Abstract
Cardiac myosin binding protein-C (cMyBP-C) is a heart muscle-specific thick filament protein. Elevated level of serum cMyBP-C is an indicator of early myocardial infarction (MI), but its value as a predictor of future cardiovascular disease is unknown. Based on the presence of significant amount of cMyBP-C in the serum of previous study subjects independent of MI, we hypothesized that circulating cMyBP-C is a sensitive indicator of ongoing cardiovascular stress and disease. To test this hypothesis, 75 men and 83 women of similar ages were recruited for a prospective study. They underwent exercise stress echocardiography to provide pre- and poststress blood samples for subsequent determination of serum cMyBP-C levels. The subjects were followed for 1 to 1.5 years. Exercise stress increased serum cMyBP-C in all subjects. Twenty-seven primary events (such as death, MI, revascularization, invasive cardiovascular procedure, or cardiovascular-related hospitalization) and 7 critical events (CE; such as death, MI, stroke, or pulmonary embolism) occurred. After adjusting for sex and cardiovascular risk factors with multivariate Cox regression, a 96% sensitive prestress cMyBP-C threshold carried a hazard ratio of 8.1 with p = 0.041 for primary events. Most subjects (6 of 7) who had CE showed normal ejection fraction on echocardiography. Pre-stress cMyBP-C demonstrated area under receiver operating curve of 0.91 and multivariate Cox regression hazard ratio of 13.8 (p = 0.000472) for CE. Thus, basal cMyBP-C levels reflected susceptibility for a variety of cardiovascular diseases. Together with its high sensitivity, cMyBP-C holds potential as a screening biomarker for the existence of severe cardiovascular diseases.
Heart disease remains as the leading cause of mortality and demonstrated a mortality rate increase from 2014 to 2015 in the United States.1 Sudden cardiac death (SCD) occurs with estimated rates of 76 to 110.8/100,000 per year.2 Coronary artery disease (CAD), arrhythmia, and dilated cardiomyopathy are major causes of SCD.2 Approximately 60% of these patients will not have a previous coronary heart disease history.3 Stroke is the fifth leading cause of death, with a statistically significant increase from 2014 to 2015 in the United States.1 Thus, finding a new method to diagnose existence of asymptomatic but severe cardiovascular diseases can be useful. Cardiac myosin binding protein-C (cMyBP-C) is a 140-kilodalton protein that resides on the thick filament of the heart muscle.4 Phosphorylated cMyBP-C enhances diastolic function,5 mediates inotropy,6 and confers heart protection during ischemia.7 Myocardial infarction (MI) and ischemic injury will cause release of cMyBP-C into the bloodstream.8–11 However, circulating cMyBP-C exists in all ages independent of ischemic injury.8,9,12,13 Consequently, we hypothesize that serum cMyBP-C is a reflector of cardiovascular disease status; therefore, cMyBP-C level can predict future cardiovascular disease events.
Methods
Institutional Review Boards of Baylor Scott & White Health Central Texas and Loyola University Chicago (LU# 206102) reviewed and approved the study protocols. Research staff recruited consecutive patients undergoing exercise stress echocardiography as ordered by their physicians from April 2015 to November 2015 at 1 clinical center in Texas. Inclusion criteria consisted of age ≥18 and ability to reach ≥85% of age-adjusted maximum heart rate. Exclusion criteria consisted of previously diagnosed heart failure and/or cardiomyopathy. Blood samples were obtained immediately before and after the exercise stress test. Research staff followed the clinical outcome via electronic records for at least 1 year. Primary outcome consisted of death, SCD, MI, revascularization, postenrollment angiogram showing critical coronary stenose(s) (≥70% stenosis in a major epicardial coronary artery), hospitalization for any cardiovascular cause (e.g., stroke), and performance of an invasive procedure for any cardiovascular cause. SCD is defined as death occuring within 1 hour of symptom onset. Separately, critical events (CE) consisted of death, MI, stroke, pulmonary embolism, arrhythmic or dysrrhythmic event requiring prompt intervention (e.g., ventricular tachycardia or complete heart block), and sudden loss of conciousness requiring hospitalization. Clinical staff who were blinded to the study read the stress echocardiography. Research staff who were blinded to the serum cMyBP-C levels adjudicated outcomes.
