Abstract
Pre-clinical studies investigated the effects of chronic exposure to nicotine on lungs, kidneys and brains using animal models. Most of these studies delivered nicotine into the circulatory and central nervous systems (CNS) through intraperitoneal injection or oral consumption methods. Few studies used inhalation machine system for nicotine delivery into brains in rodents to mimic human exposure to cigarettes. However, finding a more accurate and clinically relevant method of nicotine delivery is critical. A computerized inhalation machine has been designed (SciReq) and is currently employed in several institutions. The computerized machine delivers electronic (e)-cigarette vapor as well as tobacco smoke to rodents using marketed e-cigarette devices or tobacco cigarettes. This provides evidence about clinical effects of nicotine delivery by traditional methods (combustible cigarettes) and new methodologies (e-cigarettes) in physiological systems. Potential neurobiological mechanisms for the development of nicotine dependence have been determined recently in mice exposed to e-cigarette vapors in our laboratory using SciReq system. In this review article, the discussion focuses on the efficiency and practical applicability of using this computerized inhalation exposure system in inducing significant changes in brain protein expression and function as compared to other nicotine delivery methods. The SciReq inhalation system utilized in our laboratory and others is a method of nicotine delivery to the CNS, which has physiological relevance and mimics human inhalant exposures. Translation of the effects of inhaled nicotine on the CNS into clinical settings could provide important health considerations.
Keywords: Electronic cigarette, Tobacco cigarette, Cigarette inhalation, Nicotine, Combustible cigarette
Abbreviations: α-7nAChR, alpha-7 nicotinic acetylcholine receptor; CNS, central nervous system; e-cigarette, electronic cigarette; GLT-1, glutamate transporter-1; xCT, cystine/glutamate exchanger
1. Introduction
Parenteral routes of nicotine administration have been the standard methods used in pre-clinical nicotine delivery methods for decades (Nadal et al., 1998, Tizabi et al., 2002, Knackstedt et al., 2009, Fowler and Kenny, 2011, Wang et al., 2014). These parenteral routes include intraperitoneal, intravenous and intracerebral injections. Oral nicotine consumption is a method utilized in several studies to examine the behavioral effects of nicotine in animals (Sparks and Pauly, 1999, Adriani et al., 2002, Sari et al., 2016). However, these delivery methods have less desirable clinical and pharmacokinetic properties as compared to inhalation of nicotine (For review see (Le Houezec, 2003)). Laboratories have designed a computerized system for nicotine or tobacco smoke inhalation to investigate their effects in the body organs, including the brain.
The use of inhalation system for nicotine or tobacco cigarette smoke delivery in an animal model can provide novel evidence about the long-term effects of these chemicals on several neurotransmitters. It is important to note that exposure to nicotine through intra-striatal or subcutaneous routes of nicotine administration upregulated nicotinic acetylcholine receptors (nAChRs) in the mesocorticolimbic areas (Auta et al., 2000, Buisson and Bertrand, 2001, Alsharari et al., 2015). In addition, intravenous self- administration of nicotine via base/infusion for 21 days reduced one of the major glial glutamate transporters such as glutamate transporter 1 (GLT-1) in central reward brain regions (Knackstedt et al., 2009). These effects on the central nervous system (CNS) induced by nicotine exposure using non-clinical nicotine exposure methods may or may not be clinically relevant. Thus, using a nicotine delivery system reflective of human exposure routes is important to confirm or refute these findings. Thus, this will define addictive behavioral and neurobiological effects that may be induced by inhaled nicotine, which may mediate alterations in the function and expression of certain brain proteins. In this review article, we compared and contrasted the inhalation route with other routes of nicotine exposure on mediation of addictive effects.
Inhalation has been associated with a fast rate of nicotine absorption as compared to other routes of delivery of nicotine (For review see (Le Houezec, 2003)). In addition, bioavailability of nicotine in the brain has been reported to be higher after inhalation of cigarettes as compared to parenteral routes of nicotine delivery (Benowitz, 1990). Alterations in pharmacokinetic parameters occurred in subjects exposed to chronic inhalation of cigarette smoke-containing nicotine compared to other methods of nicotine delivery (Benowitz, 1990, Le Houezec, 2003). These differences in pharmacokinetics provide evidence that chronic nicotine inhalation may mediate alterations in key proteins involved in the development of nicotine dependence to a different extent, and in a different pattern than other routes of nicotine administration.
The inhalation exposure system (computerized inExpose machine) of nicotine has been found to be associated with several modifiable characteristics. Different electronic cigarette (e-cigarette) and tobacco cigarette brands can be used in the system (Hwang et al., 2016), and this could be clinically relevant when testing the most marketed e-cigarette and tobacco cigarette products (Fig. 1). In addition, the exposure period and duration to cigarettes can be controlled by the experimenters to mimic the actual human exposure duration and frequency to cigarettes (Hwang et al., 2016) (Fig. 1). Interestingly, other drugs of abuse can also be applied using the inhalation exposure machine, which may provide potential evidence about the effects of inhaled drugs of abuse on the body. We here shed light on the main characteristics of using the computerized inhalation exposure system in animal models compared to other routes of nicotine delivery.
