Abstract
The presence, within the human bone marrow, of cells with both endothelial and hemogenic potential has been controversial. Herein, we identify, within the human fetal bone marrow, prior to establishment of hematopoiesis, a unique APLNR+, Stro-1+ cell population, coexpressing markers of early mesodermal precursors and/or hemogenic endothelium. In adult marrow, cells expressing similar markers are also found, but at very low frequency. These adult-derived cells can be extensively culture expanded in vitro without loss of potential, they preserve a biased hemogenic transcriptional profile, and, upon in vitro induction with OCT4, assume a hematopoietic phenotype. In vivo, these cells, upon transplantation into a fetal microenvironment, contribute to the vasculature, and generate hematopoietic cells that provide multilineage repopulation upon serial transplantation. The identification of this human somatic cell population provides novel insights into human ontogenetic hematovascular potential, which could lead to a better understanding of, and new target therapies for, malignant and nonmalignant hematologic disorders.
INTRODUCTION
During ontogeny, definitive hematopoietic stem/progenitor cells (HSC) are thought to arise from vascular endothelial cells, through an endothelial-to-hematopoietic transition, a natural process that occurs in specialized embryonic hemogenic endothelial cells.1–3 These unique cells have been identified in tissues such as the dorsal aorta, the placenta, the yolk sac, and the cranial vitelline and umbilical arteries,4–8 and express CD34, VEGFR2, c-Kit, and VE-Cadherin.5, 9, 10
Studies using pluripotent stem cells demonstrated that hematovascular precursors emerge from lineage negative, APLNR+, PDGFRα+ mesodermal cells following upregulation of VEGFR2 and decrease of PDGFRα expression, and that lineage commitment towards an endothelial and/or hematopoietic phenotype initiates when VE-Cadherin expression commences.11–13
Although the hemogenic endothelium is thought to constitute a transient population of cells within the embryo that disappears during development,5 studies using murine endothelium have shown that, even in the adult, vascular cells are able to convert directly to immunocompetent HSC after forced expression of Fosb, Gfi1, Runx1, and Spi1 transcription factors,14 and that other cells present within the murine bone marrow, such as the very small embryonic-like stem cells, differentiate into the hematopoietic lineage after coculture over OP9 stromal cells.15
In humans, it has been reported that extremely rare subsets of both fetal16 and adult17 bone marrow endothelial cells are endowed with hematopoietic differentiation capacity. Thus, it is possible that the emerging bone marrow vascular network, which provides a suitable environment for the lodging of HSC emigrating from other fetal sites, contains cells with a hemato-endothelial signature that persist as a vestigial population of cells.
Here, we delineate the phenotype of cells during bone marrow ontogeny, and show that a subpopulation of cells express the G-protein–coupled APELIN receptor (APLNR), a marker of early mesoderm enriched for hemangioblast colony-forming cells, and a regulator of early human hematopoiesis.18 In addition, we demonstrate that the vast majority of APLNR+ cells co-express Stro-1+,19, 20 a marker of endothelial21 and perivascular cells,22–24 and that the APLNR+ Stro-1+ subpopulation expresses VEGFR2 and VE-cadherin, and low levels of PDGFRα. Moreover, we determined that Stro-1+, VE-cadherin+ cells are present within the developing marrow vasculature, and that, although their frequency decreases as gestation progresses, in adulthood they persist at constant levels as a rare population of cells within the bone marrow. We additionally established that cultured/expanded adult Stro1+ isolated primary cells (SIPs), although unable to generate hematopoietic colonies in semisolid media, assume a hematopoietic phenotype when induced with OCT4. In addition, SIPs, when transplanted into a fetal environment, lodge within the vasculature and perivascular compartment, and after up-regulating endogenous OCT4, they generate hematopoietic cells that are capable of serial hematopoietic reconstitution, with generation of both myeloid and lymphoid cells. Transplantation of clonally-derived SIPs into fetal animals also confirmed these results. These data have shed light on the development of vascular niches during bone marrow development, and could thus lead to a better understanding of normal hematopoiesis and/or onset of malignant and nonmalignant hematologic disorders.
MATERIALS AND METHODS
Analysis of human fetal and adult bone marrow
Human fetal bone tissues were obtained from Advanced Bioscience Resources (Alameda, CA). Human adult bone marrow (BM) was obtained from healthy adult donors after informed consent, or from commercial sources (AllCells, LLC, Alameda, CA). Fetal and adult tissues were obtained according to guidelines from the Office of Human Research Protection at Wake Forest Health Sciences, and University of Nevada, Reno.
Flow cytometric analysis of adult or fetal BM was performed using methods described in Supplementary Materials and Methods (SMM). Isolation and culture of BM-derived Stro1+ isolated primary cells (SIPs) and clonal cell populations is also detailed in SMM. In short, SIPs were isolated based on Stro-1 positivity, CD45 and GlyA negativity, and fibronectin adhesion, and clonally-derived SIPs were sorted into fibronectin-coated 96-well plates by single cell deposition using a FACSVantage (BD Biosciences, San Jose, CA). 24 hours later, wells were visually inspected by phase contrast microscopy (Olympus IX70, Melville, NY) to confirm the presence of a single cell/well. Only wells containing single cells were used to establish the clones employed in these studies. The number of wells positive for cellular growth that initially contained a single cell was 83%.
Confocal Microscopy
A detailed description of methods used appears in SMM. Confocal fluorescence images were acquired with an Olympus Fluoview 1000 or FV1200 SPECTRAL Laser scanning confocal microscope with 488, 594, and 647 nm laser excitations and an Olympus UPlanFLN- 40×/1.30 oil objective. Confocal images were taken as z-stacks with 5–9 slices per image before being projected as 2D images. Image acquisition and primary image processing were done using Fluo View™ software.
Tube formation assay
Tube formation assays were performed in 24-well plates by seeding 1×104 cells/well onto 300μl of polymerized Matrigel (BD Biosciences, Bedford, MA) at 37°C, in EGM-2 supplemented with an additional 10% FBS, as described in SMM.
