Summary
Background
Vitamin D deficiency has been associated with non‐alcoholic fatty liver disease (NAFLD). However, the role of polymorphisms determining vitamin D status remains unknown.
Objectives
The objectives of this study were to determine in UK children with biopsy‐proven NAFLD (i) their vitamin D status throughout a 12‐month period and (ii) interactions between key vitamin D‐related genetic variants (nicotinamide adenine dinucleotide synthase‐1/dehydrocholesterol reductase‐7, vitamin D receptor, group‐specific component, CYP2R1) and disease severity.
Methods
In 103 paediatric patients with NAFLD, serum 25‐hydroxyvitamin D (25OHD) levels and genotypes were determined contemporaneously to liver biopsy and examined in relation to NAFLD activity score and fibrosis stage.
Results
Only 19.2% of children had adequate vitamin D status; most had mean 25OHD levels considered deficient (<25 nmol·L−1, 25.5%) or insufficient (<50 nmol·L−1, 55.3%). Patients had significantly lower 25OHD levels in winter months (95% CI: 22.7–31.2 nmol·L−1) when compared with spring (30.5–42.1 nmol·L−1; P = 0.0089), summer (36.3–47.2 nmol·L−1; P < 0.0001) and autumn (34.2–47.5 nmol·L−1; P = 0.0003). Polymorphisms in the nicotinamide adenine dinucleotide synthase‐1/dehydrocholesterol reductase‐7 (rs3829251, rs12785878) and vitamin D receptor (rs2228570) genes were independently associated with increased steatosis; while a group‐specific component variant (rs4588) was associated with increased inflammation in liver biopsies.
Conclusions
Children with NAFLD in the UK have particularly low winter vitamin D status, with vitamin D insufficiency prevalent throughout the year. Polymorphisms in the vitamin D metabolic pathway are associated with histological severity of paediatric NAFLD.
Keywords: 25OHD, genotype, non‐alcoholic fatty liver disease, vitamin D
Abbreviations
- NAFLD
non‐alcoholic fatty liver disease
- 25OHD
25‐hydroxyvitamin D
- BMI
body mass index
- AST
aspartate transaminase
- ALT
alanine transaminase
- NAS
NAFLD activity score
- SNPs
single nucleotide polymorphisms
- NADSYN1
nicotinamide adenine dinucleotide synthase‐1
- NASH
non‐alcoholic steatohepatitis
- DHCR7
dehydrocholesterol reductase‐7
- GC
group‐specific component
- VDR
vitamin D receptor
- PNPLA3
patatin‐like phospholipase domain‐containing protein 3
Introduction
A growing body of research suggests a relationship between vitamin D deficiency and chronic liver disease, in particular non‐alcoholic fatty liver disease (NAFLD). With prevalence estimated up to 80% in obese children, NAFLD is now the most common chronic liver disease in the paediatric population and fast becoming the most common indication for liver transplantation 1. Although transplantation for NAFLD in children is exceedingly rare, the histological pattern of paediatric disease (type 2 non‐alcoholic steatohepatitis, NASH) is associated with more severe disease and possibly with a rapid rate of progression 2.
Despite the high prevalence of NAFLD however, its molecular pathogenesis remains only partially understood and targeting those who are most likely to progress is difficult. The development and progression of the disease are presumed to be multifactorial in nature and influenced by both genetic and nutritional factors 3. Lifestyle change is the only proven effective treatment for paediatric NAFLD 4 and identifying those at increased risk of progression may allow more targeted individualized therapy. Recent studies have demonstrated that low vitamin D status associates with paediatric NAFLD independently of body mass index (BMI) in Australian 5 and Italian 6 cohorts, raising the question of whether improvement in vitamin D status through diet or supplementation may be beneficial in some patients 7.
The most commonly used biomarker of vitamin D status is 25‐hydroxyvitamin D (25OHD), which is the most stable circulating form of vitamin D and reflects both dietary intake and cutaneous synthesis 8. In the UK, 25OHD status is highest in the summer and lowest during winter and spring months. Vitamin D status has a strong hereditary component with distinct sets of common gene variants, explaining some of the differences observed in vitamin D status between different ethnic populations 9, 10. Given the possible association between low vitamin D status and NAFLD, and the hereditary component of vitamin D status, the aims of this study were to determine in a multi‐ethnic cohort of children from the UK with biopsy‐proven NAFLD (i) their 25OHD status and extent of vitamin D deficiency/insufficiency throughout a given 12‐month period and (ii) interactions between key polymorphisms related to vitamin D metabolism and NAFLD disease severity.
