Abstract
Background
Bt crops will face a new ecological risk of reduced effectiveness against target-insect pests owing to the general decrease in exogenous-toxin content in Bt crops grown under elevated carbon dioxide (CO2). The method chosen to deal with this issue may affect the sustainability of transgenic crops as an effective pest management tool, especially under future atmospheric CO2 level raising.
Methods
In this study, rhizobacterias, as being one potential biological regulator to enhance nitrogen utilization efficiency of crops, was selected and the effects of Bt maize (Line IE09S034 with Cry1Ie vs. its parental line of non-Bt maize Xianyu 335) infected by Azospirillum brasilense (AB) and Azotobacter chroococcum (AC) on the development and food utilization of the target Mythimna separate under ambient and double-ambient CO2 in open-top chambers from 2016 to 2017.
Results
The results indicated that rhizobacteria infection significantly increased the larval life-span, pupal duration, relative consumption rate and approximate digestibility of M. separata, and significantly decreased the pupation rate, pupal weight, adult longevity, fecundity, relative growth rate, efficiency of conversion of digested food and efficiency of conversion of ingested food of M. separata fed on Bt maize, while here were opposite trends in development and food utilization of M. separata fed on non-Bt maize infected with AB and AC compared with the control buffer in 2016 and 2017 regardless of CO2 level.
Discussion
Simultaneously, elevated CO2 and Bt maize both had negative influence on the development and food utilization of M. separata. Presumably, CO2 concentration arising in future significantly can increase their intake of food and harm to maize crop; however, Bt maize infected with rhizobacterias can reduce the field hazards from M. separata and the application of rhizobacteria infection can enhance the resistance of Bt maize against target lepidoptera pests especially under elevated CO2.
Keywords: Elevated CO2, Growth and development, Transgenic Bt maize, Food utilization, Rhizobacteria, Mythimna separata
Introduction
With increased fossil fuel combustion and drastic changes in land utilization, the concentration of atmospheric carbon dioxide (CO2) has increased by more than 40%, from 280 to 400 ppm, between the industrial revolution and now (Ciais et al., 2013). The recent forecast indicated that atmospheric CO2 concentration will increase to approximately 900 ppm by 2,100 (Intergovernmental Panel on Climate Change (IPCC) 2014). Increasing atmospheric CO2 concentration alone can be very significant in crop production because of its direct effect on plant physiology and biochemistry (Cornelissen, 2011), and indirect effect on tri-trophic interactions involving plants, herbivores, and predators or pathogens (Robinson, Ryan & Newman, 2012; Trębicki et al., 2017). Elevated atmospheric CO2 also affects the crop production via direct or indirect impact on the physiology and feeding behavior of phytophagous insects (Zvereva & Kozlov, 2006; Massad & Dyer, 2010; O’Neill et al., 2010). These changes may then lead to more severe and frequent outbreaks of pest insects in agricultural ecosystems (Percy et al., 2002).
Several studies have shown that the elevated CO2 increased lepidopteran insect feeding and damage severity in agricultural crops (Ainsworth et al., 2007; Lindroth et al., 2001), because of the increased proportion of C:N in host plant tissue and lower nutritional quality caused by elevated CO2 (Ainsworth et al., 2007). For example, larvae of Helicoverpa armigera fed on wheat grown in elevated CO2 showed the extended larval life span and increased consumption with reduced growth rate (Chen, Wu & Ge, 2004). Transgenic maize that expresses insecticidal Cry proteins derived from the soil bacterium Bacillus thuringiensis Berliner (Bt) has been used to control target lepidopteran insects (Carrière, Crowder & Tabashnik, 2010; Huang et al., 2014; Walters et al., 2010), e.g., European corn borer Ostrinia nubilalis (Hübner), Asian corn borer O. furnacalis (Guenée) (Lepidoptera: Crambidae) and corn armyworm Mythimna separata (Lepidoptera: Noctuidae) (Guo et al., 2016; Zhang et al., 2013; Jia et al., 2016). Transgenic Bt maize has widely been adopted worldwide (Cattaneo et al., 2006; Huang et al., 2005; Hutchison et al., 2010; Lu et al., 2012). It was anticipated that the primary effect of elevated CO2 on Bt toxin production would be due to differences in N concentration in plant tissues (Coviella, Stipanovic & Trumble, 2002). Biologically relevant changes in plant defensive chemistry of Bt maize are expected to have measurable effects on the target lepidopteran pests under climate change.
