Abstract
Efforts to translate sub-anesthetic ketamine infusions into widespread clinical use have centered around developing medications with comparable neurobiological activity, but with attenuated psychoactive effects so as to minimize the risk of behavioral toxicity and abuse liability. Converging lines of research, however, suggest that some of the psychoactive effects of sub-anesthetic ketamine may have therapeutic potential. Here, we assess whether a subset of these effects – the so-called mystical-type experience – mediates the effect of ketamine on craving and cocaine use in cocaine dependent research volunteers. We found that ketamine leads to significantly greater acute mystical-type effects (by Hood Mysticism Scale: HMS), dissociation (by Clinician Administered Dissociative States Scale: CADSS), and near-death experience phenomena (by the Near-Death Experience Scale: NDES), relative to the active control midazolam. HMS score, but not the CADSS or NDES score, was found to mediate the effect of ketamine on global improvement (decreased cocaine use and craving) over the postinfusion period. This is the first controlled study to show that mystical-type phenomena, long considered to have therapeutic potential, may work to impact decision-making and behavior in a sustained manner. These data suggest that an important direction for medication development is the identification of ketamine-like pharmacotherapy that is selectively psychoactive (as opposed to free of experiential effects entirely), so that mystical-type perspectival shifts are more reliably produced and factors lending to abuse or behavioral impairment are minimized. Future research can further clarify the relationship between medication-occasioned mystical-type effects and clinical benefit for different disorders.
Keywords: addiction, cocaine, hallucinogen, ketamine, mystical experience, psychedelic, psychoactive effects
1. Introduction
The dissociative anesthetic and N-methyl-D-aspartate antagonist ketamine has demonstrated a variety of sustained psychiatric benefits when infused in the sub-anesthetic range (below 1.2 mg/kg/hr), including anxiolytic, anti-depressant, and anti-addiction effects (Dakwar et al., 2014a; Iadarola et al., 2015; Sanacora and Schatzberg, 2015). Its anti-depressant activity has been linked to the enduring promotion of neural plasticity in prefrontal regions (Li et al., 2010), perhaps influenced by a metabolite with a relatively long half-life (Zanos et al., 2016). This mechanism of promoting plasticity, which has been shown to involve the elaboration of brain-derived neurotrophic factor (BDNF) as well as glutamate modulation (Maeng et al., 2008; Liu et al., 2010), may also be involved in the effects of ketamine on other psychiatric conditions, such as anxiety and substance use disorders.
Sub-anesthetic ketamine also engenders infusion-dependent and transient psychoactive effects: a variety of dissociative, psychotomimetic, and mystical-type experiences (Perry et al., 2007; Dakwar et al., 2014b). These phenomena are thought to increase abuse liability and the risk of behavioral impairment, and researchers are therefore developing and testing agents with neural mechanisms comparable to ketamine, though without psychoactive effects, so as to increase the likelihood of widespread clinical dissemination (Sanacora and Schatzberg, 2015). A sub-set of these psychoactive effects, however, may be important to therapeutic mechanism. Dissociative phenomena have been correlated with anti-depressant activity (Luckenbaugh et al., 2014), and according to data collected in a small trial, the intensity of mystical-type experience elicited by ketamine may enhance motivation to quit drug use (Dakwar et al., 2014b). These preliminary findings suggest that the non-ordinary experiences associated with ketamine are somehow related to its efficacy, and that they are not simply epiphenomena that present more risk than benefit.
Scientific speculation concerning the role of non-ordinary experience in mental health began more than a century ago. William James was the first psychologist of note to propose that non-ordinary states of consciousness, specifically those analogous to religious or mystical experience, might harbor transformative potential, with the propensity to trigger long-term shifts in moral values and decision-making (James, 1907). Sigmund Freud was intrigued by the therapeutic potential of trance-like or hypnotic states, and oriented the process of psychoanalysis around the cultivation of a conscious state conducive to free-association, fantasy, and introspection (Freud, 1995). With the discovery in the early 20th century of the potent serotonergic hallucinogen lysergic acid diethylamide (LSD), psychiatric researchers aimed to understand how the psychoactive properties of LSD and related compounds might be utilized therapeutically (Hofmann, 1980). The psycholytic (from the Greek psyche lysis: mind dissolving) model posited that the dream-like effects of LSD might facilitate psychoanalysis by weakening the defenses that preclude introspection, recollection, and fantasy, while the psychedelic (from psyche delos: mind manifesting) model, in deference to James, aimed to therapeutically utilize the dramatic shifts in perspective on the self and on the world that might emerge during LSD administration, and which are comparable to revelatory or mystical experience (Grof, 2001). Accordingly, the psychotherapeutic framework in the latter model worked to prepare participants for the “psychedelic” experience, to provide guidance when LSD or a comparable compound was administered, and to support integration of the perspectival changes subsequently (Grof, 2001). With the criminalization of serotonergic hallucinogens (primarily due to exaggerated claims of risk and harmful effects), however, these models have fallen into disrepute and neglect, with medication-occasioned non-ordinary experiences now generally viewed as problematic effects that do little but confer abuse liability and behavioral toxicity (Grinspoon and Bakalar, 1979).
