Abstract
Aliphatic alcohols are common and bulk chemicals in organic synthesis. The site-selective functionalization of non-activated aliphatic alcohols is attractive but challenging. Herein, we report a silver-catalyzed δ-selective Csp3-H bond functionalization of abundant and inexpensive aliphatic alcohols. Valuable oximonitrile substituted alcohols are easily obtained by using well-designed sulphonyl reagents under simple and mild conditions. This protocol realizes the challenging δ-selective C–C bond formation of simple alkanols.
Aliphatic alcohols usually require pre-activation for successful functionalization of their carbon chain. Here, the authors report a silver-catalyzed δ-selective functionalization of aliphatic alcohols via Csp3-H bond cleavage under mild conditions without the need of substrate pre-activation.
Introduction
Aliphatic alcohols that are readily availble and bulk chemicals serve as important building blocks for the construction of value-added molecules for organic chemists1,2. However, the selective modification or functionalization of aliphatic alcohols on the carbon chain is very hard due to the inertness of Csp3-H bonds as well as the difficulties in the control of regioselectivity, and therefore remains a challenging issue3–6. Since the original discoveries by Barton, the remote functionalization via 1,5-Hydrogen Atom Transfer (1,5-HAT) of alkoxyl radicals has been frequently applied in different transformations. However, these methods require a pre-activation of alcohols and the corresponding precursors, such as nitrite esters7–9, peroxy compounds10–13, hypohalites14–18, N-alkoxyphthalimides19–21, N-alkoxylpyridine-2-thiones22,23, and lead(IV) alkoxides24,25, are sometimes hard to handle or prepare and usually need harsh conditions for the genaration of alkoxyl radicals (Fig. 1a). Strategies for the direct activation of simple alcohols under mild conditions are highly desirable.
Fig. 1.
The remote site-selective functionalization reactions of aliphatic alcohols. a Traditional indirect strategy to alkoxyl radical. b Alcohol-directed γ C–O bond formation. c Visible light promoted intra-molecular migration and δ-selective C–N bond formation. d This work: Ag-catalyzed direct δ-selective C–C bond functionalization
The direct generation of alkoxyl radicals from alcohols is attractive but more challenging as the bond dissociation energy of O–H bond is around 105 kcal mol–126. To address this problem, transition metal catalysis or photocatalysis were developed inducing β-scission reactions of tertiary alcohols27–35. Hartwig and coworkers pioneeringly reported a direct γ-selective C–O bond formation of alcohols with the assistance of silicon reagents by Ir-catalysis (Fig. 1b)36,37. Zhu and coworkers developed a novel intra-molecular heteroaryl migration of tertiary alcohols by photocatalysis38. With a cerium-based photocatalyst, Zuo and coworkers reported an elegant δ-selective C–N bond formation reaction of primary alcohols enabled by a ligand-to-metal charge transfer excitation (Fig. 1c)39. To the best of our knowledge, the direct and selective inter-molecular C–C bond formation of simple alcohols via distal Csp3-H activation to afford corresponding oxime ether products is still unknown.
Herein, we describe a silver-catalyzed direct δ-selective Csp3-H bond functionalization of simple and readily available alkanols under mild conditions (Fig. 1d). This reaction features the three-fold advantages: without the need of pre-activation, a simple Ag/oxidant system enables this selective Csp3-H functionalization of aliphatic alkanols; inert C–H bond activation with excellent regio-selectivities and chemo-selectivities are achived; the mild reaction conditions, broad substrate scope, and potential for further applications make this method attractive for the synthesis of valuable functionalized alcohols.
