Abstract
Introduction
Despite the different assays available for immune-risk stratification before living-donor kidney transplantation (LDKT), the precise type and number of tests to perform remain uncertain.
Methods
In a cohort of 330 consecutive LDKT patients, all of which were complement-dependent cytotoxicity (CDC)−crossmatch negative, we retrospectively analyzed the impact on main clinical outcomes of most sensitive immunoassays (complement-dependent cytotoxicity−panel-reactive antibody [CDC-PRA], flow cytometry crossmatch [FC-XM], donor-specific antibodies [DSAs], and their complement-binding capacity DSA-C3d]), together with donor/recipient HLA eplet matching. Mean follow-up was 67 months (range 24−190 months).
Results
Of 330 patients, 35 (11%) showed a CDC-PRA >20%; 17 (5%) FC-XM+; 30 (9%) DSA+, 18(5%) DSA-C3d+, with low overlapping results (10 patients positive in all donor-specific tests). Unlike HLA allele compatibility, the mean number of HLA class II eplet mismatches was higher in LDKT patients with positive baseline test results. DSA-C3d+ showed higher mean fluorescence intensity (MFI) DSA, with a cut-off MFI of 6192 accurately predicting complement fixation (area under the curve = 0.85, P = 0.008). Although all assays were associated with acute rejection (AR), only DSA-C3d+ (odds ratio [OR] = 6.64, P = 0.038) or high MFI-DSA (OR = 7.54, P = 0.038) independently predicted AR. Likewise, poorly HLA class II eplet−matched patients were at higher risk for AR, particularly patients with negative baseline test results (OR = 1.14, P = 0.019). Finally, previous AR and FC-XM+/DSA+, regardless of C3d positivity, independently predicted graft loss.
Conclusion
Combining FC-XM and solid-phase assays with the evaluation of donor/recipient HLA eplet mismatches, are most accurate tools for immune-risk stratification prior LDKT.
Keywords: acute rejection, crossmatch immunoassays, donor-specific antibodies, HLA matchmaker, immune risk stratification, living donor kidney transplantation
More than 35% of patients on a waiting list for kidney transplantation are sensitized against human leukocye antigen (HLA), having longer waiting times for an HLA-compatible transplant than nonsensitized patients.1, 2 However, the sensitization degree before transplantation and the antidonor specificity may differ according to the immunoassay used,3, 4, 5, 6 thus influencing decision making regarding establishing desensitization strategies or even leading to the withdrawal of the transplantation due to an overreaching rejection risk.
Living-donor kidney transplantation (LDKT) is the best therapeutic option for patients with end-stage renal disease.7, 8 A main advantage over deceased-donor transplantation is that a thorough evaluation of the immunological risk is more feasible due to the programmed nature of the transplant: on 1 hand, assessing the presence of preformed anti-HLA DSAs and, on the other, defining the degree of HLA−donor/recipient matching. Indeed, a broad array of assays such as complement-dependent cytotoxicity crossmatch (CDC-XM), FC-XM, and solid-phase assays detecting circulating DSA with or without complement-binding capacity (either C1q- or C3d-binding) may be performed prior to transplantation.9, 10, 11, 12 Although all of these assays are informative about alloimmune status, they may also lead to ambiguous results, not always providing overlapping evidence.13
In fact, recent reports assessing cohorts of both deceased-donor and LDKT patients showed an increased risk of acute rejection (AR) and graft loss in the presence of pretransplantation-positive FC-XM but not isolated DSA.14, 6 Conversely, other groups have described lower graft survival only with both high MFI DSA and positive FC-XM but not with low DSA levels regardless of a positive FC-XM.15 Also, inferior graft outcomes in presence of DSA, independently of CDC-XM and FC-XM have also been reported.16, 5 In addition, the refinement of solid-phase assays by measuring the complement-binding capacity of DSAs, with either C1q or C3d, has revealed a greater likelihood and severity of AR and poorer graft survival in these patients.12, 17, 18, 19 Furthermore, although better donor/recipient HLA matching at the allele level still accounts for better graft outcomes,20 the specific analysis of donor/recipient HLA mismatches at the epitope level has recently emerged as a more accurate tool for identifying the degree of donor/recipient HLA compatibility.21, 22, 23
However, the clinical value of all of these tests and their combination prior to LDKT has not been evaluated yet in the same cohort of kidney transplant patients to determine the most sensitive and specific assays for stratifying the risk of rejection and graft loss. Hence, we aimed at retrospectively investigating, in a large cohort of consecutive LDKT patients from 2 different transplant programs, the predictive value on clinical outcomes of the currently available immunoassays, alone or in combination, together with donor/recipient HLA mismatch evaluation at the eplet level. Herein we show that pretransplantation DSA, with either high-MFI or C3d-binding capacity, are independent risk factors for AR, and that the combination of preformed DSAs, regardless of their complement-binding capacity, with positive FC-XM discriminates patients with poorer graft survival. Also, a low HLA class II eplet mismatch is key to reducing the likelihood of subsequent immune activation, regardless of pretransplantation sensitization status.
Methods
Study Population
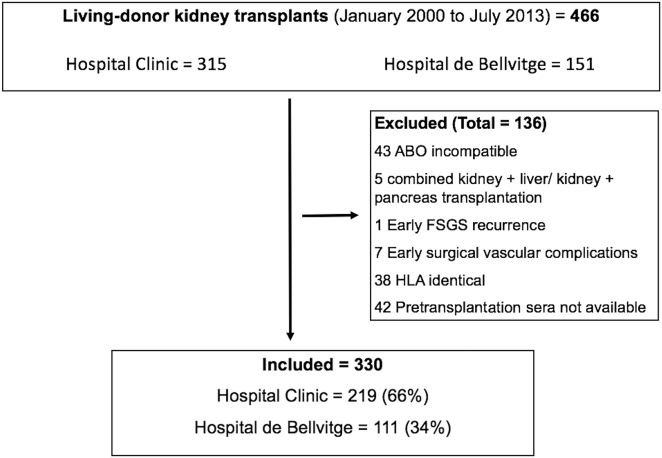
We retrospectively analyzed 330 consecutive adult LDKT patients from 2 transplant centers in Barcelona, Spain (Hospital de Bellvitge and Hospital Clínic), who underwent transplantation from 2000 until 2013. All patients with negative CDC-XM at the time of transplantation surgery and with available pretransplantation serum samples (within 4 weeks before transplantation) were evaluated with 4 immunoassays: complement-dependent cytotoxicity panel-reactive antibody CDC-PRA, FC-XM, solid-phase single antigen beads (SAB) to assess DSA and those with C3d-binding capacity (DSA-C3d). Minimum follow-up was 2 years, with a mean actuarial follow-up of 67 ± 29 months (range 24−190 months). ABO-incompatible and HLA-identical patients, those receiving other transplant organs, and those with early graft loss for surgically related complications were excluded (Figure 1). CDC-PRA and CDC-XM data were available to the clinicians before transplantation in all patients, and DSA and FC-XM in 134 patients who underwent transplantation after 2011. We retrospectively analyzed, on pretransplantation frozen samples, DSA and FC-XM in the 196 remaining patients who underwent transplantation before 2011 and DSA-C3d tests in all patients with positive DSA. All of the tests were carried out at the immunology laboratory at Hospital Clínic. The study was approved by the institutional review boards from both institutions. Informed consent was obtained from all patients.
Figure 1.
Flowchart of patients in the study cohort. ABO, blood group; FSGS, focal segmental glomerulosclerosis; HLA, human leukocyte antigen.
Outcome Definitions
Cases of AR were all biopsy proven except for 6 cases due to biopsy contraindication, and were all graded following the last Banff classification.24 Twelve patients showing borderline changes were considered as AR due to acute graft dysfunction and positive response to antirejection treatment. Graft loss was defined as either return to chronic dialysis or re-transplantation. Renal function was evaluated using the Modification of Diet in Renal Disease (MDRD) equation−estimated glomerular filtration rate (eGFR) at 6, 12, 24, and 60 months after transplantation.
