Abstract
Chronic, low-grade inflammation is a common comorbid condition in chronic kidney disease (CKD), and particularly in chronic dialysis patients. In this review, we consider the question of whether inflammation affects outcomes in dialysis patients. Levels of pro-inflammatory cytokines, as well as C-reactive protein, are elevated in chronic dialysis patients. Multiple factors likely contribute to chronic inflammatory activation in kidney disease patients including the uremic milieu, lifestyle and epigenetic influences, infectious and thrombotic events, the dialysis process, and dysbiosis. Increased inflammatory markers in both CKD and chronic dialysis patients are associated with adverse clinical outcomes including all-cause mortality, cardiovascular events, kidney disease progression, protein energy wasting and diminished motor function, cognitive impairment, as well as other adverse consequences including CKD-mineral and bone disorder, anemia, and insulin resistance. Strategies that have been shown to reduce chronic systemic inflammation in CKD and chronic dialysis patients include both pharmacological and non-pharmacological interventions. However, despite evidence that systemic inflammatory markers can be lowered in kidney disease patients treated with various strategies, evidence that this improves clinical outcomes is largely unavailable and represents an important future research direction. Overall, there is strong observational evidence that inflammation is high in chronic dialysis patients and that this is independently associated with numerous adverse clinical outcomes. Targeting inflammation represents a potentially novel and attractive strategy if it can indeed improve adverse outcomes common in this population.
Keywords: chronic dialysis, endpoints, inflammatory, kidney disease, outcomes
Prevalence of end-stage renal disease (ESRD) in the United States is rising and is associated with high morbidity and mortality, as well as healthcare expenditure.[1] Over recent decades, there has been considerable research implicating inflammation in chronic kidney disease (CKD) and ESRD, thus, inflammation could now be considered a well-established rather than a novel risk factor for clinical outcomes. In this review, we will consider the question of whether inflammation affects outcomes in chronic dialysis patients. We will discuss evidence of increased inflammation in CKD and chronic dialysis patients, the association of elevated inflammatory markers with adverse clinical outcomes, and both pharmacological and non-pharmacological strategies to reduce inflammation in CKD and chronic dialysis.
Overview of Inflammation in CKD and Chronic Dialysis
It was first proposed in 1983 that inflammation, via monocyte release of interleukin-1 (IL-1), the master cytokine of inflammation, was the basis of numerous complications in chronic dialysis.[2] It is now appreciated that the development of inflammation in CKD begins well before the need for chronic dialysis, and elevated levels of inflammatory biomarkers such as interleukin-6 (IL-6) and C-reactive protein (CRP) suggest that CKD and chronic dialysis can both be viewed as low-grade inflammatory processes.[3, 4] In CKD, inflammatory macrophages infiltrate the kidney, promoting release of pro-inflammatory cytokines including, IL-1β, tumor necrosis factor-α (TNF-α), IL-6, and interleukin-23 (IL-23).[5] While the release of pro-inflammatory cytokines may have acute beneficial effects, chronic inflammation is recognized to promote adverse consequences.[6] In fact, persistent low-grade inflammation has been recognized as an important contributor to risk of mortality, cardiovascular events, kidney disease progression, and other adverse outcomes in both CKD and chronic dialysis.[7–9]
Evidence of Increased Inflammation in CKD and Chronic Dialysis
Chronic, low-grade inflammation is regarded as a common comorbid condition in CKD, and particularly in chronic dialysis patients.[7] Several circulating markers are commonly assessed as indicators of systemic inflammation. IL-1 is a pro-inflammatory mediator of both acute and chronic inflammation, and induces synthesis and expression of hundreds of secondary inflammatory mediators.[10] IL-1β is the main form of circulating IL-1 and is initially synthesized as a precursor (pro-IL-1β) that becomes activated in the setting of a macromolecular structure known as the inflammasome,[10, 11] which is activated in CKD and perpetuates the inflammatory response.[12, 13] IL-6 is a pro-inflammatory cytokine that promotes inflammatory events through activation and proliferation of lymphocytes, differentiation of B cells, leukocyte recruitment, and induction of the acute-phase protein response in the liver.[14] IL-6 can be induced by IL-1 and by TNF-α, the latter of which is a soluble receptor primarily produced by monocytes and macrophages and elevated in states of chronic inflammation.[15] CRP is an acute phase reactant, downstream from IL-6, and is a more specific marker of plaque vulnerability and risk of cardiovascular events, with data suggesting it may play a direct role in atherogenesis rather than simply acting as a marker, as previously believed.[16]
Levels of pro-inflammatory cytokines including IL-1β,[17] IL-1 receptor antagonist (IL-1Ra),[5] TNF-α,[15, 17] and IL-6[5, 18], as well as CRP levels,[5, 18] increase with progressively declining renal function. Indeed, in CKD and chronic dialysis, markers of systemic inflammation are notably elevated, including IL-1Ra,[19] IL-1β[17], CRP[3, 4, 18, 20, 21], and IL-6[3, 4, 18]. Albumin and fibrinogen levels, two other acute-phase reactants, are lower and higher in CKD, respectively.[18, 20, 21] Overall level of inflammation appears to be generally lower in chronic peritoneal dialysis compared to hemodialysis patients.[22]
Etiology of Inflammation in CKD and Chronic Dialysis
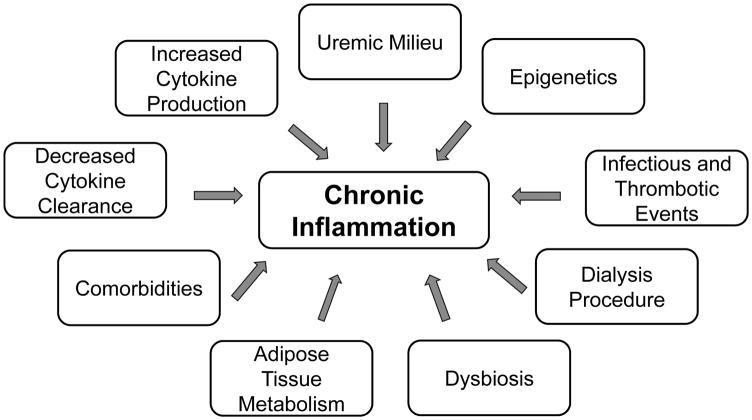
Multiple factors likely contribute to chronic inflammatory activation in kidney disease patients (Figure 1). With declining renal function, both decreased renal clearance, as well as increased production, contribute to higher levels of circulating cytokines.[6, 23] The uremic milieu also promotes oxidative stress[18] and carbonyl stress[24], both of which are highly pro-inflammatory. Epigenetic influences, resulting from the interaction between genetic background and diet, lifestyle, and environment also contribute to increased inflammation.[7] Frequent infectious and thrombotic events provide additional inflammatory stimulations, particularly in dialysis patients, including catheter-related bloodstream infections, access site infections and thrombosed intravenous fistulas and grafts.[25] The microbiological quality of the dialysate and impurities in dialysis water may also contribute to inflammation.[26, 27] Overall, the acute effect of hemodialysis on cytokine levels is controversial, with inconsistent results regarding changes in IL-1 β, TNF- α, IL-6, and interleukin-18.[28–32] It is possible that changes in plasma cytokine levels lag behind stimulation and that other factors may modify concentrations at later time points.[33] In fact, hemodialysis has been shown to acutely up-regulate transcription of pro-inflammatory cytokines.[33]
Figure 1. Factors Contributing to Increased Inflammation in Chronic Dialysis Patients.
