that involves robotic or manual placement of the impaired limbs into normal gait patterns on a slowly moving treadmill, or other ABTs (e.g., passive or functional electric stimulation cycling) [166]. Through intense repetitive practice, it is postulated that ABTs activate sublesional spinal networks that promote beneficial neuromuscular adaptations by retraining the CNS to recover task-specific motor activities, via stimulation of the central pattern generator (CPG) in the lumbosacral region of the spinal cord [166]. Indeed, a number of studies involving persons with motor-incomplete SCI indicate that BWSTT produces several functional benefits, including (1) improved temporal gait parameters associated with walking ability (e.g., increased number of steps, faster cadence, and improved muscle activation patterns) [167,168,169,170,171,172,173,174,175,176,177]; (2) improved muscle strength and rate of torque development in impaired limbs, and reduced detrimental co-activation of antagonist muscle groups [24,169,171,178,179,180]; and (3) reduced muscle atrophy [24,178,181,182,183,184] (Table 1). However, when data from well-controlled randomized clinical trials assessing BWSTT after SCI [179,185,186,187,188] are pooled, there appears to be only limited meaningful improvement in overground walking speed or distance [4,189,190,191], given that the minimal clinically important difference in walking speed is reported as 0.13 m/s in the SCI population [192]. This finding stresses the necessity of testing combinatory therapies that prime the neuromuscular system for better functional recovery when combined with BWSTT.
Table 1.
Characteristics of Bodyweight Supported Locomotor Training in Human Spinal Cord Injury Studies.
| Study | Intervention | Duration | Population | N | Outcomes |
|---|---|---|---|---|---|
| Studies Assessing Gait | |||||
| Dobkin et al., 2007 [185] | Manual-assisted BWSTT vs. OG training |
12 wk | 38 ASIA B, 107 ASIA C&D | 145 | BWSTT increased walking speed from ~0.40 m/s at 2 weeks post-entry to 0.85 m/s for ASIA C&D. OG training increased walking speed from ~0.50 at 2 weeks post-entry to 0.84 m/s for ASIA C&D. Poor walking outcomes were observed for ASIA B participants. |
| Field-Fote et al., 2001 [167] | BWSTT with ES | 3 days/wk, 12 wk | ASIA C | 19 | Walking speed increased from 0.12 to 0.21 m/s. a |
| Alcobendas-Maestro et al., 2012 [218] | Robotic-assisted BWSTT vs. OG training |
3–6 mo | ASIA C&D | 48 M/32F | Robotic-assisted BWSTT and OG training walking speed remained the same from baseline to post-training. Robotic-assisted BWSTT increased 6 min walk distance from 91 to 169 m. a |
| Alexeeva et al., 2011 [179] | BWS training on a fixed track vs. BWSTT vs. Comprehensive PT |
3 days/wk 13 wk | ASIA C&D | 30 M/5 F | BWS on a fixed track increased walking speed from 0.33 to 0.44 m/s. a BWSTT increased walking speed from 0.30 to 0.46 m/s. a PT increased walking speed from 0.41 to 0.51 m/s. a |
| Duffell et al., 2015 [202] | Robotic-assisted BWSTT | 3 days/wk, 4 wk | ASIA C&D | 19 M/7 F | Walking speed increased from ~0.55 to ~0.58 m/s. a |
| Esclarin-Ruz et al., 2014 [219] | Robotic-assisted BWSTT + OG training (LKOGT) vs. Conventional OG training (OGT) | 5 days/wk, 8 wk | ASIA C&D | 59 M/24 F | LKOGT increased walking speed from 0.48 to 0.54 m/s and 6 min walk distance from 122 to 187 m b in participants with upper motor neuron injury. LKOGT increased walking speed from 0.24 to 0.46 m/s and 6 min walk distance from 82 to 157 m b in participants with lower motor neuron injury. OGT increased walking speed from 0.36 to 0.39 m/s and 6 min walk distance from 93 to 119 m in participants with upper motor neuron injury. OGT increased walking speed from 0.28 to 0.45 m/s and 6 min walk distance from 94 to 145 m in participants with lower motor neuron injury. |
| Field-Fote et al., 2005 [220] | Manual-assisted BWSTT (TM) vs. BWSTT + ES (TS) vs. BWS OG training + ES (OGS) vs. Robotic-assisted BWSTT (LR) |
5 days/wk 12 wk | ASIA C&D | 22 M/5 F | TM increased walking speed from ~0.07 to ~0.10. TS increased walking speed from ~0.12 to ~0.16 m/s. a OG increased walking speed from ~0.14 to ~0.19 m/s. a LR increased walking speed from ~0.09 to ~0.11. |
| Field-Fote & Roach 2011 [186] | Manual-assisted BWSTT (TM) vs. BWSTT + ES (TS) vs. BWS OG training +ES (OGS) vs. Robotic-assisted BWSTT (LR) |
5 days/wk, 12 wk | ASIA C&D | 52 M/12 F | TM increased walk speed from 0.17 to 0.22 m/s a and 2 min distance from 22.1 to 23.0 m. TS increased walk speed from 0.18 to 0.23 m/s a and 2 min distance from 20.6 to 24.4 m. a OGS increased walk speed from 0.19 to 0.28 m/s a and 2 min distance from 24.0 to 38.3 m. a,b LR increased walk speed from 0.17 to 0.18 m/s and 2 min distance from 16.8 to 17.9 m. |
| Gorassini et al., 2009 [169] | Manual-assisted BWSTT | 5 days/wk, 14 wk | ASIA C&D | 14 M/3 F | In 9 responders, walking speed increased from 0.31 to 0.55 m/s. a In 8 non-responders, there was no change in walking speed. |
| Harkema et al., 2012 [170] | Manual-assisted BWSTT | 3 day/wk, 4 to 92 wks | ASIA C&D | 148M/48 F | Walking speed increased from 0.31 to 0.51 m/s a and 6 min walk distance increased from 91 to 154 m. a |
| Kapadia et al., 2014 [221] | Robotic-assisted BWSTT + ES | 3 days/wk, 16 wk | ASIA C&D | 13 M/3 F | Walking speed increased from 0.23 to 0.28 m/s and 6 min walk distance increased from 187.9 to 217.1 m. a |
| Knikou 2013 [214] | Manual-assisted BWSTT | 5 days/wk, 1.5–3.5 mo | ASIA C&D | 9 M/3 F | 6 min walk distance increased from 36.25 to 39.05 m for ASIA C and from 252 to 279.5 m for ASIA D participants. |
| Krishnan et al., 2016 [171] | Robotic-assisted BWSTT | 3 day/wk, 4 wk | ASIA C&D | 8 M/8 F | Median walking speed increased from 0.58 to 0.66 m/s. a 6 min walking distance did not change. |
| Labruyere et al., 2014 [222] | Robotic-assisted BWSTT | 4 days/wk, 4 wk | ASIA C&D | 5 M/4 F | Walking speed increased from 0.62 to 0.66 m/s. |
| Lam et al., 2015 [223] | Robotic-assisted BWSTT with resistance (LR) vs. Robotic-assisted BWSTT only (LO) | 3 days/wk, 12 wk | ASIA C&D | 9 M/6 F | LR increased walking speed from 0.29 to 0.40 m/s. LO increased walking speed from 0.33 to 0.44 m/s. |
| Lucarelli et al., 2011 [187] | BWSTT vs. Conventional gait training |
2 days/wk, 12 wk | ASIA C&D | 20 M/10 F | BWSTT increased walking speed from 0.85 to 1.25 m/s a as well as increased cadence, distance, step length and swing phase. Conventional training did not improve gait quality or speed. |
| Morrison et al., 2018 [224] | Manual-assisted BWSTT | 120 sessions | ASIA C&D | 49 M/20 F | Median walking speed increased by 0.25 m/s. a Median 6 min walk distance increased by 66 m. a |
| Niu et al., 2014 [225] | Robotic-assisted BWSTT | 3 days/wk, 4 wk | ASIA C&D | 27 M/13 F | Walking speed increased in the low-walking capacity group from 0.12 to 0.15 m/s and in the high-walking capacity group from 0.84 to 0.97 m/s. 6 min walk distance did not change. |
| Nooijen et al., 2009 [172] | Manual-assisted BWSTT vs. Manual-assisted BWSTT with ES vs. OG training with ES vs. Robotic-assisted BWSTT |
4 days/wk, 12 wk | ASIA C&D | 40 M/11 F | All therapies led to small improvements in gait quality (increased cadence and step length) with no differences among groups. |
| Potsans et al., 2004 [188] | BWSTT with ES Cross-over design: treatment-control (AB); control-treatment (BA) |
5 days/wk, 4 wk | ASIA C&D | 12 M/2 F | In AB group, walking speed increased 0.23 m/s and 6 min walk distance increased 72.2 m. In BA group, walking speed increased 0.17 m/s and 6 min walk distance increased 63.8 m. |
| Thomas et al., 2005 [180] | Manual-assisted BWSTT | 4 days/wk, 16 wk | ASIA C&D | 8 M/2 F | Walking speed increased from 0.15 to 0.53 m/s a and 6 min walk distance increased from 34.2 to 167.6 m. |
| Varoqui et al., 2014 [203] | Robotic-assisted BWSTT | 3 days/wk, 4 wk | ASIA C&D | 14 M/ 1 F | Walking speed increased from 0.56 to 0.64 m/s a and 6 min walk distance did not change (207 to 209 m). |
| Winchester et al., 2005 [226] | Robotic-assisted BWSTT | 3 days/wk 12 wk | ASIA C&D | 4 M | Walking speed increased for 3 participants from 0.0 to 0.11, 0.0 to 0.81, 0.24 to 0.62 m/s and one remained unable to ambulate. |
| Wirz et al., 2005 [175] | Robotic-assisted BWSTT | 4 days/wk, 8 wk | ASIA C&D | 18 M/2 F | Walking speed increased from ~0.37 to ~.48 m/s a and 6 min walk distance 120 to 160 m. a |
| Wu et al., 2012 [227] | 4 wk Robotic-assistance BWSTT + 4 wk Robotic-resistance BWSTT | 3 days/wk, 8 wk | ASIA D | 8 M/2 F | Walking speed increased from 0.67 to 0.76 m/s a and 6 min walk distance increased from 223 to 247 m. Step length and cadence increased. a |
| Studies Assessing Muscle | |||||
| Duffell et al., 2015 [202] | Robotic-assisted BWSTT | 3 days/wk, 4 wk | ASIA C&D | 19 M/7 F | Maximal isometric ankle dorsiflexion torque increased from 12.3 to 13.2 Nm, a but plantar flexion torque did not change (28.1 vs. 28.4 Nm). |
| Galen et al., 2014 [228] | Robotic-assisted BWSTT | 5 days/wk, 6 wk | ASIA C&D | 14 M/4 F | Percent peak torque increased 68% for hip flexion, 54% for hip extension, 93% for knee flexion and 71% for knee extension. |
| Gorassini et al., 2009 [169] | Manual-assisted BWSTT | 5 days/wk, 14 wk | ASIA C&D | 14 M/3 F | In 9 responders, peak electromyography activity increased from 67 to 135 μV in the tibialis anterior muscle and 36 to 50 μV in the hamstrings. In 8 non-responders, there was no change. |
| Jayaraman et al., 2008 [201] | Manual-assisted BWSTT | 5 days/wk, 9 wk | ASIA C&D | 4 M/1 F | Isometric knee extension strength increased 21%. Isometric plantar flexion strength increased 44%. Knee extension and plantar flexion voluntary muscle activation improved. Maximal CSA of the plantar flexors increased and 15%. |
| Krishnan et al., 2016 [171] | Robotic-assisted BWSTT | 3 day/wk, 4 wk | ASIA C&D | 8 M/8 F | BWSTT increased isometric ankle dorsiflexion by 20% and ankle plantar flexion by 22%. |
| Thomas et al., 2005 [180] | Manual-assisted BWSTT | 4 days/wk, 16 wk | ASIA C&D | 8 M/2 F | Peak electromyography activity averaged from four lower limb muscles increased during treadmill walking from 82.4 to 137.1 μV. |
| Varoqui et al., 2014 [203] | Robotic-assisted BWSTT | 3 days/wk, 4 wk | ASIA C&D | 14 M/1 F | Ankle dorsiflexion torque increased from 26.8 to 29.1 Nma and ankle plantar flexion torque increased from 10.9 to 13.5 Nm. a |
BWSTT = bodyweight supported treadmill training; CSA = cross-sectional area; ES = electrical stimulation; m = meter, min = minute; mo = month; OG = overground; PT = physical therapy; wk = week. Note: a indicates statistically different from baseline; b indicates statistically different between training groups.
Preclinical studies also utilize ABTs to evaluate mechanisms underlying activity-dependent neuroplasticity after SCI and have observed locomotor and neuromuscular improvements in response to BWSTT (Table 2) [5]. However, there are considerable differences in the reported effects of BWSTT on locomotor and neuromuscular recovery in SCI rodents, in-part due to the influence of age and gender, the mechanism of producing SCI (i.e., transection, hemisection, or contusion), delay in initiating BWSTT, duration of therapy, and the methods of assessing neuromuscular recovery [5,193]. In general, better outcomes for locomotor recovery are observed in female rats with mild to moderate contusion SCI, with training that begins seven to 14 days after injury and continues for at least eight weeks [5]. In this regard, female rodents have higher endogenous E2 than males [194], which is important given that subphysiologic and physiologic E2 treatment significantly improves open-field locomotor recovery in both male and female rodents after SCI, in-part, by reducing secondary apoptotic and inflammatory damage to the spinal cord [155].
Table 2.