Recent research with the 5HT2A agonist psilocybin has revived and partly rehabilitated the psychedelic model (Johnson et al., 2008), and secondary analyses have suggested that the intensity of mystical-type experience during psilocybin sessions correlates with improvements in anxiety or depressive symptoms, as well as in quality of life and substance use outcomes (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014; Griffiths et al., 2016). As mentioned earlier, the intensity of mystical-type experience appeared to also mediate the effect of ketamine on motivation to quit cocaine in a human research laboratory, raising the possibility that such medication-occasioned experiences might be therapeutic even outside psychedelic frameworks – that is, when individuals are not specifically prepared for and guided towards mystical-type experiences, and when therapist-guided integration of the experience does not occur. This study had several limitations, however, including a small sample size, the lack of behavioral measures, and a restricted survey of psychoactive effects (Dakwar et al, 2014b). These limitations made it difficult, moreover, to conclude that mystical-type effects do indeed constitute a mediator, as opposed to a marker of potency. The purpose of this secondary analysis is to expand on this preliminary study by revisiting a completed laboratory-based trial examining the effect of ketamine on cocaine self-administration in a larger sample of cocaine dependent research volunteers (Dakwar et al., 2017), and to investigate whether mystical-type experience mediates the anti-addiction benefits of ketamine using behavioral, in addition to subjective, outcomes. Another purpose is to examine whether ketamine produces a sub-set of mystical-type experience that has been anecdotally (though not experimentally) associated with it, the near-death experience (NDE) (Luke and Kittenis, 2005), and to explore whether NDE-type phenomena also serve to mediate efficacy. We predict that, compared to an active control (midazolam), ketamine elicits mystical-type experience, dissociative phenomena, and NDE-type effects. We also predict, in line with the central hypothesis of the “psychedelic model”, that mystical-type experience (and not dissociative phenomena) mediates the efficacy of ketamine on the main outcomes of interest: cocaine self-administration, cocaine use in the natural ecology, and cocaine craving.
2. Methods
2.1. Study Design
20 non-depressed, cocaine dependent individuals disinterested in treatment or abstinence completed this crossover trial approved by the New York State Psychiatric Institutional Review Board; complete data on psychoactive effects were available for 18 of them. After understanding research risks and providing informed consent, participants were hospitalized up to 3 times; they resided on a controlled research unit for 6 days at a time, and each hospitalization was separated by two weeks to account for carry-over effects and to assess cocaine use in the natural ecology.
Each 6-day hospitalization involved an initial 2-day washout period; a 28-minute “sample session” on Day 3 when 2 obligatory free-base cocaine doses (25 mg) were smoked to allow for assignment of value to the research cocaine and to intensify craving; a 52-minute i.v. infusion on Day 4; a 70-minute “choice session” of 5 choices (25 mg cocaine vs. $11) on Day 5; and discharge on Day 6. During the first hospitalization, participants received an infusion of normal saline so as to identify, and exclude from research, individuals who do not robustly choose cocaine prior to the active infusions (≤2 choices).
In the second and third hospitalizations, participants were randomized (1:1) to counterbalanced orderings of 52-minute sub-anesthetic infusions of ketamine (0.71 mg/kg) or of the active control midazolam (0.025 mg/kg) under double-blind conditions. Following each infusion, participants were administered a battery of measures intended to ascertain infusion-dependent psychoactive effects, including dissociative, mystical-type, and near-death experiences. No psychotherapy or behavioral treatment was provided. The primary outcome for the trial was reduction in cocaine self-administration, and secondary outcomes were reductions in craving and in cocaine use in the natural ecology.
The percent improvement (0–100%) was calculated relative to baseline for each outcome. A composite improvement score was determined by assigning proportional weight to the three domains of improvement using a calculation designated a priori, with improvement in the primary outcome – cocaine self-administration – assigned the greatest weight (0.5 of the score), while improvements in cocaine use and in craving in the natural ecology assigned less weight (0.35 and 0.15, respectively). Participants who did not use cocaine post-infusion and through the 2-week follow-up were assigned a composite score of 100%, which corresponds to maximal gain.