Methods
Study design and patients
This observational study received ethical approval from the UK National Research Ethics Service (09/H0808/15) and was conducted according to the Helsinki declaration. Anonymised clinical data were examined from patients who had attended a tertiary paediatric liver centre (King's College Hospital, London, UK) between March 2001 and July 2013; who had suspected NAFLD confirmed by biopsy and had buffy coat samples available. All patients included were less than 19 years of age at sample collection. Patients were excluded if taking vitamin D supplementation or if NAFLD was not the primary liver diagnosis. Other liver diseases were ruled out through comprehensive clinical work up including abdominal ultrasound, exclusion of viral hepatitis, Wilson disease and inborn errors of metabolism including lysosomal acid lyase deficiency. Liver biopsy was undertaken in the case of persistently abnormal liver function tests (>2 upper limit of normal aspartate transaminase [AST] or alanine transaminase [ALT]) with a duration over 6 months and/or an enlarged spleen (>1 cm above upper limit of normal for age/height) on ultrasound on two or more occasions suggesting significant liver injury. Liver biopsies were formalin‐fixed and paraffin‐embedded. Sections were stained with haematoxylin and eosin, orcein, Perl's and reticulin. Liver copper was also measured. Histological assessment was performed by a single hepato‐histopathologist who was blinded to the patient's clinical details and scored according to the Kleiner/Brunt system. Each biopsy was assigned a score for steatosis (0–3), lobular inflammation (0–3), hepatocyte ballooning (0–2) and fibrosis (0–4), and the NAFLD activity score (NAS) was calculated. BMI was calculated by dividing weight, in kilograms by height, measured with a fixed stadiometer, in meters squared.
General biochemistry and measurement of 25‐hydroxyvitamin D
Liver enzymes and lipids were analysed using standard methods on the ADVIA 2400 analyser (Siemens Healthcare Diagnostics, UK). Serum 25OHD levels were measured only once due to low sample volumes using a chemiluminescent immunoassay, the ADVIA Centaur Vitamin D Total Assay. Samples were coded, and the researcher was blinded to clinical details. The sensitivity of the assay is estimated to range from 10 nmol·L−1 to 375 nmol·L−1, while the inter‐assay variation was 4.2–11.9% and the percentage of cross reactivity with vitamin D2 and D3 was 1.1%. We used thresholds defined by the UK Department of Health 11 and the US Institute of Medicine 8 for assessing vitamin D status; 25OHD concentrations below 25 nmol·L−1 were considered deficient and below 50 nmol·L−1 as insufficient, respectively.
Genotyping
Eight candidate polymorphisms were selected based on their established influence on vitamin D status 9, 10, 12 and/or liver disease 13, 14. The variants included two intronic single nucleotide polymorphisms (SNPs) (rs12785878 and rs3829251) in the nicotinamide adenine dinucleotide (NAD) synthetase‐1 (NADSYN1) gene, responsible for the final step in the biosynthesis of NAD. The NADSYN1 gene is located immediately proximal to the dehydrocholesterol reductase‐7 (DHCR7) gene on chromosome 11q12, which encodes the rate‐limiting enzyme responsible for the reduction of 7‐dehydrocholesterol to cholesterol in the skin, making it unavailable for the synthesis of vitamin D 10. There is sufficient linkage disequilibrium in the region that SNPs in NADSYN1 could be marking genetic variation in DHCR7, and both of these SNPs have been reported associated with serum 25OHD levels in population studies 9, 15. In addition, we genotyped three SNPs, one intronic and two non‐synonymous, in the group‐specific component (GC) gene, which encodes the vitamin D binding protein, the carrier of 25OHD in serum: rs2282679, rs7041 and rs4588. Polymorphisms in the cytochrome P450, family 2, subfamily R, member 1 (CYP2R1) gene, responsible for the first hydroxylation of vitamin D to 25OHD in the liver (rs10741657), and the vitamin D receptor (VDR) gene, involved in the nuclear transportation of vitamin D (rs2228570), were also investigated. Additionally, the polymorphism that has been most commonly identified associated with NAFLD, the patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) variant rs738409, was included 13, 14.
Genomic DNA was extracted from buffy coats, and immediately stored at −80°C. Probe primer sets were designed by Applied Biosystems (Paisley, UK), and genotyping assays were run on an ABI7500 real time instrument (Applied Biosystems) using allelic discrimination through the measurement of allele‐specific fluorescence. All samples were run in duplicate with positive and no‐template controls; reproducibility was >99%.
Statistical analysis
Statistical analyses were performed using spss v22 (IBM corporation, Armonk, New York) and Prism v7 (GraphPad Software Inc., California). Significance was set at a threshold of P‐value ≤0.05. Data were checked for normality using the D'Agostino and Pearson omnibus normality test, and outliers identified by the ROUT test. Allele and genotype frequencies were tested for consistency with Hardy–Weinberg equilibrium, and the chi‐squared test was used to test the distribution of categorical variables between different genotypes and presented as specific percentages. 25OHD levels were evaluated by Independent two‐tailed t‐test. Ethnicity was classified as Caucasian and Non‐Caucasian due to low frequencies in other categories. Serum 25OHD levels were transformed using the equation of Sachs 16 and predicted August values were used for all comparisons. We have estimated that for this cohort (n = 103), considering an α error = 0.05 and SD = 19.33 nmol·L−1 in serum vitamin D levels, we had a power of >80% to detect a difference of 5.34 nmol·L−1 within this group. Linear regression analysis was used for analysing the association between biochemical variables and the strength of the association was reported as correlation coefficient (r). For multiple linear regression with interaction analysis, a two‐way anova test of unbalanced designs with interaction was performed to examine for interaction between 25OHD levels, genotype and histology, employing the Benjamini and Hochberg method to calculate an adjusted P‐value.
