Abstract
Advances in imaging technologies have allowed for the analysis of fMRI data in real-time (rtfMRI), leading to the development of neurofeedback (nf) training. This rtfMRI-nf training utilizes fMRI tomographic localization capacity to allow a person to see and regulate the localized hemodynamic signal from his or her own brain. In this review, we summarize the results of several studies that have developed and applied neurofeedback training to healthy and depressed individuals with the amygdala as the neurofeedback target and the goal to increase the hemodynamic response during positive autobiographical memory recall. We review these studies and highlight some of the challenges and advances in developing a rtfMRI-nf paradigm for broader use in psychiatric populations. The work described focuses on our line of research aiming to develop the rtfMRI-nf into an intervention, and includes a discussion of the selection of a region-of-interest for feedback, selecting a control condition, behavioral and cognitive effects of training, and predicting which participants are most likely to respond well to training. While the results of these studies are encouraging and suggest the clinical potential of amygdala rtfMRI-nf in alleviating symptoms of major depressive disorder, larger studies are warranted to confirm its efficacy.
Keywords: amygdala, autobiographical memory, emotional processing, fMRI neurofeedback, major depressive disorder
Introduction
Development of rtfMRI Neurofeedback
Real-time functional magnetic resonance imaging (rtfMRI), in which blood oxygen-level dependent (BOLD) data processing and display are performed concomitant with image acquisition(1), has enabled rtfMRI neurofeedback (rtfMRI-nf), allowing a person to see and regulate the fMRI signal from their own brain(2). rtfMRI-nf precisely localizes neurophysiological activation, allowing focal investigation of relationships between cognitive-behavioral functions and neuroplasticity changes in deep brain structures(3, 4). Recent research suggests that by using rtfMRI-nf, healthy individuals can learn to control neurophysiological activity in a variety of regions including somatomotor cortex(3), auditory cortex(5), and inferior frontal gyrus(6). Participants can learn to self-regulate brain activity with rtfMRI-nf in structures relevant to emotional processing including insula, amygdala, and ventrolateral prefrontal cortex using negative memories and imagery(7), anterior insula using positive and negative imagery(8), anterior cingulate cortex by focusing on or away from painful stimuli(9), and amygdala using negative imagery(10) or positive autobiographical memories (AMs)(11). Although these studies used healthy populations, emerging evidence suggests rtfMRI-nf has clinical utility in reducing symptoms associated with chronic pain(9), smoking cessation(12), anxiety(13), and major depressive disorder (MDD)(14, 15).
Rationale for the use of rtfMRI for Major Depressive Disorder
Major Depressive Disorder (MDD) is a chronic, disabling, and devastating condition. It is the leading cause of disability worldwide, and the US economic burden is estimated at $60 billion(16). Up to two-thirds of patients who seek standard interventions will not respond, while only one-half of patients who do will achieve sustained remission(17). The most commonly implemented psychological MDD treatment, cognitive-behavioral therapy (CBT), is most effective for mildly-to-moderately depressed patients(18), and often not effective in severely ill patients(19). Invasive treatments for patients who do not respond to initial treatments, such as electroconvulsive therapy, vagus nerve stimulation, and deep brain stimulation, pose significant risks to patients(20, 21). Novel and promising antidepressant effects seen with ketamine in treatment resistant individuals, can also cause hallucinations, high blood pressure, confusion and fear in the short term, and kidney and bladder dysfunction with chronic use(22). Therefore, there is a pressing need to research and develop novel non-invasive interventions for MDD.
Selection of the amygdala as a neurofeedback target
The choice of the amygdala is significant because of its critical role in emotional processing and responding. Interactions with a variety of cortical and subcortical areas, which together integrate and evaluate the salience of interoceptive and exteroceptive sensory stimuli, modify the response of the amygdala to specific stimuli or contexts(23). This network assigns salience to stimuli, coordinates adaptive behavioral responses(24), and modulates cognitive processing such as perception, attention, memory, and decision making(25–29).
While much attention has been focused on the amygdala’s role in processing and responding to negative/fearful emotional stimuli(25), evidence suggests the amygdala more generally influences the perceived salience of stimuli and events(29), and that amygdala BOLD activity increases to both positively and negatively valenced emotional stimuli(26, 28, 30–33). The amygdala has neuroanatomical connections with regions governing affective memory and emotion regulation, including the hippocampus, anterior cingulate, and orbitofrontal cortices(34, 35), and has reciprocal functional connectivity with these regions during tasks involved in emotion regulation(36) and emotional memory recall(37). Quantitative meta-analyses support a functional dissociation between left and right amygdala such that right amygdala is engaged in rapid and automatic detection of emotional stimuli, while left amygdala is involved in detailed and elaborate stimulus evaluation(26, 38).
In addition to its involvement in emotional processing and responding, our selection of the amygdala as a target for rtfMRI-nf also comes form the amygdala’s central role in neurobiological models of MDD, based on evidence from preclinical studies in experimental animals and neuroimaging studies in MDD patients(39). MDD-associated abnormalities are “doubly dissociated” from healthy individuals by virtue of their greater left amygdala response to negative stimuli and attenuated response to positive stimuli(33, 40), including positive AMs(41). Healthy participants showed increased left amygdala activity during positive AM recall that is not present in MDD participants, while MDD participants showed increased left amygdala activity during recall of negative AMs that is not evident in healthy participants(41). These results indicate the existence of a normative positive memory/processing bias in healthy populations that is absent in MDD patients. The emotional processing bias in depressed individuals is manifest in memory, and includes deficits in the recall of emotionally positive autobiographical memories(41–42). Low amygdala reactivity to positive stimuli could indicate less engagement during encoding of positively valenced stimuli or reduced recruitment of attentional resources that can bring emotional stimuli to conscious awareness(43), suggesting neurocognitive techniques increasing positive processing conceivably hold therapeutic potential in the clinical management of MDD.
Evidence also supports a role of the amygdala in recovery from MDD. Amygdala responsiveness to positive stimuli is correlated inversely with depression severity(40, 41). In patients remitted from MDD, amygdala activity during positive AM recall is indistinguishable from controls(41). Longitudinal designs show that following successful SSRI treatment, hyperactivation of the amygdala to negative faces decreased while activation of the amygdala to positive faces increased in a task in which faces are presented below conscious awareness(33). Increased left amygdala activity to the presentation of positive words and decreased activity in response to negative words was also seen following Cognitive Control Therapy(44). These findings suggest that decreased activation of the amygdala to positive stimuli is clinically significant and that some antidepressant drugs/cognitive therapies exert their therapeutic effect by normalizing emotional processing.
The evidence supporting a role of the amygdala in onset and recovery from MDD, taken together with evidence that the amygdala links the domains of affective experience/response and emotional memory recall, suggests that the enhancement of amygdala processing of positive stimuli via rtfMRI-nf holds therapeutic potential for depressed individuals and further justifies our selection of this region as our rtfMRI-nf target. While decreasing the amygdala response during negative AM recall may also be clinically effective (with preliminary reports focusing on amygdala hyperactivity to negative stimuli (e.g., (45–47)), our previous findings of associations between symptoms and amygdala responses to positive but not negative AMs(41, 48), led us to focus specifically on the processing of positive AMs.
