Abstract
High-level cognitive constructs, such as creativity and intelligence, entail complex and multiple processes, including cognitive control processes. Recent neurocognitive research on these constructs highlight the importance of dynamic interaction across neural network systems and the role of cognitive control processes in guiding such a dynamic interaction. How can we quantitatively examine the extent and ways in which cognitive control contributes to creativity anf intelligence? To address this question, we apply a computational network control theory (NCT) approach to structural brain imaging data acquired via diffusion tensor imaging in a large sample of participants, to examine how NCT relates to individual differences in distinct measures of creative ability and intelligence. Recent application of this theory at the neural level is built on a model of brain dynamics, which mathematically models patterns of inter-region activity propagated along the structure of an underlying network. The strength of this approach is its ability to characterize the potential role of each brain region in regulating whole-brain network function based on its anatomical fingerprint and a simplified model of node dynamics. We find that intelligence is related to the ability to “drive” the brain system into easy to reach neural states by the right inferior parietal lobe and lower integration abilities in the left retrosplenial cortex. We also find that creativity is related to the ability to “drive” the brain system into difficult to reach states by the right dorsolateral prefrontal cortex (inferior frontal junction) and higher integration abilities in sensorimotor areas. Furthermore, we found that different facets of creativity—fluency, flexibility, and originality—relate to generally similar but not identical network controllability processes. We relate our findings to general theories on intelligence and creativity.
Keywords: creativity, intelligence, network control theory, cognitive control
1. Introduction
High-level cognition entails complex and multiple processes, including cognitive control processes. For example, current theories on the creative process view it as a multistage process, involving dynamic interactions between bottom-up, automatic processes involved during idea generation; and top-down, executive control processes involved during idea evaluation (Barr, Pennycook, Stolz, & Fugelsang, 2014; Beaty, Benedek, Silvia, & Schacter, 2016; Chrysikou, in press; Sowden, Pringle, & Gabora, 2014). These theories attribute a key role to cognitive control processes in guiding creative novelty seeking and response retrieval, selection, and evaluation (Chrysikou, in press). Similarly, the neural processes related to reasoning and intelligence demand cognitive control processes, required for fluent manipulation of complex information (Hearne, Cocchi, Zalesky, & Mattingley, 2015; Hearne, Mattingley, & Cocchi, 2016; Jung & Haier, 2007). However, the exact nature of the cognitive control required in such high-level cognitive processes are still mostly unknown and debated. Here, we apply a state-of-the-art computational approach—network control theory—to quantitatively examine how different control strategies in specific brain regions relate to creativity and intelligence. Such a comparison can further elucidate the differences between these two high-level, cognitive constructs.
In the past decade, there has been a large increase in neurocognitive research on creativity, attempting to identify the main brain regions that contribute to creativity (Dietrich & Kanso, 2010; Gonen-Yaacovi et al., 2013; Jung, Mead, Carrasco, & Flores, 2013; Shen, Yuan, Liu, & Luo, 2017; Wu et al., 2015; Yoruk & Runco, 2014). These efforts have related the generation process in creativity to the Default Mode Network (DMN) and the evaluation process in creativity to the Executive Control Network (ECN; Beaty et al., 2016). The DMN is a set of midline and inferior parietal regions that activate in the absence of most external task demands (Andrews-Hanna, Smallwood, & Spreng, 2014). The DMN is associated with cognitive processes that require internally-directed or self-generated thought, including mind-wandering, future thinking, semantic memory, and mental simulation (Andrews-Hanna et al., 2014; Zabelina & Andrews-Hanna, 2016). The ECN is a set of prefrontal and posterior parietal regions that are engaged during cognitive tasks that require externally-directed attention, such as working memory, relational integration, response inhibition, and task-set switching (Zabelina & Andrews-Hanna, 2016). Recent studies have found that ECN and DMN networks cooperate in tasks that require evaluation of internal information, such as autobiographical future memory planning, emotion regulation, and mind wandering (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Gerlach, Spreng, Madore, & Schacter, 2014; Ochsner, Silvers, & Buhle, 2012; Spreng et al., 2014).
A growing amount of research relates the creative process to dynamical interactions between these two systems (Beaty, Benedek, Kaufman, & Silvia, 2015; Beaty et al., 2016; Liu et al., 2015; Pinho, de Manzano, Fransson, Eriksson, & Ullén, 2014). For example, Beaty et al. (2015) conducted a temporal functional connectivity analysis when participants either generated alternative uses or simple characteristics for common objects. The authors show DMN and ECN cooperation at later stages of the creative task, which they interpret as the ECN executing evaluation processes on ideas generated by the DMN during earlier stages of the task (Beaty et al., 2015). Such a dynamic role for the ECN is consistent with a theory on the role of the prefrontal cortex as a filtering mechanism, contingent on task demands and context (Chrysikou, Weber, & Thompson-Schill, 2014). According to this theory, cognitive control, as mediated by the prefrontal cortex, is critical to performance on tasks that rely on top-down, rule-based processes (such as idea evaluation), and can constrain performance in tasks that rely on bottom-up, spontaneous processes (such as idea generation; Chrysikou et al., 2014).
Recent neurocognitive theories also relate intelligence to similar brain networks and dynamics as those observed in creativity research (Hearne et al., 2016; Jung & Haier, 2007; Pineda-Pardo, Martínez, Román, & Colom, 2016). The dominant theory on the neural processes involved in intelligence has implicated frontal and parietal activation in relation to individual differences in intelligence, theory known as the Parieto-Frontal Integration Theory of intelligence (P-FIT; Jung & Haier, 2007). Hearne, Mattingley, and Cocchi (2016) conducted a resting state functional connectivity analysis to examine the P-FIT theory and how it relates to interactions between different neural system networks. Suprisngly, the authors found that greater connectivity between ECN (overlapping with the P-FIT theory) and DMN networks was associated with higher intelligence scores. Such an integration was also shown in a task related functional imaging study, which demonstrated how task complexity in the Wason sorting task led to higher connectivity between ECN and DMN areas (Hearne et al., 2015). Recently, Santarnecchi, Emmendorfer, and Pascual-Leone (2017) conducted a meta-analysis to examine the brain regions and networks related to fluid intelligence (Gf), the ability to apply a variety of mental operations to solve novel problems (McGrew, 2005). This analysis highlighted the interaction of attention, salience, and cognitive control networks in Gf (Santarnecchi et al., 2017). Specifically, the authors argue that individual differences in Gf are attributed to the interactions between the ventral (stimulus driven) attention network and the dorsal (goal driven) attention network (Corbetta, Patel, & Shulman, 2008). Finally, the authors found that increasing task difficulty recruited left prefrontal cortex areas (Santarnecchi et al., 2017).
The apparent parallels in the neurocognitive mechanisms supporting intelligence and creativity, along with several recent studies linking these constructs at the behavioral level (Beaty, Silvia, Nusbaum, Jauk, & Benedek, 2014; Benedek, Jauk, Sommer, Arendasy, & Neubauer, 2014; Benedek et al., 2017; Kenett, Beaty, Silvia, Anaki, & Faust, 2016; Silvia, 2015), raises interesting questions about how intelligence and creativity engage cognitive control processes in the brain. Importantly, these studies argue for an interaction between the ECN and DMN, and that the ECN exerts cognitive control mechanisms that are crucial for reasoning and creativity. However, these studies do not account for the specific control mechanisms exerted in these processes. Furthermore, they do not examine the differences in the neurocognitive processes engaged in such control processes related to creativity or intelligence. Here we present a novel computational network neuroscience method that is based on white-matter connectivity networks that may be applied to examine theoretical control processes related to different high-level cognitive processes.
Currently, the majority of the research on the neurocognitive processes of high-level cognition, such as creativity and intelligence, is conducted via functional MRI (both rest and task related; Basten, Hilger, & Fiebach, 2015; Beaty et al., 2016; Deary, Penke, & Johnson, 2010). Functional MRI is well suited in examining state level variability across participants, given that rest and task functional activity related patterns fluctuate in ways that predict similarly fluctuating cognitive measures. However, anatomical brain network analysis might be better suited for looking at trait level variability across participants, by measuring stable individual differences in their neuroanatomy that might constrain neural and psychological states. Furthermore, recent computational studies have begun to demonstrate how functional brain signals are constrained by anatomical brain connectivity (Medaglia, Huang, et al., 2016). Thus, anatomical brain connectivity can contribute to investigating state-level cognition, and to better understanding functional neural signals. This is due to the unique information embedded in the anatomical signal that is independent of the functional signal, Thus, anatomical connectivity analysis contributes to neurocognitive research on cognitive phenomena (Sotiropoulos & Zalesky, 2017).
Only a few studies have examined how white matter structural connectivity relates to creative ability (Jung, Grazioplene, Caprihan, Chavez, & Haier, 2010; Jung et al., 2013; Ryman et al., 2014; Takeuchi et al., 2017; Takeuchi et al., 2010, 2011; Wu, Zhong, & Chen, 2016). Takeuchi et al. (2010) conducted a diffusion tensor imaging study to examine the relation between white matter integrity (measured with fractional anisotropy) and creative ability. The authors show how increased integrity in white-matter anatomical connectivity in several brain areas, including the bilateral prefrontal cortex and the corpus callosum, were significantly correlated with creativity. The authors interpret the significant relation between creativity and white matter integrity in the frontal lobes via enhanced cognitive control processes, and the relation with the corpus callosum via more efficient information integration across the two hemispheres. A few have examined how white matter structural connectivity relates to intelligence (Bettcher et al., 2016; Haász et al., 2013; Ohtani et al., 2017; Ohtani et al., 2015; Penke et al., 2012). These studies, while mostly supporting the P-FIT theory of intelligence, have also found a relation between white matter integrity in the corpus callosum and intelligence (Bettcher et al., 2016) or distributed white matter tracts across the brain (Haász et al., 2013). For example, Haász et al. (2013) examined the relation of white matter integrity to different components of fluid intelligence. The authors show how higher Gf was related to general higher white matter integrity across the brain.
Thus, individual differences in such high-level cognitive constructs may be related to variance in whole brain white matter connectivity, which may facilitate efficient cognitive control processes. In the current study, we apply computational network control theory (NCT) in relation to individual differences in creativity and intelligence. This allows us to examine how whole brain structural connectivity theoretically “controls” dynamic brain processes in relation to individual differences in creativity and intelligence and whether they engage similar control processes.