Serum cMyBP-C levels were quantified in a double-blinded manner for all samples, using a previously developed sandwich enzyme-linked immunosorbent assay.14 The assay used mouse mAb against C0 domain of cMyBP-C (sc-137180 Clone E7, Santa Cruz) for capturing; rabbit polyclonal antibody against residues 2 to 14 of cMyBP-C for detection15; and serially diluted recombinant human C0C1f (40 kDa) fragment of cMyBP-C for standard curve.8 The processing staff was blinded to the clinical outcomes. cMyBP-C is expressed in units of nanogram or milliliter.
SPSS Statistics 24 (IBM Corporation, Armonk, New York) and Stata 13 (StataCorp LLC, College Station, Texas) software were used for statistical analyses. We reported results only when they reflected agreement between the software programs. We examined data for normality by histograms, quantile-quantile plots, and Shapiro-Wilk test. If needed, we used log transformation to normalize the data for parametric testing. Independent t test for independent samples or paired t test for pre- or poststress values were used for normally distributed data. Chi-square test was used for categorical data. Nonparametric tests were used for comparison when data could not fit the normal distribution (Mann-Whitney U for independent samples, related-sample Wilcoxon rank sum test for related samples). Kaplan-Meier curve with log-rank test was used for survival analyses. Cox proportional hazards regression was used to determine the effect of different variables on the outcome. Significance was set at p ≤0.05. C-statistic is the area under the receiver operating curve (AUROC). Data are shown as mean ± standard error.
Results
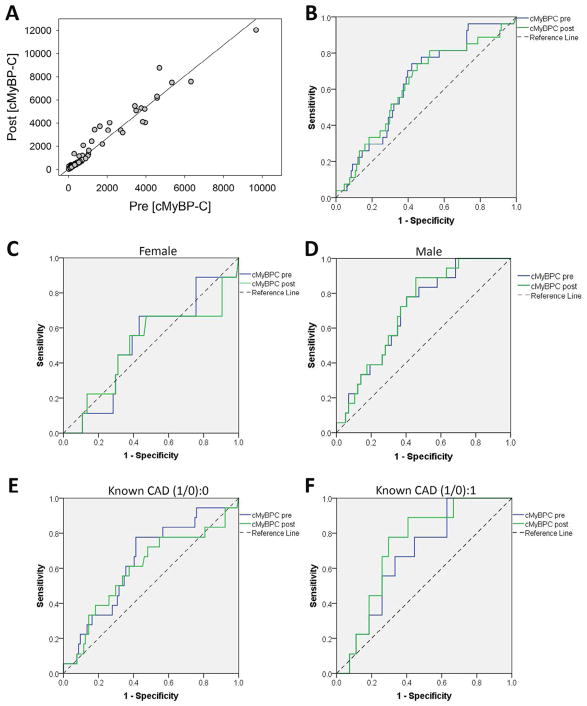
Our study enrolled 158 subjects consisting of 75 (47%) men and 83 (53%) women. Age, duration of follow-up, prevalence of diabetes, and smoking history were similar between the sexes. Men exhibited increased prevalence of CAD, hypertension, and dyslipidemia (Table 1). Exercise stress caused increases in serum cMyBP-C in all subjects (679 ± 107 to 955 ± 146, p <0.000001). Prestress cMyBP-C showed extraordinary linear correlation with poststress cMyBP-C (r = 0.979, p <0.000001) (Figure 1). Twenty-seven primary outcome events occurred (1 unexpected death during overnight sleep, 13 ischemic events, 9 arrhythmia events, 2 structural heart disease requiring intervention, and 2 vascular-neural events). Excluding subjects who experienced primary outcome event, we found that prestress cMyBP-C correlated with tissue Doppler Sa (r = 0.276, p = 0.002) but not with e′ or E/e′ (Sa: peak myocardial contraction velocity during systole; e′: peak myocardial relaxation velocity during early diastole; E: peak blood inflow to left ventricle velocity across mitral valve during early diastole). See supplement for International Classification of Diseases, Tenth Revision diagnoses that triggered exercise stress test and additional results such as correlation analyses.