HLA Typing
Recipients’ and donors’ HLA class I (A, B) and class II (DR, DQ) typing was performed by DNA-based low-resolution typing with sequence-specific primers (SSP) as previously described.25
Eplet Mismatch Analysis
The HLAMatchmaker program (Rene Duquesnoy, 2016; University of Pittsburgh Medical Center, Pittsburgh, PA) was used to assess eplet matching (4ABCEpletMatchingVs02protoype.xlsb and DRDQDPEpletMatchingVs02protoype.xlsb from http://www.epitopes.net/downloads.html). Donor and recipient typing (A, B, DR, and DQ) were converted to high resolution using a local frequency table typed by sequence-based typing. Total numbers of incompatible eplets and antibody-proven eplets were calculated.21 Here, all analyses were performed using the global number of eplet mismatches.
Humoral Alloimmune Risk Characterization
Complement-Dependent Panel-Reactive Antibody
Both CDC-PRA and CDC-XM tests were performed as previously described.26 CDC-PRA tests were performed using frozen cells obtained from blood bank donors, and CDC-XM was done with fresh donor peripheral blood mononuclear cells (PBMCs). Neither antihuman globulin nor extended incubation was used. To rule out the presence of autoantibodies, all crossmatch tests were carried out with dithiotreitol and without dithiotreitol to discard IgM, evaluating the discrepancy between techniques and by performing an autoXM. The highest CDC-PRA before transplantation was considered for the analysis. CDC-PRA > 20% was defined as positive and >80% highly HLA sensitized.
Flow-Cytometry Crossmatch
All donor PBMCs for FC-XM tests were obtained from freshly obtained blood samples. T- and B-cell FC-XM were performed using peripheral blood donor cells.11 With a scale expressing staining intensity as a linear channel value (0−1024), median channel fluorescence (MCS: median channel fluorescence shift) for antihuman IgG-F(ab)' fluorescein isothiocyanate was quantified on CD3+ T cells and CD19+ B cells. FC-XM was positive when MCS of the sample exceeded the negative control value by 3 SDs. T-cell FC-XM was considered negative when MCS was ≤25 and positive when it was ≥100. For B-cell FC-XM, an MCS value of ≤200 was considered negative and ≥300 positive.
Solid-Phase Single-Antigen Assay for DSA Characterization
All patients were tested by SAB assay to detect donor-specific anti-HLA IgG using a single-antigen class I and class II flow beads assay kit (Lifecodes, Division of Immucor, Stanford, CA). All beads showing a normalized MFI of >1500 were considered positive if (MFI/MFI lowest bead) was >5. The highest MFI value for each DSA was considered for the analysis.
C3d Complement-Binding DSA
In all patients showing DSA+, a C3d test (C3d complement-binding DSA [DSA-C3d]) was performed using a solid-phase assay and following the manufacturer’s procedures (Lifecodes; Immucor, Stanford, CA). An MFI threshold of 1500 was considered positive (DSA-C3d+). For a better comparison, DSA-IgG was analyzed using the same lot of beads in both the C3d detection and IgG SAB assay.
Statistical Analysis
All data were expressed as mean ± SD or as median and interquartile range for continuous variables, and as frequencies for categorical variables.
The number of eplet mismatches was evaluated as a continuous variable. Comparisons between groups were performed using the Pearson χ2 test for categorical data, and the Fisher test was applied when the number of cases was <5. One-way analysis of variance and t tests were used for normally distributed data, and the nonparametric Kruskal–Wallis test and Mann–Whitney U test were used for non−normally distributed data. Receiver operating characteristic (ROC) curve analysis was used to assess the specificity and sensitivity of MFI threshold predicting C3d-binding-capacity of DSA.
Bivariate correlation analyses were performed using the Pearson or Spearman test for nonparametric variables. Both univariate and multivariate logistic regression models were performed to examine the factors associated with AR.
Kaplan–Meier probabilities of graft survival and rejection-free survival were plotted and compared by different immunoassay results using log-rank tests. A Cox regression model was used to estimate hazard ratios for univariate analyses for graft survival and to compare clinical and immunological variables. Analyses of graft loss were censored for patient death. Multicollinearity was assessed using variance inflation factors.
All P values were 2-tailed, and statistical significance was fixed at P < 0.05. SPSS version 20.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA) were used for data management and analysis.
Results
Study Population
Main clinical and demographic characteristics of the study cohort are depicted in Table 1. Most patients were Caucasian/white (99%), male (66%), on dialysis therapy (82%), and receiving a first kidney transplant (82%).
Table 1.
Main demographic, clinical, and immunological characteristics of the studied population
| Demographic and clinical variables | n (%) |
|---|---|
| Recipient age, yr (mean ± SD) | 45 ± 14 |
| Donor age, yr (mean ± SD) | 52 ± 11 |
| Recipient gender, male | 218 (66) |
| Donor gender, male | 112 (34) |
| Type of donor: child to mother OR husband to wife versus other types of donor | 41 (12) |
| ESRD cause | |
| Unknown | 120 (36) |
| Glomerulopathy | 95 (29) |
| Polycystic kidney and tubulo-interstitial disease | 59(18) |
| Diabetic and hypertensive nephropathy | 32 (10) |
| Others | 24 (7) |
| Time on dialysis, mo (median, IQR) | 8, 0–27 |
| Number of transplants (1/2/≥3) | 271 (82) / 38 (12) /21 (6) |
| Induction treatment: no/rATG /anti-CD25 | 36 (11) / 114 (34) / 180 (54) |
| Immunosuppressive therapy | |
| CNI-based | 323 (98) |
| No CNIs (mTOR i) | 7 (2) |
| Desensitization treatment | 23 (7) |
| Plasmaphreresis + rituximab + i.v. Ig | 12 (50) |
| Plasmapheresis + i.v. Ig | 2 (8) |
| i.v. Ig | 8 (34) |
| Plasmapheresis | 1 (4) |
| Delayed graft function | 3 (1) |
| Acute rejection | 65 (19) |
| TCMR | 40 (71) |
| Banff grades: IA/IB/IIA/IIB/III/BL | 7 / 7 / 8 / 5 / 1 / 12 |
| ABMR | 19 (29) |
| Mean eGFR, ml/min (mean ± SD) | |
| 1 yr (n = 308) | 71.5 ± 20.2 |
| 2 yr (n = 285) | 70.3 ± 21.4 |
| 5 yr (n = 179) | 67 ± 23 |
| 1-yr Death-censored graft survival | 326 (99) |
| Actuarial death-censored graft survival | 288 (87) |
| 1-yr Patients’ survival | 324 (98) |
| Actuarial patients’ survival | 306 (93) |
| Immunological variables | |
|---|---|
| HLA allele mismatches (<3/3/>3) | 89 (27) / 99 (30) / 142 (43) |
| Class I (A, B) (0/1/2/3/4) | 5 (2) / 55 (17) / 141 (43) / 71 (22) / 58 (18) |
| Class II: (DR) (0/1/2) | 62 (19) / 175 (53) / 93 (28) |
| HLA Eplet mismatches (mean ± SD) | |
| Class I (A, B) | 11.4 ± 6 |
| Class II: –DR/–DQ | 9.6 ± 9.7 / 8 ± 7.3 |
| CDC-XM+ | 5 (1) |
| Peak CDC-PRA >20% | 35 (11) |
| 20%−80% | 27 (77) |
| >80% | 8 (23) |
| FC-XM+ | 17 (5) |
| FC-XM anti-T+ | 2 (12) |
| FC-XM anti-B+ | 11 (64) |
| FC-XM+ and T+ and B+ | 4 (24) |
| DSA+ | 30 (9) |
| Class I+ | 9 (30) |
| Class II+ | 12 (40) |
| Class I+ and II+ | 9 (30) |
| DSA-C3d+ | 18 (5) |
| Class I+ | 2 (11) |
| Class II+ | 13 (72) |
| Class I+ and II+ | 3 (17) |
ABMR, antibody-mediated rejection; BL, borderline changes; CDC-PRA, complement-dependent cytotoxicity panel-reactive antibody; CDC-XM, complement-dependent cytotoxicity crossmatch; CNI, calcineurin inhibitor; DSA, donor-specific antibody (solid phase assay); DSA-C3d, C3d binding donor-specific antibody; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FC-XM, flow cytometry crossmatch; mTOR i, mammalian target of rapamycin inhibitors; rATG, rabbit anti-thymocyte globulin (Thymoglobulin); TCMR, T-cell−mediated rejection.