Additionally, many researchers found that the nitrogen metabolism of transgenic Bt crops could affect the expression of Bt toxin protein, and stimulating plant N uptake to increase in biomass N relative to C to increase the nitrate reductase activity and Bt toxin production of Bt crops. (Stitt & Krapp, 1999; Pang et al., 2005; Gao et al., 2009). Nitrogen plays the most important role for plant growth, and it is an important complement of enzymes catalyzing and controlling reactions in plants for normal physiological processes (Richardson et al., 2009). While most of nitrogen in the environment is found in a form of nitrogen gas (N2) which approximately amounts to 78% in the atmosphere, plant available nitrogen found in soil is generally derived from fertilizer augmentation. As plants cannot use N2 directly, soil-inhabiting microbes play a significant role in nitrogen uptake by plants as they change the N2 into ammonia (Yamprai, Mala & Sinma, 2014). Azospirillum sp. and Azotobacter sp. are the two major free-living soil microbes (Biari, Gholami & Rahmani, 2008), that are economically important nitrogen-fixing bacteria in maize crop production system (Yamprai, Mala & Sinma, 2014). Thus, optimization of soil-nitrogen management offers significant potential in the utilization of soil rhizobacterias to increase Bt-crop nitrogen utilization to affect the expression of Bt toxin under elevated CO2.
Materials and Methods
Setup of CO2 levels
A two-year study (2016–2017) was conducted in six open-top chambers (i.e., OTCs; Granted Patent: ZL201120042889.1; 2.5 m in height × 3.2 m in diameter) (Chen et al., 2011) at the Innovation Research Platforms for Climate Change, Biodiversity and Pest Management (CCBPM; http://www.ccbpm.org) field laboratory in Ningjin County, Shandong Province of China (37°38′ 30.7″ N, 116°51′ 11.0″ E). A total of two CO2 levels, ambient (375 μl/L, hereafter referred to as aCO2) and elevated (750 μl/L or double-ambient, hereafter referred to as eCO2) were applied continuously from 10 June to 7 October in both years. A total of three OTCs were used for each CO2 treatment, and the CO2 concentrations in each OTC were monitored continuously and adjusted using an infrared CO2 analyzer (Ventostat 8102; Telaire Company, Goleta, CA, USA). The OTCs of elevated CO2 treatments were inflated with canned CO2 gas with 95% purity and automatically controlled by the same type of infrared CO2 analyzer (Chen, Wu & Ge, 2004). Actual mean CO2 concentrations and temperature throughout the entire experiment for both 2016 and 2017 are provided in Table 1.
Table 1. Actual mean (±SE) CO2 concentration and temperature in the open-top chambers (OTC) from seedling emergence to harvest of transgenic Bt maize and its parental line of non-Bt maize in 2016 and 2017.
| Climate factors | OTC | 2016 | 2017 | Two-way ANOVAs (F/P values) |
|---|---|---|---|---|
| CO2 (μl/L) | eCO2 OTC | 744.4 ± 3.3a, A | 748.8 ± 4.5a, A | FCO2 = 399.32, P = 0.000 |
| FYear = 2.85, P = 0.13 | ||||
| aCO2 OTC | 372.6 ± 4.7b, A | 374.5 ± 3.8b, A | FInteraction = 0.45, P = 0.52 | |
| Temperature (°C) | eCO2 OTC | 26.01 ± 0.5a, A | 26.12 ± 0.3a, A | FCO2 = 0.006, P = 0.94 |
| FYear = 3.38, P = 0.10 | ||||
| aCO2 OTC | 25.99 ± 0.4a, A | 26.11 ± 0.4a, A | FInteraction = 0.057, P = 0.82 |
Notes:
OTC: ambient-CO2 OTC (aCO2 OTC) and elevated-CO2 OTC (eCO2 OTC). Different lowercase letters indicate significantly different between the eCO2 OTC and aCO2 OTC in same year by the Duncan test at P < 0.05, respectively.
Not significantly different between 2016 and 2017 at same CO2 level or temperature by the Duncan test at P > 0.05, respectively.
Plant materials
The Bt maize cultivar (Line IE09S034, hereafter referred to as Bt) and its non-Bt parental line (cv. Xianyu 335, referred to as Xy) were both obtained from the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences. Both Bt and non-Bt lines used in this study had the similar maturity (approximately 102 d: from 10 June to 20 September) and were well adapted to the growing conditions of northern China (Guo et al., 2016; Zhang et al., 2013; Jia et al., 2016; Ling, 2010). Both maize accessions were planted in plastic buckets (diameter × height = 30 × 45 cm) filled with 20 kg autoclaved soil and 10 g compound fertilizer (N:P:K = 18:15:12), then placed them into chambers on 10 June each year.