Table 1.
Subject characteristics
| Parameter (Mean ± SD) | Male (n = 75) | Female (n = 83) | p-values |
|---|---|---|---|
| Age (years) | 59 ± 14 | 56 ± 11 | 0.119 |
| Duration of follow-up (days) | 534 ± 67 | 532 ± 73 | 0.883 |
| Known Coronary Artery Disease (1/0)* | 30 (40%) | 6 (7%) | <1.0 × 10−6 |
| Hypertension (1/0)* | 52 (69%) | 44 (53%) | 0.036 |
| Diabetes Mellitus (1/0) | 13(17%) | 16(19%) | 0.753 |
| Dyslipidemia (1/0)* | 61(81%) | 52(63%) | 0.009 |
| +Smoking history (1/0) | 15(20%) | 11(13%) | 0.253 |
| Left Ventricle End Diastolic Diameter* (cm) | 4.67 ± 0.49 | 4.49 ± 0.05 | 0.008 |
| Left Ventricle Ejection Fraction* (%) | 58 ± 6 | 61 ± 5 | 0.003 |
| e’(cm/s) | 7.54 ± 1.96 | 7.82 ± 2.23 | 0.414 |
| E/e’ | 10.49 ± 3.41 | 10.95 ± 4.17 | 0.471 |
p <0.05 between male and female.
Diabetes includes previous diagnosis of diabetes, on diabetic treatment, fasting glucose > 126 mg/dl, or hemoglobin A1C >6.5%. Dyslipidemia includes previous diagnosis of dyslipidemia of any type (e.g., hyperlipidemia or low high-density lipoprotein), being on statin, or cholesterol threshold values identified by Adult Treatment Panel-III guidelines if no previous diagnosis is present. Hypertension includes previous diagnosis of hypertension, on hypertensive medication without history of heart failure, systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg. + Smoking history is patient admitting to actively smoked within 6 months of study enrollment.
Figure 1.
Serum cMyB-C relations and receiver operating characteristic (ROCs) curves for primary outcome. (A) Prestress cMyBP-C versus poststress cMyBP-C plot, Pearson correlation r = 0.979 p = 2.4 × 10−109. (B) Area under the receiver operating curve (AUROC) prestress AUROC = 0.638, p = 0.024; poststress AUROC = 0.629, p = 0.039. (C) ROC analysis on women; prestress AUROC = 0.519, p = 0.855; poststress AUROC = 0.5, p = 1. (D) ROC analyses on men; prestress AUROC = 0.699, p = 0.011; poststress AUROC = 0.710, p = 0.008. (E) ROC analysis on subjects without previous diagnosis of coronary artery disease (CAD); prestress AUROC = 0.641, p = 0.057; poststress AUROC = 0.6, p = 0.175. (F) ROC analysis on subjects with previous diagnosis of coronary artery disease (CAD); prestress AUROC = 0.675, p = 0.121; poststress AUROC = 0.728, p = 0.043.
Receiver operating characteristic (ROC) curve analyses showed AUROCs of 0.638 for prestress and 0.626 for poststress cMyBP-C for primary outcome events. Male sex and existence of known CAD significantly increased AUROCs (Figure 1).