Maintenance immunosuppression was based mainly on calcineurin inhibitors (CNIs), mycophenolate mofetil and corticosteroids, and most patients received induction therapy with anti-CD25 monoclonal antibody (basiliximab) or rATG. Desensitization therapies including i.v. Ig (2 g/kg total dose), plasmapheresis (5 sessions), and/or rituximab (475 g/m2), alone or in combination, were used in a subgroup of patients if there was a positive result in any of the immunoassays. All tests were performed prior to these therapies.
The incidence of AR was 19% (65/330), 29% being antibody-mediated rejection (ABMR) and 71% T-cell–mediated rejection. One-year patient and death-censored graft survival were 98% and 99%. Among 330 LDKT patients, there were 22 graft losses (6.6%): 6 (27%) due to AR (4 = T-cell–mediated rejection, 1 = ABMR, 1 not biopsy proven), 7 (36%) due to chronic ABMR, 5 (23%) due to nonspecific interstitial fibrosis and tubular atrophy, and 2 (9%) due to recurrent glomerulonephritis. In 2 cases, graft biopsy could not be performed to characterize the cause of graft loss. A total of 21 patients (6%) died during the study period. The main causes of death were malignancies in 7 patients (33%), infections in 5 (20%), and cardiovascular events in 3 (12%).
Differences Between Donor/Recipient HLA Allele and Eplet Mismatches
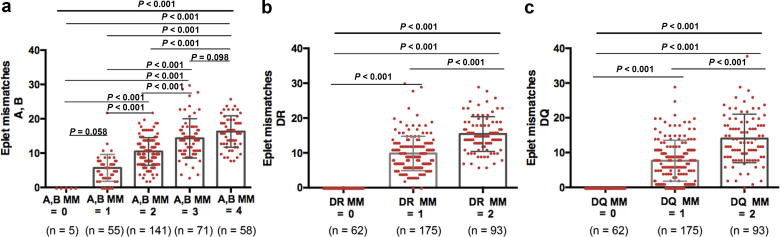
As shown in Table 1, 142 of the 330 LDKT patients (43%) showed >3 total donor/recipient HLA allele mismatches, 99 of 330 (30%) displayed 3, and 89 (27%) showed <3. The mean number of HLA class I (A, B), DR, and DQ eplet mismatches were 11.4 ± 6, 9.6 ± 9.7, and 8 ± 7.3, respectively. As illustrated in Figure 2, a progressive increase in the number of HLA eplet mismatches was observed as the number of HLA allele mismatches increased (r = 0.62, r = 0.74, and r = 0.64, for A and B, DR, and DQ mismatches, respectively; all P < 0.001).
Figure 2.
Distribution of donor-recipient human leukocyte antigen (HLA) eplet mismatches (MMs) according to the number of HLA allele MMs. (a) Distribution of donor−recipient HLA eplet MMs according to the number of class I (A, B) HLA allele MMs. (b) Distribution of donor−recipient HLA−eplet MMs according to the number of DR HLA allele MMs. (c) Distribution of donor−recipient HLA eplet MMs according to the number of DQ HLA-allele MMs. Mean ± SD HLA eplet MMs in patients with 0, 1, 2, 3, or 4 HLA allele AB MMs was 0, 5.6 ± 4, 10.5 ± 4, 14.3 ± 6, and 16.3 ± 4.5, respectively. Mean ± SD HLA eplet MMs in patients with 0, 1, or 2 HLA allele DR MMs was 0, 9.8 ± 5, and 15.3 ± 5, respectively. Mean ± SD HLA eplet MMs in patients with 0, 1, or 2 HLA allele DQ MMs was 0, 7.6 ± 6, and 14 ± 7, respectively.
Pretransplantation Immune Assays Identify Different Sensitized LDKT
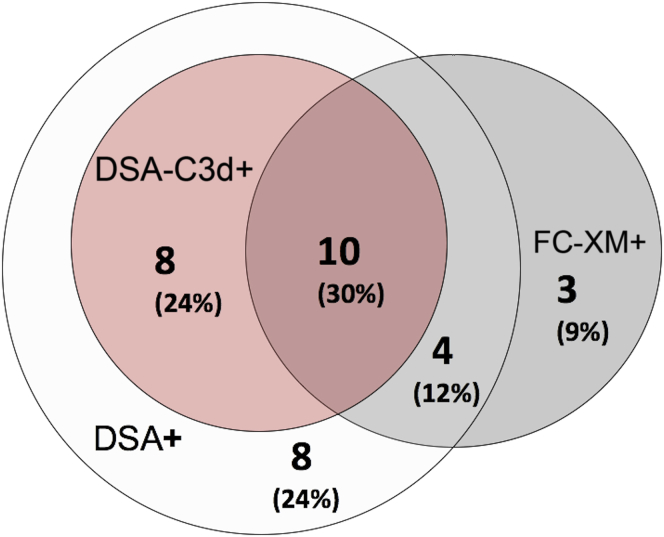
Five of 330 patients (1%) displayed a positive CDC-XM before desensitization therapy and became negative at the time of transplantation. CDC-PRA was >20% in 35 of 330 patients (11%) and >80% in 8 of 330 patients (2%). A total of 17 patients (5%) showed FC-XM+, 30 of 330 (9%) DSA+, and 18 of 330 (5%) DSA-C3d+. Thirteen patients (4%) showed more than 1 preformed DSA. As shown in Figure 3, 33 of 330 patients (10%) showed a positive result in any of the different donor-specific immunoassays: of these, only 10 of 33 (30%) had a positive result in all tests, 8 of 33 (24%) were FC-XM-/DSA+/DSA-C3d+, 4 of 33 (12%) FC-XM+/DSA+/DSA-C3d−, 8 of 33 (24%) FC-XM-/DSA+/DSA-C3d−, and 3 of 33 (9%) exclusively FC-XM+.
Figure 3.
Distribution of patients according to the presence of a positive result of the different immunoassays. In all, 33 of 330 patients (10%) showed a positive result in any of the different donor-specific immunoassays: of these 33, 10 patients (30%) showed a positive result in all tests, 8 (24%) were FC-XM−/DSA+/DSA-C3d+, 4 (12%) were FC-XM+/DSA+/DSA-C3d−, 8 (24%) were FC-XM−/DSA+/DSA-C3d−, and 3 (9%) were exclusively FC-XM+. DSA, donor-specific antibody; DSA-C3d, donor-specific antibody−C3d complement-binding; FC-XM, flow cytometry crossmatch.
Table 2 shows the main differences between the most relevant clinical and demographic characteristics and the results of the different immunoassays. As depicted, although no disparities were observed regarding donor and recipient age, gender, and cause of end-stage renal disease, patients showing positive results on the different tests more frequently had undergone transplantation previously, were females receiving an allograft from their husband or child, had a longer time on dialysis, and had more frequently received both T-cell−depletion induction and desensitization therapies.