Transplantation of SIPs into fetal sheep recipients
Fetal sheep (n=41) were injected intraperitoneally by ultrasound-guided transabdominal percutaneous injection,28, 29 with SIPs populations in QBSF serum-free media (Quality Biological, Gaithersburg, MD), at the doses indicated in the results section, at 55–62 days of gestation, and according to University of Nevada approved Institutional Animal Care and Use Committee (IACUC) guidelines.
Assessment of human donor hematopoietic cell engraftment
BM and PB from animals transplanted with human cells were analyzed for the presence of donor (human) hematopoietic cells by flow cytometry using monoclonal antibodies and methods described in SMM.
Assessment of human SIPs engraftment in secondary recipients
Stro-1+ human cells were isolated from bone marrow of secondary recipients using magnetic sorting, as described in SMM. Sorted cells were used to extract DNA to perform PCR, or for immunostaining. After confirmation by PCR that samples were positive for β-actin, PCR was performed using human-specific HLA-DQα30 and/or GAPDH primers31 as detailed in SMM.
Transplantation of CD34+CD45+ cells isolated from bone marrow of primary sheep SIPs recipients into NOD/scid/γ(c)(−/−) (NSG) mice, and flow cytometric analysis of transplanted NSG Mice
Newborn NSG mice (1–3 day old) were irradiated with 100 cGy and injected intrahepatically, as previously described,32 with human CD34+CD45+ cells isolated from bone marrow of primary sheep SIPs recipients. Details of the transplant and analysis can be found in SMM.
Adult SIPs cell transduction and induction of cellular reprogramming
Plasmid construction, lentiviral vector generation for cellular reprograming, and culture details26 can be found in detail in SMM.
Statistics and data analysis
Experimental results are presented as the mean plus/minus the standard error of the mean (SEM). We used GraphPad Prism 6 (GraphPad Software) for statistical analysis. Comparisons between experimental results were determined by two-sided non-paired Student’s t-test analysis. A p value <0.05 was considered statistically significant. We also validated the power of our statistical analysis by using the two-sided Wilcoxon Rank Sum Test for independent samples.
RESULTS
During human development, the APLNR+, Stro-1+ population contains cells with a phenotype consistent with that of hemogenic endothelial precursors
During the commitment of human pluripotent stem cells to the hematopoietic lineage, apelin receptor (APLNR), vascular endothelial growth factor receptor 2 (VEGFR2)11, 18, and platelet-derived growth factor receptor-α (PDGFRα)33 have been used to define the early mesodermal precursors with the potential to give rise to endothelial, mesenchymal, and hematopoietic cells. Subsequent mesodermal commitment to the endothelial and hematopoietic lineages proceeds through VE-cadherin+ intermediates.34 Within the human bone marrow, stromal elements with the capacity to transfer the hematopoietic microenvironment in vitro were reported to express Stro-1, an antigen discovered with an antibody generated by immunizing mice with human 34+ cells.19
Confocal microscopy of fetal bone marrow at 10±1.5 gestational weeks (gw) demonstrated that APLNR+ cells are present in the inner part of the developing fetal marrow and express Stro-1 (Fig. 1a). The continued presence of APLNR+, Stro-1+ cells was confirmed by flow cytometric analysis of cells freshly isolated from 18±1.5gw (n=3) donors (Fig. 1b). More than 90% of APLNR+, Stro-1+ cells were VE-Cadherin+, most co-expressed VEGFR2, and, in addition, a smaller percentage was PDGFRα positive (Fig. 1b). By contrast, less than half of the APLNR-, Stro-1+ cells expressed VE-Cadherin, only a small fraction expressed VEGFR2, and none were positive for PDGFRα after subtracting non-specific staining seen with the isotype control (Fig. 1b). Therefore, during fetal development, the APLNR+, Stro-1+ population contains cells with a phenotype consistent with that of hemogenic endothelial precursors.
Figure 1. APLNR+, Stro-1+ population contains cells with a phenotype consistent with that of hematovascular mesodermal precursors and/or hemogenic endothelial precursors.
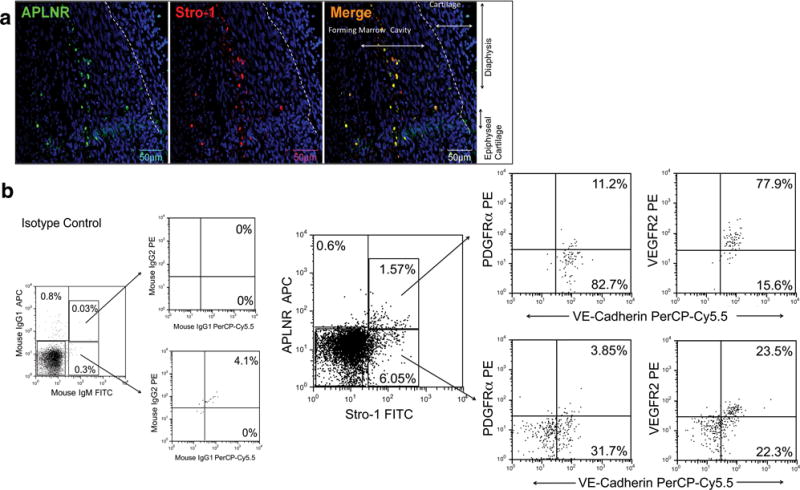
(a) APLNR+ Stro-1+ cells can be identified at 10±1.5gw in the inner part of the developing bone marrow (n=3). DAPI (blue) labels all nuclei. (b) Representative dot plots of flow cytometric evaluation of freshly isolated fetal BMMNC, demonstrating that APLNR+ cells are present, and that the majority of these cells express Stro-1 (n=3). Stro-1+ APLNR+ cells are VE-Cadherin+, express VEGFR2, and a small percentage also express PDGFRα. By contrast, Stro-1+ APLNR- cells contain a mixture of VE-Cadherin positive and negative cells, and only a small fraction express VEGFR2. Percentages depicted in the images are before subtraction of respective isotype controls (n=3). Percentage of background fluorescence obtained when staining the same population of cells with appropriate isotype control is shown in the leftmost panel.
