Abstract
Pharanois phoborhodopsin (ppR) from Natronomonas pharaonis is a transmembrane photoreceptor protein involved in negative phototaxis. Structural changes in ppR triggered by photoisomerization of the retinal chromophore are transmitted to its cognate transducer protein (pHtrII) through a cyclic photoreaction pathway involving several photointermediates. This pathway is called the photocycle. It is important to understand the detailed configurational changes of retinal during the photocycle. We previously observed one of the photointermediates (M-intermediates) by in situ photoirradiation solid-state NMR experiments. In this study, we further observed the 13C cross-polarization magic-angle-spinning NMR signals of late photointermediates such as O- and N′-intermediates by illumination with green light (520 nm). Under blue-light (365 nm) irradiation of the M-intermediates, 13C cross-polarization magic-angle-spinning NMR signals of 14- and 20-13C-labeled retinal in the O-intermediate appeared at 115.4 and 16.4 ppm and were assigned to the 13-trans, 15-syn configuration. The signals caused by the N′-intermediate appeared at 115.4 and 23.9 ppm and were assigned to the 13-cis configuration, and they were in an equilibrium state with the O-intermediate during thermal decay of the M-intermediates at −60°C. Thus, photoirradiation NMR studies revealed the photoreaction pathways from the M- to O-intermediates and the equilibrium state between the N′- and O-intermediate. Further, we evaluated the detailed retinal configurations in the O- and N′-intermediates by performing a density functional theory chemical shift calculation. The results showed that the N′-intermediate has a 63° twisted retinal state due to the 13-cis configuration. The retinal configurations of the O- and N′-intermediates were determined to be 13-trans, 15-syn, and 13-cis, respectively, based on the chemical shift values of [20-13C] and [14-13C] retinal obtained by photoirradiation solid-state NMR and density functional theory calculation.
Introduction
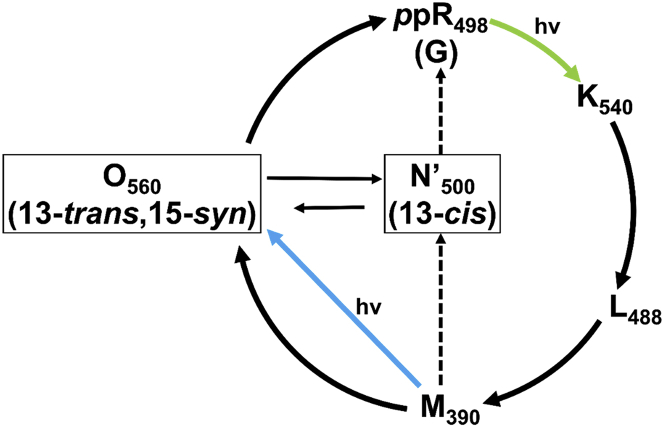
Pharaonis phoborhodopsin (ppR), also called sensory rhodopsin II (SRII), is a membrane protein isolated from the halophilic and alkaliphilic archaeal Natronomonas pharaonis that consists of seven-transmembrane α-helices with a vitamin-A aldehyde retinal as a chromophore (1). All-trans retinal is predominantly incorporated into the apoprotein of ppR. ppR functions as a photoreceptor protein by forming a 2:2 complex with the cognate two-helix transducer protein pHtrII to transmit the photosignal into the cytoplasm (2, 3, 4, 5). The ppR/pHtrII complex is involved in negative phototaxis through a cyclic photoreaction pathway called the photocycle. In the initial state, ppR has an absorbance maximum at 498 nm in the ground (G) state under dark conditions. Light absorption transforms ppR from the ground state (ppRG) to a K (540 nm)-intermediate with an absorbance maximum of 540 nm. This transformation is initiated by trans/cis photoisomerization of the retinal. The photocycle is followed by several intermediates, such as L (498 nm), M (390 nm), and O (560 nm), undergoing thermal relaxation processes. Finally, the O-intermediate thermally returns to the ppRG state (6).
The K-intermediate has a half-life of ∼1 μs, and its retinal is in the 13-cis, 15-anti configuration. Transformation of the K-intermediate provides the L-intermediate, which has a half-life of ∼30 μs and a 13-cis, 15-anti retinal configuration. Subsequently, a proton is removed from the Schiff base (SB) of the L-intermediate, resulting in transformation to the M-intermediate, which has a long half-life of ∼1.7 s. The M-intermediate has a 13-cis, 15-anti retinal configuration with a deprotonated SB. Upon reprotonation, the M-intermediate transforms into the O-intermediate with a protonated SB, which has a half-life of ∼770 ms and a 13-trans, 13-syn retinal configuration (4, 5, 6, 7). The M- and O-intermediates have long half-lives as compared with the K- and L-intermediates, and thus they are known as late-active intermediates.
Important signal transduction processes such as changes in protein structure are induced at the late step in the photocycle. These changes likely include the formation of two specific hydrogen bonds, one between Tyr199ppR and Asn74pHtrII and one between Thr189ppR and Glu43pHtrII/Ser62pHtrII, as observed in the crystal structure of the ppR/pHtrII complex in the dark state (8) and K and M states (9, 10). Thr204 is another important residue in ppR and plays a role in color tuning and in the photocycle kinetics of ppR (11). Further observations have shown that Thr204 is indispensable for the negative phototaxis function of the complex (12). There is steric hindrance in the K-intermediate between the C14-H of retinal and Thr204 (13). At the same time, a specific hydrogen bond alteration occurs between Thr204 and Tyr174 in a pHtrII-dependent manner (12). Helix movement in ppR and outward tilting of helix F during the photocycle have been suggested by various groups (14, 15, 16) and are believed to be essential steps for the activation of pHtrII. However, no helix tilting was observed in the crystal structure of the M-intermediate of the ppR/pHtrII complex (9). After tilting of the F-helix in ppR, TM2 in pHtrII rotates, transferring the signal to the phosphorylation cascade to initiate rotation of the bacterial flagellar motor, resulting in negative phototaxis.
The photocycle as described above depends on the photoisomerization of retinal. The retinal chromophore in ppR forms a covalent bond with a perfectly conserved Lys residue (Lys205) bonded to an SB. Animal rhodopsin (type II rhodopsin) photobleaches, whereas microbial rhodopsin (type I rhodopsin) retains the retinal through the photocycle. Therefore, continuous irradiation of ppR with green light results in repeated photocycles without photobleaching, and the photointermediates can be trapped in a stationary state using type I rhodopsins such as ppR (1, 5, 17).
Photoirradiation solid-state NMR spectroscopy has been used to reveal the photoactivated intermediates of retinal membrane proteins. For example, photoactivated intermediates have been characterized by 13C NMR studies of [13C] retinal and 15N NMR studies of [ζ-15N]Lys of bacteriorhodopsin (bR) (18, 19, 20, 21). The light-adapted state and the M- and N-intermediates of bR have been investigated using photoirradiation solid-state NMR spectroscopy (19, 20). The early M-intermediate, M0, and late M-intermediate, Mn, in the bR photocycle have been characterized by in situ photoirradiation solid-state NMR spectrometry (22, 23, 24). The combination of NMR spectroscopy with a dynamic nuclear polarization method revealed the heterogeneity of dark-adapted bR and distortion in the K-intermediate, and four discrete L-intermediates were detected (25, 26). In addition to bR, the photoactive site of channelrhodopsin-2 was revealed (27). Thus, we have developed an in situ photoirradiation solid-state NMR apparatus that allows irradiation of the sample with extremely high efficiency and enables observation of the photointermediates and photoreaction processes of photoreceptor membrane proteins (28, 29, 30, 31, 32, 33, 34, 35).
We previously trapped several photointermediates in the photostationary state using in situ photoirradiation solid-state NMR and observed the M-intermediates of ppR under green-light irradiation (29). Continuous irradiation of ppR with green light (520 nm) resulted in the accumulation of late-active intermediates such as multiple M-intermediates (M1, M2, and M3) because of their long lifetimes as compared with the early photointermediates such as the K- and L-intermediates. Generally, the half-lives of the late intermediates of sensory-type rhodopsin are much longer than that of ion-pump-type rhodopsins such as bR (5). It is reasonable that the longer half-life time of the photointermediates in sensory rhodopsins are accompanied by dynamic conformational changes to allow signal transmission to the transducer protein and signal amplification during the photocycle, whereas the fast photocycle is advantageous for transporting ions during the photocycle of ion-pump-type rhodopsins.
In this study, we focus on observing the late photointermediates after the M- intermediates by performing in situ photoirradiation solid-state NMR measurements using two light-emitting diode (LED) light sources, one each at 520 nm (green light) and 365 nm (blue light). We previously showed that the 13C NMR signal from [20-13C] retinal responds sensitively to changes in the 13C=14C configuration in retinal. Similarly, the 13C NMR signal from [14-13C] retinal should be sensitive to changes in the 15C=Nζ configuration in retinal. Here, we specifically detected the 13C NMR signal from [14, 20-13C]-labeled retinal in ppR. Based on the chemical shift values for [20-13C] and [14-13C] retinal in the late-active intermediates and the results of density functional theory (DFT) calculations, we discuss the detailed configurations of these intermediates.