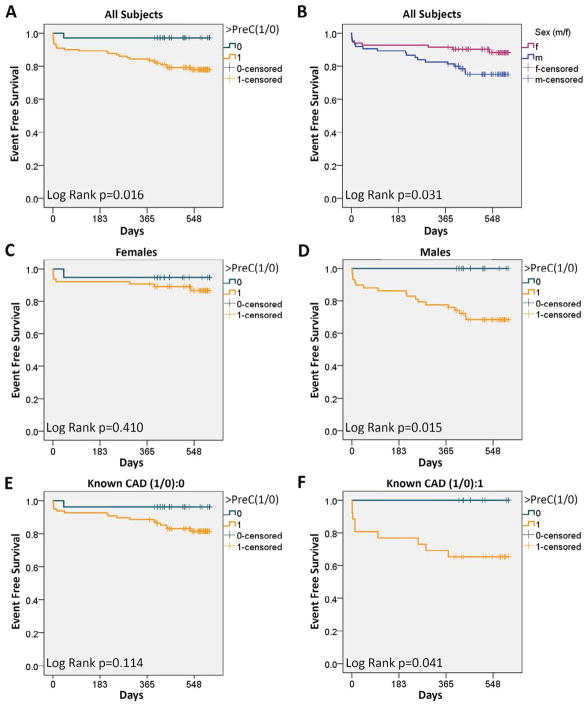
The combination of near-perfect linear pre- or poststress correlation, similar pre- or poststress ROCs, and the potential of wider applicability of prestress cMyBP-C caused us to examine if a prestress cMyBP-C threshold (PreC) could be used to screen for cardiovascular events. We chose an ROC-predicted 96% sensitive value of 127 as PreC. Subjects with cMyBP-C > PreC demonstrated decreased event-free survival (Figure 2). However, sex and existence of known CAD influenced PreC-based survival analyses (Figure 2). Overall, men exhibited lower event-free survival than did women; however, this result was confounded by men exhibiting a higher prevalence of CAD, hypertension, and dyslipidemia (Figure 2).
Figure 2.
Kaplan-Meier survival analyses for primary outcome. (A) Subjects with prestress cMyBP-C > PreC threshold exhibited decreased event-free survival (<PreC 97%, >PreC 78%, p = 0.016). (B) Men exhibited decreased event-free survival in comparison with women (men 75%, women 88%, p = 0.031). (C) Women with prestress cMyBP-C > PreC threshold did not show decreased event-free survival. (D) Men with prestress cMyBP-C > PreC threshold showed decreased event-free survival (<PreC 100%, >PreC 68%, log-rank p = 0.015). (E) Subjects without known coronary artery disease (CAD) diagnosis with cMyBP-C > PreC trended toward decreased event-free survival (<PreC 96%, >PreC 81%, p = 0.114). (F) Subjects with known CAD diagnosis with cMyBP-C > PreC demonstrated decreased event-free survival (<PreC 100%, >PreC 65%, p = 0.041).
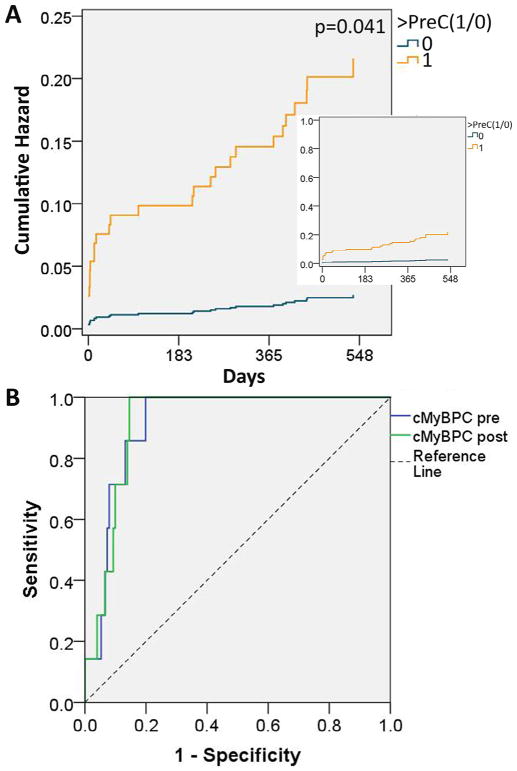
Because of potential contribution of multiple factors (e.g., sex, CAD), we performed Cox proportional hazard modeling to account for risk factors, sex, and PreC. The model showed that PreC was the only predictive factor for events at 1.5 years (hazard ratio [HR] 8.1, p = 0.041, Figure 3, Table 2). Importantly, the model showed that sex was no longer a significant factor after accounting for differences in the prevalence of CAD, hypertension, and dyslipidemia. We chose poststress cMyBP-C level of 214 as poststress threshold (PostC) based on optimization of sensitivity versus specificity. The poststress Cox regression included exercise >PostC, stress echocardiography results, and risk factors. The model showed positive exercise stress echocariography, >PostC, and smoking to be independent predictors of events (Table 2).
