Abstract
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of obesity-associated liver diseases and it has become the major cause of cirrhosis in the Western world. The high prevalence of NAFLD-associated advanced liver disease reflects both the high prevalence of obesity-related fatty liver (hepatic steatosis) and the lack of specific treatments to prevent hepatic steatosis from progressing to more serious forms of liver damage, including nonalcoholic steatohepatitis (NASH), cirrhosis, and primary liver cancer. The pathogenesis of NAFLD is complex, and not fully understood. However, compelling evidence demonstrates that dysregulation of the hedgehog (Hh) pathway is involved in both the pathogenesis of hepatic steatosis and the progression from hepatic steatosis to more serious forms of liver damage. Inhibiting Hedgehog signaling enhances hepatic steatosis, a condition which seldom results in liver-related morbidity or mortality. In contrast, excessive Hedgehog pathway activation promotes development of NASH, cirrhosis, and primary liver cancer, the major causes of liver-related deaths. Thus, suppressing excessive Hh pathway activity is a potential approach to prevent progressive liver damage in NAFLD. Various pharmacologic agents that inhibit Hh signaling are available and approved for cancer therapeutics; more are being developed to optimize the benefits and minimize the risks of inhibiting this pathway. In this review we will describe the Hh pathway, summarize the evidence for its role in NAFLD evolution, and discuss the potential role for Hh pathway inhibitors as therapies to prevent NASH, cirrhosis and liver cancer.
Keywords: Nonalcoholic fatty liver disease, hedgehog, wound-healing response, treatment
INTRODUCTION
Liver cirrhosis is responsible for over one million deaths per year, 2% of all deaths worldwide (Mokdad et al., 2014). At the turn of the century, the main causes of liver cirrhosis were viral hepatitis and alcoholic liver disease (Mokdad et al., 2014). However, since then a vaccine to prevent hepatitis B infection and anti-viral treatments that can cure hepatitis C infection have been developed, diminishing both the prevalence and incidence of cirrhosis caused by these infections. In contrast, the obesity pandemic has fueled new epidemics of diseases related to the metabolic syndrome, including nonalcoholic fatty liver disease (NAFLD). One fourth of the world’s adult population now has NAFLD (Younossi et al., 2016), a disease that was once relatively restricted to affluent Western societies, such as the United States (Clark, Brancati, and Diehl, 2003). In countries where NAFLD has been prevalent for the past couple of decades, the health burden of NAFLD is now apparent. For example, NAFLD-related cirrhosis is surpassing cirrhosis caused by viral hepatitis as the leading general indication for liver transplantation in the U.S. and (Wong et al., 2015), and it has already become the most rapidly growing cause of liver transplantation for hepatocellular carcinoma (Wong, Cheung, and Ahmed, 2014).
Nonalcoholic fatty liver (NAFL) refers to hepatocyte accumulation of lipids (aka hepatic steatosis) that can not be attributed to alcohol consumption. NAFL strongly associates with obesity and related metabolic disorders, such as insulin-resistance, diabetes mellitus, dyslipidemia and hypertension (a.k.a, the metabolic syndrome). The metabolic syndrome is defined as a “disorder of energy homeostasis” (Wikepedia) and thus, it reflects metabolic stress. The association between NAFLD and metabolic stress is complex because metabolic stress not only induces NAFLD, but NAFLD can also amplify metabolic stress. This bidirectional relationship is thought to explain why mortality due to cardiovascular disease, and non-liver cancers are increased in NAFLD patients (Musso et al., 2011).
While most individuals with isolated (“simple”) hepatic steatosis do not develop progressive liver damage, a minority (~20-30%) experience lipotoxicity, i.e., multi-factorial hepatocyte injury that results in hepatocyte death and liver inflammation, a condition dubbed nonalcoholic steatohepatitis (NASH). NASH is a dynamic condition that can regress, persist, or progress. Because chronic NASH invariably triggers wound healing responses that involve fibrogenesis and expansion of liver progenitor populations, it dramatically increases the risk for cirrhosis and primary liver cancer. (Machado and Cortez-Pinto, 2014). Epidemiological studies of NAFLD have revealed that liver-related morbidity and mortality in individuals with NASH are predicted by the severity of liver fibrosis, rather than by the degree of obesity, insulin-resistance, or hepatic steatosis (Angulo et al., 2015; Ekstedt et al., 2015; Dulai et al., 2017). These findings suggest that dysregulated repair of fatty liver damage (NASH) might distinguish the NAFLD patients who are destined to suffer bad liver outcomes from those whose liver condition will follow a relatively indolent course.
Much has been learned about the pathogenesis of NAFLD since it was first described by Ludwig et al. (Ludwig et al., 1980). However, lingering gaps in knowledge prevent optimal management of NAFLD patients because it remains impossible to accurately identify those who are at risk of progressive liver disease prospectively, and no therapy that reduces liver-specific and overall mortality has yet been discovered. In the last decade, researchers have uncovered a pivotal role for Hedgehog pathway dysregulation in NAFLD pathogenesis, particularly as a driver of disease progression to cirrhosis. Hence, Hedgehog has become a candidate target for the treatment of fibrosing NASH. This approach is immediately actionable since specific inhibitors of the pathway are already in clinical use for some cancers such as basal cell carcinoma and medulloblastoma (Guha, 2012); a phase 1 clinical trial for the treatment of hepatocellular carcinoma with an oral hedgehog inhibitor, sonidebig, is ongoing (NCT02151864); and old inexpensive drugs (e.g., the antifungal itraconazole) are known to have strong anti-hedgehog activity (Kim et al., 2010). In this article we will review knowledge about the Hedgehog signaling pathway and summarize data regarding the role of the Hedgehog pathway in the pathogenesis and progression of NAFLD, as well as preclinical evidence that targeting the Hedgehog pathway may be an effective approach to inhibit the evolution of NAFLD-related cirrhosis.
THE HEDGEHOG SIGNALING PATHWAY
Nobel Prize laureates Wieschaus and Nussland-Volhard first described the Hedgehog pathway in 1980. While using a genetic screen in Drosophila melanogaster to identify genes that regulate body plan and segmentation, they discovered that fly larvae acquired spiny denticles resembling those of the mammalian hedgehog when one gene was mutated, which they dubbed hedgehog (Nusslein-Volhard and Wieschaus, 1980). A decade later, three mammalian hedgehog counterparts were identified, and named Sonic hedgehog (Shh, after the videogame character), and Indian (Ihh) and Desert (Dhh) (after two hedgehog species) (Echelard et al., 1993). Shh and Ihh are widely expressed, whereas Dhh expression is restricted to the nervous system and testis (Merchant and Saqui-Salces, 2014).
Canonical hedgehog pathway
The four major components of the Hedgehog pathway in the fly are: the ligand (hedgehog), the receptor (Patched), the signal transducer (smoothened) and the effector transcription factor (Gli). Signaling via this pathway regulates the expression of several target genes (Figure 1). In mammals, the canonical hedgehog pathway requires the presence of the primary cilium (PC) (Arensdorf, Marada, and Ogden, 2016). Primary cilia (PC) are small immotile cilia comprised of polymerized tubulin. PC are assembled during interphase in most differentiated cells. Cells possess only one PC, which is composed by a basal body and a filamentous axoneme that projects into the extracellular space. The basal body derives from the mother centriole at the end of mitosis (Roy, 2012). Two checkpoints separate the PC from the plasma membrane: the ciliary pockets and the transition zone (Goetz, Ocbina, and Anderson, 2009). The PC is crucial for Hh pathway activation, establishing cellular compartments that regulate the pathway by controlling the entry and exit of pathway components (Ramsbottom and Pownall, 2016). Within the PC, a complex trafficking system allows movement of the different Hh pathway components along the cilia. Movement towards the tip of the PC is mediated by a kinesin motor protein-based transport system which is facilitated by the ciliary Bardet-Biedl syndrome proteins (BBS) and intraflagellar transport proteins (ITF). Movement towards the base of the PC is mediated by a dynein motor protein-based transport system which is facilitated by the BBS, ITF and Kif7 (Merchant and Saqui-Salces, 2014; Liu, Wang, and Niswander, 2005).
Figure 1. The hedgehog pathway.
A. In the absence of the hedgehog (Hh) ligand, patch (Ptch) constitutively inhibits smoothened (Smo). This prevents the dissociation of the transcription factor Gli with its inhibitor Sufu, and allows Gli sequential phosphorylation by protein kinase A (PKA), casein kinase-1 (CK1) and glycogen synthase kinase-3 (GSK). Phosphorylated Gli is ubiquitinated through the action of transducin repeat containing protein (β-TrCP), which targets Gli to proteasomal degradation. The net result is the repressor form of Gli (Gli-R), which inhibits transcription of Gli-target genes.
B. In the presence of Hh ligand, the Hh-Ptch complex is internalized and degraded. This removes the inhibitory effect of Ptch on Smo, and allows Smo entry into the primary cilium (PC). To become activated Smo is then phosphorylated through the action of CK1 and G-protein-couples receptor kinase-2 (GRK2). This allows the entry of the complex Sufu-Gli into the PC. At the tip of the PC, Sufu dissociates from Gli. Gli then returns to the cytoplasm where it undergoes partial degradation in the proteasome in an activated form. Activated Gli enters the nucleus, where it mediates transcription of Gli-target genes.
