Abstract
Background
Craving is a core symptom of addictive disorders, such as pathological gambling for example. Over the last decade, several studies have assessed the efficacy of repetitive transcranial magnetic stimulation (rTMS) in the addiction field, which triggers the dorsolateral prefrontal cortex (DLPFC) to decrease craving. The STIMJEU study investigated whether a single session of low-frequency (LF, i.e., 1 Hz) rTMS applied to the right DLPFC reduced cue-induced gambling craving in a sample of treatment-seeking pathological gamblers.
Methods
Thirty patients received both active and sham rTMS in random order and were blinded to the condition in a within-subject crossover design. Outcome measures included self-reported gambling craving (Visual Analog Scale and Gambling Craving Scale) and physiological measures (heart rate and blood pressure).
Results
The rTMS sessions were associated with a significant decrease in the gambling urge, regardless of whether the session was active or sham. When controlling cue-induced craving levels, no effects were observed on craving for active rTMS. Overall, rTMS was well-tolerated, and the credibility of the sham procedure was assessed and appeared to be appropriate.
Conclusions
We failed to demonstrate the specific efficacy of one session of LF rTMS to decrease cue-induced craving in pathological gamblers. A strong placebo-effect and rTMS parameters may partly explain these results. Yet, we are convinced that rTMS remains a promising therapeutic method. Further studies are required to examine its potential effect.
Keywords: pathological gambling, rTMS, craving, sham, non-invasive brain neurostimulation, treatment
Introduction
As both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and more recently the DSM-5 with the new term “gambling disorder” describe, pathological gambling (PG) is characterized by persistent and recurrent problematic gambling behavior leading to clinically significant impairment or distress (American Psychiatric Association [APA], 1994, 2013). The key features of PG include the loss of control over gambling and continued gambling behavior, despite significant harmful consequences. As numerous publications have previously described, the loss of control associated with addictions can result in the presence of craving (c.f., Skinner & Aubin, 2010). Craving is defined as a pressing, urgent, and irrepressible desire to give in to an addictive behavior. Beyond the mere desire to gamble, craving also includes the expectation of positive effects and the relief of negative effects as a result of this action (Young & Wohl, 2009). This persistent symptom is a consequence of specific cues, both internal (e.g., affective state) and external (e.g., visual stimuli), through classical conditioning (Koob & Volkow, 2010; Marhe, Waters, van de Wetering, & Franken, 2013; Skinner & Aubin, 2010; Sodano & Wulfert, 2010). Craving predicts relapse, even after a long period of abstinence. Therefore, therapeutic interventions must target this key symptom. The panel of interventions proposed for PG is broad, including many psychological interventions and pharmacological medications (Cowlishaw et al., 2012; Grant & Potenza, 2004; Sauvaget et al., 2015). Importantly, psychological interventions remain as the preferred treatment choice, and pharmacological interventions have smaller effect sizes and mixed findings, the most robust of which were obtained with opioid antagonists (Victorri-Vigneau et al., 2017). To date, the literature supports the short-term efficacy of specific interventions to reduce gambling behaviors and other symptoms of PG. However, the durability of this therapeutic gain is unknown.
Non-invasive brain stimulation [e.g., repeated transcranial magnetic stimulation (rTMS), deep TMS, and transcranial direct current stimulation] is a promising new avenue of treatment in the addiction field (Grall-Bronnec & Sauvaget, 2014; Jansen et al., 2013; Protasio et al., 2015; Sauvaget et al., 2015). Based on the principle of electromagnetic induction, rTMS can modulate human cortical excitability both at the site of stimulation and in remote areas (Fox et al., 1997). Substance use disorders (SUDs) and, to a lesser extent, food cravings, and certain forms of eating disorders were the main addictive disorders addressed in trials to assess the efficacy of neuromodulation techniques in reducing cravings. Cravings are underpinned by the activation of the reward and motivation circuits, involving, among others, the dorsolateral prefrontal cortex (DLPFC) (Goldman et al., 2013; Jansen et al., 2013; McBride, Barrett, Kelly, Aw, & Dagher, 2006; Volkow, Wang, Tomasi, & Baler, 2013; Wang et al., 2007; Wing, Bacher, Wu, Daskalakis, & George, 2012). Previous studies have shown that craving is associated with the hyperactivity of the right DLPFC (Wang et al., 2007). Moreover, the functional magnetic resonance imaging data have shown that pathological gamblers have an overactive right DLPFC compared with healthy participants after inducing craving with visual stimulation (Crockford, Goodyear, Edwards, Quickfall, & el-Guebaly, 2005; Van Holst, Van den Brink, Veltman, & Goudriaan, 2010). Consequently, inhibiting an overactive right DLPFC with low-frequency (LF) rTMS might decrease cravings. Therefore, rTMS applied to the DLPFC might indirectly modulate dopaminergic pathways (Addolorato, Leggio, Hopf, Diana, & Bonci, 2012) and consequently affect the symptoms of addiction (Feil & Zangen, 2010; Keck et al., 2002). Evidence is growing regarding the effects of rTMS applied to the DLPFC with regard to craving among people with substance dependence and cravings for highly palatable food (Grall-Bronnec & Sauvaget, 2014; Jansen et al., 2013; Protasio et al., 2015; Sauvaget et al., 2015).
To date, only two studies have evaluated the effect of rTMS in patients with gambling behavior. The first study tested the efficacy of rTMS on gambling urges in nine men with PG. The authors demonstrated that one active high-frequency (HF) rTMS was associated with a reduced post-game increase in the “desire to gamble” compared with a sham treatment (Zack et al., 2016). The second study used a randomized sham-controlled crossover design to examine 22 patients seeking treatment for gambling disorder. The authors concluded that a single session of HF rTMS applied over the left DLPFC reduced cue-induced cravings (Gay et al., 2017). Another exploratory, open-label study using deep TMS was conducted among five individuals with PG and did not show positive effects (Rosenberg, Klein, & Dannon, 2013). We hypothesized that applying LF rTMS to the right DLPFC would reduce cue-induced cravings. Therefore, we conducted a randomized, double-blind, sham-controlled crossover study to test whether a single application of LF rTMS over the right DLPFC, compared with sham stimulation, would temporarily reduce cue-induced cravings among treatment-seeking pathological gamblers.