In addition to VEGFR2 and VE-Cadherin,13, 35 CD3436 expression has also been used to identify populations of cells with a hemato-endothelial phenotype. Confocal analysis demonstrated that CD34+,VEGFR2+,Stro-1+ cells localize within vascular structures of fetal bone (n=3) at 10±1.5gw (Fig. 2a). Later in gestation, at 18±1.5gw (n=3) (Fig. 2b), cells co-expressing Stro-1, CD34, and VEGFR2 were also identified in specific locations within the vascular niche. Flow cytometric evaluation of freshly isolated Stro-1+ cells at 18±1.5gw (n=3) confirmed expression of VEGFR2 in 7.6–39.6% of CD34+,Stro-1+ cells (Fig. 2c). Quantification of Stro-1+ and VE-Cadherin+ cells at different gw showed that, throughout gestation, VE-Cadherin expression mirrored that of Stro-1, with both being highly expressed early in gestation, and decreasing with fetal bone maturation (Fig. 3 a,b) (n=4). The percentage of Stro-1+ cells at 10±1.5gw was 27.47±0.49% and then decreased progressively over time, to reach 7.81±0.6% at 20gw (Fig. 3b), as the marrow matured to become fully hematopoietic. Confirmation of the predominantly vascular nature of the 10±1.5gw marrow, and its evolution to a hematopoietic organ by 18±1.5gw, was also obtained by flow cytometric analysis of freshly isolated, unfractionated fetal bone marrow, demonstrating that it was only at this later time point that the majority of CD34+ cells were also CD45+ (Supplementary Fig. 1a).
Figure 2. CD34+,VEGFR2+,Stro-1+ cells localize within vascular structures of the fetal bone.
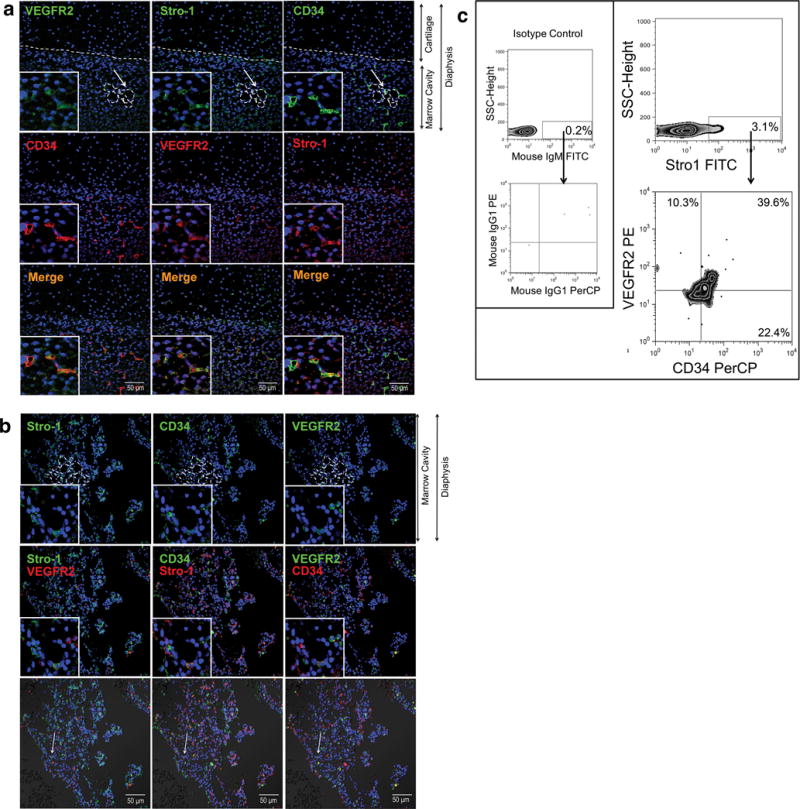
(a, b) Staining was performed using 3 different antibodies labeled with respective Alexa Fluor Dyes: Stro-1 (Alexa Fluor 647), VEGFR2 (Alexa Fluor 488), and CD34 (Alexa Fluor 594). This allowed us to visualize Stro-1, VEGFR2, and CD34 individually in rows 1 and 2, and, in combination in row 3 to determine co-expression (merged images) in vascular structures (represented as dotted lines), at (a) 10±1.5gw (n=3) and at (b)18±1.5gw (n=3). DAPI (blue) labels all nuclei. Arrows indicate features enlarged in insets. (c) Representative flow cytometric analysis (18±1.5gw) confirming that Stro-1+ cells co-express CD34 and VEGFR2 (n=3; 18±1.5gw). Percentages depicted in zebra plots were calculated after subtraction of respective isotype control. Isotype control is shown in left panel.
Figure 3. Expression of VE-Cadherin during development. In the freshly isolated adult bone marrow, APLNR+, Stro-1+, VE-Cadherin+ cells constitute a rarer population of cells.
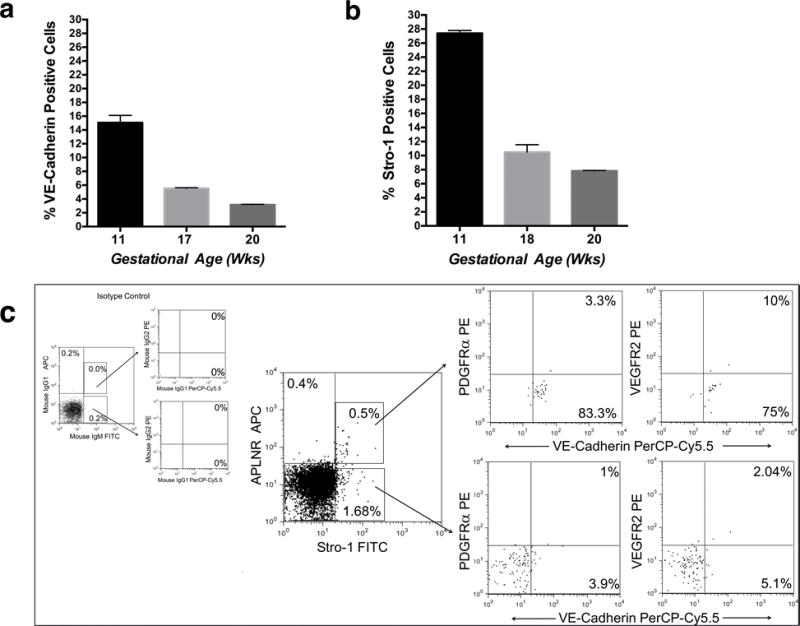
(a) Percentage of VE-Cadherin+ (n=3), and (b) Stro-1+ cells (n=3), during marrow ontogeny. (Error bars reflect SEM). (c) Representative dot plots of flow cytometric evaluation of freshly isolated adult BMMNC demonstrating that Stro1+ APLNR+ cells are still present (n=3); appropriate isotype controls are shown on the left. Stro-1+APLNR+ cells expressed VE-Cadherin, and few cells expressed VEGFR2. Percentages depicted in the images are shown after subtraction of respective isotype controls.