Hedgehog ligand (Hh) is a morphogen, i.e., it is secreted by several cells, diffuses into the extracellular space, and determines the fate of Hh-target cells as a function of its concentration (Briscoe and Therond, 2013). Hh is synthetized as a 45 kDa precursor protein, with two domains, N-terminal and C-terminal. In the endoplasmic reticulum (ER), the precursor protein undergoes autocatalytic cleavage through the action of the C-terminal domain to generate an N-terminal 19 kDa fragment (Hh-N) (Chen et al., 2011). The Hh-N fragment then undergoes sequential lipidation that is essential for its function (Ramsbottom and Pownall, 2016). Hh-N has intrinsic cholesterol transferase activity which allows covalent attachment of a cholesterol group to its carboxyl end (Porter, Young, and Beachy, 1996). Subsequently, it undergoes palmitoylation through the action of the membrane-bound O-acyltransferase (MBOAT) skinny hedgehog (SKI), resulting in a highly hydrophobic molecule (Pepinsky et al., 1998). As detailed below, Hh uses ingenious mechanisms to diffuse into the extracellular matrix despite its hydrophobicity.
Release of Hh from ligand-producing cells requires the protein dispatched (Dis) (Burke et al., 1999) which binds to the cholesterol group of Hh-N (Tukachinsky et al., 2012). A soluble glycoprotein, SCUBE-2 binds to different regions of the cholesterol group of the Hh-N and forms complexes with sheddases which cooperate with heparan sulfate proteoglycans to prune away the lipid groups in the plasma membrane that sequester Hh, freeing Hh for release into the extracellular space (Jakobs et al., 2014) as either monomers or multimeric complexes. Multimeric complexes of Hh ligand are assembled useing heparan sulfate proteoglycans as scaffolds (Goetz et al., 2006); they are more stable and more active than monomeric Hh (Goetz et al., 2006; Chen et al., 2004). In the absence of sheddases, however, multimeric complexes cannot be released to the extracellular space (Jakobs et al., 2016) and are internalized with the help of endosomal sorting complexes required for transport (ESCRT) proteins (Matusek et al., 2014), packaged into intraluminal vesicles, and released as exosomes (Parchure et al., 2015) via a mechanism that also requires Dispatched (D’Angelo et al., 2015). Lastly, Hh can be released with very low-density lipoproteins. This process is not completely understood but seems to depend on glycosylphophatidylinositol-linked glypicans (Panakova et al., 2005). Glypicans can also act as co-receptors for Hh and thus, are important modulators of the Hh signaling pathway, capable of either inhibiting signal transduction by sequestering ligand or perpetuating signaling by stabilizing the binding of Hh to its receptor (Ramsbottom and Pownall, 2016).
The 12-pass transmembrane protein patched (Ptch) is the receptor for Hh ligand. Unlike most membrane spanning receptors, Ptch can block pathway activity. In the absence of Hh ligand, it constitutively inhibits the pathway through a negative effect on the downstream effector smoothened (Smo). The mechanism by which Ptch inhibits Smo is not fully elucidated, but it does not seem to require physical interaction between the two molecules (Taipale et al., 2002). When Hh binds to Ptch, it represses Ptch, blocking its inhibitory effect on Smo, and hence allowing signaling through the pathway (Ramsbottom and Pownall, 2016). After Hh binds to Ptch, the Hh-Ptch complex is internalized and degraded (Denef et al., 2000). Ptch internalization involves a complex mechanism: Hh induces accumulation of Ptch in lipid rafts, enabling Ptch to be ubiquitinated by the E3 ubiquitin ligases Smurf1 and Smurf2, and targeted to lysosomes for degradation (Yue et al., 2014).
There are two isoforms of Ptch in mammalian cells, Ptch-1 and Ptch-2, the former being more widely expressed, and more critical for pathway activity (Carpenter et al., 1998). Three Hh co-receptors positively regulate the pathway, enhancing Hh-Ptch binding: CAM-related down-regulated by oncogened (Cdo), brother of Cdo (Boc) and growth arrest-specific (GAS)-1. To activate the signaling cascade, Hh must bind to each of these co-receptors (Izzi et al., 2011). Conversely, Hh interaction with Ptch is blocked by the soluble co-receptor hedgehog interacting protein (Hip) which binds to Hh and prevents it from coupling to Ptch. Hence, Hh-Hip interaction negatively regulates the signaling pathway (Chuang and McMahon, 1999).
Smo, a 7-pass transmembrane G protein-coupled receptor, is the signal transducer of the Hh pathway, and has been the most frequent therapeutic target for modulating Hh pathway activity. In the absence of Hh, Ptch is on the PC and blocks Smo from entering the PC (Corbit et al., 2005)) and hence, Smo localizes in intracytoplasmic vesicles. When Hh binds to and inactivates Ptc (Rohatgi, Milenkovic, and Scott, 2007), Smo can move onto the PC (Arensdorf, Marada, and Ogden, 2016). The movement of Smo onto the PC is mediated by the kinesin motor protein Kif3A via an interaction that is dependent on beta-arrestins (Kovacs et al., 2008). Accumulation of Smo in the PC requires Smo binding to the anchoring Ellis van Creveld Syndrome (EVC-2) ciliary complex (Dorn, Hughes, and Rohatgi, 2012). Smo must then be phosphorylated by casein kinase-1 (CK1) and G-protein-coupled receptor kinase-2 (GRK-2) to become fully activated (Rohatgi et al., 2009; Chen et al., 2011). The localization of Smo in the PC membrane depends on its activation state: it localizes at the base of the PC when inactive, and at the ciliary tip when active (Milenkovic et al., 2015). Smo activation can be modulated by endogenous lipids such as oxysterols (which activate Smo) and vitamin D3 (which inhibits Smo) (Bijlsma et al., 2006). Interestingly, Ptch-1 induces vitamin D3 secretion, providing another layer of Hh signaling regulation in distant cells (Bijlsma et al., 2006).
Gli proteins are transcription factors that belong to the Kruppel-like family and contain a zinc-finger DNA-binding domain (Hu et al., 2015). Mammals have 3 Gli proteins: Gli-1, Gli-2 and Gli-3. In the absence of Hh ligand, Smo is inactivated, and the transcription factor Gli is prevented from entering the nucleus, bound to a suppressor protein complex, composed of fused kinase (Fu), suppressor of fused (Sufu) and Costal-2 (Cos) (Teperino et al., 2014). Gli can then be sequentially phosphorylated by protein kinase A (PKA), glycogen synthase kinase-3 (GSK3) and CK1. Phosphorylated Gli binds to β-transducin repeat containing protein (βTrCp), and the Gli-βTrCp complex is ubiquitinated by Cul-1-base E3 ligase. Ubiquitinated Gli is targeted to the proteasome, where it can be either processed in a truncated transcription repressor (Gli-R) (Wang and Li, 2006), or it can be fully degraded (Wang, Pan, and Wang, 2010). When Smo is inactive, Gli-2 predominantly undergoes proteasomal degradation and this limits the transcription of Gli-1, a Gli-2 target gene (Ikram et al., 2004). Under these conditions, Gli-3 is efficiently processed into Gli-3-R, re-enforcing suppression of genes that would otherwise be activated by Gli-1 and/or Gli-2 (Pan and Wang, 2007). When Smo is active, Gli-2 predominately acts as transcription enhancer. This induces expression of Gli-1, which generally functions as a transcriptional activator, and Gli-1 accumulates because it does not undergo PKA-dependent proteasomal degradation. Gli-1 is a target of atypical protein kinase Cc/λ (aPKC/λ) which phosphorylates Gli-1 to enhance Gli-1 DNA binding and transcriptional activity (Atwood et al., 2013). Conversely, phosphorylation of Gli-1 by AMP-activated protein kinase (AMPK) promotes Gli-1 degradation and links cellular metabolism with the hedgehog pathway (Di Magno et al., 2016; Li et al., 2015). (Ikram et al., 2004).
Activated Smo can dissociate Gli from Sufu at the tip of the PC (Pak and Segal, 2016). Kif7, which also localizes to the tip of the PC when Smo is active, enhances Gli-Sufu localization in this compartment (He et al., 2014)(He et al., 2014)(He et al., 2014)(He et al., 2014)(He et al., 2014)(He et al., 2014)(He et al., 2014). and full-length Gli dissociates from Sufu, moves back to the cytoplasm, and enters the nucleus, where it promotes the transcription of several genes, including vascular endothelial growth factor (VEGF), angiopoietin-1 and -2 (in endothelial cells); snail, twist-2, FoxF1, α-smooth muscle actin (α-SMA), vimentin, cyclin D, interleukin (IL)-6 (in fibroblasts/myofibroblasts); and nanog, sox-2 and -9 (in stem/progenitor cells) (Merchant and Saqui-Salces, 2014; Hanna and Shevde, 2016).