Methods and Materials
Participants
This STIMJEU (STIM: stimulation; JEU: gambling) study examined pathological gamblers (defined as those with ≥5 of the DSM-IV diagnostic criteria for this condition), aged 18–70 years, who were seeking treatment at our outpatient department. Only right-handed patients were recruited given that handedness might affect rTMS research, as previously shown by Van den Eynde et al. (2010). During the first part of the recruitment period, only men were included because men predominated in the samples of outpatients seeking treatment. Finally, the research protocol was amended so that women were also eligible if they were not pregnant. In fact, women were accounted for a portion of the target population. Participants were excluded if they showed current alcohol or SUD (excluding that for nicotine), according to the DSM-IV, showed cognitive impairment, had difficulties in reading and writing in French, were pregnant, had previous rTMS treatment, had metal implants (e.g., pacemakers, metal plates, or wires), or had histories of neurological disease, epilepsy, brain injury, or brain surgery. Individuals taking medication likely to modify their seizure threshold (psychotropic medication that had not been stable for at least 7 days or benzodiazepine regardless of the duration) were also excluded.
Experimental design
This STIMJEU study used a randomized, double-blind, sham-controlled, crossover design to investigate the effect of rTMS on cue-induced gambling craving. One of the important advantages of a crossover design (i.e., a repeated/longitudinal measurement design), which features both a parallel study and a non-crossover longitudinal design, is that the influence of confounders is reduced because each patient serves as his or her own control. This study was divided into two phases. Phase 1 aimed to verify whether exposure to gambling cues significantly induces craving. In fact, we considered it as inconsistent to include patients in whom craving could not be induced. Phase 2 applied one active and one sham rTMS sessions, with a 1-week interval to avoid any carryover effect. Figure 1 illustrates the study design.
Figure 1.
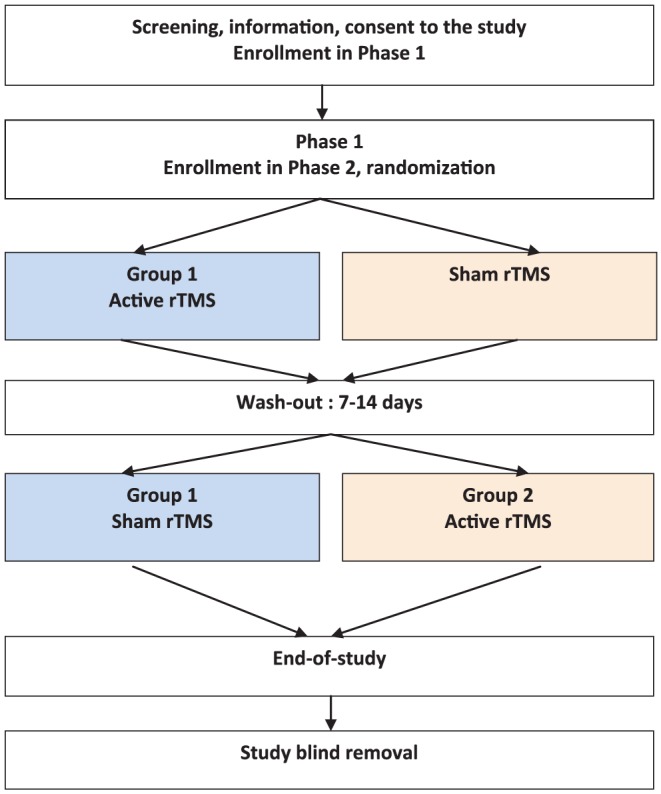
STIMJEU study design
Phase 1: Inclusion of patients with cue-induced craving
All patients underwent a semi-structured clinical interview and completed self-report questionnaires. Well-trained and experienced staff members performed this assessment, which included an evaluation of craving [intensity at baseline, measured using a Visual Analog Scale (VAS)]. For 5 min, the patients were then exposed to specific gambling cues and selected based on their own preferred game and their usual game medium (online or offline) to maximize craving induction. We applied a broad range of visual and audio stimuli to cover the diversity of the gamblers’ preferred game. For example, specialized press-and-blank betting grids were given to horse-race bettors, who also watched a race broadcasted the day before. One poker player connected to his gambler’s account on his preferred poker website. The participants were asked to imagine that they were in a real-life gambling situation, ready to bet money. We ensured that the stimuli did not induce alcohol or other substance cravings. Immediately after cue exposure, participants provided subjective ratings on a VAS. They were “definitely” included in the study, if the intensity significantly increased (i.e., by at least 50% compared with baseline). In other words, we only included patients with high reactivity to gambling cues. Finally, they were randomly allocated to the “active/sham” group or to the “sham/active” group through a web-based randomization program.
Phase 2: Brain stimulation
Patients were asked to return 1 week later for the first rTMS session. According to the crossover design, each patient received one active and one sham rTMS sessions in a random order with a 1- to 2-week interval. The wash-out period was based on a compromise among several elements: the transitory effect of rTMS on craving, the need to minimize drop-outs, and the need not to delay the start of treatment (which might bias the results) on the one hand as well as the need to avoid a carry-over effect and the availability of the patient, investigator, and rTMS equipment on the other hand. This choice was supported by the recent crossover studies conducted to assess the effect of rTMS on alcohol (Herremans et al., 2012) and food (Barth et al., 2011) cravings.
Following the motor threshold determination and the identification of the target site, a baseline measurement was performed on the patients. They were then exposed to the same individual gambling cues used during Phase 1 to induce craving. They immediately received the first rTMS session, when the craving level was at the highest, to maximize the effect of the neuromodulation technique. In fact, it was assumed that the induction of craving immediately prior to the rTMS session would create a more specific disruption of the circuits associated with craving (Amiaz, Levy, Vainiger, Grunhaus, & Zangen, 2009). The second rTMS session was planned within the next 7–14 days. This session followed the same procedure. At the end of the study, the patients were paid with €175 worth of gift vouchers for their participation.
Three investigators (AS, SB, and JMV) performed the rTMS and MGB supervised patient recruitment, the gambling challenge task, and questionnaire completion. MGB and participants were blind to the rTMS (active or sham) allocation.
rTMS procedure
Justification of the technical procedure
For the sake of full clarity, when we designed this study, the three pilot studies mentioned (Gay et al., 2017; Rosenberg et al., 2013; Zack et al., 2016) were not yet published. We chose the parameters based on the existing literature on the subject (Wang et al., 2007) and the safety guidelines concerning the use of rTMS (Rossi, Hallett, Rossini, & Pascual-Leone, 2009).