Characteristics of Bodyweight Supported Treadmill Training (BWSTT) in Rodent SCI Studies.
| Study | Sex/Age | Injury Level | Start of Training | Training Duration | BBB wk 1 | BBB End | Gait Outcome | Muscle/Electrophysiology |
|---|---|---|---|---|---|---|---|---|
| Studies Assessing BWSTT after Spinal Hemisection | ||||||||
| Maier et al., 2009 [229] | F/A | T8 | 1 wk | 8 wk | ~12 | N/R | Stepping ↑ | N/R |
| Battistuzzo et al., 2016 [230] | M/A | T10 | 1 wk | 9 wk | N/R | N/R | Kinematics ↑ | N/R |
| Battistuzzo et al., 2017 [231] | M/A | T10 | 1 wk | 9 wk | N/R | N/R | N/R | G fCSA ↑ |
| Shah et al., 2013 [232] | N/R/N/R | T10 | 5 days | 2.5 wk | N/R | N/R | Stepping ↑ | N/R |
| Goldshmit et al., 2008 [212] | N/R/A | T12 | 1 wk | 4 wk | ~3 | 11T/6C | Kinematics ↑ | G-S fCSA ↑ |
| Studies Assessing BWSTT after Mild Contusion SCI | ||||||||
| Nessler et al., 2006 [233] | F/Y | T9 | 1 wk | 12 wk | 13 | 14T/No C | N/R | N/R |
| Oh et al., 2009 [234] | M/Y | T9–10 | 1 wk | 4wk | ~4 | 13T/8C | N/R | N/R |
| Studies Assessing BWSTT after Moderate Contusion SCI | ||||||||
| Stevens et al., 2006 [197] | F/Y | T8 | 1 wk | 1 wk | ~5 | 10T/6C | N/R | Sol F ↑/fCSA ↑ |
| Liu et al., 2008 [199] | F/Y | T8 | 8 days | 12 wk | 3–7 | 15T/11C | N/R | G-S CSA ↑ |
| Nessler et al., 2006 [233] | F/Y | T9 | 1 wk | 12 wk | 9 | 11T/No C | N/R | N/R |
| Shin et al., 2014 [235] | F/Y | T9 | 1 wk | 8 wk | ~6 | ~13T/~10C | N/R | N/R |
| Wang et al., 2015 [29] | F/Y | T9 | 1 wk | 16 wk | ~7 | ~13T/~11C | N/R | N/R |
| Singh et al., 2011 [200] | F/Y | T9–10 | 1 wk | 8 wk | ~5 | ~8T/~9C | N/R | mass/bw ↑ |
| Bose et al., 2012 [198] | F/A | T8 | 1 wk | 12 wk | ~3 | ~15T/~11C | Stepping ↑ | N/R |
| Multon et al., 2003 [236] | F/A | T9 | 2–4 days | 12 wk | ~2 | 10T/8C | N/R | N/R |
| Wu et al., 2016 [237] | F/A | T10 | 1 wk | 4 wk | ~5 | ~13T/~9C | N/R | N/R |
| Foret et al., 2010 [238] | F/A | T10 | 1 day | 4 wk | ~2 | ~9T/~7C | N/R | N/R |
| Ward et al., 2014 [239] | M/Y | T8 | 2 wk | 12 wk | ~7 | ~12T/~9C | N/R | Sol EMG ↑ |
| Park et al., 2010 [240] | M/Y | T10 | 3 days | 25 days | ~2 | 11.5 | N/R | N/R |
| Liu et al., 2017 [241] | N/R/N/R | T9 | 1 wk | 2 wk | N/R | N/R | Stepping ↑ | N/R |
| Studies Assessing BWSTT after Severe Contusion SCI | ||||||||
| Hayashibe et al., 2016 [242] | F/Y | T8–9 | 1 wk | 4 wk | <1 | 16T/10C | N/R | N/R |
| Heng et al., 2009 [243] | F/Y | T9 | 42 days | 8 wk | N/R | N/R | Stepping ↑ | N/R |
| Nessler et al., 2006 [233] | F/Y | T9 | 1 wk | 12 wk | 4.5 | ~9T/No C | N/R | N/R |
| Shinozaki et al., 2016 [244] | F/Y | T10 | 6 wk | 8 wk | ~1 | 4T/3C | N/R | N/R |
| Robert et al., 2010 [245] | F/A | T7–8 | 2 wk | 2 wk | ~3 | 4T/3.5C | N/R | N/R |
| Ichiyama et al., 2009 [246] | F/A | T10 | 30 days | 8 wk | N/R | N/R | Gait not improved | N/R |
| Studies Assessing BWSTT after Spinal Transection | ||||||||
| Petruska et al., 2007 [213] | F/N | T6–8 | 16 days | 6 wk | N/R | N/R | Stepping ↑ | G EPSP ↑ |
| Tillakaratne et al., 2010 [247] | F/N | T7–8 | 26 days | 8 wk | N/R | N/R | Stepping ↑ | N/R |
| See et al., 2013 [248] | F/N | T8–9 | 3 wk | 4 wk | N/R | N/R | Kinematics ↑ | N/R |
| Timoszyk et al., 2005 [249] | F/N | N/R | 64 days | 40 days | N/R | N/R | Gait not improved | N/R |
| Zhang et al., 2007 [250] | F/Y | T8 | 5 days | 40 days | <1 | ~6.5T/~2C | N/R | N/R |
| Lee et al., 2010 [251] | F/Y | T8 | 3 wk | 42 wk | N/R | N/R | Gait not improved | N/R |
| De Leon et al., 2006 [252] | F/Y | T9 | 3 wk | 16 wk | N/R | N/R | Kinematics ↑ | N/R |
| Moshonkina et al., 2004 [253] | F/Y | T9 | 1 day | 9 wk | N/R | N/R | Kinematics ↑ | N/R |
| Kubasak et al., 2008 [254] | F/N/R | T9 | 4 wk | 20 wk | N/R | N/R | Gait not improved | N/R |
| Fouad et al., 2000 [255] | B/A | T8 | 3 days | 5 wk | ~9 | 14T/14C | Kinematics ↑ | N/R |
| Ihla et al., 2011 [196] | M/A | T8–9 | 6 days | 9 wk | N/R | N/R | N/R | Sol fCSA ↑ |
Sex: B, both females and males; F, female; M, male; N/R, not reported; Age: A, adult; N, neonate; N/R, not reported; Y, young; Injury Level: T, thoracic; Start of Training: wk, week; BBB end of training: T, trained; C, control SCI untrained; Other gait outcome: ↑ improvement; Muscle/Electrophysiology: bw, bodyweight; EPSP, evoked monosynaptic excitatory postsynaptic potentials F, force; fCSA, fiber cross-sectional area; G, gastrocnemius; G-S, gastrocnemius-soleus; ↑, increased; Sol, soleus, N/R, not reported. Note: The BBB scale ranges from 0–21 and is used to evaluate functional locomotor recovery ranging from no observable movement (0) to consistent weight-supported plantar stepping with coordinated gait (21).
5.1. Effects of ABTs on Muscle
In rodent SCI models, BWSTT produces relatively consistent improvements in muscle integrity, even following complete transection. As evidence, after spinal transection, the incorporation of BWSTT completely preserved the muscle mass:body mass ratio [195,196] and prevented ~55% of soleus fCSA loss [196]. Similarly, in a moderate-contusion SCI model, BWSTT produced 23% higher soleus fCSA and 38% higher soleus peak tetanic force in comparison with untrained SCI animals [197], with analogous effects reported by others [49,197,198,199,200]. Similarly, several case-report and cohort-studies have reported muscle responses in individuals with motor-incomplete SCI. For example, in persons with chronic SCI, four to six months of BWSTT increased vastus lateralis muscle fCSA [178,184]. Likewise, Jayaraman et al. reported that nine-weeks of BWSTT enhanced knee extensor and ankle plantar flexion voluntary muscle activation and increased plantar flexion peak torque by 43%, which accompanied a 15% increase in plantar flexor CSA [201], an important finding given that lower extremity strength is positively associated with walking function after SCI [42]. However, others have reported much less robust strength improvements [202,203] or no strength improvement [171] in response to BWSTT.
The structural improvements mentioned above appear to be influenced, in-part, by reduced catabolic signaling resulting from BWSTT. As evidence, in a rodent SCI model, three sessions of BWSTT suppressed >10 protein ubiquitination pathway genes, an effect accompanied by the full preservation of muscle mass [49]. Similarly, after spinal cord transection, five sessions of electrical stimulation of paralyzed muscle suppressed MAFbx and MuRF1 mRNA and myostatin mRNA in muscle [51]. Myostatin is a member of the transforming growth factor (TGF)-β family and a muscle-derived negative regulator of muscle growth, which acts via the activin IIB receptors [204]. Additionally, muscle regeneration occurs in response to BWSTT after SCI, evidenced by the upregulation of myogenic regulatory factors and increased expression of Pax7 positive nuclei (a marker of satellite cell activation) [63,205], suggesting that BWSTT stimulates muscle anabolic signaling pathways. In this regard, IGF-1, MGF, IGF-1R, and several IGFBPs mRNA were increased 1.5 to >10-fold in rodent muscle and IGF-1 protein expression was increased >three-fold within seven days of initiating BWSTT after moderate-contusion SCI [63]. In contrast, other reports indicate that electrical stimulation of paralyzed muscle suppressed IGFBP-4 and -5 mRNA in muscle, without altering IGF-1 or MGF mRNA after spinal transection [51,206]. Similarly, no change in circulating IGF-1 was observed in persons with chronic SCI undergoing a comprehensive physical rehabilitation program involving one to two hours per week of bodyweight-supported locomotor training [207]. As such, the role of IGF-1 signaling in the muscle regenerative response to BWSTT requires further elucidation, especially given that SCI-induced downregulation of IRS1 limits IGF-1 mediated activation of PI3K/Akt signaling in muscle acutely after SCI [51].
In addition to muscle preservation, BWSTT is reported to reduce soleus fatigue and attenuate the slow-to-fast fiber transition occurring in rodent moderate-contusion SCI models [197]. Similarly, in persons with chronic SCI, four to six months of BWSTT partially reversed the slow-to-fast fiber transition, evidenced by increased oxidative fibers and fewer type IIx (glycolytic) fibers compared with the baseline [178,184]. Interestingly, lower Mhyl, Mybph, and Myh4 gene expression (fast-twitch fiber markers) was apparent in rodent muscle within five-days of initiating BWSTT [49], indicating that reversal of the slow-to-fast fiber-type switch occurs rapidly with this physical rehabilitation modality. However, to our knowledge, changes in PGC-1α or -1β have not been evaluated in response to BWSTT after SCI. Regardless, a five-fold increase in PGC-1α mRNA in muscle was observed in men with chronic motor-complete SCI within several hours of neuromuscular electrical stimulation of quadriceps [208] and a ~two-fold increase in PGC-1α was present in men with chronic motor-complete SCI undergoing functional electric stimulation for durations of 16-weeks [209] to six-years [210], suggesting that increased PGC-1α expression may underlie the slow-to-fast fiber-type reversal occurring in response to BWSTT.
5.2. Effects of ABTs on Motoneurons
In rodent SCI models, ABTs limit injury severity, evidenced by the preservation of myelin, axons, and collagen morphology and decreased lesion volume [198], and produce a host of anatomical and functional improvements to the motor unit [29,198,200,211,212,213]. For example, after spinal cord transection, BWSTT increases axonal regrowth [212], preserves motoneuron dendritic length, increases neuromuscular junction synaptic density in the lumbar motoneuron pool [29], and produces larger group Ia afferent-evoked monosynaptic excitatory postsynaptic potentials (EPSPs) in the ankle plantar flexors, an effect that is particularly prevalent in rats regaining the greatest stepping ability [213]. Similarly, after moderate-severe contusion SCI, BWSTT produced a beneficial reduction in the sprouting of small caliber afferent fibers and improved stepping patterns while on the treadmill [200], although open-field locomotor activity did not improve in this study, suggesting some limitation to functional recovery in more severe injury scenarios. Interestingly, Singh et al. induced a spinal transection at nine weeks following contusion SCI in a subgroup of rats that previously underwent BWSTT or no training and observed that kinematic improvements were maintained in BWSTT rats and worsened in untrained rats, indicating that functional improvement resulting from BWSTT occurred via neural networks originating below the lesion level [200]. Indeed, Cote el al. reported that after spinal transection, BWSTT facilitated lumbar spine motoneuron pool recruitment via improved reflex pathways resulting from afferent input [211].
In individuals with incomplete SCI, beneficial adaptations to spinal neuronal pathways have been demonstrated in response to BWSTT. For example, the soleus H-reflex phase-dependent modulation during walking normalizes homosynaptic facilitation, reverses homosynaptic depression, and improves the presynaptic inhibition of Ia afferents [214,215,216]. However, better functional recovery has also been associated with increased ankle dorsiflexor and knee extensor maximal motor-evoked potential, a probe of corticospinal tract excitability, in persons with motor-incomplete SCI undergoing BWSTT [180] and improved ankle dorsiflexor and plantar flexor muscle co-activation patterns accompany improved walking function in response to BWSTT [171], findings that indicate greater descending corticospinal drive. In this regard, improvements in the above-mentioned factors likely stem from the reorganization of both supraspinal and spinal cord neural circuits in response to BWSTT [214,217], with further research needed to resolve whether functional adaptations rely more heavily on improved cortical or spinal circuitry in individuals with SCI.
While the molecular signals regulating BWSTT-induced motoneuron recovery after SCI require further elucidation, there is growing evidence that several inducible neurotrophic factors influence neuroplasticity [211]. For example, an upregulation of BDNF and TrkB (BDNF receptor) mRNA and protein has been observed in the skeletal muscle and spinal cord of healthy animals in response to voluntary wheel running or forced treadmill running, with BDNF protein levels increasing to a greater degree in the spinal cord than in the soleus [237,256,257]. In this regard, BDNF and TrkB both undergo retrograde transport from skeletal muscle to the spinal cord [258], an effect that may mediate locomotor recovery after SCI by improving synaptic transmission and plasticity, axon regeneration, and motoneuron survival [259]. Indeed, in rodent SCI models, BWSTT upregulates BDNF mRNA and protein in skeletal muscle and the spinal cord [29,196,198,211,237,260,261,262,263,264,265,266,267] and TrkB protein in the lumbar spinal cord [237,257]. Interestingly, Leech and Hornby also observed increased circulating BDNF in persons with motor-incomplete SCI undergoing treadmill training, with high-intensity treadmill exercise (performed at 100% peak gait speed) resulting in the larger BDNF increases than exercise performed at 33% or 66% peak gait speed [268]. In addition, BSWTT upregulated glial cell derived neurotrophic factor (GDNF) protein in the lumbar spinal cord, which was positively associated with the facilitation of motoneuron excitability [211].
