Abstract
Chlamydia muridarum induction of mouse hydrosalpinx, depending on both tubal infection and inflammation, has been used for investigating C. trachomatis pathogenesis. We now report that IL-6 both inhibits C. muridarum infection and exacerbates pathogenicity in the mouse genital tract. When intravaginally inoculated with a high dose of C. muridarum, IL-6-deficient mice developed more extensive genital tract infection with severe hydrosalpinx, suggesting that IL-6 is required for controlling the high dose infection but not essential for C. muridarum-induced pathology. However, at a low dose, IL-6-deficient mice still developed more extensive infection in the genital tract but no longer with significant pathology, suggesting that IL-6 is required for both controlling the low dose infection and exacerbating the low dose infection-induced pathology. The lack of hydrosalpinx in IL-6-deficient mice correlated with significantly reduced inflammatory infiltration in the oviduct tissue and decreased spleen CD4+ and CD8+ T cells that produce TNFα. Thus, IL-6-dependent pathways are important for both limiting chlamydial colonization in the genital tract mucosal tissues regardless of the infection doses and exacerbating chlamydial pathogenicity in the upper genital tract when IL-6-independent pathogenic mechanisms are not yet activated with a low infection dose.
Keywords: IL-6, Chlamydia muridarum, ascending infection, hydrosalpinx
1. Introduction
Chlamydial infection in the lower genital tract can ascend to the upper genital tract and cause pathologies in the upper genital tract, leading to manifestations such as pelvic inflammatory disease and infertility [1, 2]. A major underlying cause of C. trachomatis-associated tubal infertility is the long lasting fallopian tubal adhesion [3, 4]. Since C. muridarum infection in female mice can induce oviduct fibrotic blockage, resulting in hydrosalpinx and infertility [5–9], the C. muridarum genital tract infection mouse model has been extensively used for investigating the mechanisms of C. trachomatis-induced tubal pathology and immunity [10–14].
IL-6 is a multi-functional cytokine that is produced by a variety of cell types and act on multiple cell types [15]. IL-6 promotes the terminal differentiation of B cells and the survival of T cells in addition to its roles as an endogenous pyrogen [16] and an inducer of the production of acute-phase reactants [17]. IL-6 exerts its biological activities through IL-6 receptor (IL-6R) and gp130, triggering two main signaling pathways: the ERK MAPK pathway and the JAK/STAT pathway [18]. Activation of these pathways can generate functionally distinct or sometimes contradictory signals, which is why IL-6 can have both pro-inflammatory and anti-inflammatory functions [19]. A recent study has revealed that mice with T cell-specific deletion of the IL-6R α chain developed impaired Th1 cell responses in vivo, which were rescued by depletion of Tregs [20], suggesting that IL-6 signaling in effector T cells is required to overcome Treg-mediated suppression in vivo. IL-6 has been shown to play critical roles in controlling bacterial infections. IL-6 was induced at mucosal surface upon gram-negative bacterial infection [21]. Mice deficient in IL-6 became more susceptible to intracellular infection with Francisella tularensis [22] or Yersinia enterocolitica [23]. Patients who received anti-IL-6 therapy increased risk in bacterial and viral infection [24]. Besides its role in host defense, IL-6 is also an important mediator in chronic inflammatory pathology [25]. That is why anti-IL-6 therapy has been tried to treat arthritis patients [26, 27]. One of the major consequences of chronic inflammatory responses is fibrosis. IL-6 is elevated in both mice and humans with pulmonary fibrosis and is a critical mediator in paraquat-induced pulmonary fibrosis in mice [28]. Thus, IL-6 can on one hand promote host defense against bacterial infection and on the other, mediate inflammatory pathology.
The role of IL-6 in chlamydial infection and pathogenesis has been controversial. Although chlamydial organisms and proteins have been shown to induce production of IL-6 in cultured human cells [29], the role of IL-6 in chlamydial infection and pathogenesis has been evaluated mainly using mouse models. Using a mouse airway infection model, IL-6 was found to be a major cytokine produced in the mouse lung [30]. This lung IL-6 was later found to play a protective role in controlling C. muridarum infection in the airway since mice deficient in IL-6 were significantly more susceptible to C. muridarum airway infection than the wild type mice [31]. However, when mice with or without deficiency in IL-6 were compared in the genital tract after intravaginal infection with 1500 inclusion forming units (IFUs) of C. muridarum, there were no significant differences in either the live organism shedding from the mouse genital tracts or inflammatory infiltration in the genital tract tissues [32]. The question is why IL-6 deficiency increased mouse susceptibility to C. muridarum infection in the airway but not the genital tract. One possibility is that the C. muridarum dose used for the genital tract infection might be too low.
In the current study, we compared the effects of IL-6 deficiency on C. muridarum infection and pathogenicity in mouse genital tract under two different infection doses. At a high dose, IL-6-deficient mice developed more extensive genital tract infection accompanied with severe hydrosalpinx, suggesting that IL-6 is required for controlling the high dose infection but not essential for the high dose C. muridarum-induced pathology. However, at a lower dose, IL-6-deficient mice no longer developed any significant pathology although still developed more significant infection in the genital tract, suggesting that IL-6 is required for both exacerbating the low dose infection-induced pathology and controlling the low dose infection. The lack of hydrosalpinx in IL-6-deficient mice correlated with significantly reduced inflammatory infiltration in the oviduct tissue and decreased TNFα-producing T lymphocytes. Thus, we have demonstrated that IL-6-dependent pathways are important for both limiting chlamydial colonization in the genital tract mucosal tissues regardless of the infection doses and exacerbating chlamydial pathogenicity in the upper genital tract when IL-6-independent pathogenic mechanisms are not yet activated with a low infection dose.
2. Materials and Methods
2.1. Chlamydial organisms and infection
The C. muridarum organisms (Nigg3 strain, clone CMG13.32.1, ref: [33, 34]) used in the current study were propagated in HeLa cells (human cervical carcinoma epithelial cells, ATCC cat# CCL2), purified, aliquoted and stored as described previously [35, 36]. Female IL-6-deficient or IL-6−/− (B6.129S2-Il6tm1Kopf/J, Stock# 002650 with 11 generations of backcross) and IL-6-competent or wild type (C57BL/6J, stock# 000664) mice were all purchased at the age of 5 to 6 weeks old from Jackson laboratories (Bar Harbor, Maine). For mouse infection, each mouse was inoculated intravaginally with 5 × 103 (low dose) or 5 × 105 (high dose) inclusion forming units (IFUs) of live C. muridarum organisms as described previously [37]. For infection of HeLa cells in vitro, HeLa cells grown on coverslips in 24-well plates containing DMEM (GIBCO BRL, Rockville, MD) with 10% fetal calf serum (FCS; GIBCO BRL) at 370C in an incubator supplied with 5% CO2 were inoculated with C. muridarum organisms as described previously [35, 36]. The infected cultures were processed for immunofluorescence assay as described below.