Designing the Amygdala rtfMRI-nf Paradigm
Selection of a Control Task
The selection of a comparison/control intervention for rtfMRI-nf experiments is a challenge, and no consensus has been reached as to the optimal approach. Studies utilizing out of scanner control conditions(14), control conditions in which the neurofeedback bar remains static(49), or no control condition(50, 51), run the substantial risk of false positives as control participants know they are not receiving feedback, and experimenter blinding is impossible. Furthermore, previous work has found that simply instructing depressed patients to recall positive AMs actually worsens mood in MDD individuals(52), and would therefore be unethical for us to employ. Sham neurofeedback, where the participant sees BOLD activity from another participant, runs the substantial risk of participants detecting the non-contingency between their efforts and the resulting signal, allowing for easy identification of group assignment with continuous feedback. Control conditions using neurofeedback from a different region are best suited to determine a) specificity of the procedure; whether feedback from the target region is necessary for enhanced control of that region and b) whether changes in mood ratings are due to feedback from the target region or due to a placebo effect.
We employed a control condition in which subjects received rtfMRI-nf from the horizontal segment of the intraparietal sulcus, a region primarily implicated in number processing(53–56). While studies have reported increased intraparietal activity (more generally, not restricted to the horizontal segment) during emotional distancing(57), and viewing images of sexually arousing stimuli(58), potentially suggesting some involvement in emotional processing, the control condition utilized in these studies involved comparing BOLD responses to emotional versus neutral pictures(59). Therefore, a likely alternative explanation is that more attentional processes are engaged during the presentation of emotional relative to neutral pictures resulting in the increased activation. Therefore, while our control region may not be completely independent of emotional regulation, its general involvement in attentional processes does not suggest parietal activity is antagonistic to positive emotional regulation. Indeed, as participants are able to increase their parietal response during positive AM recall without significant clinical or memory effects, the use of this control condition supports the conclusion that it is gaining control over the amygdala (and related regions) that drives the clinical effects, and not simply gaining control over BOLD activity in general.
Defining the Amygdala
The amygdala region of interest was defined anatomically as a 7mm sphere in Talairach space(60) (coordinates: −21,−5,−16). ROIs can be defined anatomically using a brain atlas or anatomical landmarks, or functionally using a functional localizer scan. As individuals with MDD do not activate their amygdala to positive stimuli as healthy individuals do(41), a functional localizer scan based on positive AM recall would not have identified amygdala activity. Arguably, we could have used a different paradigm to activate the amygdala in MDD participants, such as presenting fearful stimuli. However, as emerging evidence suggests that there are different subdivisions within the amygdala that are differentially responsive to aversive and appetitive stimuli(61, 62), using fearful stimuli to localize the amygdala could have resulted in targeting the subregions uninvolved in the response to social and appetitive stimuli. Gaining control of these subregions may have been difficult to accomplish with positive AM recall and likely would not have resulted in any beneficial effects to patients.
Other studies examining the therapeutic potential of neurofeedback in MDD have used localizer scans, identified the most commonly activated voxels while processing positive stimuli, and trained patients to increase activation in these regions even more(14). The theory behind this compensatory approach is to train what is already being used to take over for impaired or lost function. While this trains patients to use the brain regions what they are already using more effectively, it is unknown if these processes are indeed compensatory or contribute to the pathology of the disorder and the difficulty MDD patients have in processing positive stimuli. We focus instead on the normalization/deficit approach, with the goal to train what is functioning abnormally to function normally.
Providing Instructions
There is a debate in the field as to whether or not to provide participants with suggested strategies for regulating the target ROI. Not providing instruction allows participants to explore different strategies and find what works for them. However, this increases the time required for learning to occur, and dysfunctional strategies may be implemented. For example, following insula neurofeedback training in patients with schizophrenia, there was enhanced recognition of disgust expressions(51). As the amygdala is involved in processing and responding to both negative and positive stimuli, participants could theoretically increase their amygdala activity while feeling fear and anxiety, which would not be a desirable outcome. For this reason, we chose to instruct participants to recall positive AMs while attempting to increase BOLD activity in the ROI.
Procedure
Under double blind conditions, participants were randomly assigned to receive rtfMRI-nf from one of two regions-of-interest defined as 7mm spheres in Talairach space(60): the left amygdala(−21,−5,−16) or the left horizontal segment of the intraparietal sulcus(−42,−48,48), (Figure 1a). Participants were informed that they would receive neurofeedback from one of two brain regions; one involved in emotional processing or another independent of emotional processing which may be difficult to regulate. They were asked to maintain the strategy of positive memory recall even if they felt it was ineffective at raising their brain activity, though they could change the positive memories utilized or the aspects of the memories focused on.
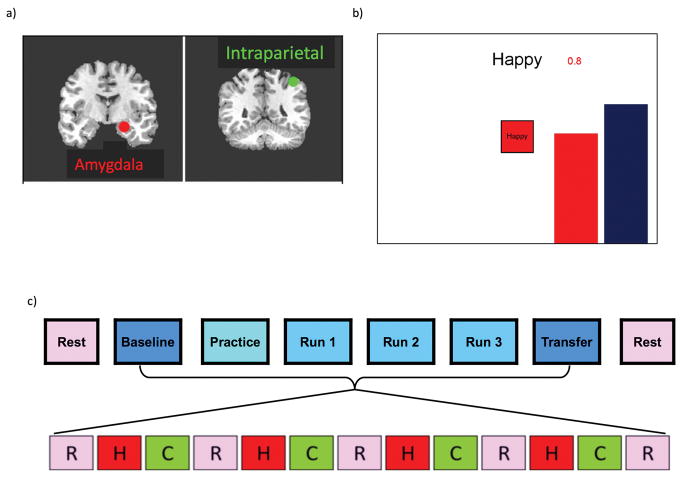
Figure 1. Design of the rtfMRI neurofeedback experiment.
a) Regions of Interest for the rtfMRI neurofeedback procedure: left amygdala (red, centered at −21, −5, −16) and left horizontal segment of the intraparietal sulcus (green, centered at −42, −48, 48). Placements are illustrated on T1-weighted coronal human brain sections in Talairach space. Following radiological notation, the left side (L) of the brain is shown on the right, and the right side (R) of the brain on the left. b) Real-time display screen for the rtfMRI neurofeedback procedure. During the Happy condition, the word “Happy,” two color bars, and a number indicating the neurofeedback signal were displayed on the screen. Participants were instructed to recall happy autobiographical memories to make themselves feel happy while trying to increase the level of the red bar representing the feedback signal from the target region to a given target level indicated by the fixed height of the blue bar. c) Protocol for the rtfMRI neurofeedback experiment. The experimental protocol consisted of eight runs each lasting 8min 40sec. Neurofeedback training consisted of alternating blocs of Rest (R, pink block), Happy (H, red block), and Count (C, green block, instructed to count backwards from 300 by a given integer), each lasting 40sec.