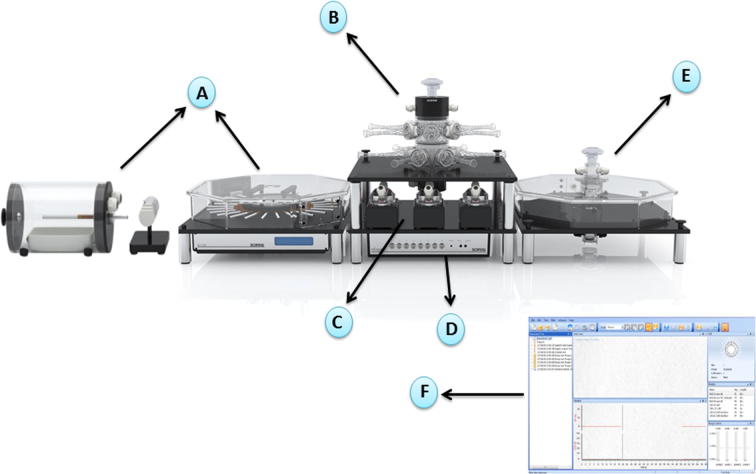
Fig. 1.
Inhalation exposure system composes of six major components. (A) Cigarette smoke generation: multiple type of cigarettes such as combustible and electronic-cigarettes can be used in the apparatus to generate smoking. (B) Nose-only exposure: animals can be placed in mask holders to provide only nose exposure to cigarettes. (C) InExpose pumps: these pumps are designed to generate smoking. (D) InExpose base unit: the base unit is connected to the software to control the exposure parameters such as exposure time and exposure duration. (E) Whole body chamber: whole body animals can be placed in chambers to expose the whole body to cigarettes. (F) Flexiware software: the exposure temperature, patterns of smoking and humidity as well as the exposure time and duration can be modified practically by the software. The image is adopted with permission from SCIREQ Scientific Respiratory Equipment Inc. (http://www.scireq.com/inexpose).
2. Comparisons of nicotine inhalation to other delivery routes
Several studies investigated the effects of nicotine on nicotinic receptors, dopaminergic and glutamatergic systems in the CNS. These studies found that nicotine exposure was able to upregulate subtypes of nAChRs in mesocorticolimbic brain regions (Buisson and Bertrand, 2001, Alsharari et al., 2015). In addition, intraperitoneal injection of nicotine increased dopamine release in part by stimulation of nAChRs (Tizabi et al., 2002, Tizabi et al., 2007). Moreover, nicotine self-administration upregulated ionotropic glutamate receptors (Wang et al., 2007, Kenny et al., 2009, Alasmari et al., 2016). In addition, intravenous self-administration of nicotine was found to reduce the expression of GLT-1 in the nucleus accumbens (Knackstedt et al., 2009). The alterations in dopaminergic and glutamatergic systems as well as nicotinic receptors following different nicotine exposure methods have been suggested to mediate the development of nicotine dependence. However, little is known about the effects of chronic exposure to nicotine exposure using inhalation of e-cigarette vapor or tobacco smoke-containing nicotine on dopaminergic system, glutamatergic system and nicotinic receptors.
A recent study from our laboratory reported that chronic inhalation of e-cigarettes vapor containing-nicotine induced alterations in the glutamatergic system in the brain of female CD1 mice (Alasmari et al., 2017). This study found that inhalation of e-cigarette vapor containing-nicotine for six months upregulated alpha 7 nAChR (α-7 nAChR) in frontal cortex and striatum in CD1 mice (Alasmari et al., 2017). It is important to note that α-7 nAChR regulates glutamate release from pre-synaptic glutamatergic neurons (Konradsson-Geuken et al., 2009). In addition, chronic inhalation of e-cigarette vapor induced downregulation of cystine/glutamate exchanger (xCT) in striatum and hippocampus as compared to a group exposed to air (Alasmari et al., 2017). xCT is an important glial protein that regulates glutamate homeostasis (Baker et al., 2002). Moreover, chronic exposure to e-cigarette vapor induced significant decrease in GLT-1 expression in the striatum. GLT-1 is glial glutamate transporter that clears the majority of extracellular glutamate (Danbolt, 2001). The reduction in the expression of GLT-1 and xCT is suggested to be associated with increase in extracellular glutamate concentration as it was found in animal model of alcohol dependence (Nemmar et al., 2013). Alterations of these proteins have been suggested previously to be involved in part in the development of nicotine dependence (For review see (Alasmari et al., 2016)).