Results
Study population
A total of 103 paediatric patients with NAFLD were enrolled in this observational study, and complete liver histology was available for 73 (patient flow diagram: Fig. S1). Patients were predominantly male (66%) and Caucasian (70%) with a median and (interquartile range) age of 13.8 (11.3, 15.7) years. Non‐Caucasians were from a diverse mix of ethnic backgrounds but predominantly from the Indian sub‐continent. Patients were usually overweight or obese for age, with a BMI z‐score of 2.1 (1.7, 2.3); while ALT levels were 57 (30, 80) IU·L−1. Population characteristics including liver histological features are summarized in Table 1.
Table 1.
Population characteristics1
| All participants (n = 103) | Caucasian (n = 72) | Non‐Caucasian (n = 25) | |
|---|---|---|---|
| Age (years) | 13.8 (11.3, 15.7) | 13.8 (11.3, 15.6) | 12.3 (9.8, 15.6) |
| Sex (% male) | 66 | 63 | 72 |
| Weight (kg) | 79.3 (63.8, 102.6) | 79.3 (63.8, 102.6) | 59.2 (51.2, 77.7) |
| Height (cm) | 164.2 (156.5, 176.8) | 164.2 (156.5, 176.8) | 159.1 (145.0, 164.5) |
| BMI (kg/m2) | 29.3 (25.8, 32.2) | 29.3 (25.7, 32.2) | 25.8 (24.3, 29) |
| BMI centile | 98 (95, 98) | 98 (95, 98) | 98 (95,98) |
| BMI z‐score | 2.1 (1.7, 2.3) | 2.1 (1.7, 2.3) | 2.0 (1.7, 2.2) |
| Cholesterol (mmol·L−1) | 4.3 (3.6, 4.9) | 4.3 (3.6, 4.9) | 4.4 (3.8, 4.6) |
| Triglycerides (mmol·L−1) | 1.4 (0.9, 2.3) | 1.4 (0.9, 2.3) | 1.8 (1.5, 2) |
| ALT (IU) | 57 (30, 80) | 57 (30, 80) | 28 (21, 43) |
| AST (IU) | 40 (30. 57) | 40 (30, 57) | 36 (26, 55) |
| GGT (IU) | 26 (19, 40) | 26 (19, 40) | 22 (17, 25) |
| Histology | |||
| Steatosis | |||
| 1 | 25 | 17 | 8 |
| 2 | 11 | 11 | 0 |
| 3 | 43 | 33 | 10 |
| Lobular Inflammation | |||
| 0 | 16 | 13 | 3 |
| 1 | 51 | 40 | 9 |
| 2 | 12 | 8 | 6 |
| Ballooning | |||
| 0 | 19 | 17 | 2 |
| 1 | 26 | 21 | 5 |
| 2 | 34 | 23 | 11 |
| Fibrosis | |||
| 0 | 3 | 3 | 0 |
| 1 | 23 | 16 | 7 |
| 2 | 22 | 21 | 4 |
| 3 | 31 | 24 | 7 |
| NAS | |||
| 1,2 | 9 | 8 | 4 |
| 3,4 | 31 | 24 | 7 |
| 5,6,7 | 39 | 37 | 7 |
Expressed as median (IQR) or % (sex).
Abbreviations: kg, kilogram; IQR, interquartile range; mmol·L−1, millimole per litre; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma glutamyl transferase; NAS, NAFLD activity score.
Vitamin D status
Patients had low vitamin D status in general, with the majority (80.8%) having mean serum 25OHD levels below the ‘insufficient’ threshold (<50 nmol·L−1) and 25% having 25OHD levels below the ‘deficient’ threshold (<25 nmol·L−1). Cycling of 25OHD levels throughout the year was evident; patients had significantly lower 25OHD levels in winter months (mean [95%CI] nmol·L−1: 26.9 [22.7–31.2]) when compared with spring (36.3 [30.5–42.1]; P = 0.0089), summer (41.8 [36.3–47.2]; P < 0.0001) and autumn (40.8 [34.2–47.5]; P = 0.0003) months (Fig. 1a). Therefore, in order to normalize for seasonality, samples were modelled using a recently published algorithm 16 to provide predicted August values, which were used in further comparisons (Fig. S2). In line with the published literature 17, non‐Caucasian patients had significantly lower mean 25OHD serum levels than Caucasian patients (mean [95%CI] nmol·L−1: 35.9 [30.1–41.7] vs. 40.7 [38.0–43.4], respectively, P = 0.0188; Fig. S3).
Figure 1.