Neurotrophin-3 (NT-3) is another endogenous neurotrophin that regulates synaptic transmission, promotes neuromuscular junction maturation, and improves the survival and function of sensory neurons via the activation of TrkC (receptor), both of which are expressed in the spinal cord [269,270,271,272,273]. Indeed, recent results from Fang et al. indicate that co-treatment with BDNF/TrkB and NT-3/TrkC promoted motor recovery in a rodent SCI model [274]. NT-3 is produced in the spinal cord and NT-3 mRNA and protein levels are upregulated after seven days of voluntary treadmill and wheel running in healthy rodents [256,262,265]. Similarly, following SCI, rodents exhibit increased NT-3 mRNA and protein in the spinal cord within seven days of initiating BWSTT [262,263,265,266]. Interestingly, Ying et al. reported that BWSTT did not increase spinal cord NT-3 protein expression in adult male rats after spinal cord hemisection [266], while Cote et al. observed increased NT-3 protein levels in the lumbar and thoracic spinal cord after four weeks of BWSTT in adult female rats with complete transection [211], suggesting that sex differences may exist in the NT-3 response to BWSTT. In this regard, E2 treatment increases NT-3 levels in several CNS regions [275], although we are unaware of any study evaluating whether spinal cord NT-3 expression is regulated via E2-mediated mechanisms. BWSTT is also reported to upregulate genes involved in neuroplasticity (e.g., Arc and Nrcam) and angiogenesis (e.g., Adam8 and Tie1), which may be important given that improved neurovascular remodeling has the potential to improve locomotor function after SCI [235].
6. Testosterone Adjuvant to BWSTT
We are unaware of published results from any randomized study evaluating T treatment as an adjuvant to BWSTT. However, at least one ongoing study is evaluating TRT as an adjuvant to electric stimulation-based resistance training in men with motor-complete SCI [276]. A single case-report has also indicated that a man with motor-complete SCI exhibited increased thigh muscle CSA in response to twice-weekly neuromuscular electric stimulation-based resistance training, when combined with adjuvant TRT [13]. In addition, our preliminary data indicates that the combination of testosterone-enanthate (TE), a long-acting T ester [277], and quadrupedal BWSTT (consisting of two 20-min bouts performed twice daily, five-days per week for seven-weeks) promoted more complete neuromuscular restoration than TE-alone in our rodent moderate-severe contusion SCI model [278]. Specifically, the incorporation of TE+BWSTT after SCI produced near-complete restoration of the sublesional muscle mass:body mass ratio, completely prevented the slow-to-fast fiber-type transition occurring in the soleus, attenuated the reduction in soleus fCSA by >50%, and maintained isolated soleus muscle force mechanics near the level of intact control animals. Impressively, 100% of SCI animals receiving TE+BWSTT regained the ability to perform occasional voluntary overground weight-supported hindlimb plantar stepping within seven-weeks of initiating therapy, while 0/11 SCI animals and only 2/10 of SCI animals receiving TE-alone regained this ability. As such, our preliminary data appear to support the contention that a combinatory strategy involving BWSTT with adjuvant TE promotes musculoskeletal and neuromuscular improvement in male rodents after moderate-severe contusion SCI.
Potential Side-Effects of TRT
TRT is approved for the treatment of hypogonadism in adult men and has been shown to increase muscle protein synthesis in women [279]. However, given its androgenic potential, chronic TRT remains controversial for women, at least when administered in the doses necessary to produce musculoskeletal benefit in males. This should not detract from TRT research in relation to SCI because men represent ~80% of new SCI cases [280] and many of these men will develop hypogonadism of an indefinite duration [91,92,93]. Regardless, careful evaluation of TRT safety and efficacy is necessary prior to implementation of this therapy after SCI.
To-date, meta-analyses have confirmed that TRT produces three adverse events (AEs) in non-neurologically impaired adult men: (1) polycythemia, which occurs in ~6% of men treated with TRT; (2) a higher number of cumulative prostate-related events (discussed below); and (3) a slight decrease in HDL cholesterol, which is of unknown clinical significance [281]. The development of polycythemia in men receiving TRT has raised concerns regarding the cardiovascular safety of TRT, especially when taken in context with findings from the Testosterone in Older Men with Mobility Limitations (TOM) trial [282] and from two retrospective studies that indicated increase cardiovascular risk in hypogonadal men receiving TRT [283,284]. In this regard, the TOM trial was discontinued upon recommendation of the Data Safety Monitoring Board due to a higher prevalence of cardiovascular-related AEs in the TRT versus placebo groups [282]. However, this trial received criticism because of the relatively poor classification of cardiovascular AEs [285]. In comparison, the NIH-funded T-trials, which represent the largest randomized clinical trials to-date evaluating the safety and efficacy of TRT, reported no differences in adjudicated cardiovascular AEs in hypogonadal men receiving TRT versus placebo [11]. Similarly, heavy scientific criticism and numerous calls for retraction [286] accompanied the retrospective studies from Vigen et al. [284] and Finkel et al. [283] that reported increased cardiovascular risk in hypogonadal men receiving TRT. Indeed, the aforementioned findings remain in direct conflict with findings from the largest meta-analyses on the topic, which reported that TRT does not increase cardiovascular risk in men enrolled in well-controlled clinical trials [287,288], and with findings from the largest retrospective study (n = 83,010 men with low T) that indicated TRT reduced all-cause mortality by 56% and MI and stroke risk by 24–36% in comparison with untreated hypogonadal men [289]. Of note, the potential cardiovascular responses to TRT have not been extensively evaluated in the SCI population. However, in a small clinical trial, La Fountaine et al. reported that QTa interval variability was higher in hypogonadal men with SCI than in eugonadal men with SCI, and that TRT normalized QTa interval variability, suggestive of reduced arrhythmia risk [290].
The risk for increased cumulative prostate-related events also merits mention because prostate growth and worsened urinary symptoms resulting from TRT have the potential to worsen bladder dysfunction, which is already common in the SCI population [291]. In this regard, the prostate-related AEs associated with TRT appear to be driven primarily by the 5α-reduction of T to DHT [8]. As evidence, we have reported that older hypogonadal men receiving TE-alone exhibited a 43% increase in prostate volume over a period of 12-months, while men receiving TE plus finasteride exhibited no change in prostate volume [102]. In comparison, 5α-reductase inhibition did not prevent the musculoskeletal or lipolytic benefits resulting from TRT in our trial [102] or others [292,293], indicating that finasteride co-treatment improves the prostate-related safety profile of TRT without inhibiting musculoskeletal efficacy in older men. The safety and efficacy of TE plus finasteride treatment in men with SCI currently remains unknown, although an ongoing clinical trial in our laboratory is evaluating this promising combinatory therapy in hypogonadal men with SCI [294]. In addition, it is important to note that a 15-yr retrospective study of 150,000 men reported that TRT was not associated with prostate cancer [295] and that no meta-analysis to-date has reported increased prostate cancer risk resulting from TRT [8].
Several less common risks are also associated with TRT, which we have previously reviewed [296], including: pain or bleeding at injection sites, skin reactions, fluid retention, breast tenderness, gynecomastia, and liver disorders. Importantly, these side-effects remain putative [8] and have not been confirmed by meta-analysis [281]. However, one potential risk that merits mention is the possibility that TRT may cause or worsen obstructive sleep apnea [297], a condition that is relatively common in tetraplegic men with SCI [298]. As such, need exists to determine TRT safety in men with SCI who display obstructive sleep apnea, especially given the higher sleep apnea prevalence in non-neurologically impaired men receiving TRT [297].
7. Conclusions
Upregulation of FOXO signaling occurs in the immediate days to weeks after SCI, an effect that likely influences the rapid muscle loss in this population. Similarly, muscle PGC-1α and -1β are reduced in response to SCI, underlying the slow-to-fast fiber change and impaired mitochondrial function subsequent to SCI. In comparison, downregulation of IGF-1 and PI3K/Akt signaling occurs more chronically after SCI. These molecular changes are precipitated by the spinal injury and subsequent disuse, but may also be exacerbated by low T that occurs in many men with SCI. T promotes muscle anabolism via direct AR-mediated genomic signaling and/or via cross-talk with IGF-1 and the PI3k/Akt signaling pathway. T may also promote anticatabolic effects in muscle by directly or indirectly suppressing FOXO signaling. Further, androgen treatment increases PGC-1α in muscle and attenuates the slow-to-fast muscle fiber-type transition occurring in response to spinal transection. In addition, T improves the preservation of motoneuron dendritic length after SCI directly via AR-mediated mechanisms and/or indirectly, following aromatization to E2. Regardless, these improvements appear to result in only minor improvement in locomotor recovery in rodents after moderate-severe contusion SCI, likely because only limited descending corticospinal input remains. In comparison, BWSTT improves descending corticospinal drive and/or facilitates reorganization of spinal cord neural circuitry below the lesion, which produces neuromuscular improvement and some restoration of musculoskeletal integrity. However, findings from well-controlled randomized clinical trials indicate that BWSTT only promotes limited clinically meaningful improvement in voluntary locomotor recovery in humans after SCI. Interestingly, activity-mediated neuromuscular plasticity appears to rely, in-part, upon the upregulation of BDNF/TrkB in the spinal cord. Androgen/AR signaling is required for BDNF/TrkB-mediated dendritic restoration in androgen sensitive motoneurons in the lumbosacral spinal cord, suggesting that T treatment may enhance activity-mediated neuromuscular plasticity after SCI. Indeed, our preliminary data supports this contention, given that adult male rodents receiving TE adjuvant to BWSTT exhibited profound musculoskeletal and neuromuscular improvement and recovery of voluntary weight-supported overground plantar stepping after moderate-severe contusion SCI. Elucidation of the molecular mechanisms underlying these neuromusculoskeletal and locomotor improvements will assist in validating our hypothesis and will improve the likelihood for the translation of this promising multimodal therapeutic strategy.
Abbreviations
| ABT | activity-based therapies |
| AR | androgen receptor |
| ARE | androgen response element |
| BDNF | brain-derived neurotrophic factor |
| BWSTT | bodyweight-supported treadmill training |
| CNS | central nervous system |
| CSA | cross-sectional area |
| DHT | dihydrotestosterone |
| E2 | estradiol |
| EPSP | excitatory postsynaptic potential |
| ER | estrogen receptor |
| ERE | estrogen response element |
| fCSA | fiber cross-sectional area |
| FOXO | forkhead box O |
| GPCR | G-protein-coupled receptor |
| GSK3β | glycogen synthase kinase 3β |
| IGF-1 | insulin-like growth factor |
| IGF-1R | insulin-like growth factor receptor |
| IGFBP | insulin-like growth factor binding protein |
| IRS1 | insulin receptor substrate-1 |
| LABC | levator ani/bulbocavernosus |
| MAFbx | muscle atrophy F-box or atrogin-1 |
| MGF | mechano growth factor |
| MHC | myosin heavy chain |
| mTOR | mammalian target of rapamycin |
| MuRF1 | muscle ring finger-1 |
| NT-3 | neurotrophin-3 |
| ORX | orchiectomy |
| PGC-1α | peroxisome proliferator-activated receptor gamma co-activator-1α |
| PGC-1β | peroxisome proliferator-activated receptor gamma co-activator-1β |
| PI3K | phosphatidyl inositol-3 kinase |
| SCI | spinal cord injury |
| SHBG | sex-hormone binding globulin |
| SNB | spinal nucleus of the bulbocavernosus |
| T | testosterone |
| TCF-4 | T-cell factor-4 |
| TE | testosterone enanthate |
| TrkB | tyrosine receptor kinase B |
| TrkC | tyrosine receptor kinase C |
| TRT | testosterone replacement therapy |
Author Contributions
Conceptualization, D.M.O., J.L., F.Y., S.E.B., and J.F.Y.; Methodology, D.M.O. and J.F.Y.; Formal Analysis, D.M.O. and J.F.Y.; Investigation, D.M.O., J.L., F.Y., S.E.B., and J.F.Y.; Resources, F.Y., S.E.B., and J.F.Y.; Data Curation, D.M.O. and J.F.Y.; Writing—Original Draft Preparation, D.M.O. and J.F.Y.; Writing— Review & Editing, J.L., F.Y., and S.E.B.; Visualization, D.M.O. and J.F.Y.; Supervision, F.Y., S.E.B., and J.F.Y.; Project Administration, D.M.O.; Funding Acquisition, F.Y. and J.F.Y.