From an engineering perspective, network control is a process in which a system is deliberately shifted or guided along a particular trajectory to support specific goals (Tang & Bassett, 2017). This guidance is usually theoretically examined by simulating injection of signals into the system via deliberate perturbations. Recently, network control theory has been applied to study the dynamics of large-scale neural systems (Gu et al., 2015; Medaglia, Gu, et al., 2016; Yan et al., 2017). A recent study applied NCT to investigate the significance of specific neurons in C. Elegans locomotion behavior. Importantly, these predictions were empirically examined and verified by ablating specific neurons identified as significant controllers (Yan et al., 2017). Thus, this study demonstrates the feasibility of this computational theoretical approach in examining control strategies and dynamics in such neural systems
Application of this theory at the human neural level is built on a mathematical model of brain dynamics in which patterns of inter-region activity are propagated along the white matter structure of an underlying network. The strength of this approach is its ability to better understand the role of each brain region in regulating whole-brain network function based explicitly on its anatomical fingerprint (Gu et al., 2015; Tang & Bassett, 2017). Investigating the controllability of neural dynamics is computationally challenging, as it requires modelling non-linear neural dynamics and the neural structural connectivity that gives rise to such dynamics (Gu et al., 2015). It has been shown that these two aspects of complexity are independent, and thus application of control theory in neuroscience is built upon anatomical connectivity networks combined with a simplified, linear model of such neural dynamics (Gu et al., 2015). This assumption of linear dynamics is commonly accepted, and is based upon prior models linking anatomical brain networks to resting state functional dynamics (Abdelnour, Voss, & Raj, 2014; Bettinardi et al., 2017; Honey et al., 2009; Honey, Thivierge, & Sporns, 2010; Muldoon et al., 2016). These prior studies have demonstrated that a moderate amount of variance in neural dynamics as measured by fMRI can be predicted from simplified linear models (Galán, 2008; Honey et al., 2009). Recently, Muldonn et al. (2016) examined the effects of regional brain stimulation on controllability of brain states using a non-linear computational model. The authors demonstrate that while the dynamics of their computational model is highly variable across participants, it is highly reproducible across multiple imaging scans. Furthermore, the authors applied their non-linear model to validate controllability measures computed based on the simplified linear model. Thus, while the forefront of computational neuroscience aims to develop methods to map the relation between structural and functional signals (Medaglia, Huang, et al., 2016), controllability measures built on a simplified linear model have proven their fruitfulness.
Recent applications of NCT to neural systems have mathematically formulated a set of three controllability metrics that quantify the contributions made by individual brain regions in “driving” the entire brain network from one state (the magnitude of neurophysiological activity across brain regions at a single time point) into another: Average, Modal, and Boundary controllability (Gu et al., 2015; Pasqualetti, Zampieri, & Bullo, 2014). Average controllability quantifies the theoretical extent to which a specific brain region can “drive” the brain into different states with little effort. Thus, brain regions with high average controllability can drive the brain into many “easy to reach” states, and it has been associated with DMN regions (Gu et al., 2015; Pasqualetti et al., 2014). Gu et al. (2015) have argued that if control energy relates to cognitive effort, and if brain regions relate to cognitive function, than brain regions with high average controllability are important in allowing the brain to move smoothly between such functions that require little effort. Modal controllability quantifies the theoretical extent to which a specific brain region can “drive” the brain into states that require substantial input energy to reach, thus considered as “difficult-to-reach” states. Brain region with high modal controllability have been associated with fronto-parietal regions (Gu et al., 2015). Boundary controllability quantifies the theoretical extent to which a specific brain region lies at the “boundary” between network sub-communities, contributing to the integration between them. Brain regions with high boundary controllability have been associated with attention systems (Gu et al., 2015). Collectively, these three theoretical control roles define different continua in brain networks: brain regions may vary in their tendency to drive the brain to or away from specific types of states or into integrated states, and are termed according to their ability to globally control the entire brain system. Without the context of behavior and the brain, these are mathematical abstractions that may hold no behavioral relevance. The extent to which these controllability roles vary across individuals may be related to behavioral and cognitive variability, which would establish a link between network control theoretic analysis and cognition (Medaglia, Gu, et al., 2016; Medaglia, Harvey, White, Bassett, & Hamilton, 2017; Tang et al., 2016).
A few recent studies have demonstrated the feasibility of applying network control theory to study cognition. Tang et al. (2016) investigated whole brain network controllability measures related to typical neurocognitive development. The authors found that the relative strength of average controllability of subcortical brain regions predicted improved cognitive performance as related to development. Medaglia et al. (2016) demonstrated how modal and boundary controllability related to individual differences in cognitive control. The authors computed whole-brain modal and boundary controllability, and related these measures to performance on a variety of tasks that demand executive control (such as the Stroop task). This study demonstrates how these controllability measures for specific brain areas (such as in frontal control areas) correlate with performance on the different tasks, and it is the first to ground cognitive control in network controllability measures. Finally, Medaglia et al. (2017) show how modal controllability of the left inferior frontal gyrus predicts volburability to transcranial magnetic brain stimulation on linguistic tasks. Furthermore, the authors show how such brain stimulation affects the boundary controllability of the left inferior frontal gyrus. Thus, the application of NCT in neurocognitive research advances our understanding of regions’ theoretical roles in driving activity across the brain as related to cognitive processes.
In the current study, we apply NCT on white-matter anatomical connectivity networks in a large sample of participants (N = 416) who completed a battery of creativity and intelligence tasks. Creativity was assessed via a battery of divergent thinking tasks (DT), the hallmark predictor of creative ability characteristics, frequently applied in creativity research (Baird et al., 2012; Runco & Acar, 2012), and predicts real-life creative ability (Plucker, 1999). Intelligence was measured by a battery of Raven’s matrices tasks, widely applied in intelligence research (Carpenter, Just, & Shell, 1990). For each participant, we extracted anatomical connectivity matrices based on diffusion tractography and compute average, modal, and boundary controllability for brain regions across the whole brain. We then examined and compared the relation of each of the controllability measures to creativity and intelligence. Next, we conducted a similar analysis to different facets of creativity based on standard measures of DT computed from participants’ performance – fluency, flexibility, and originality. This analysis allows us to quantify and compare how specific control strategies in different brain regions differentiate between intelligence and creativity in general, and between specific measures of creative ability. More generally, our study allows us to quantitatively examine theories on the roles of the DMN and ECN systems in driving brain network dynamics as related to individual differences in creativity and intelligence. While we are theoretically motivated to focus on the ECN and DMN networks, we conduct a whole-brain analysis to examine differences in controllability across all possible brain regions. This is motivated by the possibility that different brain regions actually regulate such dynamics and the functional imaging studies somhow shadow more specific interactions across different brain regions.
In line with recent research on the neurocognitive processes related to intelligence and creativity (Chrysikou, in press; Santarnecchi et al., 2017), we predict a significant positive relation between modal controllability, intelligence, and creativity in prefrontal cortex. Intuitively, individuals that have a prefrontal cortex that is highly specialized to drive the brain into difficult to reach brain states may have superior performance on tasks demanding higher cognitive control such as intelligence or creativity. Furthermore, consistent with theories on the significance of ECN and DMN interaction related to btoh creativity and intelligence (Beaty et al., 2016; Hearne et al., 2016), we predict a significant relation between boundary controllability, creativity, and intelligence in brain regions that have been implicated in coupling between ECN and DMN systems, such as the inferior frontal gyrus (Sebastian et al., 2016), or the insula (Menon, 2011). We expect that such a relation will highlight the coupling relation previously found in DMN and ECN networks in the creative process (Beaty et al., 2015) and in reasoning (Hearne et al., 2015).
2. Methods
2.1. Participants
The sample was collected as part of a large research project exploring the associations among individual differences in brain structure and function, creativity, and mental health (Chen et al., 2016; Chen et al., 2015; W. Liu et al., 2017). Participants were recruited from Southwest University by means of the campus network, advertisements on bulletin boards and leaflets, or through face-to-face communications on campus. Before enrolling in the study, each participant was screened with a set of exclusion procedures involving self-reported questionnaires as well as structured and semi-structured interviews.
The original sample included 443 participants. Seventeen participants were excluded from the final analysis due to a creativity score two standard deviations lower or higher than the average score. The remaining sample of 416 participants included 225 females (54%) with an average age of 20 years (SD = 1.26 years). All participants were required to be right-handed, and none had a history of psychiatric disorder, cognitive disability, substance abuse, or MRI contraindications. This research project was approved by the Southwest University Brain Imaging Center Institutional Review Board, and written informed consent was obtained from each participant. Participants received payment depending on time and tasks completed.
2.2. Materials
2.2.1. Behavioral Measures
Creativity Assessment
The verbal form of the Torrance Tests of Creative Thinking (TTCT; Torrance, 1966) was used to assess creativity (i.e., divergent thinking ability). The TTCT was revised in Chinese by the Shanghai Normal University (Ye, Hong, & Torrance, 1988), and the scoring guide was slightly adjusted in recent studies because some responses were produced in contemporary times that were non-existent in the original guidelines (Chen et al., 2015; Wei et al., 2014). We administered five tasks out of the Chinese version of the TTCT: generating questions; causes and consequences; improving products; alternate uses; and manipulating objects. The TTCT provides a total creativity score as well as indices and scores for evaluating different measures of creativity, assessed by divergent thinking abilities, which includes (a) fluency (the number of meaningful and relevant responses, which is associated with the ability to generate and consider several different possibilities), (b) flexibility (the number of different categories of responses, which reflects the ability to shift between conceptual fields), and (c) originality (the degree of originality of the responses, which is associated with thinking “outside of the box”).
Three trained raters scored the creative quality of all responses. The three raters majored in psychology and were blind to the goal of this research. First, they were trained to master the method of manual scoring of the responses. Then, they independently assessed all responses of 30 participants and yielded relatively uniform scoring criterion through structured discussions. This step was used to adjust the scoring guide for flexibility in the present sample, such as to how to evaluate a response that was nonexistent in the original guidelines. Finally, raters were asked to assess the responses of all participants based on these guidelines, and their inter-rater correlation coefficient was significant (ICC > .90).