Contributing factors include decreased cytokine clearance and increased production, the uremic milieu, epigenetic influences, infectious and thrombotic events, the dialysis procedure, dybiosis, adipose tissue metabolism, and prevalent comorbid conditions.
Dietary factors common in CKD, such as low dietary potassium and phosphorus, can alter the gut microbiome, leading to dysbiosis (pathogen overgrowth in the gut).[34] Metabolic alterations associated with uremia also favor dysbiosis, which promotes translocation of bacterial DNA and endotoxins to the bloodstream via colon wall inflammation and epithelial tight junction barrier breakdown (i.e., “leaky gut”), thus promoting systemic inflammation.[34, 35] Other commonly proposed mechanisms for chronic inflammation include altered adipose tissue metabolism via pro-inflammatory adipokines[36] and a high prevalence of pro-inflammatory comorbidities, such as diabetes and atherosclerotic disease.[7]
Inflammatory Markers are Predictors of Adverse Clinical Outcomes
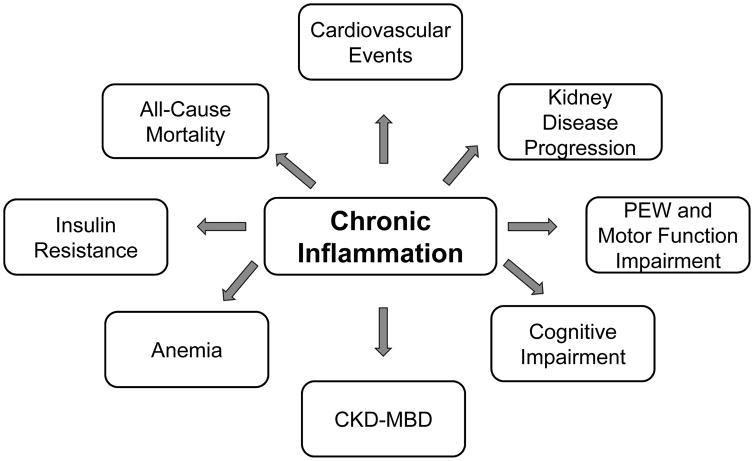
Increased inflammatory markers in CKD and chronic dialysis patients are associated with adverse clinical outcomes including all-cause mortality, cardiovascular events, kidney disease progression, protein energy wasting and diminished motor function, cognitive impairment, as well as other adverse consequences including CKD-mineral and bone disorder (CKD-MBD), anemia, and insulin resistance (Figure 2). We will review the epidemiological, as well as pre-clinical evidence, supporting these associations.
Figure 2. Adverse Clinical Outcomes Associated with Increased Inflammation in Chronic Dialysis.
Adverse clinical outcomes that have been associated with elevated systemic inflammatory markers in chronic kidney disease (CKD) and/or chronic dialysis patients include all-cause mortality, cardiovascular events, kidney disease progression, protein energy wasting (PEW) and motor function impairment, cognitive impairment, CKD-mineral bone disorder (CKD-MBD), anemia, and insulin resistance.