Determination of the resting motor threshold (rMT) and cortical target location
Following the mapping of the abductor pollicis brevis site in the right motor cortex, each patient’s rMT was established as the minimum stimulus required to induce the contraction of the right thumb at least 5 out of 10 times (Pridmore, Fernandes Filho, Nahas, Liberatos, & George, 1998). The site for stimulation of the right DLPFC was determined following the Beam’s method (Beam, Borckardt, Reeves, & George, 2009). At the second visit, the rMT procedure was repeated, and the TMS coil was located at the same site as at visit 1.
Participants were then fitted with two electrodes on their scalps just below the hairline and at the chin. Electrodes were connected to an Eco 2 transcutaneous electrical nerve stimulation device (TENS). During the rTMS, participants were instructed to close their eyes and relax.
Active rTMS procedure
The Magpro R30-incl. MagOption, along with real and sham figure-eight coils, was used to administer rTMS. Participants received frequency of 1 Hz at 120% rMT, with one train producing 360 pulses in a single 6-min session. The shortest session was elicited, given the hypothesis that craving intensity naturally diminishes over the course of a few minutes, and this decrease might instead be incorrectly attributed to a specific effect of the rTMS (Grall-Bronnec & Sauvaget, 2014). For active rTMS, the TENS was turned off (no current flowed through the scalp electrodes).
Sham rTMS procedure
Placebo stimulation was applied at the same location and frequency using the Magstim sham-coil system. For sham rTMS, the TENS was turned on at a frequency of 1 Hz and an intensity able to move the periorbital and chin muscles. To ensure the validity of the sham procedure, the same investigator performed both the active and sham procedures. Moreover, at the end of the second session (whether active or sham, depending on the randomized order), participants were asked three questions: (a) Are you able to tell which was the real session and which was the sham session?, (b) If so, when do you think you received these treatments?, and (c) How were you able to tell the difference?
Measures
Sociodemographic characteristics
We collected age and gender information.
Evaluation of craving
Craving was assessed by a VAS (the primary outcome), a gambling-related craving questionnaire, and physiological measures (the secondary outcomes). Outcome measures were tested at several time points: at baseline, immediately after gambling cue exposure/before the rTMS session, immediately after the rTMS session, and every 5 min until the craving intensity returned to the baseline level.
Visual Analog Scale (VAS)
In accordance with the previous studies (Grall-Bronnec & Sauvaget, 2014), the urge to gamble was measured using a 10-cm VAS, ranking patients’ response to the question “how much do you want to gamble right now?.” The VAS is an easy-to-use, sensitive, reproducible, and reliable instrument.
Gambling Craving Scale (GACS)
The GACS is a multidimensional scale measuring gambling-related craving comprising three subscales: anticipation, desire, and relief (Young & Wohl, 2009). It is a nine-item scale, with three items for each subscale. For the purpose of this study, we only used the three items from the desire factor (“I crave gambling right now,” “I need to gamble now,” and “I have an urge to gamble”), which represents a strong and urgent desire to gamble. In fact, the two remaining factors are more likely to reflect gambling motives.
In accordance with the previous studies, we assumed that craving assessment by a self-rated measure demonstrated high validity (Herremans et al., 2012).
Physiological measures
Heart rate and blood pressure were recorded. These measures are associated with physiological arousal and are considered as proxy measures of gambling craving (Ashrafioun & Rosenberg, 2012; Skinner & Aubin, 2010). They were relevant in this context, because they are objective and therefore complementary to the above subjective measures.
Gambling characteristics
DSM-IV PG section
Patients were included in the STIMJEU study through an interview based on the 10 DSM-IV diagnostic criteria for PG (APA, 1994). This categorical approach was completed using a dimensional approach by adding the number of positive DSM-IV criteria. Indeed, the number of diagnostic criteria met is correlated with the severity of the disorder (Toce-Gerstein, Gerstein, & Volberg, 2003).
Gambling-Related Cognitions Scale (GRCS)
The GRCS is a 23-item self-report scale that assesses a range of gambling-related cognitive biases and errors: illusion of control (GRCS-IC), predictive control (GRCS-PC), interpretative bias (GRCS-IB), gambling-related expectancies (GRCS-GE), and perceived inability to stop gambling (GRCS-IS) (Raylu & Oei, 2004).
Gambling habits
The participants were asked to define their favorite type of game, according to the classification proposed by Boutin (2010), and the medium usually used for gambling (online or offline).
Clinical characteristics
Mini International Neuropsychiatric Interview (M.I.N.I.)
The fifth version of the M.I.N.I. allows for the main axis-I psychiatric disorders of the DSM-IV and antisocial personality disorder to be explored in a quick and standardized way (Lecrubier et al., 1997). We used it to verify that the patients met the eligibility criteria, especially with regard to alcohol and SUDs.
Tolerability
A follow-up phone call was made 24 hr after the session to check whether side effects had occurred. Patients were excluded if a serious side effect occurred during a session or if they had used any psychoactive substance (excluding nicotine or their usual medication) within 12 hr prior to the session.
Statistical analysis
The main analysis followed the intent-to-treat principle (all randomized patients were analyzed according to their randomization groups). Descriptive statistical analyses were provided at baseline for categorical (number and percentage) and continuous (min–max, mean, median, and interquartile range) data.
An analysis of covariance (ANCOVA) controlling for baseline scores was performed including treatment, period, and interaction between period and treatment effects. Interaction between baseline scores and treatment effects was also included in the ANCOVA model to check that the treatment effect was not different according to the baseline score level. All models were validated using graphical residual analysis.
A power calculation indicated that 42 participants were required to detect a 2-point difference on the VAS for intergroup craving, with the standard deviation of the period differences assumed as 3.5 with 95% power and a two-tailed α of 0.05.
Ethics
The study was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, with approval from the local ethics committee. Written informed consent was collected from all participants.