Materials and Methods
Sample preparation
[14, 20-13C]-labeled retinal-ppR with a His-tag (6×His) at the C-terminal was overexpressed in Escherichia coli BL21(DE3) strain (Nippon Genetics, Toyama, Japan) in lysogeny broth medium by induction with 1 mM isopropyl-1-thio-β-D-galactoside (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) and 10 μM [14, 20- 13C]-labeled all-trans retinal (synthesized by T.O. and A.W.). Protein expression was performed at 25°C for 15 hr. To purify the sample, the cells were lysed by ultrasonication, then the proteins were solubilized using n-dodecyl-β-D-maltoside (DDM) (Dojindo Laboratories, Kumamoto, Japan) and purified with Ni-NTA column (QIAGEN, Hilden, Germany) as previously described (29, 36, 37). pHtrII (1–159) was also prepared by the same method as described previously (29). After purification, ppR was mixed with pHtrII at a 1:1 molar ratio by electrophoresis and by monitoring ultraviolet (UV)-visible absorption and thermal stability (38) of the ppR/pHtrII complex. The complex was reconstituted into membrane using DDM micelles incorporating a lipid film of L-α-egg-phosphatidylcholine (egg-PC) (Avanti Polar Lipids, Alabaster, AL) (ppR:egg-PC molar ratio of 1:30), then DDM was removed by using Bio-Beads (Bio-RAD, Hercules, CA). The reconstituted samples were suspended in 5 mM HEPES (Dojindo Laboratories), 10 mM NaCl buffer solution (pH 7) to provide ppR and ppR/pHtrII complex embedded in egg-PC lipid bilayers.
Solid-state NMR experiments
The fully hydrated sample was concentrated by centrifugation so that the molar ratio of water:lipid:protein reached 8350:30:1 with a hydrated ratio of 300%. Consequently, 66 mg of hydrated sample with 9 mg of protein was packed into a 5.0-mm-outer-diameter zirconia pencil-type rotor with a tightly sealed glass cap (JEOL Ltd., Akishima, Japan). Solid-state NMR experiments were performed on a CMX-400 Infinity (Chemagnetics, Fort Collins, CO) solid-state NMR spectrometer equipped with an in situ photoirradiation NMR system using resonance frequencies of 400 and 100 MHz for 1H and 13C nuclei, respectively. 13C cross-polarization magic-angle-spinning (CP-MAS) experiments (39) were performed using the following conditions: 13C 90° pulse of 5.4 μs, 1H decoupling amplitude of 50 kHz, temperatures of −40 and −60°C, and a MAS frequency of 4.0 kHz. Two-pulse phase modulation proton high-power decoupling (40) was employed during each acquisition. Typically, 40,000 transients were accumulated for ∼44 hr to obtain each 1D CP-MAS NMR spectrum. 13C chemical shifts were referenced to the carbonyl resonance of glycine powder at 176.03 ppm (tetramethylsilane (TMS) at 0.0 ppm).
In situ photoirradiation solid-state NMR measurements
In situ continuous photoirradiation was carried out using an optical fiber passed from outside the magnet through a tightly sealed glass cap made from a glass rod glued to a zirconia rotor (29, 30, 33). The glass rod was grained so as to provide illumination perpendicular to the rotor wall. The fluid membrane proteins were attached to the side wall of the rotor as a thin film and then light was directed perpendicular to the rotor wall from inside the spinner. If the irradiation is not perpendicular to the rotor wall, the light is completely reflected off the surface of the film sample and does not penetrate into the sample. The use of a CMX-400 Infinity NMR spectrometer (Chemagnetics) equipped with this photoirradiation system allowed us to efficiently irradiate samples in the rotor with green (520 nm) and blue (365 nm) LED light sources.
Stationary trapping of photointermediates using in situ photoirradiation solid-state NMR
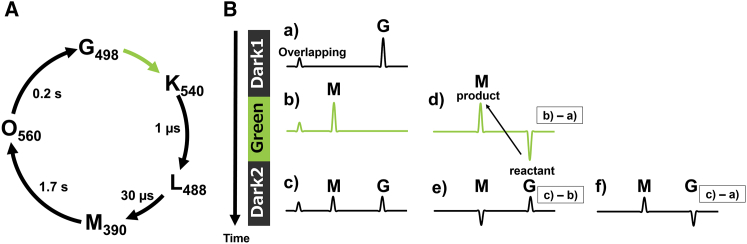
In situ photoirradiation is particularly useful for studying the photocycle of several retinal binding membrane proteins. The half-life of the M-intermediate in ppR is ∼1.7 s, which is much longer than that of the other intermediates, as shown in Fig. 1 A. Thus, continuous irradiation of a sample with green light (520 nm) (Green) traps the M-intermediates in a stationary fashion as schematically shown in Fig. 1 Bb, making it possible to detect relatively short-lived intermediates in the stationary state when their half-life differs from those of other intermediates. After the green light is turned off (Dark2), the M-intermediates relax to the G state (Fig. 1 Bc). A difference spectrum between Green and Dark1 that is observed in the dark condition (Fig. 1 Ba) (Green − Dark1; Fig. 1 Bd) indicates the photoreaction pathway from Dark1 to Green and shows that the G state is transformed to the M-intermediate. Namely, a negative peak shows the reactant, and the positive peak shows the product. Similarly, the thermal relaxation process from Green to Dark2 is shown in the difference spectrum subtracting Green from Dark2 (Dark2 − Green; Fig. 1 Be), which indicates that the M-intermediate is transformed to the G state.
Figure 1.
(A) Typical photocycle of the ppR/pHtrII complex. The M- and O-intermediates have much longer half-lives than the K- and L-intermediates. (B) The schematic experimental protocol and analysis strategy are shown. First, in situ photoirradiation solid-state NMR spectrum of the G state was observed in the dark state (Dark1) (Ba), then the spectrum of the M-intermediate under illumination with green light (Green) (Bb), and finally in the second dark state (Dark2) (Bc), in which the M-intermediate decreases and the G state increases. We analyzed the photoreaction pathway and eliminated overlapping signals by obtaining the difference spectra. The difference spectra shown are: (Bd) = (Bb) − (Ba), showing that the G state is transformed to the M-intermediate; (Be) = (Bc) − (Bb), showing that the M-intermediate is transformed to the G state; (Bf) = (Bc) − (Ba), showing that the G state is transformed to the M-intermediate. A negative peak in a difference spectrum indicates the reactant, and a positive peak indicates a product. To see this figure in color, go online.
The trapping efficiency can be increased by decreasing the temperature, because the photoreaction is a thermal process except for the photoillumination process (G → K), and therefore lowering the temperature extends the half-life of the M-intermediates. The M-intermediates can be eliminated by illuminating with 365 nm LED light, because the M-intermediates have a maximal absorbance frequency of 390 nm, which is different from the absorbance frequencies of the other intermediates. The M-intermediates can therefore be selectively eliminated by directly irradiating with 365 nm blue light, allowing detection of the activation of consecutive photoreaction processes such as the double-photon process by illuminating with multiple wavelengths. It is thus possible to select a particular intermediate by adjusting either temperature or wavelength.
Computing method to evaluate the 13C chemical shift values of retinal
13C chemical shifts of backbone Cα, C=O, and sidechain Cβ signals are significantly displaced for a variety of amino acid residues in polypeptides and proteins depending on their local conformation such as α-helix, β-sheet, or random coil (41). 13C chemical shifts of [20-13C] retinal are correlated with 13-cis/trans of 13C=14C configuration, and those of [14-13C] retinal are correlated with 15-syn/anti of 15C=Nζ configuration, as experimentally obtained from various retinal proteins and summarized in Table S1. Here, we calculate the 13C chemical shift values of retinal carbons to elucidate the relation between the 13C chemical shift values and retinal configuration.
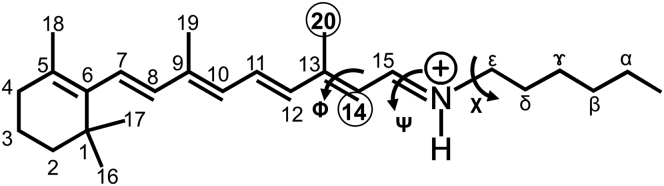
The 13C chemical shift calculations were performed using the gauge-independent atomic orbital method (42, 43, 44, 45, 46) in the Gaussian09 (47) program with the B3LYP/6-311+g∗∗ theory/basis set combination. The calculated chemical shifts were converted to ppm relative to TMS. A schematic structure of retinal, used in the chemical shift calculation, is shown in Fig. 2. Retinal binds to the protein through the side chain of Lys 205, as shown in Fig. 2. The SB was considered to be protonated in the calculation. The initial structure of the retinal was in the all-trans configuration of the crystal structure (Protein Data Bank (PDB): 1JGJ) (48), which corresponds to the G-state configuration. We investigated the configurations of the O- and N′-intermediates by evaluating the dependence of the 13C NMR chemical shift on the configuration of retinal according to the rotation of the dihedral angles of 12C-13C=14C-15C, 14C-15C=Nζ-Cε, and 15C=Nζ-Cε-Cδ, abbreviated Φ, Ψ, and Χ, respectively (Fig. 2). The geometries of the heavy atoms of retinal were fixed in each dihedral angle, and only the hydrogen atoms were optimized with the same theory/basis-set conditions before calculation of the chemical shift values. The basis-set dependence of the calculation of chemical shift was examined for the G state of retinal using several basis sets. The results are shown in Table S2. It shows that the calculated values are almost the same as those in these large basis sets. In this work, the largest basis set of 6–311 + G∗∗ in the table was used in all the calculation.