Results
Initial optimization of the reaction conditions
Oximes and oxime ethers have various synthetic applications and exist in multiple bioactive molecules40,41. In recent years, the well-designed sulfonyl oxime ether reagents showed great reactivity in alkyl radical chemistry42–46. Inspired by the recently developed silver-catalyzed radical reactions47–60 and our continues interest in developing radical reactions with sulfonyl reagents58,59, we envisioned that the sequence silver-catalyzed radical process would achieve the challenging C–H bond functionalization. To investigate our hypothesis, we initially chose A as the radical acceptor for the direct functionalization of the widely existed 1-octanol (1a). We tested several oxidants with AgNO3 (20 mol%) in CH3CN/H2O (1:1) under argon atmosphere at 50 °C (Table 1, entries 1–4). To our delight, when Na2S2O8 was used as the oxidant, the oxime ether product 2a was obtained in 37% yield (Table 1, entry 3). K2S2O8 was a bit more efficient compared with Na2S2O8 (40%, Table 1, entry 4). The yield of 2a based on the recovered starting materials increased to 71% when acetone/H2O (1:1) was employed as solvents (Table 1, entry 5). The reaction is unable to carry out without silver catalyst (Table 1, entry 6). Moreover, the solubility of silver salts is vital in this reaction as the insoluble AgI was inefficient (Table 1, entry 7). Other metal catalysts, including CuCl2, FeCl2, and MnBr2 did not show efficiency (Table 1, entries 8–10). Other solvents such as PhCF3/H2O or DMSO/H2O were inactive (Table 1, entries 11–12). Furthermore, the substituted aryl sulfonyl reagent (B, C) as well as the alkyl sulfonyl reagent D are not more effective compared with reagent A (Table 1, entries 13–15).
Table 1.
Optimization of reaction conditions

| |||||
|---|---|---|---|---|---|
| Entry | Cat (20 mol%) | Reagent | Oxidant | Solvent (1 mL/1 mL) | Yield (%)a |
| 1 | AgNO3 | A | PIDA | CH3CN/H2O | Trace |
| 2 | AgNO3 | A | Oxone | CH3CN/H2O | Trace |
| 3 | AgNO3 | A | Na2S2O8 | CH3CN/H2O | 37 |
| 4 | AgNO3 | A | K2S2O8 | CH3CN/H2O | 40 |
| 5 | AgNO3 | A | K2S2O8 | Acetone/H2O | 55(71)b |
| 6 | – | A | K2S2O8 | Acetone/H2O | n.d. |
| 7 | AgI | A | K2S2O8 | Acetone/H2O | Trace |
| 8 | CuCl2 | A | K2S2O8 | Acetone/H2O | Trace |
| 9 | FeCl2 | A | K2S2O8 | Acetone/H2O | Trace |
| 10 | MnBr2 | A | K2S2O8 | Acetone/H2O | Trace |
| 11 | AgNO3 | A | K2S2O8 | PhCF3/H2O | n.d. |
| 12 | AgNO3 | A | K2S2O8 | DMSO/H2O | Trace |
| 13 | AgNO3 | B | K2S2O8 | Acetone/H2O | 32 |
| 14 | AgNO3 | C | K2S2O8 | Acetone/H2O | 54 |
| 15 | AgNO3 | D | K2S2O8 | Acetone/H2O | 37 |
Reaction conditions: 1a (0.2 mmol), catalyst (0.04 mmol), oxidant (0.3 mmol), reagent (0.4 mmol), solvent (2 mL), stirred at 50 °C under Ar (1 atm) for 24 h
aIsolated yield
bYield based on recovered alcohols
The δ-selective functionalization of primary alkanols
With the optimized reaction conditions in hand, we started to investigate the substrate scope of this transformation. The frequently used alkanols (1a–b) reacted well to afford the corresponding oxime ether products in moderate yields (2a–b). Moreover, multiple function groups such as the halogen and azido groups are tolerated (2c–e). To our delight, better yields are obtained in the reaction of alkanols bearing an oxygen atom at the ε position, owing to the higher stability and stronger nucleophilicity of α-oxygen carbon-centered radicals than typical alkyl radicals (2f–i).
Notebaly, when we explored the aryl group substituted alkanols, the active benzylic C–H bond remained untouched in this transformation (2j–l), highlighting the excellent regio-selectivity and chemo-selectivity of the present transfrmation. Furthermore, the five-membered, six-membered, and the four-membered ring substituted alcohols reacted well to afford the corresponding oxime ethers with 51–64% yields (2m–p).