Funding
This work was supported by the Office of Research and Development, Rehabilitation Research and Development Service, Department of Veterans Affairs (1I01RX001449-01A1 and a Presidential Early Career Award for Scientists and Engineers (PECASE) #B9280-O to JFY and 1IK1RX002327-01A1 to DMO), by a Paralyzed Veterans of America (PVA) Fellowship (#2939 to FY), and by resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Beattie M.S., Farooqui A.A., Bresnahan J.C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 2.Hilton B.J., Moulson A.J., Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: Concepts and methods. Neurosci. Lett. 2017;652:3–10. doi: 10.1016/j.neulet.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., Vaccaro A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Morawietz C., Moffat F. Effects of locomotor training after incomplete spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 2013;94:2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Battistuzzo C.R., Callister R.J., Callister R., Galea M.P. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J. Neurotrauma. 2012;29:1600–1613. doi: 10.1089/neu.2011.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrman A.L., Ardolino E.M., Harkema S.J. Activity-based therapy: From basic science to clinical application for recovery after spinal cord injury. J. Neurol. Phys. Ther. 2017;41(Suppl. S3):S39–S45. doi: 10.1097/NPT.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan S.D., Nash M.S., Tefera E., Tinsley E., Blackman M.R., Groah S. Prevalence and etiology of hypogonadism in young men with chronic spinal cord injury: A cross-sectional analysis from two university-based rehabilitation centers. PM&R. 2017;9:751–760. doi: 10.1016/j.pmrj.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borst S.E., Yarrow J.F. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am. J. Physiol. Endocrinol. Metab. 2015;308:E1035–E1042. doi: 10.1152/ajpendo.00111.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A.S., Gray H., Schache A.G., Hoermann R., Lim Joon D., Zajac J.D., Pandy M.G., Grossmann M. Androgen deprivation causes selective deficits in the biomechanical leg muscle function of men during walking: A prospective case-control study. J. Cachexia Sarcopenia Muscle. 2017;8:102–112. doi: 10.1002/jcsm.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner J.W., Otzel D.M., Bowser A., Nargi D., Agarwal S., Peterson M.D., Zou B., Borst S.E., Yarrow J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle. 2018 doi: 10.1002/jcsm.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder P.J., Bhasin S., Cunningham G.R., Matsumoto A.M., Stephens-Shields A.J., Cauley J.A., Gill T.M., Barrett-Connor E., Swerdloff R.S., Wang C., et al. Effects of testosterone treatment in older men. N. Engl. J. Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauman W.A., Cirnigliaro C.M., La Fountaine M.F., Jensen A.M., Wecht J.M., Kirshblum S.C., Spungen A.M. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm. Metab. Res. 2011;43:574–579. doi: 10.1055/s-0031-1280797. [DOI] [PubMed] [Google Scholar]
- 13.Moore P.D., Gorgey A.S., Wade R.C., Khalil R.E., Lavis T.D., Khan R., Adler R.A. Neuromuscular electrical stimulation and testosterone did not influence heterotopic ossification size after spinal cord injury: A case series. World J. Clin. Cases. 2016;4:172–176. doi: 10.12998/wjcc.v4.i7.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarrow J.F., Phillips E.G., Conover C.F., Bassett T.E., Chen C., Teurlings T., Vasconez A., Alerte J., Prock H., Jiron J.M., et al. Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J. Neurotrauma. 2017;34:2972–2981. doi: 10.1089/neu.2016.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yarrow J.F., Conover C.F., Beggs L.A., Beck D.T., Otzel D.M., Balaez A., Combs S.M., Miller J.R., Ye F., Aguirre J.I., et al. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J. Neurotrauma. 2014;31:834–845. doi: 10.1089/neu.2013.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Zhao J., Zhao W., Pan J., Bauman W.A., Cardozo C.P. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J. Neurotrauma. 2012;29:1663–1675. doi: 10.1089/neu.2011.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengelaub D.R., Han Q., Liu N.K., Maczuga M.A., Szalavari V., Valencia S.A., Xu X.M. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J. Neurotrauma. 2018;35:825–841. doi: 10.1089/neu.2017.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byers J.S., Huguenard A.L., Kuruppu D., Liu N.K., Xu X.M., Sengelaub D.R. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 2012;520:2683–2696. doi: 10.1002/cne.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ung R.V., Rouleau P., Guertin P.A. Effects of co-administration of clenbuterol and testosterone propionate on skeletal muscle in paraplegic mice. J. Neurotrauma. 2010;27:1129–1142. doi: 10.1089/neu.2009.1211. [DOI] [PubMed] [Google Scholar]
- 20.Gregory C.M., Vandenborne K., Huang H.F., Ottenweller J.E., Dudley G.A. Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord. 2003;41:23–28. doi: 10.1038/sj.sc.3101370. [DOI] [PubMed] [Google Scholar]
- 21.Schiaffino S., Sandri M., Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology. 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 22.Thomas C.K., Bakels R., Klein C.S., Zijdewind I. Human spinal cord injury: Motor unit properties and behaviour. Acta Physiol. 2014;210:5–19. doi: 10.1111/apha.12153. [DOI] [PubMed] [Google Scholar]
- 23.Klein C.S., Hager-Ross C.K., Thomas C.K. Fatigue properties of human thenar motor units paralysed by chronic spinal cord injury. J. Physiol. 2006;573:161–171. doi: 10.1113/jphysiol.2005.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaraman A., Gregory C.M., Bowden M., Stevens J.E., Shah P., Behrman A.L., Vandenborne K. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord. 2006;44:680–687. doi: 10.1038/sj.sc.3101892. [DOI] [PubMed] [Google Scholar]
- 25.Hager-Ross C.K., Klein C.S., Thomas C.K. Twitch and tetanic properties of human thenar motor units paralyzed by chronic spinal cord injury. J. Neurophysiol. 2006;96:165–174. doi: 10.1152/jn.01339.2005. [DOI] [PubMed] [Google Scholar]
- 26.Barbeau H., Nadeau S., Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J. Neurotrauma. 2006;23:571–585. doi: 10.1089/neu.2006.23.571. [DOI] [PubMed] [Google Scholar]
- 27.Bose P., Parmer R., Reier P.J., Thompson F.J. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp. Neurol. 2005;191:13–23. doi: 10.1016/j.expneurol.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Gazula V.R., Roberts M., Luzzio C., Jawad A.F., Kalb R.G. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J. Comp. Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., Liu N.K., Zhang Y.P., Deng L., Lu Q.B., Shields C.B., Walker M.J., Li J., Xu X.M. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp. Neurol. 2015;271:368–378. doi: 10.1016/j.expneurol.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Biering-Sorensen B., Kristensen I.B., Kjaer M., Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve. 2009;40:499–519. doi: 10.1002/mus.21391. [DOI] [PubMed] [Google Scholar]
- 31.Moore C.D., Craven B.C., Thabane L., Laing A.C., Frank-Wilson A.W., Kontulainen S.A., Papaioannou A., Adachi J.D., Giangregorio L.M. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 2015;15:32–41. [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgey A.S., Dudley G.A. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–309. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 33.Castro M.J., Apple D.F., Jr., Hillegass E.A., Dudley G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. Occup. Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- 34.Shah P.K., Stevens J.E., Gregory C.M., Pathare N.C., Jayaraman A., Bickel S.C., Bowden M., Behrman A.L., Walter G.A., Dudley G.A., et al. Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 2006;87:772–778. doi: 10.1016/j.apmr.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 35.Castro M.J., Apple D.F., Jr., Staron R.S., Campos G.E., Dudley G.A. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J. Appl. Physiol. 1999;86:350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 36.Gregory C.M., Vandenborne K., Castro M.J., Dudley G.A. Human and rat skeletal muscle adaptations to spinal cord injury. Can. J. Appl. Physiol. 2003;28:491–500. doi: 10.1139/h03-036. [DOI] [PubMed] [Google Scholar]
- 37.Phillips E.G., Beggs L.A., Ye F., Conover C.F., Beck D.T., Otzel D.M., Ghosh P., Bassit A.C.F., Borst S.E., Yarrow J.F. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS ONE. 2018;13:e0194440. doi: 10.1371/journal.pone.0194440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talmadge R.J., Roy R.R., Edgerton V.R. Persistence of hybrid fibers in rat soleus after spinal cord transection. Anat. Rec. 1999;255:188–201. doi: 10.1002/(SICI)1097-0185(19990601)255:2<188::AID-AR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Talmadge R.J., Roy R.R., Edgerton V.R. Prominence of myosin heavy chain hybrid fibers in soleus muscle of spinal cord-transected rats. J. Appl. Physiol. 1995;78:1256–1265. doi: 10.1152/jappl.1995.78.4.1256. [DOI] [PubMed] [Google Scholar]
- 40.Thomas C.K., Zaidner E.Y., Calancie B., Broton J.G., Bigland-Ritchie B.R. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp. Neurol. 1997;148:414–423. doi: 10.1006/exnr.1997.6690. [DOI] [PubMed] [Google Scholar]
- 41.Gaviria M., Ohanna F. Variability of the fatigue response of paralyzed skeletal muscle in relation to the time after spinal cord injury: Mechanical and electrophysiological characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 1999;80:145–153. doi: 10.1007/s004210050571. [DOI] [PubMed] [Google Scholar]
- 42.DiPiro N.D., Holthaus K.D., Morgan P.J., Embry A.E., Perry L.A., Bowden M.G., Gregory C.M. Lower extremity strength is correlated with walking function after incomplete SCI. Top. Spinal Cord Inj. Rehabil. 2015;21:133–139. doi: 10.1310/sci2102-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory C.M., Bowden M.G., Jayaraman A., Shah P., Behrman A., Kautz S.A., Vandenborne K. Resistance training and locomotor recovery after incomplete spinal cord injury: A case series. Spinal Cord. 2007;45:522–530. doi: 10.1038/sj.sc.3102002. [DOI] [PubMed] [Google Scholar]
- 44.Yarar-Fisher C., Bickel C.S., Windham S.T., McLain A.B., Bamman M.M. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J. Appl. Physiol. 2013;115:756–764. doi: 10.1152/japplphysiol.00122.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarar-Fisher C., Bickel C.S., Kelly N.A., Windham S.T., McLain A.B., Bamman M.M. Mechanosensitivity may be enhanced in skeletal muscles of spinal cord-injured versus able-bodied men. Muscle Nerve. 2014;50:599–601. doi: 10.1002/mus.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panisset M.G., Galea M.P., El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal Cord. 2016;54:84–92. doi: 10.1038/sc.2015.150. [DOI] [PubMed] [Google Scholar]
- 47.Malavaki C.J., Sakkas G.K., Mitrou G.I., Kalyva A., Stefanidis I., Myburgh K.H., Karatzaferi C. Skeletal muscle atrophy: Disease-induced mechanisms may mask disuse atrophy. J. Muscle Res. Cell Motil. 2015;36:405–421. doi: 10.1007/s10974-015-9439-8. [DOI] [PubMed] [Google Scholar]
- 48.Bodine S.C., Latres E., Baumhueter S., Lai V.K., Nunez L., Clarke B.A., Poueymirou W.T., Panaro F.J., Na E., Dharmarajan K., et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 49.Baligand C., Chen Y.W., Ye F., Pandey S.N., Lai S.H., Liu M., Vandenborne K. Transcriptional pathways associated with skeletal muscle changes after spinal cord injury and treadmill locomotor training. Biomed. Res. Int. 2015;2015:387090. doi: 10.1155/2015/387090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haddad F., Roy R.R., Zhong H., Edgerton V.R., Baldwin K.M. Atrophy responses to muscle inactivity. Ii. Molecular markers of protein deficits. J. Appl. Physiol. 2003;95:791–802. doi: 10.1152/japplphysiol.01113.2002. [DOI] [PubMed] [Google Scholar]
- 51.Kim S.J., Roy R.R., Kim J.A., Zhong H., Haddad F., Baldwin K.M., Edgerton V.R. Gene expression during inactivity-induced muscle atrophy: Effects of brief bouts of a forceful contraction countermeasure. J. Appl. Physiol. 2008;105:1246–1254. doi: 10.1152/japplphysiol.90668.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urso M.L., Chen Y.W., Scrimgeour A.G., Lee P.C., Lee K.F., Clarkson P.M. Alterations in mrna expression and protein products following spinal cord injury in humans. J. Physiol. 2007;579:877–892. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeman R.J., Zhao J., Zhang Y., Zhao W., Wen X., Wu Y., Pan J., Bauman W.A., Cardozo C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch. 2009;458:525–535. doi: 10.1007/s00424-009-0643-5. [DOI] [PubMed] [Google Scholar]
- 54.Sacheck J.M., Hyatt J.P., Raffaello A., Jagoe R.T., Roy R.R., Edgerton V.R., Lecker S.H., Goldberg A.L. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 55.Thakore N.P., Samantaray S., Park S., Nozaki K., Smith J.A., Cox A., Krause J., Banik N.L. Molecular changes in sub-lesional muscle following acute phase of spinal cord injury. Neurochem. Res. 2016;41:44–52. doi: 10.1007/s11064-015-1696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye F., Baligand C., Keener J.E., Vohra R., Lim W., Ruhella A., Bose P., Daniels M., Walter G.A., Thompson F., et al. Hindlimb muscle morphology and function in a new atrophy model combining spinal cord injury and cast immobilization. J. Neurotrauma. 2013;30:227–235. doi: 10.1089/neu.2012.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond M.J., Glynn E.L., Lujan H.L., Dicarlo S.E., Rasmussen B.B. Gene and protein expression associated with protein synthesis and breakdown in paraplegic skeletal muscle. Muscle Nerve. 2008;37:505–513. doi: 10.1002/mus.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lundell L.S., Savikj M., Kostovski E., Iversen P.O., Zierath J.R., Krook A., Chibalin A.V., Widegren U. Protein translation, proteolysis and autophagy in human skeletal muscle atrophy after spinal cord injury. Acta Physiol. 2018 doi: 10.1111/apha.13051. [DOI] [PubMed] [Google Scholar]
- 59.Yarar-Fisher C., Bickel C.S., Kelly N.A., Stec M.J., Windham S.T., McLain A.B., Oster R.A., Bamman M.M. Heightened tweak-nf-kappab signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am. J. Physiol. Endocrinol. Metab. 2016;310:E754–761. doi: 10.1152/ajpendo.00240.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leger B., Senese R., Al-Khodairy A.W., Deriaz O., Gobelet C., Giacobino J.P., Russell A.P. Atrogin-1, murf1, and foxo, as well as phosphorylated gsk-3beta and 4e-bp1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve. 2009;40:69–78. doi: 10.1002/mus.21293. [DOI] [PubMed] [Google Scholar]