Figure 2.
Schematic structure of retinal covalently bonded through an SB. The letters α, β, γ, δ, and ε are the positions of the carbons of Lys205 in ppR. The circled numbers are the positions of carbons observed by 13C CP-MAS solid-state NMR. The dihedral angles Φ, Ψ, and X are defined as 12C-13C=14C-15C, 14C-15C=Nζ-Cε, and 15C=Nζ-Cε-Cδ, respectively.
Results and Discussion
Photoreaction pathways under green-light irradiation of the ppR/pHtrII complex
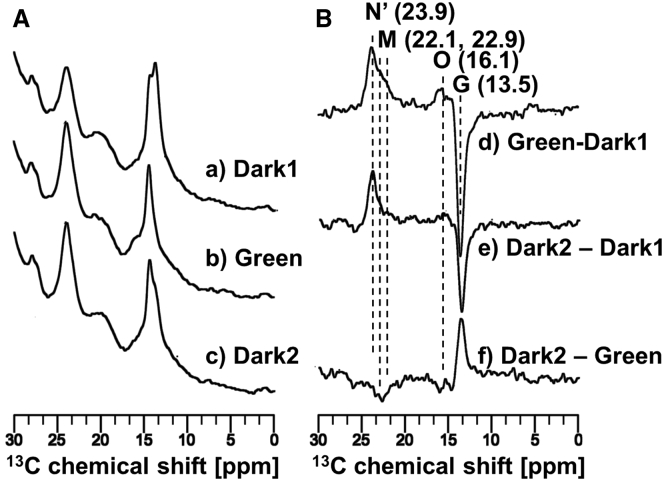
In situ photoirradiation solid-state 13C CP-MAS measurements were performed on the [20-13C]-labeled retinal-ppR/pHtrII complex at −40°C. We obtained the photoreaction pathways under illumination with green light by first observing the 13C CP-MAS NMR spectrum of the ppR/pHtrII complex in the dark condition (Dark1) at −40°C (Fig. 3 Aa; Fig. S1 Aa for full spectrum) and then the 13C CP-MAS spectrum under illumination with 520 nm green light (Fig. 3 Ab; Fig. S1 Ab for full spectrum) to observe the intermediates in stationary state.
Figure 3.
(A) 13C CP-MAS NMR spectra of the [20-13C] retinal–ppR/pHtrII complex at −40°C using a 4 kHz MAS frequency, (a) acquired under initial dark conditions (Dark1), (b) acquired under irradiation with green light (520 nm) (Green), and (c) obtained one day after turning off irradiation (Dark2). (B) The difference spectra obtained by (d) Green − Dark1 (process from Dark1 to Green), (e) Dark2 − Dark1 (process from Dark1 to Dark2), and (f) Dark2 − Green (process from Green to Dark2) are shown.
The [20-13C] retinal signals shown in Fig. 3 Aa and b heavily overlapped with the lipid signals. The difference spectrum (Green − Dark1) was therefore obtained as shown in Fig. 3 Ad and Fig. S1 Ad for full spectrum to analyze the photoreaction pathways induced by photoirradiation. The difference spectrum between the light and dark states indicated that the negative peaks correspond to the reactant state and the positive peaks correspond to the product state. Thus, the 13C CP-MAS NMR signal of the G state at 13.5 ppm decreased, and those of the M-intermediates at 22.1 and 22.9 ppm (M1 and M2), the N′-intermediate at 23.9 ppm, and the O-intermediate at 16.1 ppm all increased. We discuss the assignment of the N′- and O-intermediates in a later section. The 13C CP-MAS NMR signals of the [20-13C] retinal-ppR/pHtrII complex show the process of transforming from the G state (13.5 ppm) to a number of photointermediates. We previously reported that the G state was transformed to three kinds of M-intermediates (M1, M2, and M3) upon illumination with green light at −20°C (29). In this experiment, at −40°C, we observed photointermediates in the 13-cis form (22.9, 22.1, and 23.8 ppm), which were assigned to M1, M2, and N′-intermediate, respectively. The signal at 23.8 ppm was previously assigned to M3-intermediate. The photointermediate in the 13-trans form (16.1 ppm) was assigned to the O-intermediate. In summary, the 13-trans form product peak at 16.1 ppm can be assigned to the O-intermediate, and the 13-cis form can be assigned to the M-intermediates (22.1, 22.9 ppm) and the N′-intermediate (23.9 ppm), as discussed below. The chemical shift values are summarized in Table 1.
Table 1.
13C Chemical Shift Values of Retinal Obtained from NMR Experiments
| Chemical Shift [ppm] |
Configuration | |||
|---|---|---|---|---|
| 20-13C | 14-13C | |||
| ppR/pHtrII(−40°C) | G state | 13.5 ± 0.8 | – | 13-trans, 15-anti |
| – | O-intermediate | 16.1 ± 1.1 | – | 13-trans |
| – | M-intermediate | 22.1, 22.9 ± 1.2 | – | 13-cis, 15-anti |
| – | N′-intermediate | 23.9 ± 0.9 | – | 13-cis |
| ppR (−40°C) | G state | 13.5 ± 0.7 | 121.7 ± 0.5 | 13-trans, 15-anti |
| – | M-intermediate | 22.3 ± 1.3 | 126.8 ± 1.1 | 13-cis, 15-anti |
| ppR(−60°C) | G state | 13.6 ± 1.5 | 121.6 ± 1.7 | 13-trans, 15-anti |
| – | O-intermediate | 16.4 ± 1.3 | 115.4 ± 1.8 | 13-trans |
| – | M-intermediate | 22.6 ± 1.2 | 127.1 ± 1.7 | 13-cis, 15-anti |
| – | N′-intermediate | 23.9 ± 1.5 | 115.4 ± 1.2 | 13-cis |
Relaxation process from the green-light irradiation state to the short dark state
The green-light-irradiation state was relaxed to the dark state by the process of thermal relaxation. Fig. 3 Ac and Fig. S1 Ac for full spectrum show the 13C CP-MAS NMR spectrum of ppR/pHtrII complex obtained one day after terminating green-light irradiation. The difference spectrum shown in Fig. 3 Be and Fig. S1 Ae for full spectrum was obtained by subtracting the initial dark state (Dark1) from the second short dark state (Dark2). The peak at 23.9 ppm remained unchanged, whereas the peaks at 22.1 and 22.9 ppm decreased in intensity. The difference spectrum shown in Fig. 3 Bf and Fig. S1 Af for full spectrum was obtained by subtracting the green-light-illumination state (Green) from the second short dark state (Dark2). The peaks at 22.1, 22.9, and 16.1 ppm decreased, and the G state increased in intensity. These results indicate that the peak at 23.9 ppm is not due to the M-intermediates but rather to the N′-intermediate, and therefore the half-lives of the M-intermediates at 22.1 and 22.9 ppm and of the O-intermediate at 16.1 ppm are much shorter than that of the N′-intermediate at 23.9 ppm. Therefore, we conclude that the M- and O-intermediates relaxed to the G state, and the N′-intermediate relaxed more slowly than the M- and O-intermediates.
Photoreaction pathway from the M-intermediates to the O-intermediate
An N-like intermediate with an absorbance maximum at 500 nm was previously observed in ppR in the 13-cis form in a transient absorption study (49) that used azide to accelerate the decay of the M-intermediates (50). To confirm the existence of this N-like intermediate, the sample in our study was irradiated with near-UV (blue) light at 365 nm to eliminate the M-intermediates (51), which have a maximal absorbance of 390 nm, after the accumulation of photointermediates by irradiation with 520 nm light.
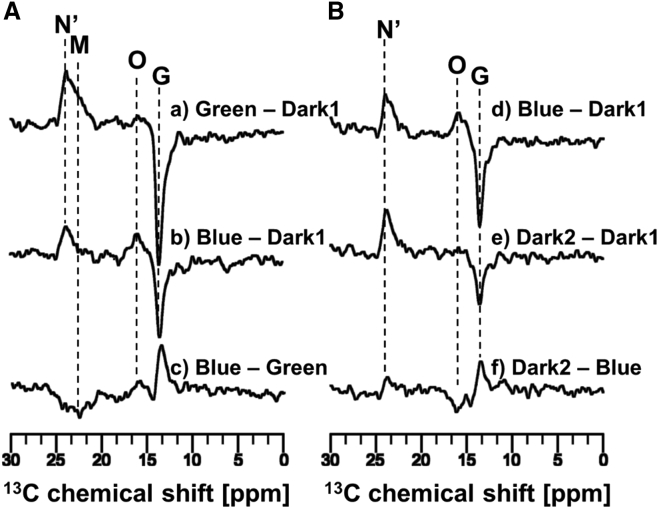
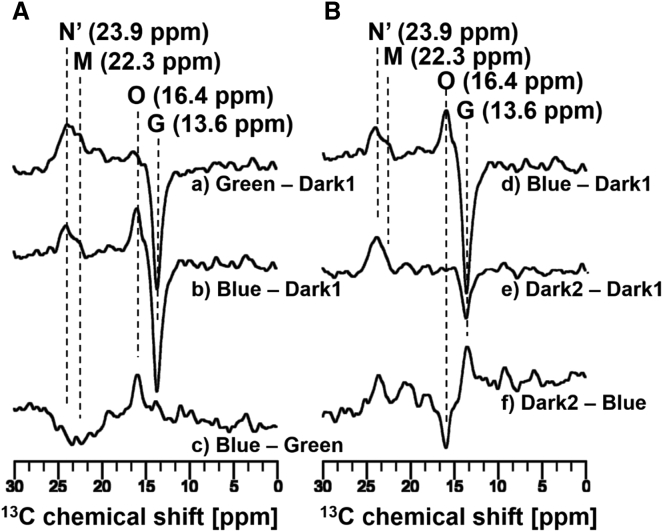
Irradiation of the ppR/pHtrII complex with 520 nm green light at −40°C converted the G state to the O-, M-, and N′-intermediates (Fig. 3 Bd and Fig. 4 Aa). We found that the signal at 23.9 ppm did not decay upon irradiation with 365 nm light (Fig. 4 Ab). As described above, M-intermediates have an absorbance maximum at 390 nm due to the deprotonated SB. Therefore, M-intermediates immediately decay upon illumination with 365 nm blue light (51), and thus the remaining signal corresponding to the 13-cis form is assigned to the N′-intermediate (23.9 ppm).
