Graphical abstract
Keywords: AL amyloidosis, Cardiac, Echocardiography, Global longitudinal strain, Regression
Highlights
-
•
The first documented case of amyloid light chain cardiac amyloidosis with normalization of average global longitudinal strain is presented.
-
•
This is further evidence of regression of cardiac amyloidosis after stem cell transplantation.
-
•
Global longitudinal strain may be a ready visual marker of prognosis, regression, and recovery.
Introduction
Cardiac involvement with amyloid light chain (AL) amyloidosis results in characteristic macroscopic structural and functional changes on transthoracic echocardiography (TTE).1, 2, 3 These changes include thickened, speckled left and right ventricular walls, dilated atria, advanced diastolic dysfunction, and trivial pericardial effusions.1, 2, 4 Global longitudinal strain (GLS) is significantly reduced, and polar maps typically show apical sparing.4, 5 Previous reports suggest that selected patients who achieve hematologic remission after chemotherapy and peripheral blood stem cell transplantation (PBSCT) may demonstrate significant regression of macroscopic cardiac features.6, 7 It has previously been suggested that GLS does not change over time after diagnosis.8, 9 We report the first case of one such patient who developed regression of two-dimensional echocardiographic abnormalities and normalization of average GLS.
Case Presentation
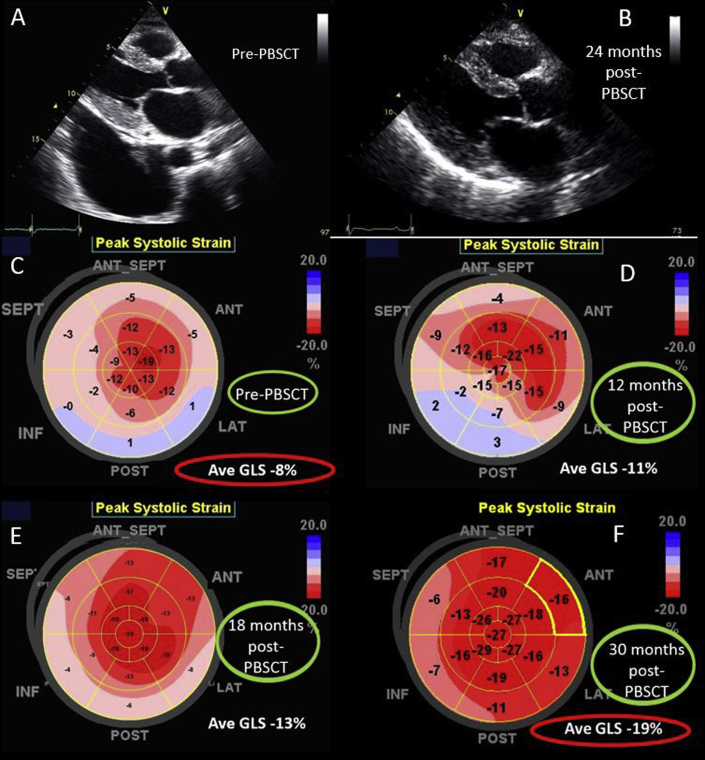
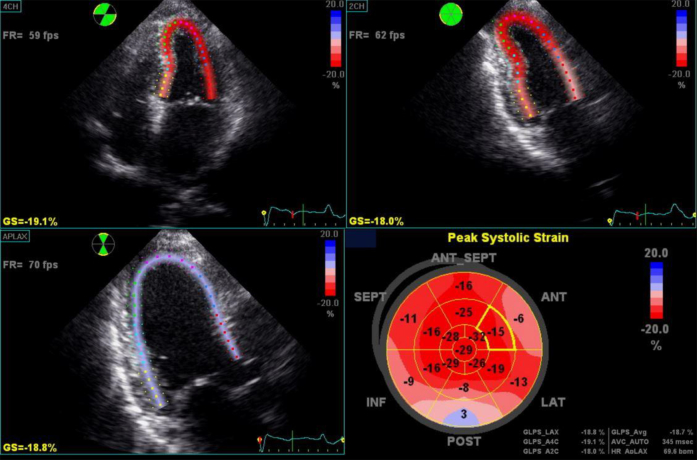
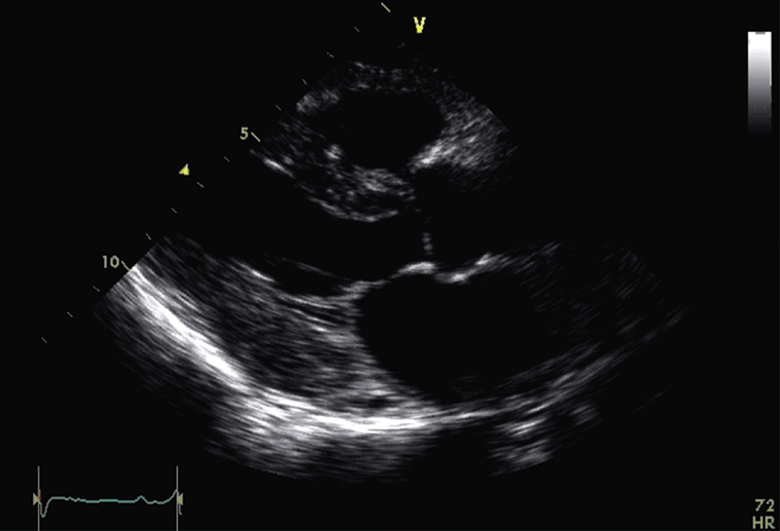
A 59-year-old woman was diagnosed with cardiac amyloidosis after a 12-month history of progressive shortness of breath and swelling of the ankles. Pretreatment TTE showed the typical findings of cardiac amyloidosis (see Figure 1A, Table 1, and Video 1). Left ventricular GLS was −8% (normal more negative than −17%), and the polar map revealed the characteristic apical sparing pattern (see Figure 1C). Subsequent investigations confirmed that she had biopsy-proven multiple myeloma with AL amyloidosis.
Figure 1.
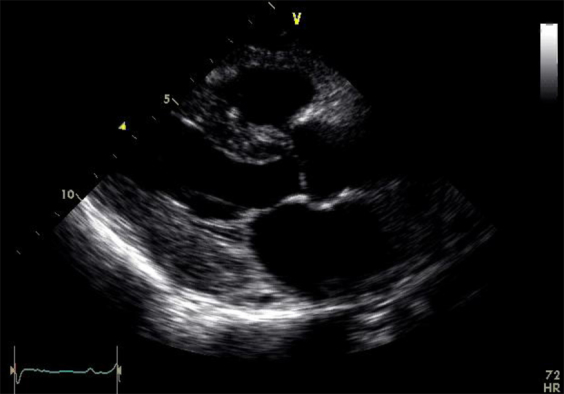
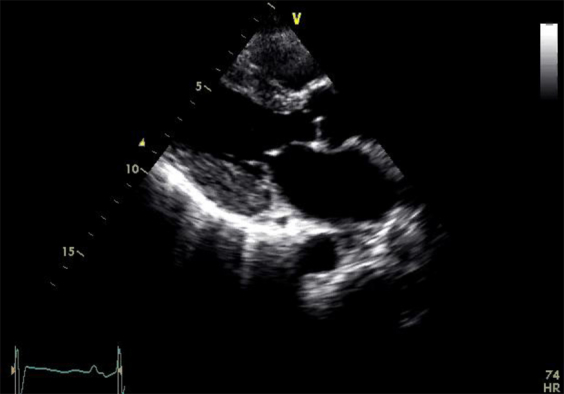
Echocardiographic images. (A) Parasternal echocardiogram before PBSCT. (B) Parasternal echocardiogram 2 years after PBSCT. (C) Polar strain map of GLS before PBSCT. (D) Polar strain map of GLS 12 months after PBSCT. (E) Polar strain map of GLS 18 months after PBSCT. (F) Polar strain map of GLS 30 months after PBSCT.
Table 1.