All-Cause Mortality
Elevated CRP[9, 37–40] and IL-6[41–44] have been repeatedly shown to independently predict all-cause mortality in chronic hemodialysis patients. In contrast, an early observational study found that albumin but not CRP was independently associated with mortality in this population.[45] IL-1 and TNF-α have additionally been associated with mortality in chronic hemodialysis patients.[43] Elevated inflammatory markers, including CRP and low albumin, also predict all-cause mortality earlier in CKD, in stage 3–4 patients.[46] Of note, evidence of an association of elevated CRP with all-cause mortality in peritoneal dialysis patients has been inconsistent, however, and may be limited by the relatively small sample sizes of the cohorts examined.[47, 48] Serial measurements of CRP improve prediction of mortality compared to a single value.[40] This is an important consideration, as inflammatory markers are subject to significant variability over time, influenced by factors including the stimulus of dialysis, acute infection, and comorbidities.[49]
Cardiovascular Outcomes
The risk of cardiovascular mortality or a cardiovascular event in CKD and chronic dialysis patients is significantly increased compared to patients without CKD, even in the presence of similar cardiovascular risk factors.[50] Evidence supports the belief that increased systemic inflammation is independently associated with cardiovascular endpoints in kidney disease patients. Indeed, high CRP levels independently predict cardiovascular mortality in chronic hemodialysis patients,[9, 37, 38] as well as patients with stage 3–4 CKD.[46] Increased IL-6 levels also predict cardiovascular mortality in chronic hemodialysis patients.[48] Similarly, elevated CRP is an independent predictor of cardiovascular events in peritoneal dialysis patients. [48]
The association of chronic systemic inflammation with cardiovascular mortality may be mediated by increased left ventricular hypertrophy, as elevated CRP levels independently predict left ventricular mass index in chronic hemodialysis patients.[51] Similarly, in pre-dialysis CKD patients, elevated CRP and IL-6 levels are associated with increased left ventricular mass index or left ventricular hypertrophy[52, 53], as well systolic dysfunction.[52] Systemic inflammation may also promote cardiovascular mortality via atherogenesis. Elevated inflammatory markers are associated with increased risk of coronary events in women with reduced estimated glomerular filtration rate (eGFR)[54], as well as carotid intimal medial thickness in pre-dialysis CKD.[8] Increased CD16+ monocytes, which promote inflammation and atherogenesis, are also independently associated with cardiovascular events in chronic dialysis patients,[55] as well as patients with pre-dialysis CKD.[56] Additionally, chronic inflammation can promote vascular calcification, via interplay with several markers of mineral metabolism.[6]
Pre-clinical studies indicate that pro-inflammatory cytokines, including IL-6 and TNF-α, may have direct atherogenic properties.[57] CRP also promotes monocyte adhesion to endothelial cells, leading to vascular smooth muscle cell proliferation and migration, and atherogenesis.[57] Pro-inflammatory cytokines can additionally promote cardiac remodeling and hypertrophy, as well as stimulate apoptosis in cardiomyocytes.[58, 59] While pro-inflammatory cytokines are not constitutively expressed in the myocardium, they are upregulated in response to myocardial injury which may also contribute to their increased circulating levels.[60]
Kidney Disease Progression
Chronic systemic inflammation also appears to be a key mechanism in kidney disease progression. Elevated inflammatory markers predict both decline in kidney function as well as incident CKD in community-based populations,[61–65] as well as decline in eGFR and progression to ESRD in patients with prevalent CKD.[66, 67] However, in the Modification of Diet in Renal Disease (MDRD) study, CRP was not an independent risk factor for progression of non-diabetic kidney disease, measured as decline in GFR.[68] Inflammatory markers do appear to predict eGFR decline in both microalbuminuric type 1 diabetics,[69] as well as progression to ESRD in type 2 diabetes.[70] Additionally, inflammatory markers associate with residual renal function in patients close to the initiation of dialysis[71] and in peritoneal dialysis patients.[72] The loss of residual renal function in peritoneal dialysis patients is also predicted by increased inflammatory markers.[73]
Mechanistically, pro-inflammatory cytokines may mediate glomerular injury, promoting monocyte and macrophage influx, mesangial cell proliferation, and fibrosis.[74–76] The Nlrp3 inflammasome is also activated during renal injury, and inflammasome-dependent cytokines contribute to kidney disease progression.[13] The activation and subsequent assembly of the inflammasome controls the production of numerous pro-inflammatory cytokines, including IL-1 β and IL-18.[13]
Protein Energy Wasting and Motor Function
Protein energy wasting, a condition characterized by multiple metabolic and nutritional derangements, is present in approximately 20–50% of chronic dialysis patients, and is characterized by loss of lean body mass and decreased visceral protein stores.[77] Cytokine-adipokine signaling plays an important role in protein energy wasting.[78] Protein energy wasting is one of the most important predictors of morbidity and mortality in CKD and ESRD.[78] Elevated inflammatory markers are also associated with reduced physical function, muscle mass, and muscle strength in the general aging population,[79] and impaired physical performance predicts all-cause mortality in patients with CKD.[80]
Physical performance, particularly lower extremity performance, is worse across the spectrum of CKD.[81] In chronic hemodialysis patients, levels of inflammatory markers are inversely related to thigh muscle area, an index of muscle wasting.[82] Additionally, higher baseline IL-1β levels are associated with an accelerated decline in an index of muscle mass over a one year follow-up period in chronic hemodialysis patients.[83] Similarly, higher baseline CRP level is associated with greater loss of lean body mass after one year of peritoneal dialysis.[84]