Latent variable analysis was applied to extract factor scores for each participant on the three DT measures (fluency, flexibility, and originality) using Mplus 7.4. a strength of this approach compared to computing averages is that it models error variance separately from true measurement variance, leading to a more robust and reliable assessment of effect size (Klein, 2011). In addition to modelling the three DT measures separately, we also specified a higher-order latent model that included these three measures as lower-order indicators. We thus examined unique relationships between the three DT measures (fluency, flexibility, and originality), as well as their combined contribution, and network controllability measures.
Intelligence assessment
To adjust for the effect of general intelligence on creativity, we assessed intelligence with the Combined Raven’s Test (CRT), a widely adopted measure administered to Chinese individuals between the ages of 5 and 75 (Li, Hu, Chen, & Jin, 1989; Qian, Wang, & Chen, 1997; Wang, Di, & Qian, 2007). The CRT is based on Raven’s Color Progressive Matrices (Raven, 1958) and Raven’s Standard Progressive Matrices (Raven, 1960). It contains 72 items in 6 segments of the CRT-RC2, corresponding to the Color Progressive Matrices lists A, AB, and B, and the Standard Progressive Matrices lists C, D, and E in the original Raven matrices. The CRT for Adults in China (CRT-AC2) has shown good reliability and validity, and the Chinese norms for CRT-AC2 was established from a sample of 2526 people (17–64) from 20 provinces in China (Qian et al., 1997).
The raw scores of the CRT are computed by summing the number of correct responses, and the distribution of participants is calculated with percentiles that vary from 0 to 100 in the different age groups. The percentiles were converted to z-scores using a z-table and the standard CRT scores (mean = 100 and SD = 15) were calculated according to the Norm for Chinese Adult by Tianjin Medical University (Qian et al., 1997; Wang et al., 2007).
2.2.2. MRI Data Acquisition
Imaging data were collected using a 12-channel head coil on a Siemens 3 T Trio scanner (Siemens Medical Systems, Erlangen, Germany) at the Brain Imaging Center, Southwest University. High-resolution, three-dimensional T1-weighted structural images were obtained using a Magnetization Prepared Rapid Acquisition Gradient-echo (MPRAGE) sequence (TR/TE = 1900 ms/2.52 ms, FA = 9°, resolution matrix = 256 × 256; slices = 176; thickness = 1.0 mm; voxel size = 1 × 1 × 1 mm3). Diffusion tensor images were obtained using a diffusion-weighted, single shot, spin echo, EPI sequence (TR/TE = 11000/98 ms, matrix = 128 × 128, field of view = 256 × 256 mm, voxel size = 2 × 2 × 2 mm3, 60 axial slices, 2 mm slice thickness, b value 1 = 0 s/mm2, b value 2 = 1000 s/mm2) in 30 directions and repeated acquisition of DWI data three times to increase the signal-to-noise (SNR).
DTI data were reconstructed in DSI Studio (www.dsi-studio.labsolver.org) using q-space diffeomorphic reconstruction (QSDR; Yeh, Wedeen, & Tseng, 2011). QSDR first reconstructs diffusion-weighted images in native space and computes the quantitative anisotropy (QA) in each voxel. These QA values are used to warp the brain to a template QA volume in Montreal Neurological Institute (MNI) space using the statistical parametric mapping nonlinear registration algorithm. Once in MNI space, spin density functions were again reconstructed with a mean diffusion distance of 1.25 mm using three fiber orientations per voxel. Fiber tracking was performed in DSI Studio with an angular cutoff of 35, step size of 1.0 mm, minimum length of 10 mm, spin density function smoothing of 0, maximum length of 400 mm and a QA threshold determined by DWI signal in the colony-stimulating factor. Deterministic fiber tracking using a modified FACT algorithm was performed until 1,000,000 streamlines were reconstructed for each individual. Anatomical scans were segmented using FreeSurfer (Fischl, 2012) and parcellated using the connectome mapping toolkit (Cammoun et al., 2012). Based on previous research (Gu et al., 2015; Hermundstad et al., 2013; Medaglia, Gu, et al., 2016), a parcellation scheme including 234 brain regions (Cammoun et al., 2012) was registered to the B0 volume from each participant’s DTI data. The B0 to MNI voxel mapping produced via QSDR was used to map region labels from native space to MNI coordinates. To extend region labels through the grey-white matter interface, the atlas was dilated by 4 mm (Cieslak & Grafton, 2014). Dilation was accomplished by filling non-labelled voxels with the statistical mode of their neighbors’ labels. In the event of a tie, one of the modes was arbitrarily selected. Each streamline was labelled according to its terminal region pair. From these data, we constructed structural connectivity networks that map streamline connections between 234 cortical and sub-cortical regions. In these anatomical connectivity matrices brain regions are defined as nodes, and a link between two nodes represents the number of streamlines connecting them, normalized for their density (Sotiropoulos & Zalesky, 2017).
2.2.3. Network controllability analysis
To study the ability of a certain brain region to influence other regions in different ways, we adopt the control theoretic notion of controllability. Controllability of a dynamical system refers to the possibility of driving the state of a dynamical system to a specific target state by means of an external control input (Tang & Bassett, 2017). Classic results in control theory ensure that controllability of the network is equivalent to the controllability Gramian matrix, which determines whether a linear system is controllable (Summers, Cortesi, & Lygeros, 2016).
Besides ensuring controllability, the eigenvalues of the controllability Gramian are a quantitative measure of the magnitude of the control input that drives a network to a desired target state, and the structure of the Gramian itself provides systematic guidelines for the selection of control regions that can theoretically optimize cognitive functions. While the magnitude of the control input may not be the unique feature to take into account when controlling brain dynamics (Kumar, Menolascino, & Ching, 2014), it allows us to better understand the relationship between the anatomical organization of the brain and its dynamics. Here, this allows us to isolate the control role of each region separately for each participant and relate it with our behavioral measures. A rigorous mathematical formulation of network controllability in brain networks can be found in Gu et al. (2015). From the Gramian matrix, different controllability measures can be computed for each node (brain region) in the network. Here, based on previous research of network controllability in brain networks, we compute for each participant and each brain region their average controllability, modal controllability, and boundary controllability (Gu et al., 2015; Medaglia, Gu, et al., 2016; Pasqualetti et al., 2014).
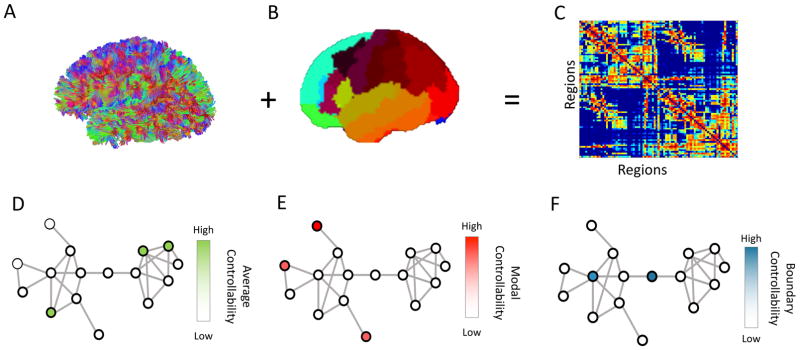
Average controllability identifies brain regions that, on average, can drive the system into different states with little effort (input energy). A state can be defined as the vector of neurophysiological activity magnitudes across brain regions at a single time point. Thus, regions with high average controllability can move the brain to many easily reachable states (Figure 1D). Thus, these regions may be important in allowing the brain to move smoothly between many cognitive functions that require little cognitive effort. Previous work has identified brain regions that demonstrate high average controllability, such as the precuneus, posterior cingulate, superior frontal, paracentral, precentral and subcortical structures (Gu et al., 2015).
Figure 1.
Overview of Methods: (A) We performed diffusion tractography for each participant, and (B) applied a whole-brain parcellation to identify anatomical divisions. (C) We constructed a anatomical connectivity matrix that represents the number of streamlines between pairs of regions, normalized by density. We defined a simplified model of brain dynamics and simulated network control to quantify (D) average, (E) modal and (F) boundary controllability for each node (brain region) in the network for each participant.
Modal controllability identifies brain regions that can drive the brain into different states that require high effort to achieve (those which require substantial input energy). Thus, regions with high modal controllability can move the brain to many difficult to reach states (Figure 1E). From a cognitive perspective, these regions may be important in switching the brain between functions that require significant cognitive effort. Previous work has identified brain regions that demonstrate high modal controllability, such as the postcentral, supramarginal, inferior parietal, pars orbitalis, medial orbitofrontal and rostral middle frontal cortices (Gu et al., 2015).
Boundary controllability identifies brain regions that can drive the system into states where different cognitive systems are either coupled or decoupled (Figure 1F). From a cognitive perspective, these regions may be important in gating, synchronizing or otherwise manipulating information across different cognitive processes. Previous work has identified brain regions that demonstrate high boundary controllability, such as the rostral middle frontal, lateral orbitofrontal, frontal pole, medial orbitofrontal, superior frontal and anterior cingulate cortices (Gu et al., 2015).
Boundary controllability identifies network nodes that lie at the boundaries between network communities, as defined across all possible levels of hierarchical modularity in a network (Tang & Bassett, 2017). As such, an initial identification of brain modules (or communities) is required. While data-driven approaches have been developed to achieve such an identification, identifying brain modular organization remains an open challenge (see Medaglia, Gu, et al., 2016). Here we chose to side step this issue and use a modular assignment that was computed via a data-driven approach that analyzed a large independent sample of resting state functional data using the same parcellation atlas. This approach, based on the method developed by Mišić et al. (2015), uses a consensus analysis to identify a partition that maximizes the modular partition of a large sample of independent datasets (Mišić et al., 2015). This partition identified 12 systems which are in line with neural systems identified in previous research (Dosenbach et al., 2010). Using this a priori independent modularity partition controls for the stochastic nature of the boundary controllability method and is justified by the identified relation between anatomical connectivity and resting state functional data (Honey et al., 2009).