Table 2.
Demographical and clinical characteristics according to the results of all immunoassays
| Variable | All negative tests (n = 278) |
CDC-PRA > 20% (n = 35) |
FC-XM+ (n = 17) |
DSA+ (n = 30) |
DSA-C3d+ (n = 18) |
Pa |
|---|---|---|---|---|---|---|
| Recipient age, yr (mean ± SD) | 45 ± 14 | 44 ± 12 | 47 ± 15 | 45 ± 14 | 48 ± 15 | 0.45 |
| Donor age (mean ± SD) | 52 ± 11 | 52 ± 12 | 52 ± 12 | 52 ± 13 | 54 ± 14 | 0.62 |
| Recipient gender: male, n (%) | 186 (67) | 25 (69) | 10 (59) | 17 (57) | 9 (50) | 0.58 |
| Donor gender: male, n (%) | 84 (30)b | 19 (53)b | 9 (53) | 19 (63)c | 12 (67)b | 0.006 |
| Donation child to mother/husband to wife versus other types of donors | 32 (12) | 3 (8) | 5 (29)b | 7 (17) | 5 (12)b | 0.06 |
| ESRD cause, n (%) | 0.43 | |||||
| Unknown | 108 (39) | 8 (23) | 2 (12) | 7 (23) | 5 (28) | |
| Glomerulopathy | 73 (26) | 16 (46) | 9 (53) | 13 (43) | 9 (50) | |
| ADPKD and TID | 47 (16) | 8 (23) | 3(18) | 7 (23) | 3 (17) | |
| DN and HTN | 29 (10) | 2 (6) | 1 (6) | 1 (3) | 0 | |
| Others | 21(8) | 1 (3) | 2 (12) | 2 (7) | 1 (6) | |
| Time on dialysis, mo (mean ± SD) | 19 ± 43b | 44 ± 47b | 36 ± 36 | 36 ± 35b | 30 ± 33 | 0.15 |
| Number of transplants (>1 versus 1), n (%) | 38 (14)c | 22 (61)c | 10 (59)c | 19 (63)c | 13 (89)c | <0.001 |
| HLA (allele) mismatches (mean ± SD) | ||||||
| Class I (A, B) | 2.4 ± 1 | 2.4 ± 0.9 | 2.3 ± 1 | 2.4 ± 0.9 | 2.4 ± 1 | 0.59 |
| Class II: DR | 1.1 ± 0.7 | 1.2 ± 0.6 | 1.3 ± 0.6 | 1.3 ± 0.5 | 1.3 ± 0.6 | 0.75 |
| HLA (eplet) mismatches (mean ± SD) | ||||||
| Class I (A, B) | 11 ± 5.9 | 11 ± 11.4 | 13 ± 11.2 | 12 ± 11.3 | 12 ± 11 | 0.81 |
| Class II: DR | 9 ± 6.6c | 12 ± 9.2c | 15 ± 9.2c | 14 ± 9c | 14 ± 9.27b | 0.004 |
| DQ | 7 ± 7.2 | 10 ± 7.8 | 13 ± 7.76c | 11 ± 7.7b | 13 ± 7.75b | 0.014 |
| Induction treatment: rATG n (%) | 82 (29)c | 22 (61)c | 15 (88)c | 24 (80)c | 16 (89)c | <0.001 |
| Desensitization therapy: yes, n (%) | 0c | 15 (42)c | 13 (76)c | 17 (57)c | 12 (67)c | <0.001 |
| DGF: yes, n (%) | 0c | 3 (8)c | 0 | 0 | 0 | <0.001 |
ADPKD, autosomal-dominant polycystic disease; CDC-PRA, complement-dependent cytotoxicity panel reactive antibody; DGF, delayed graft function; DN, diabetic nephropathy; DSA, donor-specific antibody (solid phase assay); DSA-C3d, C3d-binding donor-specific antibody; ESRD, end-stage renal disease; FC-XM, flow cytometry crossmatch; HTN, hypertensive nephropathy; rATG, rabbit anti-thymocyte globulin (Thymoglobulin); TID, tubulo-interstitial disease.
P for comparison of the distribution of patients’ characteristics according to the results of all baseline immunoassays.
P <0.05 for comparison between patients with positive and negative test results.
P <0.001 for comparison between patients with positive and negative test results.
Although no differences were observed between the mean number of HLA allele mismatches and results of pretransplantation immunoassays, a significantly higher mean number of HLA class II eplet mismatches was observed among pretransplantation-sensitized LDKT patients with positive test results.
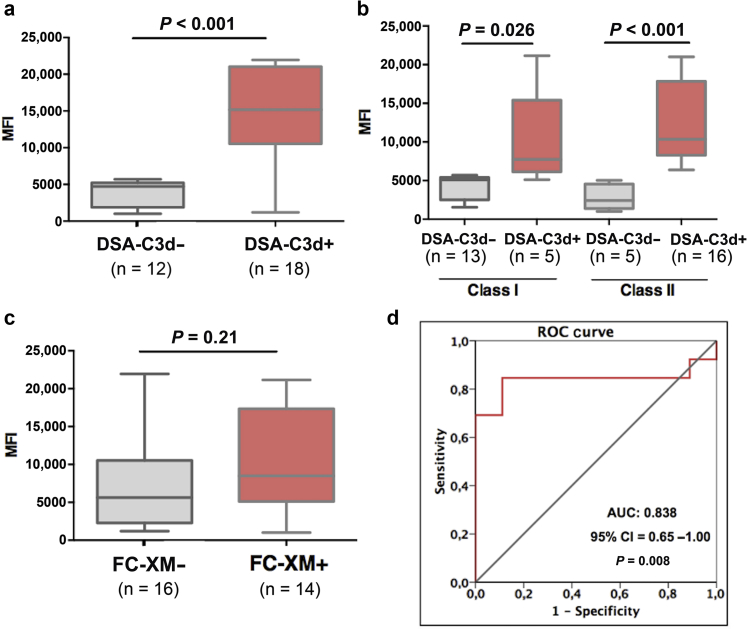
Notably, DSA-C3d+ patients displayed significantly higher mean MFI-DSA than DSA-C3d− patients (Figure 4a), as was the case for both class I and class II DSA (Figure 4b). We subsequently generated a receiver operating characteristic (ROC) curve to identify the most accurate MFI-DSA threshold differentiating DSAC3d-binding capacity. As illustrated in Figure 4d, an MFI cut-off of 6192 best discriminated C3d-binding capacity (area under the curve [AUC] = 0.838, 95% confidence interval [CI] = 0.65−1.00, P = 0.008). Although numerically higher, no statistically significant differences were observed comparing MFI-DSA between FC-XM+ and FC-XM− patients (Figure 4c).
Figure 4.
Association between mean fluorescence intensity (MFI)−donor-spectific antibody (DSA) and donor-specific antibody−C3d complement-binding (DSA-C3d) and flow cytometry crossmatch (FC-XM) positivity. (a) Comparison between mean MFI and DSA-C3d positivity (both class I and class II). (b) Comparison of MFI between either class I or class II DSA-C3d+ and DSA-C3d−. (c) Comparison between mean MFI-DSA and FC-XM positivity. (d) Receiver operating characteristic (ROC) curve analysis of MFI-DSA predicting DSA-C3d positivity. Mean MFI DSA-C3d– versus DSA-C3d+: 3799 ± 1773 versus 12,414 ± 5884, P < 0.001. Mean class I MFI DSA-C3d− versus DSA-C3d+ was 4259 ± 1612 versus 10,608 ± 7569, P = 0.026, respectively. Mean class II MFI DSA-C3d− versus DSA-C3d+ was 2801 ± 1629 versus 12,355 ± 5049, P < 0.001, respectively. Mean MFI-DSA in FC-XM− and FC-XM+ cases: 7619 ± 6358 versus 10,510 ± 6130, P = 0.21. Area under the curve (AUC): 0.838, 95% confidence interval (CI) = 0.65−1.00, P = 0.008.