2.2. Participants
We recruited participants by word of mouth, advertising, and referral. At the first contact, a standardized telephone interview was conducted. Individuals who preliminarily met entry criteria were scheduled for a first screening visit, during which they gave informed consent to undergo a full screening procedure, which has been described elsewhere (Dakwar et al., 2017). Applicants were considered eligible if they were medically healthy, non-treatment seeking cocaine dependent individuals. Individuals with physiological dependence on certain other substances (opioids, alcohol, benzodiazepines), with a history of psychotic or dissociative symptoms, with current depressive or anxiety symptoms indicative of a Diagnostic Statistical Manual, 4th edition (DSM-IV) disorder, with a first-degree family history of psychosis, with obesity (BMI > 35), or with cardiovascular or pulmonary disease were excluded. Eligible patients were scheduled for another visit during which they provided informed consent and were admitted into the protocol.
2.3. Cocaine self-administration and Infusion Procedures
The cocaine administration procedures in this study were identical to those previously used at our Institution and elsewhere, and the laboratory paradigm of self-administration was adapted from established models evaluating medication effects on abstinence initiation (Dakwar et al., 2017).
Sample sessions involved the administration of two cocaine doses (25 mg cocaine base) starting at 1 pm on Day 3 of each hospitalization, with each dose separated by 15 minutes; and on Day 5, participants underwent a session of five choices (25 mg cocaine or $11, every 15 minutes), starting at around 2 pm (about 27 hours post-infusion). Earned money was provided at discharge from the inpatient laboratory on Day 6.
In addition to the sham infusion in Inpatient Phase 1 (saline over 52 minutes), two counter-balanced active infusions were administered, each on Day 4 of Inpatient Phases 2 and 3: active control (2-min saline bolus followed by midazolam 0.025 mg/kg in saline infused over 50 minutes) or ketamine hydrochloride (0.11 mg/kg 2-min bolus followed by 0.60 mg/kg in saline over 50 minutes). Choice behavior, as well as craving and cocaine use in the natural ecology, following the sham infusion served as the baseline for determining the percent reduction in these outcomes for the active conditions. So as to minimize risk, infusions were given in a highly controlled inpatient setting.
To address expectancy effects and further protect the blind, participants were informed throughout the study that they may possibly receive any of a number of substances at each infusion, including amantadine, buspirone, d-cycloserine, ketamine, lorazepam, memantine, methamphetamine, saline, or any combination of these. This blinding procedure was intended to disguise what drug is specifically given so as to minimize expectancy. Similarly, it aimed to minimize the risk that participants would clearly identify medications. A full description of procedures and safeguards during infusions is available in previous reports (Dakwar et al., 2017, 2014a).
Participants met thrice weekly with research staff for 2 weeks following each hospitalization. They provided urine at each visit for toxicology testing; provided information on drug use; and completed various assessments and questionnaires pertaining to cocaine craving, reactivity, and side effects from the study medications.
2.4. Assessment of Psychoactive Effects
Three validated instruments were used to ascertain psychoactive effects, and trained research assistants administered them at 20 minutes following the conclusion of the infusion: 1) the 27-item Clinician Administered Dissociative States Scale (CADSS), which assesses for derealization, depersonalization, psychic fragmentation, and other dissociative states (Bremner et al., 1998); 2) the 32-item Hood Mystical Experiences Scale (HMS), which concerns several dimension of mystical experience (e.g., ineffability, unity, noesis, sense of sacredness) (Hood, 1975); and 3) the 16-item Near-Death Experiences Scale (NDES) (Greyson, 1983). The tense of the instruments was modified to query for infusion-related phenomena, as opposed to lifetime experience, consistent with other groups (Maclean et al., 2012).
2.5. Statistical Analyses and Calculation
SAS was used to carry out all analyses. First, we investigated whether ketamine (vs. midazolam) led to NDEs, dissociative phenomena, and mystical-type experiences by comparing post-infusion scores on the HMS, CADSS and NDES; and whether ketamine led to greater cumulative improvement. Paired t tests were conducted for all analyses, with 2-tailed α = 0.05.
Second, a mediation analysis was performed by treating participants as random effects and placing all psychoactive variables (HMS, NDES and CADSS scores), along with dose of ketamine received (0 to 70.5 mg, ketamine vs. midazolam), into a single multivariate analysis, with global improvement score as the dependent variable. As first proposed by Baron and Kenny (1986), mediation was supported if the hypothesized mediator was significant in the full model (p<0.05), and if the intervention effect reduced in or lost significance (p>0.05) when controlling for hypothesized mediators. The total indirect effect (mediation) was calculated by ßsi − ßmi, and the extent to which a significant mediator contributed to the indirect effect by ßs(im)* ßmm, where coefficient ßsi pertains to the intervention variable in the univariate model; ßmi to the intervention variable in the full model; ßs(im) to the intervention variable in relation to the mediator in a univariate analysis; and ßmm to the mediator variable in the full model.
