Abstract
Polycyclic indoline‐benzodiazepines can be accessed through the intermolecular reaction of Tröger bases with N‐sulfonyl‐1,2,3‐triazoles. Under RhII catalysis, α‐imino carbenes are generated and a subsequent cascade of [1,2]‐Stevens, Friedel–Crafts, Grob, and aminal formation reactions yield the polycyclic heterocycles as single isomers (d.r.>49:1, four stereocenters including two bridgehead N atoms). Further ring expansion by insertion of a second α‐imino carbene leads to elaborated polycyclic 9‐membered‐ring triazonanes.
Keywords: benzodiazepines, cascade reactions, indolines, rhodium, α-imino carbenes
N‐Sulfonyl‐1,2,3‐triazoles 1 are structural motifs that are used regularly in synthetic, biological, and medicinal chemistry.1 Significantly, triazoles 1 are in equilibrium with α‐diazo imines of type 1′, which decompose under metal catalysis to afford α‐imino carbenes A (Scheme 1, top).2 In recent years, many synthetically useful and original processes have been developed using these electrophilic intermediates, from migrations to ylide‐forming reactions and subsequent transformations.3, 4 In terms of ammonium ylide chemistry,5 few studies have been reported.6 Of interest to the current study, the synthesis of tetrasubstituted indolines through reactions of o‐vinylanilines is noteworthy.7 Herein, in a new development, the intermolecular reactivity of N‐sulfonyl‐1,2,3‐triazoles 1 with Tröger bases (TB) 2 is reported (Scheme 1, bottom). Under RhII catalysis (2 mol %),8 densely functionalized polycyclic heterocycles 3 are obtained (yields up to 85 %). After the initial addition of carbenes A to TB, the transformation involves a cascade of [1,2]‐Stevens, Friedel–Crafts, Grob and aminal formation reactions. Resulting chalice‐like products 3 are generated with high stereoselectivity (d.r.>49:1, four stereocenters including two bridgehead nitrogen atoms). When the aminal nitrogen is protected by a nosyl group, insertion of a second α‐imino carbene is possible, leading to nine‐membered triazonanes 4, albeit in moderate yields (29–34 %). In view of their unusual heterocyclic structures, products 3 and 4 should be of interest in a variety of fields, and medical chemistry in particular. These derivatives combine in a single framework both indoline and benzodiazepine skeletons, which are privileged motifs in natural product and medical chemistry (Figure 1).9, 10
Scheme 1.
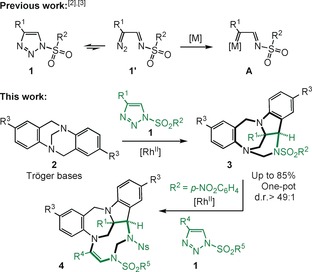
Generation of α‐imino carbenes and application to the synthesis of elaborated polycyclic indoline‐benzodiazepines.
Figure 1.
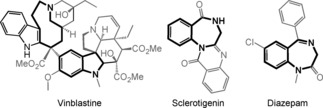
Typical examples of an indoline (left) and benzodiazepines (right).
Recently, it was shown that TB 2 (Scheme 1, bottom),11 classical diaza heterocycles prepared in one step by condensation of primary anilines with formaldehyde, react with α‐keto carbenes to afford ethano‐TB, after aminal bridge expansion.12 Drawing from the direct analogy that exists between α‐keto and α‐imino carbenes, the reactivity of 2 with intermediates A was tested. Initial experiments were performed using Tröger base 2 a (R3=Me) and N‐tosyl‐4‐phenyltriazole 1A under dirhodium catalysis (Rh2(oct)4, 1 mol %). While the reaction was mostly unproductive, a product was isolated in low yield (8 %).
This compound 3 aA presented interesting characteristics in 1H and 13C‐NMR spectroscopy and an additional mass of 271 Da compared to 2 a (equivalent to one carbene moiety). The structure of 3 aA was determined with certainty after X‐ray diffraction analysis (Figure 2). It presents an interesting chalice‐like geometry with 5‐, 6‐, and 7‐membered rings forming a rigid bowl‐shape motif above substituent R1 (here Ph), which extends from the center of the cup in an apical fashion. In Figure 2, both top and lateral views of 3 aA are represented since the geometry of compounds 3 is difficult to appreciate with a single representation.13 Overall, an interesting fusion of indoline and benzodiazepine motifs is revealed.