Figure 4.
13C CP-MAS NMR difference spectra of the [20-13C] retinal labeled ppR/pHtrII complex were measured at −40°C using 4 kHz MAS. (Aa) The difference spectrum obtained by subtracting the initial dark conditions (Dark1) from the green-light (520 nm)-illumination state (Green) (Green − Dark1) is shown. (b) The difference spectrum obtained by subtracting the initial dark conditions (Dark1) from the blue-light (365 nm)-illumination state (Blue) after the green-light stationary state (Blue − Dark1) is shown. (c) The difference spectrum obtained by Blue − Green, which is identical to (b) − (a), is shown. (Bd) The difference spectrum obtained by subtracting Dark1 from Blue (Blue − Dark1) is shown. (e) The difference spectrum obtained by subtracting Dark1 from the second dark condition (Dark2) after turning off irradiation (Dark2 − Dark1) is shown. (f) The difference spectrum obtained by subtracting Blue from Dark2 (Dark2 − Blue), which is identical to (e) − (d), is shown.
After the M-intermediates of the ppR/pHtrII complex were trapped at −40°C in a stationary manner by illumination with green light (Fig. 4 Aa), irradiation with LED light at 520 nm was switched to 365 nm (Fig. 4 Ab). During this process, the intensities of the M-intermediates decayed and the intensities of the O-intermediate and the G state increased (Fig. 4 Ac), whereas the N′-intermediate did not decay over the same period (Fig. 4 Ab). Furthermore, it is noted that the M-intermediates were transformed to the O-intermediate after irradiation with 365 nm blue light (Fig. 4 Ac). The M-intermediates are reportedly transformed back to the G state by irradiation with blue light (365 nm) (51), because the M-intermediates have a maximal absorption of 390 nm. However, it was found in this experiment that the M-intermediates are transformed to the O-intermediate upon irradiation with 365 nm blue light (Fig. 4 Ac). As described above, spectroscopic evidence for the formation of an N′-intermediate was recently obtained in a transient absorption study (50), which reported that decay of the M-intermediates does not directly produce the N′-intermediate but rather produces an O-intermediate that is in equilibrium with the N′-intermediate. Based on previously reported findings and the results of this in situ photoirradiation solid-state NMR experiments, we summarize the photoreaction cycle of the ppR/pHtrII complex as follows (Fig. 5). The M-intermediates are converted to the O-intermediate under irradiation with 365 nm blue light through a double-photon process, and the O-intermediate is then converted to the N′-intermediate until an equilibrium state is achieved in which the intensity of the N′-intermediate is higher than that of the O-intermediate (Fig. 4 Ab).
Figure 5.
The photoreaction pathway of ppR including the N′-intermediate, as revealed by in situ photoirradiation solid-state NMR measurements. The O-intermediate is in an equilibrium state with the N′-intermediate. The λ maximum of the N′-intermediate was estimated at 500 nm (50). The transition process from the M-intermediates to the O-intermediate is shown by a straight arrow. The pathway for thermal relaxation from the M-intermediates to the N′-intermediate, and subsequently from the N′-intermediate to the G state, remains unclear and thus is designated with a black dashed arrow. The general configurations of the O- and N′-intermediates were the 13-trans, 15-syn, and the 13-cis forms, respectively. To see this figure in color, go online.
It has been reported that the M-intermediate is converted to ppR′-intermediate at low temperatures of −160°C under near-UV-light irradiation and then back to ppR-state (52). This kind of ppR′-intermediate was not able to be accumulated at −40 and −60°C in this experiment. Moreover, the possibility that 13C NMR signals of ppR′-intermediate are completely overlapped with those of ppR has not been ruled out, and thus ppR′-intermediate is not identified in this experiment.
Double-photon process from the G state to the O-intermediate
We identified the transformation process from the G state to the O-intermediate by observing the difference in the 13C CP-MAS spectrum upon the blue-light (365 nm) irradiation of the G state. The 13C CP-MAS NMR spectral measurement of the [20-13C] retinal-ppR/pHtrII complex was first performed under initial dark conditions (Dark1) at −40°C. Upon switching directly from the initial dark condition (Dark1) to 365 nm blue-light illumination, the M-intermediates were converted to the O-intermediate through a double-photon process (Fig. 4 Bd). Consequently, the O-intermediate was converted to the N′-intermediate until an equilibrium state was reached (Fig. 4 Be). This pathway was clearly observed in the difference spectrum generated by subtracting the blue-light (365 nm)-illumination state from the Dark2 state (Fig. 4 Bf).
Photoreaction pathways of ppR from the G state to the M-intermediates at −40°C
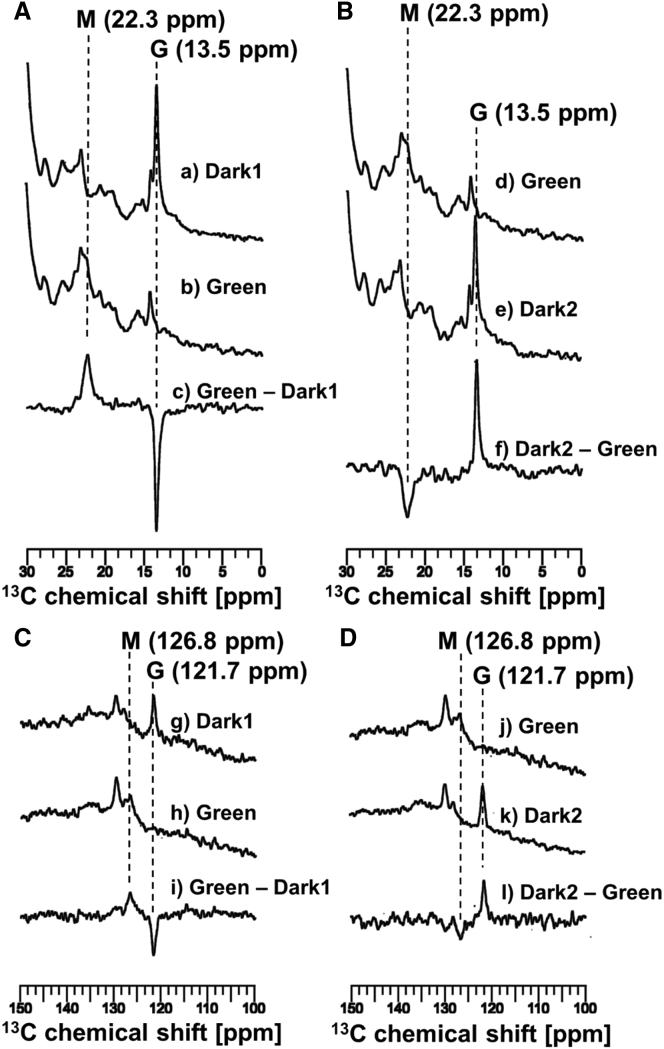
We obtained the 13C CP-MAS NMR signals of [20-13C] retinal-ppR without pHtrII reconstituted in egg-PC under the initial dark conditions (Fig. 6 Aa) followed by green-light illumination with a 520 nm LED (Fig. 6 Ab). The difference spectrum (Fig. 6 Ac) between the M-intermediates and the G state (Fig. 6 Aa and b) indicated the transformation process from the G state to the M-intermediate.
Figure 6.
13C CP-MAS NMR spectra of [20-13C, 14-13C] retinal-ppR without pHtrII at −40°C under 4 kHz MAS. (A) The 13C NMR spectra of [20-13C] retinal-ppR indicating the transformation process from the G state (13.5 ppm) to the M-intermediate (22.3 ppm), (a) acquired under initial dark conditions (Dark1) and (b) acquired under green-light (520 nm)-illumination states (Green), and (c) the difference spectrum obtained by subtracting Dark1 from Green (Green − Dark1) are shown. (B) The 13C NMR spectra of [20-13C] retinal-ppR showing the thermal relaxation process from the M-intermediate to the G state, (d) acquired under green-light-illumination states (Green) and (e) acquired under short dark conditions (Dark2) after turning off irradiation, and (f) the difference spectrum obtained by subtracting Green from Dark2 (Dark2 − Green) are shown. (C) The 13C NMR spectra showing the region from 100.0 to 150.0 ppm for the same experiment as (A), (g) acquired under initial dark conditions (Dark1) and (h) acquired under green-light-illumination states (Green), and (i) the difference spectrum obtained by subtracting Dark1 from Green (Green − Dark1) are shown. (D) The 3C NMR spectra showing the region from 100.0 to 150.0 ppm for the same experiment as (B), (j) acquired under green-light-illumination states (Green) and (k) acquired under short dark conditions (Dark2) after turning off irradiation, and (l) the difference spectrum obtained by subtracting Green from Dark2 (Dark2 − Green) are shown. The spectra shown in (C and D) indicate the transformation process from the G state (121.7 ppm) to the M-intermediate (126.8 ppm) and the thermal decay process.