3. Results
3.1. Participants
Full data on psychoactive effects and cumulative response were available for 18 participants, who had high mean baseline use and were otherwise psychiatrically uncomplicated (Table 1). All participants tolerated study procedures without adverse events, including unexpected psychiatric disturbances and initiation of ketamine or benzodiazepine misuse.
Table 1.
Participant Demographic and Morbidity Information (n=18)
| Age, Years (SD) | 49.8 (5.7) |
|---|---|
| African-American | n= 13, 72.2% |
| Hispanic | n= 4, 22.2% |
| White | n= 1, 5.6% |
| Male | n= 10, 55.6% |
| History of PTSD | n=3, 16.8% |
| Comorbid Alcohol Use Disorder | n=2, 11.2% |
| Unemployed | n= 15, 83.3% |
| Education (>12 Yrs H.S. equivalent) | n= 15, 83.3% |
| Cocaine Use Days/Past Month (SD) | 9.4 (7.1) |
| Cocaine Amount/Day, $ (SD) | 49.82 (32.21) |
3.2. Psychoactive Effects
Ketamine led to significantly greater levels of all psychoactive effects than did midazolam. On the HMS, ketamine was associated with a score of 103 (s.e.=7.8) vs. 63 (s.e.=9.8) with midazolam, p < 0.001; ketamine 10.7 (s.e.=1.6) vs. 4.3 (s.e.=0.8) midazolam on the NDES, p < 0.001; and ketamine 24.5 (s.e=3.4) and midazolam 4.9 (s.e.=1.2) on the CADSS, p < 0.001. (Figure 1).
FIGURE 1.
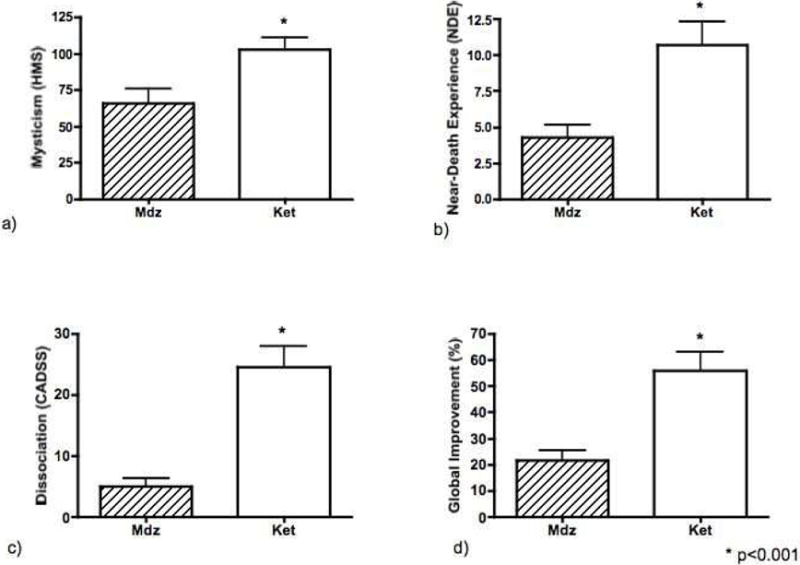
Psychoactive and Therapeutic Effects of a Single Sub-anesthetic Ketamine Infusion. Ketamine 0.71 mg/kg over 52 min (Ket) produced greater psychoactive effects on all measures than did midazolam 0.025 mg/kg over 52 min (Mdz) (a–c) as ascertained shortly after the infusion, and was also associated with greater post-infusion global improvement (d), which reflected improvement in subsequent cocaine self-administration, craving, and cocaine use in the natural ecology.
3.3. Global Improvement
Ketamine led to significantly greater global improvement (decreases in cocaine self-administration, cocaine use in the natural ecology, and cocaine craving) than did midazolam, as suggested in the primary analysis from a previous manuscript. Ketamine was associated with a score of 56% (s.e.=7%), while midazolam was associated with a score of 20.7% (s.e.=3.8%), p < 0.001.
3.4. Mediation
HMS score was the only significant mediator (ß= 0.431, p = 0.0175) (Figure 2). Ketamine dose was not significant (p=0.0562), supporting mediation. The total indirect effect was 0.2029 (=0.5676-0.3647), which explained 35.7% (=0.2029/0.5676) of total treatment effect.