- 61.Huang J., Zhu X. The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol. Res. 2016;65:547–560. doi: 10.33549/physiolres.933087. [DOI] [PubMed] [Google Scholar]
- 62.Bikle D.D., Tahimic C., Chang W., Wang Y., Philippou A., Barton E.R. Role of IGF-I signaling in muscle bone interactions. Bone. 2015;80:79–88. doi: 10.1016/j.bone.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu M., Stevens-Lapsley J.E., Jayaraman A., Ye F., Conover C., Walter G.A., Bose P., Thompson F.J., Borst S.E., Vandenborne K. Impact of treadmill locomotor training on skeletal muscle IGF1 and myogenic regulatory factors in spinal cord injured rats. Eur. J. Appl. Physiol. 2010;109:709–720. doi: 10.1007/s00421-010-1392-z. [DOI] [PubMed] [Google Scholar]
- 64.Pete G., Fuller C.R., Oldham J.M., Smith D.R., D’Ercole A.J., Kahn C.R., Lund P.K. Postnatal growth responses to insulin-like growth factor i in insulin receptor substrate-1-deficient mice. Endocrinology. 1999;140:5478–5487. doi: 10.1210/endo.140.12.7219. [DOI] [PubMed] [Google Scholar]
- 65.Tsitouras P.D., Zhong Y.G., Spungen A.M., Bauman W.A. Serum testosterone and growth hormone/insulin-like growth factor-i in adults with spinal cord injury. Horm. Metab. Res. 1995;27:287–292. doi: 10.1055/s-2007-979961. [DOI] [PubMed] [Google Scholar]
- 66.Bauman W.A., Spungen A.M., Flanagan S., Zhong Y.G., Alexander L.R., Tsitouras P.D. Blunted growth hormone response to intravenous arginine in subjects with a spinal cord injury. Horm. Metab. Res. 1994;26:152–156. doi: 10.1055/s-2007-1000798. [DOI] [PubMed] [Google Scholar]
- 67.Dreyer H.C., Glynn E.L., Lujan H.L., Fry C.S., DiCarlo S.E., Rasmussen B.B. Chronic paraplegia-induced muscle atrophy downregulates the mtor/s6k1 signaling pathway. J. Appl. Physiol. 2008;104:27–33. doi: 10.1152/japplphysiol.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorgey A.S., Gater D.R. Insulin growth factors may explain relationship between spasticity and skeletal muscle size in men with spinal cord injury. J. Rehabil. Res. Dev. 2012;49:373–380. doi: 10.1682/JRRD.2011.04.0076. [DOI] [PubMed] [Google Scholar]
- 69.Kang C., Ji L.L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012;1271:110–117. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gali Ramamoorthy T., Laverny G., Schlagowski A.I., Zoll J., Messaddeq N., Bornert J.M., Panza S., Ferry A., Geny B., Metzger D. The transcriptional coregulator pgc-1beta controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat. Commun. 2015;6:10210. doi: 10.1038/ncomms10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin J., Wu H., Tarr P.T., Zhang C.Y., Wu Z., Boss O., Michael L.F., Puigserver P., Isotani E., Olson E.N., et al. Transcriptional co-activator pgc-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 72.Kang C., Goodman C.A., Hornberger T.A., Ji L.L. PGC-1alpha overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J. 2015;29:4092–4106. doi: 10.1096/fj.14-266619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higashino K., Matsuura T., Suganuma K., Yukata K., Nishisho T., Yasui N. Early changes in muscle atrophy and muscle fiber type conversion after spinal cord transection and peripheral nerve transection in rats. J. Neuroeng. Rehabil. 2013;10:46. doi: 10.1186/1743-0003-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Y., Collier L., Qin W., Creasey G., Bauman W.A., Jarvis J., Cardozo C. Electrical stimulation modulates wnt signaling and regulates genes for the motor endplate and calcium binding in muscle of rats with spinal cord transection. BMC Neurosci. 2013;14:81. doi: 10.1186/1471-2202-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kramer D.K., Ahlsen M., Norrbom J., Jansson E., Hjeltnes N., Gustafsson T., Krook A. Human skeletal muscle fibre type variations correlate with ppar alpha, ppar delta and PGC-1 alpha mrna. Acta Physiol. 2006;188:207–216. doi: 10.1111/j.1748-1716.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 76.Baldwin K.M., Haddad F., Pandorf C.E., Roy R.R., Edgerton V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013;4:284. doi: 10.3389/fphys.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Atherton P.J., Greenhaff P.L., Phillips S.M., Bodine S.C., Adams C.M., Lang C.H. Control of skeletal muscle atrophy in response to disuse: Clinical/preclinical contentions and fallacies of evidence. Am. J. Physiol. Endocrinol. Metab. 2016;311:E594–E604. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yarrow J.F., McCoy S.C., Borst S.E. Intracrine and myotrophic roles of 5alpha-reductase and androgens: A review. Med. Sci. Sports Exerc. 2012;44:818–826. doi: 10.1249/MSS.0b013e31823bfcbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burger H.G. Androgen production in women. Fertil. Steril. 2002;77(Suppl. S4):S3–S5. doi: 10.1016/S0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 80.Labrie F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015;145:133–138. doi: 10.1016/j.jsbmb.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Conover C.F., Yarrow J.F., Garrett T.J., Ye F., Quinlivan E.P., Cannady D.F., Peterson M.D., Borst S.E. High prevalence of low serum biologically active testosterone in older male veterans. J. Am. Med. Dir. Assoc. 2017;18:366.e17–366.e24. doi: 10.1016/j.jamda.2016.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laurent M.R., Helsen C., Antonio L., Schollaert D., Joniau S., Vos M.J., Decallonne B., Hammond G.L., Vanderschueren D., Claessens F. Effects of sex hormone-binding globulin (shbg) on androgen bioactivity in vitro. Mol. Cell. Endocrinol. 2016;437:280–291. doi: 10.1016/j.mce.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 83.Thaler M.A., Seifert-Klauss V., Luppa P.B. The biomarker sex hormone-binding globulin—From established applications to emerging trends in clinical medicine. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:749–760. doi: 10.1016/j.beem.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Yarrow J.F., Wronski T.J., Borst S.E. Testosterone and adult male bone: Actions independent of 5alpha-reductase and aromatase. Exerc. Sport Sci. Rev. 2015;43:222–230. doi: 10.1249/JES.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lumbroso S., Sandillon F., Georget V., Lobaccaro J.M., Brinkmann A.O., Privat A., Sultan C. Immunohistochemical localization and immunoblotting of androgen receptor in spinal neurons of male and female rats. Eur. J. Endocrinol. 1996;134:626–632. doi: 10.1530/eje.0.1340626. [DOI] [PubMed] [Google Scholar]
- 86.Matsuura T., Ogata A., Demura T., Moriwaka F., Tashiro K., Koyanagi T., Nagashima K. Identification of androgen receptor in the rat spinal motoneurons. Immunohistochemical and immunoblotting analyses with monoclonal antibody. Neurosci. Lett. 1993;158:5–8. doi: 10.1016/0304-3940(93)90598-F. [DOI] [PubMed] [Google Scholar]
- 87.Cain M.P., Kramer S.A., Tindall D.J., Husmann D.A. Expression of androgen receptor protein within the lumbar spinal cord during ontologic development and following antiandrogen induced cryptorchidism. J. Urol. 1994;152:766–769. doi: 10.1016/S0022-5347(17)32703-9. [DOI] [PubMed] [Google Scholar]
- 88.Bauer E.R., Daxenberger A., Petri T., Sauerwein H., Meyer H.H. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. Acta Pathol. Microbiol. Immunol. Scand. 2000;108:838–846. doi: 10.1111/j.1600-0463.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 89.Almeida M., Laurent M.R., Dubois V., Claessens F., O’Brien C.A., Bouillon R., Vanderschueren D., Manolagas S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017;97:135–187. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gennari L., Nuti R., Bilezikian J.P. Aromatase activity and bone homeostasis in men. J. Clin. Endocrinol. Metab. 2004;89:5898–5907. doi: 10.1210/jc.2004-1717. [DOI] [PubMed] [Google Scholar]
- 91.Durga A., Sepahpanah F., Regozzi M., Hastings J., Crane D.A. Prevalence of testosterone deficiency after spinal cord injury. PM&R. 2011;3:929–932. doi: 10.1016/j.pmrj.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 92.Bauman W.A., La Fountaine M.F., Spungen A.M. Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord Med. 2014;37:32–39. doi: 10.1179/2045772313Y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark M.J., Schopp L.H., Mazurek M.O., Zaniletti I., Lammy A.B., Martin T.A., Thomas F.P., Acuff M.E. Testosterone levels among men with spinal cord injury: Relationship between time since injury and laboratory values. Am. J. Phys. Med. Rehabil. 2008;87:758–767. doi: 10.1097/PHM.0b013e3181837f4f. [DOI] [PubMed] [Google Scholar]
- 94.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Testicular responses to hcg stimulation at varying doses in men with spinal cord injury. Spinal Cord. 2017;55:659–663. doi: 10.1038/sc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Provocative stimulation of the hypothalamic-pituitary-testicular axis in men with spinal cord injury. Spinal Cord. 2016;54:961–966. doi: 10.1038/sc.2016.50. [DOI] [PubMed] [Google Scholar]
- 96.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Administration of increasing doses of gonadotropin-releasing hormone in men with spinal cord injury to investigate dysfunction of the hypothalamic-pituitary-gonadal axis. Spinal Cord. 2018;56:247–258. doi: 10.1038/s41393-017-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kostovski E., Iversen P.O., Birkeland K., Torjesen P.A., Hjeltnes N. Decreased levels of testosterone and gonadotrophins in men with long-standing tetraplegia. Spinal Cord. 2008;46:559–564. doi: 10.1038/sc.2008.3. [DOI] [PubMed] [Google Scholar]
- 98.Finkelstein J.S., Lee H., Burnett-Bowie S.A., Pallais J.C., Yu E.W., Borges L.F., Jones B.F., Barry C.V., Wulczyn K.E., Thomas B.J., et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schopp L.H., Clark M., Mazurek M.O., Hagglund K.J., Acuff M.E., Sherman A.K., Childers M.K. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am. J. Phys. Med. Rehabil. 2006;85:678–684. doi: 10.1097/01.phm.0000228617.94079.4a. quiz 685–677. [DOI] [PubMed] [Google Scholar]
- 100.Beggs L.A., Ye F., Ghosh P., Beck D.T., Conover C.F., Balaez A., Miller J.R., Phillips E.G., Zheng N., Williams A.A., et al. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J. Bone Miner. Res. 2015;30:681–689. doi: 10.1002/jbmr.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Y., Collier L., Pan J., Qin W., Bauman W.A., Cardozo C.P. Testosterone reduced methylprednisolone-induced muscle atrophy in spinal cord-injured rats. Spinal Cord. 2012;50:57–62. doi: 10.1038/sc.2011.91. [DOI] [PubMed] [Google Scholar]
- 102.Borst S.E., Yarrow J.F., Conover C.F., Nseyo U., Meuleman J.R., Lipinska J.A., Braith R.W., Beck D.T., Martin J.S., Morrow M., et al. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: A randomized, controlled trial. Am. J. Physiol. Endocrinol. Metab. 2014;306:E433–E442. doi: 10.1152/ajpendo.00592.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper I.S., Rynearson E.H., Mac C.C., Power M.H. The catabolic effect of trauma of the spinal cord and its investigative treatment with testosterone propionate; preliminary report. Proc. Staff Meet. Mayo Clin. 1950;25:326–330. [PubMed] [Google Scholar]
- 104.Cooper I.S., Rynearson E.H., Mac C.C., Power M.H. Testosterone propionate as a nitrogen-sparing agent after spinal cord injury. J. Am. Med. Assoc. 1951;145:549–553. doi: 10.1001/jama.1951.02920260017005. [DOI] [PubMed] [Google Scholar]
- 105.Bauman W.A., La Fountaine M.F., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M. Lean tissue mass and energy expenditure are retained in hypogonadal men with spinal cord injury after discontinuation of testosterone replacement therapy. J. Spinal Cord Med. 2015;38:38–47. doi: 10.1179/2045772314Y.0000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bennett N.C., Gardiner R.A., Hooper J.D., Johnson D.W., Gobe G.C. Molecular cell biology of androgen receptor signalling. Int. J. Biochem. Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 107.MacLean H.E., Chiu W.S., Notini A.J., Axell A.M., Davey R.A., McManus J.F., Ma C., Plant D.R., Lynch G.S., Zajac J.D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 108.Revankar C.M., Cimino D.F., Sklar L.A., Arterburn J.B., Prossnitz E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 109.Foradori C.D., Weiser M.J., Handa R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh R., Bhasin S., Braga M., Artaza J.N., Pervin S., Taylor W.E., Krishnan V., Sinha S.K., Rajavashisth T.B., Jasuja R. Regulation of myogenic differentiation by androgens: Cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–1268. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rudnicki M.A., Williams B.O. Wnt signaling in bone and muscle. Bone. 2015;80:60–66. doi: 10.1016/j.bone.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu X.H., Wu Y., Yao S., Levine A.C., Kirschenbaum A., Collier L., Bauman W.A., Cardozo C.P. Androgens up-regulate transcription of the notch inhibitor numb in c2c12 myoblasts via wnt/beta-catenin signaling to t cell factor elements in the numb promoter. J. Biol. Chem. 2013;288:17990–17998. doi: 10.1074/jbc.M113.478487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gentile M.A., Nantermet P.V., Vogel R.L., Phillips R., Holder D., Hodor P., Cheng C., Dai H., Freedman L.P., Ray W.J. Androgen-mediated improvement of body composition and muscle function involves a novel early transcriptional program including igf1, mechano growth factor, and induction of {beta}-catenin. J. Mol. Endocrinol. 2010;44:55–73. doi: 10.1677/JME-09-0048. [DOI] [PubMed] [Google Scholar]
- 114.Otto-Duessel M., Tew B.Y., Vonderfecht S., Moore R., Jones J.O. Identification of neuron selective androgen receptor inhibitors. World J. Biol. Chem. 2017;8:138–150. doi: 10.4331/wjbc.v8.i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pawlowski J.E., Ertel J.R., Allen M.P., Xu M., Butler C., Wilson E.M., Wierman M.E. Liganded androgen receptor interaction with beta-catenin: Nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 2002;277:20702–20710. doi: 10.1074/jbc.M200545200. [DOI] [PubMed] [Google Scholar]
- 116.Thomas P.S., Jr., Fraley G.S., Damian V., Woodke L.B., Zapata F., Sopher B.L., Plymate S.R., La Spada A.R. Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of x-linked spinal and bulbar muscular atrophy. Hum. Mol. Genet. 2006;15:2225–2238. doi: 10.1093/hmg/ddl148. [DOI] [PubMed] [Google Scholar]