Participants were instructed to retrieve positive autobiographical memories while attempting to increase the hemodynamic activity in the assigned region (presented as a red bar) to that of a blue bar representing the target level of activation. Each neurofeedback run consisted of alternating 40s blocks of: Rest, Happy Memories(upregulate condition; red bar shown), and Count(backwards from 300 by a given one-digit integer). For each of the Rest, Happy, and Count blocks within a run, cues were presented on the screen using both text and color icons to indicate each condition. During the Happy Memory Condition (Figure 1b), the cue “Happy” and two color bars (red, blue) were displayed on the screen. The red bar representing the neurofeedback signal was updated continuously by changing the height of the bar either upwards or downward based on the corresponding level of BOLD activity. This neurofeedback signal was also indicated by a number shown above the red bar representing the percent signal change within the target region. During this condition, participants were instructed to retrieve and contemplate positive autobiographical memories while also attempting to increase the level of the red bar to the fixed blue target bar. Because the Happy Memories condition required memory recall and rumination on those memories could potentially not be stopped quickly(63), two control conditions were implemented to distract participants’ attention from contemplating positive memories and to dampen the activation of the emotion regulation network. During the Count condition, participants saw the cue “Count” with the specific instruction to count backwards from 300 by subtracting a specified integer (9, 3, 4, 6, 7, and 9 for Baseline, Practice, Run 1, Run 2, Run 3, and the Transfer run, respectively). During the Rest condition, participants were presented with the cue “Rest” and were asked to relax and breathe regularly while looking at the display screen. No bars were displayed during the Count and Rest conditions.
The neurofeedback signal for each Happy Memory condition was computed as the fMRI percent signal change relative to the average fMRI signal for the preceding Rest block, updated every 2sec and displayed as a red bar. To reduce bar fluctuations due to noise in the fMRI signal, the bar height was computed at every time point as a moving average of the current and two preceding fMRI percent signal change values. These percent signal change values were averaged over each run and used as a performance measure.
The rtfMRI-nf procedure consisted of eight fMRI runs each lasting 8 minutes and 40 seconds (Figure 1c); a resting run, a baseline run in which no neurofeedback information was provided, a practice run, three training runs, a final transfer run in which no neurofeedback information was provided, and a final Rest run. During the Rest runs, a resting-state paradigm was employed and participants were instructed to clear their minds and not think of anything in particular while fixating on the display screen. All subsequent runs consisted of alternating blocks of Rest (5 blocks lasting 40 seconds each), Count (4 blocks lasting 40 seconds each), and Happy (4 blocks lasting 40 seconds each). The Baseline run served as a measure of amygdala activity during positive memory recall prior to rtfMRI-nf training. Participants were instructed simply to recall positive memories when the cue “Happy” appeared. No bars were presented. It is important to note that this baseline run was not part of our initial study design(11), but was added to our later studies(15, 64) in order to a) confirm the participant did indeed have blunted amygdala activity during positive AM recall and b) to provide a more valid metric of neurofeedback success – defined as the change in amygdala activity from baseline to the final transfer run.
During the Practice run, participants were given an opportunity to become comfortable with the neurofeedback procedure. For the first three Happy Memory blocks participants were instructed to recall and contemplate positive memories prepared with help from the experimenter prior to entering the fMRI environment, and then, for the last Happy condition block, to use the one memory that elevated their mood to the greatest extent. Thus, the Practice run allowed participants to accommodate to the neurofeedback task and evaluate the emotional impact of the prepared happy memories within the experimental setting.
During the subsequent three Training runs participants were encouraged to use various memories and to switch memories in order to help them raise the red bar. Because our preliminary experiments in healthy individuals indicated that the activation level of the left amygdala could be as high as a 2% BOLD signal change, the target level of the blue bar was set to 0.5%, 1.0%, 1.5% and 2.0% for PR, R1, R2, and R3, respectively. During the Transfer Run, participants were instructed to perform the same task as during neurofeedback training, but rtfMRI-nf information was not provided. The transfer run was performed to assess the transfer of the learned control and to check whether the training effect generalized to situations where no neurofeedback was available. Participants completed either one or two rtfMRI-nf sessions within a one-week period.
Data Acquisition and On-Line Analysis
Imaging was conducted using a General Electric Discovery MR750 whole-body 3 Tesla MRI scanner equipped with a custom rtfMRI neurofeedback system and MRI/fMRI imaging details can be found in(11, 65).
The image data analyses were performed using Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/). The neurofeedback was implemented using the custom real- time fMRI system utilizing the real-time features of AFNI(66) and a custom graphic user interface (GUI). The regions-of-interest, defined as described above, were transformed to the EPI image space using each subject’s high-resolution MPRAGE structural data. The resulting regions-of-interest in the EPI space contained approximately 140 voxels each. We performed a visual inspection of the regions-of-interest (both the intraparietal and amygdala regions in all participants to maintain the blind) prior to the start of neurofeedback.
Ability of Participants to Enhance their Amygdala Response
Feasibility Study – Healthy Controls
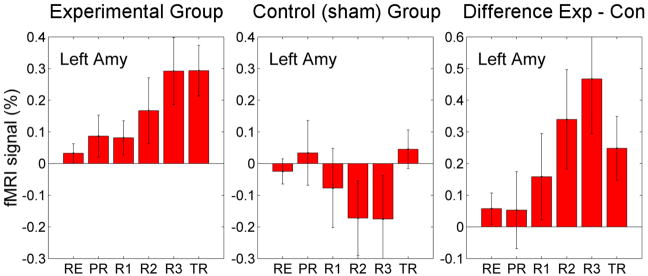
We first determined whether upregulation of amygdala activity could be achieved in healthy participants and the magnitude of signal change that could be obtained. Twenty-eight healthy male participants underwent rtfMRI-nf training to increase either their amygdala response (n=14; experimental group) or the response of the horizontal segment of the intraparietal sulcus (n=14; control group)(11). Figure 2a shows that mean BOLD activity in the amygdala increased progressively across neurofeedback runs for the experimental group, reached a 0.4% increase maximum during the final training run, and was maintained at similar levels during the transfer run. Furthermore, the experimental group had a significant linear trend, supporting a linear increase in activation of the amygdala across all runs. The control group did not demonstrate a significant change in their amygdala response over the course of training. Notably, these results are the average values during a single run; activation levels at a given Happy moment could be considerably higher, with some participants achieving a 2% signal change.
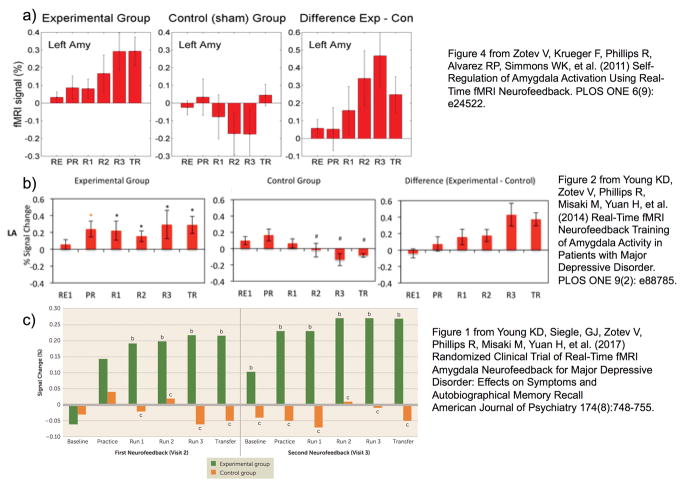
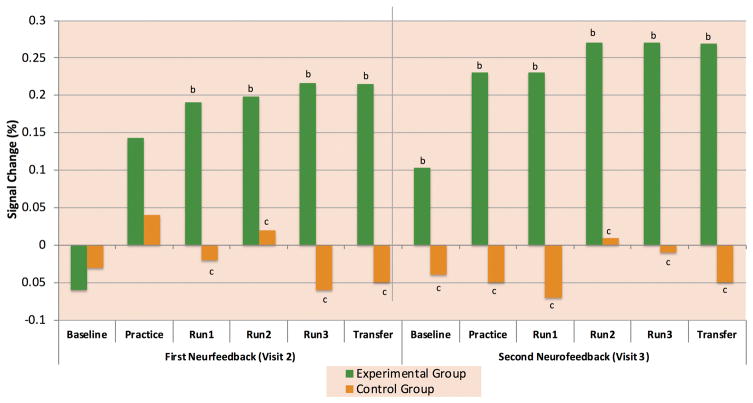
Figure 2. Learned Enhancement of Control over Amygdala BOLD fMRI Activation.