2.2.4. Analysis overview
Our analysis process is as follows (Figure 1): We defined anatomical brain networks by subdividing the entire brain into 234 anatomically distinct brain regions (network nodes) in a commonly used anatomical atlas (Cammoun et al., 2012; Daducci et al., 2012; Hagmann et al., 2008). Following prior work (Bassett, Brown, Deshpande, Carlson, & Grafton, 2011; Gu et al., 2015; Hermundstad et al., 2013; Hermundstad et al., 2014), we connected nodes (brain regions) by the number of white matter streamlines identified by a commonly used deterministic tractography algorithm (Cieslak & Grafton, 2014). This procedure results in sparse, weighted, undirected structural brain networks for each participant. To control for volume confounds between pairs of brain regions i and j, streamline counts were normalized by dividing by the sum of streamlines brain region i has, which resulted in a measure of streamline density (Medaglia, Gu, et al., 2016). Next, a simplified model of brain dynamics was applied to simulate network control and quantify average, modal, and boundary controllability for each brain region for each participant, as described above (Gu et al., 2015; Tang & Bassett, 2017). Intuitively, a node’s average and modal controllability values are negatively related (Gu et al., 2015; Wu-Yan et al., 2017). To verify this, we computed the correlations between average and modal controllability across all participants. This revealed a nearly perfect negative correlation between raw average and modal controllability scores in our dataset (average correlation = −.9992, range of correlations = −.9992 – −.9402, all p’s < .001).
We then conducted a whole-brain correlation analysis between the behavioral measures and each of the network controllability measures for all brain regions. Previous studies have shown that raw boundary scores are difficult to compare across participants. This is resolved by assigning a rank boundary value for each of the nodes. Therefore, we conducted a Spearman correlation analysis for boundary controllability and a Pearson’s correlation analysis for average and modal controllability, controlling for multiple comparisons by calculating the False Discovery Rate (FDR; Benjamini & Hochberg, 1995; Benjamini & Yekutieli, 2001) with a false positive rate of 0.05. The brain networks were then visualized via the BrainNet Viewer (http://www.nitrc.org/projects/bnv/; Xia, Wang, & He, 2013). Anatomical labels were determined using the Brainnetome Atlas (http://atlas.brainnetome.org), which uses state-of-the-art multimodal neuroimaging techniques to provide a current fine-grained, cross-validated atlas and contains information on both anatomical and functional connections (Fan et al., 2016).
3. Results
We first computed the correlations between all behavioral measures analyzed (Table 1). A small but significant negative correlation was found between the CRT and the latent measure of fluency. As expected, all three divergent thinking measures were highly correlated with each other (Table 1). Contrary to previous studies (Jauk, Benedek, Dunst, & Neubauer, 2013), no significant correlation was found between the intelligence measure, as measured with the CRT, and the compiled latent creativity, as measured with DT, measures.
Table 1.
correlation analysis between all behavioral measures
| CRT | DT | Fluency | Flexibility | Originality | |
|---|---|---|---|---|---|
|
|
|||||
| CRT | - | −.04 | −.19*** | .09 | .07 |
| DT | - | .95*** | .88*** | .90*** | |
| Fluency | - | .77*** | .80*** | ||
| Flexibility | - | .76*** | |||
| Originality | - | ||||
Note -
- p < .001
We then applied the network controllability analysis by contrasting the different measures of network controllability and brain regions in the latent CRT and DT measures. Next, we conducted the same analysis for the different DT measures – fluency, flexibility, and originality. Despite the DT measures being highly correlated, this analysis allowed us to examine any possible differences between them in relation to network controllability and creativity. Such an approach allowed us to first contrast network controllability in intelligence and creativity and then to more sensitively examine network controllability in the different dimensions of creativity that were measured.
3.1. Network controllability related to intelligence and creativity
Intelligence
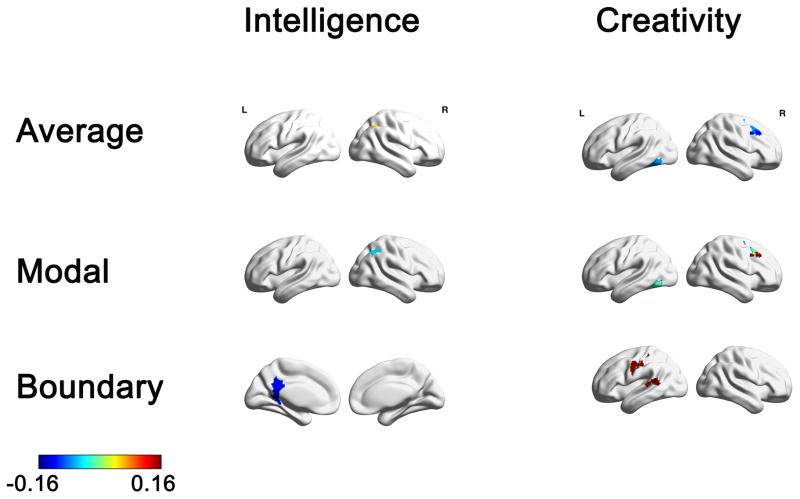
The correlation analysis between the CRT measure and the network controllability measures identified only two brain regions that survived FDR correction (Table 2 and Figure 2). A brain region within the right Inferior Parietal Lobe (IPL) exhibited a significant positive correlation with average controllability (adjusted p < .02) and a significant negative correlation with modal controllability (adjusted p < .02). Furthermore, the left Retrosplenial Cortex (RSC) exhibited a significant negative correlation with boundary controllability (adjusted p < .03).
Table 2.
Whole-brain correlation analysis between the two behavioral measures (CRT and DT) and network controllability measures (average, modal, and boundary).
| Area | Hemisphere | BA | x | y | Z | Average | Boundary | Modal | |
|---|---|---|---|---|---|---|---|---|---|
| CRT | |||||||||
| IPL | Right | 40 | 43 | −51 | 46 | .11* | −.11* | ||
| RSC | Left | 29 | −7 | −44 | 18 | −.15* | |||
|
| |||||||||
| DT | |||||||||
| IFJ | Right | 9 | 44 | 13 | 40 | −.16*** | .16*** | ||
| pMFG | Right | 8 | 35 | 17 | 53 | −.10* | .10* | ||
| Fusiform | Left | 37 | −40 | −54 | −17 | −.10* | .10* | ||
| post-central gyrus | Left | 3 | −46 | −11 | 35 | .14* | |||
| post-central gyrus | Left | 3 | −45 | −21 | 52 | .14* | |||
| post-central gyrus | Left | 4 | −57 | −16 | 44 | .14* | |||
| pSTG | Left | 22 | −53 | −44 | 10 | .14* | |||
Note – All correlation values reported survived FDR correction; x, y, z coordinates represent the peak maximal voxel in MNI space. Anatomical labels were determined using the Brainnetome Atlas (http://atlas.brainnetome.org).
- p < .05;
- p < .001
Figure 2.
Relation between individual differences in average, modal, and boundary controllability anatomical brain networks to CRT and DT. Maps highlight brain regions with significant correlation values that survived FDR correction. Warmer/colder colors indicate a positive/negative correlation between controllability and behavior.
Creativity
The correlation analysis between the latent DT measure and the network controllability measures revealed several brain regions that survived the FDR correction (Table 2 and Figure 2). This analysis revealed three brain regions that exhibited a significant negative correlation with average controllability and a significant positive correlation with modal controllability: An area in the right dorsolateral Pre-Frontal Cortex, the right Inferior Frontal Junction (IFJ; average: adjusted p < .001; modal: adjusted p < .001), the right posterior Medial Frontal Gyrus (pMFG; average: adjusted p < .04; modal: adjusted p < .04); and the left Fusiform area (average: adjusted p < .04; modal: adjusted p < .04). Furthermore, this analysis revealed a significant positive correlation with several post-central brain regions and boundary controllability (all adjusted p’s < .04) and a brain region in the left Superior Temporal Gyrus (STG; adjusted p < .03).
3.2. Network controllability related to Fluency, Flexibility, and Originality
We conducted a similar analysis on the three different DT measures: fluency, flexibility, and originality. The aim of this analysis was to examine any differences in the relation between them and network controllability measures.
Fluency
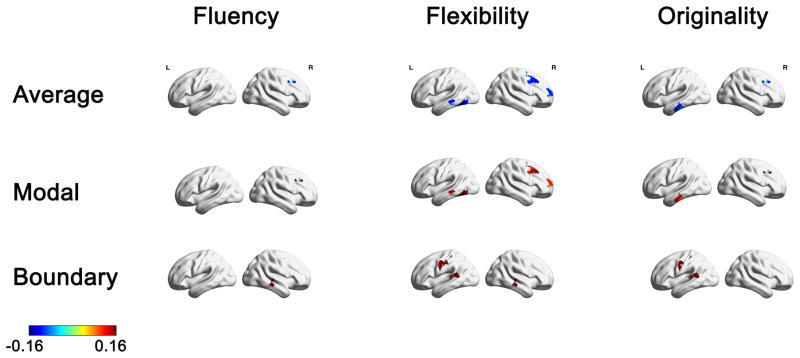
The correlation analysis between the fluency measure and the network controllability measures found two brain regions that survived FDR correction (Table 3 and Figure 3). The right IFJ exhibited a significant negative correlation with average controllability (adjusted p < .01) and a significant positive correlation with modal controllability (adjusted p < .01). Furthermore, the right Medial Temporal Gyrus (MTG) exhibited a significant positive correlation with boundary controllability (adjusted p < .02).
Table 3.