Despite the secondary carbon–hydrogen functionalization, the tertiary carbon–hydrogen is also compatible in this protocol. The alcohol 1q containing a tertiary carbon–hydrogen bond afforded 2q in a little bit low yield. We suppose that the steric hindrance of 1q blocked the radical addition process of carbon radical to sulfonyl reagent (Table 2).
Table 2.
AgNO3-catalyzed δ-selective functionalization of primary alkanols

|
Standard conditions: see entry 5, Table 1. Yields shown are isolated products
aYield based on recovered alcohols
bDetermined by 1H NMR
cAcetone/H2O (0.6 mL/0.6 mL) was used
The δ-selective functionalization of substituted alkanols
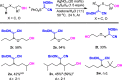
With the aforementioned results in hand, we next explored more special alkanols under the optimized conditions (Table 3). The 2-butoxyethanol and 2-ethoxyethanol reacted well to afford 2r and 2s in moderate yields, respectively. The 2-methoxyethanol derivative 2t could be isolated in 33% yield, as the primary alkyl radical is unstable compared to secondary alkyl radicals. Besides the primary alkanols, the secondary alcohol 1u is compatible in our conditions producing 2u with 2:1 of diastereoisomers. Moreover, the γ-substituted alcohol 1v afforded 2v in moderate yield. Unfortunately, the β-substituted alcohol 1w was inactive because of the gem-dimethyl effect (2w).
Table 3.
AgNO3-catalyzed δ-selective functionalization of substituted alkanols
|
Standard conditions: see entry 5, Table 1. Yields shown are isolated products
aDiastereoselectivity was determined by 1H NMR
bAcetone/H2O (0.6 mL/0.6 mL) was used
cYield based on recovered alcohols
Further application of functionalized alkanols
The oximonitrile fragment in the functionalized products is an important precursor of amidoxime which are key motifs of some fungicides, insecticides, and other bioactive compounds (Fig. 2)46. By a zinc-mediated reduction, the oxime can transform into the corresponding amine product 4 (Fig. 2)61. Through further operation, 4 could transform into tetrazoles, oxadiazoles, and even amino acid derivatives, which are valuable motifs in bioactive molecules and drugs62,63.
Fig. 2.

The chemoselective transformation of 2. The oxime ether product 2 could be converted to valuable amidoxime product 3 and α-cyanoamine product 4
Mechanistic studies
Several experiments were investigated to gain the mechanistic insight of this transformation. When stoichiometric amount of TEMPO (Fig. 3a) or BHT (Fig. 3b) was added to the reaction mixture under standard conditions, respectively, no oxime ether product was detected with the revovery of the substrate 1a. These results indicated that this silver-catalyzed transformation may undergo a radical pathway. Then, we studied the effect of the hydroxyl group. The 1-Octane 5 cannot transform to the corresponding selective functionalized product 6 under standard reaction conditions (Fig. 3c). Furthermore, the reaction of 1-methoxyoctane 7 and reagent A afforded no oxime ether product 8 (Fig. 3d). These results supported that the hydroxyl group in the alkanols is essential for the initiation of this transformation.
Fig. 3.
The mechanistic studies. a Radical scavenger experiment with TEMPO. b Radical scavenger experiment with BHT. c Reaction of n-octane under standard conditions. d Reaction of protected alcohol under standard conditions
To be emphasized, when 2-methyl-1-butanol 9 was tested, N-(benzyloxy)-2-methylbutanimidoyl cyanide 10 was obtained in 27% yield (Fig. 4). This transformation involves an alkoxyl radical-induced β-scission process20, which alternatively supports the direct alkoxyl radical generation from alcohols enabled by the current silver/oxidant catalysis.
Fig. 4.