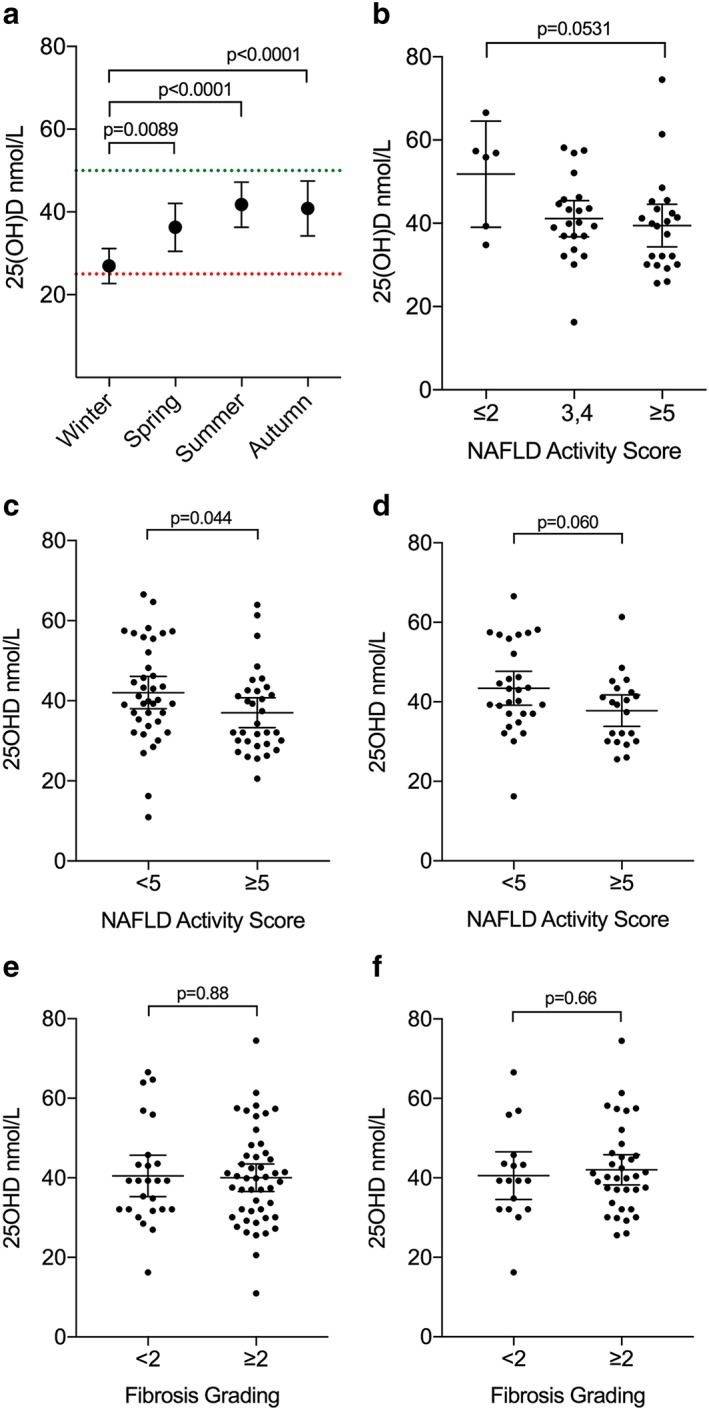
Serum 25OHD levels and histological grading. (a) Independent and repeated measures of serum 25OHD levels of cohort. Dashed lines shows accepted thresholds for deficiency (red) and insufficiency (green). (b) In Caucasian patients, Brunt/Kleiner NAS grading vs. predicted August 25OHD status demonstrates lower levels in patients with more severe disease using standard cut‐offs for simple steatosis (score 0–2), Borderline NASH (3–4) and NASH (5–8). (c) Utilizing a score of <5 and ≥5 to define severity of disease, those with less severe disease had significantly lower 25OHD in the group as a whole, with a similar trend in Caucasian patients only (d). Fibrosis grading vs. predicted August 25OHD status in all patients (e) and Caucasian patients (f) are shown. 25OHD levels are expressed around the mean ± 95% CI; data were tested for normality using the D'Agostino and Pearson omnibus normality test and analysed using an unpaired t‐test. 25OHD, 25‐hydroxyvitamin D; NASH, nonalcoholic steatohepatitis.
Caucasian patients with low NAS scores (≤2) tended to have higher levels of 25OHD when compared with patients with the highest NAS scores (≥5), although this did not reach significance due to few patients with low NAS scores in our cohort (P = 0.0531; Fig. 1b). There was a significant lower mean 25OH vitamin D level found in all children with a NAS score ≥ 5 when compared with those with a NAS score < 5, (P = 0.04; Fig. 1c). In Caucasian children only, there was a clear trend to lower vitamin D levels in those with NAS ≥5 vs. NAS ≤2 although this did not meet statistical significance (P = 0.06; Fig. 1d).
There were no differences in vitamin D levels found in those with mild (F < 2) vs. significant fibrosis (F ≥ 2) in either the group as a whole (Fig. 1e) or in Caucasian patients only (Fig. 1f). The associations between serum 25OHD levels and weight, BMI, liver enzymes ALT, AST and GGT, and cholesterol were assessed. BMI showed a moderate inverse correlation with 25OHD levels (r = −0.420, P = 0.005; Fig. S4A) while cholesterol levels showed a mild inverse correlation with 25OHD levels (r = −0.294, P = 0.018; Fig. S4B).