Whole-brain correlation analysis between the different DT measuresv (Fluency, Flexibility, and Originality) and network controllability measures (average, modal, and boundary).
| Area | Hemisphere | BA | x | y | z | Average | Boundary | Modal | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Fluency | |||||||||
| IFJ | Right | 9 | 44 | 13 | 40 | −.15*** | .15*** | ||
| MTG | Right | 21 | 60 | −27 | −7 | .15* | |||
|
| |||||||||
| Flexibility | |||||||||
| IFJ | Right | 9 | 44 | 13 | 40 | −.14*** | .14*** | ||
| aMFG | Right | 10 | 34 | 53 | 7 | −.11* | .11* | ||
| pMFG | Right | 6 | 37 | 3 | 46 | −.11* | .11* | ||
| pMFG | Right | 8 | 35 | 17 | 53 | −.11* | .11* | ||
| Fusiform | Left | 37 | −40 | −54 | −17 | −.12* | .12* | ||
| pMTG | Left | 21 | −62 | −36 | −7 | −.11* | .11* | ||
| MTG | Right | 21 | 60 | −27 | −7 | .13* | |||
| post-central gyrus | Left | 3 | −46 | −11 | 35 | .14* | |||
| post-central gyrus | Left | 3 | −45 | −21 | 52 | .14* | |||
| post-central gyrus | Left | 4 | −57 | −16 | 44 | .14* | |||
| pSTG | Left | 22 | −53 | −44 | 10 | .13* | |||
|
| |||||||||
| Originality | |||||||||
| IFJ | Right | 9 | 44 | 13 | 40 | −.14*** | .14*** | ||
| pITG | Left | 20 | −54 | −21 | −29 | −.10* | .10* | ||
| pITG | Left | 20 | −54 | −40 | −21 | −.10* | .10* | ||
| post-central gyrus | Left | 3 | −46 | −11 | 35 | .14* | |||
| post-central gyrus | Left | 3 | −45 | −21 | 52 | .14* | |||
| pSTG | Left | 22 | −53 | −44 | 10 | .13* | |||
Note – All correlation values reported survived FDR correction; x, y, z coordinates represent the peak maximal voxel in MNI space. Anatomical labels were determined using the Brainnetome Atlas (http://atlas.brainnetome.org)
- p < .05;
- p < .001
Figure 3.
Relation between individual differences in average, modal, and boundary controllability of anatomical brain networks to fluency, flexibility, and originality DT measures. Maps highlight brain regions with significant correlation values that survived FDR correction. Warmer/colder colors indicate a positive/negative correlation between controllability and behavior.
Flexibility
The correlation analysis between the flexibility measure and the network controllability measures found several brain regions that survived FDR correction (Table 3 and Figure 3). This analysis revealed several brain regions that exhibited a significant negative correlation with average controllability and a significant positive correlation with modal controllability: The right IFJ (average: adjusted p < .001; modal: adjusted p < .001), three brain regions (one anterior, two posterior) in the right MFG (average: all adjusted p’s < .03; modal: all adjusted p’s < .03), the left Fusiform (average: adjusted p < .02; modal: adjusted p < .02), and the left posterior MTG (average: adjusted p < .02; modal: adjusted p < .02). Furthermore, several brain regions exhibited a significant positive correlation with boundary controllability: The right MTG (adjusted p < .049), three brain regions in the post-central gyrus (all adjusted p’s < .04), and the left STG (adjusted p < .049).
Originality
The correlation analysis between the originality measure and the network controllability measures found several brain regions that survived FDR correction (Table 3 and Figure 3). This analysis revealed two brain regions that exhibited a significant negative correlation with average controllability and a significant positive correlation with modal controllability: The right IFJ (average: adjusted p < .001; modal: adjusted p < .001), and two brain regions in the posterior Inferior Temporal Gyrus (ITG; average: all adjusted p’s < .04; modal: all adjusted p’s < .04). Furthermore, two brain regions exhibited a significant positive correlation with boundary controllability: Two brain regions in the post-central gyrus (all adjusted p’s < .047), and the left STG (adjusted p < .045).
4. Discussion
In the current study, we applied a novel computational approach—network control theory—to quantify the relation between the role of different brain regions in theoretically “controlling” whole brain neural dynamics related to creativity and intelligence. Current research has implicated cognitive control processes in the ECN in both intelligence (Hearne et al., 2016; Jung & Haier, 2007) and creativity (Beaty et al., 2016; Chrysikou, in press; Chrysikou et al., 2014). But what specific control processes distinguish these cognitive abilities? We argue that network control theory can advance our understanding of the control processes evoked during fluid intelligence, as measured with the CRT, and creativity, as measured with a battery of divergent thinking tasks. Our approach is motivated by previous initial work that has implicated the importance of average controllability in typical development (Tang et al., 2016) and modal and boundary controllability in cognitive control tasks (Medaglia, Gu, et al., 2016; Medaglia et al., 2017). The current study extends recent applications of control theory to cognitive neuroscience by revealing differential control effects of intelligence and creativity—two high-level cognitive abilities that tap common and distinct aspects of cognitive control (Benedek, Jauk, Sommer, et al., 2014). While these abilities involve complex non linear neural dynamics, previous studies demonstrated how a simplified linear model applied in network control theory can be applied to study neural dynamics (Muldoon et al., 2016; Tang & Bassett, 2017). Finally, while our work is theoretically focused on the ECN and DMN systems, we apply here a whole brain analysis. Such an analysis is consistent with previous similar studies, and is also aimed to identify different brain regions that contribute to the neural dynamics, but are somhow obscured in functional imaging studies.
4.1. Controllability, Intelligence, and Creativity
Regarding intelligence, we found that the right IPL exhibited a positive correlation with average controllability and a negative correlation with modal controllability. Previous studies have found activation in right IPL related to mental manipulation of information and working memory tasks (Chochon, Cohen, Van De Moortele, & Dehaene, 1999; Klepousniotou, Gracco, & Pike, 2014; H. Liu et al., 2017). In accordance with the parietal-frontal theory of intelligence (Jung & Haier, 2007), the higher average controllability in the right IPL may facilitate processing of the multiple interpretations of specific CRT stimuli. In this sense, the more the IPL can drive the brain network into easy to reach states, the easier it may be to process demanding CRT stimuli.
We also found a negative correlation between intelligence and boundary controllability within the retrosplenial cortex (RSC). The RSC has been implicated in episodic memory, future simulation, spatial cognition, and context processing, and it exhibits direct connections with hippocampal, parahippocampal, and thalamic regions (Miller, Vedder, Law, & Smith, 2014; Spreng, Mar, & Kim, 2009; Vann, Aggleton, & Maguire, 2009). According to Miller, Vedder, Law, and Smith (2014), the RSC plays a role in processing complex cue associations (Miller et al., 2014), a function that appears crucial to performance on the CRT task, which requires processing complex associations between cues, and identifying a solution that adheres to such a complex relation. The negative relation between intelligence and RSC boundary controllability might reflect lower integration-segregation abilities in this area for heightened performance in the CRT, possibly due to a higher demand of more fluid spread of information across the dorsal-RSC-hippocampus stream.
Regarding creativity, we found a consistent pattern of relations between network controllability measures in specific brain regions associated with cognitive control, including a positive correlation with modal controllability and a negative correlation with average controllability. The brain region that showed the strongest correlations between modal controllability and creativity measures was a region in the right dorsolateral prefrontal cortex—the right Inferior Frontal Junction (IFJ). Located at the junction of the inferior frontal sulcus and the precentral sulcus (Muhle-Karbe et al., 2016), the left IFJ has been shown to play a general role in creative tasks (Gonen-Yaacovi et al., 2013) and has been specifically attributed to cognitive flexibility and task switching (Harding, Yücel, Harrison, Pantelis, & Breakspear, 2015; Yin, Wang, Pan, Liu, & Chen, 2016). Recently, Sebastian et al. (2016) showed that the right IFJ was associated with the detection of salient stimuli and co-activated with both the ventral and dorsal attention networks (Corbetta et al., 2008). This co-activation pattern points to its potential role as a mediator between the stimulus-driven ventral attention network and the goal-directed dorsal attention network (Corbetta et al., 2008; Levy & Wagner, 2011). Furthermore, in light of its role in switching between the ventral (stimulus based) and dorsal (goal directed) attention systems, the IFJ may serve as a link between DMN and ECN interactions, which have commonly been reported in fMRI studies (Beaty et al., 2015; Beaty et al., 2016). We found a similar pattern of relations within anterior posterior regions of the right MFG, consistent with past work implicating this region in suppressing inappropriate responses (CITE), shifting between response alternatives (Chen et al., 2016; Gonen-Yaacovi et al., 2013; Volle et al., 2012), and facilitating dynamic coupling between ECN and DMN networks during divergent thinking (Beaty et al., 2015; Beaty et al., 2016; Zhu et al., 2017).
Our analysis further revealed a similar pattern of relations in the left fusiform area. Research has shown that compared with baseline, original idea generation exhibits robust activation of the fusiform gyrus, indicating its involvement in the formation of new associations (Bechtereva et al., 2004; Chrysikou & Thompson-Schill, 2011; Ellamil, Dobson, Beeman, & Christoff, 2012). For example, Chrysikou and Thompson-Schill (2011) examined neural activity during generating common versus uncommon uses for objects. The authors found higher left fusiform activation when participants were required to generate uncommon responses, possibly reflecting a deeper retrieval process over visual features of objects. Our findings support the findings of Chrysikou and Thompson-Schill (2011) by finding a positive correlation between modal controllability and flexibility in the left fusiform area.
Finally, we found a similar pattern of network controllability effects within the left posterior MTG and ITG. The posterior MTG plays a key role semantic control processes (Binder & Desai, 2011; Davey et al., 2015; Davey et al., 2016; Noonan, Jefferies, Visser, & Lambon Ralph, 2013; Price, 2010) and is commonly implicated in studies of creativity (Gonen-Yaacovi et al., 2013; Shen et al., 2017). Recently, Davey et al. (2016) investigated the functional role of the left posterior MTG in semantic control, and showed that the left posterior MTG integrates information from the DMN and ECN. In this context, higher modal controllability in the left posterior MTG may facilitate inhibition of salient responses during verbal creativity tasks, consistent with behavioral work linking inhibitory control and divergent thinking abilities (Benedek, Jauk, Sommer, et al., 2014). The role of the posterior inferior temporal areas (BA 20) in creativity is currently unclear (Benedek, Jauk, Fink, et al., 2014; Zhu et al., 2017). A recent meta-analysis has shown how this region plays a secondary role in language processing, as a kind of marginal language processing region (Ardila, Bernal, & Rosselli, 2016). In a recent large-scale patient study, Herbet et al. (2016) investigate the anatomical factors that prevent full recovery of lexical retrieval in patients. The authors identify the importance of BA 20 and its underlying white matter tracts to lexical retrieval deficits (Herbet et al., 2016). Thus, higher modal controllability in the pITG may facilitate more lexical retrieval processes that increase originality.