2.2. Monitoring live C. muridarum organism recovery from vaginal swabs
Vaginal swabs were taken on different days after intravaginal infection to monitor C. muridarum infection in the lower genital tracts. Each swab was suspended in 500μl of ice-cold SPG followed by vortexing with glass beads, and the released organisms were titrated on HeLa cell monolayers in duplicates as described previously [38]. The total number of IFUs per swab was calculated based on the number of IFUs per field, number of fields per coverslip, dilution factors and inoculation and total sample volumes. An average was taken from the serially diluted and duplicate samples for any given swab. The calculated total number of IFUs/swab was converted into log10 and the log10 IFUs were used to calculate means and standard deviation for each group at each time point. The above titration method had a detection sensitivity of 10 live chlamydial organisms per swab because we took 50μl out from the total 500μl swab suspension for titration on a HeLa cell monolayer. Efforts were made to count any single chlamydial inclusion on the monolayer. Any vaginal swab samples with the number of live organisms below 10 were considered negative and recorded as 0.
.
2.3. Evaluating mouse genital tract tissue pathology and histological scoring
Mice were sacrificed on day 63 after infection and the mouse urogenital tract tissues were isolated. Before the tissues were removed from mouse body, an in situ gross examination was performed for evidence of uterine horn dilation and oviduct hydrosalpinx or any other abnormalities of the oviducts. The severity of oviduct hydrosalpinx was scored based on the following criteria: no hydrosalpinx (0), hydrosalpinx detectable only after amplification (1), hydrosalpinx clearly visible with naked eyes but the size is smaller than (2), equal to (3) or larger than (4) the ovary on the same side. The oviducts from left and right sides of the same mouse were scored separately and the two scores were added together as the score for the mouse. Mice identified with any positive hydrosalpinx score on either side were counted as hydrosalpinx positive, which was used for comparing the hydrosalpinx incidence rates.
The excised genital tract tissues, after photographing, were fixed in 10% neutral formalin and embedded in paraffin and serially sectioned longitudinally (with 5μm/section). Efforts were made to include cervix, both uterine horns and oviducts as well as lumenal structures of each tissue in each section. The sections were stained with hematoxylin and eosin (H&E) as described elsewhere [6]. The H&E stained sections were scored for severity of inflammation and dilation based on the modified schemes established previously [6, 39, 40]. Scoring for inflammatory cell infiltrates (at the chronic stage of infection, the infiltrates mainly contain mononuclear cells): 0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; and 4, confluent infiltration. Scoring for dilatation of oviduct: 0, no significant dilatation; 1, mild dilatation of a single cross section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation. The oviducts from left and right sides of the same mouse were scored separately and the two scores were added together as the score for the mouse. The score for each side was an average of scores from 5 random views in that side of the slide/section. Three sections (each separated by 5 sections during section collection) were used for each mouse. The scoring was carried out by researchers who were blinded for mouse sample identity.
2.4. Immunofluorescence assay
HeLa cells grown on coverslips with or without chlamydial infection were fixed and permeabilized for immunostaining as described previously [41–43]. Hoechst (blue, Sigma) was used to visualize nuclear DNA. For titrating IFUs from mouse vaginal swab and oviduct tissue homogenate samples, a mouse anti-chlamydial LPS antibody (clone# MB5H9, unpublished observation) plus a goat anti-mouse IgG conjugated with Cy3 (red; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to visualize chlamydial inclusions. All immunofluorescence-labeled samples were observed under an Olympus AX-70 fluorescence microscope equipped with multiple filter sets (Olympus, Melville, NY).
2.5. ELISA
To detect mouse anti-C. muridarum antibodies, purified C. muridarum EBs were used to coat ELISA plates as antigen. For detecting serum IgG, serum samples were collected from mice at the conclusion of the experiments. Each serum was 4-fold serially diluted starting at 1:1600 into 1:6400 and 1:25600 respectively for both IL-6−/− and wild type mice. The mouse serum IgG binding to the plate-coated C. muridarum elementary bodies (EBs) were detected with a goat anti-mouse IgG conjugated with horse radish peroxidase (HRP) enzyme (cat#31430, Thermo Scientific, Waltham, MA) plus a soluble substrate ABTS [2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, cat# 30931–67-0, Sigma-Aldrich, St. Louis, MO]. For isotyping the serum IgG, the same ELISA scheme described above was used. The serum samples from immunized mice were diluted at 1:1600 prior to application to the C. muridarum-coated plates. The mouse IgG1, IgG2a, IgG2b, IgG2c and IgG3 that bound to the plate-immobilized C. muridarum EBs were detected using the corresponding secondary antibody HRP conjugates, including goat anti-mouse IgG1 (cat# A10551), IgG2a (cat#A10685), IgG2b (cat#M32407), IgG2c (cat# pa129288) and IgG3 ((cat#m32707, all from Thermo scientific). The same substrate ABTS was used and the results were expressed raw ODs.
2.6. Flow cytometry
In order to monitor the intracellular cytokine production by T cells in mice, lymphocytes from spleens of mice with or without IL-6-deficiency were collected on day 63 after C. muridarum intravaginal infection and were re-stimulated in vitro for 24h with dendritic cells (DCs) pre-pulsed overnight with C. muridarum EBs. To Make DCs, femur and tibia bones from the same strain of mice were harvested for making bone marrow cell suspension 7 days prior to the flow cytometry assay. The bone marrow cells were cultured in GM-CSF-containing medium. The loosely attached cells that contained DCs were selectively collected on day 6 and DCs were further enriched by removing macrophages through repeated attachment. The enriched DCs were then pulsed overnight with live C. muridarum EBs at an MOI of 10. The next day, the EB-pulsed DCs are ready to present C. muridarum antigens to T cells. After the overnight in vitro antigen presentation or re-stimulation (10 to 14 hours), a Golgi stopper (Brefeldin A, B5936, Sigma-Aldrich) was added to the culture at a final concentration of 5 μg/ml) 6 hours before detecting intracellular cytokines.