Average percent signal change for the Happy-Rest condition for each run and group in a) the initial feasibility study in healthy controls (11) b) the proof-of-concept study in MDD participantsp (15) and c) the randomized clinical trial of rtfMRI-nf (64). In figure 2b, * indicates a significant difference from 0 and # a significant difference from the experimental group at p<0.05. In Figure 2c, b indicates a significant difference from 0 and c indicates a significant difference from the experimental group at p<0.05
a) Figure 4 from Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, et al. (2011) Self-Regulation of Amygdala Activation Using Real-Time fMRI Neurofeedback. PLOS ONE 6(9): e24522.
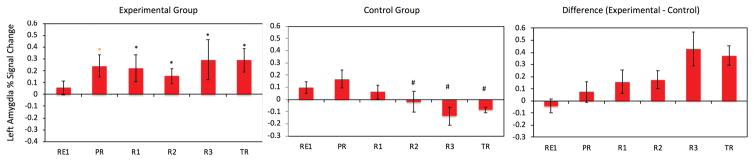
b) Figure 2 from Young KD, Zotev V, Phillips R, Misaki M, Yuan H, et al. (2014) Real-Time fMRI Neurofeedback Training of Amygdala Activity in Patients with Major Depressive Disorder. PLOS ONE 9(2): e88785.
c) Figure 1 from Young KD, Siegle, GJ, Zotev V, Phillips R, Misaki M, Yuan H, et al. (2017) Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall American Journal of Psychiatry 174(8):748–755.
To rule out potential confounding physiological factors that might contribute to the increased amygdala response(67), we examined cardiac and respiratory rates during the happy and rest conditions in the experimental and control groups. No group differences in cardiac or respiratory rate variations were found, nor did these rates differ between the regulate and rest conditions.
Pilot Study – MDD patients
Once we confirmed that healthy individuals can indeed learn to upregulate their amygdala response via rtfMRI-nf training, we conducted a small pilot study in patients with MDD to determine if they could also be trained to upregulate their amygdala response during positive AM recall(15). MDD participants were unmedicated, right-handed adults between the ages of 18–55 years who met DSM-IV-TR criteria for major depressive disorder. Exclusion criteria included current pregnancy, general MRI exclusions, serious suicidal ideation, psychosis, major medical or neurological disorders, exposure to any medication likely to influence cerebral function or blood flow within 3 weeks, and meeting DSM-IV-TR criteria for drug or alcohol abuse within the previous year or for lifetime alcohol or drug dependence (excepting nicotine). Comorbid anxiety disorders were not an exclusion, provided that MDD was the primary diagnosis.
The same design was used as in (11), with 14 participants in the experimental amygdala rtfMRI-nf group and 7 in the control parietal rtfMRI-nf group. Figure 2b shows that participants successfully regulated and increased their amygdala response relative to the control group and maintained it during the transfer run. There was also a linear decrease in amygdala activity in the control group; a finding we interpret as evidence that in the absence of amygdala neurofeedback it is difficult to maintain sustained activity in brain regions involved in positive emotion, as has been previously shown in patients with MDD(68).
The linear increase over training runs was not as pronounced as that observed in the healthy group. Furthermore, amygdala BOLD activity during practice was significantly above zero, raising the question of whether MDD participants did indeed have a blunted amygdala BOLD response during positive AM recall that needed to be corrected. To that end we added the baseline run and another rtfMRI-nf session and conducted a double-blind randomized clinical trial(64).
Clinical Trial
In the first randomized double-blind clinical trial of rtfMRI-nf training to increase amygdala BOLD activity during positive AM recall in patients with MDD (ClinicalTrials.govidentifier:NTC02079610), unmedicated adults meeting the same inclusion/exclusion criteria as for the pilot study were randomly assigned to receive two rtfMRI-nf sessions within a one-week period from the amygdala(n=19) or the parietal control region(n=17)(64). Only one participant from each group chose to withdraw from the study, indicating that this intervention is well tolerated and free from aversive side effects.
Figure 2c shows that participants in the experimental group were indeed able to increase the amygdala response over the course of the study. Particularly important to note is that at the initial baseline run, MDD participants did indeed exhibit very low or negative amygdala reactivity during positive AM recall. At the second session one week later, the amygdala response during baseline was significantly elevated relative to both the control group and the first baseline session, suggesting long-term transference of learning had occurred. Activity in the experimental group was higher during the 2nd training session than the first, suggesting that additional learning occurred with the additional training.
Effects of neurofeedback on Clinical Symptoms and Behavior
As we were able to demonstrate that both healthy and depressed individuals can be trained to upregulate their left amygdala BOLD response to positive memories via rtfMRI-nf training, we were also interested in whether this procedure altered mood, clinical symptoms, and other tasks involving cognitive and emotional processing. We therefore incorporated a variety mood ratings scales, symptom severity scales, and emotional processing/memory tasks that were administered pre and post rtfMRI-nf training.
Mood and Symptoms
As the goal of the initial study in healthy participants was to determine feasibility, we did not collect data on mood to examine changes(11). In the pilot study in depressed participants, state measures of current mood were collected pre and post rtfMRI-nf(15). As only one session was performed, only state mood ratings were examined, as clinical symptoms are measured on a longer-term scale (i.e., based on how a participant was feeling overall during the past week, not at any particular moment). The experimental group had a significant increase in state ratings of happiness and decreases in depression, anxiety, and irritation. The changes in happiness and anxiety were significantly greater in the experimental relative to the control group. These results supported the hypothesis that increasing amygdala BOLD activity during positive AM recall could indeed improve mood in patients with depression. This supported moving onto the next step of conducting a clinical trial and examining changes in clinical symptoms.
To this end we collected self-report (BDI-II) and clinical-administered (MADRS, HDRS-21) ratings of depressive symptoms one week prior to rtfMRI-nf training, during each training session, and one-week following the final training session(64). All ratings significantly decreased in the experimental group both from baseline and relative to the control group. Twelve participants in the experimental group responded to neurofeedback (defined as at least a 50% decrease in MADRS score), compared with two participants in the control group. Six participants in the experimental group and one in the control group met criteria for remission at study end (MADRS score > 10), making a number needed to treat of 4 (95% CI=2, 50). This remission rate is similar to rates seen with antidepressant medications(69) and cognitive behavioral therapy(70). Furthermore, there was a significant relationship between the ability to regulate the amygdala and the degree of improvement in clinical symptoms, providing additional support for the causal role between rtfMRI-nf learning and clinical improvement. While these results are encouraging, the follow-up period was only one week and additional studies are currently underway to determine the duration of these antidepressant effects.