Additionally, certain strains of animals prefer to consume nicotine orally only with additive appetizers such as sucrose or saccharin (Hauser et al., 2012, Sari et al., 2016). These additives are able to help with the bitter taste of nicotine (Hauser et al., 2012, Nesil et al., 2015, Sari et al., 2016). However, addition of other ingredients that are not found in cigarettes may lead to changes in neurobiological systems. Sucrose and saccharin have been found to induce alterations in the dopaminergic, glutamatergic and GABAergic systems (Khvotchev et al., 2000, Rada et al., 2005, Mitra et al., 2014). Thus, inhalation system might be a valid method to investigate the effects of chronic exposure to nicotine on key target proteins in the brain.
3. Pharmacokinetic of nicotine inhalation compared to other routes of nicotine exposure
Inhalation route can lead to high rate of absorption of nicotine through pulmonary system (Benowitz, 1990, Le Houezec, 2003). Importantly, alveolar capillaries in the lungs have large surface areas, which facilitate and enhance the rate and extent of nicotine absorption (Hirsch et al., 2005). These kinetic properties indicate the potential effects of nicotine inhalation delivery on the rate and extent of nicotine absorption, which may lead to high bioavailability of nicotine in the brain. Moreover, oral ingestion of nicotine has a lower systemic bioavailability since nicotine is metabolized pre-systemically in the liver (Le Houezec, 2003). This suggests that absorption of nicotine might be higher with inhalation method. In addition, inhalation of nicotine has been associated with a fast rate of distribution in the brain (Le Houezec, 2003). Distribution of nicotine into different tissues after smoking a cigarette has been detected in previous studies (Le Houezec, 2003, Nides, 2008, Berridge et al., 2010). Nicotine distribution in the brain and arterial blood was found immediately after smoking a cigarette (Nides, 2008, Berridge et al., 2010), and the concentration of nicotine was found to be decreased within twenty to thirty minutes in plasma (Le Houezec, 2003). Data suggest faster brain bioavailability of nicotine, which may then induce neurobiological alterations in the function of certain proteins that mediate the development of nicotine dependence (Ponzoni et al., 2015, Alasmari et al., 2017). It is important to note that the ratio of nicotine in the brain to plasma has been reported to be elevated during inhalation exposure (Ghosheh et al., 2001), and this ratio was decreased during the elimination period (Ghosheh et al., 2001). We suggest here that the brain distribution of nicotine after smoking is rapid, which indicate that the pulmonary system plays a crucial role in rapid onset of nicotine distribution into the brain.
A study from our laboratory found that inhalation of e-cigarette vapors containing nicotine for six months showed high concentration of nicotine and cotinine, a major metabolite and biomarker of nicotine, in the frontal cortex of female CD-1 mice (Alasmari et al., 2017). We suggest that nicotine is absorbed and metabolized rapidly in the body and that nicotine and its metabolites are transported into the brain. These data indicate that the computerized inhalation machine is efficient and can deliver high concentrations of nicotine into the brain. Further results from this study demonstrated that nicotine inhalation induced alterations in the expression of several target proteins, including GLT-1, xCT and α-7 nAChR, which may have a role in the development of nicotine dependence.
4. Benefits of a computerized inhalation exposure system as a nicotine delivery method
Several clinically desirable properties are controlled using the digitized inhalation exposure system (Wong, 2007, Hwang et al., 2016). Preparation of cigarette compositions is possible using the computerized system, and this applies to most brands of combustible cigarettes and e-cigarettes (Hwang et al., 2016). In addition, different concentrations of nicotine can be loaded into this system to determine potential dose-effects on vital organs. Exposure time and exposure duration to cigarettes as well as atmospheric and humidity conditions have been controlled by researchers using the computerized inhalation exposure system in animal models (Wong, 2007, Hwang et al., 2016) (Fig. 1). These factors can be modified to make identical condition to particular human exposure condition that can be investigated. For example, new e-cigarette users have puff topography more similar to conventional cigarette smokers (puff time ∼1–2 s long).
In addition to nicotine, other drugs of abuse such as marijuana, methamphetamine and cocaine can be applied in the inhalation system. This will provide clinical evidence about the neurobiological mechanisms that mediate the development of drug dependence. Importantly, alcohol consumers are more likely to smoke tobacco cigarettes (Bierut et al., 2000, Falk et al., 2008), and alcohol drinking has been suggested to increase tobacco consumption (Falk et al., 2006). As a corollary, tobacco use has a critical role in augmentation of alcohol intake (Grant et al., 2004). This suggests that there are factors that may lead to the reinforcing effects of co-abuse of alcohol and nicotine. However, in contrast to clinical studies, pre-clinical studies reported conflicting data showing that nicotine exposure can reduce or increase alcohol seeking behavior and blood alcohol concentrations (Parnell et al., 2006, Bito-Onon et al., 2011, Sari et al., 2016). One of the possible reasons for the conflicting results is that parental or oral administration of nicotine may induce pharmacokinetic interactions with ingested alcohol (Parnell et al., 2006). The methodology of nicotine exposure such as limited or continuous access is another factor that might affect the effects of nicotine exposure on alcohol intake. Thus, applying an accurate physiological nicotine delivery method through e-cigarette vapor/cigarette smoke inhalation in animal models of alcohol drinking may provide stronger clinical evidence about the effects of cigarette smoking on alcohol consumption. These may provide information about whether there are synergistic, additive or antagonizing effects of alcohol and nicotine co-exposure on neurobiological proteins that mediate polysubstance uses.