Figure 2.
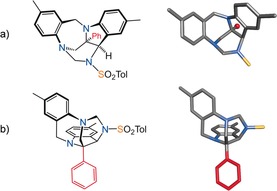
Representations of indoline‐benzodiazepine 3 aA and stick views of the crystal structure.24 a) View from above the rim (top). b) Lateral view. Part of the tosyl group is removed for clarity in the stick models. The apical phenyl group is indicated in red and replaced with a single ball in the top right representation.
With this result and characterization in hand, optimization studies were conducted to improve the synthesis of 3 aA and the results are reported in Table S1 in the Supporting Information. Using CHCl3 as the solvent and Rh2(Piv)4 (2 mol %) as the catalyst improved the outcome. Complete consumption of 2 a is achieved after 48 h at 80 °C when using a slight excess of 1A (1.5 equiv, 0.25 m). Product 3 aA is then formed in 70 % yield as a single stereoisomer.14 With these conditions, disubstituted and naphthyl derivatives 3 bA and 3 cA were obtained in 30 % and 64 % yields, respectively. In the first case, the lower yield might be due to the strain created by the methyl groups at immediate proximity to the central bicyclic core.15 Modulation of the substituents at the para position to the nitrogen atoms of TB substrates led to products 3 dA to 3 kA in moderate to good yields (45–75 %). The reactions proceed more effectively with electron donating substituents (3 dA–3 fA) than with electron‐withdrawing groups (3 gA–3 kA). A higher nucleophilic character of the nitrogen atoms is beneficial for the first elemental step of the mechanism (see below, Scheme 3, A→B).16
Scheme 3.
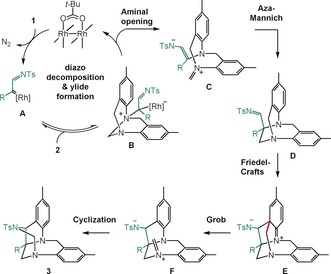
Mechanistic rationale.
The reaction was also performed with a series of N‐sulfonyl triazoles (1B–1P) and TB 2 a as substrates. An inverse electronic demand was then beneficial. In fact, with electron‐donating substituents on the triazole precursors, products 3 aB–3 aD are obtained in low to moderate yields (34–55 %), while higher yields (74–83 %) are afforded in the presence of electron‐withdrawing groups (3 aF–3 aH). This electronic preference was confirmed with four other derivatives (3 aI–3 aL); thiophene and methyl ester substituents promoted the reaction effectively while n‐propyl and 6‐methoxynaphthalene residues led to lower yields.
The influence of the sulfonyl group substituent was then further investigated. Dimethylsulfamoyl‐, mesyl (Ms)‐, and p‐nosyl (Ns)‐substituted triazoles were reacted and afforded products 3 aM, 3 aN, and 3 aO in 56 %, 61 % and 47 % yields, respectively. With 3 aO in hand, removal of the p‐nosyl group was possible by a treatment with thiophenol/K2CO3 to afford 5 in 74 % yield (Scheme 2).17 Care was also taken to verify that the process can occur under metal‐free conditions. Using Davies’ N‐sulfonyl‐4‐phthalimido triazole 1P,18 product 3 aP was formed under thermal activation, albeit in only 31 % yield. Finally, 3 lA and 3 mA were also prepared; these compounds formed as single regioisomers and are of importance for the mechanistic discussion (see below).
Scheme 2.
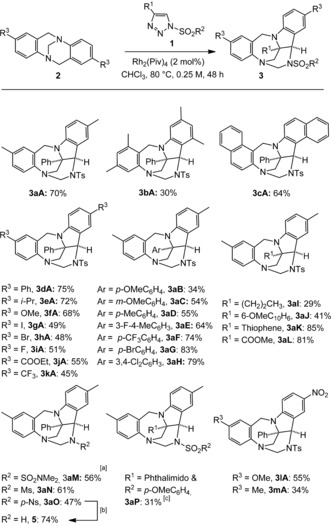
Scope of the reaction. [a] Reaction performed at 100 °C. [b] PhSH, K2CO3, CH3CN/DMSO (49:1), 50 °C, 2 h. [c] Thermal activation only.