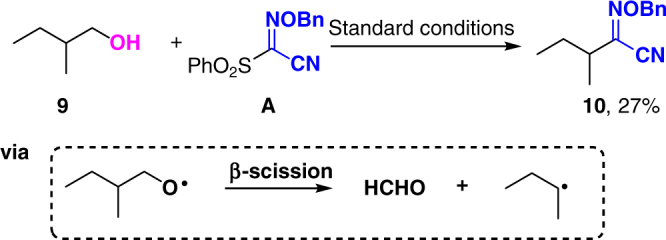
The β-scission experiment. Under standard conditions, N-(benzyloxy)-2-methylbutanimidoyl cyanide 10 could be prepared from 9 through an alkoxyl radical-induced β-scission process
Based on the aforementioned experimental results, the possible mechanism was proposed in Fig. 5. Initially, AgNO3 is oxidized to AgII. Then, intermediate I formed by coordination of alcohols 1 to AgII undergoes a homolytic cleavage process to afford alkoxyl radical II and regenarate AgI. Subsequently, the intermediate II undergoes 1,5-HAT to afford carbon radical III which is then trapped by sulphonyl reagent A to afford radical intermediate IV. The followed fragmentation produces δ-selective functionalized alkanol 2 with the release of sulponyl radical V. Finally, the sulfonyl radical is transformed to benzenesulfonic acid51.
Fig. 5.
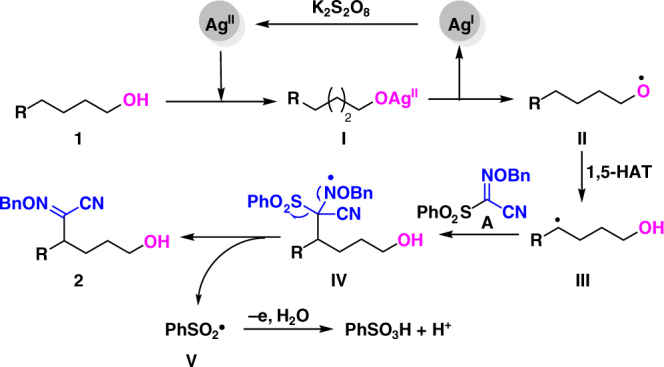
Proposed mechanism. The proposed mechanism involves a AgI/AgII catalytic cycle
Discussion
In summary, we have developed a AgNO3-catalyzed δ-selective functionalization of aliphatic alcohols via Csp3-H bond cleavage under mild conditions without pre-activation of the alcohol substrates. This atom-economical and easy handled strategy has been applied to various primary and secondary alkanols affording valuable oximonitrile substituted products with high chemo-selectivity. Mechanistic studies indicate the reaction undergoes an alkoxyl radical-mediated 1,5-HAT process. We anticipate that this discovery could inspire the development of the transformation of common aliphatic alcohols and Csp3-H functionalization.
Methods
General procedure for the functionalization of alkanols
AgNO3 (6.8 mg, 0.04 mmol), K2S2O8 (81 mg, 0.3 mmol), and reagent PhSO2C(CN)=NOBn (A) (120 mg, 0.4 mmol) were added to a 20 mL Schlenk tube under Ar. Aliphatic alcohols 1 (0.2 mmol) was added via syringe, followed by addition of acetone (1.0 mL), H2O (1.0 mL). The formed mixture was stirred at 50 °C under Ar for 24 h. After cooling to room temperature, the mixture was diluted with water (10 mL) and extracted with EA (3 × 10 mL). The combined extracts were washed with a saturated solution of NaCl (15 mL), dried over MgSO4, and evaporated in vacuo. The residue was purified by chromatography on silica gel (PE/EA = 5:1) to afford product 2.
Data availability
All data that support the findings of this study are available in the online version of this paper in the accompanying Supplementary Information (including experimental procedures, compound characterization data).
Electronic supplementary material
Acknowledgements
This work is dedicated to Professor Xiyan Lu on the occasion of his 90th birthday. Financial support from the National Natural Science Foundation of China (21632001, 21772002), the National Basic Research Program of China (973 Program) (No. 2015CB856600), the National Young Top-Notch Talent Support Program, and Peking University Health Science Center (No. BMU20160541) are greatly appreciated. We thank Xiyu Hu in this group for reproducing the results of 2c and 2s.