4.2. Boundary controllability and creativity
We found a consistent correlation between boundary controllability within sensorimotor areas and divergent thinking performance. Although this finding was unexpected, it is consistent with a cognitive embodiment perspective, which argues for the importance of sensorimotor simulations (Barsalou, 2008) in divergent thinking tasks. Sensorimotor simulations refer to “the reenactment of perceptual, motor, and introspective states acquired during experience with the world, body, and mind” (Barsalou, 2008). Such simulation mechanisms have been argued to act as a core computational factor in the brain (Binder, 2016). One such example is mental imagery, which involves the construction of conscious representations in working memory (Pearson, Naselaris, Holmes, & Kosslyn, 2015). Finally, a recent study demonstrated how semantic representations of concepts bidirectionally converges with the sensorimotor system (Ekstrand et al., 2017). Thus, mental conceptual manipularions required in DT may involve the recruitment of sensorimotor brain regions.
Neuroimaging studies have demonstrated how simulation plays a central role in conceptual representations (Binder, 2016; Binder & Desai, 2011; Binder, Desai, Graves, & Conant, 2009). Surprisingly, however, no research has directly examined the relation of sensorimotor simulations and creativity (but see Cousijn, Zanolie, Munsters, Kleibeuker, & Crone, 2014, p. 10), despite the flexible processes utilized in creativity on sensorimotor features of concepts (Barsalou, 2008; Binder, 2016). In divergent thinking tasks, the canonical measure of creative ability, participants are required to simulate and manipulate concepts in order to generate novel, alternative, or uncommon uses to them (Runco & Acar, 2012). In a recent study, Matheson, Buxbaum and Thompson-Schill (2017) examine multivariate voxel pattern activation when participants generate common and uncommon tool uses to concrete tools. The authors found that common tool use is related to categorical information in ventral stream areas while uncommon tools use is related to action and visual information in dorsal stream areas, thus demonstrating flexible activation within the “tool network” (Matheson et al., 2017). The authors interpret this flexibility as contributing to the generation of creative responses.
In the context of Matheson and colleagues, we argue that the positive correlations between sensorimotor brain regions and boundary controllability as related to individual differences in divergent thinking measures of flexibility and originality, highlight the importance of integration of sensori and motor information in relation to creativity. This may indicate that a higher role in integration across brain sub-networks in such sensorimotor areas contributes to creative ability by higher integration of ventral and dorsal “tool” areas, which may lead to more creative responses in divergent thinking tasks. Such an interpretation requires future research to further explore the role of sensorimotor simulation in DT.
We also found positive correlations between divergent thinking measures and boundary controllability within temporal regions, including the left posterior STG and right MTG. Activation in the left posterior STG has been consistently found in creativity research (Gonen-Yaacovi et al., 2013; Jung-Beeman, 2005; Mirous & Beeman, 2012; Shen et al., 2017). The left STG is considered to play a key role in integrative processing via selective access to distributed cross-modal representations, which is important in forming novel associations that are required in semantic creativity tasks (Shen et al., 2017). The right MTG has also been implicated in several creativity studies (Bashwiner, Wertz, Flores, & Jung, 2016; Chen et al., 2016; Cousijn, Koolschijn, Zanolie, Kleibeuker, & Crone, 2014; Cousijn, Zanolie, et al., 2014). For example, a recent resting-state study found that divergent thinking ability was related to increased functional connectivity between the right MTG and post-central gyrus (Cousijn, Zanolie, et al., 2014). Thus, higher boundary controllability of this region could facilitate integration with sensorimotor areas, which could in turn increase fluency and flexibility of ideas that originate from sensorimotor simulations.
Taken together, our results highlight the different control processes involved in intelligence and creativity. We found that intelligence was related to a heightened ability to drive the brain into easy-to-reach states within contextual association regions (e.g., IPL and RSC). On the other hand, creativity was related to a heightened ability to drive the brain into both easy- and difficult-to-reach states, with effects largely localized within regions that connect between bottom-up and top-down processes to integration/segregation processes in sensorimotor areas.
4.3 Limitations and Future Directions
A few limitations of the current study are worth noting. First, despite an established relation between intelligence and creativity (Jauk et al., 2013), we only found a correlation between our measure of intelligence (CRT) and divergent thinking fluency (Table 1). In a previous report of these behavioral data (from the same sample; Chen et al., 2015), the authors suggested this relation might be due to the focus of the CRT on reasoning skills, which might be more similar in our sample of college students. Importantly, studies that have found a correlation between intelligence and creativity have done so only at a latent level, based on several measures of intelligence (Beaty et al., 2014; Benedek, Jauk, Sommer, et al., 2014; Nusbaum & Silvia, 2011), and not a single measure, as in the current study. Furthermore, previous studies have also found a negative correlation between fluency and intelligence (e.g., Beaty et al., 2014). Thus, future research is needed to replicate our findings with other measures of creativity and intelligence. Finally, previous research has indicated differences in the notion of creativity between Western and Eastern cultures (Niu & Sternberg, 2002). Such differences may account for the pattern of the behavioral results. Thus, our study requires future replication in a Western society.
Another limitation concerns the method we used to measure structural connectivity, which was based on DTI data. Past research suggests that DTI may under-sample some white matter fibers, particularly those linking hemispheres or those that cross paths with other fibers (Wedeen et al., 2008). This may also partially account for relatively small correlations found in our data. Future efforts should apply diffusion spectrum imaging to improve estimates of structural network architecture.
Third, our boundary controllability analysis was based on an independent, a-priori brain modularity partition, which was based on a modularity analysis of resting-state fMRI data (Mišić et al., 2015). Future research is needed to establish a-priori partitions based on structural connectivity networks, which will increase the reliability and validity of the boundary controllability analysis.
Finally, the ability of specific nodes in a network (such as anatomical brain networks) to drive the system into a specific state has been recently questioned (Menara, Gu, Bassett, & Pasqualetti, 2017; Tu et al., 2017). In this paper, we were interested in investigating the theoretical notion of network control theory and individual differences in creativity, without committing to linking between cognitive control processes and network controllability.
In conclusion, we applied a network control theory approach to identify specific control strategies in specific brain regions as related to intelligence and creativity. We found that intelligence is related to driving the brain into easy-to-reach states within association cortices, whereas creativity is related to driving the brain into difficult-to-reach states within regions involved in DMN-ECN switching and sensorimotor processing. Our work demonstrates the strength of this computational approach and how it can shed unique light on cognitive control processes involved in cognition, and highlights the role of white matter structural connectivity on large scale functional dynamics in high-level cognition.
Acknowledgments
JDM acknowledges support from the Office of the Director at the National Institutes of Health (1-DP5-OD-021352-01). REB acknowledges support from the Imagination Institute, funded by the John Templeton Foundation (RFP-15-12). QC and JQ acknowledge support from the National Natural Science Foundation of China (31470981; 31571137), National Outstanding young people plan, the Program for the Top Young Talents by Chongqing, the Fundamental Research Funds for the Central Universities (SWU1509383). STS acknowledges support from the National Institute of Health (R01 DC009209 and R01 DC015359).
References
- Abdelnour F, Voss HU, Raj A. Network diffusion accurately models the relationship between structural and functional brain connectivity networks. Neuroimage. 2014;90:335–347. doi: 10.1016/j.neuroimage.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316(1):29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A, Bernal B, Rosselli M. How extended Is Wernicke’s area? Meta-analytic connectivity study of BA20 and integrative proposal. Neuroscience Journal. 2016;2016:4962562. doi: 10.1155/2016/4962562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JWY, Franklin MS, Schooler JW. Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science. 2012;23(10):1117–1122. doi: 10.1177/0956797612446024. [DOI] [PubMed] [Google Scholar]
- Barr N, Pennycook G, Stolz JA, Fugelsang JA. Reasoned connections: A dual-process perspective on creative thought. Thinking & Reasoning. 2014;21(1):61–75. [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bashwiner DM, Wertz CJ, Flores RA, Jung RE. Musical creativity “revealed” in brain structure: Interplay between motor, default mode, and limbic networks. Scientific Reports. 2016;6:20482. doi: 10.1038/srep20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. Conserved and variable architecture of human white matter connectivity. Neuroimage. 2011;54(2):1262–1279. doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Basten U, Hilger K, Fiebach CJ. Where smart brains are different: A quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 2015;51:10–27. [Google Scholar]
- Beaty RE, Benedek M, Kaufman SB, Silvia PJ. Default and Executive Network Coupling Supports Creative Idea Production. Scientific Reports. 2015;5:10964. doi: 10.1038/srep10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, Schacter DL. Creative Cognition and Brain Network Dynamics. Trends in Cognitive Sciences. 2016;20(2):87–95. doi: 10.1016/j.tics.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ, Nusbaum EC, Jauk E, Benedek M. The roles of associative and executive processes in creative cognition. Memory & Cognition. 2014;42(7):1–12. doi: 10.3758/s13421-014-0428-8. [DOI] [PubMed] [Google Scholar]