We further investigated whether Stro-1+ subpopulations of cells also expressed markers of established endothelium. Confocal microscopy of fetal bone at 10±1.5gw confirmed the presence of CD31+Stro-1+ cells lining vascular areas (Supplementary Fig. 1b). Evaluation of freshly isolated Stro-1+ cells from later stages in gestation (18±1.5gw; n=3), showed that 23.9–42.3% of Stro-1+,CD34+ cells co-express CD31, that 4–19.7% of Stro-1+,CD31+,CD34- cells are present, and that 7.8–19.9% of Stro-1+,CD34+ cells express the endothelial/perivascular marker CD146 (Supplementary Fig. 1c).
In order to further characterize Stro-1+ cells during development, we investigated whether these cells expressed markers associated with other stem cell phenotypes.
As can be seen in Supplementary Fig. 1c, Stro-1+, CD34+ cells express CD49f (30.8–38.3%), a marker of primitive HSC,37, as well as CD90 and CD117, markers of primitive stem cell populations within the hematopoietic and vascular lineages.
Within the freshly isolated human adult bone marrow, APLNR+, Stro-1+, VE-Cadherin+ cells constitute a rarer population of cells
We next evaluated whether human adult bone marrow APLNR+ cells expressed markers identical to those found in fetal APLNR+ cells during development. Flow cytometric analysis of freshly isolated BMMNC confirmed that APLNR+ Stro-1+ cells are still present (n=3), but are rarer in the adult than during development (Fig. 3c). Furthermore, in contrast to their fetal counterpart, only APLNR+ Stro-1+cells expressed VE-Cadherin, and VEGFR2 expression in adult APLNR+ Stro-1+ cells was significantly decreased. Also, evaluation of adult Stro-1+ cells revealed loss of expression of markers associated with primitive stem cell populations and the vascular lineage. Specifically, when compared with fetal cells (Supplementary Fig 1c) fewer Stro-1+ cells expressed CD117 and CD31, most cells that expressed CD34 were also CD31 positive, and only a very small percentage of Stro-1+ cells expressed CD49f (n=3) (Supplementary Fig. 2).
Phenotypic and functional comparison of cultured fetal and adult Stro1+ Isolated Primary cells (SIPs)
Both fetal and adult Stro-1+ cells are rare in freshly isolated bone marrow, but they can be isolated and expanded (SIPs), as adherent populations to significant numbers. Therefore, we next determined the phenotype and function of these cells after in vitro culture. Fetal and adult SIPs displayed similar phenotypic markers (Fig. 4a, Supplementary Fig 3a, and Supplementary Results), but CD34 mRNA transcripts were only detectable in fetal SIPs (Fig. 4b). TEM images comparing the morphology of these cells can be seen in Supplementary Fig. 3d. Tube formation assays demonstrated that adult and fetal SIPs at the same passage (P4), formed capillary tubes, but fetal cells were significantly more efficient than their adult counterparts (Fig. 4c). In addition, both cultured fetal and adult Stro-1+ cells expressed UEA-1, EphB2, and EphrinB2, markers associated with endothelial cells and angiogenesis38 (Supplementary Fig. 3c).
Figure 4. Comparison of cultured fetal and adult SIPs.
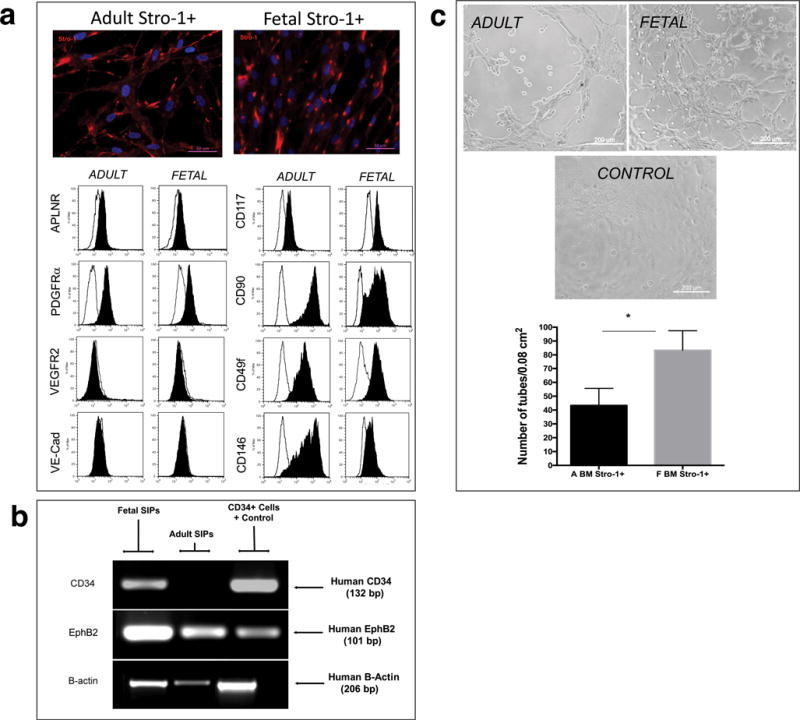
(a) Immunofluorescence and Flow cytometric analysis of Stro-1+ cultured fetal and adult cells at P4 are depicted (n=3). The black filled histogram shows fluorescent data for each specific marker, and the unfilled line marks respective isotype controls. Cell populations were considered positive if the ratio between the Median Fluorescence Intensity (MFI) for the specific marker and the MFI of the isotype control ≥ 1.5. (b) RT-PCR analysis, using human-specific primers for CD34, demonstrated that fetal, but not adult, SIPs expressed CD34. Amplification of the same samples with primers specific for human EphB2 and B-actin confirmed the presence amplifiable RNA. For each set of primers samples were run simultaneously in the same gel, but gel was cropped to improve clarity, and to show amplification of samples specific to fetal and adult BM-derived SIPs. (c) Tube formation assays confirmed that, at the same passage (P4), both cell populations were able to form capillary tubes. Cells initiated tubule formation at 2–4h of culture, which fully developed by 6h, with the presence of nodes of ≥ 4 branches; images were acquired on a Ziess Axiovert 200M and 10X magnification. Quantification of number of branch sites/nodes demonstrated fetal cells were significantly more efficient at generating tubes than their adult counterparts (n=3).