Value of Baseline Immunoassays and HLA-Eplet Mismatching Predicting AR
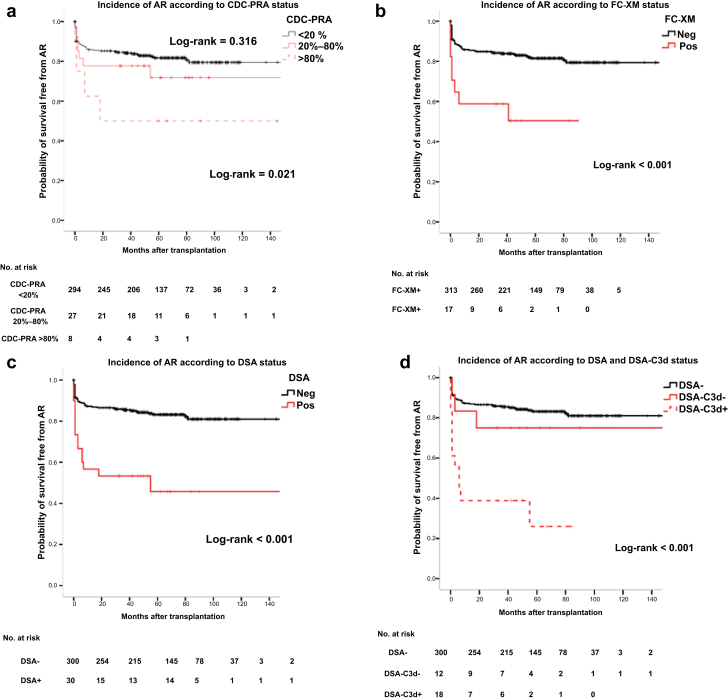
As shown in Figure 5a to d, patients with any positive assay, either CDC-PRA>20%, FC-XM+, DSA+ or DSA-C3d+, showed significantly higher incidence of AR than those with negative tests. Unlike HLA allele mismatches, mean HLA (DR)-eplet incompatibilities were higher in patients developing AR than those that did not. When analyzing the type of AR, this association was only observed for patients developing ABMR (Supplementary Figure S1).
Figure 5.
Kaplan−Meier free-survival curves for acute rejection (AR) according to the different immunoassays investigated. (a) Kaplan−Meier survival curves free from AR according to complement-dependent panel-reactive antibody (CDC-PRA) status. (b) Kaplan−Meier survival curves free from AR according to flow cytometry crossmatch (FC-XM) positivity. (c) Kaplan−Meier survival curves free from AR according to donor-specific antibody (DSA) positivity. (d) Kaplan−Meier survival curves free from AR according to donor-specific antibody−C3d complement-binding (DSA-C3d) positivity. Cumulative incidence of AR: CDC-PRA <20%: 53 (18%); CDC-PRA 20−80%: 7 (26%); CDC-PRA >80%: 4 (50%); log rank = 0.043. FC-XM–: 57 (18%); FC-XM+: 8 (47%); log rank = <0.001. DSA−: 50 (17%); DSA+: 15 (50%); log-rank <0.001. Cumulative incidence of AR: DSA−: 50 (17%); DSA-C3d−: 3 (25%); DSA-C3d+: 12 (67%); log-rank <0.001. DSA–, absence of donor-specific antibodies by solid phase assay; DSAC3d–, DSA without C3d binding capacity; DSAC3d+, DSA with C3d binding capacity; Neg, negative; Pos, positive.
When assessing the main clinical, demographic, therapeutic, and immunological variables predicting AR in a multivariate logistic regression, although desensitization therapy, high HLA(DR) eplet mismatches, and all positive pretransplantation immunoassays were associated with higher incidence of AR, only DSA-C3d+ independently predicted AR (OR = 6.64, 95% confidence interval [CI] = 1.14−36.56, P = 0.038). Likewise, when MFI-DSA >6190 or the combination of both FC-XM+ and DSA-C3d+ were evaluated in the same model rather than DSA-C3d, they also independently predicted AR (OR = 7.54, 95% CI = 1.11−50.85, P = 0.038, and OR = 4.94, 95% CI = 0.98−24.81, P = 0.05, respectively). The combination of other assays did not reach statistical significance (Table 3).
Table 3.
Univariate and multivariate binary logistical regression for AR
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Recipient age (yr) | 0.99 | 0.97–1.007 | 0.1 | |||
| Donor gender: female | 1.31 | 0.72–2.36 | 0.37 | |||
| Child to mother or husband to wife versus other types of donors | 1.19 | 0.54–2.64 | 0.66 | |||
| Time on dialysis (mo) | 1.001 | 0.99–1.007 | 0.79 | |||
| Transplant number >1 versus 1 | 1.49 | 0.79–2.79 | 0.22 | |||
| Induction treatment: rATG | 1.32 | 0.75–2.30 | 0.33 | |||
| CNI-free IS regimen: yes | 1.65 | 0.31–8.71 | 0.55 | |||
| Desensitization therapy: yes | 2.88 | 1.19–6.98 | 0.019 | 2.68 | 0.49–14.85 | 0.26 |
| CDC-XM + prior to desensitization | 2.77 | 0.45–16.94 | 0.27 | |||
| HLA allele mismatches | 1.01 | 0.84–1.21 | 0.93 | |||
| Class I | 1.082 | 0.82–1.42 | 0.57 | |||
| Class II | 1.12 | 0.75–1.69 | 0.55 | |||
| HLA eplet mismatches | ||||||
| Class I (A, B) | 1.012 | 0.966–1.060 | 0.61 | |||
| Class II: DR | 1.042 | 1.001–1.084 | 0.043 | 1.02 | 0.98–1–07 | 0.24 |
| DQ | 1.022 | 0.986–1.060 | 0.22 | |||
| CDC-PRA > 20 % | 2.08 | 0.96–4.51 | 0.06 | 1.18 | 0.35–3.99 | 0.79 |
| CDC-PRA > 80% | 4.35 | 1.06–17.89 | 0.042 | 3.01 | 0.45–20.3 | 0.26 |
| FC-XM anti T or B+ | 3.99 | 1.48–10.79 | 0.006 | 1.53 | 0.28–8.36 | 0.62 |
| DSA+ | 5.00 | 2.29–10.88 | <0.001 | 1.89 | 0.41–8.82 | 0.41 |
| DSA-C3d+ | 9.77 | 3.51–27.20 | <0.001 | 6.64 | 1.14–36.56 | 0.038 |
| DSA MFI > 6190a | 10.59 | 3.54–31.73 | <0.001 | 7.54 | 1.11–50.85 | 0.038 |
| FC-XM+/DSA+/b | 4.45 | 1.50–13.17 | 0.007 | 3.59 | 0.78–16.51 | 0.10 |
| FC-XM+/DSA-C3d+b | 6.64 | 1.81–24.26 | 0.004 | 4.94 | 0.98–24.81 | 0.05 |
CDC-XM, complement-dependent cytotoxicity crossmatch; CDC-PRA, complement-dependent cytotoxicity panel-reactive antibody; CI, confidence interval; CNI, calcineurin inhibitor; DSA, donor-specific antibody (solid phase assay); DSA-C3d, C3d-binding donor-specific antibody; FC-XM, flow cytometry crossmatch; MFI, mean fluorescence intensity; OR, odds ratio; rATG, rabbit anti-thymocyte globulin (Thymoglobulin®).