5. Comparisons of computerized inhalation (SciReq) system to other inhalation systems; validity and limitations
Cotinine has been detected in plasma and brain of female CD1 mice exposed to e-cigarette for six months using SciReq system (Alasmari et al., 2017). The study by Alsamari et al. found that plasma cotinine concentration was similar in mice exposed to e-cigarette vapor as that found in human active smokers (Hukkanen et al., 2005). This indicates that the computerized inhalation system is a valid method for nicotine delivery into the circulatory system. However, other studies found that another exposure system, a mouse pie cage Aerosol Medication Nebulizer, was also able to deliver nicotine containing e-cigarette vapors to mice such that cotinine was found in measureable concentrations in mouse plasma (Garcia-Arcos et al., 2016). Moreover, cotinine has been detected in mouse urine and brain following chronic exposure to tobacco smoke using mechanical ventilator delivery system (Ponzoni et al., 2015). However, the uses of both systems, computerized and non-computerized, have been associated with very low increase in plasma or urine cotinine level in air-control groups (Drummond et al., 2016, Alasmari et al., 2017). This effect is due to the inhalation of the environmental levels of tobacco smoke or e-cigarette vapor within the exposure area. Thus, using an efficient vacuum in both systems to clean all environmental smoke after each run is highly important. Studies found that inhalation of e-cigarette vapor using SciReq system induced reduction in the host defense as well as alterations in inflammatory cytokines and neurobiological proteins in mice (Hwang et al., 2016, Alasmari et al., 2017). These data are consistent with previous studies reporting similar effects of tobacco smoke in humans (Staley et al., 2006, Garlichs et al., 2009, Herr et al., 2009, Knackstedt et al., 2009). Reported literature showed conflicting findings demonstrating the efficiency of using other inhalation systems to investigate the effects of tobacco smoke in rodents (Matulionis, 1984, Bowles et al., 2005, Moreno-Gonzalez et al., 2013). Importantly, chronic exposure to tobacco smoke failed to induce alterations in the pulmonary manifestations in mice and rats using different smoking inhalation machines (Matulionis, 1984). This indicates that the methodology of smoke inhalation should be validated and monitored carefully.
To the best of our knowledge, few studies have investigated the effects of passive exposure to tobacco smoke in pre-clinical models (Khan et al., 2008) and finding a new protocol for passive exposure to e-cigarette or tobacco smoke is critical. It is important to consider that the rate of passive exposure to smoke is increasing recently worldwide and the long-term effect of second-hand exposure to smoke in humans is a significant health concern. Alternatively, SciReq system is flexible depending on desired exposure method, it offers eight separated champers for the whole body exposure, or more than ten restraints mesh holders for nose only exposure, which enable the researchers to run groups of animals at the same time with controlled procedures. However, non-software inhalation systems use smoke inlets connected to one or two cages for smoke exposure and each cage can handle more than one animal (Kaisar et al., 2017). This method is less accurate as compared to the SciReq system since the amount of inhaled smoke would not be similar between animals in each cage. In addition, this method is associated with increased cage-change frequency and the environmental parameters, including the humidity, temperature and the puff volume, which should be controlled and tested in each exposure run. Interestingly, SciReq system can be used for both in vivo and in vitro applications and so far there is no well-established method to investigate the effects of e-cigarette vapors on the cell lines.