- 117.Gao K., Wang Y.S., Yuan Y.J., Wan Z.H., Yao T.C., Li H.H., Tang P.F., Mei X.F. Neuroprotective effect of rapamycin on spinal cord injury via activation of the wnt/beta-catenin signaling pathway. Neural Regen. Res. 2015;10:951–957. doi: 10.4103/1673-5374.158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu G.B., Niu F.W., Zhang Y.C., Du L., Liang Z.Y., Gao Y., Yan T.Z., Nie Z.K., Gao K. Methylprednisolone promotes recovery of neurological function after spinal cord injury: Association with wnt/beta-catenin signaling pathway activation. Neural Regen. Res. 2016;11:1816–1823. doi: 10.4103/1673-5374.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao K., Shen Z., Yuan Y., Han D., Song C., Guo Y., Mei X. Simvastatin inhibits neural cell apoptosis and promotes locomotor recovery via activation of wnt/beta-catenin signaling pathway after spinal cord injury. J. Neurochem. 2016;138:139–149. doi: 10.1111/jnc.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ye F., McCoy S.C., Ross H.H., Bernardo J.A., Beharry A.W., Senf S.M., Judge A.R., Beck D.T., Conover C.F., Cannady D.F., et al. Transcriptional regulation of myotrophic actions by testosterone and trenbolone on androgen-responsive muscle. Steroids. 2014;87:59–66. doi: 10.1016/j.steroids.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Y., Bauman W.A., Blitzer R.D., Cardozo C. Testosterone-induced hypertrophy of l6 myoblasts is dependent upon erk and mtor. Biochem. Biophys. Res. Commun. 2010;400:679–683. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- 122.Wu Y., Zhao W., Zhao J., Pan J., Wu Q., Zhang Y., Bauman W.A., Cardozo C.P. Identification of androgen response elements in the insulin-like growth factor i upstream promoter. Endocrinology. 2007;148:2984–2993. doi: 10.1210/en.2006-1653. [DOI] [PubMed] [Google Scholar]
- 123.Ferrando A.A., Sheffield-Moore M., Yeckel C.W., Gilkison C., Jiang J., Achacosa A., Lieberman S.A., Tipton K., Wolfe R.R., Urban R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 124.Deane C.S., Hughes D.C., Sculthorpe N., Lewis M.P., Stewart C.E., Sharples A.P. Impaired hypertrophy in myoblasts is improved with testosterone administration. J. Steroid Biochem. Mol. Biol. 2013;138:152–161. doi: 10.1016/j.jsbmb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 125.White J.P., Gao S., Puppa M.J., Sato S., Welle S.L., Carson J.A. Testosterone regulation of akt/mtorc1/foxo3a signaling in skeletal muscle. Mol. Cell. Endocrinol. 2013;365:174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Basualto-Alarcon C., Jorquera G., Altamirano F., Jaimovich E., Estrada M. Testosterone signals through mtor and androgen receptor to induce muscle hypertrophy. Med. Sci. Sports Exerc. 2013;45:1712–1720. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 127.Xu T., Shen Y., Pink H., Triantafillou J., Stimpson S.A., Turnbull P., Han B. Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J. Steroid Biochem. Mol. Biol. 2004;92:447–454. doi: 10.1016/j.jsbmb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Serra C., Sandor N.L., Jang H., Lee D., Toraldo G., Guarneri T., Wong S., Zhang A., Guo W., Jasuja R., et al. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: Differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology. 2013;154:4594–4606. doi: 10.1210/en.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pires-Oliveira M., Maragno A.L., Parreiras-e-Silva L.T., Chiavegatti T., Gomes M.D., Godinho R.O. Testosterone represses ubiquitin ligases atrogin-1 and murf-1 expression in an androgen-sensitive rat skeletal muscle in vivo. J. Appl. Physiol. 2010;108:266–273. doi: 10.1152/japplphysiol.00490.2009. [DOI] [PubMed] [Google Scholar]
- 130.Ferrando A.A., Sheffield-Moore M., Paddon-Jones D., Wolfe R.R., Urban R.J. Differential anabolic effects of testosterone and amino acid feeding in older men. J. Clin. Endocrinol. Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- 131.Zhao W., Pan J., Wang X., Wu Y., Bauman W.A., Cardozo C.P. Expression of the muscle atrophy factor muscle atrophy f-box is suppressed by testosterone. Endocrinology. 2008;149:5449–5460. doi: 10.1210/en.2008-0664. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y., Ruggiero C.L., Bauman W.A., Cardozo C. Ankrd1 is a transcriptional repressor for the androgen receptor that is downregulated by testosterone. Biochem. Biophys. Res. Commun. 2013;437:355–360. doi: 10.1016/j.bbrc.2013.06.079. [DOI] [PubMed] [Google Scholar]
- 133.MacKrell J.G., Yaden B.C., Bullock H., Chen K., Shetler P., Bryant H.U., Krishnan V. Molecular targets of androgen signaling that characterize skeletal muscle recovery and regeneration. Nucl. Recept. Signal. 2015;13:e005. doi: 10.1621/nrs.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao J., Bauman W.A., Huang R., Caplan A.J., Cardozo C. Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manner. Steroids. 2004;69:357–366. doi: 10.1016/j.steroids.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Barbosa M.R., Shiguemoto G.E., Tomaz L.M., Ferreira F.C., Rodrigues M.F., Domingues M.M., Souza Master M.V., Canevazzi G.H., Silva-Magosso N.S., Selistre-de-Araujo H.S., et al. Resistance training and ovariectomy: Antagonic effects in mitochondrial biogenesis markers in rat skeletal muscle. Int. J. Sports Med. 2016;37:841–848. doi: 10.1055/s-0042-107247. [DOI] [PubMed] [Google Scholar]
- 136.Park Y.M., Pereira R.I., Erickson C.B., Swibas T.A., Kang C., Van Pelt R.E. Time since menopause and skeletal muscle estrogen receptors, PGC-1alpha, and ampk. Menopause. 2017;24:815–823. doi: 10.1097/GME.0000000000000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maher A.C., Akhtar M., Tarnopolsky M.A. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol. Genom. 2010;42:342–347. doi: 10.1152/physiolgenomics.00016.2010. [DOI] [PubMed] [Google Scholar]
- 138.McCully K.K., Mulcahy T.K., Ryan T.E., Zhao Q. Skeletal muscle metabolism in individuals with spinal cord injury. J. Appl. Physiol. 2011;111:143–148. doi: 10.1152/japplphysiol.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patte-Mensah C., Penning T.M., Mensah-Nyagan A.G. Anatomical and cellular localization of neuroactive 5 alpha/3 alpha-reduced steroid-synthesizing enzymes in the spinal cord. J. Comp. Neurol. 2004;477:286–299. doi: 10.1002/cne.20251. [DOI] [PubMed] [Google Scholar]
- 140.Poletti A., Coscarella A., Negri-Cesi P., Colciago A., Celotti F., Martini L. 5 alpha-reductase isozymes in the central nervous system. Steroids. 1998;63:246–251. doi: 10.1016/S0039-128X(98)00018-X. [DOI] [PubMed] [Google Scholar]
- 141.Hauser K.F., MacLusky N.J., Toran-Allerand C.D. Androgen action in fetal mouse spinal cord cultures: Metabolic and morphologic aspects. Brain Res. 1987;406:62–72. doi: 10.1016/0006-8993(87)90769-4. [DOI] [PubMed] [Google Scholar]
- 142.MacLusky N.J., Clark C.R., Shanabrough M., Naftolin F. Metabolism and binding of androgens in the spinal cord of the rat. Brain Res. 1987;422:83–91. doi: 10.1016/0006-8993(87)90542-7. [DOI] [PubMed] [Google Scholar]
- 143.Pozzi P., Bendotti C., Simeoni S., Piccioni F., Guerini V., Marron T.U., Martini L., Poletti A. Androgen 5-alpha-reductase type 2 is highly expressed and active in rat spinal cord motor neurones. J. Neuroendocrinol. 2003;15:882–887. doi: 10.1046/j.1365-2826.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 144.Hauser K.F., Toran-Allerand C.D. Androgen increases the number of cells in fetal mouse spinal cord cultures: Implications for motoneuron survival. Brain Res. 1989;485:157–164. doi: 10.1016/0006-8993(89)90677-X. [DOI] [PubMed] [Google Scholar]
- 145.Fargo K.N., Foecking E.M., Jones K.J., Sengelaub D.R. Neuroprotective actions of androgens on motoneurons. Front. Neuroendocrinol. 2009;30:130–141. doi: 10.1016/j.yfrne.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verhovshek T., Buckley K.E., Sergent M.A., Sengelaub D.R. Testosterone metabolites differentially maintain adult morphology in a sexually dimorphic neuromuscular system. Dev. Neurobiol. 2010;70:206–221. doi: 10.1002/dneu.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huguenard A.L., Fernando S.M., Monks D.A., Sengelaub D.R. Overexpression of androgen receptors in target musculature confers androgen sensitivity to motoneuron dendrites. Endocrinology. 2011;152:639–650. doi: 10.1210/en.2010-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ji Y.X., Zhao M., Liu Y.L., Chen L.S., Hao P.L., Sun C. Expression of aromatase and estrogen receptors in lumbar motoneurons of mice. Neurosci. Lett. 2017;653:7–11. doi: 10.1016/j.neulet.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 149.Rakotoarivelo C., Petite D., Lambard S., Fabre C., Rouleau C., Lumbroso S., de Weille J., Privat A., Carreau S., Mersel M. Receptors to steroid hormones and aromatase are expressed by cultured motoneurons but not by glial cells derived from rat embryo spinal cord. Neuroendocrinology. 2004;80:284–297. doi: 10.1159/000083611. [DOI] [PubMed] [Google Scholar]
- 150.Nowacek A.S., Sengelaub D.R. Estrogenic support of motoneuron dendritic growth via the neuromuscular periphery in a sexually dimorphic motor system. J. Neurobiol. 2006;66:962–976. doi: 10.1002/neu.20274. [DOI] [PubMed] [Google Scholar]
- 151.Cai Y., Chew C., Munoz F., Sengelaub D.R. Neuroprotective effects of testosterone metabolites and dependency on receptor action on the morphology of somatic motoneurons following the death of neighboring motoneurons. Dev. Neurobiol. 2017;77:691–707. doi: 10.1002/dneu.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rudolph L.M., Sengelaub D.R. Castration-induced upregulation of muscle eralpha supports estrogen sensitivity of motoneuron dendrites in a sexually dimorphic neuromuscular system. Dev. Neurobiol. 2013;73:921–935. doi: 10.1002/dneu.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakamizo T., Urushitani M., Inoue R., Shinohara A., Sawada H., Honda K., Kihara T., Akaike A., Shimohama S. Protection of cultured spinal motor neurons by estradiol. Neuroreport. 2000;11:3493–3497. doi: 10.1097/00001756-200011090-00019. [DOI] [PubMed] [Google Scholar]
- 154.Chen J., Hu R., Ge H., Duanmu W., Li Y., Xue X., Hu S., Feng H. G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol. Med. Rep. 2015;12:1733–1740. doi: 10.3892/mmr.2015.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Elkabes S., Nicot A.B. Sex steroids and neuroprotection in spinal cord injury: A review of preclinical investigations. Exp. Neurol. 2014;259:28–37. doi: 10.1016/j.expneurol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 156.Ottem E.N., Bailey D.J., Jordan C.L., Breedlove S.M. With a little help from my friends: Androgens tap bdnf signaling pathways to alter neural circuits. Neuroscience. 2013;239:124–138. doi: 10.1016/j.neuroscience.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 157.Verhovshek T., Rudolph L.M., Sengelaub D.R. Brain-derived neurotrophic factor and androgen interactions in spinal neuromuscular systems. Neuroscience. 2013;239:103–114. doi: 10.1016/j.neuroscience.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Osborne M.C., Verhovshek T., Sengelaub D.R. Androgen regulates trkb immunolabeling in spinal motoneurons. J. Neurosci. Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- 159.Ottem E.N., Beck L.A., Jordan C.L., Breedlove S.M. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase b in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- 160.Yang L.Y., Arnold A.P. Interaction of bdnf and testosterone in the regulation of adult perineal motoneurons. J. Neurobiol. 2000;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::AID-NEU2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 161.Yang L.Y., Verhovshek T., Sengelaub D.R. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- 162.Xu J., Gingras K.M., Bengston L., Di Marco A., Forger N.G. Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons. J. Neurosci. 2001;21:4366–4372. doi: 10.1523/JNEUROSCI.21-12-04366.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gill L.C., Gransee H.M., Sieck G.C., Mantilla C.B. Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons. Respir. Physiol. Neurobiol. 2016;226:128–136. doi: 10.1016/j.resp.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cardona-Rossinyol A., Mir M., Caraballo-Miralles V., Llado J., Olmos G. Neuroprotective effects of estradiol on motoneurons in a model of rat spinal cord embryonic explants. Cell. Mol. Neurobiol. 2013;33:421–432. doi: 10.1007/s10571-013-9908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin C.W., Chen B., Huang K.L., Dai Y.S., Teng H.L. Inhibition of autophagy by estradiol promotes locomotor recovery after spinal cord injury in rats. Neurosci. Bull. 2016;32:137–144. doi: 10.1007/s12264-016-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Behrman A.L., Harkema S.J. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys. Med. Rehabil. Clin. 2007;18:183–202. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 167.Field-Fote E.C. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord Injury. Arch. Phys. Med. Rehabil. 2001;82:818–824. doi: 10.1053/apmr.2001.23752. [DOI] [PubMed] [Google Scholar]
- 168.Forrest G.F., Sisto S.A., Barbeau H., Kirshblum S.C., Wilen J., Bond Q., Bentson S., Asselin P., Cirnigliaro C.M., Harkema S. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete ais-b spinal cord injury. J. Spinal Cord Med. 2008;31:509–521. doi: 10.1080/10790268.2008.11753646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gorassini M.A., Norton J.A., Nevett-Duchcherer J., Roy F.D., Yang J.F. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J. Neurophysiol. 2009;101:969–979. doi: 10.1152/jn.91131.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harkema S.J., Schmidt-Read M., Lorenz D.J., Edgerton V.R., Behrman A.L. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch. Phys. Med. Rehabil. 2012;93:1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 171.Krishnan V., Kindig M., Mirbagheri M. Robotic-assisted locomotor training enhances ankle performance in adults with incomplete spinal cord injury. J. Rehabil. Med. 2016;48:781–786. doi: 10.2340/16501977-2133. [DOI] [PubMed] [Google Scholar]
- 172.Nooijen C.F., Ter Hoeve N., Field-Fote E.C. Gait quality is improved by locomotor training in individuals with sci regardless of training approach. J. Neuroeng. Rehabil. 2009;6:36. doi: 10.1186/1743-0003-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wernig A., Muller S., Nanassy A., Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur. J. Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 174.Wirz M., Bastiaenen C., de Bie R., Dietz V. Effectiveness of automated locomotor training in patients with acute incomplete spinal cord injury: A randomized controlled multicenter trial. BMC Neurol. 2011;11:60. doi: 10.1186/1471-2377-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wirz M., Zemon D.H., Rupp R., Scheel A., Colombo G., Dietz V., Hornby T.G. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: A multicenter trial. Arch. Phys. Med. Rehabil. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 176.Jones M.L., Evans N., Tefertiller C., Backus D., Sweatman M., Tansey K., Morrison S. Activity-based therapy for recovery of walking in individuals with chronic spinal cord injury: Results from a randomized clinical trial. Arch. Phys. Med. Rehabil. 2014;95:2239–2246. doi: 10.1016/j.apmr.2014.07.400. [DOI] [PubMed] [Google Scholar]