Association of genetic variants with liver histology
Caucasian allele frequencies for all SNPs examined in this study (Table S1) were comparable with those reported for HapMap European sample population, except for PNPLA3 rs738409, where the G allele showed lower prevalence in the general population than in patients with NAFLD (minor allele frequency 0.23 in HapMap vs. 0.44 for children with NAFLD). No deviation from Hardy–Weinberg equilibrium was detected (P < 0.05 for all genotypes).
In Caucasian patients, presence of the G allele in PNPLA3 (rs738409) was strongly associated with severity of steatosis (P = 0.0125; Fig. 2a). Similarly, the NADSYN1/DHCR7 gene variant rs12785878 G allele (P = 0.0104; Fig. 2b) and NADSYN1/DHCR7 rs3829251 A allele (P = 0.0451; Fig. 2c), and VDR rs2228570 A allele (P = 0.0121; Fig. 2d) were all associated with severity of steatosis using the chi‐squared test. The PNPLA3 G allele was also associated with presence of inflammation on biopsy (P = 0.0264; Fig. 2e) as was the GC variant rs4588 (P = 0.0282), causing a base change of C > A, (Fig. 2f). The remaining variants did not show any statistically significant association with steatosis or inflammation. There were no associations between any of the gene variants examined and histological ballooning or fibrosis. In a two‐way ANOVA test of unbalanced designs with interaction analysis and accounting for multiple hypothesis testing, no strong interactions between 25OHD levels, genotype and histology were observed.
Figure 2.
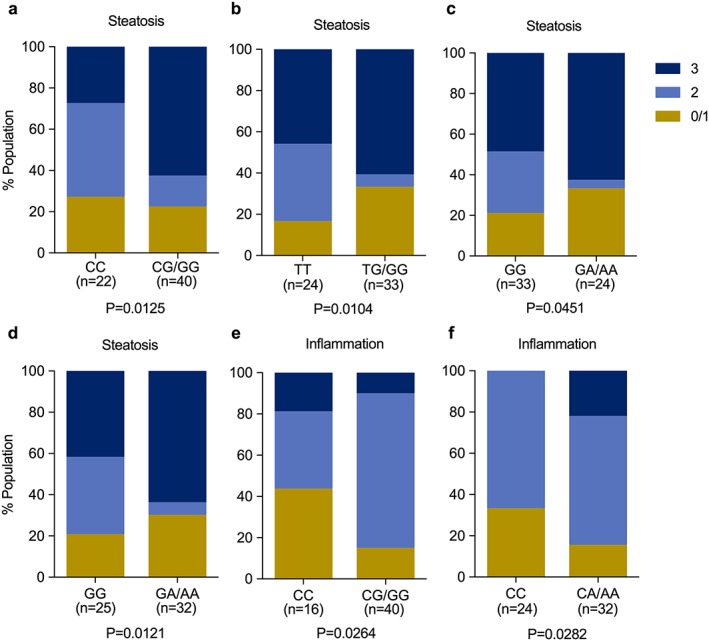
Histological scoring for steatosis in Caucasian patients in relationship to genotype for (a) PNPLA3 rs738409, (b) NADYSN1/DHCR7 rs12785878, (c) NADSYN1/DHCR7 rs3829251 and (d) VDR rs2228570. Histological scoring for inflammation in the same population in relationship to genotype for (e) PNPLA3 and (f) GC rs4588. All data are representative of population percentages and were assessed by chi‐squared test. DHCR7, dehydrocholesterol reductase‐7; GC, group‐specific component; NADSYN1, nicotinamide adenine dinucleotide synthase‐1; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; VDR, vitamin D receptor.
Discussion
To our knowledge, this is the first report to examine the relationship between vitamin D status, genetic variants known to affect vitamin D status and NAFLD histological severity in a paediatric population. The majority of children in this cohort had insufficient vitamin D levels throughout the year, with severe vitamin D deficiency during the winter months. The average serum 25OHD level in August in this patient group was 44.1 nmol·L−1, considerably lower than the 60.1 nmol·L−1 average found in the 2470 UK adolescents within the Avon Longitudinal Study of Parents and Children 18. While we used conservative cut‐offs to define deficiency and insufficiency here, it is important to note that these have been defined based on skeletal effects of vitamin D, and serum OHD levels associated with protective immunological effects are considerably higher (90–100 nmol·L−1) 19. Patients in our cohort showed an inverse correlation between both BMI and cholesterol and 25OHD levels similar to a previous report 20. Multiple factors have been implicated in the hypovitaminosis D typically observed in obesity, including reduced dietary intakes and decreased conversion pre vitamin D to active vitamin D metabolites secondary to sequestration of the fat‐soluble vitamin in excess adipose tissue 20. Importantly, and consistent with recently published data from an Italian paediatric cohort 6, serum 25OHD levels were found to be significantly lower in patients with more severe NAFLD as defined by NAS.