Comparison of echocardiographic measurements before and after cardiac regression, 24 months after PBSCT
| Measurement | Before PBSCT | 12 mo after PBSCT | 18 mo after PBSCT | 24 mo after PBSCT |
|---|---|---|---|---|
| Ejection fraction (%) | 60 | 58 | 59 | 59 |
| Septal wall (mm) | 13 | 13 | 13 | 12 |
| Posterior wall (mm) | 20 | 21 | 15 | 12 |
| Left atrial area (cm2) | 26 | 25 | 25 | 23 |
| Left atrial volume (mL/m2) | 46 | 45 | 42 | 36 |
| Diastolic function grade | 3 | 2 | 2 | 2 |
| E velocity (m/sec) | 1.2 | 1 | 1.1 | 1 |
| Declaration time (msec) | 142 | 190 | 171 | 165 |
| e′ velocity (m/sec) | 0.02 | 0.03 | 0.04 | 0.04 |
| E/e′ ratio | 60 | 33 | 28 | 25 |
Hematologic treatment involved induction with antiplasma cell therapies, including immunomodulatory agents (thalidomide and lenalidomide) and protease inhibitors (bortezomib). High-dose chemotherapy with melphalan 200 mg/m2 split over 2 days was given before PBSCT. Peripheral blood stem cell reinfusion was undertaken with the patient monitored in the coronary care unit. Inpatient care continued until engraftment and recovery were clinically apparent.
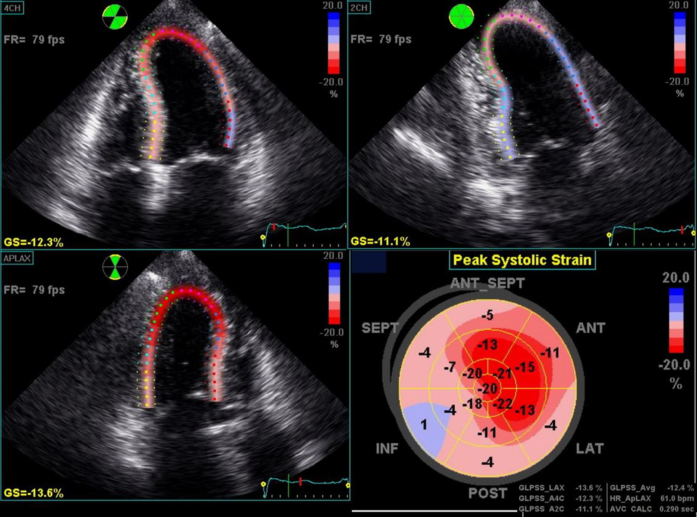
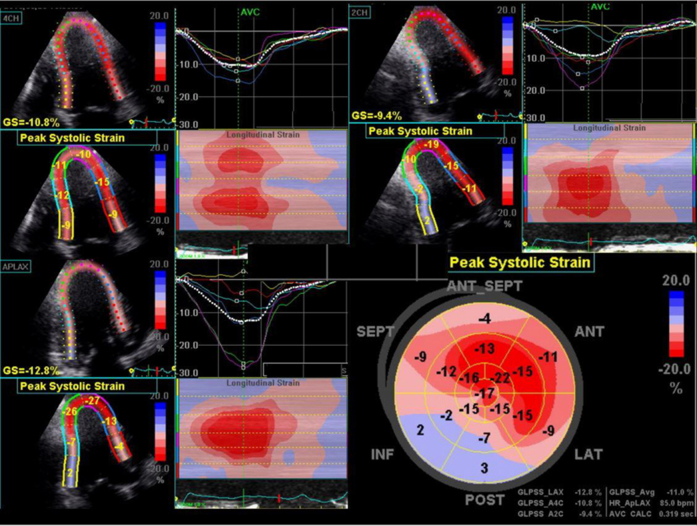
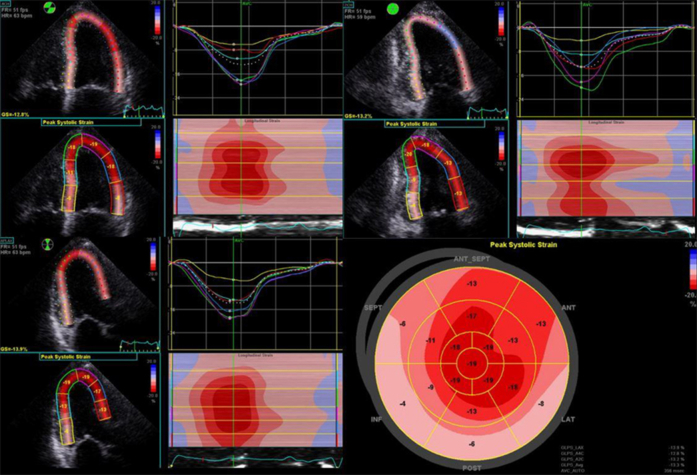
Sequential repeat TTE was performed every 6 months, using the GE Vivid 9 ultrasound system (GE Healthcare, Little Chalfont, United Kingdom) (see Videos 2-5). Pre-PBSCT TTE was performed when the patient was clinically well and 1 month after the most recent course of chemotherapy. At 2 years after PBSCT, TTE showed significant regression of the anatomic features of cardiac amyloidosis (see Figure 1A and 1B, Table 1, and Video 4). The polar strain map showed significant improvement in the apical sparing pattern (see Figure 1C–1F, and Supplemental Figures 1-8). Average GLS had improved to a “normal” value of −19%. These echocardiographic improvements corresponded to progressive improvement in her functional status, from New York Heart Association class IV before PBSCT to class I at late follow-up.
