Abstract
Introduction
Complex Regional Pain Syndrome (CRPS), a rare and severe chronic pain condition, often responds poorly to existing treatments. Previous studies demonstrated Transcranial Magnetic Stimulation (TMS) provided short-term pain relief for upper extremity CRPS.
Methods
Building on previous methodologies, we employed a TMS protocol that may lead to significant pain relief for upper and lower extremity CRPS in a nonrandomized open label pilot trial involving 21 participants. We individualized TMS coil positioning over motor cortex of somatic pain location, and administered intermittent theta-burst stimulation followed by 10 Hz high frequency stimulation using a deeper targeting coil. We assessed response (≥ 30% pain reduction) from a single session (n=5) and 5 consecutive daily sessions (n=12) and compared change in pain from baseline, after 1 treatment and 1 week post-treatment between groups using a mixed ANVOA.
Results
Both groups demonstrated significant pain reduction after 1 session and 1 week post-treatment; however, no group differences were present. From a single session, 60% of participants responded at Week 1. From 5 sessions, 58% and 50% of participants responded at Weeks 1 and 2, respectively. Two from each group achieved >50% pain reduction beyond 6–8 weeks. No serious adverse events occurred. Though headache and nausea were the most common side-effects, we urge careful monitoring to prevent seizures with this protocol.
Conclusions
We used a TMS protocol that, for the first time, led to significant pain relief in upper and lower extremity CRPS, and will soon examine our protocol in a larger, controlled trial.
Keywords: Complex regional pain syndrome, CRPS, transcranial magnetic stimulation, TMS, theta-burst stimulation, high frequency stimulation
INTRODUCTION
Complex Regional Pain Syndrome (CRPS) is a rare and severe chronic pain condition (1,2), and effective treatments options are not established for many patients (3). CRPS is defined as “continuing pain, which is disproportionate to any inciting event”, along with a variable array of signs and symptoms (sensory, vasomotor, sudomotor/edema, and/or motor/trophic features) that do not fit another diagnosis (4). A defined nerve injury is not apparent in the majority of cases (CRPS Type I), but may be noted in others (CRPS Type II) (4,5). The underlying pathophysiology is still unclear, but likely involves complex peripheral and central mechanisms (6). While some patients may have symptoms spontaneously resolve (often within the first year following the inciting event), many other patients do not see symptoms resolve or improve (1,7). A recent Cochrane Review found that the level of evidence for CRPS therapies is of low quality (3), and current treatment guidelines are based largely on expert experience, case reports, open-label trials, and pilot studies (4).
Transcranial Magnetic Stimulation (TMS), a non-invasive method for stimulating the brain, is a growing area of research for pain management (8,9). TMS is generally safe and well-tolerated (10), and stimulation of prefrontal cortex is used clinically to treat depression (11). Although key questions such as the most efficacious brain target area and stimulation parameters to extend the durability of treatment may remain, TMS of the motor cortex has shown to be a promising avenue of treatment for several chronic pain conditions (9, 12–15) and has been examined previously in CRPS patients (13,14). For example, the effects of a single-session of real or sham 10 Hz rTMS applied to the motor cortex of patients with upper limb CRPS Type I were examined (13). Compared to sham, the real rTMS session resulted in a significant decrease in pain intensity ratings immediately following stimulation; however, pain returned 45 min later and was not further examined beyond 90 min (13). A second study, also in patients with upper limb CRPS Type I, examined 10 daily sessions of either real or sham 10 Hz rTMS to the motor cortex as an adjuvant therapy to a standardized pharmacological treatment and physical therapy regimen (14). Real rTMS resulted in a significant reduction in pain intensity ratings (50.9%), approximately double that of sham rTMS (24.7%) during the ongoing, daily treatment sessions. Although differences compared to respective baselines remained one week following stimulation, treatment efficacy between groups was not sustained (14).
The main limitations of the TMS for CRPS studies reported above are the narrow participant population, only in upper limb CRPS Type I and the durability of treatment effects (13,14). Thus, our objective was to further examine TMS as a treatment option for CRPS, expanding to a wider patient population, as well as, testing the durability of TMS using a novel frequency pairing comparing single treatment to daily consecutive treatments (16). Our cohort included patients with both types of CRPS (I and II), as well as, upper or lower extremities or both. Moreover, the capability to target lower extremities is important; more than 50% of patients with CRPS have lower extremity pain in our tertiary care clinic (record review of 56 patients) and literature reviews do not indicate a dramatic difference of upper versus lower extremity occurrence or treatment response (1). We thus used a coil that allowed us to reach corticospinal representations associated with the lower extremities on the motor cortex, which was not done in prior studies likely due to device constraints (only recently have deep coils been adequately cooled to allow extended high-frequency stimulation without overheating). We also combined intermittent theta burst and 10 Hz high-frequency stimulation, as Lefaucheur’s group has reported that this gives improved results in neuropathic pain (16).