A rationale for the observed reactivity is outlined in Scheme 3. In the presence of RhII catalysts and Rh2(Piv)4 in particular, N‐sulfonyl triazoles 1 generate α‐imino carbenes A by nitrogen extrusion.2 These electrophilic intermediates react with Tröger bases 2 to form nitrogen ylides B. The quaternization of the nitrogen atom leads to ring opening of the aminal bridge to form intermediates of type C. These species react intramolecularly in an aza‐Mannich reaction and cyclize to form sulfonyl imines D. The overall process from 1 to D corresponds to a formal [1,2]‐Stevens rearrangement.12a Then, induced by the spatial proximity between the electrophilic imine and the electron‐rich aniline, a Friedel–Crafts cyclization occurs to form intermediate E. In this rigid moiety, the alignment of the nitrogen lone pair with the σ* orbital of the adjacent C−C bond (highlighted in red) leads to a Grob fragmentation19 and the formation of intermediates F. A final intramolecular trapping of the new iminium species by the nitrogen of the sulfonamide group leads to final aminal formation and products 3.
In the above rationale, the ylide formation (A→B) is presented as a reversible step. This was established by the regioselective formation of 3 lA and 3 mA, in which nitrogen atoms para to the nitro group of 2 l and 2 m—the more electron poor and less nucleophilic N atoms—have reacted with carbenes A.20 This result, in contradiction with the general reactivity (Scheme 2),16 can be however rationalized by a Curtin–Hammet analysis.12a It is proposed that carbenes A react preferentially with the more electron‐rich nitrogen atoms (para to the MeO or Me groups, step A→BI) but the subsequent aminal opening is disfavored since it would lead to electronically unstable intermediates CI (Scheme 4, top). Opportunely, upon ylide equilibration, reaction of A with the less nucleophilic (disfavored) nitrogen occurs (→BII). The subsequent aminal opening forms intermediates CII and their higher stability constitutes the kinetic and thermodynamic driving force of the process. Importantly, in the reactions with 1P performed under thermal activation,18 TB 2 l and 2 m react to afford 1:1 mixtures of regioisomers (see Schemes S1–S2 in the Supporting Information). In these metal‐free processes, intermediates BIII and BIV are probably formed in equimolar ratios and do not interconvert (Scheme 4, bottom). The presence of the RhII catalyst is thus essential to ensure the reversibility of the ylide formation.
Scheme 4.
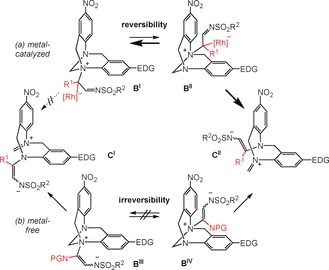
a) Reversible ylide formation under metal‐catalyzed conditions and preferred formation of electronically‐stabilized iminium CII. b) Formation of iminium intermediates CI and CII under (kinetic) metal‐free conditions. EDG=Electron‐donating group (OMe or Me), NPG=N‐phthalimido proup.
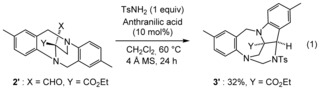
Of importance for the mechanistic understanding, aldehyde‐substituted ethano‐TB 2′ was also prepared (see the Supporting Information) and reacted with tosyl amine in the presence of anthranilic acid (10 mol %).21 The corresponding iminium D could not be observed in 1H NMR spectroscopy, but a slow and steady formation of polycyclic product 3′ (32 %) was observed [Eq. 1]. This experiment suggests that, once formed, intermediates D react rapidly in the Friedel–Crafts and subsequent reactions to form products 3.
Finally, with p‐nosyl‐substituted derivative 3 aO in hand, the possibility to form benzodiazepine‐indoline adducts containing a 9‐membered 1,3,6‐triazonane ring was recognized (Scheme 5).22 Treatment of 3 aO with an excess of 1A, 1B, or 1O (3 equiv) in CHCl3 (0.25 m) at 80 °C (48 h) and Rh2(Piv)4 as catalyst (2 mol %) afforded products 4A, 4B and 4O in moderate yields (29–34 %). The structure of compounds 4 was unambiguously determined by X‐ray crystallography. Mechanistically, the most nucleophilic and less‐hindered tertiary nitrogen atom of 3O reacts with carbenes A (generated in situ) to yield ylides G (Scheme 5, bottom). Then, aminal opening (formation of iminium intermediates H) and intramolecular nucleophilic closures lead to products 4.