This contrasts with a recent report on adult patients with NAFLD by Patel et al. that showed no relationship with vitamin D levels, or the hepatic expression of genes involved in vitamin D metabolism, and presence or severity of NAFLD. 21 Other studies in adult patients with NAFLD have shown conflicting results, some demonstrating a correlation between NAFLD and nonalcoholic NASH severity, with lower levels of vitamin D 22 and some not showing any association 23. A meta‐analysis including 974 adult patients did not show an association between vitamin D (25OHD) levels and NAFLD 24. Vitamin D levels alone are necessarily variable, and the degree to which they vary over the several decades in which NAFLD may progress is unknown. Indeed, recent work shows genetic variation determines serum levels of vitamin D binding protein thereby influencing the levels of bound and ‘free’ or bioavailable 25OHD 25. A limitation to our study is that, similar to the genome‐wide association studies, total 25OHD levels were measured in serum and we cannot account for bioavailability here. Likewise, hepatic expression of genes responsible for vitamin D metabolism may well be altered or switched off in the context of significant liver injury. Genetic susceptibility to vitamin D alteration, either in absorption, conversion to the active form or its availability are all potential confounders to interpreting serum 25OHD levels; the measurement of effects on the end‐receptor may be more consistent.
We examined candidate polymorphisms in genes involved in the vitamin D metabolic pathway that had previously been identified by genome‐wide association or population studies as associated with either vitamin D status or liver disease. Our results show that, in Caucasian children with biopsy‐confirmed NAFLD in the UK, variants of the NADSYN1/DHCR7 (rs12785878, rs3829251) and VDR (rs2228570) genes are independently associated with increased steatosis, while a GC variant (rs4588) was associated with increased inflammation. Interestingly, this rs4588 SNP influences the serum level of vitamin D binding protein, which was not measured here and may dramatically influence the amount of bioavailable hormone 25. On the other hand, no associations were found between a variant in the CYP2R1 gene (rs2228570) and two other variants of the GC gene (rs2282679 and rs7041). In line with reports in American and Italian paediatric NAFLD cohorts 13, 14, we found presence of the risk (G) allele for the PNPLA3 rs738409 variant was associated with increased steatosis and inflammation scores. However, we found no significant associations between our investigated SNPs in the vitamin D metabolic pathway and serum 25OHD levels that have previously been reported in other populations 9, 15. This could be a reflection of the ethnically heterogeneous population studied and/or the small sample size.
Nicotinamide adenine dinucleotide synthase‐1 encodes the enzyme responsible for the final step in the biosynthesis of NAD, which is used in the production of cholesterol in the skin from 7‐dehydrocholesterol, making it unavailable for the vitamin D synthetic pathway 15. It is in such close proximity to the DHCR7 gene, which encodes the rate‐limiting enzyme responsible for the reduction of 7‐dehydrocholesterol to cholesterol in the skin, that the polymorphisms studied here (rs12785878 and rs3829251) may influence the function of either gene. In our study, patients presenting with at least one copy of either rare variants showed increased steatosis grading. Thus, NADSYN1/DHCR7 may play a role in development of NAFLD, and previously, the rs12785878 rare allele has been increased liver stiffness as assessed by transient elastography 26. Although these SNPs have been associated with decreased circulating 25OHD concentrations, when examining this genotype against 25OHD status in our study, results were likely confounded by small frequency of the rare genotypes and limited size of our cohort relative to population studies, restricting the conclusions that can be drawn.
In a similar manner, the GC gene encodes the vitamin D binding protein, which is synthesized in the liver and is responsible for the binding and transport of vitamin D and its metabolites 15. The most widely studied SNPs include rs4588 and rs7041, which are 11 base pairs apart from one another, described as being in moderate linkage disequilibrium and shown to be consistently associated with 25OHD status. However, in this present study, no SNPs in the GC gene were associated with lower 25OHD status. This could be due to the small sample size or different ethnic groups. Interestingly, SNP genotyping in our study did demonstrate increased inflammation grade in the presence of the rs4588 A allele. Relatedly, previous study of the expression of VDR in patients with NASH identified an inverse correlation with steatosis severity, lobular inflammation and the NAS 27. Our study shows an association between presence of the rs2228570 risk allele and increased steatosis.
By analysing both serum levels and known variants that may contribute to the metabolism and action of vitamin D, this study attempts to define some of those who may have a susceptibility to NAFLD at a young age. The antifibrotic effects of vitamin D in human hepatic stellate cells have previously been demonstrated 28, and a valid question is whether vitamin D treatment may have anti‐fibrotic therapeutic potential in paediatric NAFLD 7. While the efficacy of vitamin D supplementation on endothelial function in obese children has recently been examined 29, studies with liver endpoints are lacking. The limitations of this study need to be addressed in future research. The sample size was limited to a relatively small number of patients who had undergone liver biopsy. Significantly, greater numbers are likely to be required to detect some of the more modest associations with various variants. Obese controls are not included in the study although HAPmap is used to determine population frequencies of the variants. Additionally, patient and family sociodemographic details and deprivation index were not investigated; these are potential confounding factors for body mass and 25OHD levels in children 30 and would be useful for future studies. Levels of vitamin D were normalized to August values, and comparison is made with children from the Avon Longitudinal Study of Parents and Children but not matched for BMI. Levels of vitamin D are necessarily cross‐sectional and are likely to be dynamic, and so an attempt is made with this study to describe underlying susceptibilities to the development and progression of liver injury via vitamin D metabolism rather than rely on levels alone to explain disease.