There are practical challenges to conducting research for patients with CRPS. The incidence of CRPS is quite low in the general population (~20 in 100,000) (1,2) and low even in tertiary pain specialty clinics (~50 in 2000, record review in our clinic). Ethical and practical considerations are required to engage patients in research. We thus chose to conduct an open-label nonrandomized adjunctive treatment trial, to characterize effects in patients that had significant pain despite stably-maintained treatments.
METHODS
Participants
Recruitment and Screening
Participants were recruited from across the United States, and were eligible to participate if they met “Budapest” Clinical Diagnostic Criteria for CRPS (4) and had pain greater than 3/10 average on a numerical rating scale (NRS). Additionally, participants were screened for TMS and MRI tolerability and safety and maintained their current pain management plans and pain medications throughout the trial (see Table 1). Informed consent was collected and research conducted under the approval of the Stanford Institutional Review Board. We completed an initial pre-pilot study of 4 participants (ClinicalTrials.gov Identifier: NCT01926119) to assess feasibility which led to a larger pilot trial of 21 participants (ClinicalTrials.gov Identifier: NCT02067273). Throughout the manuscript, we will refer to the larger 21 participant trial primarily, as the data collection variables were modified between the projects and were not easily combined.
Table 1.
Participant Characterization
| Patient Number | Gender | Age | CRPS Type (for treated limb) | Etiology | Pain Location(s) & Treatment Target Limb | Pain Duration (years) | Baseline Pain (VAS) | Current Pain Medications |
|---|---|---|---|---|---|---|---|---|
| 1 Day of TMS Treatment | ||||||||
| 5 | M | 54 | I | Fracture | RUE | 7.0 | 3.1 | Nortriptyline, Pregabalin |
| 10 | F | 54 | II (Peroneal Nerve) | Crush | LLE* + RLE | 8.5 | 8.8 | Gabapentin, Methadone, Mexiletine |
| 18 | M | 26 | I | Crush | RLE | 4.5 | 8.0 | Gabapentin |
| 19 | F | 43 | II (Ulnar Nerve) | Surgery | LUE | 5.0 | 7.4 | Ibuprofen, Percocet |
| 20 | F | 41 | I | Crush | RUE* + RLE | 4.5 | 8.6 | Clonidine, Morphine, Percocet |
| 21 | F | 71 | II (Radial Nerve) | Surgery | LUE* + RUE | 3.5 | 5.3 | Pregabalin |
| 5 Days of TMS Treatment | ||||||||
| 1 | F | 48 | I | Surgery | LUE | 4.5 | 6.6 | Endocet, Fentanly |
| 2 | F | 51 | I | Surgery | RUE | 5.5 | 5.8 | Naproxen, Pregabalin |
| 3 | F | 47 | II (Posterior Tibial) | Surgery | LLE | 2.0 | 9.1 | Cyclobenzaprine, Tramadol, Vicodin |
| 4 | F | 55 | I | Surgery | RLE | 4.5 | 4.0 | Acetaminophen, Ibuprofen, Percocet |
| 6 | F | 43 | I | Medication-Induced | LLE* + RLE | 1.0 | 5.8 | None |
| 7 | F | 76 | I | Surgery | LLE* + RLE | 7.0 | 8.3 | Celecoxib, Clonidine, Endocet, Gabapentin, Oxycodone |
| 8 | F | 51 | II (Ulnar Nerve) | Surgery | RUE | 15.5 | 7.3 | Ketamine |
| 9 | F | 27 | II (Ulnar Nerve) | Crush | RUE | 4.0 | 4.6 | Acetaminophen, Gabapentin, Naproxen, Percocet, Tramadol |
| 11 | F | 33 | II (Posterior Tibial) | Crush | RLE | 1.0 | 5.6 | Gabapentin, Low Dose Naltrexone |
| 12 | F | 23 | II (Sural Nerve) | Surgery | LLE* + RLE + BUE | 4.5 | 5.9 | Ketamine, Pregabalin, Topiramate |
| 13 | F | 45 | II (Ulnar Nerve) | Crush | RUE | 1.0 | 5.6 | Low Dose Naltrexone, Nabumetone |
| 14 | F | 39 | I | Surgery | LLE + RLE* + BUE | 9.5 | 8.9 | Gabapentin |
| 15 | F | 30 | I | Fracture | LLE + RLE* + BUE | 10.5 | 7.8 | Amitryptiline, Ibuprofen, Pregabalin |
| 16 | F | 38 | I | Surgery | RLE | 0.5 | 6.2 | Buprofen, Duloxetine, Norco, Pregabalin |
| 17 | F | 30 | I | Crush | RLE* + RUE | 10.0 | 6.2 | Dextromethorphan, Tizanidine, Tramadol |
R = Right, L = Left, B = Bilateral
UE = upper extremity, LE = lower extremity
= TMS Target Region if multiple pain locations
Treatment Group Selection
Our goal, in this pilot trial, was to provide an accessible intervention to a population with active CRPS and limited treatment options. In order to accommodate travel and scheduling needs, we designed the study to be open-label and non-randomized and allowed participants to choose between 1 or 5 day treatment regimens.