For flow cytometry labeling, the in vitro-restimulated lymphocytes were blocked with a rat anti-mouse Fc receptor (CD16/CD32) antibody (clone 2.4G2, BD Biosciences, San Jose, CA) prior to immune staining. The blocked lymphocytes were labeled with anti-CD4 (PerCP/Cy5.5, RM4–5, eBiolegend, San Diego, CA) and anti-CD8p (Efluor@450, eBioH35–17.2, eBioscience, San Diego, CA) antibodies. Following permeabilization with an intracellular staining buffer (Biolegend), antibodies against various cytokines were added: anti-TNFα (FITC conjugated, clone MP6-XT22, eBioscience), anti-IFNγ (PE/Cy7 conjugated, clone XMG1.2, eBiolegend), anti-IL-5 (APC, TRFX5, eBiolegend), and anti-IL-13 (PE, eBio13A, eBioscience). After all antibody staining, the samples were measured using LSRII flow cytometer (BD Bioscience) for counting the number of cells stained with a given combination of antibodies as described previously ([44, 45]. The data were analyzed using software FlowJo.
2.7. Statistical analyses
All data including the time courses for the number of live organisms (IFUs), genome copies and infection rates were compared using “area-under-the-curve or AUC” or “days to clearance” between two groups of mice using Wilcoxon rank sum test. For AUC calculation from the IFU data, total number of Log10 IFUs collected from vaginal or rectal swabs of a given mouse over the entire time course was used to represent the AUC for that mouse for the purpose of comparison between the cytokine-deficient and -competent C. muridarum-infected groups. For AUC calculation from the % of mice positive for IFU data, the % of mice positive for IFU at each time point was used as an independent AUC variable in a given group. Thus, the number of the time points observed in a given experiment determined the group sample size for the purpose of comparison between groups. In order to compare the days to clearance between the plasmid-deficient and -competent C. muridarum-infected groups, the number of days for a given mouse to remain positive for IFUs was used as the “days to clearance” for that mouse. The pathology score data were also analyzed with Wilcoxon rank sum test. The Fisher’s Exact test was used to analyze category data including the % of mice positive for live organism shedding or hydrosalpinx.
3. Results
3.1. Mice deficient in IL-6 develop more extensive infection with severe hydrosalpinx following intravaginal infection with C. muridarum at a high dose.
To evaluate the role of IL-6-mediated signaling pathways in C. muridarum infection in the genital tract, we compared live organism shedding courses between mice with or without IL-6 deficiency following intravaginal inoculation with C. muridarum at a dose of 5 × 105 IFUs per mouse (Fig. 1). We found that IL-6−/− mice permitted more extensive C. muridarum colonization. Although both IL-6−/− and wild type mice shed similar levels of live organisms during the early stage (the first 2 weeks) of the infection course, two weeks later, the levels of live organisms recovered from the IL-6−/− mice on days 21, 28 and 35 were significantly higher than those of the wild type C57BL/6J mice. As a result, the overall extent of live organism shedding, days to clearance and number of mice positive for live organism shedding between the two groups of mice were all significantly different, suggesting that IL-6-mediated signaling pathways are critical for limiting C. muridarum infection in the genital tracts during the late stage of the infection course. To evaluate the role of IL-6-mediated signaling pathways in C. muridarum pathogenicity in the genital tract, we further compared hydrosalpinx between the same two groups of mice on day 63 (Fig. 2). There was no significant difference in either hydrosalpinx scores (p>0.05, Wilcoxon) or incidences (p>0.05, Fisher’s Exact) between the wild type and IL-6−/− mice.
Fig.1. Comparison of live organism shedding from the genital tract between mice with or without IL-6 deficiency following C. muridarum infection at a high dose.
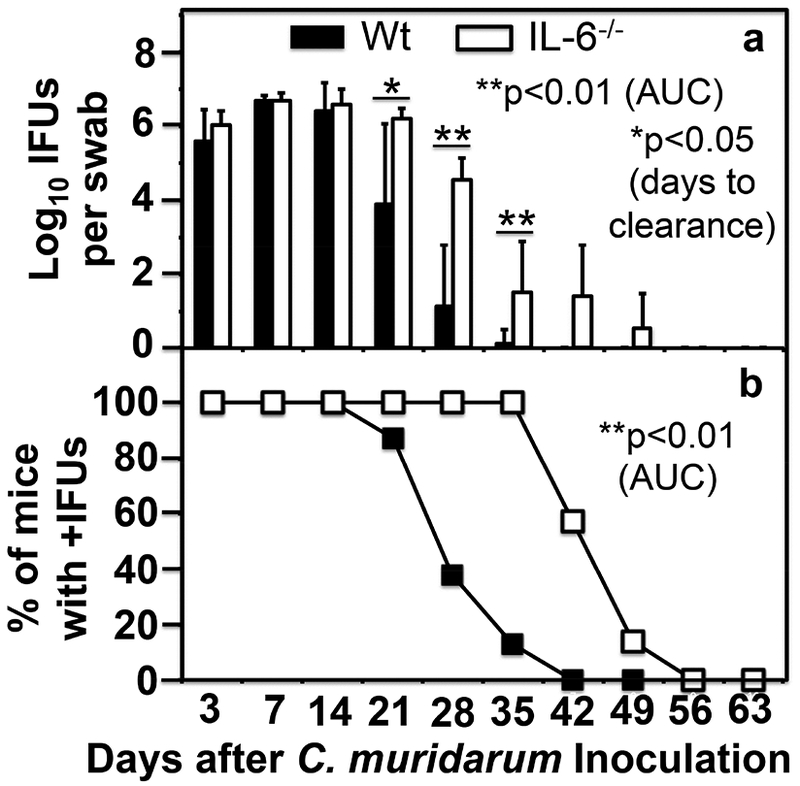
Groups of female C57BL/6J mice without (solid bar or square, wild type or Wt, n=8) or with (open bar or square, IL-6−/−,,n=7) deficiency in IL-6 were inoculated intravaginally with 5 × 105 IFUs of C. muridarum. Live C. muridarum organisms were monitored from the vaginal swabs taken on different days after the inoculation as shown along the X-axis. The results were expressed as Log10 IFUs (panel a) or % of mice that were still positive for shedding live organisms (b) as shown along the Y-axis. Note that IL-6−/− mice permitted more extensive C. muridarum colonization (on days 21, *p<0.05, Wilcoxon; 28 and 35, **p<0.01 after inoculation) than the Wt C57BL/6J mice in terms of both the extent of live organism shedding (a) and number of mice positive for live organism shedding (b), p<0.01 for both, Wilcoxon, area under curve or AUC, Wt vs. IL-6−/−.