Results
Description of the participants
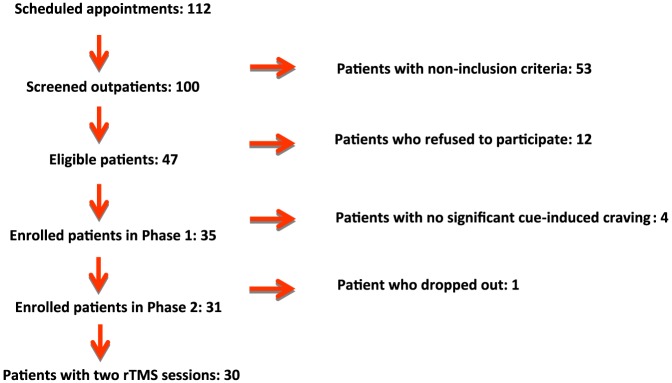
As Figure 2 shows, 112 problem gamblers called our department to make an appointment between March 2012 and December 2014. A total of 100 individuals were screened for this study, and 47 met the inclusion criteria. The reasons for non-inclusion are summarized in Table 1. Finally, 31 patients were enrolled, one of whom dropped out just before receiving the first treatment. Thirty completed the study and received two rTMS sessions. The baseline characteristics of the sample are described in Table 2.
Figure 2.
Flow chart of the STIMJEU study
Table 1.
Frequency of non-inclusion criteria
| Number of patients | |
|---|---|
| Screened | 100 |
| Eligible | 47 |
| Non-eligible | 53 |
| Non-inclusion criteria | |
| Current AUD or SUD | 16 |
| Women (during the first part of the enrollment period) | 10 |
| Non-attendance to the inclusion visit | 7 |
| rTMS contraindication (seizure and Parkinson’s disease) | 6 |
| PG DSM-IV diagnostic criteria <5 | 6 |
| Left-handed | 4 |
| Current benzodiazepine medication | 3 |
| Age ≥70 years | 1 |
Note. AUD: alcohol use disorder; PG: pathological gambling; SUD: substance use disorder; rTMS: repetitive transcranial magnetic stimulation.
Table 2.
Baseline characteristics of the patients according to their randomization group
| Median [lower quartile (Q1)−upper quartile (Q3)] or number (%) | ||
|---|---|---|
| Group 1 active rTMS–sham rTMS (n = 15) | Group 2 sham rTMS–active rTMS (n = 16) | |
| Sociodemographic characteristics | ||
| Sex (% males) | 14 (93.33%) | 13 (81.25%) |
| Age (years) | 33 (28–42) | 39 (34.5–56) |
| Gambling characteristics | ||
| Favorite type of game | ||
| “Pure chance games” | 6 (40.00%) | 7 (43.75%) |
| “Chance games with pseudo skills” | 4 (26.67%) | 8 (50.00%) |
| “Chance games with elements of skills” | 5 (33.33%) | 1 (6.25%) |
| Usual medium of game | ||
| Offline | 10 (66.67%) | 13 (81.25%) |
| Online | 5 (33.33%) | 3 (18.75%) |
| Severity of pathological gambling (number of DSM-IV diagnostic criteria) | 7 (6–8) | 6 (5–7) |
| Disease history (years) | 5 (2–9) | 9 (5–17) |
| Gambling-related cognitions | ||
| GRCS total score (/161) | 79 (66–95) | 85 (72–104) |
| GRCS-GE (/28) | 16 (14–21) | 14 (12–22) |
| GRCS-IC (/28) | 4 (4–8) | 7 (4–17) |
| GRCS-PC (/42) | 20 (16–24) | 22 (16–26) |
| GRCS-IS (/35) | 27 (17–29) | 25 (20–29) |
| GRCS-IB (/28) | 17 (11–23) | 18 (10–20) |
| Craving | ||
| Baseline | 3.00 (1–5) | 4.25 (1.25–5.25) |
| Cue-induced | 7.00 (6–10) | 8.5 (6.5–10) |
Note. GRCS: Gambling Related Cognitions Scale; GRCS-GE: GRCS-Gambling-related Expectancies; GRCS-IC: GRCS-Illusion of Control; GRCS-PC: GRCS-Predictive Control; GRCS-IS: GRCS-Inability to Stop gambling; GRCS-IB: GRCS-Interpretative Bias; rTMS: repetitive transcranial magnetic stimulation.
Immediate rTMS effects on craving
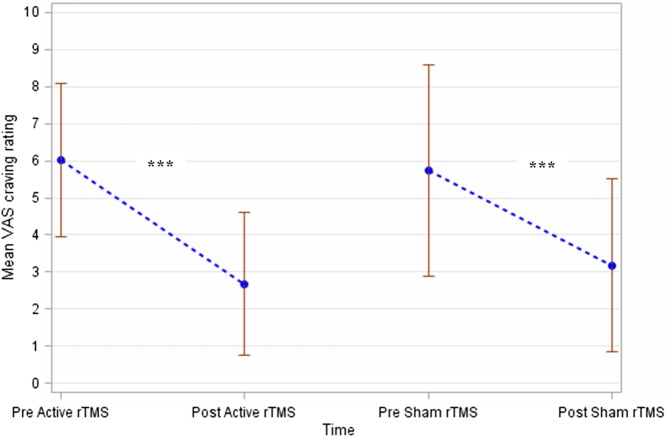
As Figure 3 shows, the neuromodulation sessions were associated with a significant decrease in the primary outcome “urge to gamble” (active session: p value = .0003 and sham session: p value < .0001). However, the “Active rTMS” and “Sham rTMS” groups showed no difference with regard to score change (p value = .18). These results were adjusted to the level of cue-induced craving assessed using the VAS (p value < .0001). The interaction between the baseline score and the treatment effect was not significant (p value = .71) and was removed from the ANCOVA model. Importantly, no interaction was found between period and treatment (p value = .94). In other words, the order of rTMS sessions did not affect the blinding of the sham versus active rTMS sessions.
Figure 3.
Evolution of the craving intensity according to the active versus sham rTMS sessions
Furthermore, the change in the secondary outcomes GACS-desire, heart rate, and systolic/diastolic blood pressure did not significantly differ between the two groups (p values = .70, .63, .36, and .43, respectively; Table 3).
Table 3.