The canonical Hh pathway is highly regulated. Once the Hh pathway is activated, it initiates mechanisms of self-restraint. For example, 3 Hh inhibitors, Ptch, Hip and Foxa2 are direct transcriptional targets of Gli-2. In addition to the inhibitory actions of Ptch and Hip on pathway activity (see above), Foxa2 directly inhibits Gli-2 transcript (Mavromatakis et al., 2011). Like P-AMPK (which increases during energy depletion), protein kinase A (PKA) and cAMP (sensors of glucose deprivation) also negatively regulate Hh signaling. PKA and GSK3 sequentially phosphorylate Sufu to stabilize Sufu in a complex with Gli-2 and Gli-3. This blocks nuclear localization of the Gli factors and suppresses expression of Gli-target genes (Chen et al., 2011). When Smo is inactive, the orphan G-protein-coupled receptor Gpr161 also localizes in the PC and activates adenylcyclase, increasing local cAMP levels and PKA activity to assure the Hh pathway remains inactive (Mukhopadhyay et al., 2013). When Hh binds to Ptch and Smo enters the PC, Gpr161 leaves the PC, cAMP levels decrease, and reduced PKA activity amplifies Hh pathway activation. The Smo-induced removal of Gpr161 from the PC is dependent of β-arrestin (Pal et al., 2016). Furthermore, ciliary accumulation of Gpr161 is modulated by the lipid composition of the PC membrane, which is kept relatively enriched in Pi(4)P by the action of the cilium-located phosphatase Inpp5e. Inpp5e dephosphorylates Pi(4,5)P2 to generate Pi(4)P and thus Pi(4,5)P2 accumulates when Inpp5e activity is inhibited., Pi(4,5)P2 recruits Tubby-like protein 3 (Tulp3) and IFP to the PC, and these proteins promote trafficking of Gpr161 into the PC, resulting in inhibition of the Hh pathway (Chavez et al., 2015; Garcia-Gonzalo et al., 2015). The Hh pathway can also be regulated by factors that can modulate Gli activity independent of Smo. For example, transforming growth factor (TGF)-β can directly activate Gli-2 (Dennler et al., 2007; Johnson et al., 2011). Osteopontin, a Gli-target gene, also inhibits GSK3β, thus promoting Gli activation (Das, Samant, and Shevde, 2013). K-ras seems to induce, whereas p53 and Notch-target Hes-1 repress, Gli-1 expression in a Smo-independent manner (Nolan-Stevaux et al., 2009; Stecca and Ruiz i Altaba, 2009; Schreck et al., 2010). Finally, Pi3K-AKT signaling enhances, while protein kinase Cδ inhibits, Gli-1 transcriptional activity (Stecca et al., 2007; Cai et al., 2009).
Noncanonical hedgehog pathway
Two types of noncanonical Hh signaling have been described: type 1, which is Ptch-dependent but Smo independent; and type 2, which is Smo-dependent, but Hh-ligand and PC-independent. In type 1 signaling, Ptch-1 can trigger apoptosis through activation of caspase-3 when Hh ligand is removed, and restoring binding of Hh ligand to Ptch inhibits this process (Chinchilla et al., 2010). In the absence of Hh ligands, Patch-1 can also inhibit proliferation by preventing nuclear localization of cyclin D. This anti-proliferative effect of Patch-1 is also inhibited by Hh ligand (Barnes et al., 2001).
In type 2 signaling, Smo’s Gαi activity regulates cellular processes such as metabolism, proliferation, calcium flux and migration (Teperino et al., 2014; Polizio et al., 2011; Belgacem and Borodinsky, 2011; Teperino et al., 2012). For example, Smo can induce a Warburg-like effect promoting a glycolytic metabolism in muscle and adipose tissue through a calcium-AMPK kinase axis (Teperino et al., 2012). Independently of Hh ligands or Ptc, Smo can also stimulate GTPases, which promote cytoskeletal rearrangement and induce migration of fibroblasts and endothelial cells (Polizio et al., 2011; Bijlsma et al., 2007; Razumilava et al., 2014).
PHYSIOLOGY OF THE HEDGEHOG PATHWAY IN THE LIVER
During embryogenesis, the Hh pathway regulates growth, differentiation, patterning, and vascularization in multiple tissues, including liver (Hanna and Shevde, 2016; Deutsch et al., 2001). The actual role of Hh pathway in liver embryogenesis is not completely understood, but Shh is highly expressed in the ventral foregut endoderm which will differentiate into the hepatic bud, and Shh expression fades as the liver bud forms. Later in development there is transient expression of Hh in the hepatoblasts that disappears once hepatoblasts differentiate into hepatocytes (Omenetti et al., 2011).
Thereafter, the pathway gradually shuts down in the liver by adolescence (Omenetti et al., 2011). Hence, Hh ligand is barely expressed and the Hh pathway is relatively dormant in healthy adult liver (Sicklick et al., 2006). However, during hepatic regeneration after partial hepatectomy (Ochoa et al., 2010), acute liver injury (Pratap et al., 2010) or in response to chronic liver injury (Omenetti et al., 2008), the Hh pathway strongly reactivates and acquires a major role in the wound healing response (Michelotti et al., 2013).
NASH is a form of obesity-related chronic liver disease that results when fat accumulated in hepatocytes induces lipotoxicity (Machado and Diehl, 2016), causing tissue injury and related proinflammatory, profibrogenic and procarcinogenic responses. The injured and dying liver cells release signals that elicit a multi-faceted wound-healing response that: i) mobilizes an inflammatory reaction to clear dead and irrepairably-injured cells; ii) stimulates vasculogenesis to optimize blood flow to the damaged parenchyma; iii) activates hepatic stellate cells to remodel the matrix; and iv) induces outgrowth of the progenitors to replace the lipotoxic hepatocytes that died. These various aspects of the response must be coordinated perfectly for wound healing to be effective, and they must ultimately terminate when repair has successfully regenerated healthy hepatic parenchyma. Hence, recovery from NASH requires a dynamic dialogue among various types of cells that fulfill different missions in the wound healing process. The Hh pathway plays an essential role in orchestrating the proper reconstruction of damaged adult liver, just as it assures the appropriate formation of various tissues during embryogenesis. However, when the pathway becomes dysregulated or cannot be shut-down because noxious stimuli are persistent, sustained Hh signaling perpetuates wound healing activities, resulting in chronic inflammation, vasculogenesis, fibrogenesis, and progenitor cell accumulation. Over time, this increases the risk for cirrhosis and primary liver cancer (Machado and Diehl, 2016).
During the past decade, research has revealed how the Hh pathway becomes activated in the liver during NAFLD and shown that this controls the progression of NASH (see below). These insights are particularly provocative given growing evidence that obesity itself may reflect a state of generally sub-normal Hh activity. In several species, for example, globally inhibiting Hh pathway activity promotes obesity, and stimulating Hh signaling inhibits adipogenesis (Fleury et al., 2016). Although more research is needed to clarify tissue-specific mechanisms that control Hh signaling, local availability of Hh ligands appears to be an important variable in the liver.
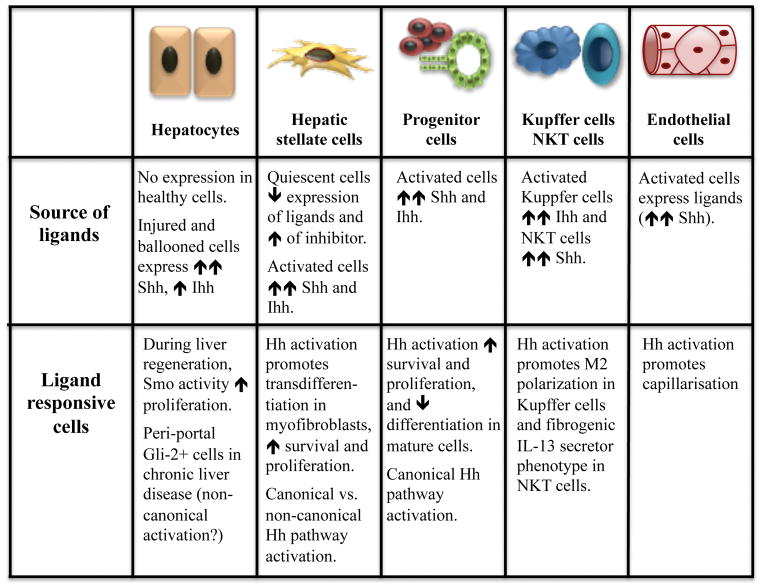
Healthy adult hepatocytes generally do not produce Hh ligands (Sicklick et al., 2006) but hepatic synthesis of Hh ligands increases dramatically in many types of liver injury, including NASH. In NASH, the primary sources of Hh ligands are the injured hepatic epithelial cells, particularly lipotoxic hepatocytes (Rangwala et al., 2011; Guy et al., 2012) and reactive cholangiocytes (Natarajan et al., 2014; Natarajan et al., 2017). In hepatocytes, fatty acid toxicity provokes ER and apoptotic-stress, resulting in disruption of the cytoskeleton and swelling (a.k.a., ballooning). Ballooned hepatocytes are the hallmark of hepatocyte injury in NASH and immuno-stained human NASH livers readily demonstrate Hh ligand accumulation in such cells. Further, the Hh-positive hepatocytes in human NASH are typically surrounded by nonparenchymal cells with Gli2-positive nuclei, suggesting that the injured liver epithelial cells are activating Hh signaling in the adjacent stroma (Guy et al., 2012). In vitro models proved that expression of Sonic and Indian Hh mRNAs and proteins are induced in ballooned hepatocytes and showed that these cells release biologically active Hh ligands that are able to trigger Hh signaling in Hh-target cells (Rangwala et al., 2011; Kakisaka et al., 2012; Machado et al., 2014). Further, treatments that improve liver injury and hepatocyte ballooning in humans with NASH also reduce hepatic accumulation of both Hh-positive hepatocytes and Gli2-positive stromal cells (Guy et al., 2014). Other types of cells that accumulate in injured livers during hepatic wound healing are also capable of producing Hh ligands, such as immune cells (e.g., NKT cells (Syn et al., 2012) and macrophages (Pereira et al., 2013)), activated progenitor cells (Jung et al., 2007), activated hepatic stellate cells/myofibroblasts (Sicklick et al., 2005; Yang et al., 2008; Choi et al., 2009) and activated liver sinusoidal endothelial cells (Xie et al., 2013). Hence, local production of Hh ligands by injured liver epithelial cells, as well as by cells that are mobilized to repair liver injury, is able to initiate Hh signaling in neighboring cells, even in obese individuals who may maintain low Hh pathway activity in other tissues. Conversely, eliminating lipotoxic stress in hepatocytes of such subjects removes Hh-generating hepatocytes. This abolishes a key stimulus for Hh pathway activation in Hh-responsive cells, and rapidly permits hepatic Hh signaling to subside to its typically low level of basal activity. Decreasing/inactivating Hh-producing inflammatory cells, such as NKT cells and macrophages, would further help inhibit disease progression (Kwon et al., 2016).