Participant Characterization
Prior to treatment, we characterized each patient by collecting medical history, administering pain-related questionnaires, and administering a CRPS assessment to confirm the diagnosis. A trained clinical researcher evaluated participants using the Complex Regional Pain Syndrome Severity Score, a validated tool as described by Harden et al. (17) to determine the presence or absence of the following signs and symptoms: hyperalgesia, mechanical allodynia, temperature asymmetry, color asymmetry, asymmetric edema, sweating asymmetry, dystrophic changes in the hair/nails/skin, and finally motor abnormalities including tremor, dystonia, weakness, decreased range of motion. Average daily pain ratings using a 0–10 visual analog scale (VAS) were collected prospectively on a daily basis for at least 3 days prior to the first day of treatment to establish an average baseline level of pain in the CRPS-affected limb. Patient characteristics and treatment target data is provided in Table 1. Information regarding prior treatments and positive response rates to those treatments, defined as participants indicating “treatments helped,” is provided in Figure 1.
Figure 1.
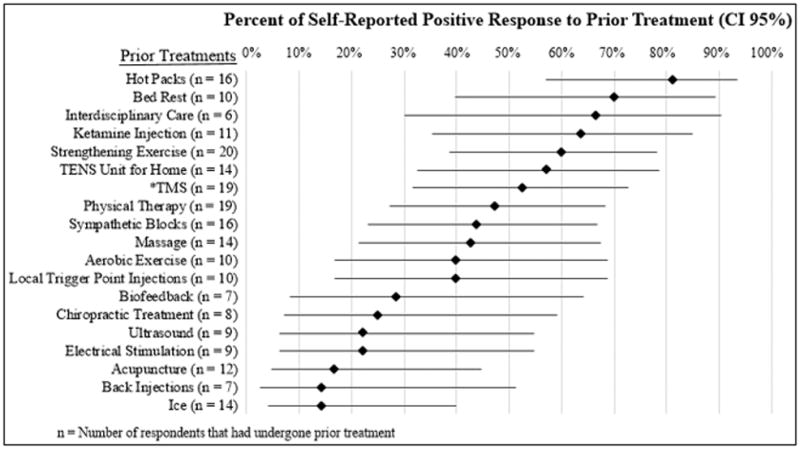
Comparative Response Relative to Prior Treatments
TMS Procedures
Anatomical Localization Mapping
When feasible, we acquired T1-weighted structural MRI scans of participants to aid TMS placement (3.0T G.E. MRI, 1mm3 resolution). If not feasible due to safety or comfort, we used a template MRI scan to ensure TMS positioning remained in the same location throughout treatment. We used a Brainsight TMS neuronavigation system (Rogue Research, Montreal), with markers on both the patient’s head and on the TMS coil, to guide TMS treatment. This neuronavigation system allows for reproducibility between sessions from the initial target selection in a 3-dimensional representation of the participant’s head. In our protocol, we marked the bridge of the nose, the tip of the nose, and the tragi of ears bilaterally as markers. Using these 3D reference points in each participant, we could reproduce TMS placement consistently across treatment sessions. While an MRI scan aided targeting cues by showing actual motor cortex in the reference system, the template approach without MRI was still sufficient to create a target location from the initial muscle response target. We targeted TMS over motor cortex, positioned to stimulate the primary muscle group in the affected region. In the event that a participant had multiple affected regions, we targeted the region of the motor cortex that was associated with the most-affected CRPS area (see Table 1).
Target Identification and Resting Motor Threshold
A cooled DB-80 bent figure-8 coil (MagVenture, Atlanta) was used for all patients, which allowed deeper penetration into motor cortex. The stimulator used was a MagPro X100 (Magventure, Atlanta). The coil was oriented with anterior-to-posterior current flow. To determine the motor hotspot, the front-parietal cortex was stimulated with 50% stimulator output per pulse and the coil was advanced in 1 cm increments in a grid-like fashion. Each active motor response was annotated and saved in Brainsight as hotspots. To determine the maximal hotspot associated with the patients most-affected CRPS extremity, we placed the coil centrally to the mapped area with the most specific motor response and repeated the grid procedure until we found the site that produced the most specific and robust motor activity. We also used this protocol to define the abductor pollicus brevis motor hotspot in each patient as a corollary to the painful limb. We used EMG in participants to define the resting motor threshold (RMT) as tolerated. For participants that were unable to tolerate EMG due to adhesive allergies related to the surface electrodes, so we relied on visual inspection of muscle activity.
Once the hotspot was determined we then recorded the target using the Brainsight software; the structural details of the MRI, aside from those superficial structures used in targeting, were not used to determine the motor hotspot. Resting motor threshold (RMT) was determined using the TMS Motor Threshold Assessment Tool (MTAT 2.0), a well-established maximum likelihood parameter estimation by sequential testing (ML-PEST) tool for estimating the likely RMT (18). To determine the RMT, single pulses beginning at a 35% of maximal stimulator output intensity were delivered with the stimulation parameter changing to determine the RMT per the ML-PEST protocol as previously described (18). A positive response was defined as a ≥50 microvolt change above baseline in the targeted muscle. When EMG was not used, a positive response was defined by a visual contraction of the targeted muscle..