Figure 3.
Cumulative hazard for primary outcome. (A) Cox hazard regression was used to account for age, sex, known coronary artery disease, diabetes, dyslipidemia, hypertension, smoking history, and prestress cMyBP-C > PreC. Only cMyBP-C > PreC was found to be a significant predictor of events with hazard ratio of 8.1, p = 0.041. Curves show that probability for occurrence of event (cumulative hazard) increases with progress of time. (B) Receiver operating characteristic curve for critical events. Area under the receiver operating curves (AUROCs) are 0.914 (p = 0.000218) for prestress cMyBP-C and 0.917 (p = 0.000197) for poststress cMyBP-C.
Table 2.
Cox multivariate regression for primary outcome
| 95% CI
|
|||||
|---|---|---|---|---|---|
| Variable | B | p value | Hazard ratio | Lower | Upper |
| Pre-stress Model | |||||
| Age (years) | 0.005 | 0.778 | 1.005 | 0.970 | 1.042 |
| Sex (m/f) | −0.641 | 0.144 | 0.527 | 0.223 | 1.246 |
| Known CAD (1/0) | 0.302 | 0.553 | 1.353 | 0.499 | 3.664 |
| Hypertension (1/0) | 0.373 | 0.485 | 1.453 | 0.510 | 4.139 |
| Dyslipidemia (1/0) | 0.095 | 0.866 | 1.100 | 0.364 | 3.322 |
| Diabetes (1/0) | 0.707 | 0.132 | 2.028 | 0.808 | 5.089 |
| +Smoke (1/0) | 0.639 | 0.214 | 1.895 | 0.692 | 5.190 |
| >PreC (1/0)* | 2.093 | 0.041 | 8.107 | 1.093 | 60.098 |
| Post-stress Model | |||||
| Age (years) | −0.023 | 0.226 | 0.977 | 0.941 | 1.015 |
| Sex (m/f) | −0.417 | 0.358 | 0.659 | 0.271 | 1.604 |
| Known CAD (1/0) | 0.156 | 0.768 | 1.169 | 0.414 | 3.300 |
| Hypertension (1/0) | 0.892 | 0.135 | 2.440 | 0.759 | 7.847 |
| Dyslipidemia (1/0) | −0.515 | 0.392 | 0.597 | 0.184 | 1.943 |
| Diabetes (1/0) | 0.580 | 0.252 | 1.785 | 0.663 | 4.810 |
| +Smoke (1/0)* | 1.591 | 0.007 | 4.907 | 1.551 | 15.529 |
| >PostC (1/0)* | 1.563 | 0.002 | 4.773 | 1.755 | 12.980 |
| + Exercise Stress Echo (1/0)* | 2.530 | <0.001 | 12.553 | 4.378 | 35.995 |
p <0.05 denotes statistically significant contributing factor. 95% confidence interval (CI) for the hazard ratios are also provided.
Multivariate Cox proportional hazards modeling was used to identify predictors of occurrence of primary outcome events. B is the Cox coefficient. Hazard ratio is (probability of event occurrence with condition)/(probability of event occurrence without condition). Diabetes includes previous diagnosis of diabetes, on diabetic treatment, fasting glucose >126 mg/dl, or hemoglobin A1C >6.5%. Dyslipidemia includes previous diagnosis of dyslipidemia of any type (e.g., hyperlipidemia or low high-density lipoprotein), being on statin, or cholesterol threshold values identified by Adult Treatment Panel-III guidelines if no previous diagnosis is present. Hypertension includes previous diagnosis of hypertension, on hypertensive medication without history of heart failure, systolic blood pressure >140 mm Hg, or diastolic blood pressure >90 mm Hg. + Smoking history is patient admitting to actively smoked within 6 months of study enrollment. PreC (127 ng/ml) is the threshold used for prestress cMyBP-C and PostC (214 ng/ml) is the threshold used for poststress cMyBP-C.