6. Comparisons of computerized inhalation (SciReq) system to other inhalation systems; system characteristics and exposure parameters
Although studies reported that inhalation of nicotine induced changes in the rodent biological systems using computerized and non-computerized inhalation methods (Talukder et al., 2011, Drummond et al., 2016, Hwang et al., 2016, Alasmari et al., 2017, Franck et al., 2017, Ma et al., 2018), the use of computerized system is more convenient and accurate. Unlike other non-software inhalation systems, the computerized system, SciReq, composes of several parts that work together on a continuous and cooperative manner (Alasmari et al., 2017). The base unit is controlled by software and this unit can handle up to four pumps. The base unit provides precise exposure parameters (temperature, humidity and exposure duration) sent from the software. Additionally, each system pump is connected to a smoking generating apparatus, and this indicates that different experimental groups can run in the system at the same time. These pumps produce time-flow pattern of smoke and in the same time it can test the atmosphere of the smoke generation machine. The atmosphere is monitored by a filter chamber that grabs the particulates, which are then analyzed chemically or gravimetrically. The capacity of atmosphere (0–100%) can be measured by qualitative particulate transducers. The Flexiware software is Windows-based software in which the users can save, monitor and modify the input data. The software enables the researchers to create their own profiles and the input data in each profile can be exported and stored in a Microsoft Excel style. In addition, the computerized software provides options for the type smoke delivery (constant/random) per unit of time. The software measures the lung elastane and resistance to the smoke as well as carbon monoxide (CO) and puff volume precisely (Fahmy et al., 2010, Robichaud et al., 2015). In addition, the Flexiware software analyzes lung functions, including the ventilation rate and alveolar pressure as well as the lung volume (Herrmann et al., 2017). In contrast, non-computerized inhalation method requires a soap bubble flow meter and CO monitor to measure puff volume and CO, respectively (Tsuji et al., 2013). Additionally, non-digitalized smoke inhalation system measures the particle size of the substances via a cascade impactor (Tsuji et al., 2013). However, the computerized system composes several nebulizers that produce different particle sizes of aerosols of prepared mixture solutions (Phillips et al., 2017). The nebulizer generates minimal heat or force to the solutions and delivers different types of substances such as nanoparticle suspensions or solutions, ovalbumin and DNA fragments (Novali et al., 2015). One type of nebulizer produces a standard particle size (4–6 µm), while the other nebulizer produces fine particle sizes (2.5 – 4 µm) of aerosols (Devos et al., 2017). Custom-mesh nebulizer can be used to produce large particle sizes. Nanoparticles can be aerosolized by the Aerogen nebulizer (Phillips et al., 2017).
Alternatively, non-software apparatus can handle only one cigarette and the researchers should change the cigarette after each run (Kaisar et al., 2017). SCIREQ system offers two different champers for smoking generations, single cigarette apparatus and cigarette smoking robot (CSR) (Ogunwale et al., 2017). The CSR can handle up to 24 cigarettes with automatic smoke ejection for several hours without human intervention. The smoking generation apparatus is supplied with a special adaptor for e-cigarette solutions. Two different ways, nose-only or whole body exposure, can be applied in the machine to expose animals to e-cigarette vapor or tobacco smoke (Fig. 1). The nose-only exposure method reduces the systemic effects of nicotine or other substances that penetrate into the body through skin or eye (Oyabu et al., 2016). This is also associated with fast changeover of the atmosphere mimicking human smoking exposure (Pauluhn and Thiel, 2007). However, the nose-only method of exposure requires restraining the animals in soft-mesh restraints, which is stressful for the animals. Alternatively, whole body exposure method allows animals to move freely throughout the chambers, which minimizes the stress. Moreover, desired concentrations of nicotine can be generated using whole body exposure supplied with aerosol generation devices. But the whole body exposure method can lead to deposition of the inhalant on the eyes and fur (Nemmar et al., 2013, Oyabu et al., 2016). Additionally, gastrointestinal delivery of the nicotine and other chemicals has been reported in animals exposed to cigarettes in whole-body exposure apparatus (Oyabu et al., 2016). In this apparatus, rodents huddle and hide their noses from the inhalants. Thus, it is harder to measure the exact amount of e-cigarette vapor/tobacco smoke that are inhaled by the rodents compared to nose-only exposure. Finally, SCIREQ system offers a new method to expose the tobacco smoke to cell lines. Cell plates can be placed in the whole body champers and the tobacco smoke or e-cigarette vapor is driven across the liquid phase of air-liquid interface where the cells grow. Two different ways, main-stream smoke and side-stream smoke can be used in this system to expose the cells to tobacco smoke. Main-stream smoke requires a buffer chamber that distributes the puffs on all sides and the pump pulls the smoke outside the system. Side-stream smoke provides more constant delivery of smoke.
7. Conclusion
Establishment of a physiological exposure method of nicotine delivery in animal models through inhalation of cigarette vapor/smoke may lead to new clinical considerations. Other non-inhalation methods of nicotine delivery have been historically used to evaluate nicotinic effects on CNS pathways and physiologic changes throughout the body. However, these methods may have less desirable clinical pharmacokinetics of nicotine as compared to inhalation of e-cigarette vapor/cigarette smoke-containing nicotine. The computerized inhalation machine may allow investigators to modify the exposure dose of nicotine and the exposure duration to the cigarettes/e-cigarettes. Moreover, investigators can use any type of cigarettes/e-cigarettes, which can provide information about the effects of marketed e-cigarette devices or tobacco cigarettes in relevant animal models. The machine may be used to determine the neurobiological effects of other abused drugs that are given through inhalation such as marijuana, methamphetamine and cocaine. The favorable characteristics of this system make it highly that pre-clinical data obtained from using the inhalation system will be translatable to clinical stages of investigation. The computerized inhalation system controls several factors involved in the laboratory experiments and this system monitors the exposure parameters automatically with less users’ interventions as compared to non-computerized inhalation systems.