Fig.2. Comparison of hydrosalpinx development between mice with or without IL-6 deficiency following C. muridarum infection at a high dose.
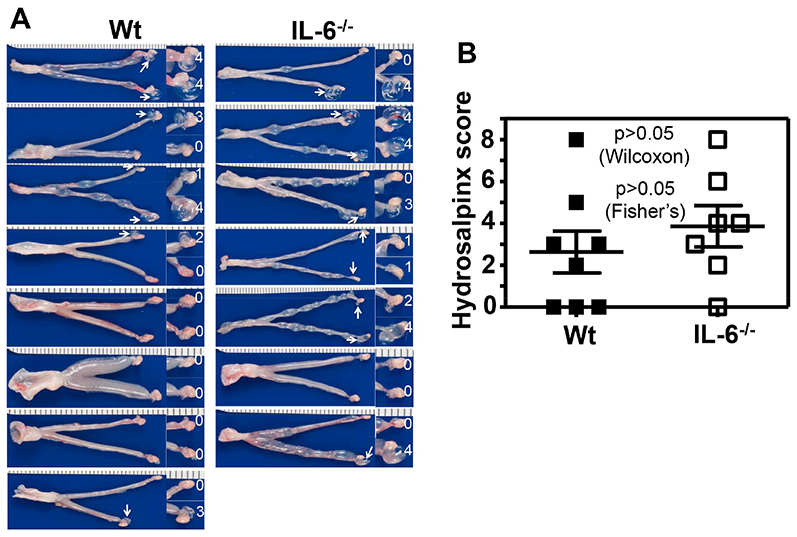
Groups of female C57BL/6J mice without (left column, wild type or Wt, n=8) or with (right column, IL-6−/−,,n=7) deficiency in IL-6 were inoculated intravaginally with 5 × 105 IFUs of C. muridarum as described in Fig.1 legend. On day 63, all mice were sacrificed for observing upper genital tract pathology hydrosalpinx. (A) Each individual mouse genital tract was shown with the vagina pointing to the left and oviduct/ovary to the right side. Oviduct with hydrosalpinx was indicated with a white arrow and the oviduct/ovary region was magnified shown on the right with the corresponding hydrosalpinx scores indicated. (B) Hydrosalpinx scores (p>0.05, Wilcoxon) and incidences (p>0.05, Fisher’s Exact) were compared between Wt (solid bar) and IL-6−/− (open bar) mice.
3.2. Mice deficient in IL-6 still develop more extensive infection but without significant hydrosalpinx following intravaginal infection with C. muridarum at a low dose.
To increase the sensitivity of mouse genital tract responses to C. muridarum infection, we lowered the C. muridarum infection dose by 100 folds to 5 × 103 IFUs and then compared the live organism shedding from the genital tract between mice with or without IL-6 deficiency (Fig. 3). We found that IL-6−/− mice still permitted more extensive C. muridarum infection two weeks after inoculation, including days 14 (p<0.01), 21, 28 & 35 (p<0.05). As a result, the overall shedding course of the IL-6−/− mice was significantly more severe than that of the wild type C57BL/6J mice in terms of the extent of live organism shedding (p<0.01, AUC, Wilcoxon) but not the days to clearance or number of mice positive for live organism shedding. When the pathology hydrosalpinx was compared macroscopically on day 63 (Fig. 4), we found that IL-6−/− mice failed to develop any significant hydrosalpinx while the wild type mice did so under the same infection condition (scores, p<0.05, Wilcoxon) despite the fact that IL-6−/− mice developed more extensive infection in the genital tract. Thus, under a low infection dose, the roles of IL-6-dependent signaling pathways in both limiting chlamydial infection and exacerbating pathology have become more obvious. The lack of hydrosalpinx in IL-6−/− mice was further validated under microscopy by semi-quantitating the oviduct dilation (Fig. 5A & B). We found that oviduct dilation scores in IL-6−/− mice were significantly lower than those of the C57BL/6J mice (p<0.05, Wilcoxon).
Fig.3. Comparison of live organism shedding from the genital tract between mice with or without IL-6 deficiency following C. muridarum infection at a low dose.
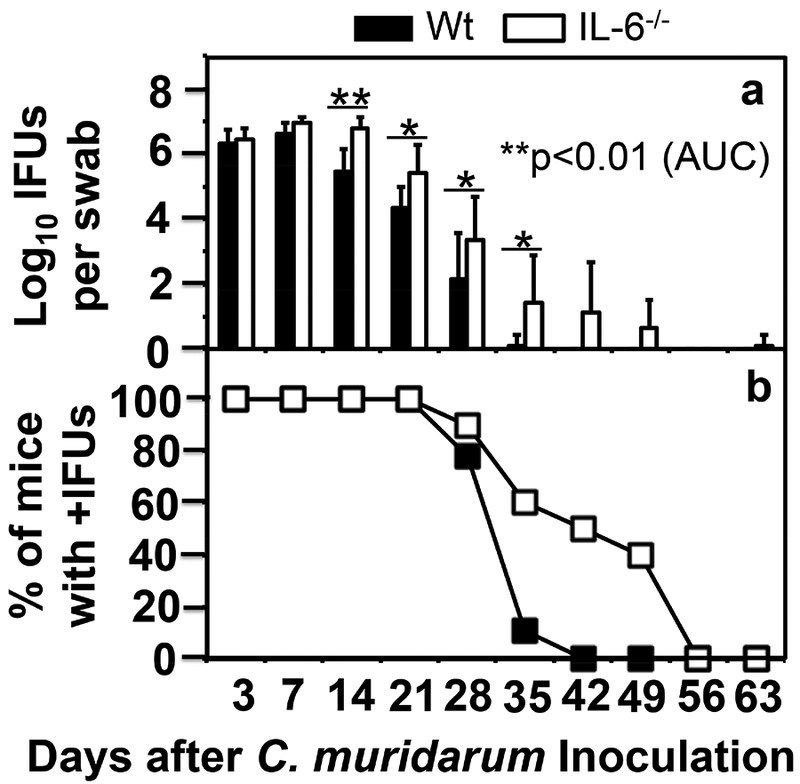
Groups of female C57BL/6J mice without (solid bar or square, wild type or Wt, n=9) or with (open bar or square, IL-6−/−,,n=10) deficiency in IL-6 were inoculated intravaginally with 5 × 103 IFUs of C. muridarum. Live C. muridarum organisms were monitored from vaginal swabs taken on different days after the inoculation as shown along the X-axis. The results were expressed as Log10 IFUs (panel a) or % of mice that were still positive for shedding live organisms (b) as shown along the Y-axis. Note that IL-6−/− mice permitted more extensive C. muridarum colonization (on days 14, **p<0.01, or 21, 28 & 35, *p<0.05 for all, Wilcoxon, after inoculation) than the Wt C57BL/6J mice in terms of the extent of live organism shedding (a, p<0.01, Wilcoxon, when area under curve or AUC was compared with that of the IL-6−/− mice) but not the number of mice positive for live organism shedding (b, p>0.05, Wilcoxon, area under curve or AUC).