FIGURE 2.
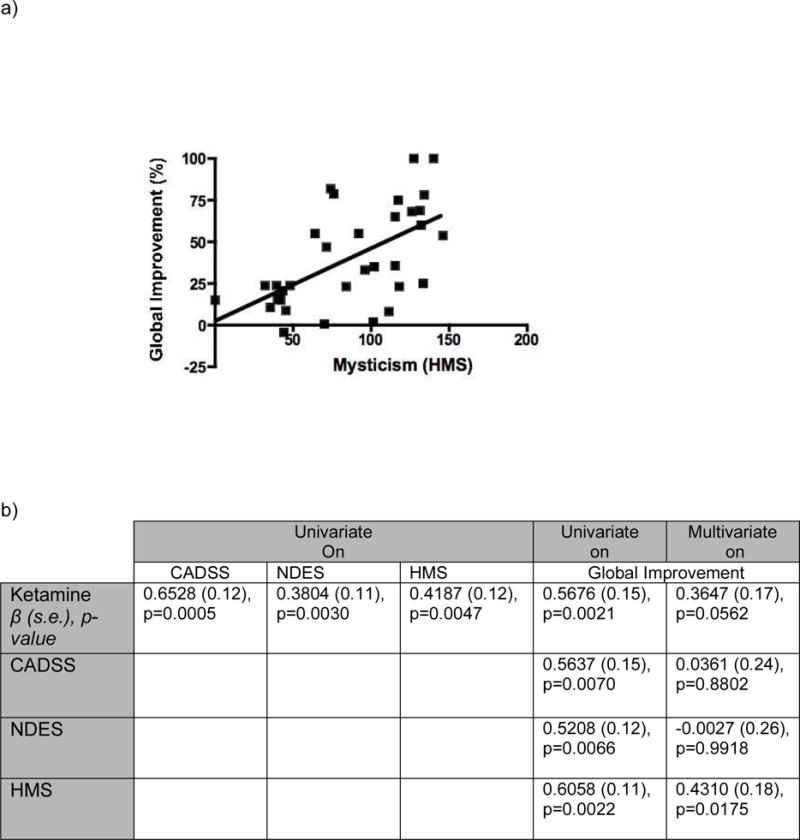
(a) Regression plot assessing correlation between mystical experience and global improvement, and (b) β values calculated from univariate regressions examining the effect of ketamine on psychoactive effects, and from univariate/multivariate analyses with respect to change in dependent variable (global improvement) for ketamine-induced dissociation (CADSS score), mystical-type experience (HMS score), and near-death experience related phenomena (NDES), as well as for ketamine dose received, ranging from 0 to 70.5 mg (midazolam vs. ketamine infusions). The correlation between mystical-type experience and global improvement is represented in (a), and was significant in a multivariate analysis, β = 0.431, p = 0.0175 (b), suggesting that mystical-type phenomena mediate the benefits of ketamine in this sample.
4. Discussion
As predicted, improvements in cocaine self-administration, cocaine use in the natural ecology, and cocaine craving were found to be mediated by HMS score, suggesting that mystical-type phenomena played a central role in the behavioral impact of ketamine. We also showed that ketamine elicits a variety of psychoactive effects, all of which were transient and well tolerated within our administration procedures, with no incidence of behavioral toxicity or the emergence of misuse.
These findings contribute to our growing understanding of the neural and experiential complexity of ketamine. Until recently, the psychoactive effects of ketamine have been characterized primarily in relation to its use as a drug model of psychopathology. As a known psychotomimetic and dissociative anesthetic, sub-anesthetic ketamine has been long recognized to lead to dose-dependent and transient dissociative and psychotic-like phenomena (Eickoff et al., 2014; Frohlich and Van Horn, 2013; Perry et al., 2007). As the past two decades have seen growing interest in the therapeutic uses of sub-anesthetic ketamine, researchers have devoted greater attention to its potentially beneficial neurobiological and psychoactive effects. An early precedent was set by Kruptisky et al. (1997, 2002), who evaluated ketamine more than 30 years ago within the context of “psychedelic therapy,” hypothesizing that ketamine leads to mystical-type effects that might be leveraged therapeutically for alcohol and opioid use disorders. This group remains isolated in its psychedelic orientation, however, and most researchers at present focus on the neurobiological mechanisms of ketamine while dismissing its psychoactive properties as unwanted and problematic side-effects (Sanacora and Schatzberg, 2015).
