Abstract
Background
Low skeletal muscle radiodensity (SMD) is related to higher mortality in several cancers but the association with colorectal cancer (CRC) prognosis is unclear.
Methods
This observational study included 3,262 men and women from the Kaiser Permanente Northern California population diagnosed from 2006–2011 with stages I–III CRC. We evaluated hazard ratios for all-cause and CRC-specific mortality of low SMD, assessed by computed tomography using optimal stratification, relative to patients with normal SMD. We also evaluated the cross-classification of categories of low vs. normal SMD and muscle mass (MM), with outcomes.
Results
Median follow-up was 6.9 years. Optimal stratification cutpoints for SMD were 32.5 in women and 35.5 in men. In multivariate-adjusted analyses, compared to those with normal SMD levels, CRC patients with low SMD showed higher overall (hazard ratio [HR]=1.61, 95% confidence interval [CI]:1.36–1.90) and CRC-specific (HR=1.74, 95% CI:1.38–2.21) mortality. Patients with low SMD and low MM (i.e., sarcopenia) had the highest overall (HR=2.02, 95% CI: 1.65–2.47) and CRC-specific (HR=2.54, 95% CI: 1.91–3.37) mortality rates.
Conclusion
In patients with CRC, those with low SMD had elevated risks of disease-specific and overall mortality, independent of MM or adiposity. Clinical practice should incorporate body composition measures into the evaluation of CRC patient health status.
Keywords: Body composition, muscle radiodensity, muscle radiodensity, myosteatosis, muscle, adiposity, colorectal cancer, survival, mortality
Introduction
Colorectal cancer (CRC) is the fourth most common cancer diagnosed and the second leading cause of cancer-related mortality in the United States each year1. An increasing number of investigations have examined associations between markers of body composition, including skeletal muscle radiodensity (SMD), i.e., the radiodensity of skeletal muscle measured by computerized tomography (CT) and cancer prognosis. Sarcopenia (low muscle mass) and myosteatosis, i.e., the fatty infiltration into muscle which governs SMD, are normal by-products of aging, the latter related to higher levels of body fat, but are exacerbated by disease and cancer treatments2–6 and thus are common in cancer patients7,8. Several studies have found that low SMD is associated with poorer cancer prognosis generally9 and more specifically in patients with lung10, breast11, pancreatic10,12, and ovarian13 cancers. The influence of SMD in CRC patients is, however, unclear.
Findings have been highly mixed. Boer noted no association of either psoas or abdominal SMD and survival in 91 patients with resectable colon cancer14 though Blauwhoff-Buskermolen and colleagues reported a significant association of abdominal SMD and overall survival in 67 metastatic colorectal cancer patients but only after multiple adjustment15. By contrast, in 322 patients with primary operable colorectal cancer, McSorley and colleagues found a significant association between SMD and disease-specific survival in univariate analysis but no significant association after multiple adjustment16. Malietzis et al. reported nonsignificant, elevated associations between low abdominal SMD and overall and disease-specific survival in 805 CRC patients17 whereas Sabel and colleagues found significant associations of psoas-area SMD and both disease-specific and overall survival in 302 colon cancer patients18. Because low SMD has been related to higher systemic inflammatory response16,19, metabolic dysregulation20,21, and post-surgical complications14, we would expect low SMD to be related to poorer CRC prognosis. However, associations in previous studies, while suggestive, are equivocal due in large part to small study size. Furthermore, as indicated, methods of body composition assessment differed across studies impeding direct comparison.
Therefore, using computed tomography (CT) scans, collected as a routine part of clinical care to help diagnose CRC patients, as well as electronic medical record (EMR) data within the Kaiser Permanente Northern California (KPNC) population, we evaluated body composition and examined the effect of SMD on overall and CRC-specific mortality in 3,262 stage I–III CRC patients diagnosed at KPNC from 2006–2011. We used CT scans assessed at the L3 vertebra because of the high correlation of L3 with whole body values22. We further considered the combined influence of low SMD and low MM on CRC outcomes.