Scheme 5.
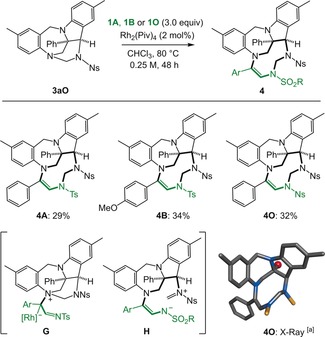
Synthesis of benzodiazepine‐indoline triazonanes 4 and probable intermediates G and H. [a] Part of the sulfonyl groups are removed for clarity; the apical phenyl group is replaced with a red single ball.24
In summary, polycyclic indoline‐benzodiazepines 3 were generated in a single step through the direct intermolecular reaction of TB with N‐sulfonyl‐1,2,3‐triazoles. Initiated by the formation of α‐imino carbenes under RhII catalysis, a cascade of [1,2]‐Stevens, Friedel–Crafts, Grob, and aminal formation reactions occurs to yield the triaza polycycles as single isomers (d.r.>49:1, four stereocenters including two bridgehead N atoms). Mechanistic insight was obtained, demonstrating, for instance, the importance of metal‐bound ylides to explain the regioselectivity of certain reactions. Further ring expansions by insertion of a second α‐imino carbene are possible, resulting in elaborated polycyclic 9‐membered ring triazonanes 4. Products of type 3 or 4 present interesting skeleton and geometries that ought to be tested in medicinal chemistry, and their preparation is difficult to imagine by other routes.23
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the University of Geneva and the Swiss NSF for financial support. We also acknowledge the contributions of the Sciences Mass Spectrometry (SMS) platform at the Faculty of Sciences, University of Geneva.
A. Bosmani, A. Guarnieri-Ibáñez, S. Goudedranche, C. Besnard, J. Lacour, Angew. Chem. Int. Ed. 2018, 57, 7151.
Contributor Information
Alessandro Bosmani, http://www.unige.ch/sciences/chiorg/lacour/.
Prof. Jérôme Lacour, Email: jerome.lacour@unige.ch.
References
- 1.
- 1a. Kolb H. C., Finn M. G., Sharpless K. B., Angew. Chem. Int. Ed. 2001, 40, 2004–2021; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2001, 113, 2056–2075; [Google Scholar]
- 1b. Lewis W. G., Green L. G., Grynszpan F., Radić Z., Carlier P. R., Taylor P., Finn M. G., Sharpless K. B., Angew. Chem. Int. Ed. 2002, 41, 1053–1057; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 1095–1099; [Google Scholar]
- 1c. Amblard F., Cho J. H., Schinazi R. F., Chem. Rev. 2009, 109, 4207–4220; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1d. Le Droumaguet C., Wang C., Wang Q., Chem. Soc. Rev. 2010, 39, 1233–1239; [DOI] [PubMed] [Google Scholar]
- 1e. Thirumurugan P., Matosiuk D., Jozwiak K., Chem. Rev. 2013, 113, 4905–4979. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Harmon R. E., Stanley F., Gupta S. K., Johnson J., J. Org. Chem. 1970, 35, 3444–3448; [Google Scholar]
- 2b. Harmon R. E., Earl R. A., Gupta S. K., J. Chem. Soc. D 1971, 296–297. [Google Scholar]
- 3.