In the multivariate model for acute rejection evaluating the impact of each test individually (adjusted for desensitization therapy and human leukocyte antigen [HLA] DR-eplet mismatches), DSA+ also appears as an independent variable (OR = 5.1, 95% CI = 1.81–14.41, P = 0.002). Both DSA-C3d+ and DSA MFI > 6190 are independently correlated (OR = 12.0, 95% CI = 2.98–48.34, P < 0.001; OR = 15.6, 95% CI = 2.97–81.88, P = 0.001, respectively).
Analysis adjusted for desensitization therapy, HLA-DR eplet mismatches, CDC-PRA > 20%, CDC-PRA > 80%, FC-XM+, DSA+.
Analysis adjusted for desensitization therapy, HLA-DR eplet mismatches, CDC-PRA > 20%, CDC-PRA > 80%.
When breaking down DSA type, both class I and class II DSA (C3d-binding and non−C3d-binding) were associated with increased risk of AR on univariate analysis, and only C3d-binding DSA (predominantly class II) was independently correlated (data not shown).
The predictive values of each assay for AR, as well as either for ABMR or T-cell–mediated rejection, showed high specificity and negative predictive value for those tests providing antidonor reactivity (FC-XM, DSA and DSA-C3d) (Supplementary Table S1).
Interestingly, when excluding from the analysis all patients with any positive immunoassay at baseline, high HLA(DR) eplet mismatches independently predicted higher risk of ABMR (OR = 1.14, 95% CI = 1.02−1.27, P = 0.02). During ABMR events in this subgroup, most of the patients (57%) developed anti-DR antibodies de novo (Supplementary Table S2).
Pretransplantation Immune Sensitization and Kidney Graft Function Progression
As illustrated in Supplementary Figure S2, patients experiencing AR showed lower 12-month, 24-month, and 5-year eGFR than those who did not. Similarly, DSA+ and DSA-C3d+ patients displayed worse graft function progression at 1, 2, and 5 years than DSA− and DSA-C3d− patients. Conversely, no differences were observed according to CDC-PRA and FC-XM tests.
Pretransplantation Immunoassays and Graft and Patient Survival
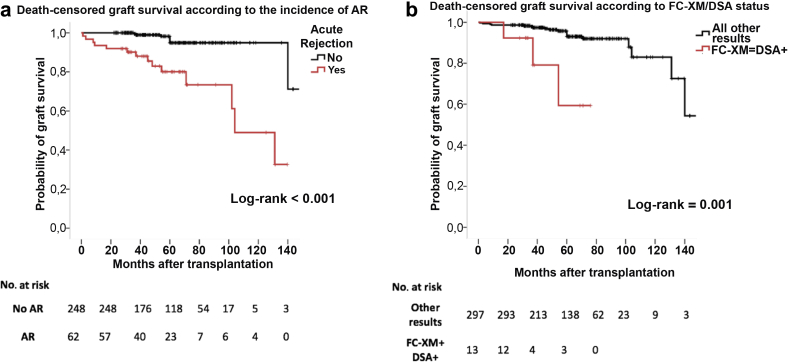
Death-censored graft survival was significantly poorer in patients who experienced AR than among those who did not, as well as among patients showing a double-positive test as FC-XM+/DSA+ (Figure 6a and b). On univariate and multivariate Cox-regression analyses (Table 4), whereas young recipient age, previous transplantations, low 6-month eGFR, AR, and a positive result in any immunoassay were associated with graft loss, only AR (HR = 6.68, 95% CI = 2.51−17.78, P < 0.001), young age (HR = 0.95, 95% CI = 0.92−0.99, P = 0.028), and low 6-month eGFR (HR = 0.96, 95% CI = 0.93−0.99, P = 0.005) independently predicted graft loss.
Figure 6.
Kaplan−Meier free-survival curves of death-censored graft survival. (a) Kaplan−Meier free-survival curve for death-censored graft survival according to acute rejection (AR). (b) Kaplan−Meier free-survival curve for death-censored graft survival according to flow cytometry crossmatch (FC-XM)/donor-specific antibody (DSA). Cumulative incidence of death-censored graft loss: no AR: 8 (3%); AR: 14 (23%); log-rank <0.001. All other results: 19 (6%); FC-XM/DSA+: 3 (23%); log-rank = 0.001.
Table 4.
Univariate and multivariate Cox regression for death-censored graft loss
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Recipient age (yr) | 0.96 | 0.93–1.00 | 0.051 | 0.95 | 0.92–0.99 | 0.028 |
| Donor age (yr) | 1.015 | 0.98–1.053 | 0.42 | |||
| Time on dialysis (mo) | 1.006 | 0.998–1.013 | 0.13 | |||
| Transplant number >1 versus 1 | 2.23 | 0.93–5.35 | 0.071 | 1.97 | 0.71–5.44 | 0.19 |
| Child to mother or husband to wife versus other types of donors | 2.23 | 0.29–16.70 | 0.43 | |||
| eGFR 6 mo (ml/min per 1.73 m2) | 0.97 | 0.94–0.99 | 0.009 | 0.96 | 0.93–0.99 | 0.005 |
| HLA allele mismatches | 0.93 | 0.69–1.27 | 0.67 | |||
| Class I | 1.03 | 0.65–1.62 | 0.89 | |||
| Class II | 0.79 | 0.43–1.45 | 0.44 | |||
| HLA eplet mismatches | ||||||
| Class I (A, B) | 1.03 | 0.95–1.11 | 0.48 | |||
| Class II: DR | 1.01 | 0.927–1.09 | 0.86 | |||
| DQ | 1.01 | 0.93–1.09 | 0.78 | |||
| Induction treatment: rATG | 1.62 | 0.63–4.13 | 0.31 | |||
| Desensitization therapy: yes | 3.81 | 1.09–13.30 | 0.036 | 3.57 | 0.52–24.4 | 0.19 |
| CDC-XM + prior to desensitization | 3.44 | 0.46–25.92 | 0.23 | |||
| Acute rejection | 8.47 | 3.39–21.12 | <0.001 | 6.68 | 2.51–17.78 | <0.001 |
| CDC-PRA > 20% | 3.01 | 1.19–7.64 | 0.020 | 1.59 | 0.45–5.60 | 0.48 |
| CDC-PRA >80% | 1.028 | 1.13–8.08 | 0.97 | |||
| FC-XM anti T or B+ | 5.81 | 1.66–20.34 | 0.006 | 3.43 | 0.58–20.27 | 0.17 |
| DSA+ | 4.42 | 1.69–11.52 | 0.002 | 2.23 | 0.50–18.38 | 0.23 |
| DSA-C3d+ | 4.31 | 1.24–14.99 | 0.022 | 2.61 | 0.36–18.57 | 0.34 |
| DSA MFI > 6190a | 5.07 | 1.46–17.67 | 0.011 | 2.20 | 0.33–14.81 | 0.42 |
| FC-XM+/DSA+b | 6.56 | 1.88–22.86 | 0.003 | 3.99 | 0.86–18.66 | 0.07 |
| FC-XM+/DSA-C3d+b | 5.67 | 1.29–24.87 | 0.021 | 2.89 | 0.52–15.99 | 0.22 |
CDC-PRA, complement-dependent cytotoxicity panel-reactive antibody; CDC-XM, complement-dependent cytotoxicity crossmatch; CI, confidence interval; DSA, donorspecific antibody (solid phase assay); DSA-C3d, C3d-binding donor-specific antibody; eGFR, estimated glomerular filtration rate; FC-XM, flow cytometry crossmatch; HR, hazard ratio; MFI, mean fluorescence intensity; rATG, rabbit anti-thymocyte globulin (Thymoglobulin).
In the multivariate Cox regression model excluding acute rejection and analyzing all tests individually (adjusted for recipient age, transplant number, eGFR 6 mo, and desensitization therapy), only FC-XM+/DSA+ was independently predicting graft loss (HR = 8.01, 95% CI = 1.27–50.48, P = 0.027).