METHODS
Study Population
The study population consisted of all patients ages 18–80 years from KPNC diagnosed from 2006–2011 with stage I–III invasive CRC whose cancer was confirmed by computed tomography (CT), who received surgery, and for whom an electronic weight and height were available at diagnosis. Study participants have been previously described23. Case ascertainment began in 2006, one year after weights routinely became available in the EMR. A third of the Northern California population are KP members; members represent the underlying population except at socioeconomic extremes24. 49.9% of study participants were female and 50.1% male. A waiver of written informed consent was obtained and the study was approved by the KPNC and University of Alberta institutional review boards.
Data Collection
Body composition assessment and CT image analysis
Body composition was measured from CT scans (96% contrast vs. non-contrast images) taken within four months of diagnosis and prior to treatment with (neoadjuvant or adjuvant) chemotherapy or radiation (median = 0.2 months, range from −2.0 to 3.8 months); 82% of CT scans occurred prior to surgery. Using SliceOmatic Software version 5.0 (TomoVision, Montreal, Quebec, Canada), a single, trained researcher (JX) quantified the cross-sectional area of muscle and adipose in centimeters squared (cm2) at the third lumbar vertebra (L3), a vertebral landmark previously validated and utilized in studies of cancer patients25. Single-slice abdominal cross-sectional areas at the L3 vertebra have been strongly correlated with whole body volumes of muscle and adipose tissue22. Skeletal muscle areas included rectus abdominus, erector spinae muscles, quadratus lumborum, psoas, and internal, transverse and external oblique muscle groups. Using pre-established thresholds of Hounsfield units26,27, we assessed MM, and adipose tissue was segmented to distinguish visceral (intra-abdominal) adipose tissue (VAT), subcutaneous adipose tissue (SAT) and intramuscular adipose tissue (IMAT). SMD was assessed as mean Hounsfield units across muscle area measured at the L3 vertebra. The coefficient of variation for paired observations for SMD was 0.7%.
Clinical variables and endpoints
KPNC Cancer Registry data and the EMR were reviewed for information on prognostic factors, including disease stage, tumor characteristics, surgical procedures, and treatment (chemotherapy, radiation therapy). Data on overall and CRC-specific mortality were obtained from the KPNC computerized mortality file, which is comprised of data from the California State Department of Vital Statistics, U.S. Social Security Administration, and KPNC utilization data sources. Colorectal cancer death was attributed to persons if CRC was listed as a cause of death on the death certificate.
Other covariate data
EMR data were accessed for information on numerous potential confounding variables including sociodemographic variables (self-reported race is included in the EMR) and smoking status. The Charlson-Deyo index28 was used to measure comorbidity. Height and weight were measured by a medical assistant at each medical visit. Partitioned BMI was computed in kilograms per height in meter squared removing kilograms of muscle and adipose from the measure of weight; partitioned BMI thus included body weight due to organ, bone, and water weight and was analyzed continuously. BMI closest to the CT scan (median=0.0 months) was used in analyses.
Statistical analysis
We examined covariate distributions by low vs. normal SMD, evaluating differences using χ2 tests. Cox proportional hazards regression was used to examine associations between SMD at the time of diagnosis, and all-cause and CRC-specific mortality. Follow-up time was computed in years from the date of diagnosis.
We initially evaluated tertiles and quartiles of SMD with outcomes. We also explored possible nonlinear relationships between SMD and survival, nonparametrically, and by sex, with restricted cubic splines29, a technique enabling specification of a relationship between two variables when the function is nonlinear. Tests for nonlinearity used the likelihood ratio test, comparing the model with the linear term to one with linear and cubic spline terms. Because each of these analyses provided strong evidence of a threshold effect of SMD with outcomes, we used optimal stratification30,31 to generate sex-specific cutpoints to distinguish patients at higher mortality risk. All subsequent analyses used the dichotomous (low vs. normal) SMD variable. We defined patients to have low SMD if values fell below the cutpoints and normal SMD if patient values were greater than or equal to computed cutpoints.
We evaluated time to failure using Kaplan-Meier curves, comparing survival in patients with low and normal SMD using log-rank tests. We subsequently compared Cox models controlling for age, race, sex, to those adjusted additionally for stage, grade, cancer site (distal colon, proximal colon, and rectal), treatment, partitioned BMI, and smoking. Models were further adjusted for tertiles of VAT and SAT. We specifically did not include IMAT in adjustment to avoid collinearity since SMD levels are governed by IMAT levels.