Cultured Adult SIPs generate hematopoietic cells in vivo
Transcriptome analysis of adult SIPs demonstrated that they express some hemangioblast- and hematopoietic-associated genes (Supplementary Results and Supplementary Fig. 3d.) Therefore, we next investigated the ability of SIPs to generate hematopoietic cells in vivo. SIPs expanded in vitro (P3–5) were transplanted into a total of 10 sheep fetuses at the following doses: 3.25×105/fetus (n=3), 1.3×106/fetus (n=3), and 1×107/fetus (n=4). Analysis of hematopoietic engraftment, as determined by the total percentage of human myeloid and lymphoid cells at 2 months post-transplant, is shown in Fig. 5a. In the PB, the percentage of human hematopoietic cells varied from 0.47 to 6.54%, and in BM from 0.68 to 10.72%. While all transplanted animals harbored donor-derived lymphocytes, human myeloid and lymphoid cells were concurrently detected in 9 out of 10 of the recipients. Lymphoid and myeloid engraftment, with reconstitution of both granulocytic and erythroid compartments, was detected in 50% of the animals, and 90% of recipients had donor-derived lymphoid and erythroid cells. Human CD34+ cells were found in 60% of the transplanted animals, at levels ranging from 0.02 to 1.65%, and their levels did not directly correlate with either overall or multilineage engraftment. The overall percentage of engraftment within the different hematopoietic lineages of these primary recipients is detailed in the left bar of Fig. 5b, and representative images of flow cytometric analysis appear in Supplementary Figs. 4 and 5a. RFP-positive cells could readily be detected in the PB of in one additional animal that received SIPs transduced with an MSCV-Neo-RFP vector. (Supplementary Fig. 5b).
Figure 5. Adult SIPs are serially transplantable, and converted in vivo to HSC with multilineage differentiation capability in primary and secondary recipients.
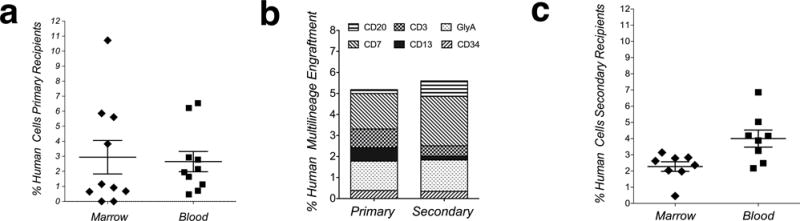
(a) Percentage of total human hematopoietic (myeloid and lymphoid) engraftment at 2 months post-transplant in animals transplanted with SIPs (n=10); each mark represents levels of engraftment within one animal. (b) Overall percentage of engraftment within the different hematopoietic lineages. (c) HSC generated in primary recipients are serially transplantable. Analysis of human hematopoietic engraftment in blood and marrow in secondary recipients (n=8), as determined by the percentage of human myeloid and lymphoid cells at 2 months post-transplant, is shown.
In order to investigate whether serially-transplantable HSC were being generated from SIPs within primary recipients, we performed serial transplantation studies, in which BM from primary SIPs recipients was transplanted into 8 secondary fetal sheep recipients.
Analysis of human hematopoietic engraftment in PB and BM in secondary recipients, as determined by the percentage of human myeloid and lymphoid cells at 2 months post-transplant, is shown in Fig. 5c. Human hematopoietic cells were detected in the PB and BM of all of the transplanted animals. The range of human hematopoietic cells was 2.17–6.86% and 0.46–3.14% in the PB and BM, respectively. All transplanted animals harbored donor-derived lymphocytes and erythroid cells, while 80% had lymphocytes, granulocytes, and erythrocytes. Human CD34+ cells (0.09–1.07%) were present in 60% of the transplanted animals. The overall percentage of engraftment within the different hematopoietic lineages of the secondary recipients is detailed in the right bar of Fig. 5b and in Supplementary Fig. 4.
In addition, human CD34+CD45+ cells, isolated from frozen bone marrow of primary SIPs recipients, were capable of engrafting 2 of 3 NSG mice for at least 8 wks, as assessed by the presence of human CD45+ cells (Fig. 6a and Supplementary Results)
Figure 6. Adult SIPs are serially transplantable.