The association between CKD and reduced physical function may be mediated by the effects of inflammation on musculoskeletal function.[80] These changes include stimulation of protein degradation/muscle catabolism and skeletal muscle wasting,[78, 85] suppressed appetite,[86, 87], enhanced resting energy expenditure,[88] and suppression of anabolic hormones such as growth hormone.[89] Uremia may further impair muscle metabolism by augmenting CKD-associated inflammation, oxidative stress, and insulin resistance.[81, 85] Of note, results of a small, randomized, placebo-controlled trial suggest that inhibiting inflammation via an IL-1 trap may improve endurance in stage 3–4 CKD.[90]
Cognitive Function
Cognitive impairment is also common in kidney disease patients. The prevalence of cognitive impairment in ESRD patients is at least twice that of age-matched adults in the general population, and cognitive impairment is already evident in early stages of CKD.[91] CKD is recognized as an independent risk factor for incident cognitive impairment[92, 93] and cognitive decline.[94, 95] Cognitive impairment is also associated with severity of CKD, with increased impairment on tests including the modified mini-mental state examination and trail making test part B with decreasing kidney function.[96] Importantly, cognitive impairment and dementia are associated with increased risk of death in chronic dialysis patients[97, 98], as well as decreased quality of life.[99]
Elevated systemic markers of inflammation are independently associated with cognitive decline in the general population;[100–102] however, results are not consistent across all studies.[103, 104] Limited available evidence also suggests an association of inflammation with diminished cognitive function in CKD and chronic dialysis patients.[105–107] In chronic hemodialysis patients, IL-6 and TNF-α inversely correlate with the cognitive function subscale of the Kidney Disease Quality of Life questionnaire, suggesting a link between inflammation and cognitive impairment.[105] CRP levels are also independently associated with cognitive performance on the mini-mental state examination before and after a hemodialysis session.[106] Recently, in the Chronic Renal Insufficiency Cohort (CRIC) study, higher CRP, IL-1β, and fibrinogen levels were associated with increased risk of impaired attention during the approximately 6 year follow-up period in individuals with baseline CKD.[107]
Mechanistically, the association of systemic inflammation with cognitive impairment may be mediated by a high prevalence of cardiovascular risk factors known to associate with cognitive impairment, such as diabetes, hypertension, and hypercholesterolemia, as well as depression and medication use.[91] Anemia is an additional risk factor for cognitive impairment that may play a role in this association.[108] The dialysis process may also promote cognitive impairment, due to hemodialysis-related hypotension, microembolization, and cerebral edema.[91] However, acutely, a single dialysis session has been shown to improve neuropsychological testing, including logical and visual memory, psychomotor speed, executive function, and concentration, suggesting a reversible component of cognitive impairment in chronic dialysis patients.[109]
Other Adverse Consequences
Low-grade systemic inflammation has also been shown to associate with other adverse clinical parameters in chronic hemodialysis patients. Numerous epidemiological studies have demonstrated an independent association between inflammation and disturbed markers of mineral metabolism.[110–113] Additionally, in vitro and in vivo studies suggest direct regulation of fibroblast growth factor 23[114–117] and 1,25-dihydroxyvitamin D[118] production by inflammation. However, directly inhibiting inflammation with an IL-1 trap does not change markers of CKD-MBD in patients with stage 3–4 CKD.[90] Inflammation also contributes to anemia and erythropoietin resistance,[119] mediated by decreased erythropoietin production[120], decreased stimulatory activity of erythropoietin [121], and increased production of hepcidin.[122] Inflammation may additionally play a pathogenic role in the development of insulin resistance,[123] as pro-inflammatory cytokines promote decreased skeletal muscle insulin-stimulated glucose uptake.[124]
Strategies to Reduce Chronic Inflammation
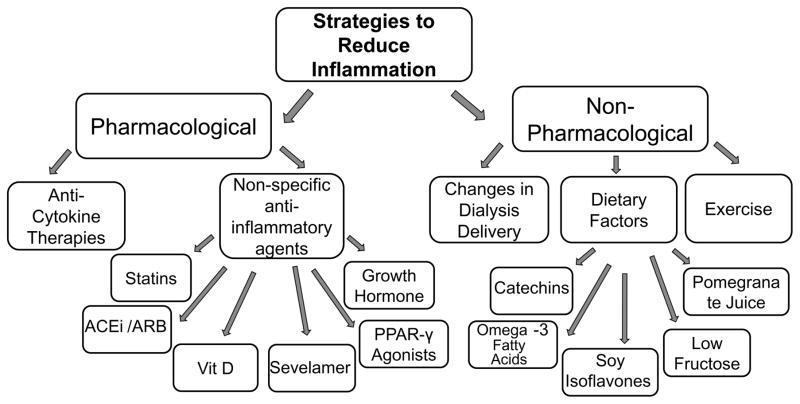
Strategies that have been shown to reduce chronic systemic inflammation in CKD and chronic dialysis patients include both pharmacological and non-pharmacological interventions (Figure 3). We will review briefly clinical trials that have evaluated strategies to reduce systemic inflammation in kidney disease patients.
Figure 3. Pharmacological and Non-Pharmacological Strategies to Reduce Inflammation in Chronic Dialysis.
Strategies shown to reduce systemic inflammatory markers in chronic kidney disease and/or chronic dialysis patients include pharmacological and non-pharmacological strategies. Pharmacological strategies that have been evaluated are specific anti-cytokine therapies, as well as non-specific agents with anti-inflammatory properties, including statins, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), cholecalciferol (vit D), sevelamer, peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, and growth hormone. Non-pharmacological strategies that have been shown to lower systemic inflammatory markers include changes in dialysis delivery, dietary factors (catechins [green tea extract], omega-3 fatty acids, soy isoflavones, low fructose, and pomegranate juice), and exercise.
Pharmacological Strategies
Despite scientific interest in trials targeting inflammation in patients with kidney disease for decades, direct anti-cytokine therapies have only been tested very recently in this population. In 2011, four weeks of IL-1β antagonism with anakinra was shown to be efficacious to reduce systemic inflammation, as evidenced by reduced CRP levels[125], and also improved adiponectin levels,[126] in a small study of chronic hemodialysis patients. More recently, in a randomized, double-blind trial in stage 3–4 CKD patients, twelve weeks of treatment with the IL-1 trap rilonacept also reduced CRP levels as compared to placebo[127]. Notably, IL-1 inhibition also improved brachial artery flow-mediated dilation, an index of vascular endothelial function and independent predictor of incident cardiovascular events and mortality.[128] Furthermore, this improvement was associated with reduced vascular oxidative stress, and the drug was well-tolerated. These studies support the need for future trials evaluating the safety and efficacy of direct anti-cytokine therapies for reducing renal and cardiovascular morbidity and mortality in patients with kidney disease. Of note, phase 1 studies of an IL-6 monoclonal antibody in CKD and chronic dialysis patients with chronic inflammation are also currently underway (NCT03126318 and NCT02868229).