We simultaneously evaluated associations of SMD and MM to determine independent effects of muscle components on survival. To consider the influence of body phenotypes on outcomes, we further evaluated the cross-classification of dichotomous SMD with normal vs. low MM (i.e., sarcopenia, the definition based on our previous work32) with the reference category including those with both normal SMD and normal MM.
Finally, we conducted analyses of SMD and outcomes stratified by sex, age (<64 vs. ≥64 years), race, stage, BMI (18.5–<25 25–<30, 30+ kg/m2), comorbidity, treatment status, and CRC site. Heterogeneity in associations in stratified analyses were examined via introduction of cross-product terms for the dichotomous SMD variable and stratification variables in regression models with evaluation of significance by likelihood ratio χ2 tests. We conducted tests of proportionality with variable by time interactions. Tests of statistical significance were two-sided. Significant results denote p-values≤0.05.
RESULTS
Of the 3,262 CRC patients, 879 died with 451 deaths from CRC. Follow-up ranged from 0–10.9 years, with a median 6.9 years follow-up.
Baseline characteristics
Examining covariates, age, adiposity, BMI, comorbidity, and smoking were inversely related and MM was directly related to SMD. Whites and Hispanics had lower SMD than Blacks or Asians/Pacific Islanders. Proximal cancers were more common among those with low SMD and rectal cancers were more common in those with high SMD. Patients with low SMD were less likely to receive radiation or chemotherapy. Sex and stage were unrelated to SMD (Table 1).
Table 1.
Characteristics of CSCANS Cohort, by level of Muscle Radiodensity
| Muscle radiodensity | |||||||
|---|---|---|---|---|---|---|---|
| Total n=3,262 N |
Mean SMD |
N | Low n=966 Row% |
N | Normal n=2,296 Row% |
pvalue | |
| Overall | 27.3 (5.7–35.5) | 44.0 (32.5–72.1) | |||||
| Gender | |||||||
| Men | 1634 | 40.6 | 463 | 28.3 | 1171 | 71.7 | 0.11 |
| Women | 1628 | 37.5 | 503 | 30.9 | 1125 | 69.1 | |
| Age at diagnosis | |||||||
| <50 | 432 | 47.9 | 23 | 5.3 | 409 | 94.7 | <0.0001 |
| 50–<60 | 806 | 42.3 | 123 | 15.3 | 683 | 84.7 | |
| 60–<70 | 941 | 38.4 | 261 | 27.7 | 680 | 72.3 | |
| >=70 | 1083 | 33.6 | 559 | 51.6 | 524 | 48.4 | |
| Race/Ethnicity | |||||||
| White | 2118 | 37.5 | 743 | 35.1 | 1375 | 64.9 | <0.0001 |
| Black | 234 | 41.5 | 40 | 17.1 | 194 | 82.9 | |
| Hispanic | 365 | 38.6 | 118 | 32.3 | 247 | 67.7 | |
| API | 520 | 44.7 | 59 | 11.3 | 461 | 88.6 | |