The cellular targets of Hh ligands in the liver are somewhat uncertain because it appears that the Hh-responsiveness of liver-resident cells is influenced by various factors, including circadian periodicity, age, and health (Borjigin et al., 1999; Duesterdieck-Zellmer et al., 2015). Mature hepatocytes in healthy adult livers are believed to be Hh unresponsive (Sicklick et al., 2006) because they do not have a PC (Wheatley, Wang, and Strugnell, 1996).. However, some peri-portal hepatocytes exhibit nuclear Gli-2 in fatty livers, consistent with induction of Hh signaling (Fleig et al., 2007; Syn et al., 2009; Swiderska-Syn et al., 2013). It is possible that Gli-2 expression in those hepatocytes might have resulted from non-canonical (i.e., Smo- independent) activation of Gli-2 (Grzelak et al., 2014; Grzelak et al., 2017) by factors other than Hh ligands, such as TGF-β (Dennler et al., 2007; Johnson et al., 2011). Alternatively, the Gli-2-positive hepatocytes might be the immature progeny of Hh-responsive progenitor cells that still retain PC that permit ligand-depended canonical activation of the Hh pathway (Ochoa et al., 2010). Finally, perhaps hepatocytes can acquire a PC when injured, similar to endothelial cells which do not express PC in the resting state but acquire a PC when exposed to increased hydrostatic pressure (Ke and Yang, 2014).
In any case, Smoothened is definitely activated in some hepatocytes following liver injury because cyclopamine, a direct Smoothened antagonist, inhibits proliferative activity in hepatocytes harvested from regenerating livers after partial hepatectomy (Ochoa et al., 2010). Further, Gli-expressing hepatocytes have been shown to release other Hh-inducible factors, such as osteopontin, that promote inflammation and fibrogenesis (Kwon et al., 2016). Hepatic progenitor cells strongly react to canonical Hh pathway activation with increased proliferation and viability but decreased hepatocytic differentiation (Sicklick et al., 2006; Fleig et al., 2007; Syn et al., 2009; Grzelak et al., 2017; Jung et al., 2010). Hh-responsive progenitor cells also release many factors that can promote the wound-healing response, such as platelet-derived growth factor (PDGF) and osteopontin which activate hepatic stellate cells (Omenetti et al., 2008; Syn et al., 2011), and proinflammatory chemokines (for example, CXCL16 and osteopontin) (Omenetti et al., 2009) which recruit immune cells to the liver. Also, hepatic stellate cells are strongly activated by Hh ligands, becoming more proliferative, myofibroblastic, and fibrogenic (Yang et al., 2008; Choi et al., 2009). Sinusoidal endothelial cells respond to Hh with loss of fenestration and a vasoconstriction-inducing phenotype, which promotes capillarization and vascular remodeling (Xie et al., 2013). Lastly, immune cells (e.g., NKT cells and macrophages) respond to Hh by becoming more pro-fibrogenic and promoting immune tolerance (Pereira et al., 2013; Syn et al., 2009).
In conclusion, NASH (like other forms of chronic liver disease) evokes a wound healing response that is coordinated by crosstalk among different types of cells that are involved in tissue repair. The Hh pathway orchestrates the orderly activation all of these cells and regulates their functions so that healthy hepatic parenchyma is regenerated. Hedgehog producing cells, such as dying hepatocytes and inflammatory macrophages, activate and increase the pool of hedgehog responsive cells, including hepatic progenitor cells and hepatic stellate cells. This suggests that defective repair (i.e., cirrhosis and liver cancer) result from dysregulated Hh signaling and positions the Hh pathway as a major therapeutic target in NAFLD.
HEDGEHOG PATHWAY IN NONALCOHOLIC FATTY LIVER DISEASE
Although sustained and excessive Hh pathway activity plays a pivotal role in driving the progression of NAFLD to fibrosing NASH, liver cirrhosis and hepatocellular carcinoma (see below), transient Hh pathway activation is necessary to recover from NASH, and some basal threshold of Hh activity may be necessary to prevent hepatic steatosis. Viewed from this perspective, Hh signaling might be necessary to prevent the onset of NAFLD and thus, Hh pathway activation could be a compensatory response to ectopic fat accumulation in the liver.
Matz-Soja et al. showed that conditional liver-specific deletion of Smo in adult mice downregulated the Hh pathway and induced liver steatosis. The steatogenic effect of Gli suppression resulted from a skewing of lipid metabolism towards lipogenesis through the induction of lipogenic factors such as sterol regulatory element-binding protein (SREBP)-1c and adiponutrin/patatin-like phospholipase domain-containing protein-3 (PNPLA-3). Furthermore, activation of the Hh pathway (through SUFU knockdown, small molecule Shh agonists or Gli overexpression) reversed the steatogenic phenotype in hepatocytes derived from genetic models of obesity-related NAFLD, i.e., ob/ob mice and melanocortin-4 knockout mice (Matz-Soja et al., 2016).
Once hepatocyte lipotoxicity develops, Hh signaling is increased and evidence that failure to constrain Hh pathway activity appropriately drives progression of fibrosing NASH is stricking. Briefly, the Hh pathway has been studied in different animal models of NASH, including genetically obese leptin-deficient ob/ob mice and wild type mice with NASH induced by feeding either diets deficient in methionine and choline or diets enriched with various fats (Fleig et al., 2007; Syn et al., 2009; Hirsova et al., 2013; Machado et al., 2015; Wang et al., 2016). In all models, hepatic Hh pathway activation has been observed consistently. Similarly, in human NASH the Hh pathway is activated and the level of pathway activity correlates with the severity of liver damage (as assessed by numbers of ballooned hepatocytes, levels of portal inflammation, markers of liver repair (aka accumulation of progenitor cells and activated myofibroblasts), and fibrosis severity) (Guy et al., 2012; Grzelak et al., 2017; Syn et al., 2009; Machado et al., 2015).
As mentioned earlier, the main source of Shh is the injured ballooned hepatocyte in human NASH (Guy et al., 2012). The number of Shh-expressing hepatocytes correlates with fibrosis severity, and the severity of the ductular reaction (Guy et al., 2012; Machado et al., 2015) which is known to associate with fibrogenesis and carcinogenesis (Richardson et al., 2007; Ye et al., 2014). Once the Hh pathway is activated, it can induce a strong self-perpetuating response. Indeed, Chung et al., evaluated a transgenic mouse model that initially achieved Shh expression in only 2–5% of hepatocytes, and observed advanced liver fibrosis after 6 months and hepatocarcinogenesis after one year (Chung et al., 2015). At these late time points, Gli-2-positive (Hedgehog responsive) cells included not only ductular progenitor cells, hepatic stellate cells, and immune cells but also 30–50% of the hepatocytes (Chung et al., 2015). Genetically modified mice with Ptch haploinsufficiency also have enhanced Hh pathway activation. Such mice develop worse liver disease and fibrosis in different models of NASH (Syn et al., 2009; Syn et al., 2011; Syn et al., 2010). Conversely, mice with conditional deletion of Smo exhibit inhibited Hh signaling and are protected from liver disease in different models of NASH (Michelotti et al., 2013; Kwon et al., 2016).