Author contributions
Y.Z. and N.J. conceived and designed the experiments; Y.Z. carried out most of experiments; Y.Z., K.H., J.P., X.Q., X.L., Q.Q., J.W., X.W., L.Z., and N.J. analyzed data; Y.Z. and N.J. wrote the paper; N.J. directed the project.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05014-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luk HT, Mondelli C, Ferre DC, Stewart JA, Perez-Ramirez J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017;46:1358–1426. doi: 10.1039/C6CS00324A. [DOI] [PubMed] [Google Scholar]
- 2.Stefane B, Pozgan F. Metal-catalyzed transfer hydrogenation of ketones. Top. Curr. Chem. 2016;374:1–67. doi: 10.1007/s41061-015-0002-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Engle KM, Wang DH, Yu JQ. Palladium(II)-catalyzed C-H activation/C-C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newhouse T, Baran PS. If C-H bonds could talk: selective C-H bond oxidation. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi J, Yamaguchi AD, Itami K. C-H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 7.Barton DHR, Beaton JM, Geller LE, Pechet MM. A new photochemical reaction. J. Am. Chem. Soc. 1960;82:2640–2641. doi: 10.1021/ja01495a061. [DOI] [Google Scholar]
- 8.Barton DHR, Beaton JM. A synthesis of aldosterone acetate. J. Am. Chem. Soc. 1960;82:2641–2641. doi: 10.1021/ja01495a062. [DOI] [Google Scholar]
- 9.Barton DHR, Beaton JM, Geller LE, Pechet MM. A new photochemical reaction 1. J. Am. Chem. Soc. 1961;83:4076–4083. doi: 10.1021/ja01480a030. [DOI] [Google Scholar]
- 10.Cekovic Z, Green MM. Formation of remote double-bonds by ferrous sulfate cupric acetate promoted decomposition of alkyl hydroperoxides. J. Am. Chem. Soc. 1974;96:3000–3002. doi: 10.1021/ja00816a059. [DOI] [Google Scholar]
- 11.Cekovic Z, Dimitrijevic L, Djokic G, Srnic T. Remote functionalization by ferrous ion cupric ion induced decomposition of alkyl hydroperoxides. Tetrahedron. 1979;35:2021–2026. doi: 10.1016/S0040-4020(01)88972-9. [DOI] [Google Scholar]
- 12.Cekovic Z, Cvetkovic M. Functionalization of the delta-carbon atom by the ferrous ion induced decomposition of alkyl hydroperoxides in the presence of cupric salts. Tetrahedron Lett. 1982;23:3791–3794. doi: 10.1016/S0040-4039(00)87708-4. [DOI] [Google Scholar]
- 13.Too PC, Tnay YL, Chiba S. Copper-catalyzed aerobic aliphatic C-H oxygenation with hydroperoxides. Beilstein. J. Org. Chem. 2013;9:1217–1225. doi: 10.3762/bjoc.9.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walling C, Padwa A. Positive halogen compounds. VII. Intramolecular chlorinations with long chain hypochlorites. J. Am. Chem. Soc. 1963;85:1597–1601. doi: 10.1021/ja00894a013. [DOI] [Google Scholar]
- 15.Walling C, Bristol D. Delta-chloro alcohols and tetrahydrofurans from primary and secondary alkyl hypochlorites. J. Org. Chem. 1972;37:3514–3516. doi: 10.1021/jo00795a026. [DOI] [Google Scholar]
- 16.Walling C, Clark RT. Reactions of primary and secondary alkoxy radicals derived from hypochlorites. J. Am. Chem. Soc. 1974;96:4530–4534. doi: 10.1021/ja00821a028. [DOI] [Google Scholar]
- 17.Martin A, Salazar JA, Suarez E. Synthesis of chiral spiroacetals from carbohydrates. J. Org. Chem. 1996;61:3999–4006. doi: 10.1021/jo960060g. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, Perez-Martin I, Suarez E. Intramolecular hydrogen abstraction promoted by amidyl radicals. Evidence for electronic factors in the nucleophilic cyclization of ambident amides to oxocarbenium ions. Org. Lett. 2005;7:2027–2030. doi: 10.1021/ol050526u. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S., Lee, T. A. & Song, Y. Facile generation of alkoxyl radicals from N-alkoxyphthalimides Synlett, 471–472 (1998).