| Other | 21 | 38.5 | 5 | 23.8 | 16 | 76.2 | |
| Stage | |||||||
| I | 979 | 39.3 | 279 | 28.5 | 700 | 71.5 | 0.25 |
| II | 1030 | 38.4 | 325 | 31.5 | 705 | 68.5 | |
| III | 1253 | 39.4 | 362 | 28. 9 | 891 | 71.1 | |
| Grade | |||||||
| Well differentiated | 226 | 38.1 | 81 | 35.8 | 145 | 64.2 | 0.02 |
| Moderate | 2466 | 39.1 | 725 | 29.4 | 1741 | 70.6 | |
| Poor/Undiff | 408 | 38.6 | 126 | 30.9 | 282 | 69.1 | |
| Unknown | 162 | 41.4 | 34 | 21.0 | 128 | 79.0 | |
| Chemotherapy* | |||||||
| No | 1559 | 37.8 | 537 | 34.4 | 1022 | 65.6 | <0.0001 |
| Yes | 1703 | 40.2 | 429 | 25.2 | 1274 | 74.8 | |
| Radiation* | |||||||
| No | 2755 | 38.5 | 863 | 31.3 | 1892 | 68.7 | <0.0001 |
| Yes | 506 | 42.3 | 103 | 20.4 | 403 | 79.6 | |
| BMI | |||||||
| 18.5–<25.0 | 1007 | 42.3 | 25 | 16.0 | 131 | 84.0 | <0.0001 |
| 25.0–<30.0 | 1164 | 39.9 | 176 | 19.3 | 736 | 80.7 | |
| >=30.0 | 1030 | 34.6 | 223 | 57.9 | 162 | 42.1 | |
| Charlson comorbidity score | |||||||
| 0 | 1770 | 41.0 | 380 | 21.7 | 1390 | 78.5 | <0.0001 |
| 1–2 | 946 | 37.0 | 354 | 37.4 | 592 | 62.6 | |
| >=3 | 321 | 32.1 | 183 | 57.1 | 138 | 43.0 | |
| Missing | 225 | 42.1 | 49 | 21.8 | 176 | 78.2 | |
| Cancer site | |||||||
| Proximal | 1436 | 36.9 | 537 | 37.4 | 899 | 62.6 | <0.0001 |
| Distal | 879 | 39.6 | 233 | 26.5 | 646 | 73.5 | |
| Rectal | 947 | 41.8 | 196 | 20.7 | 751 | 79.3 | |
| Smoking | |||||||
| Never | 1516 | 40.7 | 343 | 22.6 | 1173 | 77.4 | <0.0001 |
| Former | 1347 | 37.4 | 495 | 36.7 | 852 | 63.2 | |
| Current | 396 | 38.5 | 128 | 32.3 | 268 | 67.7 | |
| Muscle Tertiles | |||||||
| Low | 1086 | 37.8 | 381 | 35.1 | 705 | 64.9 | <0.0001 |
| Middle | 1088 | 39.5 | 309 | 28.4 | 779 | 71.6 | |
| High | 1088 | 39.8 | 276 | 25.4 | 812 | 74.6 | |
| SF Tertiles | |||||||
| Low | 1086 | 42.8 | 198 | 18.2 | 888 | 81. 8 | <0.0001 |
| Middle | 1089 | 39.3 | 298 | 27.4 | 791 | 72.6 | |
| High | 1087 | 35.0 | 470 | 43.2 | 617 | 56.8 | |
| VF Tertiles | |||||||
| Low | 1087 | 44.6 | 127 | 11.74 | 960 | 88.3 | <0.0001 |
| Middle | 1087 | 39.1 | 293 | 27.0 | 794 | 73.0 | |
| High | 1088 | 33.4 | 546 | 50.2 | 542 | 49.8 | |
Radiation and chemotherapy could be neoadjuvant or adjuvant.
Categorization of SMD
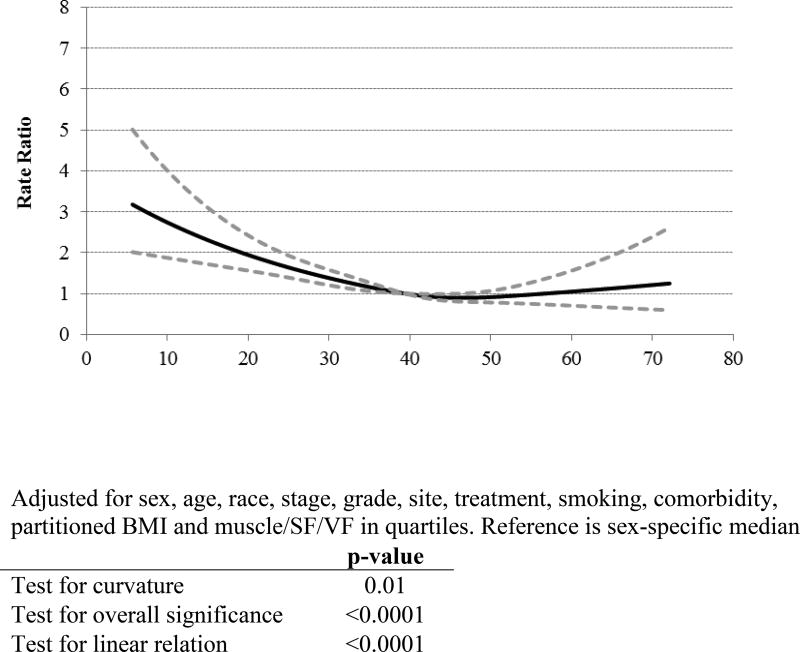
As indicated, analyses of SMD categories (Table 2) as well as spline analysis (Figure 1) each showed that associations between SMD and all-cause, as well as CRC-specific mortality, were best characterized as a threshold effect. Using optimal stratification30,31, sex-specific cutpoints were 32.5 in women and 35.5 in men.