Other trials have demonstrated reduced inflammatory markers in CKD and chronic dialysis patients treated with non-specific therapies with anti-inflammatory properties, including statins,[129] cholecalciferol,[130] ACEi/ARB,[131] sevelamer,[132] and peroxisome proliferator-activated receptor-gamma agonists[133]. Growth hormone supplementation also decreases CRP levels in chronic hemodialysis patients.[134] However, despite multiple observations that systemic inflammatory markers can be lowered in kidney disease patients treated with various pharmacological strategies, evidence that this improves clinical outcomes is largely unavailable. In addition to reducing CRP levels, one year of cholecalciferol treatment reduced left ventricular mass index in chronic hemodialysis patients; however, no placebo group was included in this study.[130] Short-term treatment with sevelamer, a non-calcium-based phosphate binder, improved vascular endothelial function measured by brachial artery flow-mediated dilation, in addition to lowering CRP levels, in stage 4 CKD patients.[130]
Notably, in the very recently completed Canakimumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), which enrolled over 10,000 patients with stable coronary artery disease and elevated CRP levels, the IL-1β inhibitor canakinumab significantly reduced the risk of major cardiovascular events by 15%.[135] However, patients with severe CKD (eGFR<30 ml/min/1.73m2) were excluded from enrollment, thus results are not applicable to patients with advanced CKD or requiring chronic dialysis, who are at high risk for poor cardiovascular outcomes.
Non-Pharmacological Strategies
Several non-pharmacological strategies have also shown efficacy in reducing systemic inflammation in CKD and chronic dialysis patients. These include dietary factors such as catechins (green tea extract),[136] omega-3 fatty acids,[137] soy isoflavones,[138] low dietary fructose,[139] and pomegranate juice.[140] Increased physical activity can also reduce markers of chronic inflammation in patients with CKD.[141] Changes in the delivery of dialysis in chronic hemodialysis patients, including the use of ultrapure dialysate[142] and short daily dialysis[143] also reduce inflammatory markers. However, in general, data supporting the idea that reducing inflammation through non-pharmacological approaches improves clinical endpoints are lacking. One year of pomegranate juice did reduce second hospitalization due to infectious cause as compared to placebo in chronic hemodialysis patients.[140] Additionally, short daily dialysis reduces left ventricular mass index compared to conventional hemodialysis; however, these improvements may be due to decreases in volume and pressure loading, as well as reduced serum phosphorus, rather than reductions in systemic inflammation.[143]
Our Opinion
In our opinion, there is strong evidence that inflammation is high in chronic dialysis patients and that this independently predicts numerous adverse clinical outcomes. However, the evidence for a role of inflammation in affecting outcomes is limited by the fact that most of the available evidence is epidemiological in nature, with some additional support provided by mechanistic animal studies. There is also evidence that various interventions, ranging from specific anti-cytokine therapies to non-pharmacological strategies, can reduce circulating inflammatory markers in CKD and chronic dialysis patients. However, data that reducing systemic inflammation improves clinical outcomes are currently lacking; this area represents an important future research direction. Data from the CANTOS trial suggests that reducing inflammation may indeed reduce adverse outcomes in kidney disease patients, but prospective trials in populations of individuals with reduced eGFR, and particularly chronic dialysis patients, are needed. Given the high morbidity and mortality in these patients, targeting inflammation represents a potentially novel and attractive strategy if it can indeed be shown to improve their adverse outcomes.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K01DK103678.
Footnotes
Disclosures
None.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017;69:A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson LW, Koch KM, Dinarello CA, Shaldon S. Haemodialysis hypotension: the interleukin hypothesis. Blood Purif. 1983;1:3–8. [Google Scholar]
- 3.Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 4.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S49–55. doi: 10.2215/CJN.02720409. [DOI] [PubMed] [Google Scholar]
- 7.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stutz A, Golenbock DT, Latz E. Inflammasomes: too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 13.Vilaysane A, Chun J, Seamone ME, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecoits-Filho R, Lindholm B, Axelsson J, Stenvinkel P. Update on interleukin-6 and its role in chronic renal failure. Nephrol Dial Transplant. 2003;18:1042–1045. doi: 10.1093/ndt/gfg111. [DOI] [PubMed] [Google Scholar]
- 15.Brockhaus M, Bar-Khayim Y, Gurwicz S, Frensdorff A, Haran N. Plasma tumor necrosis factor soluble receptors in chronic renal failure. Kidney Int. 1992;42:663–667. doi: 10.1038/ki.1992.332. [DOI] [PubMed] [Google Scholar]
- 16.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 17.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–892. [PubMed] [Google Scholar]
- 18.Panichi V, Migliori M, De Pietro S, et al. C-reactive protein and interleukin-6 levels are related to renal function in predialytic chronic renal failure. Nephron. 2002;91:594–600. doi: 10.1159/000065018. [DOI] [PubMed] [Google Scholar]
- 19.Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1 beta, TNF alpha and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 1994;45:890–896. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 20.Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Haubitz M, Brunkhorst R, Wrenger E, Froese P, Schulze M, Koch KM. Chronic induction of C-reactive protein by hemodialysis, but not by peritoneal dialysis therapy. Perit Dial Int. 1996;16:158–162. [PubMed] [Google Scholar]
- 23.Rosengren BI, Sagstad SJ, Karlsen TV, Wiig H. Isolation of interstitial fluid and demonstration of local proinflammatory cytokine production and increased absorptive gradient in chronic peritoneal dialysis. Am J Physiol Renal Physiol. 2013;304:F198–206. doi: 10.1152/ajprenal.00293.2012. [DOI] [PubMed] [Google Scholar]