- Bechtereva NP, Korotkov AD, Pakhomov SVk, Roudas MS, Starchenko MG, Medvedev SV. PET study of brain maintenance of verbal creative activity. International Journal of Psychophysiology. 2004;53(1):11–20. doi: 10.1016/j.ijpsycho.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Fink A, Koschutnig K, Reishofer G, Ebner F, Neubauer AC. To create or to recall? Neural mechanisms underlying the generation of creative new ideas. NeuroImage. 2014;88:125–133. doi: 10.1016/j.neuroimage.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Jauk E, Sommer M, Arendasy M, Neubauer AC. Intelligence, creativity, and cognitive control: The common and differential involvement of executive functions in intelligence and creativity. Intelligence. 2014;46:73–83. doi: 10.1016/j.intell.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Kenett YN, Umdasch K, Anaki D, Faust M, Neubauer AC. How semantic memory structure and intelligence contribute to creative thought: a network science approach. Thinking & Reasoning. 2017;23(2):158–183. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of statistics. 2001;29(4):1165–1188. [Google Scholar]
- Bettcher BM, Mungas D, Patel N, Elofson J, Dutt S, Wynn M, Watson CL, Stephens M, Walsh CM, Kramer JH. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia. 2016;85:100–109. doi: 10.1016/j.neuropsychologia.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinardi RG, Deco G, Karlaftis VM, Van Hartevelt TJ, Fernandes HM, Kourtzi Z, Kringelbach ML, Zamora-López G. How structure sculpts function: Unveiling the contribution of anatomical connectivity to the brain’s spontaneous correlation structure. Chaos: An Interdisciplinary Journal of Nonlinear Science. 2017;27(4):047409. doi: 10.1063/1.4980099. [DOI] [PubMed] [Google Scholar]
- Binder JR. In defense of abstract conceptual representations. Psychonomic Bulletin & Review. 2016;23(4):1096–1108. doi: 10.3758/s13423-015-0909-1. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammoun L, Gigandet X, Meskaldji D, Thiran JP, Sporns O, Do KQ, Maeder P, Meuli R, Hagmann P. Mapping the human connectome at multiple scales with diffusion spectrum MRI. Journal of Neuroscience Methods. 2012;203(2):386–397. doi: 10.1016/j.jneumeth.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychological Review. 1990;97(3):404. [PubMed] [Google Scholar]
- Chen Q, Beaty RE, Wei D, Yang J, Sun J, Liu W, Yang W, Zhang Q, Qiu J. Longitudinal Alterations of Frontoparietal and Frontotemporal Networks Predict Future Creative Cognitive Ability. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw353. [DOI] [PubMed] [Google Scholar]
- Chen QL, Xu T, Yang WJ, Li YD, Sun JZ, Wang KC, Beaty RE, Zhang QL, Zuo XN, Qiu J. Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia. 2015;75:441–449. doi: 10.1016/j.neuropsychologia.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Chochon F, Cohen L, Van De Moortele PF, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of cognitive neuroscience. 1999;11(6):617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG. The costs and benefits of cognitive control for creativity. In: Jung RE, Vartanian O, editors. The Cambridge Handbook of the Neuroscience of Creativity. New York, NY: Cambridge University Press; (in press) [Google Scholar]
- Chrysikou EG, Thompson-Schill SL. Dissociable brain states linked to common and creative object use. Human Brain Mapping. 2011;32(4):665–675. doi: 10.1002/hbm.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysikou EG, Weber MJ, Thompson-Schill SL. A matched filter hypothesis for cognitive control. Neuropsychologia. 2014;62:341–355. doi: 10.1016/j.neuropsychologia.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak M, Grafton ST. Local termination pattern analysis: a tool for comparing white matter morphology. Brain Imaging and Behavior. 2014;8(2):292–299. doi: 10.1007/s11682-013-9254-z. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Koolschijn PCMP, Zanolie K, Kleibeuker SW, Crone EA. The relation between gray matter morphology and divergent thinking in adolescents and young adults. PLoS ONE. 2014;9(12):e114619. doi: 10.1371/journal.pone.0114619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Zanolie K, Munsters RJM, Kleibeuker SW, Crone EA. The relation between resting state connectivity and creativity in adolescents before and after training. PLoS ONE. 2014;9(9):e105780. doi: 10.1371/journal.pone.0105780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Gerhard S, Griffa A, Lemkaddem A, Cammoun L, Gigandet X, Meuli R, Hagmann P, Thiran JP. The Connectome Mapper: An Open-Source Processing Pipeline to Map Connectomes with MRI. PLOS ONE. 2012;7(12):e48121. doi: 10.1371/journal.pone.0048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, Jefferies E. Automatic and controlled semantic retrieval: TMS reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. The Journal of Neuroscience. 2015;35(46):15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, Krieger-Redwood K, Bernhardt BC, Smallwood J, Jefferies E. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. NeuroImage. 2016;137:165–177. doi: 10.1016/j.neuroimage.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature Reviews Neuroscience. 2010;11(3):201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136(5):822–848. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand C, Neudorf J, Lorentz E, Gould L, Mickleborough M, Borowsky R. More than a feeling: The bidirectional convergence of semantic visual object and somatosensory processing. Acta Psychologica. 2017;181(Supplement C):1–9. doi: 10.1016/j.actpsy.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. NeuroImage. 2012;59(2):1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T. The human brainnetome atlas: A new brain atlas based on connectional architecture. Cerebral Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán RF. On how network architecture determines the dominant patterns of spontaneous neural activity. PloS one. 2008;3(5):e2148. doi: 10.1371/journal.pone.0002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience. 2014;9(12):1942–1951. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen-Yaacovi G, de Souza LC, Levy R, Urbanski M, Josse G, Volle E. Rostral and caudal prefrontal contribution to creativity: A meta-analysis of functional imaging data. Frontiers in Human Neuroscience. 2013;7:465. doi: 10.3389/fnhum.2013.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, Medaglia JD, Vettel JM, Miller MB, Grafton ST, Bassett DS. Controllability of structural brain networks. Nature Communications. 2015:6. doi: 10.1038/ncomms9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage. 2013;83:372–383. doi: 10.1016/j.neuroimage.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Yücel M, Harrison BJ, Pantelis C, Breakspear M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. NeuroImage. 2015;106:144–153. doi: 10.1016/j.neuroimage.2014.11.039. [DOI] [PubMed] [Google Scholar]
- Hearne L, Cocchi L, Zalesky A, Mattingley JB. Interactions between default mode and control networks as a function of increasing cognitive reasoning complexity. Human Brain Mapping. 2015;36(7):2719–2731. doi: 10.1002/hbm.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearne LJ, Mattingley JB, Cocchi L. Functional brain networks related to individual differences in human intelligence at rest. Scientific Reports. 2016;6:32328. doi: 10.1038/srep32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Moritz-Gasser S, Boiseau M, Duvaux S, Cochereau J, Duffau H. Converging evidence for a cortico-subcortical network mediating lexical retrieval. Brain. 2016;139(11):3007–3021. doi: 10.1093/brain/aww220. [DOI] [PubMed] [Google Scholar]
- Hermundstad AM, Bassett DS, Brown KS, Aminoff EM, Clewett D, Freeman S, Frithsen A, Johnson A, Tipper CM, Miller MB. Structural foundations of resting-state and task-based functional connectivity in the human brain. Proceedings of the National Academy of Sciences. 2013;110(15):6169–6174. doi: 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad AM, Brown KS, Bassett DS, Aminoff EM, Frithsen A, Johnson A, Tipper CM, Miller MB, Grafton ST, Carlson JM. Structurally-constrained relationships between cognitive states in the human brain. PLoS Comput Biol. 2014;10(5):e1003591. doi: 10.1371/journal.pcbi.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage. 2010;52(3):766–776. doi: 10.1016/j.neuroimage.2010.01.071. [DOI] [PubMed] [Google Scholar]
- Jauk E, Benedek M, Dunst B, Neubauer AC. The relationship between intelligence and creativity: New support for the threshold hypothesis by means of empirical breakpoint detection. Intelligence. 2013;41(4):212–221. doi: 10.1016/j.intell.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ. White matter integrity, creativity, and psychopathology: Disentangling constructs with diffusion tensor imaging. PLoS ONE. 2010;5(3):e9818. doi: 10.1371/journal.pone.0009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30(02):135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA. The structure of creative cognition in the human brain. Frontiers in Human Neuroscience. 2013;7:330. doi: 10.3389/fnhum.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in cognitive sciences. 2005;9(11):512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kenett YN, Beaty RE, Silvia PJ, Anaki D, Faust M. Structure and flexibility: Investigating the relation between the structure of the mental lexicon, fluid intelligence, and creative achievement. Psychology of Aesthetics, Creativity, and the Arts. 2016;10(4):377–388. [Google Scholar]
- Klein RB. convergence of structural equation modeling and multilevel modeling. In: Williams M, Vogt WP, editors. The SAGE Handbook of Innovation in Social Research Methods. Thousand Oaks, CA: SAGE Publishig; 2011. pp. 562–589. [Google Scholar]
- Klepousniotou E, Gracco VL, Pike GB. Pathways to lexical ambiguity: fMRI evidence for bilateral fronto-parietal involvement in language processing. Brain and Language. 2014;131:56–64. doi: 10.1016/j.bandl.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Kumar G, Menolascino D, Ching S. Input novelty as a control metric for time varying linear systems. 2014 arXiv preprint. [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224(1):40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hu K, Chen G, Jin Y. The handbook of Combined Raven’s Test in Chinese version. Shanghai: East China Normal University; 1989. [Google Scholar]
- Liu H, Yu H, Li Y, Qin W, Xu L, Yu C, Liang M. An energy-efficient intrinsic functional organization of human working memory: A resting-state functional connectivity study. Behavioural Brain Research. 2017;316:66–73. doi: 10.1016/j.bbr.2016.08.046. [DOI] [PubMed] [Google Scholar]
- Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, Braun AR. Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Human Brain Mapping. 2015;36(9):3351–3372. doi: 10.1002/hbm.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wei D, Chen Q, Yang W, Meng J, Wu G, Bi T, Zhang Q, Zuo XN, Qiu J. Longitudinal test-retest neuroimaging data from healthy young adults in southwest China. Scientific Data. 2017;4:170017. doi: 10.1038/sdata.2017.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson HE, Buxbaum LJ, Thompson-Schill SL. Differential tuning of ventral and dorsal streams during the generation of common and. Journal of Cognitive Neuroscience. 2017;29(11):1791–1802. doi: 10.1162/jocn_a_01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew KS. The Cattell-Horn-Carroll theory of cognitive abilities: Past, present, and future. In: Flanagan DP, Harrison PL, editors. Contemporary intellectual assessment: Theories, tests, and issues. New York, NY, US: Guilford Press; 2005. pp. 136–181. [Google Scholar]