Fig.4. Comparison of hydrosalpinx development between mice with or without IL-6 deficiency following C. muridarum infection at a low dose.
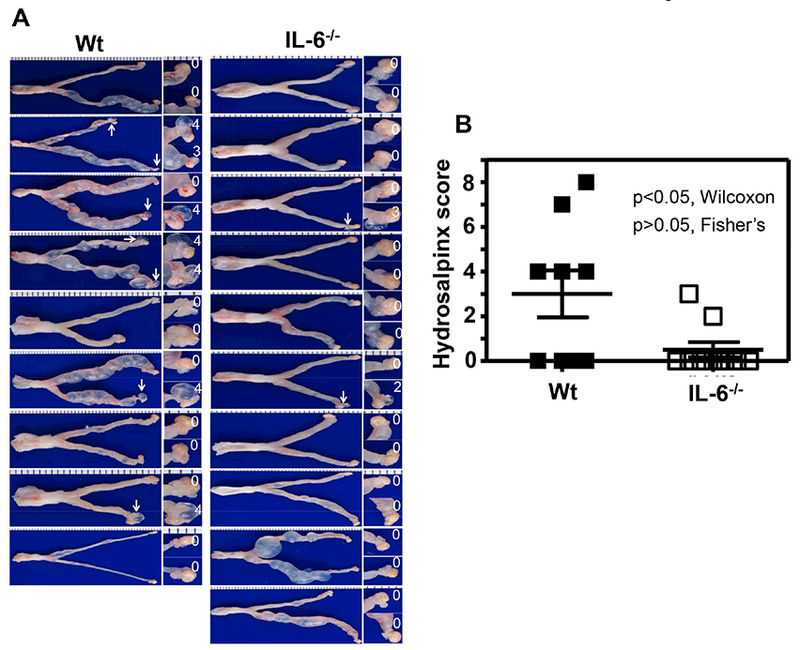
Groups of female C57BL/6J mice without (left column, wild type or Wt, n=9) or with (right column, IL-6−/−,,n=10) deficiency in IL-6 were inoculated intravaginally with 5 × 103 IFUs of C. muridarum as described in Fig.3 legend. On day 63, all mice were sacrificed for observing upper genital tract pathology hydrosalpinx. (A) Each individual mouse genital tract was shown with the vagina pointing to the left and oviduct/ovary to the right side. Oviduct with hydrosalpinx was indicated with a white arrow and the oviduct/ovary region was magnified shown on the right with the corresponding hydrosalpinx scores indicated. (B) Hydrosalpinx scores (p<0.05, Wilcoxon) and incidences (p>0.05, Fisher’s Exact) were compared between Wt (solid bar) and IL-6−/− (open bar) mice.
Fig.5. Comparison of oviduct dilation and inflammatory infiltration between mice with or without IL-6 deficiency following C. muridarum infection at a low dose.
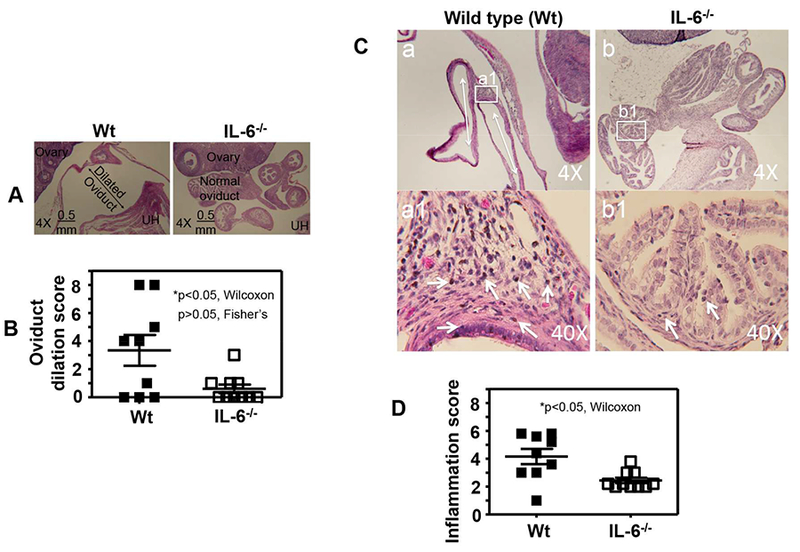
Groups of female C57BL/6J mice were inoculated intravaginally with 5 × 103 IFUs of C. muridarum as described in Fig.4 legend. On day 63, following documenting the macroscopic pathology hydrosalpinx, the same genital tract tissues were used to make sections for microscopic evaluation of oviduct dilation using the criteria described in the materials and methods section. (A) Representative microscopic images of the oviduct area acquired under a 4× objective lens from the mouse groups without (left column, wild type or Wt, n=9) or with (right column, IL-6−/−,,n=10) deficiency in IL-6 were shown with the dilated and normal oviduct, ovary and uterine horn (UH) marked. (B) Oviduct dilation scores (p<0.05, Wilcoxon) and incidences (p>0.05, Fisher’s Exact) were compared between Wt (solid bar) and IL-6−/− (open bar) mice. (C) Representative microscopic images of the oviduct area acquired under 4× or 40× objective lens from the mouse groups without (left column, wild type or Wt, n=9) or with (right column, IL-6−/−,, n=10) deficiency in IL-6 were shown with inflammatory infiltrates marked. The tissue areas that were used to semiquantitate inflammation under 40× were marked in the 4× images. (D) Oviduct inflammatory scores were compared between Wt (solid bar) and IL-6−/− (open bar) mice (*p<0.05, Wilcoxon).