Both >PreC and >PostC showed greater sensitivity for predicting primary outcome than echocardiography (Table 3). Exercise stress echocardiography achieved sensitivity of 77% and specificity of 88% for identifying significant CAD. Significant CAD is defined as death traceable to ischemia, MI, revascularization, or stenosis ≥70% in a major epicardial coronary artery. Therefore, exercise stress echocardiography performed as expected, and its low sensitivity for primary outcome could not be attributed to unusually poor execution.
Table 3.
Diagnostic performance for primary outcome event
| Pre-stress [cMyBP-C] > PreC | Post-stress [cMyBP-C] > PostC | (+) Exercise stress echocardiography | |
|---|---|---|---|
| Sensitivity | 96% | 81% | 52% |
| Specificity | 27% | 48% | 90% |
| Positive Predictive Value | 21% | 24% | 52% |
| Negative Predictive Value | 97% | 93% | 90% |
PreC (127 ng/ml) is the threshold used for prestress cMyBP-C and PostC (214 ng/ml) is the threshold used for poststress cMyBP-C.
Seven CE occurred (1 death during sleep, 1 MI, 1 stroke, 1 pulmonary embolism, 1 complete heart block, 1 ventricular tachycardia, and 1 seizure). The subject who died demonstrated prestress cMyBP-C >10-fold PreC and negative stress echocardiography for ischemia. Subjects who had neurologic events (stroke, seizure) also demonstrated >10-fold PreC. Relating prestress and poststress cMyBP-C to CEs produced ROCs with high AUROCs of 0.914 and 0.917, respectively (Figure 3). Cox regression showed that log10 transformation of prestress cMyBP-C was the only factor that affected CE outcome at prestress status (Table 4). Cox regression of poststress status showed that log10 (poststress cMyBP-C) was the only factor that affected CE occurrence (Table 4). The majority (5 of 7) of CEs were not caused by CAD. These Cox regression results show that every 10-fold rise of cMyBP-C increased risk of suffering a major event from a variety of cardiovascular diseases events by ~14-fold.
Table 4.
Cox multivariate regression for critical events
| 95% CI
|
|||||
|---|---|---|---|---|---|
| Variable | B | p value | Hazard Ratio | Lower | Upper |
| Pre-stress Model | |||||
| Age (year) | 0.007 | 0.833 | 1.007 | 0.940 | 1.079 |
| Sex (m/f) | −1.379 | 0.198 | 0.252 | 0.031 | 2.060 |
| Known CAD (1/0) | 0.994 | 0.453 | 2.701 | 0.202 | 36.096 |
| Hypertension (1/0) | 0.934 | 0.393 | 2.543 | 0.299 | 21.617 |
| Hyperlipidemia (1/0) | 1.044 | 0.346 | 2.840 | 0.324 | 24.894 |
| Diabetes (1/0) | 0.276 | 0.830 | 1.317 | 0.107 | 16.283 |
| Smoke (1/0) | 0.311 | 0.824 | 1.364 | 0.089 | 20.898 |
| Log10([cMyBP-C])*a | 2.625 | <0.001 | 13.799 | 3.169 | 60.091 |
| Post-stress Model | |||||
| Age (year) | −0.016 | 0.703 | .984 | 0.907 | 1.068 |
| Sex (m/f) | −0.999 | 0.394 | .368 | 0.037 | 3.659 |
| Known CAD (1/0) | 1.072 | 0.443 | 2.923 | 0.188 | 45.359 |
| Hypertension (1/0) | 0.966 | 0.388 | 2.627 | 0.294 | 23.514 |
| Hyperlipidemia (1/0) | 1.471 | 0.227 | 4.355 | 0.399 | 47.473 |
| Diabetes (1/0) | 0.914 | 0.549 | 2.493 | 0.125 | 49.639 |
| Smoke (1/0) | 0.523 | 0.717 | 1.687 | 0.100 | 28.604 |
| Log10([cMyBP-C])* | 2.663 | 0.001 | 14.339 | 3.146 | 65.346 |
| + Exercise Stress Echo (1/0) | 2.135 | 0.086 | 8.454 | 0.740 | 96.626 |
p <0.05 denotes statistically significant contributing factor. 95% confidence interval (CI) for the hazard ratios are also provided (aactual *p = 0.000472).