- 24.Aveles PR, Criminacio CR, Goncalves S, et al. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin Pract. 2010;116:c294–299. doi: 10.1159/000318792. [DOI] [PubMed] [Google Scholar]
- 25.Nassar GM. Preventing and treating inflammation: role of dialysis access management. Semin Dial. 2013;26:28–30. doi: 10.1111/sdi.12023. [DOI] [PubMed] [Google Scholar]
- 26.Santoro A, Mancini E. Is hemodiafiltration the technical solution to chronic inflammation affecting hemodialysis patients? Kidney Int. 2014;86:235–237. doi: 10.1038/ki.2014.81. [DOI] [PubMed] [Google Scholar]
- 27.Panichi V, Paoletti S, Consani C. Inflammatory pattern in hemodiafiltration. Contrib Nephrol. 2008;161:185–190. doi: 10.1159/000130676. [DOI] [PubMed] [Google Scholar]
- 28.Grooteman MP, Nube MJ, Daha MR, et al. Cytokine profiles during clinical high-flux dialysis: no evidence for cytokine generation by circulating monocytes. J Am Soc Nephrol. 1997;8:1745–1754. doi: 10.1681/ASN.V8111745. [DOI] [PubMed] [Google Scholar]
- 29.Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39:954–960. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 30.Raj DS, Shah H, Shah VO, et al. Markers of inflammation, proteolysis, and apoptosis in ESRD. Am J Kidney Dis. 2003;42:1212–1220. doi: 10.1053/j.ajkd.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Tarakcioglu M, Erbagci AB, Usalan C, Deveci R, Kocabas R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediators Inflamm. 2003;12:15–19. doi: 10.1080/0962935031000096935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzanatos HA, Agroyannis B, Chondros C, et al. Cytokine release and serum lipoprotein (a) alterations during hemodialysis. Artif Organs. 2000;24:329–333. doi: 10.1046/j.1525-1594.2000.06483.x. [DOI] [PubMed] [Google Scholar]
- 33.Friedrich B, Alexander D, Janessa A, Haring HU, Lang F, Risler T. Acute effects of hemodialysis on cytokine transcription profiles: evidence for C-reactive protein-dependency of mediator induction. Kidney Int. 2006;70:2124–2130. doi: 10.1038/sj.ki.5001865. [DOI] [PubMed] [Google Scholar]
- 34.Lau WL, Kalantar-Zadeh K, Vaziri ND. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron. 2015;130:92–98. doi: 10.1159/000381990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 36.Iglesias P, Diez JJ. Adipose tissue in renal disease: clinical significance and prognostic implications. Nephrol Dial Transplant. 2010;25:2066–2077. doi: 10.1093/ndt/gfq246. [DOI] [PubMed] [Google Scholar]
- 37.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 38.Wanner C, Zimmermann J, Schwedler S, Metzger T. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002:99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 39.deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 40.Snaedal S, Heimburger O, Qureshi AR, et al. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis. 2009;53:1024–1033. doi: 10.1053/j.ajkd.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Rao M, Guo D, Perianayagam MC, et al. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2005;45:324–333. doi: 10.1053/j.ajkd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 43.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beberashvili I, Sinuani I, Azar A, et al. IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2253–2263. doi: 10.2215/CJN.01770211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998;54:627–636. doi: 10.1046/j.1523-1755.1998.00032.x. [DOI] [PubMed] [Google Scholar]
- 46.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 47.Noh H, Lee SW, Kang SW, et al. Serum C-reactive protein: a predictor of mortality in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1998;18:387–394. [PubMed] [Google Scholar]
- 48.Herzig KA, Purdie DM, Chang W, et al. Is C-reactive protein a useful predictor of outcome in peritoneal dialysis patients? J Am Soc Nephrol. 2001;12:814–821. doi: 10.1681/ASN.V124814. [DOI] [PubMed] [Google Scholar]
- 49.Meuwese CL, Stenvinkel P, Dekker FW, Carrero JJ. Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat Rev Nephrol. 2011;7:166–176. doi: 10.1038/nrneph.2011.2. [DOI] [PubMed] [Google Scholar]
- 50.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 51.Monfared A, Salari A, Kazemnezhad E, et al. Association of left ventricular hypertrophy with high-sensitive C-reactive protein in hemodialysis patients. Int Urol Nephrol. 2013;45:1679–1686. doi: 10.1007/s11255-012-0375-x. [DOI] [PubMed] [Google Scholar]
- 52.Gupta J, Dominic EA, Fink JC, et al. Association between Inflammation and Cardiac Geometry in Chronic Kidney Disease: Findings from the CRIC Study. PLoS One. 2015;10:e0124772. doi: 10.1371/journal.pone.0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ioannou K, Stel VS, Dounousi E, et al. Inflammation, Endothelial Dysfunction and Increased Left Ventricular Mass in Chronic Kidney Disease (CKD) Patients: A Longitudinal Study. PLoS One. 2015;10:e0138461. doi: 10.1371/journal.pone.0138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight EL, Rimm EB, Pai JK, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. J Am Soc Nephrol. 2004;15:1897–1903. doi: 10.1097/01.asn.0000128966.55133.69. [DOI] [PubMed] [Google Scholar]
- 55.Heine GH, Ulrich C, Seibert E, et al. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 56.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 57.Wang AY. Consequences of chronic inflammation in peritoneal dialysis. Semin Nephrol. 2011;31:159–171. doi: 10.1016/j.semnephrol.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation. 1997;95:1247–1252. doi: 10.1161/01.cir.95.5.1247. [DOI] [PubMed] [Google Scholar]
- 59.Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart Fail Rev. 2001;6:95–103. doi: 10.1023/a:1011401825680. [DOI] [PubMed] [Google Scholar]
- 60.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 61.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 62.Bash LD, Erlinger TP, Coresh J, Marsh-Manzi J, Folsom AR, Astor BC. Inflammation, hemostasis, and the risk of kidney function decline in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2009;53:596–605. doi: 10.1053/j.ajkd.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 65.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60:225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 67.Amdur RL, Feldman HI, Gupta J, et al. Inflammation and Progression of CKD: The CRIC Study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarnak MJ, Poindexter A, Wang SR, et al. Serum C-reactive protein and leptin as predictors of kidney disease progression in the Modification of Diet in Renal Disease Study. Kidney Int. 2002;62:2208–2215. doi: 10.1046/j.1523-1755.2002.00677.x. [DOI] [PubMed] [Google Scholar]