- 20.Zhang J, Li Y, Zhang F, Hu C, Chen Y. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)-H functionalization under mild reaction conditions. Angew. Chem. Int. Ed. 2016;55:1872–1875. doi: 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Harms K, Meggers E. Catalytic asymmetric Csp3-H functionalization under photoredox conditions by radical translocation and stereocontrolled alkene addition. Angew. Chem. Int. Ed. 2016;55:13495–13498. doi: 10.1002/anie.201607305. [DOI] [PubMed] [Google Scholar]
- 22.Beckwith ALJ, Hay BP. Generation of alkoxy radicals from N-alkoxypyridinethiones. J. Am. Chem. Soc. 1988;110:4415–4416. doi: 10.1021/ja00221a051. [DOI] [Google Scholar]
- 23.Hartung J, Gallou F. Ring closure reactions of substituted 4-pentenyl-1-oxy radicals. The stereoselective synthesis of functionalized disubstituted tetrahydrofurans. J. Org. Chem. 1995;60:6706–6716. doi: 10.1021/jo00126a021. [DOI] [Google Scholar]
- 24.Heusler K, Kalvoda J. Intramolecular free-radical reactions. Angew. Chem. Int. Ed. 1964;3:525–596. doi: 10.1002/anie.196405251. [DOI] [Google Scholar]
- 25.Mihailović, M. Lj. & Čeković, Ž. Intramolecular oxidative cyclization of alcohols with lead tetraacetate. Synthesis, 209–224 (1970).
- 26.Blanksby SJ, Ellison GB. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003;36:255–263. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Fan X, Yu J, Zhu C. Silver-catalyzed ring-opening strategy for the synthesis of β- and γ-fluorinated ketones. J. Am. Chem. Soc. 2015;137:3490–3493. doi: 10.1021/jacs.5b00939. [DOI] [PubMed] [Google Scholar]
- 28.Ren R, Zhao H, Huan L, Zhu C. Manganese-catalyzed oxidative azidation of cyclobutanols: regiospecific synthesis of alkyl azides by C-C bond cleavage. Angew. Chem. Int. Ed. 2015;54:12692–12696. doi: 10.1002/anie.201506578. [DOI] [PubMed] [Google Scholar]
- 29.Ren R, Wu Z, Xu Y, Zhu C. C-C bond-forming strategy by manganese-catalyzed oxidative ring-opening cyanation and ethynylation of cyclobutanol derivatives. Angew. Chem. Int. Ed. 2016;55:2866–2869. doi: 10.1002/anie.201510973. [DOI] [PubMed] [Google Scholar]
- 30.Jia K, Zhang F, Huang H, Chen Y. Visible-light-induced alkoxyl radical generation enables selective C(sp3)–C(sp3) bond cleavage and functionalizations. J. Am. Chem. Soc. 2016;138:1514–1517. doi: 10.1021/jacs.5b13066. [DOI] [PubMed] [Google Scholar]
- 31.Yayla HG, Wang H, Tarantino KT, Orbe HS, Knowles RR. Catalytic ring-opening of cyclic alcohols enabled by PCET activation of strong O–H bonds. J. Am. Chem. Soc. 2016;138:10794–10797. doi: 10.1021/jacs.6b06517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo JJ, et al. Photocatalytic C-C bond cleavage and amination of cycloalkanols by cerium(III) chloride complex. Angew. Chem. Int. Ed. 2016;55:15319–15322. doi: 10.1002/anie.201609035. [DOI] [PubMed] [Google Scholar]
- 33.Jia K, Pan Y, Chen Y. Selective carbonyl-C(sp3) bond cleavage to construct Ynamides, Ynoates, and Ynones by photoredox catalysis. Angew. Chem. Int. Ed. 2017;56:2478–2481. doi: 10.1002/anie.201611897. [DOI] [PubMed] [Google Scholar]
- 34.Wang YF, Chiba S. Mn(III)-mediated reactions of cyclopropanols with vinyl azides: synthesis of pyridine and 2-azabicyclo[3.3.1]non-2-en-1-ol derivatives. J. Am. Chem. Soc. 2009;131:12570–12572. doi: 10.1021/ja905110c. [DOI] [PubMed] [Google Scholar]