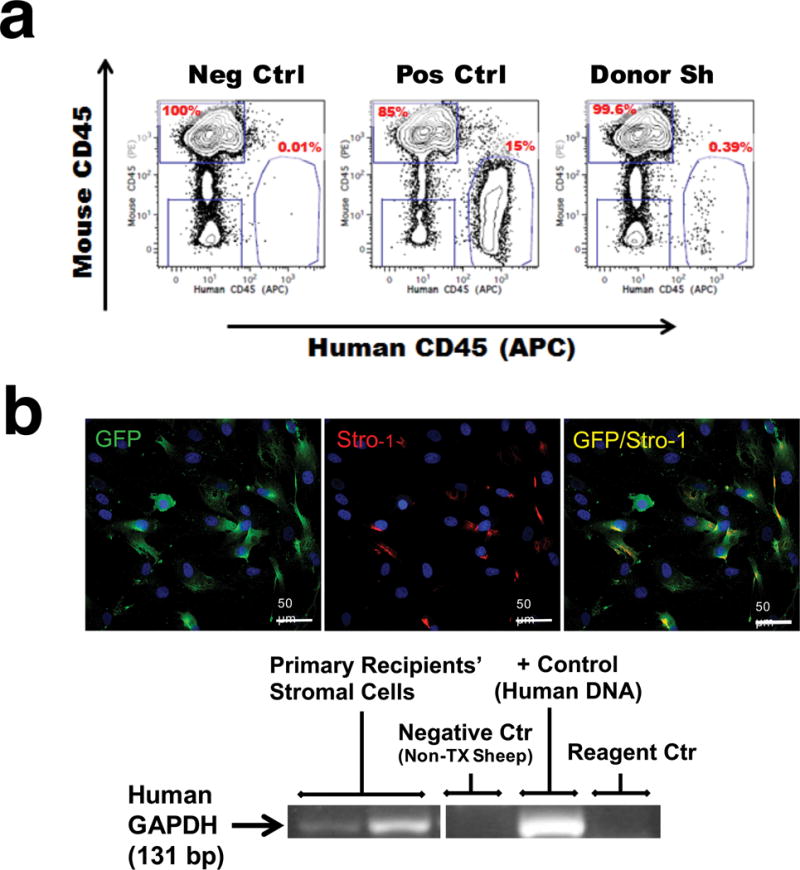
(a) CD34+CD45+ cells isolated from bone marrow of primary SIPs recipients (labeled as “Donor Sh”) were transplanted into NSG mice (n=3). Cells were gated based upon FSC, SSC, Ter119-, and 7AAD-. Positive control animals received CD34+CD117+ human mobilized peripheral blood stem cells; negative controls were non-transplanted animals. Percentages shown are based on the total number of human CD45+ cells in the plot. (b) Upper panel: SIPs remain in BM of transplanted animals. At 2.5 years post-transplant, BM from 2 of the secondary recipients that had received SIPs transduced with a lentivector encoding GFP, were sorted based on Stro-1 and grown in culture. Human-derived Stro-1+, GFP+ cells were detected in culture. Images were acquired with a Zeiss Axiovert 200M at 20X magnification. DAPI (blue) labels all nuclei. Lower panel: PCR analysis, using human-specific primers for GAPDH demonstrated that SIPs sorted from primary recipients were of human origin. Samples were run simultaneously in the same gel, but the gel was cropped to improve clarity and show amplification of samples specific to cultured cells.
SIPs persist in the bone marrow of the transplanted animals
To determine if all SIPs converted to hematopoietic cells, or some had persisted in the bone marrow of the transplanted animals as SIPs, we collected BM from 2 primary recipients that had received SIPs transduced with a lentivector encoding GFP, and transplanted these cells into 2 additional secondary recipients at a dose of 3.7×106 cells/fetus. Stro-1+ cells were sorted from the BM of secondary recipients (n=2) at 30 months post-transplant, and grown in culture. As can be seen in Fig. 6b, GFP-positive human SIPs were detected in culture. The human origin of these cells was further confirmed by PCR analysis, using human-specific primers for GAPDH.
Evaluation of clonally-derived SIPs for their ability to generate vascular cells and HSC after transplantation into fetal sheep
To show that the observed donor-derived hematopoiesis did not arise from undetectable levels of hematopoietic cells in culture, clonally-derived SIPs, obtained by deposition of single cells in 96 well plates, were expanded until sufficient cells were obtained. Phenotypic characterization of the various resultant clones demonstrated these cells to be similar to non-clonally derived SIPs (data not shown). Each clonally-derived SIP population was transplanted into one fetal sheep at a dose of 106cells/fetus (n=8). Evaluation of the recipients at 75 days post-transplantation for the presence of human hematopoiesis showed that all 8 transplanted SIP clones generated hematopoietic cells (Fig. 7a, and Supplementary Fig. 4) in the BM (1.25–7.63%), and PB (8–13.73%), including both myeloid and lymphoid cells, as determined by flow cytometry (Fig. 7b left column). In 88% of the animals, donor-derived contribution to the granulocytic and erythroid compartments was also detected, and CD34+ cells were detected in all transplanted animals (0.22±0.05%), (Supplementary Fig. 5a lower panel). PCR analysis, using human-specific primers for HLA-DQα, demonstrated that methylcellulose colonies (CFC) grown from primary recipients that received clonally-derived SIPs were of human origin (Supplementary Fig. 5c).
Figure 7. Clonally-derived SIPs convert to serially transplantable HSC after transplantation into fetal sheep.
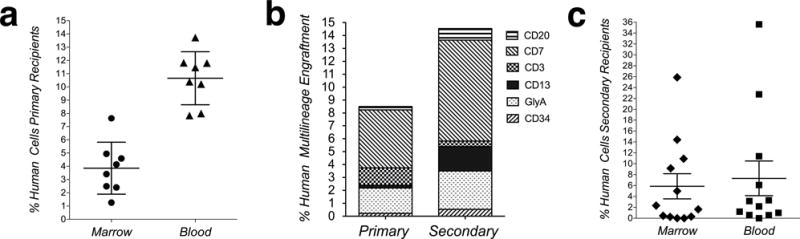
(a) Percentage of total human hematopoietic (myeloid and lymphoid) engraftment at 75 days post-transplantation in animals transplanted with clonally-derived SIPs (n=8); each mark represents levels of engraftment within one animal. (b) Clonally-derived SIPs generated HSC with multilineage differentiation capability in primary and secondary recipients; overall percentage of engraftment within the different hematopoietic lineages. (c) HSC generated from clonally-derived SIPs are serially transplantable; human hematopoietic engraftment in blood and marrow in secondary animals (n=12), as determined by the percentage of human myeloid and lymphoid cells at 2 months post-transplant.