Effect of the rTMS on the primary and secondary outcome measures
| Active rTMS (N = 30) | Sham rTMS (N = 30) | ||
|---|---|---|---|
| Mean (standard deviation) | p value | ||
| VAS: “urge to gamble” (0–10) | 2.62 (±0.33) | 3.25 (±0.33) | .18 |
| GACS: “desire” factor (1–7) | 1.89 (±1.14) | 1.97 (±1.14) | .70 |
| Heart rate (bpm) | 76.38 (±1.50) | 77.40 (±1.47) | .63 |
| Systolic blood pressure (mmHg) | 131.89 (±1.73) | 134.21 (±1.73) | .36 |
| Diastolic blood pressure (mmHg) | 82.63 (±1.11) | 83.87 (±1.11) | .43 |
Note. GACS: Gambling Craving Scale; VAS: Visual Analog Scale; rTMS: repetitive transcranial magnetic stimulation.
Credibility of the sham rTMS procedure
About 29 of the 30 participants answered the three questions assessing the validity of the sham procedure. About 10 patients admitted that they were unable to tell the difference between the active and the sham sessions, whereas the remaining 19 asserted the opposite. Of these patients, 7 failed to identify the nature of the sessions, but 12 succeeded; 10 guessed the order of the sessions based on how their skin felt and 2 based on the differential effect that the sessions had on their craving.
Procedure safety
No serious adverse reactions were observed during the study. Around 17 patients reported one or several non-serious (and mostly expected) adverse reactions, including headache (17 times), unpleasant sensations (4 times), anxiety (once), asthenia (once), sleep disturbance (once), nausea (once), and foot muscle cramps (once).
Discussion
This study examined the effect of a single LF rTMS session applied to the right DLPFC on cue-induced craving among a sample of pathological gamblers seeking treatment. To the best of our knowledge, the STIMJEU study is the first crossover, randomized, and controlled rTMS trial to be conducted and has the largest sample size of this type of addicted patients (30 patients). In fact, the sample size of the rTMS studies among patients with SUD is 22.2 on average (Jansen et al., 2013), and previous TMS studies in the gambling field included 5 (Rosenberg et al., 2013), 9 (Zack et al., 2016), and 22 (Gay et al., 2017) patients.
Effect of rTMS on gambling craving
The neuromodulation sessions were associated with a significant decrease in the urge to gamble, regardless of whether the session was active or sham rTMS. After controlling for cue-induced craving level, no effects were observed for active rTMS on craving.
To explain the unspecific treatment effect of this study, several hypotheses can be considered, especially regarding the placebo effect, the natural course of craving, and the stimulation parameters. First, a high placebo response is frequently reported among clinical addiction trials (Litten et al., 2013), especially concerning PG (Grant, Odlaug, & Schreiber, 2014). Low symptom severity and short disease history, which were the cases for the current participants, are strong predictors of the placebo response (Weimer, Colloca, & Enck, 2015). For these reasons, we assume that the placebo effect was strong, which might explain why we were unable to demonstrate a significant difference between sham and active rTMS, most likely because of a lack of power. In the case of a high placebo effect, a significant difference between the active and placebo treatment is more difficult to demonstrate. This strong placebo effect matches the previous results by Rosenberg et al. (2013) where rapid but short-lasting effects were primarily considered as a sham effect. Second, although we ensured that the sessions were as brief as possible, we cannot exclude the fact that craving decreased rapidly and spontaneously. In effect, the natural course of craving remains poorly understood and fluctuates over time under the influence of many factors (Grall-Bronnec & Sauvaget, 2014). However, previous studies have shown that craving can remain strong up to 150 min after a substance-cue presentation and then gradually decrease in intensity (Heishman, Lee, Taylor, & Singleton, 2010; Lundahl & Greenwald, 2016). We hypothesize that, in our sample, the natural course of craving was highly heterogeneous and patient-dependent. Another possible explanation of the fact that both active and sham rTMS resulted in decreased craving regarding the time course of craving is the phenomenon of regression toward the mean. Indeed, as the rTMS was applied at the moment when the craving level was at the highest, the next measure of the craving level was very likely to decrease at a later time point. Considering a “random” time point in the course of craving when applying rTMS would be an interesting alternative for future studies to avoid this bias. Third, the stimulation parameters might not have been optimal, especially the number of pulses, leading to an insufficient therapeutic effect (Grall-Bronnec & Sauvaget, 2014). Similarly, the choice of the treatment location (right DLPFC) and frequency (LF) can give rise to some criticism. According to previous authors, the increased activation of the DLPFC is associated with cognitive control, whereas cue exposure and craving are associated with increased activity in the regions implicated in emotion (Goudriaan, de Ruiter, van den Brink, Oosterlaan, & Veltman, 2010; Kober et al., 2010). Inhibiting the right DLPFC through LF rTMS might reduce the cognitive regulation of craving, thereby explaining the negative findings of our study. However, craving did not increase after rTMS in our study. Crockford and his colleagues demonstrated that PG participants exhibited increased activity in the right DLPFC when exposed to visual gambling sensory cues (which was the case in this study). Those authors assumed that the cues, through the activation of DLPFC networks, involved the use of working memory in coding external events into internal representations and volitional scanning (Crockford et al., 2005).
Our findings conflict with the previous studies that assessed the efficacy of rTMS in the field of addictions but corroborate those of others (Barth et al., 2011; Herremans et al., 2012; Hoppner, Broese, Wendler, Berger, & Thome, 2011; Van den Eynde et al., 2010; Walpoth et al., 2008). To the best of our knowledge, only three studies have explored the effects of TMS in pathological gamblers (Gay et al., 2017; Rosenberg et al., 2013; Zack et al., 2016). Rosenberg and colleagues were the first to explore the neuromodulation treatment option for PG in an open-label study. Five patients underwent 15 sessions of LF (1 Hz) deep TMS to the left DLPFC with an H-coil. Despite initial improvement, the authors failed to demonstrate treatment efficacy (Rosenberg et al., 2013). The small sample size, the treatment parameters (including treatment location and frequency), the lack of a control condition, and the possible inhibition of the right DLPFC were cited as limitations. In a recent study, Zack and colleagues explored the respective effects of one session of HF rTMS on the medial PFC and one session of continuous theta burst stimulation (cTBS) on the right DLPFC with regard to decision making, cognitive control, gambling reinforcement, and physiological arousal. Nine participants received active or sham treatments at weekly intervals. A slot machine was used as the reinforcing stimulus. The authors found that both rTMS and cTBS reduced gambling reinforcement but not impulsive choice. Relative to sham, active rTMS reduced the post-game increase in craving. These latter results are not in line with ours, although the authors inhibited the right DLPFC in one arm of their study. However, they did not directly evaluate the effect of rTMS on craving. Finally, a recent study of patients with gambling disorder reported decreased cue-induced cravings following a single session of HF rTMS applied over the left DLPFC (Gay et al., 2017). However, the authors failed to show a significant effect of rTMS on gambling behavior. A comparison between the findings of these three studies and ours must be made carefully because of the different frameworks (clinical vs. experimental) and the different designs of the studies (stimulation before or after gambling-cue exposure, type of TMS, LF vs. HF rTMS, etc.).