Discussion
Previously, a diagnosis of cardiac amyloidosis resulted in survival of 5 months.2, 3 High-dose chemotherapy and PBSCT therapies have resulted in hematologic remission and were previously shown in a small case series to result in regression of the classical echocardiographic changes of cardiac amyloidosis. This cardiac regression occurred approximately 2 years after PBSCT.6 This is in contrast to the previously held belief that cardiac amyloid infiltration is irreversible.2, 3
GLS permits quantification of left ventricular function, with greater sensitivity than ejection fraction.10 Strain rate imaging has also been shown to be useful in assessing diastolic dysfunction but was not superior to E/e′ ratio.11 GLS has been shown to be an excellent marker of prognosis in cardiac amyloidosis, and polar maps show a classical apical sparing pattern.8, 9, 12
The improvement in GLS may be related to treatment of the circulating amyloidogenic proteins but is more likely to be due to alleviation of the underlying disease. Hematologic parameters normalized early in the process (<6 months after PBSCT). GLS was essentially unchanged at that time. Cardiac improvements take temporally longer than the hematologic changes to manifest, and the changes in GLS reflect that time frame.
In this case, the changes in diastolic function and the anatomic changes (regression in wall thickness) occurred at a slower rate than the changes in GLS. The greater sensitivity of GLS over ejection fraction has resulted in the suggestion that it is useful as an earlier marker of change for many cardiomyopathies.10, 11 It may be that it is also an earlier marker of change in cardiac amyloidosis.
This is the first documented case of a patient with biopsy-proven AL cardiac amyloidosis showing remission of the hematologic process, regression of echocardiographic anatomic features of disease, and normalization of averaged GLS.
Conclusions
GLS is an important marker of cardiac mechanical function and has become established as pathognomonic of infiltration in AL cardiac amyloidosis, with the classical apical sparing pattern.8, 9, 12 It may now also prove to be a useful marker of regression and recovery after PBSCT.
Footnotes
Conflicts of Interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.case.2016.12.002.
Supplementary Data
TTE before PBSCT (MP4 video of Figure 1A).
TTE 12 months after PBSCT.
TTE 18 months after PBSCT.
TTE 24 months after PBSCT (MP4 video of Figure 1B).
PLA 30 months after PBSCT.
Supplemental Figure 1.
Pre-PBSCT.
Supplemental Figure 2.
12 months post-PBSCT.
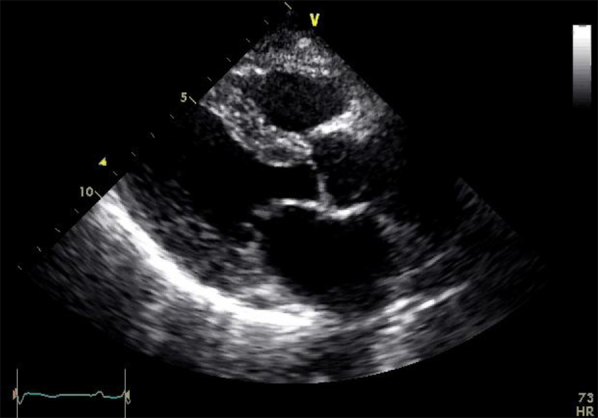
Supplemental Figure 3.