The 13C CP-MAS NMR spectra of [20-13C] retinal-ppR under the green-light illumination with a 520 nm LED (Fig. 6 Bd) and in the subsequent dark condition (Dark2) (Fig. 6 Be) and the difference spectrum (Fig. 6 Bf) between the Dark2 condition and the green-light condition (Fig. 6 Bd and e) indicated that the M-intermediate transformed to the G state via a thermal-relaxation decay process at −40°C in ppR alone.
The 13C NMR spectra of [14-13C] retinal-ppR (Fig. 6, C and D) and the difference spectrum also indicated the process G state → M-intermediate → G state, as for [20-13C] retinal-ppR. In addition, the chemical shift values of [14-13C] retinal-ppR were 121.7 ppm (G state) and 126.8 ppm (M-intermediate) at −40°C (Table 1).
Photoreaction pathways from the M-intermediate to the O-intermediate in ppR
The M-, N′-, and O-intermediates were observed under continuous green-light (520 nm) illumination at −40°C using ppR/pHtrII complex. In contrast to the case of ppR/pHtrII, in the ppR alone, the N′- and O-intermediates could not be trapped at −40°C, indicating shorter half-lives than those of the ppR/pHtrII complex. As expected, these intermediates were successfully trapped at −60°C (Fig. 7 Aa).
Figure 7.
13C CP-MAS NMR signals of [20-13C] retinal-ppR alone at −60°C under 4 kHz MAS. (A) The difference spectra of the [20-13C] retinal-ppR were obtained (a) by subtracting the initial dark conditions (Dark1) from the green-light-illumination (520 nm) state (Green) (Green − Dark1); (b) by subtracting Dark1 from the blue-light (365 nm)-illumination state (Blue) (Blue − Dark1); and (c) by subtracting Green from Blue (Blue − Green). The difference spectrum shown in (a) indicates the transformation process from the G state (13.6 ppm) to the M- (22.3 ppm), N′- (23.9 ppm), and O-intermediates (16.4 ppm) upon green-light (520 nm) illumination. The difference spectra shown in (b) and (c) indicate that the O-intermediate (16.4 ppm) was trapped upon the subsequent blue-light (365 nm) illumination. (B) The difference spectra of [20-13C] retinal-ppR obtained (d) by subtracting Dark1 from Blue (Blue − Dark1); (e) by subtracting Dark1 from the short dark conditions after turning off irradiation (Dark2) (Dark2 − Dark1); and (f) by subtracting Blue from Dark2 (Dark2 − Blue) are shown.
In situ photoirradiation solid-state 13C CP-MAS measurements were performed on [20-13C] retinal-ppR alone at −60°C. Under illumination with green light, the difference 13C CP-MAS NMR spectrum of [20-13C] retinal-ppR (green-light illumination (Green) − initial dark condition (Dark1)) indicated the transformation process from the G state (13.6 ppm) to the O-, M-, and N′-intermediates (16.4, 22.3, and 23.9 ppm) (Fig. 7 Aa). After stationary trapping of these photointermediates, the light source was switched from 520 to 360 nm. The result indicated that the signal of the M-intermediates (22.6 ppm) decreased and the signal of the O-intermediate (16.4 ppm) increased (Fig. 7 Ab). The absorbance maximum of the M-intermediate was 395 nm, and thus this result indicated that the M-intermediates were transformed to the O-intermediate by irradiation with 365 nm blue light (Fig. 7 Ac).
Relaxation process from the O-intermediate to the N′-intermediate
We obtained the relaxation process from the O-intermediate to the N′-intermediate from the difference spectra shown in Fig. 7 B. The difference 13C CP-MAS NMR spectrum of [20-13C] retinal-ppR between the blue (365 nm) state (Blue) and initial dark conditions (Dark1) indicated that the product photointermediates were the O- and N′-intermediates in the blue-light (365 nm)-illumination state. By turning off the irradiation, the O-intermediate was converted to the N′-intermediate, as with the ppR/pHtrII complex (Fig. 7 Be). This pathway was clearly observed in the difference spectrum generated by subtracting the blue-light (365 nm)-illumination state from the Dark2 state (Fig. 7 Bf).
Chemical shift values of [14-13C] retinal for the intermediates in ppR
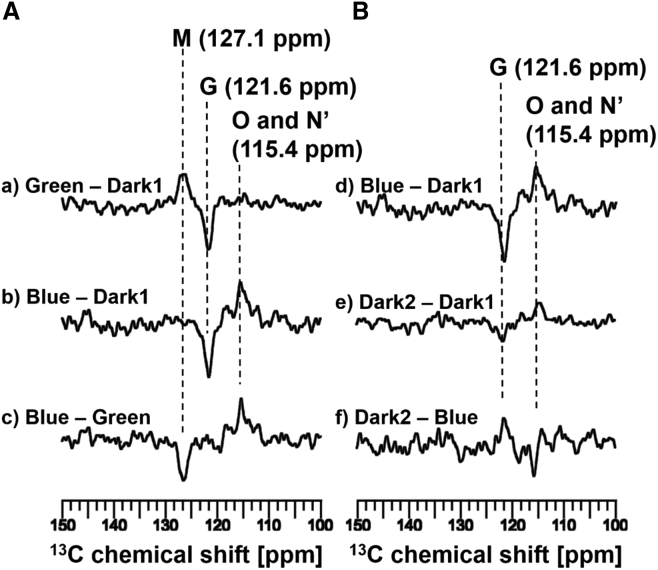
We revealed the configurations of the O- and N′-intermediate by measuring the 13C CP-MAS NMR spectra of [14-13C] retinal-ppR. The chemical shift values of [14-13C] retinal contain important information regarding the 15-syn/anti configuration (20, 33). The spectra shown in Fig. 8 show the region from 100.0 to 150.0 ppm for the same experiments as shown in Fig. 7 and indicate the chemical shifts of [14-13C] retinal-ppR. At −60°C, in situ photoirradiation solid-state 13C CP-MAS spectra were observed for [14-13C] retinal-ppR, and the signal at 127.1 ppm was assigned to [14-13C] retinal in the M-intermediates resulting from a transformation process from the G state to the M-intermediates under green-light (520 nm) illumination (Fig. 8 Aa). Subsequent blue-light (365 nm) illumination resulted in an intense signal from the O-intermediate, as with the signal of [20-13C] retinal-ppR shown in Fig. 7. Therefore, the main positive peak in the difference spectrum (Fig. 8 Ab) was assigned to the O-intermediate, and the chemical shift value of [14-13C] retinal was 115.4 ppm. As discussed above, the M-intermediates transform to the O-intermediate upon blue-light (365 nm) illumination, and thus the chemical shift value at 115.4 ppm was indeed due to the O-intermediate (Fig. 8 Ac). The spectrum shown in Fig. 8 Bd also indicated that the 115.4 ppm signal is assignable to the O-intermediate because of the double photon process from the G state to the O-intermediate upon blue-light (365 nm) illumination.
Figure 8.
13C CP-MAS NMR signals of [14-13C] retinal-ppR alone at −60°C under 4 kHz MAS. (A) The difference spectra of [14-13C] retinal-ppR obtained (a) by subtracting the initial dark conditions (Dark1) from the green-light (520 nm)-illumination states (Green) (Green − Dark1); (b) by subtracting Dark1 from the blue-light (365 nm)-illumination state (Blue) (Blue − Dark1); and (c) subtracting Green from Blue (Blue − Green) are shown. The difference spectrum shown in (a) indicates the transformation process from the G state (121.6 ppm) to the M-intermediates (127.1 ppm) upon green-light (520 nm) illumination. The difference spectra shown in (b) and (c) indicate the transition process from the M-intermediates to the O-intermediate (115.4 ppm) upon subsequent blue-light (365 nm) illumination. (B) The difference spectra of [14-13C] retinal-ppR obtained (d) by subtracting Dark1 from Blue (Blue − Dark1); (e) by subtracting Dark1 from the short dark conditions after turning off irradiation (Dark2) (Dark2 − Dark1); and (f) by subtracting Blue from Dark2 (Dark2 − Blue) are shown. The difference spectra shown in (e) and (f) indicate that the remaining signal at 115.4 ppm belongs to the N′-intermediate.
The difference spectrum shown in Fig. 8 Be clearly indicated that the 115.4 ppm signal also belonged to the N′-intermediate, because the N′-intermediate showed a very long half-life and the signal intensity remained unchanged at −60°C, as shown in Fig. 7b and e. In addition, the thermal relaxation pathway of the O-intermediate was observed as a decrease in the signal at 115.4 ppm (Fig. 8 Bf).