- Medaglia JD, Gu S, Pasqualetti F, Ashare RL, Lerman C, Kable J, Bassett DS. Cognitive control in the controllable connectome. 2016 arXiv preprint. [Google Scholar]
- Medaglia JD, Harvey DY, White N, Bassett DS, Hamilton RH. Network controllability in the IFG relates to controlled language variability and susceptibility to TMS. 2017 doi: 10.1523/JNEUROSCI.0092-17.2018. arXiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medaglia JD, Huang W, Karuza EA, Thompson-Schill SL, Ribeiro A, Bassett DS. Functional alignment with anatomical networks is associated with cognitive flexibility. 2016 doi: 10.1038/s41562-017-0260-9. arXiv preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menara T, Gu S, Bassett DS, Pasqualetti F. On structural controllability of symmetric (brain) networks. 2017 arXiv preprint. [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Miller AMP, Vedder LC, Law LM, Smith DM. Cues, context, and long-term memory: The role of the retrosplenial cortex in spatial cognition. Frontiers in Human Neuroscience. 2014;8(586):586. doi: 10.3389/fnhum.2014.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirous HJ, Beeman M. Bilateral processing and affect in creative language comprehension. In: Faust M, editor. The Handbook of the Neuropsychology of Language. Oxford, UK: Blackwell Publishing; 2012. pp. 319–341. [Google Scholar]
- Mišić B, Betzel Richard F, Nematzadeh A, Goñi J, Griffa A, Hagmann P, Flammini A, Ahn YY, Sporns O. Cooperative and competitive spreading dynamics on the human connectome. Neuron. 2015;86(6):1518–1529. doi: 10.1016/j.neuron.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB. Co-Activation-Based Parcellation of the Lateral Prefrontal Cortex Delineates the Inferior Frontal Junction Area. Cerebral Cortex. 2016;26(5):2225–2241. doi: 10.1093/cercor/bhv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon SF, Pasqualetti F, Gu S, Cieslak M, Grafton ST, Vettel JM, Bassett DS. Stimulation-Based Control of Dynamic Brain Networks. PLOS Computational Biology. 2016;12(9):e1005076. doi: 10.1371/journal.pcbi.1005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Sternberg R. Contemporary studies on the concept of creativity: The East and the West. The Journal of Creative Behavior. 2002;36(4):269–288. [Google Scholar]
- Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. Journal of Cognitive Neuroscience. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Nusbaum EC, Silvia PJ. Are intelligence and creativity really so different?: Fluid intelligence, executive processes, and strategy use in divergent thinking. Intelligence. 2011;39(1):36–45. [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani T, Nestor PG, Bouix S, Newell D, Melonakos ED, McCarley RW, Shenton ME, Kubicki M. Exploring the neural substrates of attentional control and human intelligence: Diffusion tensor imaging of prefrontal white matter tractography in healthy cognition. Neuroscience. 2017;341:52–60. doi: 10.1016/j.neuroscience.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Nestor PG, Bouix S, Saito Y, Hosokawa T, Kubicki M. Medial Frontal White and Gray Matter Contributions to General Intelligence. PLOS ONE. 2015;9(12):e112691. doi: 10.1371/journal.pone.0112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti F, Zampieri S, Bullo F. Controllability metrics, limitations and algorithms for complex networks. IEEE Transactions on Control of Network Systems. 2014;1(1):40–52. [Google Scholar]
- Pearson J, Naselaris T, Holmes EA, Kosslyn SM. Mental Imagery: Functional Mechanisms and Clinical Applications. Trends in Cognitive Sciences. 2015;19(10):590–602. doi: 10.1016/j.tics.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Maniega SM, Bastin ME, Valdes Hernandez MC, Murray C, Royle NA, Starr JM, Wardlaw JM, Deary IJ. Brain white matter tract integrity as a neural foundation for general intelligence. Molecular Psychiatry. 2012;17(10):1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- Pineda-Pardo JA, Martínez K, Román FJ, Colom R. Structural efficiency within a parieto-frontal network and cognitive differences. Intelligence. 2016;54:105–116. [Google Scholar]
- Pinho AL, de Manzano Ö, Fransson P, Eriksson H, Ullén F. Connecting to create: Expertise in musical improvisation Is associated with increased functional connectivity between premotor and prefrontal areas. The Journal of Neuroscience. 2014;34(18):6156–6163. doi: 10.1523/JNEUROSCI.4769-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucker JA. Is the proof in the pudding? Reanalyses of Torrance’s (1958 to present) longitudinal data. Creativity Research Journal. 1999;12(2):103–114. [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Qian M, Wang D, Chen Z. The development of norm of the Combined Raven’s test (CRT-AC2) for Chinese adult. Chinese Journal of Controlling Endemic Diseases. 1997;12:215–217. [Google Scholar]
- Raven JC. Guide to using the coloured progressive matrices. London, UK: HK Lewis; 1958. [Google Scholar]
- Raven JC. Guide to the standard progressive matrices: sets A, B, C, D and E. London, UK: HK Lewis; 1960. [Google Scholar]
- Runco MA, Acar S. Divergent thinking as an indicator of creative potential. Creativity Research Journal. 2012;24(1):66–75. [Google Scholar]
- Ryman SG, van den Heuvel MP, Yeo RA, Caprihan A, Carrasco J, Vakhtin AA, Flores RA, Wertz C, Jung RE. Sex differences in the relationship between white matter connectivity and creativity. NeuroImage. 2014;101:380–389. doi: 10.1016/j.neuroimage.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Emmendorfer A, Pascual-Leone A. Dissecting the parieto-frontal correlates of fluid intelligence: A comprehensive ALE meta-analysis study. Intelligence. 2017;63:9–28. [Google Scholar]
- Sebastian A, Jung P, Neuhoff J, Wibral M, Fox PT, Lieb K, Fries P, Eickhoff SB, Tüscher O, Mobascher A. Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Structure and Function. 2016;221(3):1635–1651. doi: 10.1007/s00429-015-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Yuan Y, Liu C, Luo J. The roles of the temporal lobe in creative insight: an integrated review. Thinking & Reasoning. 2017:1–55. [Google Scholar]
- Silvia PJ. Intelligence and creativity are pretty similar after all. Educational Psychology Review. 2015;27(4):1–8. [Google Scholar]
- Sotiropoulos SN, Zalesky A. Building connectomes using diffusion MRI: Why, how and but. NMR in Biomedicine. 2017 doi: 10.1002/nbm.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden PT, Pringle A, Gabora L. The shifting sands of creative thinking: Connections to dual-process theory. Thinking & Reasoning. 2014;21(1):40–60. [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh WM, Turner GR. Goal-congruent default network activity facilitates cognitive control. Journal of Neuroscience. 2014;34(42):14108–14114. doi: 10.1523/JNEUROSCI.2815-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Summers TH, Cortesi FL, Lygeros J. On submodularity and controllability in complex dynamical networks. IEEE Transactions on Control of Network Systems. 2016;3(1):91–101. [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, Sekiguchi A, Iizuka K, Yamamoto Y, Hanawa S, Araki T, Makoto Miyauchi C, Shinada T, Sakaki K, Sassa Y, Nozawa T, Ikeda S, Yokota S, Daniele M, Kawashima R. Creative females have larger white matter structures: Evidence from a large sample study. Human Brain Mapping. 2017;38(1):414–430. doi: 10.1002/hbm.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage. 2010;51(1):11–18. doi: 10.1016/j.neuroimage.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. Verbal working memory performance correlates with regional white matter structures in the frontoparietal regions. Neuropsychologia. 2011;49(12):3466–3473. doi: 10.1016/j.neuropsychologia.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Tang E, Bassett DS. Control of Dynamics in Brain Networks. 2017 arXiv preprint. [Google Scholar]
- Tang E, Giusti C, Baum G, Gu S, Kahn AE, Roalf D, Moore TM, Ruparel K, Gur RC, Gur RE. Structural drivers of diverse neural dynamics and their evolution across development. 2016 arXiv preprint. [Google Scholar]
- Torrance EP. Torrance test of creative thinking. Bensenville, IL: Scholastic Testing Service, Inc; 1966. [Google Scholar]
- Tu C, Rocha RP, Corbetta M, Zampieri S, Zoezi M, Suweis S. Warnings and caveats in brain controllability. 2017 doi: 10.1016/j.neuroimage.2018.04.010. arXiv preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10(11):792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Volle E, de Lacy Costello A, Coates LM, McGuire C, Towgood K, Gilbert S, Kinkingnehun S, McNeil JE, Greenwood R, Papps B, van den Broeck M, Burgess PW. Dissociation between verbal response initiation and suppression after prefrontal lesions. Cerebral Cortex. 2012;22(10):2428–2440. doi: 10.1093/cercor/bhr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Di M, Qian M. A report on the third revision of combined raven’s test (CRT-C3) for children in China. Chinese Journal of Clinical Psychology. 2007;15(6):559–568. [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J. Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex. 2014;51:92–102. doi: 10.1016/j.cortex.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Wu C, Zhong S, Chen H. Discriminating the difference between remote and close association with relation to hite-matter structural connectivity. PLoS One. 2016;11(10):e0165053. doi: 10.1371/journal.pone.0165053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Zhang Q, Zhang M, Qiu J. A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping. 2015;36(7):2703–2718. doi: 10.1002/hbm.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Yan E, Betzel RF, Tang E, Gu S, Pasqualetti F, Bassett DS. Benchmarking measures of network controllability on canonical graph models. 2017 arXiv preprint. [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Vértes PE, Towlson EK, Chew YL, Walker DS, Schafer WR, Barabási A-L. Network control principles predict neuron function in the Caenorhabditis elegans connectome. Nature. 2017 doi: 10.1038/nature24056. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Hong D, Torrance EP. Cross cultural comparion of creative thinking between Chinese and American students using Torrance Test. Chinese Journal of Applied Psychology. 1988;3:22–29. [Google Scholar]
- Yeh FC, Wedeen VJ, Tseng WYI. Estimation of fiber orientation and spin density distribution by diffusion deconvolution. Neuroimage. 2011;55(3):1054–1062. doi: 10.1016/j.neuroimage.2010.11.087. [DOI] [PubMed] [Google Scholar]
- Yin S, Wang T, Pan W, Liu Y, Chen A. Task-switching cost and intrinsic functional connectivity in the human brain: Toward understanding individual differences in cognitive flexibility. PLoS ONE. 2016;10(12):e0145826. doi: 10.1371/journal.pone.0145826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoruk S, Runco MA. The neuroscience of divergent thinking. ANS: Journal for Neurocognitive Research. 2014;56(1–2):1–16. [Google Scholar]
- Zabelina DL, Andrews-Hanna JR. Dynamic network interactions supporting internally-oriented cognition. Current opinion in neurobiology. 2016;40:86–93. doi: 10.1016/j.conb.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen Q, Xia L, Beaty RE, Yang W, Tian F, Sun J, Cao G, Zhang Q, Chen X, Qiu J. Common and distinct brain networks underlying verbal and visual creativity. Human Brain Mapping. 2017;38(4):2094–2111. doi: 10.1002/hbm.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]