Two previous studies have concerned themselves with whether or not ketamine elicits mystical-type experiences (Dakwar et al., 2014b; Lofwall et al., 2006). The first examined low, intramuscular (IM) doses in healthy volunteers, and concluded that ketamine, compared to a control, did not significantly generate mystical-type effects (Lofwall et al., 2006). The generalizability of these findings may be limited by a few methodological issues. The IM route leads to less bioavailability than does the IV route, and the doses tested were already substantially lower (0.2 and 0.4 mg/kg) than what had demonstrated therapeutic effects by the IV route in depressed individuals. The second study, cited earlier, examined the mystical-type effects of two sub-anesthetic IV doses of ketamine in cocaine dependent individuals and found that ketamine significantly leads to dose-dependent increases in such phenomena (Dakwar et al., 2014b). This study improved on the former by examining doses of therapeutic value (in both cocaine users and in depressed individuals), administered via the IV route, but it was limited by a small sample size and a restricted survey of mystical-type effects, with the questionnaire abbreviated to 9 items. The present study, by concerning itself with a larger sample size and by using the full HMS, constitutes the strongest evidence to date that ketamine occasions mystical-type phenomena.
A sub-set of mystical-type effects, the NDE, has also been anecdotally linked to ketamine, but without having been investigated empirically (Luke and Kittenis, 2005). NDEs have long been proposed to have therapeutic potential, with individuals who experience them naturalistically in the context of medical illness or trauma reporting enduring improvements in outlook, life satisfaction, and decision-making (Royse and Badger, 2017). NDE-type experiences have also been linked to hallucinogens, and perhaps because of the predominant dissociative effects of ketamine, which can resemble the out-of-body experiences reported with NDEs, ketamine has been thought to be foremost among hallucinogens in occasioning this type of experience (Luke and Kittenis, 2005). While this analysis is not designed to compare ketamine to other hallucinogens in propensity to elicit NDEs, these data strongly suggest that ketamine is effective at producing NDE-type experiences using a validated measure, with a duration comparable to the other psychoactive effects assessed.
The finding that NDE-type phenomena did not serve as a mediator, despite being recognized as a species of mystical-type experience (Pennachio, 1986), deserves exploration, as it may illuminate the features of mystical-type experiences that might be most relevant to behavioral change. NDE- and mystical-type experiences have many features in common, notably a notion of having contacted sacred or ineffable dimensions, feelings of joy or great peace, and a sense of experiencing the world as a unified whole (Pennachio, 1986). The HMES additionally focuses on shifts in ontology – or in the nature of being – that, according to some psychologists (James, 1907; Miller and C’de Baca, 2001; Wong, 2010), may be directly impactful on values, decision-making, and behavior. These include a deeper orientation to and renewed view of being-in-the-world, as well as a refreshed sense of wonder, humility, and respect towards existence. While such experiences might also characterize NDEs, they are not directly queried for with the NDES questionnaire. This difference in reported phenomenology might account for why mystical-type experiences, but not phenomena related to NDEs, appeared to mediate the anti-addiction benefits of ketamine in this sample. This suggests that the opportunity for existential reappraisal afforded by mystical-type experience may be a key component of its behavioral efficacy.
The mediating role of mystical-type experience is consistent with a previous report (Dakwar et al., 2014b), and appears to extend here to behavioral outcomes, as opposed to self-reported measures alone (e.g., mood, life-satisfaction, or motivation to change). The use of behavioral outcomes, and of different assessments to ascertain an array of psychoactive effects, were also methodologically important, as they helped to guard against the confounds that might occur when participants are inclined to self-report in the same direction on multiple measures. Further, the behavioral outcomes were directly observed and quantified by blinded staff in a laboratory setting. It is therefore the first indication, under controlled and rigorous conditions, that medication-occasioned mystical-type experiences might serve to effect behavioral change, a hypothesis that had informed early research with serotonergic hallucinogens, especially in substance use treatment (Grinspoon and Bakalar, 1979), but which had not been tested empirically. It also provides more evidence that the efficacy of ketamine might be related to certain of its psychoactive effects, and suggests that it does not exert therapeutic activity via neural mechanisms alone (e.g., glutamate modulation), with all alterations in consciousness epiphenomenal or problematic (Sanacora and Schatzberg, 2015). Of note, these mystical-type experiences mediated benefit even though no preparation, guidance, or post-infusion psychotherapy, as in the psychedelic model, occurred.
This study also adds to a growing body of evidence showing the safety of a single sub-anesthetic ketamine infusion in diverse psychiatric populations (Short et al., 2017). By adequately screening and preparing individuals, administering infusions under controlled conditions, and providing post-infusion support, investigators can effectively minimize the risks of ketamine, such as its abuse liability and behavioral toxicity, while also allowing for its possibly beneficial psychoactive effects to safely emerge (Dakwar et al., 2017, 2014a, 2014b; Perry et al., 2007). The present findings suggest that this safety record extends to individuals with substance use disorders as well, who may have an increased vulnerability to developing problematic substance use.