Soil nitrogen-fixing bacteria and infection of maize seeds
Lyophilized Azospirillum brasilense (strain number ACCC 10103) and Azotobacter chroococcum (strain number ACCC 10006) were provided by Agriculture Culture Collection of China (ACCC) in plastic tubes (3 cm in diameter and 15 cm in height) with bacterial growth medium. Both species of rhizobacterias were grown in liquid medium at 28 °C under continuous shaking (200 rpm) until they reached an absorbance of 1.008 (A. brasilense) and 1.005 (A. chroococcum) at a wavelength of 600 nm. Before inoculation, the culture was centrifuged, and the supernatant was discarded, and the pellet of cells was re-suspended in the liquid medium to a density of 108 copies per milliliter. The seeds of both Bt and non-Bt maize were infected with A. brasilense and A. chroococcum cultures each, and the inoculation doses were all adjusted to a final volume of 10 ml for each seed. After inoculation, all the treated seeds were maintained under sterile laminar air flow for 2 h at 28 °C (Cassán et al., 2009). Bacteria inoculation treatments consisted of three types of rhizobacteria infection, including (1) seeds infected with A. brasilense (referred to as AB); (2) seeds infected with A. chroococcum (referred to as AC); and (3) non-infected seeds (control) treated with a final volume of buffer solution (referred to as CK). The entire experiment, thus, consisted of 12 treatments, including two CO2 levels (aCO2 and eCO2), two maize cultivars (Bt and Xy), and three rhizobacteria infections (AB, AC, and CK), replicate six time. Each pot serves as one replication. Specifically, six buckets for each maize cultivar (Bt and Xy) and three rhizobacteria inoculations (6 buckets per transgenic treatment × 2 transgenic treatments × 3 inoculation treatments = 36 buckets) were placed randomly in each CO2 chamber (ambient and double-ambient CO2), and three maize seeds were sown in each bucket at 2 cm soil depth. No pesticides were applied during the entire experimental period and the manual weeding keep the maize buckets weed-free during the experiment. The rhizosphere soil was sampled from each bucket one-day before planting, 14 days after planting, and at harvest and measured the relative density of A. brasilense and A. chroococcum using RT-PCR (Tables 2 and 3) (Jiang et al., 2017).
Table 2. Sequence specific primers of rhizobacterias, Azospirillum brasilense (AB) and Azotobacter chroococcum (AC) for qRT-PCR.
| Primer | Sequence (5′-3′) | GenBank accession | Description |
|---|---|---|---|
| AB-4 | Forward: CAAGGGCACCATCCCGAC | X51500.1 | A. brasilense NifH gene |
| Reverse: CTGCTGCTCCTCCGACT | |||
| AC-2 | Forward: GTGACCCGAAAGCTGACTCC | EU693338.1 | A. chroococcum nifH gene |
| Reverse: CCACCTTCAGCACGTCTTCC |
Table 3. The rhizosphere soil densities of rhizobacterias inoculated in the potted soil of transgenic Bt maize and its parental line of non-Bt maize grown under ambient and elevated CO2 in 2016 and 2017.