This is the first study conducted in a UK paediatric population to find a relationship between genetic variations in the vitamin D metabolic pathway and liver histology severity. While the sample size and limited number of candidate polymorphisms investigated are potential limitations, liver biopsy is the criterion standard to define NAFLD and histological analysis is essential to stage disease. In addition, paediatric NAFLD has distinct histological differences to adult NAFLD including the distinction between type 1 and type 2 NASH, and findings in the adult population cannot be simply translated to children.
In conclusion, UK paediatric patients with NAFLD have extremely low winter vitamin D status, with vitamin D insufficiency prevalent throughout the year. Polymorphisms in the vitamin D metabolic pathway were associated with histological severity in patients with NAFLD. These data suggest a possible genetic link/consequence of vitamin D status on disease severity, although a possible influence of the presence of steatosis/inflammation on vitamin D metabolism needs also to be considered. Although supplementation with vitamin D has not been demonstrated an effective intervention in the limited studies performed in adult patients with NAFLD to date, further research is warranted into whether targeted supplementation in paediatric populations may be indicated.
Conflict of interest statement
S. L. N. has undertaken consultancy work for the following companies: Nestle, Kellogg's, Yoplait, The Yoghurt Council, and is Research Director for D3Tex Ltd that holds the UK Patent (GGC Patent Pending) for the use of any UVB material for preventing vitamin D deficiency. There are no conflicts of interest to declare for the other authors listed.
Author contributions
P. S. G. performed experiments, collected and analysed data and helped with manuscript preparation. A. Q. reviewed and scored histology data and helped with critical review of the manuscript. A. D. helped with the study design and with critical review of the manuscript. H. W. did the statistical analysis and helped with manuscript preparation. S. L. N. helped with study design and with critical review of the manuscript. K. H. H. contributed to the study design, assisted with data interpretation and with critical review of the manuscript. E. F. contributed to the study design, collection of data and manuscript preparation. J. B. M. contributed to study design, supervision of data analysis and preparation of manuscript. All authors have seen and have approved the final draft submitted.
Supporting information
Figure S1. Participant data analysis flow diagram
Figure S2: Modelled population 25(OH)D levels using Sachs algorithm. Data are presented as mean ± 95% CI. n = 71 for each month.
Figure S3. August 25(OH)D serum levels in Caucasian and Non‐Caucasian patients. Data are expressed around the mean. Data were tested for normality using the D'Agostino & Pearson omnibus normality test and analysed using Mann–Whitney test.
Figure S4. BMI and serum cholesterol levels in Caucasian patients. BMI (A; n = 45) and serum cholesterol levels (B; n = 65) versus 25(OH)D levels. Data were tested for normality using the D'Agostino‐Pearson omnibus normality test and Spearman correlation was performed demonstrating a weak but significant inverse correlation.
Table S1. Genotype distributions
Acknowledgements
The authors are grateful to the patients, their families and the clinical teams for their commitment to the cohort study. The authors would also like to thank the Liver Molecular Genetics team at King's College Hospital for use of their equipment for DNA extraction, and we are also indebted to the King's Pediatric Liver Biobank for samples. This work was supported by the Children's Liver Disease Foundation, with a studentship grant (NL1753‐Ph.D.) to P. S. G.
Gibson P. S., Quaglia A., Dhawan A., Wu H., Lanham‐New S., Hart K. H., Fitzpatrick E., and Moore J. B. (2018) Vitamin D status and associated genetic polymorphisms in a cohort of UK children with non‐alcoholic fatty liver disease, Pediatric Obesity, 13, 433–441, https://doi.org/10.1111/ijpo.12293.