Acknowledgments
Acknowledgments
The authors would like to thank SCIREQ Scientific Respiratory Equipment Inc. for providing us with inExpose machine image used in Fig. 1.
Funding
The review article was written during the period of funding supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism, R01HL137052-01 (L.C.A. PI) from the NIH NHLBI, Beginning Grant-in-Aid 16BGIA27790079 (L.C.A. PI) from the American Heart Association, Daniel O’Connor Scholar Award P30DK079337 (L.C.A. PI) from the UAB-UCSD O’Brien Center, ATS Foundation Award (L.C.A. PI), High Impact Award 26IP-0040 from the California TRDRP (L.C.A. Co-I), with additional salary support from the VA San Diego Healthcare System (L.C.A.). Fawaz Alasmari is supported by a scholarship from King Saud University.
Conflict of Interest
The authors have no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adriani W., Macri S., Pacifici R., Laviola G. Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behavioural Brain Res. 2002;134:21–30. doi: 10.1016/s0166-4328(01)00448-x. [DOI] [PubMed] [Google Scholar]
- Alasmari F., Al-Rejaie S.S., AlSharari S.D., Sari Y. Targeting glutamate homeostasis for potential treatment of nicotine dependence. Brain Res. Bullet. 2016;121:1–8. doi: 10.1016/j.brainresbull.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F., Alexander L.E.C., Nelson J.A., Schiefer I.T., Breen E., Drummond C.A., Sari Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;77:1–8. doi: 10.1016/j.pnpbp.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari S.D., King J.R., Nordman J.C., Muldoon P.P., Jackson A., Zhu A.Z., Tyndale R.F., Kabbani N., Damaj M.I. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta J., Lecca D., Nelson M., Guidotti A., Overstreet D.H., Costa E., Javaid J.I. Expression and function of striatal nAChRs differ in the flinders sensitive (FSL) and resistant (FRL) rat lines. Neuropharmacology. 2000;39:2624–2631. doi: 10.1016/s0028-3908(00)00082-4. [DOI] [PubMed] [Google Scholar]
- Baker D.A., Xi Z.X., Shen H., Swanson C.J., Kalivas P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci: Off. J. Soc. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N.L. Clinical pharmacology of inhaled drugs of abuse: implications in understanding nicotine dependence. NIDA Res. Monograph. 1990;99:12–29. [PubMed] [Google Scholar]
- Berridge M.S., Apana S.M., Nagano K.K., Berridge C.E., Leisure G.P., Boswell M.V. Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology. 2010;209:383–394. doi: 10.1007/s00213-010-1809-8. [DOI] [PubMed] [Google Scholar]
- Bierut L.J., Schuckit M.A., Hesselbrock V., Reich T. Co-occurring risk factors for alcohol dependence and habitual smoking. Alcohol Res. Health: The J. Nat. Institute Alcohol Abuse Alcoholism. 2000;24:233–241. [PMC free article] [PubMed] [Google Scholar]
- Bito-Onon J.J., Simms J.A., Chatterjee S., Holgate J., Bartlett S.E. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addiction Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles K.S., Horohov D.W., Paulsen D.B., LeBlanc C.J., Littlefield-Chabaud M.A., Ahlert T., Ahlert K., Pourciau S.S., Penn A. Exposure of adult mice to environmental tobacco smoke fails to enhance the immune response to inhaled antigen. Inhalat. Toxicol. 2005;17:43–51. doi: 10.1080/08958370590885690. [DOI] [PubMed] [Google Scholar]
- Buisson B., Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. J. Neurosci.: Off. J. Soc. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt N.C. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Devos F.C., Maaske A., Robichaud A., Pollaris L., Seys S., Lopez C.A., Verbeken E., Tenbusch M., Lories R., Nemery B. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respiratory Res. 2017;18:123. doi: 10.1186/s12931-017-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond C.A., Crotty Alexander L.E., Haller S.T., Fan X., Xie J.X., Kennedy D.J., Liu J., Yan Y., Hernandez D.-A., Mathew D.P. Cigarette smoking causes epigenetic changes associated with cardiorenal fibrosis. Physiolog. Genomics. 2016;48:950–960. doi: 10.1152/physiolgenomics.00070.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy B., Ding L., You D., Lomnicki S., Dellinger B., Cormier S.A. In vitro and in vivo assessment of pulmonary risk associated with exposure to combustion generated fine particles. Environment. Toxicol. Pharmacol. 2010;29:173–182. doi: 10.1016/j.etap.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk D., H-y Yi, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and drug use and disorders. Alcohol Research Health: J. Nat. Institute Alcohol Abuse Alcoholism. 2008;31:100–110. [PMC free article] [PubMed] [Google Scholar]