| Measure matters | Rhizobacteria infections | 2016 (AB; AC copies/g) | 2017 (AB; AC copies/g) | |
|---|---|---|---|---|
| Sampled soil before maize planting | 5.53 ± 0.24 105; 4.47 ± 0.12 105 | 5.61 ± 0.11 105; 4.33 ± 0.17 105 | ||
| Sampled soil at the maize seedling after 14 days | AB | aCO2-Bt | 8.46 ± 0.24 1011; 4.48 ± 0.26 105 | 8.40 ± 0.28 1011; 4.44 ± 0.11 105 |
| aCO2-Xy | 8.25 ± 0.26 1011; 4.21 ± 0.08 105 | 8.69 ± 0.23 1011; 4.56 ± 0.22 105 | ||
| eCO2-Bt | 8.36 ± 0.19 1011; 4.43 ± 0.15 105 | 8.59 ± 0.21 1011; 4.47 ± 0.17 105 | ||
| eCO2-Xy | 8.70 ± 0.27 1011; 4.58 ± 0.29 105 | 8.24 ± 0.12 1011; 4.34 ± 0.27 105 | ||
| AC | aCO2-Bt | 5.54 ± 0.25 105; 7.37 ± 0.29 1011 | 5.70 ± 0.28 105; 7.40 ± 0.26 1011 | |
| aCO2-Xy | 5.73 ± 0.24 105; 7.29 ± 0.17 1011 | 5.36 ± 0.22 105; 7.66 ± 0.25 1011 | ||
| eCO2-Bt | 5.62 ± 0.30 105; 7.71 ± 0.15 1011 | 5.13 ± 0.04 105; 7.32 ± 0.13 1011 | ||
| eCO2-Xy | 5.46 ± 0.28 105; 7.59 ± 0.17 1011 | 5.42 ± 0.13 105; 7.57 ± 0.22 1011 | ||
| CK | aCO2-Bt | 5.71 ± 0.20 105; 4.52 ± 0.21 105 | 5.92 ± 0.08 105; 4.67 ± 0.17 105 | |
| aCO2-Xy | 5.50 ± 0.29 105; 4.24 ± 0.15 105 | 5.33 ± 0.18 105; 4.31 ± 0.13 105 | ||
| eCO2-Bt | 5.46 ± 0.08 105; 4.26 ± 0.18 105 | 5.62 ± 0.31 105; 4.48 ± 0.21 105 | ||
| eCO2-Xy | 5.46 ± 0.18 105; 4.76 ± 0.23 105 | 5.47 ± 0.17 105; 4.21 ± 0.09 105 | ||
| Sampled soil at the maize harvest | AB | aCO2-Bt | 8.50 ± 0.19 1011b; 4.65 ± 0.21 105 | 8.39 ± 0.26 1011b; 4.01 ± 0.26 105 |
| aCO2-Xy | 8.44 ± 0.15 1011b; 4.11 ± 0.23 105 | 8.65 ± 0.19 1011b; 4.30 ± 0.18 105 | ||
| eCO2-Bt | 9.81 ± 0.23 1011a; 4.13 ± 0.17 105 | 1.09 ± 0.04 1012a; 4.67 ± 0.20 105 | ||
| eCO2-Xy | 9.98 ± 0.25 1011a; 4.49 ± 0.22 105 | 1.02 ± 0.03 1012a; 4.89 ± 0.23 105 | ||
| AC | aCO2-Bt | 5.57 ± 0.31 105; 7.27 ± 0.26 1011b | 5.40 ± 0.08 105; 7.30 ± 0.14 1011b | |
| aCO2-Xy | 5.99 ± 0.25 105; 7.49 ± 0.19 1011b | 4.97 ± 0.15 105; 7.24 ± 0.19 1011b | ||
| eCO2-Bt | 4.89 ± 0.27 105; 8.98 ± 0.15 1011a | 5.94 ± 0.14 105; 9.07 ± 0.12 1011a | ||
| eCO2-Xy | 5.33 ± 0.10 105; 8.96 ± 0.21 1011a | 5.77 ± 0.12 105; 9.03 ± 0.18 1011a | ||
| CK | aCO2-Bt | 5.15 ± 0.35 105; 4.65 ± 0.23 105 | 5.39 ± 0.08 105; 4.56 ± 0.22 105 | |
| aCO2-Xy | 5.49 ± 0.19 105; 4.37 ± 0.33 105 | 4.97 ± 0.16 105; 4.66 ± 0.15 105 | ||
| eCO2-Bt | 5.59 ± 0.14 105; 4.76 ± 0.11 105 | 4.88 ± 0.25 105; 4.64 ± 0.17 105 | ||
| eCO2-Xy | 5.12 ± 0.14 105; 4.69 ± 0.05 105 | 5.13 ± 0.13 105; 4.24 ± 0.10 105 | ||
Note:
Rhizobacteria infections: A. brasilense (AB) and A. chroococcum (AC) vs. the control buffer solution (CK). CO2 levels: ambient CO2 (aCO2) and elevated CO2 (eCO2). Transgenic treatment: Bt maize (Bt) and non-Bt maize (Xy). Different lowercase letters indicate significantly different between ambient CO2 and elevated CO2 for same maize cultivar in same year by the Duncan test at P < 0.05, respectively.