Table 2.
Muscle radiodensity and mortality
| Overall Mortality | CRC Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMD Range |
At-risk | Events | HR | Lower bound, 95% CI |
Upper bound, 95% CI |
Events | HR | Lower bound, 95% CI |
Upper bound, 95% CI |
|
| Low SMD by sex-specific optimal stratification | 879 | 451 | ||||||||
| Normal | 32.5 – 72.1 | 2296 | 481 | 259 | ||||||
| Low | 5.7 – 35.5 | 966 | 398 | 1.61 | 1.36 | 1.90 | 192 | 1.74 | 1.38 | 2.21 |
| Quartiles of SMD | ||||||||||
| High | 44.4 – 72.1 | 815 | 153 | 93 | ||||||
| Mid-High | 37.7 – 47.2 | 816 | 177 | 0.94 | 0.75 | 1.18 | 89 | 0.94 | 0.68 | 1.28 |
| Mid-Low | 30.8 – 41.0 | 816 | 201 | 0.95 | 0.75 | 1.21 | 109 | 1.10 | 0.79 | 1.53 |
| Low | 5.7 – 34.6 | 815 | 348 | 1.50 | 1.15 | 1.94 | 160 | 1.63 | 1.13 | 2.36 |
| p for trend across quartiles | 0.001 | 0.003 | ||||||||
| p for trend (continuous) | <0.0001 | 0.0001 | ||||||||
"SMD"=skeletal muscle radiodensity (HU); HR=hazard ratio
Models simultaneously adjusted for muscle mass, visceral and subcutaneous adiposity in quartiles
Additionally adjusted for age at diagnosis (continuous), sex (ref=Male), race (ref=White), site (ref=Proximal), stage (ref=I), grade (ref=Well differentiated), chemotherapy (ref=No), radiation (ref=No), smoking history (ref=Never), comorbidity score (ref=0) and partitioned BMI (continuous)
Figure 1.
Restricted Cubic Spline for Muscle Radiodensity and Overall Mortality
SMD, all-cause and CRC-specific mortality
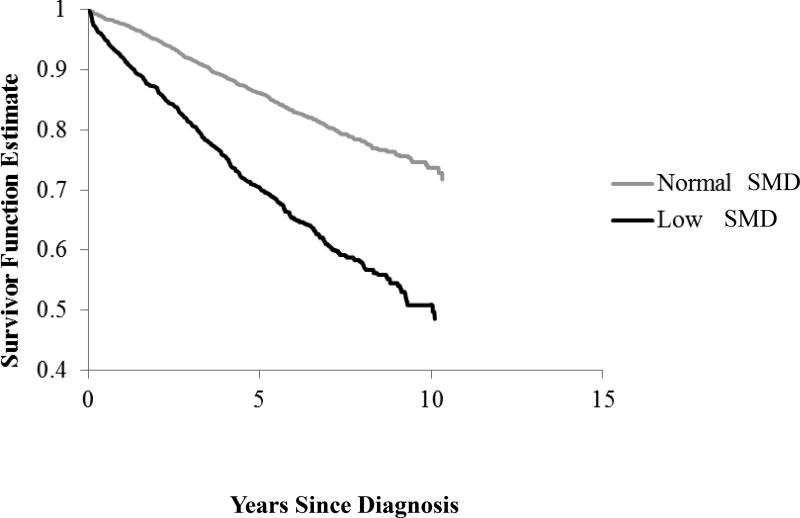
The Kaplan-Meier curve (Figure 2) showed that patients with low SMD had worse overall survival than those with normal SMD (log rank p<0.0001). In models adjusted for age, sex, and race, low SMD was associated with elevated risks of CRC-specific and overall mortality. Multivariable-adjusted results were qualitatively similar. Compared to those with normal SMD, CRC patients with low SMD showed higher overall (hazard ratio [HR]=1.61, 95% confidence interval [CI]:1.36–1.90) and CRC-specific (HR=1.74, 95% CI:1.38–2.21) mortality (Table 2).
Figure 2.