- 177.Knikou M. Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast. 2012;2012:254948. doi: 10.1155/2012/254948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Adams M.M., Ditor D.S., Tarnopolsky M.A., Phillips S.M., McCartney N., Hicks A.L. The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: A case study. J. Spinal Cord Med. 2006;29:167–171. doi: 10.1080/10790268.2006.11753860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alexeeva N., Sames C., Jacobs P.L., Hobday L., Distasio M.M., Mitchell S.A., Calancie B. Comparison of training methods to improve walking in persons with chronic spinal cord injury: A randomized clinical trial. J. Spinal Cord Med. 2011;34:362–379. doi: 10.1179/2045772311Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thomas S.L., Gorassini M.A. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J. Neurophysiol. 2005;94:2844–2855. doi: 10.1152/jn.00532.2005. [DOI] [PubMed] [Google Scholar]
- 181.Coupaud S., Jack L.P., Hunt K.J., Allan D.B. Muscle and bone adaptations after treadmill training in incomplete spinal cord injury: A case study using peripheral quantitative computed tomography. J. Musculoskelet. Neuronal Interact. 2009;9:288–297. [PubMed] [Google Scholar]
- 182.Giangregorio L., McCartney N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J. Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Giangregorio L.M., Hicks A.L., Webber C.E., Phillips S.M., Craven B.C., Bugaresti J.M., McCartney N. Body weight supported treadmill training in acute spinal cord injury: Impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- 184.Stewart B.G., Tarnopolsky M.A., Hicks A.L., McCartney N., Mahoney D.J., Staron R.S., Phillips S.M. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30:61–68. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- 185.Dobkin B., Barbeau H., Deforge D., Ditunno J., Elashoff R., Apple D., Basso M., Behrman A., Harkema S., Saulino M., et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: The multicenter randomized spinal cord injury locomotor trial. NeuroRehabil. Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Field-Fote E.C., Roach K.E. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: A randomized clinical trial. Phys. Ther. 2011;91:48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lucareli P.R., Lima M.O., Lima F.P., de Almeida J.G., Brech G.C., D’Andrea Greve J.M. Gait analysis following treadmill training with body weight support versus conventional physical therapy: A prospective randomized controlled single blind study. Spinal Cord. 2011;49:1001–1007. doi: 10.1038/sc.2011.37. [DOI] [PubMed] [Google Scholar]
- 188.Postans N.J., Hasler J.P., Granat M.H., Maxwell D.J. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2004;85:604–610. doi: 10.1016/j.apmr.2003.08.083. [DOI] [PubMed] [Google Scholar]
- 189.Mehrholz J., Kugler J., Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst. Rev. 2012;11:CD006676. doi: 10.1002/14651858.CD006676.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nam K.Y., Kim H.J., Kwon B.S., Park J.W., Lee H.J., Yoo A. Robot-assisted gait training (lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017;14:24. doi: 10.1186/s12984-017-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wessels M., Lucas C., Eriks I., de Groot S. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: A systematic review. J. Rehabil. Med. 2010;42:513–519. doi: 10.2340/16501977-0525. [DOI] [PubMed] [Google Scholar]
- 192.Lam T., Noonan V.K., Eng J.J. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246–254. doi: 10.1038/sj.sc.3102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.De Leon R.D., Dy C.J. What did we learn from the animal studies of body weight-supported treadmill training and where do we go from here? J. Neurotrauma. 2017;34:1744–1750. doi: 10.1089/neu.2016.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yarrow J.F., Conover C.F., Lipinska J.A., Santillana C.A., Wronski T.J., Borst S.E. Methods to quantify sex steroid hormones in bone: Applications to the study of androgen ablation and administration. Am. J. Physiol. Endocrinol. Metab. 2010;299:E841–E847. doi: 10.1152/ajpendo.00384.2010. [DOI] [PubMed] [Google Scholar]
- 195.Nessler J.A., Moustafa-Bayoumi M., Soto D., Duhon J.E., Schmitt R. Robot applied stance loading increases hindlimb muscle mass and stepping kinetics in a rat model of spinal cord injury; Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Boston, MA, USA. 30 August–3 September 2011; pp. 4145–4148. [DOI] [PubMed] [Google Scholar]
- 196.Ilha J., da Cunha N.B., Jaeger M., de Souza D.F., Nascimento P.S., Marcuzzo S., Figueiro M., Gottfried C., Achaval M. Treadmill step training-induced adaptive muscular plasticity in a chronic paraplegia model. Neurosci. Lett. 2011;492:170–174. doi: 10.1016/j.neulet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 197.Stevens J.E., Liu M., Bose P., O’Steen W.A., Thompson F.J., Anderson D.K., Vandenborne K. Changes in soleus muscle function and fiber morphology with one week of locomotor training in spinal cord contusion injured rats. J. Neurotrauma. 2006;23:1671–1681. doi: 10.1089/neu.2006.23.1671. [DOI] [PubMed] [Google Scholar]
- 198.Bose P.K., Hou J., Parmer R., Reier P.J., Thompson F.J. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front. Physiol. 2012;3:258. doi: 10.3389/fphys.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu M., Bose P., Walter G.A., Thompson F.J., Vandenborne K. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord. 2008;46:488–493. doi: 10.1038/sj.sc.3102169. [DOI] [PubMed] [Google Scholar]
- 200.Singh A., Balasubramanian S., Murray M., Lemay M., Houle J. Role of spared pathways in locomotor recovery after body-weight-supported treadmill training in contused rats. J. Neurotrauma. 2011;28:2405–2416. doi: 10.1089/neu.2010.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jayaraman A., Shah P., Gregory C., Bowden M., Stevens J., Bishop M., Walter G., Behrman A., Vandenborne K. Locomotor training and muscle function after incomplete spinal cord injury: Case series. J. Spinal Cord Med. 2008;31:185–193. doi: 10.1080/10790268.2008.11760710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Duffell L.D., Brown G.L., Mirbagheri M.M. Facilitatory effects of anti-spastic medication on robotic locomotor training in people with chronic incomplete spinal cord injury. J. Neuroeng. Rehabil. 2015;12:29. doi: 10.1186/s12984-015-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Varoqui D., Niu X., Mirbagheri M.M. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J. Neuroeng. Rehabil. 2014;11:46. doi: 10.1186/1743-0003-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cardozo C.P., Graham Z.A. Muscle-bone interactions: Movement in the field of mechano-humoral coupling of muscle and bone. Ann. N. Y. Acad. Sci. 2017;1402:10–17. doi: 10.1111/nyas.13411. [DOI] [PubMed] [Google Scholar]
- 205.Jayaraman A., Liu M., Ye F., Walter G.A., Vandenborne K. Regenerative responses in slow- and fast-twitch muscles following moderate contusion spinal cord injury and locomotor training. Eur. J. Appl. Physiol. 2013;113:191–200. doi: 10.1007/s00421-012-2429-2. [DOI] [PubMed] [Google Scholar]
- 206.Dupont-Versteegden E.E., Murphy R.J., Houle J.D., Gurley C.M., Peterson C.A. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am. J. Physiol. Cell Physiol. 2000;279:C1677–C1684. doi: 10.1152/ajpcell.2000.279.6.C1677. [DOI] [PubMed] [Google Scholar]
- 207.Astorino T.A., Harness E.T., Witzke K.A. Chronic activity-based therapy does not improve body composition, insulin-like growth factor-i, adiponectin, or myostatin in persons with spinal cord injury. J. Spinal Cord Med. 2015;38:615–625. doi: 10.1179/2045772314Y.0000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Petrie M., Suneja M., Shields R.K. Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J. Appl. Physiol. 2015;118:723–731. doi: 10.1152/japplphysiol.00628.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gorgey A.S., Graham Z.A., Bauman W.A., Cardozo C., Gater D.R. Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J. Spinal Cord Med. 2017;40:439–448. doi: 10.1080/10790268.2016.1229397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Adams C.M., Suneja M., Dudley-Javoroski S., Shields R.K. Altered mrna expression after long-term soleus electrical stimulation training in humans with paralysis. Muscle Nerve. 2011;43:65–75. doi: 10.1002/mus.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cote M.P., Azzam G.A., Lemay M.A., Zhukareva V., Houle J.D. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J. Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goldshmit Y., Lythgo N., Galea M.P., Turnley A.M. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J. Neurotrauma. 2008;25:449–465. doi: 10.1089/neu.2007.0392. [DOI] [PubMed] [Google Scholar]
- 213.Petruska J.C., Ichiyama R.M., Jindrich D.L., Crown E.D., Tansey K.E., Roy R.R., Edgerton V.R., Mendell L.M. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Knikou M. Neurophysiological characteristics of human leg muscle action potentials evoked by transcutaneous magnetic stimulation of the spine. Bioelectromagnetics. 2013;34:200–210. doi: 10.1002/bem.21768. [DOI] [PubMed] [Google Scholar]
- 215.Knikou M., Mummidisetty C.K. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J. Neurophysiol. 2014;111:2264–2275. doi: 10.1152/jn.00871.2013. [DOI] [PubMed] [Google Scholar]
- 216.Smith A.C., Mummidisetty C.K., Rymer W.Z., Knikou M. Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J. Neurophysiol. 2014;112:2164–2175. doi: 10.1152/jn.00308.2014. [DOI] [PubMed] [Google Scholar]
- 217.Smith A.C., Knikou M. A review on locomotor training after spinal cord injury: Reorganization of spinal neuronal circuits and recovery of motor function. Neural Plast. 2016;2016:1216258. doi: 10.1155/2016/1216258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Alcobendas-Maestro M., Esclarin-Ruz A., Casado-Lopez R.M., Munoz-Gonzalez A., Perez-Mateos G., Gonzalez-Valdizan E., Martin J.L. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: Randomized controlled trial. NeuroRehabil. Neural Repair. 2012;26:1058–1063. doi: 10.1177/1545968312448232. [DOI] [PubMed] [Google Scholar]
- 219.Esclarin-Ruz A., Alcobendas-Maestro M., Casado-Lopez R., Perez-Mateos G., Florido-Sanchez M.A., Gonzalez-Valdizan E., Martin J.L. A comparison of robotic walking therapy and conventional walking therapy in individuals with upper versus lower motor neuron lesions: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2014;95:1023–1031. doi: 10.1016/j.apmr.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 220.Field-Fote E.C., Lindley S.D., Sherman A.L. Locomotor training approaches for individuals with spinal cord injury: A preliminary report of walking-related outcomes. J. Neurol. Phys. Ther. 2005;29:127–137. doi: 10.1097/01.NPT.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- 221.Kapadia N., Masani K., Catharine Craven B., Giangregorio L.M., Hitzig S.L., Richards K., Popovic M.R. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: Effects on walking competency. J. Spinal Cord Med. 2014;37:511–524. doi: 10.1179/2045772314Y.0000000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Labruyere R., van Hedel H.J. Strength training versus robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study in patients depending on walking assistance. J. Neuroeng. Rehabil. 2014;11:4. doi: 10.1186/1743-0003-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lam T., Pauhl K., Ferguson A., Malik R.N., Krassioukov A., Eng J.J. Training with robot-applied resistance in people with motor-incomplete spinal cord injury: Pilot study. J. Rehabil. Res. Dev. 2015;52:113–129. doi: 10.1682/JRRD.2014.03.0090. [DOI] [PubMed] [Google Scholar]
- 224.Morrison S.A., Lorenz D., Eskay C.P., Forrest G.F., Basso D.M. Longitudinal recovery and reduced costs after 120 sessions of locomotor training for motor incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 2018;99:555–562. doi: 10.1016/j.apmr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 225.Niu X., Varoqui D., Kindig M., Mirbagheri M.M. Prediction of gait recovery in spinal cord injured individuals trained with robotic gait orthosis. J. Neuroeng. Rehabil. 2014;11:42. doi: 10.1186/1743-0003-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Winchester P., McColl R., Querry R., Foreman N., Mosby J., Tansey K., Williamson J. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. NeuroRehabil. Neural Repair. 2005;19:313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- 227.Wu M., Landry J.M., Schmit B.D., Hornby T.G., Yen S.C. Robotic resistance treadmill training improves locomotor function in human spinal cord injury: A pilot study. Arch. Phys. Med. Rehabil. 2012;93:782–789. doi: 10.1016/j.apmr.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 228.Galen S.S., Clarke C.J., McLean A.N., Allan D.B., Conway B.A. Isometric hip and knee torque measurements as an outcome measure in robot assisted gait training. NeuroRehabilitation. 2014;34:287–295. doi: 10.3233/NRE-131042. [DOI] [PubMed] [Google Scholar]
- 229.Maier I.C., Ichiyama R.M., Courtine G., Schnell L., Lavrov I., Edgerton V.R., Schwab M.E. Differential effects of anti-nogo-a antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- 230.Battistuzzo C.R., Rank M.M., Flynn J.R., Morgan D.L., Callister R., Callister R.J., Galea M.P. Gait recovery following spinal cord injury in mice: Limited effect of treadmill training. J. Spinal Cord Med. 2016;39:335–343. doi: 10.1080/10790268.2015.1133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Battistuzzo C.R., Rank M.M., Flynn J.R., Morgan D.L., Callister R., Callister R.J., Galea M.P. Effects of treadmill training on hindlimb muscles of spinal cord-injured mice. Muscle Nerve. 2017;55:232–242. doi: 10.1002/mus.25211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shah P.K., Garcia-Alias G., Choe J., Gad P., Gerasimenko Y., Tillakaratne N., Zhong H., Roy R.R., Edgerton V.R. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain. 2013;136:3362–3377. doi: 10.1093/brain/awt265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nessler J.A., De Leon R.D., Sharp K., Kwak E., Minakata K., Reinkensmeyer D.J. Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: Characterization of intrinsic recovery and comparison with bbb. J. Neurotrauma. 2006;23:882–896. doi: 10.1089/neu.2006.23.882. [DOI] [PubMed] [Google Scholar]