Conformation of retinal in the late photointermediates
We have assigned the 13C NMR chemical shift values for the late photointermediates M-, O-, and N′-intermediates (Table 1). The configuration of the M-intermediates was clearly shown to be 13-cis, 15-anti by comparing this result with previous studies of Salinibacter sensory rhodopsin I (SrSRI), bR, and its mutant Y185F-bR (20, 30, 33) (Table S1). Furthermore, the deprotonated SB state in the M-intermediates was obtained as a result of the fast transformation process initiated by blue-light (365 nm) illumination. This comparison and our previous studies in ppR and SrSRI (30, 31) allow the 13C=14C configuration of retinal in the O- and N′-intermediates to be assigned to the 13-trans and 13-cis forms, respectively. The 15C=Nζ configuration is more challenging, because the value of 115.4 ppm is the boundary region between the 15-syn and 15-anti forms. For example, in the study of Y185F-bR, the CS∗ intermediate has been assigned to the 15-syn form, and the chemical shift value of [14-13C] retinal was 115.3 ppm (33). On the other hand, the N-intermediate of wild-type bR has been assigned to the 15-anti form, and the chemical shift value of [14-13C] retinal was 115.2 ppm (53) (Table S1). The following is a detailed discussion of the configurations of the O- and N′-intermediates as elucidated by DFT calculation.
Determination of the dihedral angles Φ for the O- and N′-intermediates
We elucidated the configuration of the O- and N′-intermediates by investigating the relationship between the 13C NMR chemical shift values and configuration by using DFT calculation. The crystal structure (PDB: 1JGJ) (48) corresponding to the G state was used as a starting configuration and has the dihedral angles Φ = −177, Ψ = −175, and Χ = −66°. The chemical shift values of 20-13C and 14-13C for this configuration were calculated to be 17.5 and 128.1 ppm, respectively, and these values are shown in Table 2. Touw et al. (54) previously calculated the chemical shift at 20-13C for all-trans retinal to be 18.0 ppm (54), which is very similar to the value obtained in this work. However, these calculated values show some discrepancy with the experimental values of 13.6 and 121.6 ppm, with the calculated chemical shift values being 3.9 and 6.5 ppm larger than the experimental 20-13C and 14-13C values, respectively. Touw et al. (54) also showed a similar discrepancy. Therefore, in the following discussion, the calculated values will be discussed with corrected values by adding 3.9 and 6.5 ppm to the experimental values for 20-13C and 14-13C in retinal, respectively.
Table 2.
13C Chemical Shift Values of Retinal Calculated by DFT and the Conformation of Retinal in Several Photointermediates
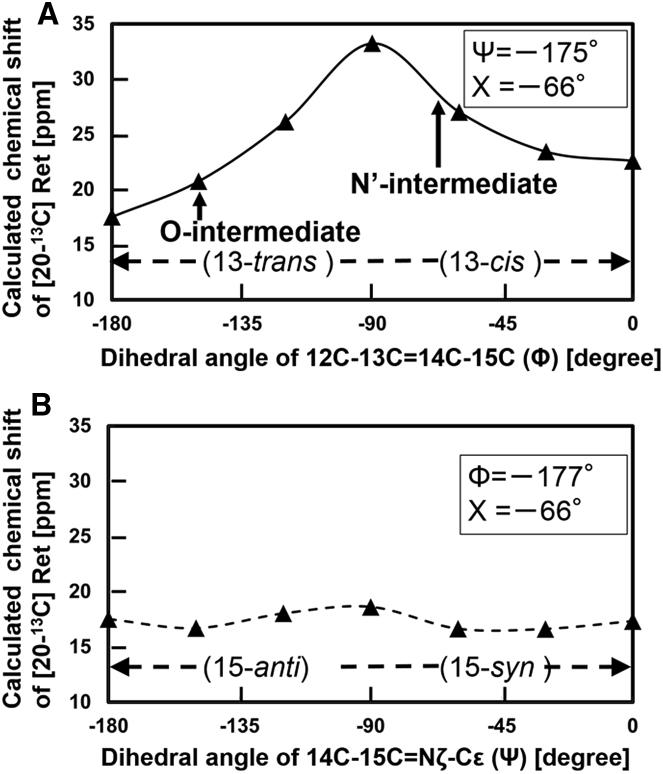
The dependence of the chemical shift value of 20-13C in retinal on the dihedral angles Φ and Ψ were investigated, and the results are shown in Fig. 9, A and B, respectively. The chemical shift at 20-13C in retinal drastically changes according to the rotation of dihedral angle Φ, but it is almost independent of the change in the dihedral angle Ψ. These results indicate that the value of 20-13C can be used to determine the configuration with dihedral angle Φ. The experimental chemical shift values of 20-13C for the O- and N′-intermediates were 16.4 and 23.9 ppm, and thus the corrected values of these conformations should be 20.3 and 27.8 ppm, respectively. The positions of these values are shown in Fig. 9 A. The corresponding dihedral angle Φ for the O-intermediate configuration was estimated as −150°, and for the N′-intermediate it was estimated as −63°, as shown in Table 2.
Figure 9.
The dependency of the chemical shift value of 20-13C in retinal on the dihedral angles Φ and Ψ is shown in (A) and (B), respectively. In (A), only the dihedral angle Φ was rotated from the trans (−180°) to the cis (0°) configuration by setting Ψ and Χ at −175 and −66°, respectively (48). In (B), only the dihedral angle Ψ was rotated from the anti (−180°) to the syn (0°) configuration by fixing Φ and Χ to −177 and −66°, respectively (48). The calculated chemical shift values were converted to ppm from TMS.
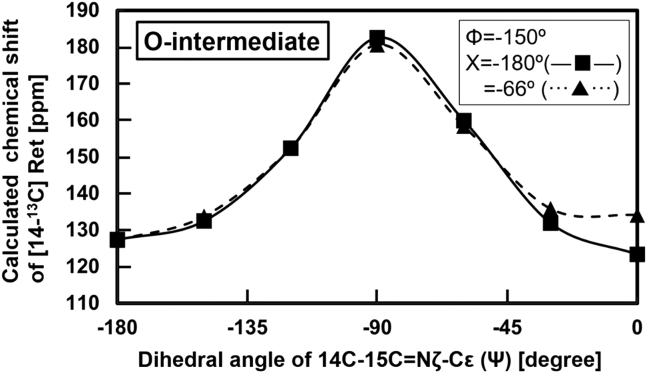
Determination of the dihedral angle Ψ for the O-intermediate
The dihedral angle Φ in the O-intermediate was estimated to be −150°, and thus the dihedral angle Ψ was investigated for this intermediate next. The dependence of the chemical shift value of 14-13C in retinal on the dihedral angle Ψ was evaluated by setting Φ at −150° and Χ at −66°. The results are shown in Fig. 10 (dashed line) and indicate that the chemical shift value for the 15-syn form was larger than that of the 15-anti form. However, previous experiments showed the opposite. For example, the values of 14-13C for 15-anti and 15-syn were 122.0 and 110.5 ppm for bR as shown in Table S1, and thus the calculated chemical shift of the syn-state should be upfield compared to that of the anti-state.
Figure 10.
The chemical shift value of 14-13C in retinal was calculated by changing the dihedral angle Ψ. The Φ angle was fixed at −150°, as estimated for the O-intermediate conformation discussed in Fig. 9. The Χ angle was set at the crystal conformation (48) of −66° in (▴), and the trans conformation (48) of −180° in (Χ).
We resolved this discrepancy by conducting calculations using the trans configuration for dihedral angle Χ (Χ = −180°), because in this configuration, we would expect a γ-effect between the 14-13C proton and the ε proton (55). The results are also shown in Fig. 10 and indicate that the chemical shift value of 14-13C in the syn conformation (Ψ = 0°) changed by −4.0 ppm from anti (Ψ = −180°) configuration (Fig. 10, dashed line). This change in chemical shift allowed estimation of the configuration of the O-intermediate. The experimental chemical shift value of 14-13C of the O-intermediate was 115.4 ppm, as summarized in Table 1, and thus the offset value of this configurations should be 121.9 ppm, corresponding to a dihedral angle Ψ of 0°. The configuration of the O-intermediate can thus be determined to have the dihedral angles Φ and Ψ of −150 and 0°, respectively, as listed in Table 2. These results indicated that the configuration of the O-intermediate was, in general, in the 13-trans and 15-syn form.