- 69.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19:789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 72.Stompor T, Zdzienicka A, Motyka M, Dembinska-Kiec A, Davies SJ, Sulowicz W. Selected growth factors in peritoneal dialysis: their relationship to markers of inflammation, dialysis adequacy, residual renal function, and peritoneal membrane transport. Perit Dial Int. 2002;22:670–676. [PubMed] [Google Scholar]
- 73.Palomo-Pinon S, Mora-Villalpando CJ, Del Carmen Prado-Uribe M, et al. Inflammation and myocardial damage markers influence loss of residual renal function in peritoneal dialysis patients. Arch Med Res. 2014;45:484–488. doi: 10.1016/j.arcmed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Karkar AM, Smith J, Pusey CD. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant. 2001;16:518–524. doi: 10.1093/ndt/16.3.518. [DOI] [PubMed] [Google Scholar]
- 75.Tipping PG, Leong TW, Holdsworth SR. Tumor necrosis factor production by glomerular macrophages in anti-glomerular basement membrane glomerulonephritis in rabbits. Lab Invest. 1991;65:272–279. [PubMed] [Google Scholar]
- 76.Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010:S22–26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pupim LB, Cuppari L, Ikizler TA. Nutrition and metabolism in kidney disease. Semin Nephrol. 2006;26:134–157. doi: 10.1016/j.semnephrol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Raj DS, Sun Y, Tzamaloukas AH. Hypercatabolism in dialysis patients. Curr Opin Nephrol Hypertens. 2008;17:589–594. doi: 10.1097/MNH.0b013e32830d5bfa. [DOI] [PubMed] [Google Scholar]
- 79.Nicklas BJ, Brinkley TE. Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev. 2009;37:165–170. doi: 10.1097/JES.0b013e3181b7b3d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between Physical Performance and All-Cause Mortality in CKD. J Am Soc Nephrol. 2013;24:822–830. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiner DE, Seliger SL. Cognitive and physical function in chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23:291–297. doi: 10.1097/01.mnh.0000444821.87873.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaizu Y, Ohkawa S, Odamaki M, et al. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis. 2003;42:295–302. doi: 10.1016/s0272-6386(03)00654-1. [DOI] [PubMed] [Google Scholar]
- 83.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 84.Stenvinkel P, Lindholm B, Lonnqvist F, Katzarski K, Heimburger O. Increases in serum leptin levels during peritoneal dialysis are associated with inflammation and a decrease in lean body mass. J Am Soc Nephrol. 2000;11:1303–1309. doi: 10.1681/ASN.V1171303. [DOI] [PubMed] [Google Scholar]
- 85.da Costa JA, Ikizler TA. Inflammation and insulin resistance as novel mechanisms of wasting in chronic dialysis patients. Semin Dial. 2009;22:652–657. doi: 10.1111/j.1525-139X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 86.Aguilera A, Codoceo R, Selgas R, et al. Anorexigen (TNF-alpha, cholecystokinin) and orexigen (neuropeptide Y) plasma levels in peritoneal dialysis (PD) patients: their relationship with nutritional parameters. Nephrol Dial Transplant. 1998;13:1476–1483. doi: 10.1093/ndt/13.6.1476. [DOI] [PubMed] [Google Scholar]
- 87.Mak RH, Cheung W, Cone RD, Marks DL. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int. 2006;69:794–797. doi: 10.1038/sj.ki.5000182. [DOI] [PubMed] [Google Scholar]
- 88.Utaka S, Avesani CM, Draibe SA, Kamimura MA, Andreoni S, Cuppari L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am J Clin Nutr. 2005;82:801–805. doi: 10.1093/ajcn/82.4.801. [DOI] [PubMed] [Google Scholar]
- 89.Meuwese CL, Carrero JJ, Stenvinkel P. Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol. 2011;171:120–126. doi: 10.1159/000327228. [DOI] [PubMed] [Google Scholar]
- 90.Nowak KL, Hung A, Ikizler TA, et al. Interleukin-1 inhibition, chronic kidney disease-mineral and bone disorder, and physical function. Clin Nephrol. 2017;88:132–143. doi: 10.5414/CN109122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial. 2008;21:29–37. doi: 10.1111/j.1525-139X.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 92.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 93.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 94.Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol. 2009;20:2427–2432. doi: 10.1681/ASN.2008101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc. 2008;56:2082–2088. doi: 10.1111/j.1532-5415.2008.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 97.Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–2548. doi: 10.1093/ndt/gfl275. [DOI] [PubMed] [Google Scholar]
- 98.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56:693–703. doi: 10.1053/j.ajkd.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Gokal R. Quality of life in patients undergoing renal replacement therapy. Kidney Int Suppl. 1993;40:S23–27. [PubMed] [Google Scholar]
- 100.Schram MT, Euser SM, de Craen AJ, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 101.Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 102.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 103.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 104.Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol A Biol Sci Med Sci. 2008;63:50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montinaro V, Iaffaldano GP, Granata S, et al. Emotional symptoms, quality of life and cytokine profile in hemodialysis patients. Clin Nephrol. 2010;73:36–43. doi: 10.5414/cnp73036. [DOI] [PubMed] [Google Scholar]
- 106.Kaltsatou A, Kouidi E, Kimiskidis VK, et al. The impact of inflammation and autonomic nervous system activity on cognitive impairment during a hemodialysis session. J Clin Exp Nephrol. 2016;1:14–22. [Google Scholar]
- 107.Kurella Tamura M, Tam K, Vittinghoff E, et al. Inflammatory Markers and Risk for Cognitive Decline in Chronic Kidney Disease: The CRIC Study. Kidney Int Rep. 2017;2:192–200. doi: 10.1016/j.ekir.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480–486. doi: 10.1038/ki.1990.229. [DOI] [PubMed] [Google Scholar]
- 109.Schneider SM, Malecki AK, Muller K, et al. Effect of a single dialysis session on cognitive function in CKD5D patients: a prospective clinical study. Nephrol Dial Transplant. 2015;30:1551–1559. doi: 10.1093/ndt/gfv213. [DOI] [PubMed] [Google Scholar]