Insect source and rearing
The colony of armyworm M. separata was originated from a population collected in maize fields in Kangbao County, Hebei province of China (41.87°N, 114.6°E) in the summer of 2014, and fed on artificial diet and maintained for more than 10 generations in climate-controlled growth chambers (GDN-400D-4; Ningbo Southeast Instrument Co., Ltd., Ningbo, China) at 26 ± 1 °C, 65 ± 5% RH, and 14: 10 h L/D photoperiod. The same rearing conditions were maintained for the following experiments. Newly-hatched larvae were randomly selected from the above colony of M. separata and fed on artificial diet (Bi, 1981) until the second instar larvae, and then the third instar larvae were individually fed on excised leaves of the experimental plants growing in CO2 chambers. Feeding trials were conducted in plastic dish (6 cm in diameter and 1.6 cm in height) and the experimental leaves were randomly selected from six buckets for each of the 12 experimental treatment combinations (2 transgenic treatments × 2 CO2 treatments × 3 bacteria inoculations) during the tasseling stage until pupation. Sample size for the M. separata larval feeding trial consisted of 20 larvae (sample unit size) with five replicates for each of the 12 treatment combinations (i.e., 1,200 larvae evaluated for the entire study). Because of the cannibalism among the late instar larvae of M. separata (Jiang et al., 2016; Ali et al., 2016; Liu et al., 2017), the sampled larvae were reared separately in the Petri dish until pupation.
Development and reproduction of M. separata
Larval development was evaluated from third instar to pupation by way of observing each individual petri dish every 8 h and recording the timing of larval ecdysis, pupation, and emergence of M. separata moths. After eclosion, the newly emerged moths were paired (female: male = 1:1) for mating in a metal frame screen cage (length × width × height = 35 × 35 × 40 cm), and the paired moths were fed with a 10% honey solution provided on a large cotton wick in a single plastic cup (diameter × height = 8 × 20 cm) covered with cotton net yarn butter paper for oviposition. The cotton net yarn and butter paper were replaced every day. Moth survivorship and oviposition were recorded daily until both moths from each pair died.
Food utilization of the larvae of M. separata
Each third instar test larvae of M. separate was weighed at the initiation of the feeding trial by using an electronic balance (AL104; METTLER-TOLEDO, Greifensee, Switzerland). Total accumulated feces from third instar until the larva entered pupal stage (sixth instar), sixth instar larval weight, and the remaining leaves were also weighed. The food utilization indices of M. separata included the relative growth rate (RGR), relative consumption rate (RCR), approximate digestibility (AD), efficiency of conversion of ingested food (ECI) and efficiency of conversion of digested food (ECD) (Chen, Ge & Parajulee, 2005a; Chen et al., 2005b). Formulas for calculation of the measured indices were adapted from Chen et al. (2005b).
Data analysis
All data were analyzed using the statistical software SPSS 19.0 (2015; SPSS Institute, Chicago, IL, USA). Four-way analysis of variance was used to analyze the effects of CO2 levels (elevated vs. ambient), transgenic treatment (Bt maize vs. non-Bt maize), rhizobacteria infection (AB and AC vs. CK), sampling years (2016 vs. 2017), and the interactions on the measured indices of growth, development, and reproduction, including larval life-span, pupation rate, pupal weight, pupal duration, adult longevity and fecundity of M. separata. The measured food utilization indices were analyzed by using an analysis of covariance with initial weight of M. separata (i.e., third instar larva) as a covariate for RCR and RGR, while food consumption was a covariate for ECI and AD to correct the effect of variation in the growth and food assimilation of M. separata (Raubenheimer & Simpson, 1992); food assimilated was also used as a covariate to analyze the ECD parameter (Hägele & Rowell-Rahier, 1999). The assumption of a parallel slope between covariate and dependent variable was satisfied for each analysis. Treatment means were separated by using the Duncan-test to examine significant difference at P < 0.05.
Results
Effects of CO2 level, transgenic treatment, and rhizobacteria infection on the rhizosphere soil densities of A. brasilense and A. chroococcum in different sampling period
Significant effects of rhizobacteria infection (P < 0.001) were observed on the measured rhizosphere soil densities of A. brasilense (AB) and A. chroococcum (AC) 14 days after maize planting. Compared with ambient CO2, elevated CO2 significantly increased the rhizosphere soil densities of both A. brasilense and A. chroococcum; compared with the control buffer solution (CK), rhizobacteria infection significantly increased the rhizosphere soil densities of A. brasilense and A. chroococcum (P < 0.001; Table 3). CO2 level and rhizobacteria infection both significantly affected the densities of A. brasilense and A. chroococcum in rhizosphere soil at maize harvest (Table 4).