Memory
In addition to assessing clinical symptoms, another goal of the clinical trial was to determine whether amygdala reactivity to non-autobiographical emotional stimuli was altered by amygdala rtfMRI-nf. As participants were using positive autobiographical memories to activate their amygdala, we examined whether the rtfMRI-nf training altered autobiographical memory recall. It is a well-replicated finding in the literature that patients with MDD have autobiographical memory deficits, characterized by overgeneral memory recall – defined as decreased recall of specific AMs (a memory for an event that occurred at an identifiable time and place and which did not last over 24h; ex: I went grocery shopping last Sunday) and increased recall of general, categorical AMs (summaries or categories of events without reference to a single episode; ex: I go grocery shopping every weekend)(71).
Overgeneral memory recall, especially for positive events, is an enduring cognitive deficit observed in patients with depression(71) that is not addressed by current treatments(72), and reportedly confers vulnerability to persistent depressive episodes(73). Following two rtfMRI-nf sessions, participants in the experimental group had an increase in the percent of specific memories recalled and a decrease in the percent of overgeneral memories recalled(64). The effect was predominately attributable to changes in positive memories. AM overgenerality is considered a pathological construct that predisposes(74) and maintains(75) depressive episodes. The improvement in AM specificity following amygdala rtfMRI-nf suggests this intervention may reverse this deficit. Furthermore, the change in amygdala activity was a mediator of the association between symptom improvement and increased positive specific AM recall, suggesting that recalling more positive specific memories can reduce depressive symptoms, but more so when the amygdala is engaged.
Specificity and Generalization to Other Positive Stimuli
In order to further determine the clinical potential of this intervention, we examined whether responses to other positive stimuli (faces and words) would be altered following amygdala rtfMRI-nf to examine whether the training altered other cognitive deficits and biases. To this end we examined pre and post amygdala responses to emotional faces as well as reaction time and accuracy on tasks of emotional processing.
Participants completed the P1vital Oxford Emotional Test Battery (available from http://www.p1vital.com/Oxford%20Emotional%20Test%20Battery/ETB_tasks.html) outside of the scanner one week before and one week following completion of the rtfMRI-nf protocol. This test battery has been widely used to study changes in emotional processing following standard antidepressant administration(76, 77), with results showing decreased reaction times and improved accuracy and memory for positive stimuli and increased reaction times and decreased accuracy and memory for negative stimuli(76, 78, 79). This suggests that antidepressant treatments work in part by increasing positive emotional processing.
We found similar changes in positive processing as observed following antidepressant pharmacotherapy(80). We observed decreased reaction times following amygdala rtfMRI-nf training to identifying positive facial emotions on the Facial Expression Recognition Task and to classifying self-referential words as positive on the Emotional Categorization Task. Furthermore we found increased attention toward positive faces and decreased attention toward negative faces during the Facial Dot Probe Task in the amygdala rtfMRI-nf relative to the control rtfMRI-nf group(80).
Finally, we examined the specificity of our procedure and examined whether the amygdala rtfMRI-nf procedure resulted in non-specific increases in amygdala activity to any emotional stimuli (which would not be desirable in individuals with MDD), whether responses to positive stimuli were enhanced without altering responses to negative stimuli, or whether training normalized the response by increasing activity to positive and decreasing activity to negative stimuli. To this end participants completed a backward masking that has been established as a paradigm that differentially alters amygdala hemodynamic activity to the presentation of happy and sad face stimuli presented below conscious awareness(33). Using this paradigm we observed an increase in the amygdala hemodynamic response to positive faces following amygdala rtfMRI-nf, as expected. We also observed a decrease in the amygdala hemodynamic response to negative faces in the rtfMRI-nf group relative both to their own baseline and the control rtfMRI-nf group, suggesting that amygdala rtfMRI-nf normalizes the amygdala response to emotional stimuli(80). This finding suggests that training the amygdala response in one direction (upregulate to positive) at least partly generalized to the processing of other types of emotional stimuli in the amygdala and that patients learned to adaptively regulate their amygdala response rather than to increase it nonspecifically.
Effects of neurofeedback on other brain regions
The amygdala is strongly connected to a wide variety of brain regions which play key roles in controlling one’s emotional, motivational, and social behavior(62, 81). Amygdala-medial prefrontal cortex (mPFC) connectivity is considered particularly important for emotion regulation both with respect to decreasing negative affect(36) and increasing reward behaviors(82). Furthermore, aberrant amygdala-prefrontal connectivity is prevalent in MDD(83, 84) and may underlie impairments in affect regulation(83). Therefore, while increasing voluntary control of the amygdala via rtfMRI-nf training results in clinical improvements, these improvements may be due to changes in how the brain communicates and regulates emotion. To this end we have examined both whole brain changes in activity following rtfMRI-nf in response to positive AM recall, as well as regional changes in functional connectivity with the amygdala.
In healthy males, amygdala rtfMRI-nf relative to control rtfMRI-nf resulted in increased activity during positive AM recall in the superior frontal gyrus, ventrolateral and ventromedial prefrontal cottices, amygdala, parahippocampal gyrus, pregenual anterior cingulate and posterior cingulate cortices(11). We further analyzed the resulting network interactions within these regions, which demonstrated the importance of prefrontal control of the amygdala during rtfMRI-nf training(85). In our pilot study with MDD participants, we found increased activity during the happy condition in similar regions as observed in healthy individuals: the amygdala, parahippocampal gyrus, anterior and posterior cingulate cortices, and ventrolateral prefrontal cortex. Additional significance was observed in the dorsomedial prefrontal and orbitofrontal cortices and thalamus(15). We also recorded EEG during rtfMRI-nf training and found that successful upregulation of the amygdala BOLD signal also normalizes frontal EEG asymmetry in the alpha band and increases EEG coherences over left frontal areas, with both measures associated with depressive symptom reductions(86). These observations suggest a normalization of abnormal PFC responding in depression. In our randomized clinical trial we again found increased activity following rtfMRI-nf in similar prefrontal-limbic regions: anterior cingulate cortex, insula, precuneus, amygdala/parahippocampal complex, and middle frontal gyrus(64).
Overall, our results suggest that the ability to maintain elevated amygdala activity during positive memory recall following rtfMRI-nf training engaged a prefrontal-temporal cortical-limbic network implicated in emotion processing and memory recall(86–88). Many of these regions share extensive anatomical and functional connections with the amygdala and are recruited during emotional learning (including the medial prefrontal cortex(87)), and in the modulation of emotional processes (including the anterior cingulate;(89)). These regions also form part of the core network recruited during AM recall(88). This pattern suggests that rtfMRI-nf from the amygdala is not dependent on a single brain region, but upon a network, and that training conceivably enhances the affective or attentional significance of these memories. Several of these regions, including the insula, anterior cingulate cortex, and ventrolateral prefrontal cortex are consistently activated during self-regulation in rtfMRI studies independent of the targeted region-of-interest(90) and are likely involved in the regulation/learning process and are not specific to our specific rtfMRI-nf procedure.