3.3. Mice deficient in IL-6 exhibit reduced inflammatory infiltrates in the oviduct tissues
We further compared oviduct tissue inflammatory infiltration between mice with or without IL-6 deficiency infected with C. muridarum at a low dose (Fig. 5C & D). Following documentation of the oviduct dilation under microscopy, the same tissue sections were carefully evaluated for inflammatory infiltration using the criteria described in the materials and methods section. We found that most inflammatory infiltrates were clustered in the tissue associated with the dilated oviduct wall. Since the severely dilated oviduct wall is squeezed very thin with epithelial layer and muscle layer packed in a few cell layers, there are hardly any inflammatory cells left. When we compared the inflammatory infiltration between mice with or without IL-6 deficiency, we found that IL-6−/− mice developed significantly reduced inflammatory infiltration comparing to the wild type mice. It is not clear at this moment how the oviduct tissue inflammation contributes to the development and maintenance of oviduct dilation and hydrosalpinx.
3.4. Mice deficient in IL-6 display reduced T cells that produce TNFα or IFNγ
We further compared the systemic antibody responses to C. muridarum infection between mice with or without IL-6 deficiency (Fig. 6A). We found that both groups of mice developed robust antibody responses with a dominant IgG2 isotype. There was no significant difference between these two groups of mice. We also compared the intracellular cytokine profiles of CD4+ and CD8+ T cells in the spleens between these two groups of mice using flow cytometry (Fig. 6B). We found that the numbers of CD8+ T cells that produce TNFα or IFNγ but not IL-5 or IL-13 were significantly lower in mice deficient in IL-6 than those of wild type mice in response to an in vitro re-stimulation with C. muridarum-pulsed DCs. This also held true for the CD4+ T cells except that there was no significant difference in the number of CD4+ T cells that produce IFNγ. It is worth noting that although the difference in IFNγ-producing CD4+ T cells between the wild type and IL-6-deficient mice was not statistically different, the trend of decrease in the IL-6-deficient mice was obvious. The observation that IL-6−/− mice developed fewer CD4+ or CD8+ T cells that produce TNFα or IFNγ in response to an in vitro re-stimulation with C. muridarum suggests that IL-6 deficiency may reduce the development of pro-inflammatory T cells, which is consistent with the previous observations that IL-6−/− mice were more susceptible to bacterial infections [22, 23].
Fig.6. Comparison of anti-C. muridarum antibody production and T cell responses between mice with or without IL-6 deficiency following C. muridarum infection at a low dose.
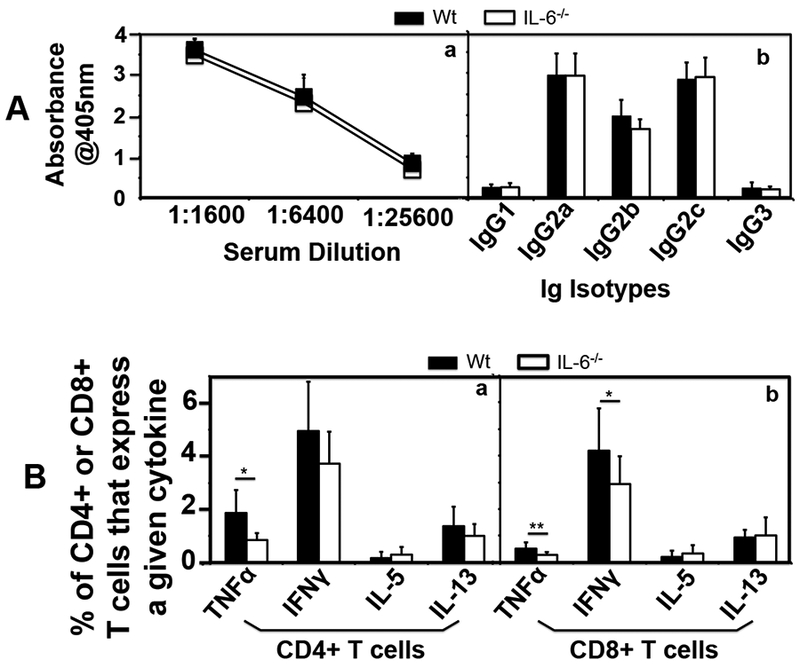
Groups of female C57BL/6J mice without (solid square or bar, wild type or Wt, n=9) or with (open square or bar IL-6−/−,, n=10) deficiency in IL-6 were inoculated intravaginally with 5 × 103 IFUs of C. muridarum as described in Fig. 3 legend. (A) On day 63, blood was collected for measuring anti-C. muridarum antibodies in sera using ELISA. The results were expressed as absorbance acquired at the wavelength of 405nm. Total IgG antibodies were measured after the serially diluted serum samples were added to the C. muridarum EB-coated 96 well plates (a) while the serum IgG isotypes were measured using the 1:1600 diluted mouse serum samples and the corresponding mouse IgG isotype-specific HRP conjugates (b). Note that there was no significant difference in the levels of either total IgG or IgG isotypes between Wt and IL-6−/− groups. All mice developed IgG2 isotype-dominant anti-C. muridarum antibody responses. (B) Splenocytes from the same mice were collected for measuring intracellular cytokines (as listed along the X-axis) using flow cytometry from both CD4+ (panel a) and CD8+ (b) T cells following in vitro restimulation with C. muridarum organism-pulsed DCs. The results were expressed as % of CD4+ or CD8+ T cells that produced a given cytokine. Note that wild type mice developed more CD4+ or CD8+ T cells that produce TNFα or IFNγ in response to an in vitro re-stimulation (*p<0.05, **p<0.01). Please note that although the difference in IFNγ-producing CD4+ T cells between the wild type and IL-6-deficient mice was not statistically different, the trend of decrease in IFNγ-producing CD4+ T cells was obvious in the IL-6-deficient mice.