Analysis adjusted for recipient age, transplant number, eGFR 6 mo, desensitization therapy, acute rejection, CDC-PRA>20%, FC-XM+, DSA+.
Analysis adjusted for recipient age, transplant number, eGFR 6 mo, desensitization therapy, acute rejection, and CDC-PRA > 20%.
When main clinical, demographic, and immunological variables were evaluated for their influence predicting patient death, only younger recipient age was significantly associated with lower risk of death (OR = 0.93, 95% CI = 0.90−0.97, P < 0.001).
Conclusion
Although a number of immunoassays assessing the degree of anti-HLA immune sensitization and a more accurate HLA-matching approach evaluating HLA epitope compatibility have emerged in the last decades, there is no clear consensus regarding the type and number of immune tests that more efficiently may discriminate kidney transplant candidates with poorer graft outcomes. Here, evaluating a large cohort of LDKT recipients from 2 different transplant programs, we first show that although all current immunoassays are capable of identifying transplant candidates with different degrees of humoral sensitization, they display a rather poor overlap among them. Furthermore, although a positive result of any of the tests was associated with a higher incidence of AR, only DSA-C3d independently predicted high risk of AR, thus highlighting the greater aggressiveness of such preformed DSAs leading to AR. In line with previous works, high MFI-DSAs more likely fixed complement and displayed a stronger AR risk, particularly ABMR. However, the presence of a pretransplantation DSA, regardless of its complement-binding capacity, together with a positive FC-XM, were the strongest correlates of allograft loss, suggesting persistent alloimmune activation over time despite chronic immunosuppression.
An important finding in our study is that despite the fact that no differences were observed regarding HLA allele matching and higher rejection risk, most sensitized individuals were poorly matched at the HLA eplet level. This might be of great importance, particularly among this high-risk population, as poor matching at this molecular level might increase the likelihood of DSA binding to true immunogenic donor epitopes and thus lead to allograft rejection. In this regard, we found that the higher the mean donor/recipient HLA eplet mismatch number, the higher the incidence of AR, particularly ABMR. Remarkably, the importance of optimal matching at this level, was also replicated among nonsensitized LDKT patients, in whom a higher donor/recipient HLA-DR eplet mismatch correlated with a significantly higher incidence of ABMR.
In agreement with previous works3, 4, 5, 6, 14, 15, 16 although in our cohort all immunoassays were associated with a higher incidence of AR, the respective discrimination capacity significantly varied among them. All of these tests are currently being performed in most transplant programs around the world. Therefore, in our analysis, we assessed in multivariate models the different immunological tests investigated. In fact, although all of them evaluate the degree of anti-HLA humoral sensitization, they all provide different insights regarding the biological mechanisms by which the humoral immune response might be activated, and thus differently predict the immunological risk of transplant patients.
Nevertheless, clearly overlapping immune tests such as DSA/C3d+ and DSA with MFI >6190, or the 2 combinations of FC-XM+/DSA+ and FC-XM+/DSA-C3d+, were analyzed in different models, as they might provide the same biological information and thus overfit the model.
Indeed, we first observed that patients displaying a positive test result at baseline shared similar clinical backgrounds such as a longer dialysis time, previous transplantations, female sex with previous pregnancies, and receipt of a transplant from donors with whom they had previously been exposed to alloantigens, such as husband or child to wife/mother, respectively.
However, although all tests had similarly high negative predictive value, FC-XM+ and DSA-C3d+ showed the greatest specificity in predicting AR. This observation strongly suggests the greater aggressiveness of such preformed DSAs as compared to those not fixing complement despite similar immunosuppression; thus, guided preventive strategies would be highly recommended.27 In our study, patients receiving desensitization therapy because of any positive pretransplantation immunoassay result appeared to be at high risk for AR, even if they achieved a negative CDC-XM after such a preventive strategy. However, despite this greater AR risk, desensitization therapy per se did not have an impact on graft or patient survival, thus highlighting the relevance of the result of the immunoassay performed after desensitization, which could guide the decision to go further into transplantation or to reconsider alternative approaches such as paired-exchange donation programs.
Although pretransplantation sensitization, regardless of the type of immunoassay used, was associated with poorer graft survival, only low 6-month eGFR, previous AR, and FC-XM+/DSA+ were independent predictors of graft loss. These data suggest the need for considering these 2 tests as main immunoassays for immune risk stratification before transplantation.
Our study has some limitations. As previously reported, not all DSA with high MFI fix complement, and conversely, some low MFI-DSA are capable of binding complement in vitro, due to a prozone effect that may lead to falsely low MFI in the presence of a high load of antibodies per bead. A titration or DSA IgG subclass characterization may overcome such a limitation, although this would be costly and labor intensive for daily clinical practice.28, 29, 30 Also, 3 patients displayed a very mild but positive FC-XM without any detectable DSA. Although our main hypothesis is that they were all false-positive test results, 1 patient displayed a single anti-C HLA antibody with a very low MFI (500); thus, in the absence of the HLA C antigen donor type, we cannot exclude the presence of a potential DSA in this patient. The retrospective nature of the study and the fact that the results of some immunoassays were known before transplantation may weaken the impact of our findings. However, the large and consecutive cohort of LDKT patients from 2 different transplant centers, and the multivariate statistical models controlling for main immunologic, clinical, therapeutic, and demographic variables, significantly counterbalance these drawbacks. In addition, although high-resolution HLA typing was not available in this work to enumerate donor/recipient eplet mismatches and was inferred using a local frequency table typed by sequence-based typing, a strong correlation between high- and low-resolution typing predicting the development of de novo DSA has been previously shown, thus suggesting that immunogenic epitope mismmatches might also be inferred by using low-resolution HLA typing.21 Furthermore, as our patient population was highly homogeneous in terms of ethnicity, this significantly reduces the difference in this estimation approach. Finally, the large number of variables analyzed in the multivariate model could have possibly hidden an interaction among them. However, our results were confirmed using separate models assessing each test individually.
In summary, solid-phase antibody identification and flow cytometry crossmatch assays are the 2 main tests that are highly warranted for a compelling stratification of immune risk prior to transplantation. Moreover, special caution should taken in patients displaying high MFI-DSA, as they may be more likely to develop posttransplantation AR despite receiving strong immunosuppression. Finally, a more accurate donor/recipient HLA-matching evaluation at the HLA-DR eplet level is highly recommended to reduce the risk of posttransplantation alloimmune activation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was funded by 2 national Spanish grants from the Spanish Institute of Health (ISCiii; PI13/01263 and PI16/01321), Federer funding, a way to build Europe. Also, this work was partially supported by the European commission grant BIO-DRIM (No.FP7/2007-2017). OB did also have an intensification research grant from the National Institute of Health (ISCiii; INT15/00112). We also would like to acknowledge our Biobank unit for the careful management of all our samples. We are thankful to Cristian Tebe (Statistical department, IDIBELL Bellvitge Biomedical Research Institute, Barcelona) for his help in the statistical analyses. MM received a fellowship grant from ESOT (European Society for Organ Transplantation).
Acknowledgments
Author Contributions
MM and EM collected the data, performed data analysis and interpretation and contributed to the writing of the manuscript. EM, IR, AM, NM, DC, FD, SGV, JMC, JMG, and OB contributed to data collection and clinical follow-up of the included patients. ERM and JM carried out and supervised the immunological tests described in the study. OB designed the study, contributed to the writing of the manuscript, revised the final version and supervised the project. All authors discussed the results and commented on the manuscript.
Footnotes
Table S1. Accuracy of the different humoral alloimmunoassays predicting acute rejection and different types of acute rejection.
Table S2. Univariate and multivariate binary logistical regression for ABMR excluding patients with positive pretransplantation humoral immunoassays (PRA<20%; FC-XM−; DSA−; DSA-C3d−: 278 patients).