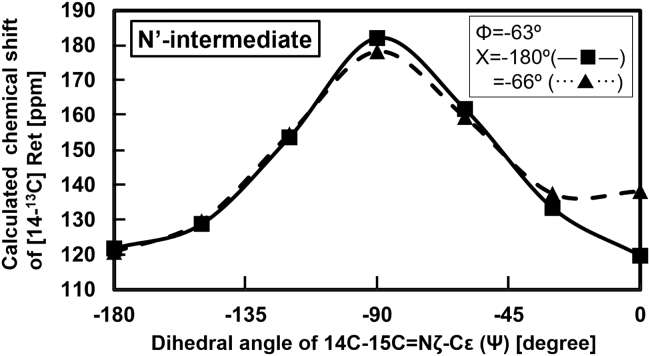
Determination of the dihedral angle Ψ for the N′-intermediate
The dihedral angle of Ψ was estimated in a manner similar to that discussed above. The conformation of the N′-intermediate was calculated using the dependence of the chemical shift value of 14-13C in retinal on the dihedral angle Ψ, and the results are shown in Fig. 11. The dihedral angle Φ in the N′-intermediate was set at −63° as estimated in Fig. 9 A. Two sets of values were used in the calculations for the dihedral angle Χ: the crystal value of −66° and the trans form value of −180°, and both the results are shown in Fig. 11. The curves of the chemical shifts allowed estimation of the conformation of the N′-intermediate. The chemical shift value of 14-13C of the N′- intermediate was 115.4 ppm, as summarized in Table 1, and thus the offset value of this conformation should be 121.9 ppm. However, three points correspond to the dihedral angle Ψ in Fig. 11: the dihedral angles Ψ and Χ are (Ψ = −175° and Χ = −66°), (Ψ = −175° and Χ = −180°), and (Ψ = −17° and Χ = −180°). Although the dihedral angle Φ in the N′-intermediate was determined as −63°, the remaining dihedral angles Ψ and Χ could be any of these three possibilities.
Figure 11.
The chemical shift value of 14-13C in retinal was calculated by changing the dihedral angle Ψ and fixing Φ at −63°, as estimated for the N′-intermediate configuration discussed in Fig. 9. The Χ angle was set at the crystal configuration (48) of −66° in (▴), and the trans conformation (48) of −180° in (Χ).
Conclusions
It is demonstrated that in situ photoirradiation solid-state NMR spectroscopy allows us to observe the photointermediates in the stationary state and determine the photoreaction pathways of photoreceptor membrane proteins such as the ppR/pHtrII complex system to gain insight into the signal transduction mechanism.
We observed the M-, O-, and N′-intermediates from ppR and ppR/pHtrII complex reconstituted in egg-PC membrane by irradiation with green light using in situ photoirradiation solid-state NMR. Changes in the 13C CP-MAS NMR signals resulting from switching the wavelength of light used for continuous irradiation from green light (520 nm) to blue light (365 nm) revealed the pathways of the late photoactive intermediates from the M-intermediates to the O-intermediate. Interestingly, one of the multiple M-intermediates previously reported was identified as an N-like intermediate reported in bR, which we designated the N′-intermediate. The N′-intermediate is produced from the O-intermediate in ppR, whereas the N-intermediate is produced from M-intermediate in bR. This result indicates that large structural changes of the proteins are necessary to produce the N′-intermediate, which is in an equilibrium state with the O-intermediate. In addition, the half-life of the N′-intermediate for the ppR/pHtrII complex was shown to be longer than that for ppR. In the physiological point of view, we suggested that the N′-intermediate might play important role in signaling processes as a preamplitude mechanism because it has a long half-life and is in equilibrium with the O-intermediate. Detailed configurations of the O- and N′-intermediates were revealed to be 13-trans, 15-syn, and 13-cis, respectively, by NMR experiments and DFT calculation and showed that the chemical shift values of [14-13C] retinal are useful for determining the 15-syn or 15-anti configuration. The results of our DFT calculation are the first step of the trial to find the conformation of the intermediate states of the retinal, which would be difficult to get only from the experimental work. Further refinement of the calculation will be needed to confirm the proposed intermediate conformations in future.
Author Contributions
Y.M. performed NMR measurements, DFT calculation, and analyses, and wrote the article. I.K. and A.N. designed NMR experiments and wrote the article. K.U. performed DFT calculation and wrote the article. T.O. and A.W. prepared labeled retinal. N.K. and Y.S. designed sample preparation and gave valuable discussion on the article.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research in an Innovative Area (16H00756 to A.N., 25104005 to Y.S., and 16H00828 to I.K.), a Grant-in-Aid for Scientific Research (C) (15K06963 to A.N.) and Research (B) (15H04363 to Y.S. and 15H04336 to I.K.), and a Grant-in-Aid for Japan Society for the Promotion of Research as Research Fellowship (16J00073 to Y.M.) from the Ministry of Culture, Sports, Science and Technology of Japan. This work was also supported by the Japan Society and Technology-CREST and the Japan Agency for Medical Research and Development grants to Y.S. The authors thank the Research Center for Computational Science, Okazaki, Japan, for use of their computing facilities to perform a part of the calculation described in this work.
Editor: Timothy Cross.
Footnotes
One figure and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30669-6.
Contributor Information
Izuru Kawamura, Email: izuruk@ynu.ac.jp.
Kazuyoshi Ueda, Email: ueda-kazuyoshi-cz@ynu.ac.jp.
Akira Naito, Email: naito@ynu.ac.jp.
Supporting Material
References
- 1.Govorunova E.G., Sineshchekov O.A., Spudich J.L. Microbial Rhodopsins: diversity, mechanisms, and optogenetic applications. Annu. Rev. Biochem. 2017;86:845–872. doi: 10.1146/annurev-biochem-101910-144233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoff W.D., Jung K.H., Spudich J.L. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu. Rev. Biophys. Biomol. Struct. 1997;26:223–258. doi: 10.1146/annurev.biophys.26.1.223. [DOI] [PubMed] [Google Scholar]
- 3.Spudich J.L., Luecke H. Sensory rhodopsin II: functional insights from structure. Curr. Opin. Struct. Biol. 2002;12:540–546. doi: 10.1016/s0959-440x(02)00359-7. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki D., Irieda H., Sudo Y. Phototactic and chemotactic signal transduction by transmembrane receptors and transducers in microorganisms. Sensors (Basel) 2010;10:4010–4039. doi: 10.3390/s100404010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue K., Tsukamoto T., Sudo Y. Molecular and evolutionary aspects of microbial sensory rhodopsins. Biochim. Biophys. Acta. 2014;1837:562–577. doi: 10.1016/j.bbabio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Kamo N., Shimono K., Sudo Y. Photochemistry and photoinduced proton-transfer by pharaonis phoborhodopsin. Biochemistry (Mosc.) 2001;66:1277–1282. doi: 10.1023/a:1013187403599. [DOI] [PubMed] [Google Scholar]
- 7.Imamoto Y., Shichida Y., Yoshizawa T. Chromophore configuration of pharaonis phoborhodopsin and its isomerization on photon absorption. Biochemistry. 1992;31:2523–2528. doi: 10.1021/bi00124a012. [DOI] [PubMed] [Google Scholar]
- 8.Gordeliy V.I., Labahn J., Engelhard M. Molecular basis of transmembrane signalling by sensory rhodopsin II-transducer complex. Nature. 2002;419:484–487. doi: 10.1038/nature01109. [DOI] [PubMed] [Google Scholar]
- 9.Moukhametzianov R., Klare J.P., Gordeliy V.I. Development of the signal in sensory rhodopsin and its transfer to the cognate transducer. Nature. 2006;440:115–119. doi: 10.1038/nature04520. [DOI] [PubMed] [Google Scholar]
- 10.Ishchenko A., Round E., Gordeliy V. New insights on signal propagation by sensory rhodopsin II/transducer complex. Sci. Rep. 2017;7:41811. doi: 10.1038/srep41811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimono K., Hayashi T., Kamo N. Importance of the broad regional interaction for spectral tuning in Natronobacterium pharaonis phoborhodopsin (sensory rhodopsin II) J. Biol. Chem. 2003;278:23882–23889. doi: 10.1074/jbc.M301200200. [DOI] [PubMed] [Google Scholar]
- 12.Sudo Y., Furutani Y., Spudich J.L. Functional importance of the interhelical hydrogen bond between Thr204 and Tyr174 of sensory rhodopsin II and its alteration during the signaling process. J. Biol. Chem. 2006;281:34239–34245. doi: 10.1074/jbc.M605907200. [DOI] [PubMed] [Google Scholar]
- 13.Sudo Y., Furutani Y., Kandori H. Steric constraint in the primary photoproduct of an archaeal rhodopsin from regiospecific perturbation of C-D stretching vibration of the retinyl chromophore. J. Am. Chem. Soc. 2005;127:16036–16037. doi: 10.1021/ja056203a. [DOI] [PubMed] [Google Scholar]
- 14.Wegener A.A., Chizhov I., Steinhoff H.J. Time-resolved detection of transient movement of helix F in spin-labelled pharaonis sensory rhodopsin II. J. Mol. Biol. 2000;301:881–891. doi: 10.1006/jmbi.2000.4008. [DOI] [PubMed] [Google Scholar]
- 15.Spudich J.L. Variations on a molecular switch: transport and sensory signalling by archaeal rhodopsins. Mol. Microbiol. 1998;28:1051–1058. doi: 10.1046/j.1365-2958.1998.00859.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H., Sudo Y., Kamo N. Transient movement of helix F revealed by photo-induced inactivation by reaction of a bulky SH-reagent to cysteine-introduced pharaonis phoborhodopsin (sensory rhodopsin II) Photochem. Photobiol. Sci. 2004;3:537–542. doi: 10.1039/b315454h. [DOI] [PubMed] [Google Scholar]