- 35.Wang YF, Toh KK, Ng EPJ, Chiba S. Mn(III)-mediated formal [3+3]-annulation of vinyl azides and cyclopropanols: a divergent synthesis of azaheterocycles. J. Am. Chem. Soc. 2011;133:6411–6421. doi: 10.1021/ja200879w. [DOI] [PubMed] [Google Scholar]
- 36.Simmons EM, Hartwig JF. Catalytic functionalization of unactivated primary C-H bonds directed by an alcohol. Nature. 2012;483:70–73. doi: 10.1038/nature10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Driess M, Hartwig JF. Iridium-catalyzed regioselective silylation of secondary alkyl C–H bonds for the synthesis of 1,3-diols. J. Am. Chem. Soc. 2014;136:6586–6589. doi: 10.1021/ja5026479. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, et al. Tertiary-alcohol-directed functionalization of remote C(sp3)-H bonds by sequential hydrogen atom and heteroaryl migrations. Angew. Chem. Int. Ed. 2018;57:1640–1644. doi: 10.1002/anie.201709025. [DOI] [PubMed] [Google Scholar]
- 39.Hu A, et al. δ-selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2018;140:1612–1616. doi: 10.1021/jacs.7b13131. [DOI] [PubMed] [Google Scholar]
- 40.Mikhaleva AI, Zaitsev AB, Trofimov BA. Oximes as reagents. Russ. Chem. Rev. 2006;75:797–823. doi: 10.1070/RC2006v075n09ABEH003594. [DOI] [Google Scholar]
- 41.Kobayashi S, Ishitani H. Catalytic enantioselective addition to imines. Chem. Rev. 1999;99:1069–1094. doi: 10.1021/cr980414z. [DOI] [PubMed] [Google Scholar]
- 42.Kim S, Lee IY, Yoon JY, Oh DH. Novel radical reaction of phenylsulfonyl oxime ethers. A free radical acylation approach. J. Am. Chem. Soc. 1996;118:5138–5139. doi: 10.1021/ja9600993. [DOI] [Google Scholar]
- 43.Ryu I, et al. New radical cascade reactions incorporating multiple one-carbon radical synthons: a versatile synthetic methodology for vicinal singly and doubly acylated oxime ethers. J. Am. Chem. Soc. 1999;121:12190–12191. doi: 10.1021/ja992125d. [DOI] [Google Scholar]
- 44.Kim S, Song HJ, Choi TL, Yoon JY. Tin-free radical acylation reactions with methanesulfonyl oxime ether. Angew. Chem. Int. Ed. 2001;40:2524–2526. doi: 10.1002/1521-3773(20010702)40:13<2524::AID-ANIE2524>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Kim SG, Lim CJ. Tin-free radical-mediated C-C-bond formations with alkyl allyl sulfones as radical precursors. Angew. Chem. Int. Ed. 2002;41:3265–3267. doi: 10.1002/1521-3773(20020902)41:17<3265::AID-ANIE3265>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 46.Gaspar B, Carreira EM. Cobalt catalyzed functionalization of unactivated alkenes: regioselective reductive C−C bond forming reactions. J. Am. Chem. Soc. 2009;131:13214–13215. doi: 10.1021/ja904856k. [DOI] [PubMed] [Google Scholar]
- 47.Fang G, Cong X, Zanoni G, Liu Q, Bi XH. Silver-based radical reactions: development and insights. Adv. Synth. Catal. 2017;359:1422–1502. doi: 10.1002/adsc.201601179. [DOI] [Google Scholar]
- 48.Zheng QZ, Jiao N. Ag-catalyzed C-H/C-C bond functionalization. Chem. Soc. Rev. 2016;45:4590–4627. doi: 10.1039/C6CS00107F. [DOI] [PubMed] [Google Scholar]
- 49.Tan X, et al. Silver-catalyzed decarboxylative trifluoromethylation of aliphatic carboxylic acids. J. Am. Chem. Soc. 2017;139:12430–12433. doi: 10.1021/jacs.7b07944. [DOI] [PubMed] [Google Scholar]