TMS Protocol
Stimulation was administered starting with intermittent theta burst (iTBS) delivered at 70% RMT (iTBS is a burst of 3 pulses at 50Hz repeated every 200ms, each train lasts for 2s and repeated every 10s, 600 pulses total) and followed immediately by 10 Hz stimulation delivered at 80% RMT (10Hz for 10s with an intertrain interval of 30s, 2000 pulses total) for a total of 2600 pulses (16). We carefully monitored for muscle activity throughout treatment, using EMG and/or visual observation. If any concerning muscle activity was detected (non-spurious, synchronous with stimulation that increased in strength), we reduced the TMS intensity (~5–10% of MT) and reassessed for further adjustments at the end of each train (reduced stimulation strength if muscle activity was still evident or increased strength by small steps if no muscle activity observed).
Outcome Measures and Statistical Analysis
Pain was assessed before and after each treatment session using both a formal VAS, as well as a quick verbal 0–10 numerical rating scale (NRS) for immediate characterization at the start and stop of stimulation. Post-treatment pain was also assessed at the one-week follow-up visit and via weekly electronic surveys. Our primary outcome was whether the reduction in pain from baseline (BL), at post-TMS treatment of 1 session and at the 1-week follow-up differed between participants that received 1 or 5 TMS treatment sessions. The data were slightly skewed and kurtotic, but did not significantly differ from normality. The Shapiro-Wilk test of normality revealed that the data are approximately normally distributed, p > 0.05, thus parametric analyses were conducted. Specifically, a mixed Analysis of Variance (ANOVA) was conducted with time as the within-subjects factor and group as the between-subjects factor. Following significant main effects, Bonferroni-adjusted pairwise comparisons were examined.
Our secondary outcome measure was magnitude of treatment response, defined as ≥30% reduction in pain from baseline (19). This defined treatment response is larger than the estimated placebo response in a prior trial of TMS for CRPS (14). We further defined a “major response” as ≥50% reduction in pain, which was the demonstrated treatment effect from the same TMS for CRPS trial (14). If participants responded to treatment, we continued to track their pain scores via weekly surveys for up to 4 months post intervention until response was lost for at least 2 weeks. Last, to examine differences in pain from baseline to post-TMS session 5 and from baseline to Week 2 for the participants that received 5 TMS treatment sessions, paired-samples t-tests were conducted with significance set at p < 0.025. All statistical analyses were conducted using SPSS version 21 (IBM Corp., Armonk, NY, USA). Information of participants that withdrew from the study is included in Table 1 and 3; however, their data was not included in the group averages, response rate reporting, or the statistical analyses.
Table 3.
Stimulation Parameters and Adverse Events
| Patient Number | TMS Output Adjusted | Adverse Events | Withdrawal Reason |
|---|---|---|---|
| Pre-Pilot: 5 Days of Treatment | |||
|
| |||
| Pilot-1 | Not noted | Headache, Nausea with Vomiting | |
| Pilot-2 | Not noted | Headache | |
| Pilot-3 | Not noted | Headache, Nausea with Vomiting | Scheduling |
| Pilot-4 | Not noted | Headache | |
| 1 Day of TMS Treatment | |||
|
| |||
| 5 | 1 Session | None Reported | |
| 10 | 1 Session | None Reported | |
| 18 | 1 Session | None Reported | |
| 19 | 1 Session | None Reported | |
| 20 | 1 Session | Head Pain | Head Pain |
| 21 | 1 Session | None Reported | |
| 5 Days of TMS Treatment | |||
|
| |||
| 1 | 2 Sessions | None Reported | |
| 2 | 4 Sessions | None Reported | |
| 3 | 2 Sessions | Painful Muscle Spasms | Muscle Spasms |
| 4 | 3 Sessions | Painful Muscle Spasms, Headache, Fatigue | |
| 6 | 5 Sessions | None Reported | |
| 7 | 3 Sessions | Procedural Discomfort | |
| 8 | 0 Sessions | Headache | Head Pain |
| 9 | 5 Sessions | EMG electrode induced pain, Mild Head Pain | |
| 11 | 0 Sessions | Mild Head Pain | |
| 12 | None Reported | Availability | |
| 13 | 1 Session | Mild Head, Neck, and Gingival Pain | |
| 14 | 2 Sessions | Increased Pain in All Affected Limbs | |
| 15 | 1 Session | Head and Neck Pain, Nausea (Patient predisposed due to TMD†) | |
| 16 | 3 Sessions | None Reported | |
| 17 | 3 Sessions | Head Pain | |
Temporomandibular Joint Disorders
Adjusted outputs ~5–10% of intensity
Results
Participants
A total of 21 individuals (19 female and 2 male) participated in the trial. Mean age was 44.0 years (range: 23 – 76 years, SD = 13.9 years), and mean pain duration was 5.4 years (range: 0.5 – 15.5 years, SD = 3.8 years). At baseline, mean pain intensity was 6.6 on a 10-point VAS scale (range: 3.1 – 9.1, SD = 1.7). Of the 21 individuals, based on scheduling constraints and patient preference, 6 patients were enrolled for a single TMS session while 15 patients were enrolled for 5 consecutive sessions of TMS over 5 days. One patient did not complete the single TMS session protocol, due to intolerable head pain. Three patients did not complete the 5 TMS sessions protocol, 1 of which withdrew mid-protocol due to adverse events and 1 due to adverse head pain related to stimulation, and 1 by personal preference due to scheduling conflicts.