Multivariate Cox proportional hazards modeling was used to identify predictors of occurrence of critical events. B is the Cox coefficient. Logarithm base 10 (Log10) transformation was used to remap cMyBP-C from very skewed distribution toward normal distribution and to provide effects of every 10-fold change in cMyBP-C level.
Discussion
This is the first study demonstrating that basal serum cMyBP-C level could predict future cardiovascular events. All subjects exhibited basal circulating level of cMyBP-C that increased with exercise stress. Very similar ROCs and near-perfect pre- or poststress correlation suggested that basal and poststress cMyBP-C carry similar diagnostic potential. Cox regression showed that prestress cMyBP-C > PreC is the only factor that affected primary cardiovascular disease outcome with HR of 8.1, and prestress Log10(cMyBP-C) is the only factor that affected critical cardiovascular events with HR of 13.799 in age, sex, and cardiovascular risk factors at basal condition. Thus, basal cMyBP-C holds potential as a screening biomarker for existence of severe cardiovascular diseases.
Circulating cMyBP-C may provide cardiac protection during systemic stress. Subjects who had CEs showed higher prestress cMyBP-C (in ng/ml, −CE 569 ± 90, +CE 3070 ± 1178, p <0.0001). However, most (6 of 7) of these subjects demonstrated normal ejection fraction on echocardiography; therefore, circulating cMyBP-C did not strictly follow cardiac damage, but rather reflected overall cardiovascular disease stress. A subgroup of 9 normal subjects (no cardiovascular risk factors, normal stress echocardiography, no occurrence of events) demonstrated lower prestress cMyBP-C levels than the rest of the study cohort (in ng/ml, normal 205 ± 40, all others 708 ± 113, p <0.001). This also suggested basal cMyBP-C reflected cardiovascular disease severity. Interestingly, stress-induced % change in cMyBP-C inversely correlated with exercise duration (r = −0.180, p = 0.041), but did not correlate with peak heart rate × peak systolic pressure product in event-free subjects. This suggested that subjects who have better cardiovascular condition release less cMyBP-C under stress. Natriuretic peptides reflecting heart failure severity16 and serving as an effective treatment for heart failure17 provide a good example of heart protein release with cardioprotective effect. Thus, heart protection through the release of cMyBP-C during stress can explain rising cMyBP-C with exercise and, hence, the correlation of basal cMyBP-C with significant cardiovascular-neural diseases.
This study has limitations. Enrolling subjects who are undergoing exercise stress echocardiography may have biased the study with a cohort that has higher risk of cardiovascular events than the general population. Cohort size (n = 158) is also small. Subjects had access to multiple different health-care systems at the study location; therefore, using electronic records at only 1 health-care facility for follow-up may have missed events. Short duration of follow-up (1.5 year) and small number of subjects (158) likely limited the ability of the established 10-year cardiovascular risk factors18 to show significant effects. Thus, a much larger study with longer follow-up that is conducted within a primary care setting with scheduled follow-up survey(s) is needed to verify the current study findings for the general population.
In conclusion, our prospective study results showed that serum cMyBP-C reflected cardiovascular diseases severity in patients undergoing exercise stress echocardiography to predict occurrence of events; however, a much larger study is needed to generalize our findings.
Supplementary Material
Acknowledgments
Baylor Scott & White Health—Central Texas, Internal Medicine Department and Division of Cardiology provided clinical services support.