Figure S1. Comparison of mean number of eplet mismatches in patients experiencing acute rejection episodes. (A) Acute rejection. (B) T-cell−mediated rejection. (C) Antibody-mediated rejection.
Figure S2. Kidney allograft function progression according to the different immunoassays. (A) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to incidence of acute rejection. (B) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<20% or >20%). (C) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<80% or >80%). (D) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to FC-XM positivity. (E) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA positivity. (F) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA-C3d positivity.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Accuracy of the different humoral alloimmunoassays predicting acute rejection and different types of acute rejection.
Univariate and multivariate binary logistical regression for ABMR excluding patients with positive pretransplantation humoral immunoassays (PRA<20%; FC-XM−; DSA−; DSA-C3d−: 278 patients).
Comparison of mean number of eplet mismatches in patients experiencing acute rejection episodes. (A) Acute rejection. (B) T-cell−mediated rejection. (C) Antibody-mediated rejection.
Kidney allograft function progression according to the different immunoassays. (A) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to incidence of acute rejection. (B) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<20% or >20%). (C) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<80% or >80%). (D) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to FC-XM positivity. (E) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA positivity. (F) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA-C3d positivity.
References
- 1.Hart A., Smith J.M., Skeans M.A. OPTN/SRTR 2015 annual data report: kidney. Am J Transplant. 2017;17(suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pruthi R., Hilton R., Pankhurst L. UK Renal Registry 16th annual report: chapter 4. Demography of patients waitlisted for renal transplantation in the UK: national and centre-specific analyses. Nephron Clin Pract. 2013;125:81–98. doi: 10.1159/000360023. [DOI] [PubMed] [Google Scholar]
- 3.Couzi L., Araujo C., Guidicelli G. Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 2011;91:527–535. doi: 10.1097/TP.0b013e31820794bb. [DOI] [PubMed] [Google Scholar]
- 4.Eng H.S., Bennett G., Tsiopelas E. Anti-HLA donor-specific antibodies detected in positive B-cell crossmatches by Luminex predict late graft loss. Am J Transplant. 2008;8:2335–2342. doi: 10.1111/j.1600-6143.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohan S., Palanisamy A., Tsapepas D. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orandi B.J., Garonzik-Wang J.M., Massie A.B. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014;14:1573–1580. doi: 10.1111/ajt.12786. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 8.Port F.K., Wolfe R.A., Mauger E.A. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. [PubMed] [Google Scholar]
- 9.Patel R., Terasaki P.I. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735–739. doi: 10.1056/NEJM196904032801401. [DOI] [PubMed] [Google Scholar]
- 10.Mahoney R.J., Ault K.A., Given S.R. The flow cytometric crossmatch and early renal transplant loss. Transplantation. 1990;49:527–535. doi: 10.1097/00007890-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Karpinski M., Rush D., Jeffery J. Flow cytometric crossmatching in primary renal transplant recipients with a negative anti-human globulin enhanced cytotoxicity crossmatch. J Am Soc Nephrol. 2001;12:2807–2814. doi: 10.1681/ASN.V12122807. [DOI] [PubMed] [Google Scholar]
- 12.Loupy A., Lefaucheur C., Vernerey D. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 13.Schinstock C.A., Gandhi M.J., Stegall M.D. Interpreting anti-HLA antibody testing data: a practical guide for physicians. Transplantation. 2016;100:1619–1628. doi: 10.1097/TP.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachelet T., Visentin J., Guidicelli G. Anti-HLA donor-specific antibodies are not created equally. Don’t forget the flow…. Transpl Int. 2016;29:508–510. doi: 10.1111/tri.12745. [DOI] [PubMed] [Google Scholar]
- 15.Schinstock C., Gandhi M., Cheungpasitporn W. Kidney transplant with low levels of DSA or low positive B-flow crossmatch. Transplantation. 2017;101:2429–2439. doi: 10.1097/TP.0000000000001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adebiyi O.O., Gralla J., Klem P. Clinical significance of pretransplant donor-specific antibodies in the setting of negative cell-based flow cytometry crossmatching in kidney transplant recipients. Am J Transplant. 2016;16:3458–3467. doi: 10.1111/ajt.13848. [DOI] [PubMed] [Google Scholar]
- 17.Sicard A., Ducreux S., Rabeyrin M. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol. 2015;26:457–467. doi: 10.1681/ASN.2013101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malheiro J., Tafulo S., Dias L. Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation. Transpl Int. 2016;30:347–359. doi: 10.1111/tri.12873. [DOI] [PubMed] [Google Scholar]
- 19.Viglietti D., Loupy A., Vernerey D. Value of donor–specific anti–HLA antibody monitoring and characterization for risk stratification of kidney allograft loss. J Am Soc Nephrol. 2017;28:702–715. doi: 10.1681/ASN.2016030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams R.C., Opelz G., McGarvey C.J. The risk of transplant failure with HLA mismatch in first adult kidney allografts from deceased donors. Transplantation. 2016;100:1094–1102. doi: 10.1097/TP.0000000000001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiebe C., Pochinco D., Blydt-Hansen T.D. Class II HLA epitope matching—a strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant. 2013;13:3114–3122. doi: 10.1111/ajt.12478. [DOI] [PubMed] [Google Scholar]
- 22.Lachmann N., Niemann M., Reinke P. Donor recipient matching based on predicted recognizable HLA epitopes predicts the incidence of de novo donor-specific HLA antibodies following renal transplantation. Am J Transplant. 2017;17:3076–3086. doi: 10.1111/ajt.14393. [DOI] [PubMed] [Google Scholar]
- 23.Wiebe C., Rush D.N., Nevins T.E. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol. 2017;28:3353–3362. doi: 10.1681/ASN.2017030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas M., Sis B., Racusen L.C. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 25.Baxter-Lowe L.A. HLA-DR sequence-based typing. In: Hahn A., editor. American Society for Histocompatability and Immunogenetics Laboratory Manual. 4th ed. American Society for Histocompatability and Immunogenetics; Mt. Laurel, NJ: 2000. pp. 8.1–8.6. [Google Scholar]
- 26.Jordan S.C., Tyan D., Stablein D. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. doi: 10.1097/01.ASN.0000145878.92906.9F. [DOI] [PubMed] [Google Scholar]
- 27.Lefaucheur C., Viglietti D., Hidalgo L.G. Complement-activating anti-HLA antibodies in kidney transplantation: allograft gene expression profiling and response to treatment. J Am Soc Nephrol. 2018;29:620–635. doi: 10.1681/ASN.2017050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claisse G., Absi L., Cognasse F. Relationship between mean fluorescence intensity and C1q/C3d-fixing capacities of anti-HLA antibodies. Hum Immunol. 2017;78:336–341. doi: 10.1016/j.humimm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Zeevi A., Lunz J., Feingold B. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32:98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tambur A.R., Herrera N.D., Haarberg K.M.K. Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant. 2015;15:2421–2430. doi: 10.1111/ajt.13295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Accuracy of the different humoral alloimmunoassays predicting acute rejection and different types of acute rejection.
Univariate and multivariate binary logistical regression for ABMR excluding patients with positive pretransplantation humoral immunoassays (PRA<20%; FC-XM−; DSA−; DSA-C3d−: 278 patients).
Comparison of mean number of eplet mismatches in patients experiencing acute rejection episodes. (A) Acute rejection. (B) T-cell−mediated rejection. (C) Antibody-mediated rejection.
Kidney allograft function progression according to the different immunoassays. (A) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to incidence of acute rejection. (B) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<20% or >20%). (C) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to CDC-PRA (<80% or >80%). (D) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to FC-XM positivity. (E) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA positivity. (F) Comparison between mean eGFR at 12 months, 24 months, and 5 years according to DSA-C3d positivity.