- Falk D.E., Yi H., Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders. Alcohol Res. Health: J. Nat. Institute Alcohol Abuse Alcoholism. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fowler C.D., Kenny P.J. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck F., Benatti B., Andia D., Cirano F., Casarin R., Corrêa M., Ribeiro F. Impact of resveratrol on bone repair in rats exposed to cigarette smoke inhalation: histomorphometric and bone-related gene expression analysis. Internat. J. Oral Maxillofac. Surg. 2017 doi: 10.1016/j.ijom.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Arcos I., Geraghty P., Baumlin N., Campos M., Dabo A.J., Jundi B., Cummins N., Eden E., Grosche A., Salathe M. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71:1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlichs C., Cicha I., Raaz D., Meyer L., Stumpf C., Klinghammer L., Yilmaz A., Daniel W. CD40/CD154 system and pro-inflammatory cytokines in young healthy male smokers without additional risk factors for atherosclerosis. Inflamm. Res. 2009;58:306–311. doi: 10.1007/s00011-008-8084-8. [DOI] [PubMed] [Google Scholar]
- Ghosheh O.A., Dwoskin L.P., Miller D.K., Crooks P.A. Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2'-(14)C]nicotine. Drug Metabolism Disposit.: Biol. Fate Chem. 2001;29:645–651. [PubMed] [Google Scholar]
- Grant B.F., Hasin D.S., Chou S.P., Stinson F.S., Dawson D.A. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archiv. General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hauser S.R., Katner S.N., Deehan G.A., Jr., Ding Z.M., Toalston J.E., Scott B.J., Bell R.L., McBride W.J., Rodd Z.A. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcoholism, Clin. Exp. Res. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr, C., Beisswenger, C., Hess, C., Kandler, K., Suttorp, N., Welte, T., Schröder, J-M., Vogelmeier, C., Group RBftCS. (2009) Suppression of pulmonary innate host defence in smokers. Thorax 64:144–149. [DOI] [PubMed]
- Herrmann F.E., Wollin L., Wirth J., Gantner F., Lämmle B., Wex E. Olodaterol shows anti-fibrotic efficacy in in vitro and in vivo models of pulmonary fibrosis. British J. Pharmacol. 2017;174:3848–3864. doi: 10.1111/bph.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch F.R., Bunn P.A., Mulshine J.L., Kato H. CRC Press; 2005. IASLC textbook of prevention and early detection of Lung cancer. [Google Scholar]
- Hukkanen J., Jacob P., Benowitz N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hwang J.H., Lyes M., Sladewski K., Enany S., McEachern E., Mathew D.P., Das S., Moshensky A., Bapat S., Pride D.T. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016:1–13. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- Kaisar M.A., Kallem R.R., Sajja R.K., Sifat A.E., Cucullo L. A convenient UHPLC-MS/MS method for routine monitoring of plasma and brain levels of nicotine and cotinine as a tool to validate newly developed preclinical smoking model in mouse. BMC Neurosci. 2017;18:71. doi: 10.1186/s12868-017-0389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny P.J., Chartoff E., Roberto M., Carlezon W.A., Jr., Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacol.: Off. Publicat. Am. College Neuropsychopharmacol. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.M., Khan M.Y., Minhas L.A. Effect of passive tobacco smoking on fertility of female mice. J. College Physicians Surgeons-Pakistan: JCPSP. 2008;18:708–712. [PubMed] [Google Scholar]
- Khvotchev M., Lonart G., Sudhof T.C. Role of calcium in neurotransmitter release evoked by alpha-latrotoxin or hypertonic sucrose. Neuroscience. 2000;101:793–802. doi: 10.1016/s0306-4522(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Knackstedt L.A., LaRowe S., Mardikian P., Malcolm R., Upadhyaya H., Hedden S., Markou A., Kalivas P.W. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsson-Geuken A., Gash C.R., Alexander K., Pomerleau F., Huettl P., Gerhardt G.A., Bruno J.P. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Houezec J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. Internat. J. Tuberculosis lung Disease: Off. J. Internat. Union against Tuberculosis Lung Dis. 2003;7:811–819. [PubMed] [Google Scholar]
- Ma S., Wang C., Zhao B., Ren X., Tian S., Wang J., Zhang C., Shao Y., Qiu M., Wang X. Tandem mass tags labeled quantitative proteomics to study the effect of tobacco smoke exposure on the rat lung. Biochim. Biophys. Acta (BBA)-Proteins Proteomics. 2018;1866:496–506. doi: 10.1016/j.bbapap.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Matulionis D. Effects of cigarette smoke generated by different smoking machines on pulmonary macrophages of mice and rats. J. Analyt. Toxicol. 1984;8:187–191. doi: 10.1093/jat/8.4.187. [DOI] [PubMed] [Google Scholar]
- Mitra A., Lenglos C., Timofeeva E. Activation of GABAA and GABAB receptors in the lateral septum increases sucrose intake by differential stimulation of sucrose licking activity. Behav. Brain Res. 2014;273:82–88. doi: 10.1016/j.bbr.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Moreno-Gonzalez I., Estrada L.D., Sanchez-Mejias E., Soto C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat. Communicat. 2013;4:1495. doi: 10.1038/ncomms2494. [DOI] [PubMed] [Google Scholar]