An important limitation is that it is beyond the scope of this analysis to draw conclusions regarding mechanism. While the data suggest that mystical-type effects are not simply a marker of ketamine potency given that other markers of potency (i.e., dose, dissociation) failed to enjoy such a role, it is not possible to assert that these experiences causally led to the benefits observed. It is conceivable, for example, that mystical-type phenomena provide a particularly sensitive, experiential indicator of the neurobiological efficacy of ketamine. Neurobiological mechanisms common to agents eliciting mystical-type phenomena, and which may also be involved in psychiatric efficacy, include the promotion of prefrontal neurogenesis, alterations in default-mode network (DMN) connectivity, and increased synchrony between the anti-correlated networks (Carhart-Harris et al., 2016; Catlow et al., 2013; Lebedev et al., 2015; Li et al., 2010; Liu et al., 2010; Sanacora and Schatzberg, 2015; Scheidegger et al., 2012; Vollenweider and Kometer, 2010). Interestingly, DMN alterations associated with psilocybin administration also appear to be correlated with hallmarks of mystical-type experience, such as the dissolution of the sense of self (Lebedev et al., 2015). It is therefore possible that these mechanisms, singly or collectively, might serve to produce both any therapeutic activity and mystical-type phenomena, with the experience itself enjoying a secondary or peripheral role.
This possibility notwithstanding, there is strong reason to regard mystical-type experiences as providing their own direct benefit. It has been documented widely that the changes in perspective and decision-making following such experience continue to be felt, as well as personally attributed to the event, months to years later (Griffiths et al., 2008; James, 1907; Miller and C’de Baca, 2001). It is difficult to understand such persistent impact without appreciating the experiential manner in which mystical-type perspectival shifts come to be uniquely interpreted, invested with enduring significance, and integrated into daily life as a consistent set of attitudes, choices, and behaviors. The literature on medication-occasioned altered states is strikingly consistent in conjecturing such a casual role for mystical-type experience (Bogenschutz et al., 2015; Garcia-Romeu et al., 2014; Griffiths et al., 2016, 2008; Grinspoon and Bakalar, 1979; Grof, 2001), which is congruent with reports of the transformative role these (often spontaneous) experiences have in the lives of visionaries, religious figures, or ordinary individuals (James, 1907; Miller and C’de Baca, 2001). It is therefore compelling to attribute a similar impact to such experiences here, and to consider how these experiences can be more reliably and safely generated by pharmacotherapy, as well as how they might be optimized and harnessed in clinical settings so as to facilitate psychiatric efficacy (Johnson et al., 2008; Studerus et al., 2012).
5. Conclusions
This is the first rigorous, controlled study to show that medication-occasioned mystical-type phenomena, long considered to have therapeutic potential, may serve to impact decision-making and behavior in a sustained manner, even in the absence of behavioral treatment or psychotherapy. These data suggest that an important line of inquiry is the identification of ketamine-like medications that are not bereft of psychoactive potency altogether, but which are more selective in their phenomenological profile so that mystical-type effects are enhanced and characteristics lending to abuse or behavioral toxicity are minimized. Future studies might also aim to further examine the links between psychoactive effects and their neural correlates in ketamine and related agents, to elucidate the particular mystical-type phenomena that might be most likely to mediate benefits for different disorders, and to better understand the relationship between neural activity, subjective experience, and clinical benefit.
Highlights.
Different lines of evidence suggest that certain psychoactive effects may be relevant to the therapeutic activity of ketamine and related compounds (such as serotonergic hallucinogens).
Mystical-type phenomena, but not other psychoactive effects, were found to mediate the therapeutic impact of ketamine on cocaine dependent individuals.
These data suggest that it is important to identify ketamine-like medications that are not bereft of psychoactive potency altogether, but which are more likely to produce mystical-type effects specifically.
Acknowledgments
Funding: The authors thanks the National Institute on Drug Abuse for funding this study through grants DA035472 and DA031771 awarded to Dr. Dakwar. The authors also thank the New York State Psychiatric Institute for salary support and the provision of resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: E.D. obtained funding for the study, oversaw study procedures, and wrote the original manuscript; C.H., E.N., R.F., M.H., and F.L. provided additional supervision over study procedures, assisted in data analysis, and edited the manuscript. All authors approved the final version of the manuscript.
Disclosure: There are no conflicts of interest to report.