In addition to whole brain changes in activity, we have also explored regional changes in amygdala functional connectivity in our studies. In healthy males, amygdala functional connectivity increased with several regions over the course of amygdala rtfMRI-nf training. These regions included the medial frontal polar cortex, dorsomedial prefrontal cortex, pregenual anterior cingulate cortex, and superior frontal gyrus(11). A support vector autoregrssion analysis of the effective connectivity of this network suggests that the anterior cingulate cortex exerted significant directional effects on the amygdala(85). When we examined amygdala functional connectivity changes in MDD participants in the clinical trial, we found increased connectivity over the course of the study between the amygdala and multiple prefrontal cortical (right inferior frontal gyrus/lateral orbital cortex, dorsal ACC and ventrolateral PFC, left medial frontopolar cortex, bilateral medial PFC) and striatal regions (bilateral putamen, right caudate), as well as the right insula, cerebellum, and bilateral thalamus and precuneus(91). Two connections – amygdala-precuneus and amygdala inferior frontal gyrus – were significantly correlated with improvement in depressive symptoms after controlling for baseline severity and amygdala activity change, and amygdala-precuneus connectivity was also significantly correlated with regulation success. Amygdala-IFG connectivity was not associated with regulation success.
The results from these two populations (healthy males and MDD patients) found similar regional changes in amygdala functional connectivity, but implicated different regions as critical for the training effects to occur. This is likely due to the different methods employed –the study in healthy controls was interested in what changed with neurofeedback and what was driving the change in amygdala activity, while the clinical trial sought to determine which functional connectivity changes contributed to the observed clinical effects. Therefore, while the ACC appears critical for gaining control over the amygdala, the increase in precuneus-amygdala connectivity appears critical for both neurofeedback regulation success and symptom improvement. These results suggest additional targets for rtfMRI-nf interventions, and also that connectivity rtfMRI-nf may be even more effective than single region feedback.
Predicting Response to Feedback
Examining correlates of neurofeedback success could help guide future efforts towards understanding who this treatment might be effective for. Therefore, we examined correlations between neurofeedback success in the amygdala rtfMRI-nf groups and demographic and clinical characteristics. In each study (healthy males, pilot, clinical trial) there was a significant relationship between the ability to regulate the amygdala (either the average % signal over the course of training(11, 15) or the difference from baseline to transfer(80)) was significantly associated with performance on the Toronto Alexthymia Scale (TAS). Individuals with more difficulty in identifying and describing emotions, regardless of diagnosis, had more difficulty regulating their amygdala, suggesting that some level of emotional insight is necessary for this rtfMRI-nf training paradigm. There were no other factors that predicted response to feedback; however, these samples were relatively small and testing in larger, more heterogeneous samples may reveal additional factors that are associated with neurofeedback success. Additionally, activation of several regions, including the anterior insula and basal ganglia, has been found to occur during rtfMRI-nf learning independent of the targeted region of interest(90). It is therefore possible that activity in these and other regions would also be predictive of whether or not someone can learn to regulate a hemodynamic signal, and research would benefit from including baseline activations of these regions as predictors of regulation success. Of note, there was a trend towards a significant inverse correlation between baseline amygdala activity in our clinical trial and regulation success (r=−0.39, p=0.08), suggesting that the training is more effective in those individuals who also have an abnormal amygdala response and supporting our approach of normalizing activity rather than increasing activity in compensatory mechanisms.
In healthy males, the training effect was also inversely correlated with participants’ susceptibility to anger, suggesting that healthy individuals’ performance during neurofeedback training may be inversely correlated with their sensitivity to other people’s negative emotions(11). This correlation was not significant in the patients with MDD. Instead the length of the current depressive episode was inversely correlated with the training effect(15, 80), suggesting that the success of amygdala rtfMRI-nf may be dependent upon targeting patients early in the course of their depressive episode. This is consistent with previous research reporting that patients within a year of onset of their current depressive episode are more likely to respond to treatment than those whose episodes were of longer duration(92).
Future Directions
While the research we have done thus far suggests a strong clinical potential of increasing amygdala activity during positive AM recall via rtfMRI-nf, there is still much work that needs to be done to translate this into a clinical treatment available to patients with MDD. While we have found mood enhancing effects of rtfMRI-nf following a single session(15), additional improvements both in terms of mechanism engagement (amygdala BOLD signal during positive AM recall) and clinical symptoms were observed following a 2nd session(64). Studies attempting to train brain regions implicated in emotional processing via rtfMRI-nf in mood-disordered individuals have provided between 1–5 separate training sessions(14, 45, 93, 94), yet no examination of dose response has been conducted. Determining a sufficient number of rtfMRI sessions is critical for treatment development(95), and therefore efforts are now underway to determine whether additional sessions provide additional benefit in terms of target engagement and whether the number of sessions can be tailored using baseline characteristics (including those previously found to be associated with neurofeedback success such as length of current episode and difficulty identifying feelings(15)).
Additionally, further replication in larger, more heterogeneous samples that include medicated individuals is necessary to determine the subpopulations or characteristics for whom this intervention is best suited. We were initially hesitant to include patients taking antidepressant medications, as studies have generally reported that SSRIs result in reduced limbic reactivity to emotional stimuli, raising the concern that SSRIs may increase anhedonic symptoms(96). However, emerging evidence suggests that SSRIs normalize limbic reactivity to emotional stimuli, rather than dampening it more generally (33, 97). We are therefore including individuals on antidepressant medications in our ongoing studies.
Determining the duration of symptom improvements is also critical to establish in order to fully understand the clinical potential of this intervention. We previously demonstrated clinical effects last at least one-week following the final training session. However, whether the duration of these clinical effects is comparable to other antidepressant interventions was not yet studied. Therefore, we are currently following patients for 3 months, a duration commonly used in antidepressant clinical trails.
Finally, we are also working to determine whether this intervention would be best utilized as an augmentation to existing therapies. For example, as antidepressant medications take up to four weeks to result in clinical improvement(98), and as we have observed large changes in depression ratings over the course of two weeks in those receiving amygdala rtfMRI-nf, augmenting the start of an antidepressant regime with amygdala rtfMRI-nf sessions may ‘bridge the gap’ between prescription and mood improvement. Cognitive behavioral therapy (CBT) may also benefit from augmentation with amygdala rtfMRI-nf. A fundamental component of CBT for MDD involves the restructuring of thought and emotional processing towards affectively positive and away from affectively negative thoughts, feelings, and memories(99). Newer cognitive therapies specifically target positive affect and increasing patients’ ability to sustain positive affect over time(100). The continued focus on improving positive affect as a component of CBT provides a clear rationale to train MDD patients to more effectively access positive autobiographical memories in the generation of positive mood. To this end we are conducting a randomized clinical trial examining clinical outcome following CBT augmented with amygdala rtfMRI-nf relative to CBT augmented with control rtfMRI-nf (Grant 4R00MH101235-03; clinicaltrails.gov identifier NCT02709161). We theorize that by training patients to put their brain into a state where they are better able to process positive AMs, that it will be easier to learn and incorporate the tenants of CBT and that this will lead to a quicker and more sustained response to CBT.
Conclusions
Collectively, these studies show that both healthy individuals and unmedicated patients with MDD can significantly increase their amygdala response to positive AM recall via rtfMRI-nf training. Furthermore, they are able to maintain this response following training and in the absence of neurofeedback information suggesting both short term (same-session) and long-term (between session baselines) transference of learning/ability to maintain this learned response in the absence of neurofeedback information.
In patients with MDD, significant improvements in clinical symptoms are observed, along with improvements in memory and attention for positive stimuli. Though changes in the processing of negative stimuli were also evident following neurofeedback training, only changes to positive stimuli were associated with measures of clinical improvement and neurofeedback success. This suggests the enhancement of the processing of positive emotional information, rather than suppression of the processing of negative, that underlies the clinical effects of our amygdala neurofeedback paradigm.