Transplantation into secondary fetal sheep recipients (n=12) confirmed that the hematopoietic cells generated from clonally-derived SIPs in the primary fetal sheep recipients were able to engraft secondary recipients; the total percentage of human cells in the PB (0.57–35%) and BM (0.45–25%), as detected by flow cytometry, is depicted in Fig. 7c and Supplementary Fig. 4. CD34+ cells were present in 60% of these secondary recipients (0.53±0.14), and the animals with the highest levels of engraftment also exhibited expansion of either the erythroid (Gly-A) or lymphoid (CD7) lineage (Fig. 7b right column).
We also investigated whether clonally-derived SIPs could contribute to both the vascular niche and hematopoietic cells after transplant. Confocal microscopy on sections of bone marrow harvested from animals transplanted with a clone of SIPs that had been transduced with a lentiviral vector encoding GFP demonstrate that Stro-1+GFP+ cells can readily be identified contributing to the vasculature of the transplanted animal (Supplementary Fig. 6a). Flow cytometric analysis using an antibody against GFP demonstrated GFP-positive cells in the BM of these animals (Supplementary Fig. 6b). Confirmation this clonal cell population also gave rise to cells within the hematopoietic lineage was obtained by flow cytometric analysis, which demonstrated the presence of human hematopoietic cells (Supplementary Fig. 6c).
Oct4 induces SIPs towards a hematopoietic phenotype in vitro
After determining that human OCT4 was expressed in the marrow of all transplanted animals, to varying degrees (Supplementary Figure 7a), we next performed in vitro studies to examine whether over-expression of OCT4 could induce SIPs towards a hematopoietic phenotype.26 Data presented in the supplementary results, and Supplementary Figure 7b,c demonstrate that in vitro induction of SIPs using a lentivector encoding OCT4 led the generation of colonies co-expressing CD45 and CD34.
DISCUSSION
In humans, little evidence has been provided that a cell population with hemogenic properties exists within the fetal bone marrow, but this possibility has not been entirely excluded.39 Morphologic studies investigating human fetal bone marrow hematopoietic ontogeny have shown that the development of the vascular bed occurs between 9–10.5gw, and precedes establishment of hematopoiesis.40, 41 Here, we provide the first evidence that, at these early time points of gestation, APLNR, a marker of mesangiohematopoietic mesoderm11, 18 is expressed by cells that also express Stro-1 in the inner part of the developing bone marrow. APLNR+ Stro-1+ cells are also VEGFR2bright,PDGFRαLow/−, a repertoire of markers associated with hematovascular mesodermal precursors. The concomitant expression of VE-Cadherin, a marker connected with the emergence of hemogenic endothelial precursors13 by APLNR+, Stro-1+ cells, indicates that these cells have a phenotype fitting that of late hematovascular mesodermal precursors/emerging hemogenic endothelial precursors.
The use of CD34 as another marker of hematovascular cells,36 to delineate in vivo, the sites where Stro-1+ cells localize during fetal BM development, demonstrated that Stro-1+,VEGFR2+,CD34+ are present within the vascular niche of the developing bone. We also showed, for the first time, that although the percentage of Stro-1+ cells decreases during development to constitute a small population within the adult bone marrow, a subpopulation of Stro-1+ cells within the adult still expresses APLNR, VE-Cadherin, and VEGFR2.
After ex-vivo expansion, characterization of fetal and adult SIPs showed that expression of VEGFR2+ and VE-Cadherin was lost, when compared to freshly isolated cells, while the expression of PDGFRα was increased. Nevertheless, neither fetal nor adult SIPs were able to produce hematopoietic colonies upon methylcellulose culture, which is in agreement with what has previously been described for hematovascular mesodermal precursors and hemogenic endothelial precursors.13 Prior studies showed that the hematopoietic and endothelial potential of hematovascular mesodermal precursors could be demonstrated after co-culture with OP9 cells.13, 15 Because co-culture of SIPs with OP9 cells did not reveal their hematopoietic potential, here we used an in vivo fetal model to test the hemogenic and vasculogenic potential of in vitro expanded SIPs. These studies show that these adult cells generated hematopoietic cells in vivo, and that SIPs were capable of multilineage hematopoietic reconstitution of primary and secondary sheep and mice recipients. Further support that the inherent hemogenic potential is unique to SIPs comes from studies in which we showed that transplantation of other human BM-derived stromal cells which were not isolated based upon Stro-1-positivity, were unable to generate detectable levels of hematopoietic cells in this same fetal model.42 In addition, in vitro induction of SIPs using a lentivector encoding OCT4 led to the generation of colonies co-expressing CD45 and CD34. The fact that, in SIPs-transplanted recipients, there was a strong bias towards lymphoid engraftment, despite the presence of myeloid cells, raised the possibility of the contamination of SIPs with primitive long-lasting lymphoid cells. The data demonstrating that clonally-derived SIPs were also able to generate HSC that were serially-transplantable rules out this possibility, and provides further proof that SIPs have the ability to convert to hematopoietic cells, if primed with the necessary factors. In addition, we show for the first time, that clonally-derived SIPs also contribute to the vascular elements.
In conclusion, these studies have demonstrated the existence of an expandable population of adult human somatic cells, whose ontogenetic phenotype parallels that described for hematovascular mesodermal precursors and/or hemogenic endothelial precursors,13 and show these cells have the ability to contribute to vascularization and generate hematopoietic cells upon transplantation. The identification of this human somatic cell population provides novel insights into human ontogenetic hematovascular potential, which could lead to a better understanding of malignant and nonmalignant hematologic disorders.
Supplementary Material
Acknowledgments
This work was supported by NHLBI R01HL097623 and Intramural Pilot program of Wake Forest School of Medicine.