Group Comparison of TMS Treatment Effect
A mixed ANOVA was conducted to examine whether 5 TMS treatment sessions produced a greater reduction in pain from baseline, immediately following the first treatment session and Week 1 follow-up compared to 1 treatment session. The analysis revealed a significant main effect of time, F(2,30) = 9.34, p = 0.001, but failed to reveal a significant main effect of treatment length, F(1,15) = 0.05, p = 0.82 and a significant interaction, F(3,3) = 2.05, p = 0.15. Bonferroni corrected post hoc tests showed that compared to baseline pain ratings, TMS treatment significantly reduced pain immediately following treatment (p = 0.002) and one week later (p = 0.024), although there was no difference in the magnitude of treatment response between 1 and 5 TMS treatment sessions at the 1 week follow-up.
TMS Treatment Response Rates
Given that each TMS treatment group demonstrated a significant reduction of pain over time, we characterized the magnitude of treatment response, see Table 2. Immediately following the single session of TMS treatment, participants demonstrated a 60% response rate (and 40% major response rate). At the Week 1 follow-up, there was still a 60% response rate (and a 60% major response rate). For participants that received 5 TMS treatment sessions, there was a 42% response rate (and 17% major response rate) immediately following the first day of treatment which increased to a 58% response rate (and 42% major response rate) immediately following the fifth treatment session (t(11) = 3.98, p = 0.002; baseline to immediate post-treatment session 5). At the Week 1 follow-up, there was still a 58% response rate (and a 25% major response rate). Similarly, at the Week 2 follow-up, participants continued to show a 50% response rate (and a 25% major response rate) and reported pain scores continued to be significantly different compared to baseline, t(11) = 3.16, p = 0.009.
Table 2.
Treatment Response and Length
| Patient Number | Pre-TMS Baseline Pain | Post-TMS Session 1 Pain | Post-TMS Session 5 Pain | Follow Up Week 1 Pain | Follow Up Week 2 Pain | Response Duration (Weeks) |
|---|---|---|---|---|---|---|
| 1 Day of TMS Treatment | ||||||
|
| ||||||
| 5 | 3.1 | 2.3 | 1.4** | 1.6* | 14+ | |
| 10 | 8.8 | 7.2 | 8.8 | 9.4 | - | |
| 18 | 8 | 5.4* | 9.1 | - | 0 | |
| 19 | 7.4 | 0.0** | 2.7** | 3.1** | ~ 6+ | |
| 20 (w) | 8.6(w) | (w) | (w) | (w) | (w) | |
| 21 | 5.3 | 0.5** | 1.0** | - | ~ 8+ | |
|
| ||||||
| Pain Average | 6.50 | 3.10 | 4.60 | 4.70 | ||
| Response Rate | 0.60 | 0.60 | 0.40 | |||
|
| ||||||
| 5 Days of TMS Treatment | ||||||
|
| ||||||
| 1 | 6.6 | 1.4** | 0.6** | 0.9** | 1.5** | 3 |
| 2 | 5.8 | 5.5 | 3.9* | 3.5* | 3.3* | 4 |
| 3 (w) | 9.1(w) | 10(w) | (w) | (w) | (w) | (w) |
| 4 | 4 | 1.7** | 1.1** | 2.5* | 2.0* | ~ 4 |
| 6 | 5.8 | 6.3 | 3.0* | 7.4 | 5.5 | 0 |
| 7 | 8.3 | 6 | 7.9 | 5.4* | 7.2 | 1 |
| 8 (w) | 7.3(w) | 8(w) | (w) | 8.5(w) | 7.6(w) | (w) |
| 9 | 4.6 | 2.6* | 3.9 | 4.3 | 3.8 | 0 |
| 11 | 5.6 | 5.4 | 5.2 | 4.9 | 6.6 | - |
| 12 (w) | 5.9(w) | (w) | (w) | (w) | (w) | (w) |
| 13 | 5.6 | 3.0* | 2.7** | 3.9* | 1.4** | ~ 16+ |
| 14 | 8.9 | 7.5 | 9.1 | 9.6 | 9.8 | - |
| 15 | 7.8 | 7.1 | 6.1 | 6.6 | 6.3 | - |
| 16 | 6.2 | 4.0* | 0.0** | 0.0** | 0.0** | 14+ |
| 17 | 6.2 | 8.2 | 0.4** | 2.6** | 3.3* | 2 |
|
| ||||||
| Pain Average | 6.30 | 4.90 | 3.66 | 4.30 | 4.20 | |
| Response Rate | 0.42 | 0.58 | 0.58 | 0.50 | ||
= Response ( > 30% Decrease in Pain from Pre-TMS Baseline)
= Major Response ( > 50% Decrease in Pain from Pre-TMS Baseline)
~ is estimated with some missing weekly pain reports
Pain Averages includes only patients that completed the trial.