Excessive Hh pathway activity has also been implicated in liver carcinogenesis. In different animal models of hepatocellular carcinoma, the Hh pathway is activated in pre-cancerous tissues and cancerous tumors (Cai et al., 2016). In precursor cancerous cells, Shh promotes proliferation through induction of cyclin B1 and CDK1 mitotic proteins (Cai et al., 2016), while preventing apoptosis (Zhang et al., 2014; Wang et al., 2013). Hh also induces an epithelial to mesenchymal transition in malignant cells, favoring migration and invasiveness (Chen et al., 2014; Byrne et al., 2015). Finally, cancer-associated stromal cells release Hh ligands that promote malignant cell proliferation, angiogenesis and metastasis (Chan et al., 2014; Liu et al., 2016; Li et al., 2016). In human hepatocellular carcinoma, excessive Hh signaling occurs relatively frequently (Sicklick et al., 2006; Cheng et al., 2009; Al-Bahrani et al., 2015; Dugum et al., 2016), and the level of Hh pathway activity positively correlates with tumor size, invasion, chemoresistance, metastasis and mortality (Sicklick et al., 2006; Cheng et al., 2009; Chen et al., 2011; Lu et al., 2012; Che et al., 2012). Finally, adenomas that associate with obesity and hepatic steatosis also tend to have an overly active Hh pathway (Nault et al., 2017), and adenoma transformation is one possible mechanism for carcinogenesis in non-cirrhotic NAFLD (Michelotti, Machado, and Diehl, 2013).
In summary, while the role of the Hh pathway in the initial stages of NAFLD (i.e. isolated steatosis) is not fully understood, it is consistently activated in NASH and the best-studied pathway for regulating NASH-related fibrosis progression and hepatocarcinogenesis. (Machado and Diehl, 2016). As such, it is an attractive therapeutic target for the management of patients with progressive NAFLD.
HEDGEHOG PATHWAY AS A THERAPEUTIC TARGET IN NONALCOHOLIC FATTY LIVER DISEASE
In humans, improvement in NASH histological activity correlates with a decrease in the activation of the Hh pathway, suggesting a therapeutic role of targeting Hh in NASH. A major clinical trial in NASH, the PIVENS trial (Pioglitazone, Vitamin E for Non-alcoholic Steatohepatitis), showed histological improvement of NASH in patients who responded to treatment. A post-hoc analysis of PIVENS evaluated pre and post-treatment biopsies from 59 patients treated with either vitamin E or placebo, and showed that biochemical and histological response, including a decrease in liver fibrosis, strongly correlated with loss of Shh expressing hepatocytes (Guy et al., 2014).
The Hh pathway can be pharmacologically targeted through inhibition of Hh, inhibition of Smo, or inhibition of Gli transcriptional activity (Figure 2). When considering therapeutic targeting of the Hh pathway, it is important to recall that some tissues require Hh signaling to avoid toxicity, such as gonads, gastrointestinal tract, and bone marrow (Pak and Segal, 2016).
Figure 2.
Pharmacological targeting of the hedgehog pathway.
In preclinical studies, a monoclonal antibody against Hh (5E1) was able to inhibit activation and proliferation of hepatic stellate cells (Sicklick et al., 2005), as well as the Shh-dependent paracrine effects of hepatic stellate cells on the proliferation of progenitor cells (Michelotti et al., 2013) and on the metabolic reprogramming and proliferation of malignant hepatoma cells (Chan et al., 2012). Shh can also be inhibited by robotnikinin, a small molecule that binds Shh, preventing it from engaging Ptch-1 and hence, inhibiting activation of the Hh pathway (Stanton et al., 2009). Although robotnikinin was discovered in a small-molecule microarray-based screen in 2009, there is lack of preclinical or clinical experience with robotnikinin in liver cells.
Inhibition of Smo is the best-studied approach to target the Hh pathway and this strategy has moved to clinical applications. Cyclopamine was the first available Smo antagonist. It was first identified in the late 1960s, from the observation of several birth defects (for example craniofacial deformities including cyclopia) in the offspring of lambs that ingested corn lilies (Veratrum californicum) in the Western US (Lee et al., 2014). From those lilies, investigators isolated the steroidal alkaloid cyclopamine. Dr. Philip Beachy et al., showed that cyclopamine inhibited the Hh pathway by directly binding to, and inhibiting, Smo (Cooper et al., 1998; Incardona et al., 1998). Chemically, cyclopamine has some characteristics that limit its clinical utility; for example it has low water solubility, and it is not stable in acid conditions (such as the stomach pH) (Pak and Segal, 2016). A semisynthetic derivative, 3-keto-N-(aminoethyl-amicaproyl-dehydrocinnamoyl) cyclopamine-2 (KAAD-cyclopamine) has increased solubility and activity, being 10–20 fold more potent than cyclopamine (Taipale et al., 2000). Syn et al. studied the effect of cyclopamine in the methionine-choline deficient diet supplemented with ethionine mouse NASH model, and showed decreased expression of mesenchymal and fibrogenic genes, suggesting a potential role in the prevention of NASH-related fibrogenesis (Syn et al., 2009). Furthermore, in different mouse models of hepatocellular carcinoma, treatment with cyclopamine decreased tumor size (Jeng et al., 2012) and increased radiosensitivity (Tsai et al., 2015). A synthetic cyclopamine analogue, saridegib or IPI-926, achieved improved stability, kinetic profile and potency as compared to cyclopamine (Peluso et al., 2014). Phase I and II clinical trials of saridegib in basal cell carcinoma showed potent anti-tumoral effect, but saridegib showed no effect or deleterious effects in patients with myelofibrosis, chondrosarcoma or pancreatic cancer (Jimeno et al., 2013; Ko et al., 2016; Sasaki et al., 2015).
Vismodegib (also called GDC-0449) is a potent Smo antagonist that was identified by high-throughput in vitro screening (Sandhiya et al., 2013) by Curis Genentech. The US Food and Drug Administration (FDA) approved it for the treatment of advanced basal-cell carcinoma in January 2012 (Guha, 2012). Vismodegib is well-absorbed orally, but has several drug interactions: P-glycoprotein inhibitors such as macrolides increase vismodegib adverse effects and inhibitors of gastric acid secretion decrease vismodegib bioavailability. Vismodegib has shown anti-fibrogenic and anti-inflammatory effects in different mouse models of NASH (Kwon et al., 2016; Hirsova et al., 2013). Furthermore, vismodegib was also strongly anti-fibrotic in the Mdr2 deficient mouse model (Philips et al., 2011). This is relevant for NASH because Mdr2 deficient mice have altered bile acid homeostasis and bile acids are known to have an important role in NASH pathogenesis (Arab et al., 2017). Vismodegib also showed anti-carcinogenic effects in different animal models of hepatocellular carcinoma, decreasing tumor size, invasion, angiogenesis and metastatic disease (Philips et al., 2011; Jeng et al., 2015; Pinter et al., 2013). Vismodegib has been studied in clinical trials for basal cell carcinoma, medulloblastoma, gastrointestinal tract cancers, chondrosarcoma, pancreatic, lung and hematological cancer. The best effects were noted in basal cell carcinoma, with phase IV studies showing good response rates, although with frequent acquired resistance due to de novo mutations (Rimkus et al., 2016). There are several other small molecules that inhibit Smo, under study, including sonidegib (also called erismodegib or LDE225), glasdegib (PF-04449913) and taladegib (LY2940680). Sonidegib also has FDA-approval for basal cell carcinoma and is currently being tested on patients with advanced hepatocellular carcinoma (NCT02151864). Furthermore, sonidegib has demonstrated anti-fibrotic and anti-inflammatory actions in a mouse model of NASH (Kwon et al., 2016). In addition, itraconazole, an old, commonly used and inexpensive anti-fungal drug, has anti-Smo activity mediated via direct binding to Smo (Kim et al., 2010). It is currently being studied in clinical trials for different solid tumors and has shown anti-fibrotic effects in animal models of lung fibrosis (Naranjo et al., 2010; Naranjo et al., 2011). No studies have been done yet in other fibrotic diseases.
Finally, GANTs are Gli antagonists that act in the final step of the Hh pathway (Lauth et al., 2007). GANT-58 and GANT-61 both inhibit Gli-mediated gene activation, however the latter is more specific for Gli and more potent in the inhibition of Gli-mediated transcriptional activity (Rimkus et al., 2016). GANT-61 can induce autophagy, apoptosis and cytotoxicity in hepatocellular cells in vitro and can inhibit hepatocellular tumor formation and growth in SCID mice (Wang et al., 2013). GANTs have not been evaluated in clinical trials (Rimkus et al., 2016).