Credibility of the sham procedure
Only 12 of 30 patients guessed the order of the sessions correctly, suggesting that the sham condition was reasonably matched to the real TMS, with respect to face and scalp sensations. This rate is in keeping with previous results (40%) (Barth et al., 2011) but lower than other results reported with regard to rTMS randomized clinical trials of patients with depression (more than 50%) (Berlim, Broadbent, & Van den Eynde, 2013) and higher than the results of Gay et al. (2017), who found that less than 25% of the participants correctly guessed the rTMS allocation, even though they used the same sham method as ours. These discrepancies suggest that both human and technical factors influence the patients’ ability to identify the difference between sham and active sessions. To better explore this phenomenon, we asked the patients to justify their choice. A large majority based their decision on skin sensations, suggesting the influence of the placebo effect. These findings suggest that it is difficult to develop a reliable sham rTMS method, and this result should be reported along with the blinding success rate in any randomized control trial using rTMS. Moreover, because current sham methods might inadequately mimic real rTMS, the blinding and bias estimations of the treatment effects might be only partially successful (Broadbent et al., 2011). The expression of craving is eminently subjective, and its intensity can be over- or underestimated if the placebo method has not been optimized (Brunoni, Lopes, Kaptchuk, & Fregni, 2009). Thus, we cannot exclude the possibility of significant bias given the sham method we used with regard to the results of this study.
Study strengths and weaknesses
These results must be viewed in the context of several limitations, which were described above to explain the negative results of our trial. We add that we failed to recruit 42 participants as indicated by the power calculation. However, we decided to end the study because of recruitment difficulties (only 47 of 100 screened patients were eligible, because we chose strict inclusion criteria to demonstrate a specific effect of rTMS, and a quarter of those contacted had refused to participate) and because our sample size was significantly larger than those of most other studies. Nevertheless, it is possible that our analyses lacked power. In addition, the number of patients who did not meet the inclusion criteria suggests that the current results might not be generalized to the entire population of pathological gamblers. However, these limits are compensated by the strengths of the study. As a precautionary measure, only patients with high reactivity to gambling cues were included, and the rTMS sessions were applied when the craving level was at its highest to maximize the effect of the neuromodulation technique. To the best of our knowledge, previous studies on this topic have not verified whether the patients were craving inducible. In this study, four patients were not craving inducible and were therefore not included. Furthermore, we planned to evaluate craving using several methods to better capture the complexity of the craving concept. This study assessed several dimensions: feelings, cognitions, and physiological correlates. In a recent publication, we showed that most of the studies assessing the efficacy of rTMS on craving and addictive behaviors used a VAS as an outcome measure and exclusively in 8 of 18 cases (Grall-Bronnec & Sauvaget, 2014). Only three studies combined several measures, both subjective and objective (Amiaz et al., 2009; Eichhammer et al., 2003; Li et al., 2013). In addition, patients with alcohol or other SUDs were not eligible to avoid confusing the craving to gamble and that for alcohol or other substances. This criterion represented the main reason for non-inclusion in this study.
In conclusion, we failed to demonstrate the efficacy of one session of LF rTMS to decrease the cue-induced cravings of pathological gamblers. Nevertheless, we are convinced that rTMS remains a promising therapeutic method. Additional studies with more patients are needed to examine the potential effect of rTMS and determine the best technical parameters. A placebo lead-in should be used to exclude placebo responders. Furthermore, several daily sessions of rTMS might be required to induce long-lasting effects and change gambling behaviors. In a previous review, we indicated that a design with repeated daily sessions of rTMS over a period of several weeks should be used in addictive disorders, considering that repeated, frequent sessions will result in changes in cerebral neuroplasticity and generate a long-lasting effect (Grall-Bronnec & Sauvaget, 2014).
Acknowledgements
The authors would like to thank D. Drapier, C. Nauczyciel, B. Rocher, and J.-L. Vénisse for their valuable contributions.
Funding Statement
Funding sources: The STIMJEU study was funded by a grant from the University Hospital of Nantes (Internal call for tenders, 2011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contribution
AS, VS-R, and MG-B: study concept and design. AS and MG-B: analysis and interpretation of data. AG and VS-R: statistical analysis. AS and MG-B: obtained funding. AS and MG-B: study supervision. AS, AG, VS-R, and MG-B: drafting of manuscript. AS, SB, AG, JL, AP, PV, J-MV, VS-R, and MG-B: critical revision. AS, VS-R, and MG-B have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors report no financial or other relationship relevant to the subject of this article.