4. Discussion
Although IL-6 is known to play critical roles in both host defense against bacterial infections [22–24] and chronic inflammatory pathologies [25–27], the role of IL-6 in preventing chlamydial infection was demonstrated only in the mouse airway [31] but not the genital tract [32]. In the current study, we re-evaluated the role of IL-6 using the C. muridarum genital tract infection mouse model and have presented evidence that IL-6 can affect both C. muridarum replication and pathogenicity in the mouse genital tract. First, following an intravaginal inoculation with C. muridarum at a high dose (5×105 IFUs), mice deficient in IL-6 developed more extensive infection in the genital tract, including higher titers of live organisms recovered at multiple time points two weeks after the initial infection and significantly longer shedding courses comparing to the wild type control mice. Second, the extensive infection in the IL-6-deficient mice did not translate into significantly increased pathology in terms of either hydrosalpinx incidence or severity score, suggesting that host inflammatory pathology responses were not in proportion to the extent of the infection. Third, when we reduced the initial inoculation dose by 100 folds to 5×103 IFUs, the IL-6-deficient mice were no longer able to develop any significant hydrosalpinx while the wild type C57BL/6J mice still developed significant hydrosalpinx under the same infection condition, demonstrating that IL-6-mediated signaling pathways are necessary for mice to develop hydrosalpinx in response to the low dose infection. Fourth, the conclusion on the role of IL-6 in C. muridarum induction of hydrosalpinx was further strengthened by the observation that the IL-6-deficient mice still developed significantly enhanced infection in the genital tract despite the lack of pathology. Fifth, under the low inoculation dose condition, the lack of pathology in the IL-6-deficient mice correlated with the decreased inflammatory infiltration in the genital tract tissues and reduced C. muridarum-specific T cells that produce TNFα. Finally, the extended infection with C. muridarum in the genital tract of the IL-6-deficient mice also correlated with reduced C. muridarum-specific CD4+ T cells that produce IFNγ (although the reduction was not statistically significant). This finding is consistent with the both observed prolonged live organism shedding courses observed in the IL-6-deficient mice (Figs. 1 & 3) and the fact that IL-6 is known to promote T cell survival and intrinsically help T cells to overcome the suppression by Treg [20].
The current study by demonstrating a critical role of IL-6 in limiting C. muridarum infection in the genital tract regardless of the initial infection doses has provided a model for further addressing the mechanisms by which IL-6-dependent pathways contribute to host defense against chlamydial infection in the genital tract. Although IL-6 was initially discovered as a B cell growth factor, its role in regulating B cell immunity has not been clearly elucidated. Consistently, data from our current study did not seem to reveal any significant correlation between the enhanced C. muridarum replication and any compromised anti-C. muridarum antibody production. However, there was an obvious correlation between the extended infectious courses and reduced C. muridarum-specific CD4+ T cells that produce IFNγ in the IL-6-deficient mice. It is well known that IFNγ-producing CD4+ T cells are the most critical component for controlling C. muridarum infection [46]. IL-6 not only promotes T cell survival but also help T cells to overcome the suppression of Treg [20]. Thus, the compromised Th1 development to C. muridarum infection in the IL-6-deficient mice may explain why these mice permitted significantly more C. muridarum infection in the genital tract. Although IL-6 is also known to regulate innate immunity, our current data seems to support a role of IL-6 in adaptive immunity. This is because there was no significant difference in live organism shedding on either days 3 or 7 after the initial inoculation between mice with or without IL-6 deficiency. The extended C. muridarum growth in IL-6-deficient mice occurred two weeks after the initial infection, suggesting that IL-6 affected C. muridarum infection via adaptive immunity. It will be interesting in investigating whether the T cell intrinsic role of IL-6 to help Th1 cells to overcome the Treg suppression as revealed by Nish et al [20] is also critical for IL-6 controlling C. muridarum infectivity.
Despite the ease to induce hydrosalpinx in mice by genital tract C. muridarum, the inflammatory mechanisms by which C. muridarum induces oviduct pathology remain unclear. The current study has demonstrated that mice deficient in IL-6 failed to develop any significant hydrosalpinx in response to a low dose infection, suggesting that under this infection condition, an IL-6-dependent or mediated signaling pathway has become a major mechanism by which C. muridarum induced hydrosalpinx. However, the IL-6-dependent mechanism can be replaced by IL-6-independent mechanisms when the infection was increased by 100× (comparing Figs. 2 vs. 4). Thus, the current study has developed an infection dose-controllable model system for further investigating the mechanisms by which the genital tract C. muridarum organisms enhance hydrosalpinx. The lack of hydrosalpinx in the IL-6-deficient mice correlated with the reduction in T cells that produce TNFα, suggesting a critical role of TNFα-dependent mechanism in C. muridarum pathogenic mechanisms. Indeed, TNFa [47, 48], especially those produced by CD8+ T cells [49], has been shown to significantly contribute to C. muridarum induction of hydrosalpinx. It will be interesting to test whether supplement of C. muridarum-specific CD8+ T cells that produce TNFa can rescue the IL-6-deficient mice to develop hydrosalpinx.
It is worth noting that although the C. muridarum infection in the mouse genital tract model has been extensively used for studying C. trachomatis infection and pathogenesis in humans, there are many distinct differences between these two. Thus, caveats should be considered when applying the knowledge learnt from the murine model to human chlamydial infection. At this moment, the role of IL-6 in C. trachomatis infection and pathogenesis in women is largely unknown. However, it is clear that patients who receive anti-IL-6 immunotherapy are more susceptible to infections [24], suggesting a critical role of IL-6 in controlling microbial infection in humans. Thus, it may be worth the efforts in carrying our clinical studies by comparing the levels of systemic and local IL-6 in women with or without C. trachomatis infection or tubal factor infertility. If IL-6 is important for human defense to chlamydial infection, we can expect a reverse correlation between IL-6 level and frequency of C. trachomatis infection in women. Since IL-6 has been associated with inflammatory pathology and fibrosis [26–28], it is possible that women with tubal factor infertility may display an elevated level of IL-6. Genetic association of IL-6 polymorphisms with various chronic inflammation and fibrosis has been established [50, 51]. Similarly, it will be interesting to test whether there is an association of IL-6 polymorphisms with fallopian adhesions/tubal factor infertility. These human subject fdstudies may provide direct evidence on the role of IL-6 in C. trachomatis infection and pathogenesis in women.