These studies collectively a) support a role for the amygdala in positive emotional processing and memory recall, b) suggest reduced amygdala hemodynamic activity to positive memories may be an important mechanism interfering with recovery from MDD, and c) provide a neuroscience based intervention with potential as a treatment for MDD. Further research is needed to determine if these results can be broadly replicated and who this treatment is best suited for.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health/National Institute of Mental Health under Award Number K99MH101235, by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, and by the Laureate Institute for Brain Research and the William K. Warren Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosures: WCD is currently an employee of Janssen Pharmaceuticals, LLC, of Johnson & Johnson, Inc. The other authors have no financial conflicts of interest or disclosures to report. Drs. Young and Bodurka take responsibility for the integrity of the data and accuracy of the data analysis. All authors had full access to all the data in the study.
Author Contributions: JB designed and implemented the study protocol. JB, KY, VZ, WCD, RP contributed to the conception of the study. VZ, JB, KY, MM developed data analysis methods. KY, VZ, RP and MM conducted data analysis, and KY, JB drafted the manuscript and figures.
References
- 1.Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med. 1995;33:230–236. doi: 10.1002/mrm.1910330213. [DOI] [PubMed] [Google Scholar]
- 2.deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9:720–729. doi: 10.1038/nrn2414. [DOI] [PubMed] [Google Scholar]
- 3.deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage. 2004;21:436–443. doi: 10.1016/j.neuroimage.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 4.Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007;25:989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Yoo SS, O’Leary HM, Fairneny T, Chen NK, Panych LP, Park H, Jolesz FA. Increasing cortical activity in auditory areas through neurofeedback functional magnetic resonance imaging. Neuroreport. 2006;17:1273–1278. doi: 10.1097/01.wnr.0000227996.53540.22. [DOI] [PubMed] [Google Scholar]
- 6.Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N. Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum Brain Mapp. 2009;30:1605–1614. doi: 10.1002/hbm.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. Neurofeedback: A promising tool for the self-regulation of emotion networks. Neuroimage. 2010;49:1066–1072. doi: 10.1016/j.neuroimage.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage. 2007;35:1238–1246. doi: 10.1016/j.neuroimage.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 9.deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A. 2005;102:18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage. 2003;18:760–768. doi: 10.1016/s1053-8119(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 11.Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, Bellgowan P, Drevets WC, Bodurka J. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. 2011;6:e24522. doi: 10.1371/journal.pone.0024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartwell KJ, Hanlon CA, Li X, Borckardt JJ, Canterberry M, Prisciandaro JJ, Moran-Santa Maria MM, LeMatty T, George MS, Brady KT. Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J Psychiatry Neurosci. 2016;41:48–55. doi: 10.1503/jpn.140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zilverstand A, Sorger B, Sarkheil P, Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front Behav Neurosci. 2015;9:148. doi: 10.3389/fnbeh.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R. Real-time self-regulation of emotion networks in patients with depression. PLoS One. 2012;7:e38115. doi: 10.1371/journal.pone.0038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One. 2014;9:e88785. doi: 10.1371/journal.pone.0088785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. The World Health Report 2004 - Changing History. Geneva: 2004. [Google Scholar]
- 17.Cain RA. Navigating the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study: practical outcomes and implications for depression treatment in primary care. Prim Care. 2007;34:505–519. vi. doi: 10.1016/j.pop.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Merrill KA, Tolbert VE, Wade WA. Effectiveness of cognitive therapy for depression in a community mental health center: a benchmarking study. J Consult Clin Psychol. 2003;71:404–409. doi: 10.1037/0022-006x.71.2.404. [DOI] [PubMed] [Google Scholar]
- 19.Elkin I, Gibbons RD, Shea MT, Sotsky SM, Watkins JT, Pilkonis PA, Hedeker D. Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1995;63:841–847. doi: 10.1037//0022-006x.63.5.841. [DOI] [PubMed] [Google Scholar]
- 20.Merkl A, Heuser I, Bajbouj M. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol. 2009;219:20–26. doi: 10.1016/j.expneurol.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Mohr P, Rodriguez M, Slavickova A, Hanka J. The application of vagus nerve stimulation and deep brain stimulation in depression. Neuropsychobiology. 2011;64:170–181. doi: 10.1159/000325225. [DOI] [PubMed] [Google Scholar]
- 22.Kirby T. Ketamine for depression: the highs and lows. Lancet Psychiatry. 2015;2:783–784. doi: 10.1016/S2215-0366(15)00392-2. [DOI] [PubMed] [Google Scholar]
- 23.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 24.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Whalen PJ, Phelps EA, editors. The human amygdala. New York, NY: Guildford Press; 2009. [Google Scholar]
- 28.Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 30.Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 32.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 35.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 36.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Drevets W, Price J, Furey M. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67:155–160. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Young KD, Siegle GJ, Bodurka J, Drevets WC. Amygdala Activity During Autobiographical Memory Recall in Depressed and Vulnerable Individuals: Association With Symptom Severity and Autobiographical Overgenerality. Am J Psychiatry. 2016;173:78–89. doi: 10.1176/appi.ajp.2015.15010119. [DOI] [PubMed] [Google Scholar]
- 42.Young KD, Bellgowan PS, Bodurka J, Drevets WC. Behavioral and neurophysiological correlates of autobiographical memory deficits in patients with depression and individuals at high risk for depression. JAMA Psychiatry. 2013;70:698–708. doi: 10.1001/jamapsychiatry.2013.1189. [DOI] [PubMed] [Google Scholar]
- 43.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 44.Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral Therapies in the 21st Century: Summary of an Emerging Field and an Extended Example of Cognitive Control Training for Depression. Cognitive Therapy and Research. 2007;31:235–262. [Google Scholar]
- 45.Bruhl AB, Scherpiet S, Sulzer J, Stampfli P, Seifritz E, Herwig U. Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: a proof-of-concept study. Brain Topogr. 2014;27:138–148. doi: 10.1007/s10548-013-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paret C, Kluetsch R, Ruf M, Demirakca T, Hoesterey S, Ende G, Schmahl C. Down-regulation of amygdala activation with real-time fMRI neurofeedback in a healthy female sample. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Theberge J, Neufeld RW, McKinnon MC, Reiss J, Jetly R, Lanius RA. The neurobiology of emotion regulation in posttraumatic stress disorder: Amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. 2017;38:541–560. doi: 10.1002/hbm.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young KD, Drevets WC, Bodurka J, Preskorn SS. Amygdala activity during autobiographical memory recall as a biomarker for residual symptoms in patients remitted from depression. Psychiatry Res. 2016;248:159–161. doi: 10.1016/j.pscychresns.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci. 2011;11:44–51. doi: 10.3758/s13415-010-0010-1. [DOI] [PubMed] [Google Scholar]