CONCLUSIONS
NAFLD has become pandemic, with an increasing prevalence paralleling the increase in obesity and related metabolic disturbances. Currently, we do not have effective treatment for NAFLD, although we now know that patients with NAFLD-related liver fibrosis are at risk of progression to end-stage liver disease and pre-mature death. Both NAFLD and the Hh pathway were first described in 1980. This coincidence is intriguing since the Hh pathway is both heavily lipid-regulated and the best-studied driver pathway for fibrogenesis in NAFLD. Preclinical data consistently support a causal role for excessive Hh pathway activation in NAFLD progression to cirrhosis. Overly active Hh signaling is also involved in the pathogenesis of primary liver cancer. Those preclinical data are supported by association studies in human NAFLD. As such, the Hh pathway is an obvious therapeutic target to prevent bad liver outcomes in NAFLD. Hh signaling can be blocked pharmacologically at different steps of the pathway. Many drugs are under clinical investigation, and some of them are already approved to treat different malignant diseases. Preclinical studies also suggest that inhibiting the Hh pathway with these agents improves fibrosing NASH and its complications, namely hepatocellular carcinoma. To date, however, there have been no clinical trials of Hh inhibitors in NAFLD. This likely reflects the suspicion that the window for therapeutic efficacy may be narrow since the Hh pathway is highly complex, tightly regulated and critical for controlling growth responses that are necessary to maintain tissue homeostasis, as well as to repair tissue damage. Thus, it remains to be determined if long-term inhibition of the pathway is possible and safe. Further research is necessary to address this issue because there is a desperate need for therapies that can safely and effectively prevent/treat NASH-related cirrhosis and liver cancer.
Abbreviations
- AMPK
AMP-activated protein kinase
- aPKC/λ
atypical protein kinase-Cc/λ
- BBS
Bardet-Biedl syndrome proteins
- Boc
brother of Cdo
- Cdo
CAM-related down-regulated by oncogened
- CK-1
casein kinase-1
- Dhh
Desert hedgehog
- Dis
dispateched
- ER
endoplasmic reticulum
- ESCRT
endosomal sorting complexes required for transport
- GAS
growth arrest-specific
- GRK-2
G-protein-coupled receptor kinase-2
- GSK3
glycogen synthase kinase-3
- Hh
hedgehog
- Hip
hedgehog interacting protein
- Ihh
Indian hedgehog
- IL
interleukin
- ITF
intraflagellar transport proteins
- MBOAT
membrane-bound O-acyltransferase
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PC
primary cilium
- PDGF
platelet derived groth factor
- PKA
protein kinase A
- Ptch
patched
- Shh
Sonic hedgehog
- SKI
skinny hedgehog
- SMA
smooth muscle actin
- Smo
smoothened
- Sufu
suppressor of fused
- TGF
transforming growth factor
- TrCp
transducin repeat containing protein
- VEGF
vascular endothelial growth factor
References
- 1.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 4.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 6.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 7.Machado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World journal of gastroenterology: WJG. 2014;20:12956–80. doi: 10.3748/wjg.v20.i36.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97 e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 10.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: Systematic Review and Meta-analysis. Hepatology. 2017;65:1557–65. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 12.Guha M. Hedgehog inhibitor gets landmark skin cancer approval, but questions remain for wider potential. Nature reviews. Drug discovery. 2012;11:257–8. doi: 10.1038/nrd3714. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–99. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 15.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–30. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 16.Merchant JL, Saqui-Salces M. Inhibition of Hedgehog signaling in the gastrointestinal tract: targeting the cancer microenvironment. Cancer treatment reviews. 2014;40:12–21. doi: 10.1016/j.ctrv.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arensdorf AM, Marada S, Ogden SK. Smoothened Regulation: A Tale of Two Signals. Trends Pharmacol Sci. 2016;37:62–72. doi: 10.1016/j.tips.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S. Cilia and Hedgehog: when and how was their marriage solemnized? Differentiation; research in biological diversity. 2012;83:S43–8. doi: 10.1016/j.diff.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Goetz SC, Ocbina PJ, Anderson KV. The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol. 2009;94:199–222. doi: 10.1016/S0091-679X(08)94010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsbottom SA, Pownall ME. Regulation of Hedgehog Signalling Inside and Outside the Cell. J Dev Biol. 2016;4:23. doi: 10.3390/jdb4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–11. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 22.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nature reviews. Molecular cell biology. 2013;14:416–29. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Tukachinsky H, Huang CH, Jao C, Chu YR, Tang HY, Mueller B, Schulman S, Rapoport TA, Salic A. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. The Journal of cell biology. 2011;192:825–38. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–9. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 25.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A. Identification of a palmitic acid-modified form of human Sonic hedgehog. The Journal of biological chemistry. 1998;273:14037–45. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 26.Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–15. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- 27.Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2:308–20. doi: 10.1016/j.celrep.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobs P, Exner S, Schurmann S, Pickhinke U, Bandari S, Ortmann C, Kupich S, Schulz P, Hansen U, Seidler DG, Grobe K. Scube2 enhances proteolytic Shh processing from the surface of Shh-producing cells. J Cell Sci. 2014;127:1726–37. doi: 10.1242/jcs.137695. [DOI] [PubMed] [Google Scholar]
- 29.Goetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ. A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. The Journal of biological chemistry. 2006;281:4087–93. doi: 10.1074/jbc.M511427200. [DOI] [PubMed] [Google Scholar]
- 30.Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes & development. 2004;18:641–59. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakobs P, Schulz P, Ortmann C, Schurmann S, Exner S, Rebollido-Rios R, Dreier R, Seidler DG, Grobe K. Bridging the gap: heparan sulfate and Scube2 assemble Sonic hedgehog release complexes at the surface of producing cells. Scientific reports. 2016;6:26435. doi: 10.1038/srep26435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matusek T, Wendler F, Poles S, Pizette S, D’Angelo G, Furthauer M, Therond PP. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516:99–103. doi: 10.1038/nature13847. [DOI] [PubMed] [Google Scholar]
- 33.Parchure A, Vyas N, Ferguson C, Parton RG, Mayor S. Oligomerization and endocytosis of Hedgehog is necessary for its efficient exovesicular secretion. Mol Biol Cell. 2015;26:4700–17. doi: 10.1091/mbc.E15-09-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Angelo G, Matusek T, Pizette S, Therond PP. Endocytosis of Hedgehog through dispatched regulates long-range signaling. Dev Cell. 2015;32:290–303. doi: 10.1016/j.devcel.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 36.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–7. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 37.Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–31. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 38.Yue S, Tang LY, Tang Y, Tang Y, Shen QH, Ding J, Chen Y, Zhang Z, Yu TT, Zhang YE, Cheng SY. Requirement of Smurf-mediated endocytosis of Patched1 in sonic hedgehog signal reception. Elife. 2014:3. doi: 10.7554/eLife.02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13630–4. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–21. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 42.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 43.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–81. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell. 2012;23:823–35. doi: 10.1016/j.devcel.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3196–201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, Jiang J. Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol. 2011;9:e1001083. doi: 10.1371/journal.pbio.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milenkovic L, Weiss LE, Yoon J, Roth TL, Su YS, Sahl SJ, Scott MP, Moerner WE. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8320–5. doi: 10.1073/pnas.1510094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu L, Lin X, Lu H, Chen B, Bai Y. An overview of hedgehog signaling in fibrosis. Molecular pharmacology. 2015;87:174–82. doi: 10.1124/mol.114.095141. [DOI] [PubMed] [Google Scholar]
- 51.Teperino R, Aberger F, Esterbauer H, Riobo N, Pospisilik JA. Canonical and non-canonical Hedgehog signalling and the control of metabolism. Seminars in cell & developmental biology. 2014;33:81–92. doi: 10.1016/j.semcdb.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:33–8. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137:2001–9. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. The Journal of investigative dermatology. 2004;122:1503–9. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 55.Pan Y, Wang B. A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome. The Journal of biological chemistry. 2007;282:10846–52. doi: 10.1074/jbc.M608599200. [DOI] [PubMed] [Google Scholar]