References
- Addolorato G., Leggio L., Hopf F. W., Diana M., Bonci A. (2012). Novel therapeutic strategies for alcohol and drug addiction: Focus on GABA, ion channels and transcranial magnetic stimulation. Neuropsychopharmacology, 37(1), 163–177. doi:10.1038/npp.2011.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R., Levy D., Vainiger D., Grunhaus L., Zangen A. (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction, 104(4), 653–660. doi:10.1111/j.1360-0443.2008.02448.x [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association [APA]. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association [APA]. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashrafioun L., Rosenberg H. (2012). Methods of assessing craving to gamble: A narrative review. Psychology of Addictive Behaviour, 26(3), 536–549. doi:10.1037/a0026367 [DOI] [PubMed] [Google Scholar]
- Barth K. S., Rydin-Gray S., Kose S., Borckardt J. J., O’Neil P. M., Shaw D., Madan A., Budak A., George M. S. (2011). Food cravings and the effects of left prefrontal repetitive transcranial magnetic stimulation using an improved sham condition. Front Psychiatry, 2, 9. doi:10.3389/fpsyt.2011.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam W., Borckardt J. J., Reeves S. T., George M. S. (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulation, 2(1), 50–54. doi:10.1016/j.brs.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim M. T., Broadbent H. J., Van den Eynde F. (2013). Blinding integrity in randomized sham-controlled trials of repetitive transcranial magnetic stimulation for major depression: A systematic review and meta-analysis. The International Journal of Neuropsychopharmacology, 16(5), 1173–1181. doi:10.1017/S1461145712001691 [DOI] [PubMed] [Google Scholar]
- Boutin C. (2010). Le jeu: Chance ou stratégie? Choisir librement la place du jeu dans votre vie [Gambling: Chance or strategy? Freely choose the role of gambling in your life]. Montreal, QC: Les Editions de l’Homme. [Google Scholar]
- Broadbent H. J., van den Eynde F., Guillaume S., Hanif E. L., Stahl D., David A. S., Campbell I. C., Schmidt U. (2011). Blinding success of rTMS applied to the dorsolateral prefrontal cortex in randomised sham-controlled trials: A systematic review. World Journal of Biological Psychiatry, 12(4), 240–248. doi:10.3109/15622975.2010.541281 [DOI] [PubMed] [Google Scholar]
- Brunoni A. R., Lopes M., Kaptchuk T. J., Fregni F. (2009). Placebo response of non-pharmacological and pharmacological trials in major depression: A systematic review and meta-analysis. PLoS One, 4(3), e4824. doi:10.1371/journal.pone.0004824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowlishaw S., Merkouris S., Dowling N., Anderson C., Jackson A., Thomas S. (2012). Psychological therapies for pathological and problem gambling. Cochrane Database of Systematic Reviews, 11, CD008937. doi:10.1002/14651858.CD008937.pub2 [DOI] [PubMed] [Google Scholar]
- Crockford D. N., Goodyear B., Edwards J., Quickfall J., el-Guebaly N. (2005). Cue-induced brain activity in pathological gamblers. Biological Psychiatry, 58(10), 787–795. doi:10.1016/j.biopsych.2005.04.037 [DOI] [PubMed] [Google Scholar]
- Eichhammer P., Johann M., Kharraz A., Binder H., Pittrow D., Wodarz N., Hajak G. (2003). High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. Journal of Clinical Psychiatry, 64(8), 951–953. doi:10.4088/JCP.v64n0815 [DOI] [PubMed] [Google Scholar]
- Feil J., Zangen A. (2010). Brain stimulation in the study and treatment of addiction. Neuroscience and Biobehavioral Reviews, 34(4), 559–574. doi:10.1016/j.neubiorev.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Fox P., Ingham R., George M. S., Mayberg H., Ingham J., Roby J., Martin C., Jerabek P. (1997). Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport, 8(12), 2787–2791. doi:10.1097/00001756-199708180-00027 [DOI] [PubMed] [Google Scholar]
- Gay A., Boutet C., Sigaud T., Kamgoue A., Sevos J., Brunelin J., Massoubre C. (2017). A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. European Psychiatry, 41, 68–74. doi:10.1016/j.eurpsy.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Goldman M., Szucs-Reed R. P., Jagannathan K., Ehrman R. N., Wang Z., Li Y., Suh J. J., Kampman K., O’Brien C. P., Childress A. R., Franklin T. R. (2013). Reward-related brain response and craving correlates of marijuana cue exposure: A preliminary study in treatment-seeking marijuana-dependent subjects. Journal of Addiction Medicine, 7(1), 8–16. doi:10.1097/ADM.0b013e318273863a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A. E., de Ruiter M. B., van den Brink W., Oosterlaan J., Veltman D. J. (2010). Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addiction Biology, 15(4), 491–503. doi:10.1111/j.1369-1600.2010.00242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall-Bronnec M., Sauvaget A. (2014). The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: A critical literature review of efficacy, technical and methodological considerations. Neuroscience and Biobehavioral Reviews, 47, 592–613. doi:10.1016/j.neubiorev.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Grant J. E., Odlaug B. L., Schreiber L. R. (2014). Pharmacological treatments in pathological gambling. British Journal of Clinical Pharmacology, 77(2), 375–381. doi:10.1111/j.1365-2125.2012.04457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. E., Potenza M. N. (2004). Pathological gambling. A clinical guide to treatment. Arlington, VA: American Psychiatric Publishing, Inc. [Google Scholar]
- Heishman S. J., Lee D. C., Taylor R. C., Singleton E. G. (2010). Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of tobacco deprivation and sex. Experimental and Clinical Psychopharmacology, 18(3), 245–256. doi:10.1037/a0019401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans S. C., Baeken C., Vanderbruggen N., Vanderhasselt M. A., Zeeuws D., Santermans L., De Raedt R. (2012). No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug and Alcohol Dependence, 120(1–3), 209–213. doi:10.1016/j.drugalcdep.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Hoppner J., Broese T., Wendler L., Berger C., Thome J. (2011). Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. The World Journal of Biological Psychiatry, 12(Suppl 1), 57–62. doi:10.3109/15622975.2011.598383 [DOI] [PubMed] [Google Scholar]
- Jansen J. M., Daams J. G., Koeter M. W., Veltman D. J., van den Brink W., Goudriaan A. E. (2013). Effects of non-invasive neurostimulation on craving: A meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2472–2480. doi:10.1016/j.neubiorev.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Keck M. E., Welt T., Muller M. B., Erhardt A., Ohl F., Toschi N., Holsboer F., Sillaber I. (2002). Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology, 43(1), 101–109. doi:10.1016/S0028-3908(02)00069-2 [DOI] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E. F., Weber J., Mischel W., Hart C. L., Ochsner K. N. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of National Academy of Sciences of the United States of America, 107(33), 14811–14816. doi:10.1073/pnas.1007779107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. doi:10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D., Weiller E., Amorim P., Bonora I., Harnett Sheehan K., Janavs J., Dunbar G. C. (1997). The Mini International Neuropsychiatric Interview (MINI), a short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry, 12(5), 224–231. doi:10.1016/S0924-9338(97)83296-8 [Google Scholar]