Funding: This work was supported by the National Institutes of Health, Bethesda, Maryland (R01 HL130356, R01 HL105826, and K02 HL114749 to SS; K08HL114877 to CWT); the American Heart Association Midwest Postdoctoral Fellowship (13POST14720024 to SG); the American Heart Association Grant-in-Aid (14GRNT20490025 to SS and CWT); and Cardiovascular Genome-Phenome Study (15CVGPSD27020012) and Catalyst Award (17CCRG33671128) to SS.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found, in the online version, https://doi.org/10.1016/j.amjcard.2017.07.042.
References
- 1.Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States. NCHS Data Brief. 2015;2016:1–8. [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Ni H, Coady S, Rosamond W, Folsom AR, Chambless L, Russell SD, Sorlie PD. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;157:46–52. doi: 10.1016/j.ahj.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas PC, Liu Y, Abdalla MI, Thomas CM, Kidwell DT, Dusio GF, Mukhopadhyay D, Kumar R, Baker KM, Mitchell BM, Powers PA, Fitzsimons DP, Patel BG, Warren CM, Solaro RJ, Moss RL, Tong CW. Phosphorylation of cardiac Myosin-binding protein-C is a critical mediator of diastolic function. Circ Heart Fail. 2015;8:582–594. doi: 10.1161/CIRCHEARTFAILURE.114.001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong CW, Wu X, Liu Y, Rosas PC, Sadayappan S, Hudmon A, Muthuchamy M, Powers PA, Valdivia HH, Moss RL. Phosphoregulation of cardiac inotropy via myosin binding protein-C during increased pacing frequency or beta1-adrenergic stimulation. Circ Heart Fail. 2015;8:595–604. doi: 10.1161/CIRCHEARTFAILURE.114.001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein c phosphorylation is cardioprotective. Proc Natl Acad Sci USA. 2006;103:16918–16923. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govindan S, McElligott A, Muthusamy S, Nair N, Barefield D, Martin JL, Gongora E, Greis KD, Luther PK, Winegrad S, Henderson KK, Sadayappan S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuster DW, Cardenas-Ospina A, Miller L, Liebetrau C, Troidl C, Nef HM, Mollmann H, Hamm CW, Pieper KS, Mahaffey KW, Kleiman NS, Stuyvers BD, Marian AJ, Sadayappan S. Release kinetics of circulating cardiac myosin binding protein-C following cardiac injury. Am J Physiol Heart Circ Physiol. 2014;306:H547–H556. doi: 10.1152/ajpheart.00846.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaier TE, Anand A, Shah AS, Mills NL, Marber M. Temporal relationship between cardiac Myosin-binding protein C and cardiac troponin I in type 1 myocardial infarction. Clin Chem. 2016;62:1153–1155. doi: 10.1373/clinchem.2016.257188. [DOI] [PubMed] [Google Scholar]
- 11.Govindan S, Kuster DW, Lin B, Kahn DJ, Jeske WP, Walenga JM, Leya F, Hoppensteadt D, Fareed J, Sadayappan S. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am J Cardiovasc Dis. 2013;3:60–70. [PMC free article] [PubMed] [Google Scholar]
- 12.El Amrousy D, Hodeib H, Suliman G, Hablas N, Salama ER, Esam A. Diagnostic and prognostic value of plasma levels of cardiac myosin binding protein-C as a novel biomarker in heart failure. Pediatr Cardiol. 2016;38:418–424. doi: 10.1007/s00246-016-1532-2. [DOI] [PubMed] [Google Scholar]
- 13.Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, Mollmann H, Nef H, Sicard P, Kailey B, Devaraj R, Redwood SR, Kunst G, Weber E, Marber MS. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110:23. doi: 10.1007/s00395-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuster DW, Barefield D, Govindan S, Sadayappan S. A sensitive and specific quantitation method for determination of serum cardiac myosin binding protein-C by electrochemiluminescence immunoassay. J Vis Exp. 2013;78:50786. doi: 10.3791/50786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 18.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.