Contributor Information
E. Fitzpatrick, Email: emer.fitzpatrick@kcl.ac.uk
J. B. Moore, Email: j.b.moore@leeds.ac.uk.
References
- 1. Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr 2013; 13: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunt EM, Kleiner DE, Wilson LA, et al Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD‐Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009; 49: 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore JB. Non‐alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc 2010; 69: 211–220. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, et al The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2017; 67: 328–357. [DOI] [PubMed] [Google Scholar]
- 5. Black LJ, Jacoby P, She Ping‐Delfos WC, et al Low serum 25‐hydroxyvitamin D concentrations associate with non‐alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol 2014; 29: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 6. Nobili V, Giorgio V, Liccardo D, et al Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur J Endocrinol 2014; 170: 547–553. [DOI] [PubMed] [Google Scholar]
- 7. Nobili V, Reif S. Vitamin D and liver fibrosis: let's start soon before it's too late. Gut 2015; 64: 698–699. [DOI] [PubMed] [Google Scholar]
- 8. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Institute of Medicine 2011. [PubMed] [Google Scholar]
- 9. Wang TJ, Zhang F, Richards JB, et al Common genetic determinants of vitamin D insufficiency: a genome‐wide association study. Lancet 2010; 376: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu L, Sheng H, Li H, et al Associations between common variants in GC and DHCR7/NADSYN1 and vitamin D concentration in Chinese Hans. Hum Genet 2012; 131: 505–512. [DOI] [PubMed] [Google Scholar]
- 11. Department of Health . Nutrition and Bone Health: With Particular Reference To Calcium and Vitamin D No. 49. The Stationary Office: London, 1998. [Google Scholar]
- 12. Nissen J, Rasmussen LB, Ravn‐Haren G, et al Common variants in CYP2R1 and GC genes predict vitamin D concentrations in healthy Danish children and adults. PloS One 2014; 9: e89907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, Nash CRN. The association of genetic variability in patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology 2010; 52: 894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Santoro N, Kursawe R, D'Adamo E, et al A common variant in the patatin‐like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology 2010; 52: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn J, Yu K, Stolzenberg‐Solomon R, et al Genome‐wide association study of circulating vitamin D levels. Hum Mol Gen 2010; 19: 2739–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sachs MC, Shoben A, Levin GP, et al Estimating mean annual 25‐hydroxyvitamin D concentrations from single measurements: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr 2013; 97: 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Au LE, Economos CD, Goodman E, Must A, Chomitz VR, Sacheck JM. Vitamin D intake and serum vitamin D in ethnically diverse urban schoolchildren. Public Health Nutr 2012; 15: 2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams DM, Fraser A, Sayers A, et al Associations of childhood 25‐hydroxyvitamin D2 and D3 and cardiovascular risk factors in adolescence: prospective findings from the Avon Longitudinal Study of Parents and Children. Eur J Prev Cardiol 2014; 21: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bischoff‐Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson‐Hughes B. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006; 84: 18–28. [DOI] [PubMed] [Google Scholar]
- 20. Stein EM, Strain G, Sinha N, et al Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009; 71: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel YA, Henao R, Moylan CA, et al Vitamin D is not associated with severity in NAFLD: results of a paired clinical and gene expression profile analysis. Am J Gastroenterol 2016; 111: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nelson JE, Roth CL, Wilson LA, et al Vitamin D deficiency is associated with increased risk of non‐alcoholic steatohepatitis in adults with non‐alcoholic fatty liver disease: possible role for MAPK and NF‐kappaB? Am J Gastroenterol 2016; 111: 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bril F, Maximos M, Portillo‐Sanchez P, et al Relationship of vitamin D with insulin resistance and disease severity in non‐alcoholic steatohepatitis. J Hepatol 2015; 62: 405–411. [DOI] [PubMed] [Google Scholar]
- 24. Jaruvongvanich V, Ahuja W, Sanguankeo A, Wijarnpreecha K, Upala S. Vitamin D and histologic severity of nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Dig Liver Dis 2017; 49: 618–622. [DOI] [PubMed] [Google Scholar]
- 25. Powe CE, Evans MK, Wenger J, et al Vitamin D‐binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013; 369: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Wang X, Liu Y, et al The GC, CYP2R1 and DHCR7 genes are associated with vitamin D levels in northeastern Han Chinese children. Swiss Med Wkly 2012; w13636: 142. [DOI] [PubMed] [Google Scholar]
- 27. Barchetta I, Carotti S, Labbadia G, et al Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology 2012; 56: 2180–2187. [DOI] [PubMed] [Google Scholar]
- 28. Beilfuss A, Sowa JP, Sydor S, et al Vitamin D counteracts fibrogenic TGF‐beta signalling in human hepatic stellate cells both receptor‐dependently and independently. Gut 2015; 64: 791–799. [DOI] [PubMed] [Google Scholar]
- 29. Javed A, Kullo IJ, Balagopal PB, Kumar S. Effect of vitamin D3 treatment on endothelial function in obese adolescents. Pediatr Obes 2016; 11: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hazell TJ, Gallo S, Vanstone CA, Agellon S, Rodd C, Weiler HA. Vitamin D supplementation trial in infancy: body composition effects at 3 years of age in a prospective follow‐up study from Montreal. Pediatr Obes. 2017; 12: 38–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Participant data analysis flow diagram
Figure S2: Modelled population 25(OH)D levels using Sachs algorithm. Data are presented as mean ± 95% CI. n = 71 for each month.
Figure S3. August 25(OH)D serum levels in Caucasian and Non‐Caucasian patients. Data are expressed around the mean. Data were tested for normality using the D'Agostino & Pearson omnibus normality test and analysed using Mann–Whitney test.
Figure S4. BMI and serum cholesterol levels in Caucasian patients. BMI (A; n = 45) and serum cholesterol levels (B; n = 65) versus 25(OH)D levels. Data were tested for normality using the D'Agostino‐Pearson omnibus normality test and Spearman correlation was performed demonstrating a weak but significant inverse correlation.
Table S1. Genotype distributions