- Li X., Hartwell K. J., Owens M., Lematty T., Borckardt J. J., Hanlon C. A., Brady K. T., George M. S. (2013). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biological Psychiatry, 73(8), 714–720. doi:10.1016/j.biopsych.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten R. Z., Castle I. J., Falk D., Ryan M., Fertig J., Chen C. M., Yi H. Y. (2013). The placebo effect in clinical trials for alcohol dependence: An exploratory analysis of 51 naltrexone and acamprosate studies. Alcoholism, Clinical and Experimental Research, 37(12), 2128–2137. doi:10.1111/acer.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl L. H., Greenwald M. K. (2016). Magnitude and duration of cue-induced craving for marijuana in volunteers with cannabis use disorder. Drug and Alcohol Dependence, 166, 143–149. doi:10.1016/j.drugalcdep.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R., Waters A. J., van de Wetering B. J., Franken I. H. (2013). Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: An ecological momentary assessment study. Journal of Consulting and Clinical Psychology, 81(1), 1–12. doi:10.1037/a0030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D., Barrett S. P., Kelly J. T., Aw A., Dagher A. (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology, 31(12), 2728–2738. doi:10.1038/sj.npp.1301075 [DOI] [PubMed] [Google Scholar]
- Pridmore S., Fernandes Filho J. A., Nahas Z., Liberatos C., George M. S. (1998). Motor threshold in transcranial magnetic stimulation: A comparison of a neurophysiological method and a visualization of movement method. The Journal of ECT, 14(1), 25–27. doi:10.1097/00124509-199803000-00004 [PubMed] [Google Scholar]
- Protasio M. I., da Silva J. P., Arias-Carrion O., Nardi A. E., Machado S., Cruz M. S. (2015). Repetitive transcranial magnetic stimulation to treat substance use disorders and compulsive behavior. CNS & Neurological Disorders Drug Targets, 14(3), 331–340. doi:10.2174/1871527314666150318114043 [DOI] [PubMed] [Google Scholar]
- Raylu N., Oei T. P. (2004). The Gambling Related Cognitions Scale (GRCS): Development, confirmatory factor validation and psychometric properties. Addiction, 99(6), 757–769. doi:10.1111/j.1360-0443.2004.00753.x [DOI] [PubMed] [Google Scholar]
- Rosenberg O., Klein L. D., Dannon P. N. (2013). Deep transcranial magnetic stimulation for the treatment of pathological gambling. Psychiatry Research, 206(1), 111–113. doi:10.1016/j.psychres.2012.09.045 [DOI] [PubMed] [Google Scholar]
- Rossi S., Hallett M., Rossini P. M., Pascual-Leone A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. doi:10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvaget A., Trojak B., Bulteau S., Jimenez-Murcia S., Fernandez-Aranda F., Wolz I., Menchón J. M., Achab S., Vanelle J. M., Grall-Bronnec M. (2015). Transcranial direct current stimulation (tDCS) in behavioral and food addiction: A systematic review of efficacy, technical, and methodological issues. Frontiers in Neuroscience, 9, 349. doi:10.3389/fnins.2015.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. D., Aubin H. J. (2010). Craving’s place in addiction theory: Contributions of the major models. Neuroscience and Biobehavioral Reviews, 34(4), 606–623. doi:10.1016/j.neubiorev.2009.11.024 [DOI] [PubMed] [Google Scholar]
- Sodano R., Wulfert E. (2010). Cue reactivity in active pathological, abstinent pathological, and regular gamblers. Journal of Gambling Studies, 26(1), 53–65. doi:10.1007/s10899-009-9146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toce-Gerstein M., Gerstein D. R., Volberg R. A. (2003). A hierarchy of gambling disorders in the community. Addiction, 98(12), 1661–1672. doi:10.1111/j.1360-0443.2003.00545.x [DOI] [PubMed] [Google Scholar]
- Van den Eynde F., Broadbent H., Guillaume S., Claudino A., Campbell I. C., Schmidt U. (2010). Handedness, repetitive transcranial magnetic stimulation and bulimic disorders. European Psychiatry, 27(4), 290–293. doi:10.1016/j.eurpsy.2010.08.015 [DOI] [PubMed] [Google Scholar]
- Van Holst R. J., Van den Brink W., Veltman D. J., Goudriaan A. E. (2010). Brain imaging studies in pathological gambling. Current Psychiatry Report, 12(5), 418–425. doi:10.1007/s11920-010-0141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorri-Vigneau C., Spiers A., Caillet P., Bruneau M., Challet-Bouju G., Grall-Bronnec M. (2017). Opioid antagonists for pharmacological treatment of gambling disorder: Are they relevant? Current Neuropharmacology. Advance online publication. doi:10.2174/1570159X15666170718144058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Tomasi D., Baler R. D. (2013). The addictive dimensionality of obesity. Biological Psychiatry, 73(9), 811–818. doi:10.1016/j.biopsych.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walpoth M., Hoertnagl C., Mangweth-Matzek B., Kemmler G., Hinterholzl J., Conca A., Hausmann A. (2008). Repetitive transcranial magnetic stimulation in bulimia nervosa: Preliminary results of a single-centre, randomised, double-blind, sham-controlled trial in female outpatients. Psychotherapy and Psychosomatics, 77(1), 57–60. doi:10.1159/000110061 [DOI] [PubMed] [Google Scholar]
- Wang Z., Faith M., Patterson F., Tang K., Kerrin K., Wileyto E. P., Detre J. A., Lerman C. (2007). Neural substrates of abstinence-induced cigarette cravings in chronic smokers. The Journal of Neuroscience, 27(51), 14035–14040. doi:10.1523/JNEUROSCI.2966-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer K., Colloca L., Enck P. (2015). Placebo effects in psychiatry: Mediators and moderators. Lancet Psychiatry, 2(3), 246–257. doi:10.1016/S2215-0366(14)00092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing V. C., Bacher I., Wu B. S., Daskalakis Z. J., George T. P. (2012). High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophrenia Research, 139(1–3), 264–266. doi:10.1016/j.schres.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Young M. M., Wohl M. J. (2009). The Gambling Craving Scale: Psychometric validation and behavioral outcomes. Psychological Addiction Behavior, 23(3), 512–522. doi:10.1037/a0015043 [DOI] [PubMed] [Google Scholar]
- Zack M., Cho S. S., Parlee J., Jacobs M., Li C., Boileau I., Strafella A. (2016). Effects of high frequency repeated transcranial magnetic stimulation and continuous theta burst stimulation on gambling reinforcement, delay discounting, and stroop interference in men with pathological gambling. Brain Stimulation, 9(6), 867–875. doi:10.1016/j.brs.2016.06.003 [DOI] [PubMed] [Google Scholar]