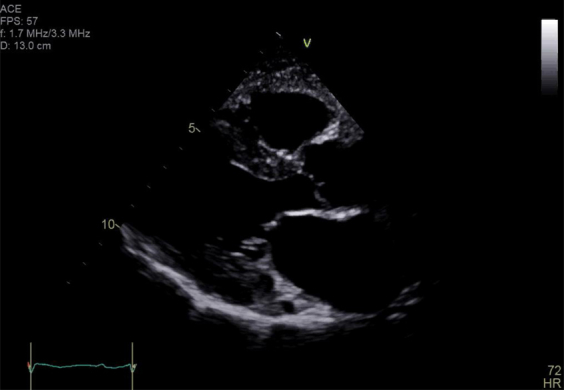
18 months post-PBSCT.
Supplemental Figure 4.
24 months post-PBSCT.
Supplemental Figure 5.
Pre-PBSCT.
Supplemental Figure 6.
12 months post-PBSCT.
Supplemental Figure 7.
18 months post-PBSCT.
Supplemental Figure 8.
24 months post-PBSCT.
References
- 1.Fitzgerald B.T., Scalia G.M., Cain P.A., Garcia M.J., Thomas J.D. Left atrial size—another differentiator for cardiac amyloidosis. Heart Lung Circ. 2011;20:574–578. doi: 10.1016/j.hlc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Falk R.H., Comenzo R.L., Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 3.Falk R.H. Cardiac amyloidosis—a treatable disease, often overlooked. Circulation. 2011;124:1079–1085. doi: 10.1161/CIRCULATIONAHA.110.010447. [DOI] [PubMed] [Google Scholar]
- 4.Klein A.L., Oh J., Miller F.A., Seward J.B., Tajik A.J. Two-dimensional and Doppler echocardiographic assessment of infiltrative cardiomyopathy. J Am Soc Echocardiogr. 1988;1:48–59. doi: 10.1016/s0894-7317(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 5.Phelan D., Collier P., Thavendiranathan P., Popović Z.B., Hanna M., Plana J.C. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald B.T., Bashford J., Scalia G.M. The return of the normal heart: resolution of cardiac amyloidosis after chemotherapy and bone marrow transplantation. Heart Lung Circ. 2013;22:655–660. doi: 10.1016/j.hlc.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Meier-Ewert H.K., Sanchorawala V., Berk J., Finn K.T., Skinner M., Seldin D.C. Regression of cardiac wall thickness following chemotherapy and stem cell transplantation for light chain (AL) amyloidosis. Amyloid. 2011;18:130–131. doi: 10.3109/13506129.2011.574354048. [DOI] [PubMed] [Google Scholar]
- 8.Hu K., Liu D., Nordbeck P., Cikes M., Störk S., Kramer B. Impact of monitoring longitudinal systolic strain changes during serial echocardiography on outcome in patients with AL amyloidosis. Int J Cardiovasc Imaging. 2015;31:1401–1412. doi: 10.1007/s10554-015-0711-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu D., Hu K., Störk S., Herrmann S., Kramer B., Cikes M. Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS One. 2014;9:e115910. doi: 10.1371/journal.pone.0115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smiseth O.A., Torp H., Opdahl A., Haugaa K.H., Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasner M., Gaub R., Sinning D., Westermann D., Steendijk P., Hoffmann W. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur J Echocardiogr. 2010;11:743–751. doi: 10.1093/ejechocard/jeq060. [DOI] [PubMed] [Google Scholar]
- 12.Salinaro F, Meier-Ewert HK, Miller E, Pandey S, Sanchorawala V, Berk JL, et al. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis [published online December 24, 2016]. Eur Heart J Cardiovasc Imaging. http://dx.doi.org/10.1093/ehjci/jew298. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TTE before PBSCT (MP4 video of Figure 1A).
TTE 12 months after PBSCT.
TTE 18 months after PBSCT.
TTE 24 months after PBSCT (MP4 video of Figure 1B).
PLA 30 months after PBSCT.