Footnotes
None of the authors have any competing financial interests to disclose
References
- 1.Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2006 Jan 1;107(1):106–110. doi: 10.1182/blood-2005-05-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solaimani Kartalaei P, Yamada-Inagawa T, Vink CS, de Pater E, van der Linden R, Marks-Bluth J, et al. Whole-transcriptome analysis of endothelial to hematopoietic stem cell transition reveals a requirement for Gpr56 in HSC generation. The Journal of experimental medicine. 2015 Jan 12;212(1):93–106. doi: 10.1084/jem.20140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009 Feb 12;457(7231):887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008 Mar 6;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008 Dec 4;3(6):625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbord P, Tavian M, Coulombel L, Luton D, San Clemente H, Humeau L, et al. Early ontogeny of the human hematopoietic system. C R Seances Soc Biol Fil. 1995;189(4):601–609. [PubMed] [Google Scholar]
- 7.Swiers G, Baumann C, O’Rourke J, Giannoulatou E, Taylor S, Joshi A, et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nature communications. 2013;4:2924. doi: 10.1038/ncomms3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lievre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998 Nov;125(22):4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- 9.Tamura H, Okamoto S, Iwatsuki K, Futamata Y, Tanaka K, Nakayama Y, et al. In vivo differentiation of stem cells in the aorta-gonad-mesonephros region of mouse embryo and adult bone marrow. Experimental hematology. 2002 Aug;30(8):957–966. doi: 10.1016/s0301-472x(02)00822-6. [DOI] [PubMed] [Google Scholar]
- 10.Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood. 2003 Oct 1;102(7):2436–2443. doi: 10.1182/blood-2003-01-0118. [DOI] [PubMed] [Google Scholar]
- 11.Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010 Dec 3;7(6):718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka H, Takakura N, Nishikawa S, Tsuchida K, Kodama H, Kunisada T, et al. Expressions of PDGF receptor alpha, c-Kit and Flk1 genes clustering in mouse chromosome 5 define distinct subsets of nascent mesodermal cells. Dev Growth Differ. 1997 Dec;39(6):729–740. doi: 10.1046/j.1440-169x.1997.t01-5-00009.x. [DOI] [PubMed] [Google Scholar]
- 13.Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012 Sep 27;2(3):553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lis R, Karrasch CC, Poulos MG, Kunar B, Redmond D, Duran JGB, et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017 May 25;545(7655):439–445. doi: 10.1038/nature22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC, et al. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011 Feb;39(2):225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oberlin E, Tavian M, Blazsek I, Peault B. Blood-forming potential of vascular endothelium in the human embryo. Development. 2002 Sep;129(17):4147–4157. doi: 10.1242/dev.129.17.4147. [DOI] [PubMed] [Google Scholar]
- 17.Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, et al. Identification of the hemangioblast in postnatal life. Blood. 2002 Nov 1;100(9):3203–3208. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- 18.Yu QC, Hirst CE, Costa M, Ng ES, Schiesser JV, Gertow K, et al. APELIN promotes hematopoiesis from human embryonic stem cells. Blood. 2012 Jun 28;119(26):6243–6254. doi: 10.1182/blood-2011-12-396093. [DOI] [PubMed] [Google Scholar]
- 19.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991 Jul 1;78(1):55–62. [PubMed] [Google Scholar]
- 20.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003 May 1;116(Pt 9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 21.Ning H, Lin G, Lue TF, Lin CS. Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochemical and biophysical research communications. 2011 Sep 23;413(2):353–357. doi: 10.1016/j.bbrc.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003 Apr;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 23.Sanada C, Kuo CJ, Colletti EJ, Soland M, Mokhatari S, Knovich MA, et al. Mesenchymal stem cells contribute to endogenous FVIIIc production. J Cell Physiol. 2012 Oct 5; doi: 10.1002/jcp.24247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soland MA, Keyes LR, Bayne R, Moon J, Porada CD, St Jeor S, et al. Perivascular stromal cells as a potential reservoir of human cytomegalovirus. Am J Transplant. 2014 Apr;14(4):820–830. doi: 10.1111/ajt.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007 Dec 21;318(5858):1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 26.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010 Nov 25;468(7323):521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 27.Panopoulos AD, Belmonte JC. Induced pluripotent stem cells in clinical hematology: potentials, progress, and remaining obstacles. Curr Opin Hematol. 2012 Jul;19(4):256–260. doi: 10.1097/MOH.0b013e328353c78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, et al. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007 Dec;46(6):1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 29.Shaw SW, Bollini S, Nader KA, Gastadello A, Mehta V, Filppi E, et al. Autologous transplantation of amniotic fluid-derived mesenchymal stem cells into sheep fetuses. Cell Transplant. 2011;20(7):1015–1031. doi: 10.3727/096368910X543402. [DOI] [PubMed] [Google Scholar]
- 30.Crapnell KB, Almeida-Porada G, Khaiboullina S, St Jeor SC, Zanjani ED. Human haematopoietic stem cells that mediate long-term in vivo engraftment are not susceptible to infection by human cytomegalovirus. British journal of haematology. 2004 Mar;124(5):676–684. doi: 10.1111/j.1365-2141.2004.04827.x. [DOI] [PubMed] [Google Scholar]
- 31.Goodrich AD, Ersek A, Varain NM, Groza D, Cenariu M, Thain DS, et al. In vivo generation of beta-cell-like cells from CD34(+) cells differentiated from human embryonic stem cells. Exp Hematol. 2010 Jun;38(6):516–525 e514. doi: 10.1016/j.exphem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takenaga M, Fukumoto M, Hori Y. Regulated Nodal signaling promotes differentiation of the definitive endoderm and mesoderm from ES cells. J Cell Sci. 2007 Jun 15;120(Pt 12):2078–2090. doi: 10.1242/jcs.004127. [DOI] [PubMed] [Google Scholar]
- 34.Slukvin II. Hematopoietic specification from human pluripotent stem cells: current advances and challenges toward de novo generation of hematopoietic stem cells. Blood. 2013 Dec 12;122(25):4035–4046. doi: 10.1182/blood-2013-07-474825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturgeon CM, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nature biotechnology. 2014 Jun;32(6):554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytometry A. 2013 Jan;83(1):114–126. doi: 10.1002/cyto.a.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011 Jul 8;333(6039):218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 38.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998 May 29;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 39.Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012 May 24;119(21):4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996 May 15;87(10):4109–4119. [PubMed] [Google Scholar]
- 41.Tavian M, Cortes F, Charbord P, Labastie MC, Peault B. Emergence of the haematopoietic system in the human embryo and foetus. Haematologica. 1999 Jun;84(Suppl EHA-4):1–3. [PubMed] [Google Scholar]
- 42.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000 Jun 1;95(11):3620–3627. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