- 3a. Chattopadhyay B., Gevorgyan V., Angew. Chem. Int. Ed. 2012, 51, 862–872; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 886–896; [Google Scholar]
- 3b. Gulevich A. V., Gevorgyan V., Angew. Chem. Int. Ed. 2013, 52, 1371–1373; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 1411–1413; [Google Scholar]
- 3c. Davies H. M., Alford J. S., Chem. Soc. Rev. 2014, 43, 5151–5162; [DOI] [PubMed] [Google Scholar]
- 3d. Anbarasan P., Yadagiri D., Rajasekar S., Synthesis 2014, 46, 3004–3023; [Google Scholar]
- 3e. Wang Y., Lei X., Tang Y., Synlett 2015, 26, 2051–2059; [Google Scholar]
- 3f. Jiang Y., Sun R., Tang X.-Y., Shi M., Chem. Eur. J. 2016, 22, 17910–17924. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Chuprakov S., Hwang F. W., Gevorgyan V., Angew. Chem. Int. Ed. 2007, 46, 4757–4759; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 4841–4843; [Google Scholar]
- 4b. Chuprakov S., Kwok S. W., Zhang L., Lercher L., Fokin V. V., J. Am. Chem. Soc. 2009, 131, 18034–18035; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4c. Chuprakov S., Malik J. A., Zibinsky M., Fokin V. V., J. Am. Chem. Soc. 2011, 133, 10352–10355; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4d. Zibinsky M., Fokin V. V., Org. Lett. 2011, 13, 4870–4872; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4e. Yadagiri D., Anbarasan P., Chem. Eur. J. 2013, 19, 15115–15119; [DOI] [PubMed] [Google Scholar]
- 4f. Schultz E. E., Sarpong R., J. Am. Chem. Soc. 2013, 135, 4696–4699; [DOI] [PubMed] [Google Scholar]
- 4g. Miura T., Tanaka T., Matsumoto K., Murakami M., Chem. Eur. J. 2014, 20, 16078–16082; [DOI] [PubMed] [Google Scholar]
- 4h. Miura T., Nakamuro T., Liang C.-J., Murakami M., J. Am. Chem. Soc. 2014, 136, 15905–15908; [DOI] [PubMed] [Google Scholar]
- 4i. Medina F., Besnard C., Lacour J., Org. Lett. 2014, 16, 3232–3235; [DOI] [PubMed] [Google Scholar]
- 4j. Miura T., Fujimoto Y., Funakoshi Y., Murakami M., Angew. Chem. Int. Ed. 2015, 54, 9967–9970; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 10105–10108; [Google Scholar]
- 4k. Lindsay V. N. G., Viart H. M. F., Sarpong R., J. Am. Chem. Soc. 2015, 137, 8368–8371; [DOI] [PubMed] [Google Scholar]
- 4l. Yang Y., Zhou M.-B., Ouyang X.-H., Pi R., Song R.-J., Li J.-H., Angew. Chem. Int. Ed. 2015, 54, 6595–6599; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6695–6699; [Google Scholar]
- 4m. Xu H.-D., Xu K., Zhou H., Jia Z.-H., Wu H., Lu X.-L., Shen M.-H., Synthesis 2015, 47, 641–646; [Google Scholar]
- 4n. Kubiak R. W., Mighion J. D., Wilkerson-Hill S. M., Alford J. S., Yoshidomi T., Davies H. M. L., Org. Lett. 2016, 18, 3118–3121; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4o. Guarnieri-Ibáñez A., Medina F., Besnard C., Kidd S. L., Spring D. R., Lacour J., Chem. Sci. 2017, 8, 5713–5720; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4p. Miura T., Nakamuro T., Stewart S. G., Nagata Y., Murakami M., Angew. Chem. Int. Ed. 2017, 56, 3334–3338; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 3382–3386; [Google Scholar]
- 4q. Miura T., Zhao Q., Murakami M., Angew. Chem. Int. Ed. 2017, 56, 16645–16649; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16872–16876. [Google Scholar]
- 5. Sweeney J. B., Chem. Soc. Rev. 2009, 38, 1027–1038. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Chuprakov S., Kwok S. W., Fokin V. V., J. Am. Chem. Soc. 2013, 135, 4652–4655; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Jeon H. J., Jung D. J., Kim J. H., Kim Y., Bouffard J., Lee S.-g., J. Org. Chem. 2014, 79, 9865–9871; [DOI] [PubMed] [Google Scholar]
- 6c. Lee D. J., Han H. S., Shin J., Yoo E. J., J. Am. Chem. Soc. 2014, 136, 11606–11609; [DOI] [PubMed] [Google Scholar]
- 6d. Lee D. J., Ko D., Yoo E. J., Angew. Chem. Int. Ed. 2015, 54, 13715–13718; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 13919–13922; [Google Scholar]
- 6e. Lei X., Li L., He Y.-P., Tang Y., Org. Lett. 2015, 17, 5224–5227; [DOI] [PubMed] [Google Scholar]
- 6f. Xu H.-D., Jia Z.-H., Xu K., Zhou H., Shen M.-H., Org. Lett. 2015, 17, 66–69; [DOI] [PubMed] [Google Scholar]
- 6g. Ryu T., Baek Y., Lee P. H., J. Org. Chem. 2015, 80, 2376–2383; [DOI] [PubMed] [Google Scholar]
- 6h. Zhao Y.-Z., Yang H.-B., Tang X.-Y., Shi M., Chem. Eur. J. 2015, 21, 3562–3566; [DOI] [PubMed] [Google Scholar]
- 6i. Wang Y., Lei X., Tang Y., Chem. Commun. 2015, 51, 4507–4510; [DOI] [PubMed] [Google Scholar]
- 6j. Rostovskii N. V., Ruvinskaya J. O., Novikov M. S., Khlebnikov A. F., Smetanin I. A., Agafonova A. V., J. Org. Chem. 2017, 82, 256–268; [DOI] [PubMed] [Google Scholar]
- 6k. Fu L., Davies H. M. L., Org. Lett. 2017, 19, 1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yadagiri D., Reddy A. C. S., Anbarasan P., Chem. Sci. 2016, 7, 5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.