- 50.Ning Y, Ji Q, Liao P, Anderson EA, Bi X. Silver-catalyzed stereoselective aminosulfonylation of alkynes. Angew. Chem. Int. Ed. 2017;56:13805–13808. doi: 10.1002/anie.201705122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Wang X, Li Z, Cui L, Li C. Silver-catalyzed decarboxylative radical azidation of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 2015;137:9820–9823. doi: 10.1021/jacs.5b06821. [DOI] [PubMed] [Google Scholar]
- 52.Qiu JK, et al. Catalytic dual 1,1-H-abstraction/insertion for domino spirocyclizations. J. Am. Chem. Soc. 2015;137:8928–8931. doi: 10.1021/jacs.5b05735. [DOI] [PubMed] [Google Scholar]
- 53.Li Z, Wang Z, Zhu L, Tan X, Li C. Silver-catalyzed radical fluorination of alkylboronates in aqueous solution. J. Am. Chem. Soc. 2014;136:16439–16443. doi: 10.1021/ja509548z. [DOI] [PubMed] [Google Scholar]
- 54.Hu F, Shao X, Zhu D, Lu L, Shen Q. Silver-catalyzed decarboxylative trifluoromethylthiolation of aliphatic carboxylic acids in aqueous emulsion. Angew. Chem. Int. Ed. 2014;53:6105–6109. doi: 10.1002/anie.201402573. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Song L, Li C. Silver-catalyzed radical aminofluorination of unactivated alkenes in aqueous media. J. Am. Chem. Soc. 2013;135:4640–4643. doi: 10.1021/ja400124t. [DOI] [PubMed] [Google Scholar]
- 56.Yin F, Wang Z, Li Z, Li C. Silver-catalyzed decarboxylative fluorination of aliphatic carboxylic acids in aqueous solution. J. Am. Chem. Soc. 2012;134:10401–10404. doi: 10.1021/ja3048255. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, et al. Silver-catalyzed decarboxylative chlorination of aliphatic carboxylic acids. J. Am. Chem. Soc. 2012;134:4258–4263. doi: 10.1021/ja210361z. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Y, et al. Silver-catalyzed decarboxylative azidation of aliphatic carboxylic acids. Org. Lett. 2015;17:4702–4705. doi: 10.1021/acs.orglett.5b02155. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Wen X, Song S, Jiao N. Silver-catalyzed radical transformation of aliphatic carboxylic acids to oxime ethers. ACS Catal. 2016;6:6465–6472. doi: 10.1021/acscatal.6b02074. [DOI] [Google Scholar]
- 60.Kan J, Huang S, Lin J, Zhang M, Su W. Silver-catalyzed arylation of (hetero)arenes by oxidative decarboxylation of aromatic carboxylic acids. Angew. Chem. Int. Ed. 2015;54:2199–2203. doi: 10.1002/anie.201408630. [DOI] [PubMed] [Google Scholar]
- 61.Mo, K. et al. Chemo- and stereoselective reduction of beta-keto-alpha-oximino nitriles by using baker’s yeast. Eur. J. Org. Chem. 2015, 1137–1143 (2015).
- 62.Bailey MD, Halmos T, Goudreau N, Lescop E, Llinas-Brunet M. Novel azapeptide inhibitors of hepatitis C virus serine protease. J. Med. Chem. 2004;47:3788–3799. doi: 10.1021/jm049864b. [DOI] [PubMed] [Google Scholar]
- 63.Trabanco AA, Cid JM, Lavreysen H, Macdonald GJ, Tresadern G. Progress in the developement of positive allosteric modulators of the metabotropic glutamate receptor 2. Curr. Med. Chem. 2011;18:47–68. doi: 10.2174/092986711793979706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available in the online version of this paper in the accompanying Supplementary Information (including experimental procedures, compound characterization data).