Response Rate is number of responders over total completed.
Length of Treatment Response
The progressive pain decrease and rebound in time observed in the pre-pilot study, led us to include the length of treatment response (decrease in pain scores from baseline) as a key measure. For the 5 individuals with 1 day of TMS, we tracked the length of treatment response for the 4 participants that had a response at some point in the trial (Table 2). One individual had a delayed response that lasted over 14 weeks, one individual had an immediate response that did not last to the 1 week follow up, and two individuals achieved a major response lasting beyond 6 or 8 weeks (estimated, as some reports missed and not continued beyond 6 or 8 weeks). For the 12 individuals with 5 days of TMS, we tracked the length of treatment response for the 9 individuals that had a response at some point in the trial (Table 2). Two of those individuals demonstrated responses only immediately following treatment, five individuals had estimated response ranging between 1 – 4 weeks, and two individuals reported a major response beyond 14 or 16 weeks.
Characterization of Prior Treatments
As we do not have a placebo control in this trial, it is important to note the extensive treatment history for our patients. We asked each participant to report on previous treatments tried, and to indicate whether those treatments helped symptoms, had no effect, or made symptoms worse. Figure 1 shows the positive response rates, defined as participants indicating “treatment helped,” to all treatments tried by at least 6 participants (with 95% confidence intervals calculated by the Wilson procedure), to compare with the positive response rate to TMS at the week 1 follow up (n = 21 combined from the single session protocol and the 5 sessions protocol of TMS treatment; “helped symptoms” operationalized as a treatment response). Despite all the various treatments tried, we note that all participants entered the study with high levels of pain despite remaining on their current treatment regimens. Thus, some participants experienced substantial and long-lasting relief beyond other treatments.
Adverse Events
Adverse events for the pre-pilot study and the pilot trial are listed in Table 3 and results discussed below include all participants, withdrawn and completers. In the pre-pilot study, three out of 4 participants report headache following stimulation, with 2 of those reporting nausea and vomiting approximately 6–8 hours after treatment. Nausea and vomiting resolved within 24 hours, and no focal neurological findings were reported upon assessment via communication with a protocol supervising physician.
In the pre-pilot study, the most common adverse events for these participants were head pain and headache, with a few participants also reporting neck pain. Two participants had TMS or procedurally-induced muscle spasms in the affected areas. Nausea, but not vomiting, was reported in the pilot trial by only one participant. No participants experienced a seizure; however, we had to adjust stimulation strength for 18 out of the 21 participants due to evidence of cortical excitability and spread during treatment. Approximately 10% of adjustments occurred during the iTBS portion and 90% of adjustments occurred during the subsequent 10 Hz portion.
DISCUSSION
Summary
This is the first study to demonstrate significant pain relief from TMS for participants with CRPS Type I and II. In the largest and most diverse CRPS population tested to date, we found treatment responses across disease type (CRPS I and II) and somatic location (upper and lower extremities). Our protocol involved individual targeting of primary motor cortex (mapped to the participant’s most affected extremity), using theta-burst primed high-frequency stimulation (16). Following 5 days of treatment, we found a very promising response rate (58% of participants at Week 1 follow up, 50% of participants at Week 2 follow up) for individuals that have failed numerous other treatment approaches. Interestingly, similar response rates were found in participants that received only one day of treatment (60% of participants at Week 1 follow up). Length of response in both cohorts(although not significantly different between treatment session lengths) is also promising, and future work should examine additional maintenance sessions (similar to depression treatment) and integration with interdisciplinary care.
Effects in an Expanded Patient Cohort
The rate of response is notable for our cohort, and the dramatic difference between responders versus non-responders should be further explored. Our study indicates that TMS may have benefit for a broad range of patients, as we are the first TMS study to include participants with Type II CRPS and participants with lower extremity pain. While this was not a blinded placebo-controlled study; our response rate is consistent with the 58% rate reported in a controlled study (14), We also note that patients in our study had a large range in pain duration and extremely variable responses from numerous other treatments with the majority of reported previous treatments providing a positive response less than half the time.
The mechanism of pain relief through motor cortex stimulation is unclear, although motor cortex plays an important role in the perception of pain (12). Separate from the magnitude of response, our observational impression was that there is a bimodal split between responders and non-responders. This bimodal response or non-response may have significance with regard to underlying etiology or number of limbs affected. For those with multiple limbs affected, simply targeting the multiple cortical regions associated with each limb for treatment, may also increase efficacy of treatment response. Better characterization of these groups (and larger sample sizes) and the examination of multiple target treatments would be useful for understanding mechanisms and customizing treatment plans (6) and is something we plan to do in the future.