- 50.Cannon R, Lubar J, Congedo M, Thornton K, Towler K, Hutchens T. The effects of neurofeedback training in the cognitive division of the anterior cingulate gyrus. Int J Neurosci. 2007;117:337–357. doi: 10.1080/00207450500514003. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz S, Lee S, Soekadar SR, Caria A, Veit R, Kircher T, Birbaumer N, Sitaram R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum Brain Mapp. 2013;34:200–212. doi: 10.1002/hbm.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J Abnorm Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- 53.Newman SD, Willoughby G, Pruce B. The effect of problem structure on problem-solving: an fMRI study of word versus number problems. Brain Res. 2011;1410:77–88. doi: 10.1016/j.brainres.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 54.Fias W, Lammertyn J, Caessens B, Orban GA. Processing of abstract ordinal knowledge in the horizontal segment of the intraparietal sulcus. J Neurosci. 2007;27:8952–8956. doi: 10.1523/JNEUROSCI.2076-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- 56.Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- 57.Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise K, Pizzarello S, Dorantes C, Tecuta L, Guerreri S, Goodman M, New A, Flory J, Siever LJ. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mouras H, Stoleru S, Bittoun J, Glutron D, Pelegrini-Issac M, Paradis AL, Burnod Y. Brain processing of visual sexual stimuli in healthy men: a functional magnetic resonance imaging study. Neuroimage. 2003;20:855–869. doi: 10.1016/S1053-8119(03)00408-7. [DOI] [PubMed] [Google Scholar]
- 59.Mourao-Miranda J, Volchan E, Moll J, de Oliveira-Souza R, Oliveira L, Bramati I, Gattass R, Pessoa L. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20:1955–1963. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 61.Prevost C, McCabe JA, Jessup RK, Bossaerts P, O’Doherty JP. Differentiable contributions of human amygdalar subregions in the computations underlying reward and avoidance learning. Eur J Neurosci. 2011;34:134–145. doi: 10.1111/j.1460-9568.2011.07686.x. [DOI] [PubMed] [Google Scholar]
- 62.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 64.Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J. Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am J Psychiatry. 2017;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bodurka J, Bandettini P. Real-time software for monitoring MRI scanner operation. Neuroimage. 2008;41:S85. [Google Scholar]
- 66.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 67.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, Gottfried JA. Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J Neurosci. 2016;36:12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, Crombie I. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: a meta-analysis. Ann Fam Med. 2005;3:449–456. doi: 10.1370/afm.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196:173–178. doi: 10.1192/bjp.bp.109.066001. [DOI] [PubMed] [Google Scholar]
- 71.Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychol Bull. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nandrino JL, Pezard L, Poste A, Reveillere C, Beaune D. Autobiographical memory in major depression: a comparison between first-episode and recurrent patients. Psychopathology. 2002;35:335–340. doi: 10.1159/000068591. [DOI] [PubMed] [Google Scholar]
- 73.Peeters F, Wessel I, Merckelbach H, Boon-Vermeeren M. Autobiographical memory specificity and the course of major depressive disorder. Compr Psychiatry. 2002;43:344–350. doi: 10.1053/comp.2002.34635. [DOI] [PubMed] [Google Scholar]
- 74.Gibbs B, Rude S. Overgeneral Autobiographical Memory as Depression Vulnerability. Cognitive Therapy and Research. 2004;28:511–526. [Google Scholar]
- 75.Brittlebank AD, Scott J, Williams JM, Ferrier IN. Autobiographical memory in depression: state or trait marker? Br J Psychiatry. 1993;162:118–121. doi: 10.1192/bjp.162.1.118. [DOI] [PubMed] [Google Scholar]
- 76.Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- 77.Harmer CJ, Cowen PJ. ‘It’s the way that you look at it’--a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120407. doi: 10.1098/rstb.2012.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21:684–690. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- 79.Murphy SE, Longhitano C, Ayres RE, Cowen PJ, Harmer CJ. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology (Berl) 2006;187:121–130. doi: 10.1007/s00213-006-0401-8. [DOI] [PubMed] [Google Scholar]
- 80.Young KD, Misaki M, Harmer CJ, Victor T, Zotev V, Phillips R, Siegle GJ, Drevets WC, Bodurka J. Real-Time fMRI Amygdala Neurofeedback Changes Positive Information Processing in Major Depressive Disorder. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seo DO, Funderburk SC, Bhatti DL, Motard LE, Newbold D, Girven KS, McCall JG, Krashes M, Sparta DR, Bruchas MR. A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. J Neurosci. 2016;36:10831–10842. doi: 10.1523/JNEUROSCI.1164-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, Vandekar SN, Durbin T, Shinohara RT, Sheline YI. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol Psychiatry. 2016;21:894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zotev V, Phillips R, Young KD, Drevets WC, Bodurka J. Prefrontal control of the amygdala during real-time fMRI neurofeedback training of emotion regulation. PLoS One. 2013;8:e79184. doi: 10.1371/journal.pone.0079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zotev V, Yuan H, Misaki M, Phillips R, Young KD, Feldner MT, Bodurka J. Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. Neuroimage Clin. 2016;11:224–238. doi: 10.1016/j.nicl.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 90.Emmert K, Kopel R, Sulzer J, Bruhl AB, Berman BD, Linden DE, Horovitz SG, Breimhorst M, Caria A, Frank S, Johnston S, Long Z, Paret C, Robineau F, Veit R, Bartsch A, Beckmann CF, Van De Ville D, Haller S. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? Neuroimage. 2016;124:806–812. doi: 10.1016/j.neuroimage.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 91.Young KD, Siegle GJ, Misaki M, Zotev V, Phillips R, Drevets WC, Bodurka J. Altered task-based and resting-state amygdala functional connectivity following real-time fMRI amygdala neurofeedback training in major depressive disorder. Neuroimage: Clinical. doi: 10.1016/j.nicl.2017.12.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelsey JE. Achieving remission in major depressive disorder: the first step to long-term recovery. J Am Osteopath Assoc. 2004;104:S6–10. [PubMed] [Google Scholar]
- 93.Gerin MI, Fichtenholtz H, Roy A, Walsh CJ, Krystal JH, Southwick S, Hampson M. Real-Time fMRI Neurofeedback with War Veterans with Chronic PTSD: A Feasibility Study. Front Psychiatry. 2016;7:111. doi: 10.3389/fpsyt.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Sacchet MD, Gotlib IH. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. 2016;249:91–96. doi: 10.1016/j.pscychresns.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stoeckel LE, Garrison KA, Ghosh S, Wighton P, Hanlon CA, Gilman JM, Greer S, Turk-Browne NB, deBettencourt MT, Scheinost D, Craddock C, Thompson T, Calderon V, Bauer CC, George M, Breiter HC, Whitfield-Gabrieli S, Gabrieli JD, LaConte SM, Hirshberg L, Brewer JA, Hampson M, Van Der Kouwe A, Mackey S, Evins AE. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuroimage Clin. 2014;5:245–255. doi: 10.1016/j.nicl.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sansone RA, Sansone LA. SSRI-Induced Indifference. Psychiatry (Edgmont) 2010;7:14–18. [PMC free article] [PubMed] [Google Scholar]
- 97.Godlewska BR, Browning M, Norbury R, Cowen PJ, Harmer CJ. Early changes in emotional processing as a marker of clinical response to SSRI treatment in depression. Transl Psychiatry. 2016;6:e957. doi: 10.1038/tp.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, Zarate CA. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals (Basel) 2010;3:19–41. doi: 10.3390/ph3010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beck A. Cognitive Therapy and Emotional Disorders. New York: Penguin; 1993. [Google Scholar]
- 100.McMakin DL, Siegle GJ, Shirk SR. Positive Affect Stimulation and Sustainment (PASS) Module for Depressed Mood: A preliminary investigation of treatment-related effects. Cognit Ther Res. 2011;35:217–226. doi: 10.1007/s10608-010-9311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]