Kaplan-Meier Curve of Muscle Radiodensity and Overall Mortality
Cross-classification of SMD and MM
Evident in analyses with simultaneous adjustment for SMD and MM, low SMD and sarcopenia (low MM) were each independently associated with higher overall and CRC-specific mortality. In analyses of the cross-classification of SMD and MM, the highest mortality risks were seen in those with both low SMD and sarcopenia compared with the reference (normal SMD/normal MM), true in both men and women (Table 3), consistent with an additive, rather than multiplicative, effect (p-value, test for interaction=0.30).
Table 3.
Cross-classification of muscle radiodensity (HU) and sarcopenia
| Overall Mortality | CRC Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| At- risk |
Events | HR | Lower bound, 95% CI |
Upper bound, 95% CI |
Events | HR | Lower bound, 95% CI |
Upper bound, 95% CI |
|
| SMD and Sarcopenia | |||||||||
| Normal SMD, no sarcopenia | 1482 | 264 | Referent | 146 | Referent | ||||
| Normal SMD, sarcopenia | 814 | 217 | 1.30 | 1.07 | 1.57 | 113 | 1.42 | 1.09 | 1.84 |
| Low SMD, no sarcopenia | 397 | 142 | 1.63 | 1.30 | 2.05 | 62 | 1.57 | 1.13 | 2.20 |
| Low SMD, sarcopenia | 569 | 256 | 2.02 | 1.65 | 2.47 | 130 | 2.54 | 1.91 | 3.37 |
"SMD"=skeletal muscle radiodensity (HU); HR=hazard ratio
SMD and Sarcopenia model adjusted for visceral and subcutaneous adipose tissue in quartiles
Models additionally adjusted for age at diagnosis (continuous), sex (ref=Male), race (ref=White), site (ref=Proximal), stage (ref=I), grade (ref=well), chemotherapy (ref=No), radiation (ref=No), smoking history (ref=Never), comorbidity score (ref=0) and partitioned BMI (continuous)
Stratified analyses
We noted a stronger association between low SMD and mortality in patients less than vs. greater than or equal to 64 years of age (p-value, test for interaction=0.04). We noted a slightly weaker association in stage II vs. stage I and III patients (p-interaction=0.09). There was little evidence of effect modification by sex (Table 4) or other variables (data not shown).
Table 4.
Muscle radiodensity (HU) and overall mortality, by patient characteristics
| Overall Mortality | |||||||
|---|---|---|---|---|---|---|---|
| Normal | Low SMD | ||||||
|
| |||||||
| At- risk |
Events | HR | Lower bound, 95% CI |
Upper bound, 95% CI |
p for interaction | ||
| Sex | |||||||
| Male | 1634 | 469 | Referent | 1.57 | 1.25 | 1.97 | 0.57 |
| Female | 1628 | 410 | Referent | 1.63 | 1.28 | 2.07 | |
| Age at diagnosis, years | |||||||
| <64 | 1627 | 312 | Referent | 2.43 | 1.77 | 3.32 | 0.04 |
| >=64 | 1635 | 567 | Referent | 1.57 | 1.30 | 1.90 | |
| Stage | |||||||
| I | 979 | 171 | Referent | 2.01 | 1.39 | 2.89 | 0.09 |
| II | 1030 | 255 | Referent | 1.28 | 0.95 | 1.73 | |
| III | 1253 | 453 | Referent | 1.57 | 1.23 | 2.00 | |
With stratification on grade, stage, and treatment with chemotherapy, proportional hazards assumptions were met.
DISCUSSION
Consistent with hypotheses, CRC patients with low SMD at diagnosis had worse overall and CRC-specific prognosis compared to those with normal SMD. Patients with both low SMD and sarcopenia had the highest overall and CRC-specific mortality risks. These findings, in the largest CRC cohort to date with data on body composition, provide support that low SMD, as well as phenotypes based on combinations of SMD and MM, are important prognostic factors in CRC patients.
Our results clarify and represent an advance over findings from previous studies of SMD and prognosis in patients with CRC14–18. Findings in previous studies, ranging from 67 to 805 patients, have suggested a possible relationship but they have been inconclusive due largely to insufficient power. In fact, in the largest previous study to examine SMD and CRC prognosis, Malietzis et al. found no significant association between myosteatosis (low SMD) and overall or CRC-specific survival17 (N=805) even though risks appeared elevated among patients with low SMD. Our findings confirm, and provide strong support for, an association of low SMD with both overall and CRC-specific mortality. The stronger association in younger patients in this population further suggests that low SMD may better differentiate CRC mortality risk in younger vs. older patients given that SMD levels decline with age.