- 234.Oh M.J., Seo T.B., Kwon K.B., Yoon S.J., Elzi D.J., Kim B.G., Namgung U. Axonal outgrowth and erk1/2 activation by training after spinal cord injury in rats. J. Neurotrauma. 2009;26:2071–2082. doi: 10.1089/neu.2008.0800. [DOI] [PubMed] [Google Scholar]
- 235.Shin H.Y., Kim H., Kwon M.J., Hwang D.H., Lee K., Kim B.G. Molecular and cellular changes in the lumbar spinal cord following thoracic injury: Regulation by treadmill locomotor training. PLoS ONE. 2014;9:e88215. doi: 10.1371/journal.pone.0088215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Multon S., Franzen R., Poirrier A.L., Scholtes F., Schoenen J. The effect of treadmill training on motor recovery after a partial spinal cord compression-injury in the adult rat. J. Neurotrauma. 2003;20:699–706. doi: 10.1089/089771503767869935. [DOI] [PubMed] [Google Scholar]
- 237.Wu Q., Cao Y., Dong C., Wang H., Wang Q., Tong W., Li X., Shan C., Wang T. Neuromuscular interaction is required for neurotrophins-mediated locomotor recovery following treadmill training in rat spinal cord injury. PeerJ. 2016;4:e2025. doi: 10.7717/peerj.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Foret A., Quertainmont R., Botman O., Bouhy D., Amabili P., Brook G., Schoenen J., Franzen R. Stem cells in the adult rat spinal cord: Plasticity after injury and treadmill training exercise. J. Neurochem. 2010;112:762–772. doi: 10.1111/j.1471-4159.2009.06500.x. [DOI] [PubMed] [Google Scholar]
- 239.Ward P.J., Herrity A.N., Smith R.R., Willhite A., Harrison B.J., Petruska J.C., Harkema S.J., Hubscher C.H. Novel multi-system functional gains via task specific training in spinal cord injured male rats. J. Neurotrauma. 2014;31:819–833. doi: 10.1089/neu.2013.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park K., Lee Y., Park S., Lee S., Hong Y., Kil Lee S. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal Res. 2010;48:270–281. doi: 10.1111/j.1600-079X.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 241.Liu Q., Zhang B., Liu C., Zhao D. Molecular mechanisms underlying the positive role of treadmill training in locomotor recovery after spinal cord injury. Spinal Cord. 2017;55:441–446. doi: 10.1038/sc.2016.134. [DOI] [PubMed] [Google Scholar]
- 242.Hayashibe M., Homma T., Fujimoto K., Oi T., Yagi N., Kashihara M., Nishikawa N., Ishizumi Y., Abe S., Hashimoto H., et al. Locomotor improvement of spinal cord-injured rats through treadmill training by forced plantar placement of hind paws. Spinal Cord. 2016;54:521–529. doi: 10.1038/sc.2015.186. [DOI] [PubMed] [Google Scholar]
- 243.Heng C., de Leon R.D. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp. Neurol. 2009;216:139–147. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Shinozaki M., Iwanami A., Fujiyoshi K., Tashiro S., Kitamura K., Shibata S., Fujita H., Nakamura M., Okano H. Combined treatment with chondroitinase abc and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci. Res. 2016;113:37–47. doi: 10.1016/j.neures.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 245.Robert A.A., Al Jadid M.S., Bin Afif S., Al Sowyed A.A., Al-Mubarak S. The effects of different rehabilitation strategies on the functional recovery of spinal cord injured rats: An experimental study. Spine. 2010;35:E1273–E1277. doi: 10.1097/BRS.0b013e3181e3fc5f. [DOI] [PubMed] [Google Scholar]
- 246.Ichiyama R., Potuzak M., Balak M., Kalderon N., Edgerton V.R. Enhanced motor function by training in spinal cord contused rats following radiation therapy. PLoS ONE. 2009;4:e6862. doi: 10.1371/journal.pone.0006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tillakaratne N.J., Guu J.J., de Leon R.D., Bigbee A.J., London N.J., Zhong H., Ziegler M.D., Joynes R.L., Roy R.R., Edgerton V.R. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience. 2010;166:23–33. doi: 10.1016/j.neuroscience.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.See P.A., de Leon R.D. Robotic loading during treadmill training enhances locomotor recovery in rats spinally transected as neonates. J. Neurophysiol. 2013;110:760–767. doi: 10.1152/jn.01099.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Timoszyk W.K., Nessler J.A., Acosta C., Roy R.R., Edgerton V.R., Reinkensmeyer D.J., de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 250.Zhang Y., Ji S.R., Wu C.Y., Fan X.H., Zhou H.J., Liu G.L. Observation of locomotor functional recovery in adult complete spinal rats with bwstt using semiquantitative and qualitative methods. Spinal Cord. 2007;45:496–501. doi: 10.1038/sj.sc.3102013. [DOI] [PubMed] [Google Scholar]
- 251.Lee Y.S., Zdunowski S., Edgerton V.R., Roy R.R., Zhong H., Hsiao I., Lin V.W. Improvement of gait patterns in step-trained, complete spinal cord-transected rats treated with a peripheral nerve graft and acidic fibroblast growth factor. Exp. Neurol. 2010;224:429–437. doi: 10.1016/j.expneurol.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.De Leon R.D., Acosta C.N. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrauma. 2006;23:1147–1163. doi: 10.1089/neu.2006.23.1147. [DOI] [PubMed] [Google Scholar]
- 253.Moshonkina T.R., Gilerovich E.G., Fedorova E.A., Avelev V.D., Gerasimenko Y.P., Otellin V.A. Morphofunctional basis for recovery of locomotor movements in rats with completely crossed spinal cord. Bull. Exp. Biol. Med. 2004;138:198–201. doi: 10.1023/B:BEBM.0000048388.76324.9c. [DOI] [PubMed] [Google Scholar]
- 254.Kubasak M.D., Jindrich D.L., Zhong H., Takeoka A., McFarland K.C., Munoz-Quiles C., Roy R.R., Edgerton V.R., Ramon-Cueto A., Phelps P.E. Oeg implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fouad K., Metz G.A., Merkler D., Dietz V., Schwab M.E. Treadmill training in incomplete spinal cord injured rats. Behav. Brain Res. 2000;115:107–113. doi: 10.1016/S0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 256.Gomez-Pinilla F., Ying Z., Roy R.R., Molteni R., Edgerton V.R. Voluntary exercise induces a bdnf-mediated mechanism that promotes neuroplasticity. J. Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 257.Skup M., Dwornik A., Macias M., Sulejczak D., Wiater M., Czarkowska-Bauch J. Long-term locomotor training up-regulates trkb(fl) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- 258.Koliatsos V.E., Clatterbuck R.E., Winslow J.W., Cayouette M.H., Price D.L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10:359–367. doi: 10.1016/0896-6273(93)90326-M. [DOI] [PubMed] [Google Scholar]
- 259.Blum R., Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology. 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- 260.De Leon R.D., See P.A., Chow C.H. Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. J. Neurotrauma. 2011;28:1021–1033. doi: 10.1089/neu.2010.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dupont-Versteegden E.E., Houle J.D., Dennis R.A., Zhang J., Knox M., Wagoner G., Peterson C.A. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- 262.Gomez-Pinilla F., Ying Z., Opazo P., Roy R.R., Edgerton V.R. Differential regulation by exercise of bdnf and nt-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 263.Hutchinson K.J., Gomez-Pinilla F., Crowe M.J., Ying Z., Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 264.Macias M., Nowicka D., Czupryn A., Sulejczak D., Skup M., Skangiel-Kramska J., Czarkowska-Bauch J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 2009;10:144. doi: 10.1186/1471-2202-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ying Z., Roy R.R., Edgerton V.R., Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor trkc in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/S0006-8993(03)03258-X. [DOI] [PubMed] [Google Scholar]
- 266.Ying Z., Roy R.R., Edgerton V.R., Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 267.Ying Z., Roy R.R., Zhong H., Zdunowski S., Edgerton V.R., Gomez-Pinilla F. Bdnf-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Leech K.A., Hornby T.G. High-intensity locomotor exercise increases brain-derived neurotrophic factor in individuals with incomplete spinal cord injury. J. Neurotrauma. 2017;34:1240–1248. doi: 10.1089/neu.2016.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Farinas I., Jones K.R., Backus C., Wang X.Y., Reichardt L.F. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 270.Frisen J., Arvidsson U., Lindholm T., Fried K., Verge V.M., Cullheim S., Hokfelt T., Risling M. Trkc expression in the injured rat spinal cord. Neuroreport. 1993;5:349–352. doi: 10.1097/00001756-199312000-00043. [DOI] [PubMed] [Google Scholar]
- 271.Lohof A.M., Ip N.Y., Poo M.M. Potentiation of developing neuromuscular synapses by the neurotrophins nt-3 and bdnf. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 272.Scarisbrick I.A., Isackson P.J., Windebank A.J. Differential expression of brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 in the adult rat spinal cord: Regulation by the glutamate receptor agonist kainic acid. J. Neurosci. 1999;19:7757–7769. doi: 10.1523/JNEUROSCI.19-18-07757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang T., Xie K., Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J. Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fang H., Liu C., Yang M., Li H., Zhang F., Zhang W., Zhang J. Neurotrophic factor and trk signaling mechanisms underlying the promotion of motor recovery after acute spinal cord injury in rats. Exp. Ther. Med. 2017;14:652–656. doi: 10.3892/etm.2017.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bimonte-Nelson H.A., Nelson M.E., Granholm A.C. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 276.Gorgey A.S., Khalil R.E., Gill R., O’Brien L.C., Lavis T., Castillo T., Cifu D.X., Savas J., Khan R., Cardozo C., et al. Effects of testosterone and evoked resistance exercise after spinal cord injury (terex-sci): Study protocol for a randomised controlled trial. BMJ Open. 2017;7:e014125. doi: 10.1136/bmjopen-2016-014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yarrow J.F., Conover C.F., Purandare A.V., Bhakta A.M., Zheng N., Conrad B., Altman M.K., Franz S.E., Wronski T.J., Borst S.E. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1213–E1222. doi: 10.1152/ajpendo.90640.2008. [DOI] [PubMed] [Google Scholar]
- 278.Yarrow J.F., Phillips E.G., Kok H.J., Conover C.F., Basset T.E., Vasconez A., Alerte J., Prock H., Flores M., Borst S.E., et al. Locomotor training with adjuvant testosterone promotes activity-mediated neuromuscular plasticity in spinal cord injured rats. Med. Sci. Sports Exerc. 2017;49:1038. doi: 10.1249/01.mss.0000519850.83264.58. [DOI] [Google Scholar]
- 279.Wang X., Smith G.I., Patterson B.W., Reeds D.N., Kampelman J., Magkos F., Mittendorfer B. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am. J. Physiol. Endocrinol. Metab. 2012;302:E740–746. doi: 10.1152/ajpendo.00533.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Spinal Cord Injury (SCI) Facts and Figures at a Glance. [(accessed on 25 May 2018)]; Available online: https://www.nscisc.uab.edu/Public/Facts%202016.pdf.
- 281.Calof O.M., Singh A.B., Lee M.L., Kenny A.M., Urban R.J., Tenover J.L., Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:1451–1457. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 282.Basaria S., Coviello A.D., Travison T.G., Storer T.W., Farwell W.R., Jette A.M., Eder R., Tennstedt S., Ulloor J., Zhang A., et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Finkle W.D., Greenland S., Ridgeway G.K., Adams J.L., Frasco M.A., Cook M.B., Fraumeni J.F., Jr., Hoover R.N. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Vigen R., O’Donnell C.I., Baron A.E., Grunwald G.K., Maddox T.M., Bradley S.M., Barqawi A., Woning G., Wierman M.E., Plomondon M.E., et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 285.Morgentaler A., Lunenfeld B. Testosterone and cardiovascular risk: World’s experts take unprecedented action to correct misinformation. Aging Male. 2014;17:63–65. doi: 10.3109/13685538.2014.913413. [DOI] [PubMed] [Google Scholar]
- 286.Traish A.M., Guay A.T., Morgentaler A. Death by testosterone? We think not! J. Sex. Med. 2014;11:624–629. doi: 10.1111/jsm.12464. [DOI] [PubMed] [Google Scholar]
- 287.Borst S.E., Shuster J.J., Zou B., Ye F., Jia H., Wokhlu A., Yarrow J.F. Cardiovascular risks and elevation of serum dht vary by route of testosterone administration: A systematic review and meta-analysis. BMC Med. 2014;12:211. doi: 10.1186/s12916-014-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Onasanya O., Iyer G., Lucas E., Lin D., Singh S., Alexander G.C. Association between exogenous testosterone and cardiovascular events: An overview of systematic reviews. Lancet Diabetes Endocrinol. 2016;4:943–956. doi: 10.1016/S2213-8587(16)30215-7. [DOI] [PubMed] [Google Scholar]
- 289.Sharma R., Oni O.A., Gupta K., Chen G., Sharma M., Dawn B., Parashara D., Savin V.J., Ambrose J.A., Barua R.S. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur. Heart J. 2015;36:2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 290.La Fountaine M.F., Wecht J.M., Cirnigliaro C.M., Kirshblum S.C., Spungen A.M., Bauman W.A. Testosterone replacement therapy improves qtavi in hypogonadal men with spinal cord injury. Neuroendocrinology. 2013;97:341–346. doi: 10.1159/000347070. [DOI] [PubMed] [Google Scholar]
- 291.Hamid R., Averbeck M.A., Chiang H., Garcia A., Al Mousa R.T., Oh S.J., Patel A., Plata M., Del Popolo G. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J. Urol. 2018 doi: 10.1007/s00345-018-2301-z. [DOI] [PubMed] [Google Scholar]
- 292.Page S.T., Amory J.K., Bowman F.D., Anawalt B.D., Matsumoto A.M., Bremner W.J., Tenover J.L. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 293.Amory J.K., Watts N.B., Easley K.A., Sutton P.R., Anawalt B.D., Matsumoto A.M., Bremner W.J., Tenover J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 294.Clinicaltrials.Gov. [(accessed on 25 May 2018)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02248701.
- 295.Kaplan A.L., Hu J.C. Use of testosterone replacement therapy in the united states and its effect on subsequent prostate cancer outcomes. Urology. 2013;82:321–326. doi: 10.1016/j.urology.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 296.Jia H., Sullivan C.T., McCoy S.C., Yarrow J.F., Morrow M., Borst S.E. Review of health risks of low testosterone and testosterone administration. World J. Clin. Cases. 2015;3:338–344. doi: 10.12998/wjcc.v3.i4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Cole A.P., Hanske J., Jiang W., Kwon N.K., Lipsitz S.R., Kathrins M., Learn P.A., Sun M., Haider A.H., Basaria S., et al. Impact of testosterone replacement therapy on thromboembolism, heart disease and obstructive sleep apnoea in men. BJU Int. 2018;121:811–818. doi: 10.1111/bju.14149. [DOI] [PubMed] [Google Scholar]
- 298.Chiodo A.E., Sitrin R.G., Bauman K.A. Sleep disordered breathing in spinal cord injury: A systematic review. J. Spinal Cord Med. 2016;39:374–382. doi: 10.1080/10790268.2015.1126449. [DOI] [PMC free article] [PubMed] [Google Scholar]