- 56.Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494:484–8. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Magno L, Basile A, Coni S, Manni S, Sdruscia G, D’Amico D, Antonucci L, Infante P, De Smaele E, Cucchi D, Ferretti E, Di Marcotullio L, Screpanti I, Canettieri G. The energy sensor AMPK regulates Hedgehog signaling in human cells through a unique Gli1 metabolic checkpoint. Oncotarget. 2016;7:9538–49. doi: 10.18632/oncotarget.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li YH, Luo J, Mosley YY, Hedrick VE, Paul LN, Chang J, Zhang G, Wang YK, Banko MR, Brunet A, Kuang S, Wu JL, Chang CJ, Scott MP, Yang JY. AMP-Activated Protein Kinase Directly Phosphorylates and Destabilizes Hedgehog Pathway Transcription Factor GLI1 in Medulloblastoma. Cell Rep. 2015;12:599–609. doi: 10.1016/j.celrep.2015.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pak E, Segal RA. Hedgehog Signal Transduction: Key Players, Oncogenic Drivers, and Cancer Therapy. Dev Cell. 2016;38:333–44. doi: 10.1016/j.devcel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He M, Subramanian R, Bangs F, Omelchenko T, Liem KF, Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16:663–72. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanna A, Shevde LA. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Cancer. 2016;15:24. doi: 10.1186/s12943-016-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mavromatakis YE, Lin W, Metzakopian E, Ferri AL, Yan CH, Sasaki H, Whisett J, Ang SL. Foxa1 and Foxa2 positively and negatively regulate Shh signalling to specify ventral midbrain progenitor identity. Mechanisms of development. 2011;128:90–103. doi: 10.1016/j.mod.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Yue S, Xie L, Pu XH, Jin T, Cheng SY. Dual Phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. The Journal of biological chemistry. 2011;286:13502–11. doi: 10.1074/jbc.M110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–23. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 65.Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S. Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. The Journal of cell biology. 2016;212:861–75. doi: 10.1083/jcb.201506132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann SN. Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev Cell. 2015;34:338–50. doi: 10.1016/j.devcel.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G, 3rd, Abedin M, Schurmans S, Inoue T, Reiter JF. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev Cell. 2015;34:400–9. doi: 10.1016/j.devcel.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer research. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 69.Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, Oyajobi BO, Matrisian LM, Mundy GR, Sterling JA. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer research. 2011;71:822–31. doi: 10.1158/0008-5472.CAN-10-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das S, Samant RS, Shevde LA. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to Smoothened-targeting Hedgehog inhibition. The Journal of biological chemistry. 2013;288:11824–33. doi: 10.1074/jbc.M112.432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes & development. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. The EMBO journal. 2009;28:663–76. doi: 10.1038/emboj.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:6060–70. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Q, Li J, Gao T, Xie J, Evers BM. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. The Journal of biological chemistry. 2009;284:2150–8. doi: 10.1074/jbc.M803235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell cycle. 2010;9:570–79. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 77.Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. The EMBO journal. 2001;20:2214–23. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. The Journal of biological chemistry. 2011;286:19589–96. doi: 10.1074/jbc.M110.197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4482–7. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, Dalgaard K, Selvaraj M, Gaster M, Lee-Young RS, Febbraio MA, Knauf C, Cani PD, Aberger F, Penninger JM, Pospisilik JA, Esterbauer H. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–26. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 81.Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA. Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites. Cellular signalling. 2007;19:2596–604. doi: 10.1016/j.cellsig.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Razumilava N, Gradilone SA, Smoot RL, Mertens JC, Bronk SF, Sirica AE, Gores GJ. Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma. Journal of hepatology. 2014;60:599–605. doi: 10.1016/j.jhep.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 84.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, Choi SS, Alpini G, Schwarz KB, Diehl AM, Whitington PF. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–58. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, Reid LM, Diehl AM. Hedgehog signaling maintains resident hepatic progenitors throughout life. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G859–70. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 86.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–23. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pratap A, Panakanti R, Yang N, Eason JD, Mahato RI. Inhibition of endogenous hedgehog signaling protects against acute liver injury after ischemia reperfusion. Pharmaceutical research. 2010;27:2492–504. doi: 10.1007/s11095-010-0246-z. [DOI] [PubMed] [Google Scholar]
- 88.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–82. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 89.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, Premont R, Yang L, Syn WK, Metzger D, Diehl AM. Smoothened is a master regulator of adult liver repair. The Journal of clinical investigation. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fleury A, Hoch L, Martinez MC, Faure H, Taddei M, Petricci E, Manetti F, Girard N, Mann A, Jacques C, Larghero J, Ruat M, Andriantsitohaina R, Le Lay S. Hedgehog associated to microparticles inhibits adipocyte differentiation via a non-canonical pathway. Scientific reports. 2016;6:23479. doi: 10.1038/srep23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM. Increased production of sonic hedgehog by ballooned hepatocytes. The Journal of pathology. 2011;224:401–10. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, Diehl AM, Nash CRN. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Natarajan SK, Ingham SA, Mohr AM, Wehrkamp CJ, Ray A, Roy S, Cazanave SC, Phillippi MA, Mott JL. Saturated free fatty acids induce cholangiocyte lipoapoptosis. Hepatology. 2014;60:1942–56. doi: 10.1002/hep.27175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Natarajan SK, Stringham BA, Mohr AM, Wehrkamp CJ, Lu S, Phillippi MA, Harrison-Findik D, Mott JL. FoxO3 increases miR-34a to cause palmitate-induced cholangiocyte lipoapoptosis. J Lipid Res. 2017;58:866–75. doi: 10.1194/jlr.M071357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, Gores GJ. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. Journal of hepatology. 2012;57:844–51. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Machado MV, Michelotti GA, Pereira TA, Boursier J, Kruger L, Swiderska-Syn M, Karaca G, Xie G, Bohnic B, Lindblom KR, Johnson E, Kornbluth S, Diehl AM. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut. 2015;64:1148–57. doi: 10.1136/gutjnl-2014-307362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guy CD, Suzuki A, Abdelmalek MF, Burchette JL, Diehl AM for the NC. Treatment response in the PIVENS trial is associated with decreased hedgehog pathway activity. Hepatology. 2014 doi: 10.1002/hep.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, Philips G, Chan IS, Karaca GF, de Pereira TA, Chen Y, Mi Z, Kuo PC, Choi SS, Guy CD, Abdelmalek MF, Diehl AM. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. 2012;61:1323–9. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira TA, Xie G, Choi SS, Syn WK, Voieta I, Lu J, Chan IS, Swiderska M, Amaral KB, Antunes CM, Secor WE, Witek RP, Lambertucci JR, Pereira FL, Diehl AM. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver international: official journal of the International Association for the Study of the Liver. 2013;33:149–61. doi: 10.1111/liv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–6. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 102.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS, Rojkind M, Diehl AM. Role for hedgehog signaling in hepatic stellate cell activation and viability. Laboratory investigation; a journal of technical methods and pathology. 2005;85:1368–80. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 103.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. Journal of hepatology. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA, Rojkind M, Diehl AM. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. American journal of physiology Gastrointestinal and liver physiology. 2009;297:G1093–106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, Chen Y, Diehl AM. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2013;62:299–309. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borjigin J, Deng J, Wang MM, Li X, Blackshaw S, Snyder SH. Circadian rhythm of patched1 transcription in the pineal regulated by adrenergic stimulation and cAMP. The Journal of biological chemistry. 1999;274:35012–5. doi: 10.1074/jbc.274.49.35012. [DOI] [PubMed] [Google Scholar]
- 107.Duesterdieck-Zellmer K, Semevolos S, Kinsley M, Riddick T. Age-related differential gene and protein expression in postnatal cartilage canal and osteochondral junction chondrocytes. Gene Expr Patterns. 2015;17:1–10. doi: 10.1016/j.gep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell biology international. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 109.Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, Huang J, Sicklick JK, Diehl AM. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Laboratory investigation; a journal of technical methods and pathology. 2007;87:1227–39. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 110.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, Teaberry V, Choi SS, Conde-Vancells J, Karaca GF, Diehl AM. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–88 e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, Diehl AM. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology. 2013;57:1814–25. doi: 10.1002/hep.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JE, Watkins DN, Warner FJ, Shackel NA, McCaughan GW. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. Journal of hepatology. 2014;60:143–51. doi: 10.1016/j.jhep.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 113.Grzelak CA, Sigglekow ND, Tirnitz-Parker JE, Hamson EJ, Warren A, Maneck B, Chen J, Patkunanathan B, Boland J, Cheng R, Shackel NA, Seth D, Bowen DG, Martelotto LG, Watkins DN, McCaughan GW. Widespread GLI expression but limited canonical hedgehog signaling restricted to the ductular reaction in human chronic liver disease. PloS one. 2017;12:e0171480. doi: 10.1371/journal.pone.0171480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ke YN, Yang WX. Primary cilium: an elaborate structure that blocks cell division? Gene. 2014 doi: 10.1016/j.gene.2014.06.050. [DOI] [PubMed] [Google Scholar]