In non-cancer populations33,34 and in cancer patients19,35, low SMD promotes higher systemic inflammatory response16,19 and insulin resistance20,21. Inflammation and metabolic derangements stimulate tumor cell proliferation36,37 and lead to worse cancer survival. Low SMD is also related to higher post-surgical complications14, which are related to elevated CRC mortality. Though this could be in part to the association with adiposity which has been associated with poorer wound healing38, the independent association of SMD with mortality, adjusted for adiposity, suggests other mechanisms which remain to be explored7.
As expected, the combination of both low SMD and sarcopenia predicted elevated mortality in CRC patients, consistent with an additive effect. Sarcopenia has predicted higher mortality in many cancers including CRC patients as seen in our recent study32. Sarcopenia has also been related to higher systemic inflammatory response16,19,35, metabolic dysregulation39–41, and post-surgical complications14 and the effects of each of these muscle abnormalities appear to be independent predictors of outcomes in CRC patients.
A study strength was the ability to examine body composition parameters at diagnosis prior to treatment. A great strength was the ability to evaluate associations in a large population-based cohort of 3,262 CRC patients, ensuring sufficient power to examine associations. Other study strengths include a large sample size, data on treatment and comorbidities, and follow-up to 10.9 years.
A study limitation, it is not possible to clearly determine whether low SMD influences or is a consequence of tumor progression though strong associations of SMD at diagnosis and outcomes even in stage I patients in the study provides some credence that the effect may not be entirely explained by reverse causation. A possible concern, most (96%) patients had contrast vs. non-contrast scans; SMD levels may be higher in scans with contrast42. This could lead to a higher numeric value at which the threshold of low SMD is defined. However, this should not influence the relative ranking of SMD in patients and thus should not influence overall associations. We did not have information on optimization of treatment and quality measures such as surgical margins or extensiveness of nodal resection. Another potential limitation was the lack of information on functional status which is often included in assessments of sarcopenia in aging populations though this information is not typically included in assessment of body composition in cancer populations. Other potential concerns are the inclusion of CT scans months from diagnosis or after surgery. However, when we conducted sensitivity analyses restricting analyses to patients with scans ≤ 1 month from diagnosis, or to patients with scans prior to surgery, results were qualitatively similar (data not shown). An additional limitation, as is true in all observational studies, residual confounding is possible though we were able to adjust for a larger set of covariates than most analyses of body composition and cancer outcomes.
In summary, low SMD was associated with elevated all-cause and CRC-specific mortality in a large population of stage I–III CRC patients. Studies are needed to understand the mechanisms underlying these results. Regardless, body composition markers are prognostic of outcomes in CRC patients and should be incorporated into clinical assessments of patient health status.
Acknowledgments
Acknowledgment of funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant R01CA175011, PI: Caan) and (grant K07 CA187403, PI: Kroenke).
Footnotes
Author contributions: CH Kroenke contributed to conceptualization and design of the study, analysis, writing and interpretation. C Prado and J Meyerhardt contributed to study design, interpretation, and editing. J Xiao contributed to data collection, interpretation, and editing. E Weltzien contributed to analysis and editing. EM Cespedes Feliciano contributed to design, interpretation, and editing. B Caan contributed to study design, interpretation, analysis, and editing. All authors of this research paper have approved the final version submitted.