Length of Treatment Response
Durability of pain relief has been a particular concern in other trials exploring the use of TMS as a potential treatment (9), and this study demonstrates that it is possible to generate long-lasting effects. In the first ever trial of single-session TMS for CRPS (13), one participant still had significant pain relief at the last time point (90 minutes) despite subsequent interpretations that the effect was short lived. We were surprised in our study to see long-lasting pain alleviating effects from only a single-session in some of our participants, but this may be due to LTP-like effects with the combined 10 Hz rTMS and iTBS (20,21,16,10) or to the intervention facilitating functional recovery (4). It should be noted that our study does not allow for direct comparison of a durable treatment effect of combined use of high frequency rTMS (HFr TMS) and iTBS given the lack of appropriate control with HFr TMS alone. A second multi-session study of 10 Hz rTMS did not find a continued treatment group effect at 1-week follow-up, but researchers anecdotally report that 1 participant was pain free at the 3-month follow-up (14). Our study supports the possibility of long-term effects, but the optimal number and spacing of treatments may need individualization. We aimed to characterize effects for pain relief following only 1 session of treatment versus 5 days of treatment sessions, hypothesizing that 5 days would result in a greater response and length of pain relief (22). Surprisingly, we did not find a difference between the magnitude and length of pain alleviation, between 1 versus 5 sessions of treatment. However, given our limited sample size, further work is still needed to establish the optimal approach to achieve and maintain effects.
TMS protocol distinction (Resting versus Active Motor Threshold)
One distinction in the methods section of this study as compared to the work of Huang et al., (23) and Lefaucher et al., (16), is that we used resting motor threshold as opposed to active motor threshold to inform stimulation intensity parameters. One reason to preclude the use of AMT specifically in our participant population is that as per Rossi et al., (10) (TMS consensus safety paper section 4.3.3) the RMT is approximately 20% higher than the AMT and we adjusted the intensity of the TBS by this amount in each participant using the RMT obtained to more closely mirror these original parameters that employed 90% AMT as the stimulation intensity. In our study we used 70% RMT to reduce the risk of hyperstimulation with TBS and the possible increased risk of cortical excitability spread and seizures. Second, the heterogeneous nature of the TMS targeting that corresponded to the myotomal nerve distributions serving the affected limbs in the patient population (variable limb locations) made it difficult from a practical standpoint to obtain AMT in this study. Last, CRPS is known to be exacerbated with physical exertion of the affected limb. Given the need to employ muscle activation in the motor unit subserved by the motor cortex target during acquisition of AMT, we opted to use the more standard RMT in all participants to ensure reliable and reproducible threshold targets without the risk of exacerbating the baseline pain of our patient population.
Adverse Events
Treatment was generally well tolerated, with no serious adverse events reported. Head pain is common with TMS, although nausea and vomiting are not (10). It is possible patient characteristics (history of migraines) or other factors contributed to these events; and we further note that a link between forehead hyperalgesia and motion sickness has been characterized in a subgroup of patients with CRPS (24). The timing of nausea (several hours post-treatment) is particularly curious, and the delay might reflect extended neural effects. It is also possible factors such as travel or acute illness contributed to some cases of nausea. While no seizures occurred during this study, we do warn that failure to monitor and adjust treatment with this protocol could potentially increase risks. A seizure was reported in a prior treatment study of TMS for CRPS (14), and we did observe the initiation of cortical spread requiring close monitoring and quick reduction in stimulation power. Our monitoring and immediate adjustments (~5–10% of intensity) likely helped prevent seizures. We note it may be challenging to monitor patients with intrinsic muscle movements (myoclonus, dystonia or medication tremors), and EMG when tolerated can be valuable for distinguishing the rhythmic patterns of TMS induced effects.
Limitations
CRPS is a rare condition with an estimated prevalence of 20 in 100,000 (1,2), and as such, traditional study designs may be limited by sample size and patient needs (e.g. travel and concurrent treatment plans). Thus, this current study does not meet the level of evidence required for research on more common pain conditions (sample size, blinding, placebo-controls, etc.). However, CRPS is characterized by severe continuous pain, and the reported immediate and sustained analgesic effects are less likely to occur from unrelated fluctuations in pain. This study is an informal comparative effectiveness trial, as these participants had inadequate relief from other concurrent treatments. We do recognize that treatment expectancy and placebo effect may account for some of the response observed given that both the experimenters and participants know active treatment is administered in an open label design. However, as previously noted, the response rates reported herein are similar to those in a sham-controlled trial for CRPS (14). Another potential limitation of this study is that our sample was predominantly women, although CRPS is estimated to be more prevalent in women (2). Given the rarity of the condition and the distance that participants travel for treatment, formal comparative efficacy trials are needed for this population. We note that this pilot trial will inform future research studies for CRPS treatments ideally with adequately powered randomized controlled trials that establish efficacy of the treatment in CRPS and guide future clinical application.