- 110.Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. doi: 10.2215/CJN.13281211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manghat P, Fraser WD, Wierzbicki AS, Fogelman I, Goldsmith DJ, Hampson G. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos Int. 2010;21:1853–1861. doi: 10.1007/s00198-009-1142-4. [DOI] [PubMed] [Google Scholar]
- 112.Navarro-Gonzalez JF, Mora-Fernandez C, Muros M, Herrera H, Garcia J. Mineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional study. Clin J Am Soc Nephrol. 2009;4:1646–1654. doi: 10.2215/CJN.02420409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutierrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10:e0122885. doi: 10.1371/journal.pone.0122885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.David V, Martin A, Isakova T, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89:135–146. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Francis C, David V. Inflammation regulates fibroblast growth factor 23 production. Curr Opin Nephrol Hypertens. 2016;25:325–332. doi: 10.1097/MNH.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ito N, Wijenayaka AR, Prideaux M, et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–218. doi: 10.1016/j.mce.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 117.David V, Francis C, Babitt JL. Ironing out the crosstalk between FGF23 and inflammation. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00359.2016. ajprenal 00359 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ebert R, Jovanovic M, Ulmer M, et al. Down-regulation by nuclear factor kappaB of human 25-hydroxyvitamin D3 1alpha-hydroxylase promoter. Mol Endocrinol. 2004;18:2440–2450. doi: 10.1210/me.2002-0441. [DOI] [PubMed] [Google Scholar]
- 119.Kovesdy CP. How can erythropoeitin-stimulating agent use be reduced in chronic dialysis patients?: Can reduction of inflammation improve ESA dose response? Semin Dial. 2013;26:540–542. doi: 10.1111/sdi.12107. [DOI] [PubMed] [Google Scholar]
- 120.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555–559. doi: 10.1089/jir.1998.18.555. [DOI] [PubMed] [Google Scholar]
- 121.Wagner M, Alam A, Zimmermann J, et al. Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:1573–1579. doi: 10.2215/CJN.00380111. [DOI] [PubMed] [Google Scholar]
- 122.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55:726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 124.Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R. Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes. 2001;50:1102–1109. doi: 10.2337/diabetes.50.5.1102. [DOI] [PubMed] [Google Scholar]
- 125.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA. IL-1beta receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol. 2011;22:437–442. doi: 10.1681/ASN.2010070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hung AM, Limkunakul C, Placido JS, et al. Administration of IL-1ra improves adiponectin levels in chronic hemodialysis patients. J Nephrol. 2014;27:681–688. doi: 10.1007/s40620-014-0070-3. [DOI] [PubMed] [Google Scholar]
- 127.Nowak KL, Chonchol M, Ikizler TA, et al. IL-1 Inhibition and Vascular Function in CKD. J Am Soc Nephrol. 2017;28:971–980. doi: 10.1681/ASN.2016040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 129.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 130.Matias PJ, Jorge C, Ferreira C, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–911. doi: 10.2215/CJN.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Vinuesa SG, Goicoechea M, Kanter J, et al. Insulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: effects of angiotensin II blockade. J Am Soc Nephrol. 2006;17:S206–212. doi: 10.1681/ASN.2006080916. [DOI] [PubMed] [Google Scholar]
- 132.Caglar K, Yilmaz MI, Saglam M, et al. Short-term treatment with sevelamer increases serum fetuin-a concentration and improves endothelial dysfunction in chronic kidney disease stage 4 patients. Clin J Am Soc Nephrol. 2008;3:61–68. doi: 10.2215/CJN.02810707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wong TY, Szeto CC, Chow KM, Leung CB, Lam CW, Li PK. Rosiglitazone reduces insulin requirement and C-reactive protein levels in type 2 diabetic patients receiving peritoneal dialysis. Am J Kidney Dis. 2005;46:713–719. doi: 10.1053/j.ajkd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 134.Kopple JD, Cheung AK, Christiansen JS, et al. OPPORTUNITY™: a large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol Dial Transplant. 2011;26:4095–4103. doi: 10.1093/ndt/gfr363. [DOI] [PubMed] [Google Scholar]
- 135.Ridke PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic disease. New Engl J Med. 2017 doi: 10.1056/NEJMoa1707914. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 136.Hsu SP, Wu MS, Yang CC, et al. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines. Am J Clin Nutr. 2007;86:1539–1547. doi: 10.1093/ajcn/86.5.1539. [DOI] [PubMed] [Google Scholar]
- 137.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr. 2007;17:296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 138.Fanti P, Asmis R, Stephenson TJ, Sawaya BP, Franke AA. Positive effect of dietary soy in ESRD patients with systemic inflammation--correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]
- 139.Brymora A, Flisinski M, Johnson RJ, Goszka G, Stefanska A, Manitius J. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol Dial Transplant. 2012;27:608–612. doi: 10.1093/ndt/gfr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shema-Didi L, Sela S, Ore L, et al. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med. 2012;53:297–304. doi: 10.1016/j.freeradbiomed.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 141.Viana JL, Kosmadakis GC, Watson EL, et al. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol. 2014;25:2121–2130. doi: 10.1681/ASN.2013070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Susantitaphong P, Riella C, Jaber BL. Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: a meta-analysis. Nephrol Dial Transplant. 2013;28:438–446. doi: 10.1093/ndt/gfs514. [DOI] [PubMed] [Google Scholar]
- 143.Ayus JC, Mizani MR, Achinger SG, Thadhani R, Go AS, Lee S. Effects of short daily versus conventional hemodialysis on left ventricular hypertrophy and inflammatory markers: a prospective, controlled study. J Am Soc Nephrol. 2005;16:2778–2788. doi: 10.1681/ASN.2005040392. [DOI] [PubMed] [Google Scholar]