The authors report no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. [Google Scholar]
- 2.Villasenor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(4):1133S–7S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 5.Torres ML, Hartmann LC, Cliby WA, et al. Nutritional status, CT body composition measures and survival in ovarian cancer. Gynecol Oncol. 2013;129(3):548–53. doi: 10.1016/j.ygyno.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Rothney MP, Catapano AL, Xia J, et al. Abdominal visceral fat measurement using dual-energy X-ray: association with cardiometabolic risk factors. Obesity (Silver Spring) 2013;21(9):1798–802. doi: 10.1002/oby.20223. [DOI] [PubMed] [Google Scholar]
- 7.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210(3):489–97. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 9.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 10.Akahori T, Sho M, Kinoshita S, et al. Prognostic Significance of Muscle Attenuation in Pancreatic Cancer Patients Treated with Neoadjuvant Chemoradiotherapy. World J Surg. 2015;39(12):2975–82. doi: 10.1007/s00268-015-3205-3. [DOI] [PubMed] [Google Scholar]
- 11.Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MC, Levin MD. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast. 2017;31:9–15. doi: 10.1016/j.breast.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 12.van Dijk DP, Bakens MJ, Coolsen MM, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317–26. doi: 10.1002/jcsm.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aust S, Knogler T, Pils D, et al. Skeletal Muscle Depletion and Markers for Cancer Cachexia Are Strong Prognostic Factors in Epithelial Ovarian Cancer. PLoS One. 2015;10(10):e0140403. doi: 10.1371/journal.pone.0140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boer BC, de Graaff F, Brusse-Keizer M, et al. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis. 2016;31(6):1117–24. doi: 10.1007/s00384-016-2538-1. [DOI] [PubMed] [Google Scholar]
- 15.Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, et al. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34(12):1339–44. doi: 10.1200/JCO.2015.63.6043. [DOI] [PubMed] [Google Scholar]
- 16.McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr. 2017 doi: 10.1016/j.clnu.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Malietzis G, Currie AC, Athanasiou T, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. The British journal of surgery. 2016;103(5):572–80. doi: 10.1002/bjs.10075. [DOI] [PubMed] [Google Scholar]
- 18.Sabel MS, Terjimanian M, Conlon AS, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol. 2013;108(3):169–75. doi: 10.1002/jso.23366. [DOI] [PubMed] [Google Scholar]
- 19.Malietzis G, Johns N, Al-Hassi HO, et al. Low Muscularity and Myosteatosis Is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg. 2016;263(2):320–5. doi: 10.1097/SLA.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 20.Miljkovic I, Cauley JA, Wang PY, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 2013;21(10):2118–25. doi: 10.1002/oby.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 23.Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study) Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon NP. Characteristics of Adult Health Plan Members in Kaiser Permanente’s Northern California Region, as Estimated from the 2011 Member Health Survey. Oakland, CA: Division of Research, Kaiser Permanente Medical Care Program; 2013. [Google Scholar]
- 25.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–75. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 27.Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998;351(9106):871–5. doi: 10.1016/S0140-6736(97)11518-5. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 30.Lausen B, Schumacher M. Maximally Selected Rank Statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 31.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Computational Statistics and Data Analysis. 1999;30:253–70. [Google Scholar]
- 32.Caan BJ, Meyerhardt JM, Kroenke CH, et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival. Cancer Epi Biomarker Prev. 2017 doi: 10.1158/1055-9965.EPI-17-0200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoico E, Corzato F, Bambace C, et al. Myosteatosis and myofibrosis: relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr. 2013;57(3):411–6. doi: 10.1016/j.archger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoico E, Rossi A, Di Francesco V, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65(3):295–9. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7(8):e41883. doi: 10.1371/journal.pone.0041883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan W, Shen X, Lei J, et al. Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int. 2014;2014:461917. doi: 10.1155/2014/461917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierpont YN, Dinh TP, Salas RE, et al. Obesity and surgical wound healing: a current review. ISRN Obes. 2014;2014:638936. doi: 10.1155/2014/638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang SY, Lim GE, Kim YK, et al. Association between Sarcopenic Obesity and Metabolic Syndrome in Postmenopausal Women: A Cross-sectional Study Based on the Korean National Health and Nutritional Examination Surveys from 2008 to 2011. J Bone Metab. 2017;24(1):9–14. doi: 10.11005/jbm.2017.24.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Hong YP, Shin HJ, Lee W. Associations of Sarcopenia and Sarcopenic Obesity With Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J Prev Med Public Health. 2016;49(1):35–44. doi: 10.3961/jpmph.15.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract. 2013;7(4):e301–7. doi: 10.1016/j.orcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Rollins KE, Javanmard-Emamghissi H, Awwad A, Macdonald IA, Fearon KCH, Lobo DN. Body composition measurement using computed tomography: Does the phase of the scan matter? Nutrition. 2017;41:37–44. doi: 10.1016/j.nut.2017.02.011. [DOI] [PubMed] [Google Scholar]