Recommendations and Conclusions
Our research shows that TMS can provide relief for many patients suffering from CRPS, particularly for patients who have not responded well to conventional medical therapies. TMS administration should be conducted with great care, with careful monitoring for cortical excitability and spread that would suggest seizure potential. Future work is needed to refine and customize treatment for each patient, and we note that TMS may be enhanced by integration into an interdisciplinary care treatment plan (4). In conclusion, our open-label non-randomized pilot trial demonstrated significant pain relief in individuals with CRPS Type I or II affecting upper and/or lower extremities using a TMS protocol of theta-burst primed high-frequency stimulation individually targeted over the motor cortex of the somatic pain location. Based on these findings, we plan to formally examine the efficacy and durability of this TMS protocol for CRPS patients in a randomized, placebo-controlled trial.
Acknowledgments
Financial Support: Funding by Rocky Mountain CRPS/RSD Non-Profit Organization, Feldman Family Foundation, The Kenneth Rainin Foundation, Stanford Medical Research Program (Gaertner), and NIH NIDA 5K23DA031808 (Johnson), NCCIH 1K23AT008477 (Kong), and NIH NIDA 5K24DA029262 (Mackey).
The authors would like to thank Rebecca McCue, Jen Hah, and Drew Sturgeon for administrative, medical, and advisory support.
Footnotes
Authorship Statement: Mark Gaertner, Jiang-Ti Kong, Sean Mackey, Kevin Johnson, and Alyssa Foote were responsible for the study design. Mark Gaertner, Jiang-Ti Kong, Kevin Johnson and Alyssa Foote were responsible for study execution. Mark Gaertner, Kevin Johnson, Sean Mackey and Kristen Scherrer were responsible for Data Analysis and interpretation. Mark Gaertner, Jiang-Ti Kong, Kevin Johnson and Kristen Scherrer were responsible for manuscript preparation. All authors approved the final submitted manuscript.
Potential conflicts of interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13(3):242–265. doi: 10.1016/j.autrev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Rockett M. Diagnosis, mechanisms and treatment of complex regional pain syndrome. Curr Opin Anaesthesiol. 2014;27(5):494–500. doi: 10.1097/ACO.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell NE, Wand BM, McAuley J, Marston L, Moseley GL. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev. 2013;4:CD009416. doi: 10.1002/14651858.CD009416.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, et al. Reflex Sympathetic Dystrophy Syndrome A. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med. 2013;14(2):180–229. doi: 10.1111/pme.12033. [DOI] [PubMed] [Google Scholar]
- 5.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81(1–2):147–154. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 6.Gierthmuhlen J, Binder A, Baron R. Mechanism-based treatment in complex regional pain syndromes. Nat Rev Neurol. 2014;10(9):518–528. doi: 10.1038/nrneurol.2014.140. [DOI] [PubMed] [Google Scholar]
- 7.Bean DJ, Johnson MH, Kydd RR. The outcome of complex regional pain syndrome type 1: a systematic review. J Pain. 2014;15(7):677–690. doi: 10.1016/j.jpain.2014.01.500. [DOI] [PubMed] [Google Scholar]
- 8.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26(1):13–18. doi: 10.1097/YCO.0b013e32835ab46d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Cortical representation of pain in primary sensory-motor areas (S1/M1)--a study using intracortical recordings in humans. Hum Brain Mapp. 2013;34(10):2655–2668. doi: 10.1002/hbm.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pleger B, Janssen F, Schwenkreis P, Volker B, Maier C, Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356(2):87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 14.Picarelli H, Teixeira MJ, de Andrade DC, Myczkowski ML, Luvisotto TB, Yeng LT, et al. Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. 2010;11(11):1203–1210. doi: 10.1016/j.jpain.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Velasco F, Carrillo-Ruiz JD, Castro G, Arguelles C, Velasco AL, Kassian A, et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain. 2009;147(1–3):91–98. doi: 10.1016/j.pain.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, Ciampi de Andrade D, et al. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain. 2012;16(10):1403–1413. doi: 10.1002/j.1532-2149.2012.00150.x. [DOI] [PubMed] [Google Scholar]
- 17.Harden RN, Maihofner C, Abousaad E, Vatine JJ, Kirsling A, Perez RSGM, Kuroda M, Brunner F, Stanton-Hicks M, Marinus J, van Hilten JJ, Mackey S, Birklein F, Schlereth T, Mailis-Gagnon A, Graciosa J, Connoly SB, Dayanim D, Massey M, Frank H, Livshitz A, Bruehl S. A prospective, multisite, international validation of the Complex Regional Pain Syndrome Severity Score. Pain. 2017 Aug;158(8):1430–1436. doi: 10.1097/j.pain.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 18.Borckardt JJ, Nahas Z, Koola J, George MS. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J ECT. 2006;22(3):169–175. doi: 10.1097/01.yct.0000235923.52741.72. [DOI] [PubMed] [Google Scholar]
- 19.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 20.Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586(16):3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565(Pt 3):945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Long lasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen LF, Drummond PD. Optokinetic stimulation increases limb pain and forehead hyperalgesia in complex regional pain syndrome. Eur J Pain. 2015;19(6):781–788. doi: 10.1002/ejp.602. [DOI] [PubMed] [Google Scholar]