- 8a. Doyle M. P., Forbes D. C., Chem. Rev. 1998, 98, 911–936; [DOI] [PubMed] [Google Scholar]
- 8b. Deng Y., Qiu H., Srinivas H. D., Doyle M. P., Curr. Org. Chem. 2016, 20, 61–81. [Google Scholar]
- 9.In relation to compounds 3 and 4, vinblastine is an interesting example since it contains not only the indoline skeleton but also a 9-membered ring in the second fragment.
- 10.
- 10a. Archer G. A., Sternbach L. H., Chem. Rev. 1968, 68, 747–784; [Google Scholar]
- 10b. Ishikura M., Yamada K., Abe T., Nat. Prod. Rep. 2010, 27, 1630–1680. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Tröger J., J. Prakt. Chem. 1887, 36, 225–245; [Google Scholar]
- 11b. Prelog V., Wieland P., Helv. Chim. Acta 1944, 27, 1127–1134; [Google Scholar]
- 11c. Sergeyev S., Helv. Chim. Acta 2009, 92, 415–444; [Google Scholar]
- 11d. Rúnarsson Ö. V., Artacho J., Wärnmark K., Eur. J. Org. Chem. 2012, 7015–7041. [Google Scholar]
- 12.The aminal opening is induced by the N-atom quaternization. See the following references and discussion therein:
- 12a. Sharma A., Guénée L., Naubron J.-V., Lacour J., Angew. Chem. Int. Ed. 2011, 50, 3677–3680; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 3761–3764; [Google Scholar]
- 12b. Sharma A., Besnard C., Guénee L., Lacour J., Org. Biomol. Chem. 2012, 10, 966–969. [DOI] [PubMed] [Google Scholar]
- 13.A third stereochemical representation of compounds 3 is provided in Scheme 3.
- 14.In TB and derivatives, the bridgehead nitrogen atoms are stereogenic and configurationally stable under neutral conditions.
- 15.With TB-carrying substituents ortho to the nitrogen atoms, only traces of the desired products were observed with very small conversion of starting materials.
- 16.With two p-nitro substituents, only traces of conversion are observed.
- 17. Fukuyama T., Jow C.-K., Cheung M., Tetrahedron Lett. 1995, 36, 6373–6374. [Google Scholar]
- 18. Alford J. S., Davies H. M. L., Org. Lett. 2012, 14, 6020–6023. [DOI] [PubMed] [Google Scholar]
- 19. Grob C. A., Baumann W., Helv. Chim. Acta 1955, 38, 594–610. [Google Scholar]
- 20.Unsymmetrically substituted TB derivatives can be used as mechanistic probes. See Ref. [12a] and Bosmani A., Pujari S. A., Besnard C., Guénée L., Poblador-Bahamonde A. I., Lacour J., Chem. Eur. J. 2017, 23, 8678–8684. [DOI] [PubMed] [Google Scholar]
- 21. Morales S., Guijarro F. G., García Ruano J. L., Cid M. B., J. Am. Chem. Soc. 2014, 136, 1082–1089. [DOI] [PubMed] [Google Scholar]
- 22.Compound 4O was first identified in the crude mixture of the reaction of 1O with 2 a as a byproduct.
- 23. Zhou Y., Li J., Ji X., Zhou W., Zhang X., Qian W., Jiang H., Liu H., J. Org. Chem. 2011, 76, 1239–1249. [DOI] [PubMed] [Google Scholar]
- 24. https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/?????? 1832809 (4 O), 1832810 (3 aA), and 1832811 (3 lA) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