References
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. J Psychopharmacol. 2015;29:289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/a:1024465317902. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams LT, Williams TM, Bolstridge M, Sessa B, Mcgonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding A, Nutt DJ. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113:4853–4858. doi: 10.1073/pnas.1518377113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez-Ramos J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res. 2013;228:481–491. doi: 10.1007/s00221-013-3579-0. [DOI] [PubMed] [Google Scholar]
- Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL. The Effects of Subanesthetic Ketamine Infusions on Motivation to Quit and Cue-Induced Craving in Cocaine-Dependent Research Volunteers. Biol Psychiatry. 2014a;76:40–46. doi: 10.1016/j.biopsych.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Anerella C, Hart C, Levin F, Mathew S, Nunes E. Therapeutic infusions of ketamine: Do the psychoactive effects matter? Drug and Alcohol Dependence. 2014b;136:153–157. doi: 10.1016/j.drugalcdep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW. Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry. 2016;22:76–81. doi: 10.1038/mp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional Segregation of the Human Dorsomedial Prefrontal Cortex. Cerebral Cortex. 2014;26:304–321. doi: 10.1093/cercor/bhu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. Introductory lectures on psycho-analysis: (1915–1917) Hogarth Press; London: 1995. [Google Scholar]
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2013;28:287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu A, Griffiths R, Johnson M. Psilocybin-Occasioned Mystical Experiences in the Treatment of Tobacco Addiction. Current Drug Abuse Reviews. 2015;7:157–164. doi: 10.2174/1874473708666150107121331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greyson B. The near-death experience scale: Construction, reliability, and validity. J Nerv Ment Dis. 1983;171:369–75. doi: 10.1097/00005053-198306000-00007. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, Mccann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22:621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Psychedelic drugs reconsidered. Basic; New York: 1979. [Google Scholar]
- Grof S. LSD Psychotherapy. MAPS; Sarasota: 2001. [Google Scholar]
- Hofmann A. LSD, my problem child. McGraw-Hill; New York: 1980. [Google Scholar]
- Hood RW. The Construction and Preliminary Validation of a Measure of Reported Mystical Experience. J Sci Study Relig. 1975;14:29. doi: 10.2307/1384454. [DOI] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Voort JLV, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The varieties of religious experience: a study in human nature. Oxford World’s Classics; Oxford: 1907. [Google Scholar]
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Grinenko AY. Ketamine Psychedelic Therapy (KPT): A Review of the Results of Ten Years of Research. J of Psychoactive Drugs. 1997;29:165–183. doi: 10.1080/02791072.1997.10400185. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanova T, Dunaevsky I, Strassman R, Grinenko A. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abuse Treat. 2002;23:273–283. doi: 10.1016/s0740-5472(02)00275-1. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Lövdén M, Rosenthal G, Feilding A, Nutt DJ, Carhart-Harris RL. Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Hum Brain Mapp. 2015;36:3137–3153. doi: 10.1002/hbm.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA. Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke DP, Kittenis M. A preliminary survey of paranormal experiences with psychoactive drugs. J Parapsychology. 2005;69:305. [Google Scholar]
- Maclean KA, Leoutsakos JMS, Johnson MW, Griffiths RR. Factor Analysis of the Mystical Experience Questionnaire: A Study of Experiences Occasioned by the Hallucinogen Psilocybin. J Sci Study of Relig. 2012;51:721–737. doi: 10.1111/j.1468-5906.2012.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, Mccammon J, Chen G, Manji HK. Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Miller WR, C’de Baca J. Quantum change: when epiphanies and sudden insights transform ordinary lives. Guilford; New York: 2001. [Google Scholar]
- Pennachio J. Near-death experience as mystical experience. J Relig Health. 1986;25:64–72. doi: 10.1007/bf01533055. [DOI] [PubMed] [Google Scholar]
- Perry EB, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O’Donnell E, Krystal JH, D’Souza DC. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology. 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- Royse D, Badger K. Near-death experiences, posttraumatic growth, and life satisfaction among burn survivors. Social Work in Health Care. 2017;56:155–168. doi: 10.1080/00981389.2016.1265627. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Schatzberg AF. Ketamine: Promising Path or False Prophecy in the Development of Novel Therapeutics for Mood Disorders? Neuropsychopharmacology. 2014;40:259–267. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E. Ketamine Decreases Resting State Functional Network Connectivity in Healthy Subjects: Implications for Antidepressant Drug Action. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. The Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of Psilocybin Response in Healthy Volunteers. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11:642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Wong PTP. Meaning therapy: an integrative and positive existential psychotherapy. J Contemp Psychother. 2010;40:85–99. [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Jr, Z CA, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]