- Nadal R., Chappell A., Samson H. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol. Clin. Exp. Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- Nemmar A., Raza H., Subramaniyan D., Yasin J., John A., Ali B.H., Kazzam E.E. Short-term systemic effects of nose-only cigarette smoke exposure in mice: role of oxidative stress. Cell. Physiol. Biochem. 2013;31:15–24. doi: 10.1159/000343345. [DOI] [PubMed] [Google Scholar]
- Nesil T., Kanit L., Pogun S. Bitter taste and nicotine preference: evidence for sex differences in rats. Am. J. Drug Alcohol Abuse. 2015;41:57–67. doi: 10.3109/00952990.2014.990091. [DOI] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am. J. Med. 2008;121:S20–S31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Novali M., Shalaby K.H., Robichaud A., Benedetti A., Fereydoonzad L., McGovern T.K., Schuessler T.F., Martin J.G. Mechanical consequences of allergic induced remodeling on mice airway resistance and compressibility. Respiratory Physiol. Neurobiol. 2015;218:11–20. doi: 10.1016/j.resp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Ogunwale M.A., Chen Y., Theis W.S., Nantz M.H., Conklin D.J., Fu X.-A. A novel method of nicotine quantification in electronic cigarette liquids and aerosols. Anal. Methods. 2017;9:4261–4266. doi: 10.1039/C7AY00501F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyabu T., Morimoto Y., Izumi H., Yoshiura Y., Tomonaga T., Lee B.-W., Okada T., Myojo T., Shimada M., Kubo M. Comparison between whole-body inhalation and nose-only inhalation on the deposition and health effects of nanoparticles. Environment. Health Prevent. Med. 2016;21:42–48. doi: 10.1007/s12199-015-0493-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell S.E., West J.R., Chen W.J. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcoholism, Clin. Exp. Res. 2006;30:1408–1413. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Pauluhn J., Thiel A. A simple approach to validation of directed-flow nose-only inhalation chambers. J. Appl. Toxicol. 2007;27:160–167. doi: 10.1002/jat.1188. [DOI] [PubMed] [Google Scholar]
- Phillips, J.E., Zhang, X., Johnston, J.A., (2017). Dry Powder and Nebulized Aerosol Inhalation of Pharmaceuticals Delivered to Mice Using a Nose-only Exposure System. JoVE (Journal of Visualized Experiments) e55454-e55454. [DOI] [PMC free article] [PubMed]
- Ponzoni L., Moretti M., Sala M., Fasoli F., Mucchietto V., Lucini V., Cannazza G., Gallesi G., Castellana C.N., Clementi F., Zoli M., Gotti C., Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Rada P., Avena N.M., Hoebel B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Robichaud A., Fereydoonzad L., Urovitch I.B., Brunet J.-D. Comparative study of three flexiVent system configurations using mechanical test loads. Exp. lung Res. 2015;41:84–92. doi: 10.3109/01902148.2014.971921. [DOI] [PubMed] [Google Scholar]
- Sari Y., Toalston J.E., Rao P.S., Bell R.L. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience. 2016;326:117–125. doi: 10.1016/j.neuroscience.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J.A., Pauly J.R. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology. 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Staley J.K., Krishnan-Sarin S., Cosgrove K.P., Krantzler E., Frohlich E., Perry E., Dubin J.A., Estok K., Brenner E., Baldwin R.M. Human tobacco smokers in early abstinence have higher levels of β2* nicotinic acetylcholine receptors than nonsmokers. J. Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder M.H., Johnson W.M., Varadharaj S., Lian J., Kearns P.N., El-Mahdy M.A., Liu X., Zweier J.L. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol.-Heart Circulat. Physiol. 2011;300:H388–H396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y., Bai L., Copeland R.L., Taylor R.E. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcoholism. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Tizabi Y., Copeland R.L., Louis V.A., Taylor R.E. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Tsuji H., Fujimoto H., Matsuura D., Nishino T., Lee K.M., Yoshimura H. Comparison of biological responses in rats under various cigarette smoke exposure conditions. J. Toxicol. Pathol. 2013;26:159–174. doi: 10.1293/tox.26.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Chen H., Steketee J.D., Sharp B.M. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacol.: Off. Publicat. Am. College Neuropsychopharmacol. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Wang B., Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Frontiers Behav. Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.A. Inhalation exposure systems: design, methods and operation. Toxicol. Pathol. 2007;35:3–14. doi: 10.1080/01926230601060017. [DOI] [PubMed] [Google Scholar]