- 115.Kwon H, Song K, Han C, Chen W, Wang Y, Dash S, Lim K, Wu T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease (NAFLD) Hepatology. 2016;63:1155–69. doi: 10.1002/hep.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–15. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–27. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Syn WK, Witek RP, Curbishley SM, Jung Y, Choi SS, Enrich B, Omenetti A, Agboola KM, Fearing CM, Tilg H, Adams DH, Diehl AM. Role for hedgehog pathway in regulating growth and function of invariant NKT cells. European journal of immunology. 2009;39:1879–92. doi: 10.1002/eji.200838890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matz-Soja M, Rennert C, Schonefeld K, Aleithe S, Boettger J, Schmidt-Heck W, Weiss TS, Hovhannisyan A, Zellmer S, Kloting N, Schulz A, Kratzsch J, Guthke R, Gebhardt R. Hedgehog signaling is a potent regulator of liver lipid metabolism and reveals a GLI-code associated with steatosis. Elife. 2016:5. doi: 10.7554/eLife.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PloS one. 2013;8:e70599. doi: 10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Machado MV, Michelotti GA, Xie G, Almeida Pereira T, Boursier J, Bohnic B, Guy CD, Diehl AM. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PloS one. 2015;10:e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF, Tabas I. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016;24:848–62. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, Diehl AM. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. Journal of hepatology. 2015;63:962–70. doi: 10.1016/j.jhep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 126.Ye F, Jing YY, Guo SW, Yu GF, Fan QM, Qu FF, Gao L, Yang Y, Wu D, Meng Y, Yu FH, Wei LX. Proliferative ductular reactions correlate with hepatic progenitor cell and predict recurrence in HCC patients after curative resection. Cell & bioscience. 2014;4:50. doi: 10.1186/2045-3701-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chung SI, Moon H, Ju HL, Cho KJ, Kim DY, Han KH, Eun JW, Nam SW, Ribback S, Dombrowski F, Calvisi DF, Ro SW. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. Journal of hepatology. 2015;64:618–27. doi: 10.1016/j.jhep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai H, Li H, Li J, Li X, Li Y, Shi Y, Wang D. Sonic hedgehog signaling pathway mediates development of hepatocellular carcinoma. Tumour Biol. 2016 doi: 10.1007/s13277-016-5463-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 130.Zhang DW, Li HY, Lau WY, Cao LQ, Li Y, Jiang XF, Yang XW, Xue P. Gli2 silencing enhances TRAIL-induced apoptosis and reduces tumor growth in human hepatoma cells in vivo. Cancer biology & therapy. 2014;15:1667–76. doi: 10.4161/15384047.2014.972286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Han C, Lu L, Magliato S, Wu T. Hedgehog signaling pathway regulates autophagy in human hepatocellular carcinoma cells. Hepatology. 2013;58:995–1010. doi: 10.1002/hep.26394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen JS, Li HS, Huang JQ, Zhang LJ, Chen XL, Wang Q, Lei J, Feng JT, Liu Q, Huang XH. Down-regulation of Gli-1 inhibits hepatocellular carcinoma cell migration and invasion. Mol Cell Biochem. 2014;393:283–91. doi: 10.1007/s11010-014-2071-x. [DOI] [PubMed] [Google Scholar]
- 133.Byrne S, Dlamini N, Lumsden D, Pitt M, Zaharieva I, Muntoni F, King A, Robert L, Jungbluth H. SIL1-related Marinesco-Sjoegren syndrome (MSS) with associated motor neuronopathy and bradykinetic movement disorder. Neuromuscul Disord. 2015;25:585–8. doi: 10.1016/j.nmd.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 134.Chan IS, Guy CD, Machado MV, Wank A, Kadiyala V, Michelotti G, Choi S, Swiderska-Syn M, Karaca G, Pereira TA, Yip-Schneider MT, Max Schmidt C, Diehl AM. Alcohol activates the hedgehog pathway and induces related procarcinogenic processes in the alcohol-preferring rat model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2014;38:787–800. doi: 10.1111/acer.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer letters. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 136.Li W, Miao S, Miao M, Li R, Cao X, Zhang K, Huang G, Fu B. Hedgehog Signaling Activation in Hepatic Stellate Cells Promotes Angiogenesis and Vascular Mimicry in Hepatocellular Carcinoma. Cancer Invest. 2016;34:424–30. doi: 10.1080/07357907.2016.1227442. [DOI] [PubMed] [Google Scholar]
- 137.Sicklick JK, Li YX, Jayaraman A, Kannangai R, Qi Y, Vivekanandan P, Ludlow JW, Owzar K, Chen W, Torbenson MS, Diehl AM. Dysregulation of the Hedgehog pathway in human hepatocarcinogenesis. Carcinogenesis. 2006;27:748–57. doi: 10.1093/carcin/bgi292. [DOI] [PubMed] [Google Scholar]
- 138.Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ, Chen Y. Role of Hedgehog signaling pathway in proliferation and invasiveness of hepatocellular carcinoma cells. International journal of oncology. 2009;34:829–36. doi: 10.3892/ijo_00000209. [DOI] [PubMed] [Google Scholar]
- 139.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential Expression of Sonic Hedgehog Protein in Human Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Pathol Oncol Res. 2015;21:901–8. doi: 10.1007/s12253-015-9918-7. [DOI] [PubMed] [Google Scholar]
- 140.Dugum M, Hanouneh I, McIntyre T, Pai R, Aucejo F, Eghtesad B, Zein N. Sonic hedgehog signaling in hepatocellular carcinoma: A pilot study. Mol Clin Oncol. 2016;4:369–74. doi: 10.3892/mco.2016.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. Journal of hepatology. 2011;55:838–45. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu JT, Zhao WD, He W, Wei W. Hedgehog signaling pathway mediates invasion and metastasis of hepatocellular carcinoma via ERK pathway. Acta pharmacologica Sinica. 2012;33:691–700. doi: 10.1038/aps.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Che L, Yuan YH, Jia J, Ren J. Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2012;24:323–31. doi: 10.3978/j.issn.1000-9604.2012.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S, Bioulac-Sage P, Letouze E, Zucman-Rossi J Investigators G. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology. 2017;152:880–94 e6. doi: 10.1053/j.gastro.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 145.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nature reviews. Gastroenterology & hepatology. 2013;10:656–65. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 146.Chan IS, Guy CD, Chen Y, Lu J, Swiderska-Syn M, Michelotti GA, Karaca G, Xie G, Kruger L, Syn WK, Anderson BR, Pereira TA, Choi SS, Baldwin AS, Diehl AM. Paracrine Hedgehog signaling drives metabolic changes in hepatocellular carcinoma. Cancer research. 2012;72:6344–50. doi: 10.1158/0008-5472.CAN-12-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, Taveras KM, Hyman JM, Lee SW, Koehler AN, Chen JK, Fox JL, Mandinova A, Schreiber SL. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–6. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee ST, Welch KD, Panter KE, Gardner DR, Garrossian M, Chang CW. Cyclopamine: from cyclops lambs to cancer treatment. J Agric Food Chem. 2014;62:7355–62. doi: 10.1021/jf5005622. [DOI] [PubMed] [Google Scholar]
- 149.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 150.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–62. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 151.Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 152.Jeng KS, Sheen IS, Jeng WJ, Yu MC, Tsai HH, Chang FY, Su JC. Blockade of the sonic hedgehog pathway effectively inhibits the growth of hepatoma in mice: An in vivo study. Oncology letters. 2012;4:1158–62. doi: 10.3892/ol.2012.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tsai CL, Hsu FM, Tzen KY, Liu WL, Cheng AL, Cheng JC. Sonic Hedgehog inhibition as a strategy to augment radiosensitivity of hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2015;30:1317–24. doi: 10.1111/jgh.12931. [DOI] [PubMed] [Google Scholar]
- 154.Peluso MO, Campbell VT, Harari JA, Tibbitts TT, Proctor JL, Whitebread N, Conley JM, White KF, Kutok JL, Read MA, McGovern K, Faia KL. Impact of the Smoothened inhibitor, IPI-926, on smoothened ciliary localization and Hedgehog pathway activity. PloS one. 2014;9:e90534. doi: 10.1371/journal.pone.0090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jimeno A, Weiss GJ, Miller WH, Jr, Gettinger S, Eigl BJ, Chang AL, Dunbar J, Devens S, Faia K, Skliris G, Kutok J, Lewis KD, Tibes R, Sharfman WH, Ross RW, Rudin CM. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19:2766–74. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, Venook AP, Kindler HL. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016;45:370–5. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sasaki K, Gotlib JR, Mesa RA, Newberry KJ, Ravandi F, Cortes JE, Kelly P, Kutok JL, Kantarjian HM, Verstovsek S. Phase II evaluation of IPI-926, an oral Hedgehog inhibitor, in patients with myelofibrosis. Leuk Lymphoma. 2015;56:2092–7. doi: 10.3109/10428194.2014.984703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sandhiya S, Melvin G, Kumar SS, Dkhar SA. The dawn of hedgehog inhibitors: Vismodegib. J Pharmacol Pharmacother. 2013;4:4–7. doi: 10.4103/0976-500X.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn WK, Jung Y, Witek RP, Choi S, Michelotti GA, Rangwala F, Merkle E, Lascola C, Diehl AM. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PloS one. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–62. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jeng KS, Jeng CJ, Jeng WJ, Sheen IS, Chang CF, Hsiau HI, Hung ZH, Yu MC, Chang FY. Sonic hedgehog pathway inhibitor mitigates mouse hepatocellular carcinoma. Am J Surg. 2015;210:554–60. doi: 10.1016/j.amjsurg.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 162.Pinter M, Sieghart W, Schmid M, Dauser B, Prager G, Dienes HP, Trauner M, Peck-Radosavljevic M. Hedgehog inhibition reduces angiogenesis by downregulation of tumoral VEGF-A expression in hepatocellular carcinoma. United European Gastroenterol J. 2013;1:265–75. doi: 10.1177/2050640613496605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers (Basel) 2016;8:E22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naranjo TW, Lopera DE, Diaz-Granados LR, Duque JJ, Restrepo A, Cano LE. Histopathologic and immunologic effects of the itraconazole treatment in a murine model of chronic pulmonary paracoccidioidomycosis. Microbes Infect. 2010;12:1153–62. doi: 10.1016/j.micinf.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 165.Naranjo TW, Lopera DE, Diaz-Granados LR, Duque JJ, Restrepo AM, Cano LE. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm Pharmacol Ther. 2011;24:81–91. doi: 10.1016/j.pupt.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 166.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]