- 17.Ernst O.P., Lodowski D.T., Kandori H. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S.O., de Groot H.J., Griffin R.G. Structure and protein environment of the retinal chromophore in light- and dark-adapted bacteriorhodopsin studied by solid-state NMR. Biochemistry. 1989;28:8897–8904. doi: 10.1021/bi00448a032. [DOI] [PubMed] [Google Scholar]
- 19.McDermott A.E., Thompson L.K., Griffin R.G. Mechanism of proton pumping in bacteriorhodopsin by solid-state NMR: the protonation state of tyrosine in the light-adapted and M states. Biochemistry. 1991;30:8366–8371. doi: 10.1021/bi00098a013. [DOI] [PubMed] [Google Scholar]
- 20.Farrar M.R., Lakshmi K.V., Herzfeld J. Solid state NMR study of [ε-13C]Lys-bacteriorhodopsin: Schiff base photoisomerization. Biophys. J. 1993;65:310–315. doi: 10.1016/S0006-3495(93)81065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groot H.J., Smith S.O., Herzfeld J. Solid-state 13C and 15N NMR study of the low pH forms of bacteriorhodopsin. Biochemistry. 1990;29:6873–6883. doi: 10.1021/bi00481a017. [DOI] [PubMed] [Google Scholar]
- 22.Hu J.G., Sun B.Q., Herzfeld J. Early and late M intermediates in the bacteriorhodopsin photocycle: a solid-state NMR study. Biochemistry. 1998;37:8088–8096. doi: 10.1021/bi973168e. [DOI] [PubMed] [Google Scholar]
- 23.Petkova A.T., Hatanaka M., Herzfeld J. Tryptophan interactions in bacteriorhodopsin: a heteronuclear solid-state NMR study. Biochemistry. 2002;41:2429–2437. doi: 10.1021/bi012127m. [DOI] [PubMed] [Google Scholar]
- 24.Hu J.G., Sun B.Q., Herzfeld J. The predischarge chromophore in bacteriorhodopsin: a 15N solid-state NMR study of the L photointermediate. Biochemistry. 1997;36:9316–9322. doi: 10.1021/bi970416y. [DOI] [PubMed] [Google Scholar]
- 25.Mak-Jurkauskas M.L., Bajaj V.S., Herzfeld J. Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR. Proc. Natl. Acad. Sci. USA. 2008;105:883–888. doi: 10.1073/pnas.0706156105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj V.S., Mak-Jurkauskas M.L., Griffin R.G. Functional and shunt states of bacteriorhodopsin resolved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc. Natl. Acad. Sci. USA. 2009;106:9244–9249. doi: 10.1073/pnas.0900908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker-Baldus J., Bamann C., Glaubitz C. Enlightening the photoactive site of channelrhodopsin-2 by DNP-enhanced solid-state NMR spectroscopy. Proc. Natl. Acad. Sci. USA. 2015;112:9896–9901. doi: 10.1073/pnas.1507713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura I., Kihara N., Naito A. Solid-state NMR studies of two backbone conformations at Tyr185 as a function of retinal configurations in the dark, light, and pressure adapted bacteriorhodopsins. J. Am. Chem. Soc. 2007;129:1016–1017. doi: 10.1021/ja0664887. [DOI] [PubMed] [Google Scholar]
- 29.Tomonaga Y., Hidaka T., Naito A. An active photoreceptor intermediate revealed by in situ photoirradiated solid-state NMR spectroscopy. Biophys. J. 2011;101:L50–L52. doi: 10.1016/j.bpj.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yomoda H., Makino Y., Naito A. Color-discriminating retinal configurations of sensory rhodopsin I by photo-irradiation solid-state NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2014;53:6960–6964. doi: 10.1002/anie.201309258. [DOI] [PubMed] [Google Scholar]
- 31.Naito A., Kawamura I. Photoactivated structural changes in photoreceptor membrane proteins as revealed by in situ photoirradiation solid-state NMR spectroscopy. In: Separovic F., Naito A., editors. Advances in Biological Solid-State NMR: Proteins and Membrane-Active Peptides. Royal Society of Chemistry; 2014. pp. 387–404. [Google Scholar]
- 32.Naito A., Kawamura I., Javkhlantugs N. Recent solid-state NMR studies of membrane-bound peptides and proteins. Annu. Rep. NMR Spectrosc. 2015;86:334–411. [Google Scholar]
- 33.Oshima K., Shigeta A., Naito A. Characterization of photo-intermediates in the photo-reaction pathways of a bacteriorhodopsin Y185F mutant using in situ photo-irradiation solid-state NMR spectroscopy. Photochem. Photobiol. Sci. 2015;14:1694–1702. doi: 10.1039/c5pp00154d. [DOI] [PubMed] [Google Scholar]
- 34.Naito A., Makino Y., Kawamura I. Photoirradiatiion and microwave irradiation NMR spectroscopy. In: Nuclear Magnetic Resonance Socirty of Japan The., editor. Experimental Approaches of NMR Spectroscopy. Springer; 2018. pp. 135–170. [Google Scholar]
- 35.Naito A., Makino Y., Kawamura I. In-situ photo irradiation solid-state NMR spectroscopy applied to retinal-binding membrane proteins. In: Webb G.A., editor. Modern Magnetic Resonance. Springer; 2017. pp. 1–22. [Google Scholar]
- 36.Kitajima-Ihara T., Furutani Y., Sudo Y. Salinibacter sensory rhodopsin: sensory rhodopsin I-like protein from a eubacterium. J. Biol. Chem. 2008;283:23533–23541. doi: 10.1074/jbc.M802990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawamura I., Yoshida H., Naito A. Dynamics change of phoborhodopsin and transducer by activation: study using D75N mutant of the receptor by site-directed solid-state 13C NMR. Photochem. Photobiol. 2008;84:921–930. doi: 10.1111/j.1751-1097.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 38.Sudo Y., Yamabi M., Kamo N. Interaction of Natronobacterium pharaonis phoborhodopsin (sensory rhodopsin II) with its cognate transducer probed by increase in the thermal stability. Photochem. Photobiol. 2003;78:511–516. doi: 10.1562/0031-8655(2003)078<0511:ionpps>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer J., Stejskal E.O. Carbon-13 nuclear magnetic resonance of polymers spinning at the magic angle. J. Am. Chem. Soc. 1976;98:1031–1032. [Google Scholar]
- 40.Bennett A.E., Rienstra C.M., Griffin R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 41.Saitô H., Ando I., Ramamoorthy A. Chemical shift tensor - the heart of NMR: insights into biological aspects of proteins. Prog. Nucl. Magn. Reson. Spectrosc. 2010;57:181–228. doi: 10.1016/j.pnmrs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.London F. Théorie quantique des courants interatomiques dans les combinaisons aromatiques. J. Phys. Radium. 1937;8:397–409. [Google Scholar]
- 43.Ditchfield R. Molecular orbital theory of magnetic shielding and magnetic susceptibility. J. Chem. Phys. 1972;56:5688–5691. [Google Scholar]
- 44.Ditchfield R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. Chemical shifts. Mol. Phys. 1974;27:789–807. [Google Scholar]
- 45.Woliński K., Sadlej A.J. Self-consistent perturbation theory open-shell states in perturbation-dependent non-orthogonal basis sets. Mol. Phys. 1980;41:1419–1430. [Google Scholar]
- 46.Wolinski K., Hinton J.F., Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990;112:8251–8260. [Google Scholar]
- 47.Frisch M.J., Trucks G.W., Pople J.A. Gaussian, Inc.; Wallingford, CT: 2004. Gaussian 09, Revision A.02. [Google Scholar]
- 48.Luecke H., Schobert B., Spudich J.L. Crystal structure of sensory rhodopsin II at 2.4 angstroms: insights into color tuning and transducer interaction. Science. 2001;293:1499–1503. doi: 10.1126/science.1062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chizhov I., Schmies G., Engelhard M. The photophobic receptor from Natronobacterium pharaonis: temperature and pH dependencies of the photocycle of sensory rhodopsin II. Biophys. J. 1998;75:999–1009. doi: 10.1016/S0006-3495(98)77588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tateishi Y., Abe T., Unno M. Spectroscopic evidence for the formation of an N intermediate during the photocycle of sensory rhodopsin II (phoborhodopsin) from Natronobacterium pharaonis. Biochemistry. 2011;50:2135–2143. doi: 10.1021/bi1019572. [DOI] [PubMed] [Google Scholar]
- 51.Roy S., Kikukawa T., Kamo N. All-optical switching in Pharaonis phoborhodopsin protein molecules. IEEE Trans. Nanobioscience. 2006;5:178–187. doi: 10.1109/tnb.2006.880828. [DOI] [PubMed] [Google Scholar]
- 52.Balashov S.P., Sumi M., Kamo N. The M intermediate of Pharaonis phoborhodopsin is photoactive. Biophys. J. 2000;78:3150–3159. doi: 10.1016/S0006-3495(00)76851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakshmi K.V., Farrar M.R., Herzfeld J. Solid state 13C and 15N NMR investigations of the N intermediate of bacteriorhodopsin. Biochemistry. 1994;33:8853–8857. doi: 10.1021/bi00196a001. [DOI] [PubMed] [Google Scholar]
- 54.Touw S.I.E., De Groot H.J.M., Buda F. DFT calculations of the1H chemical shifts and13C chemical shift tensors of retinal isomers. J. Mol. Struct. THEOCHEM. 2004;711:141–147. [Google Scholar]
- 55.Harbison G.S., Smith S.O., Griffin R.G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc. Natl. Acad. Sci. USA. 1984;81:1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.