Acknowledgement
This work was supported in part by grants US National Institutes of Health (R01AI121989 to GZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Brunham RC, Maclean IW, Binns B, Peeling RW. Chlamydia trachomatis: its role in tubal infertility. The Journal of infectious diseases 1985;152:1275–82. [DOI] [PubMed] [Google Scholar]
- [2].Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, et al. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstetrics and gynecology 2012;119:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, et al. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 2011;96:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharma M, Sethi S, Daftari S, Malhotra S. Evidence of chlamydial infection in infertile women with fallopian tube obstruction. Indian J Pathol Microbiol 2003;46:680–3. [PubMed] [Google Scholar]
- [5].de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infection and immunity 1994;62:2094–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, et al. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 2005;32:49–56. [DOI] [PubMed] [Google Scholar]
- [7].Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infection and immunity 1997;65:2145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. Bioluminescence Imaging of Chlamydia muridarum Ascending Infection in Mice. PloS one 2014;9: e101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, et al. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infection and immunity 2014;82:2688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cotter TW, Meng Q, Shen ZL, Zhang YX, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infection and immunity 1995;63:4704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infection and immunity 2005;73:8153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live Chlamydia trachomatis infection. Infection and immunity 1999;67:1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Murthy AK, Sharma J, Coalson JJ, Zhong G, Arulanandam BP. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell Immunol 2004;230:56–64. [DOI] [PubMed] [Google Scholar]
- [14].Morrison SG, Morrison RP. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. Journal of immunology 2005;175:7536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lotz M Interleukin-6: a comprehensive review. Cancer Treat Res 1995;80:209–33. [DOI] [PubMed] [Google Scholar]
- [16].Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res 2004;10:201–22. [DOI] [PubMed] [Google Scholar]
- [17].Gervois P, Kleemann R, Pilon A, Percevault F, Koenig W, Staels B, et al. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. The Journal of biological chemistry 2004;279:16154–60. [DOI] [PubMed] [Google Scholar]
- [18].Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 2014;70:11–20. [DOI] [PubMed] [Google Scholar]
- [19].Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011;1813:878–88. [DOI] [PubMed] [Google Scholar]
- [20].Nish SA, Schenten D, Wunderlich FT, Pope SD, Gao Y, Hoshi N, et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. Elife 2014;3:e01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Man P, van Kooten C, Aarden L, Engberg I, Linder H, Svanborg Eden C. Interleukin-6 induced at mucosal surfaces by gram-negative bacterial infection. Infection and immunity 1989;57:3383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kurtz SL, Foreman O, Bosio CM, Anver MR, Elkins KL. Interleukin-6 is essential for primary resistance to Francisella tularensis live vaccine strain infection. Infection and immunity 2013;81:585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dube PH, Handley SA, Lewis J, Miller VL. Protective role of interleukin-6 during Yersinia enterocolitica infection is mediated through the modulation of inflammatory cytokines. Infection and immunity 2004;72:3561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edwards CJ. IL-6 inhibition and infection: treating patients with tocilizumab. Rheumatology (Oxford) 2012;51:769–70. [DOI] [PubMed] [Google Scholar]
- [25].Gabay C Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8 Suppl 2:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis and rheumatism 2006;54:2817–29. [DOI] [PubMed] [Google Scholar]
- [27].Smolen JS, Maini RN. Interleukin-6: a new therapeutic target. Arthritis Res Ther 2006;8 Suppl 2:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hu L, Yu Y, Huang H, Fan H, Hu L, Yin C, et al. Epigenetic Regulation of Interleukin 6 by Histone Acetylation in Macrophages and Its Role in Paraquat-Induced Pulmonary Fibrosis. Frontiers in immunology 2016;7:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cunningham K, Stansfield SH, Patel P, Menon S, Kienzle V, Allan JA, et al. The IL-6 response to Chlamydia from primary reproductive epithelial cells is highly variable and may be involved in differential susceptibility to the immunopathological consequences of chlamydial infection. BMC Immunol 2013;14:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Magee DM, Smith JG, Bleicker CA, Carter CJ, Bonewald LF, Schachter J, et al. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and −6. Infection and immunity 1992;60:1217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Williams DM, Grubbs BG, Darville T, Kelly K, Rank RG. A role for interleukin-6 in host defense against murine Chlamydia trachomatis infection. Infection and immunity 1998;66:4564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Perry LL, Feilzer K, Caldwell HD. Neither interleukin-6 nor inducible nitric oxide synthase is required for clearance of Chlamydia trachomatis from the murine genital tract epithelium. Infection and immunity 1998;66:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, et al. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infection and immunity 2015;83:1881–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, et al. The Chromosome-Encoded Hypothetical Protein TC0668 Is an Upper Genital Tract Pathogenicity Factor of Chlamydia muridarum. Infection and immunity 2016;84:467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen C, Chen D, Sharma J, Cheng W, Zhong Y, Liu K, et al. The hypothetical protein CT813 is localized in the Chlamydia trachomatis inclusion membrane and is immunogenic in women urogenitally infected with C. trachomatis. Infection and immunity 2006;74:4826–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infection and immunity 2008;76:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, et al. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PloS one 2014;9:e95076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen D, Lei L, Lu C, Galaleldeen A, Hart PJ, Zhong G. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. Journal of bacteriology 2010;192:6017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011;331:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sun X, Yang Z, Zhang H, Dai J, Chen J, Tang L, et al. Chlamydia muridarum induction of glandular duct dilation in mice. Infection and immunity 2015;83:2327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Z, Lu C, Peng B, Zeng H, Zhou Z, Wu Y, et al. Induction of protective immunity against Chlamydia muridarum intravaginal infection with a chlamydial glycogen phosphorylase. PloS one 2012;7:e32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhong G, Fan P, Ji H, Dong F, Huang Y. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. The Journal of experimental medicine 2001; 193:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol 1997;54:159–69. [DOI] [PubMed] [Google Scholar]
- [44].Zhong G, Reis e Sousa C, Germain RN. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc Natl Acad Sci U S A 1997;94:13856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. The Journal of experimental medicine 1997;186:673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infection and immunity 2001;69:2643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dong X, Liu Y, Chang X, Lei L, Zhong G. Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection. Infection and immunity 2014;82:1833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Manam S, Thomas JD, Li W, Maladore A, Schripsema JH, Ramsey KH, et al. Tumor Necrosis Factor (TNF) Receptor Superfamily Member 1b on CD8+ T Cells and TNF Receptor Superfamily Member 1a on Non-CD8+ T Cells Contribute Significantly to Upper Genital Tract Pathology Following Chlamydial Infection. The Journal of infectious diseases 2015;211:2014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infection and immunity 2011;79:2928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Giannitrapani L, Soresi M, Balasus D, Licata A, Montalto G. Genetic association of interleukin-6 polymorphism (−174 G/C) with chronic liver diseases and hepatocellular carcinoma. World J Gastroenterol 2013;19:2449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Falleti E, Fabris C, Vandelli C, Colletta C, Cussigh A, Smirne C, et al. polymorphisms of interleukin-6 modulate fibrosis progression in mild chronic hepatitis Immunol 2010;71:999–1004. [DOI] [PubMed] [Google Scholar]