Table 4. Four-way ANOVA on the rhizosphere soil densities of rhizobacterias inoculated in the potted soil of Bt maize and its parental line of non-Bt maize grown under ambient and elevated CO2 in 2016 and 2017.
| Impact factors | Sampled soil at the maize seedling after 14 days | Sampled soil at the maize harvest | ||
|---|---|---|---|---|
| AB | AC | AB | AC | |
| Ya | 0.00/0.99 | 0.002/0.96 | 0.97/0.33 | 1.13/0.29 |
| Cv.b | 0.32/0.574 | 0.36/0.55 | 0.070/0.79 | 0.080/0.78 |
| CO2c | 0.19/0.89 | 0.80/0.38 | 26.01/<0.001*** | 331.16/<0.001*** |
| Rhizobacteriad | 2555.00/<0.001*** | 1380.37/<0.001*** | 1311.83/<0.001*** | 2080.71/<0.001*** |
| Y × Cv. | 0.62/0.44 | 1.96/0.17 | 0.18/0.673 | 0.53/0.47 |
| Y × CO2 | 1.21/0.28 | 2.50/0.12 | 0.74/0.40 | 0.032/0.86 |
| Y × Rhizobacteria | 0.00/1.00 | 0.02/0.99 | 0.97/0.39 | 1.13/0.33 |
| Cv. × CO2 | 0.35/0.55 | 0.010/0.92 | 0.32/0.57 | 0.36/0.55 |
| Cv. × Rhizobacteria | 0.32/0.73 | 0.36/0.70 | 0.070/0.93 | 0.80/0.93 |
| CO2 × Rhizobacteria | 0.019/0.98 | 0.80/0.45 | 26.01/<0.001*** | 331.16/<0.001*** |
| Y × Cv. × CO2 | 5.99/0.018* | 0.06/0.94 | 0.82/0.37 | 0.63/0.43 |
| Y × Cv. × Rhizobacteria | 0.62/0.54 | 1.96/0.15 | 0.18/0.84 | 0.53/0.59 |
| Y × CO2 × Rhizobacteria | 1.21/0.31 | 2.50/0.093 | 0.74/0.49 | 0.032/0.97 |
| Cv. × CO2 × Rhizobacteria | 0.35/0.70 | 0.10/0.99 | 0.32/0.73 | 0.36/0.70 |
| Y × Cv. × CO2 × Rhizobacteria | 5.99/0.05 | 0.006/0.99 | 0.82/0.45 | 0.63/0.54 |
Effects of CO2 level, transgenic treatment, and rhizobacteria infection on the development and reproduction of M. separata
Carbon dioxide level and transgenic treatment both significantly affected the larval life-span, pupation rate, pupal weight and duration, adult longevity, and fecundity in M. separata fed on both Bt and non-Bt maize infected with A. brasilense and A. chroococcum (P < 0.001). However, the rhizobacteria infection significantly affected the larval life-span, pupal duration (P < 0.05) and fecundity (P < 0.001) of M. separata fed on both transgenic treatments and at both CO2 levels (Table 5).
Table 5. Four-way ANOVA on the development and reproduction of Mythimna separata fed on Bt and non-Bt maize infected with A. brasilense and A. chroococcum under ambient and elevated CO2 in 2016 and 2017.
| Impact factors | Larval life-span (day (n = 828)) | Pupation rate (% (n = 828)) | Pupal weight (g (n = 663)) | Pupal duration (day (n = 663)) | Adult longevity (day (n = 576)) | Fecundity (eggs per female (n = 198)) |
|---|---|---|---|---|---|---|
| Ya | 1.62/0.32 | 0.94/0.71 | 1.21/0.62 | 0.11/0.90 | 1.65/0.33 | 2.99/0.10 |
| Cv.b | 1662.03/<0.001*** | 329.16/<0.001*** | 275.04/<0.001*** | 229.78/<0.001*** | 450.27/<0.001*** | 2032.99/<0.001*** |
| CO2c | 62.13/<0.001*** | 55.53/<0.001*** | 12.44/0.001** | 19.02/<0.001*** | 41.62/<0.001*** | 278.70/<0.001*** |
| Rhizobacteriad | 3.64/0.034* | 2.53/0.077 | 1.20/0.63 | 7.04/0.011* | 0.35/0.70 | 27.54/<0.001*** |
| Y × Cv. | 9.22/0.004** | 2.22/0.14 | 2.22/0.14 | 1.98/0.32 | 3.10/0.054 | 0.102/0.75 |
| Y × CO2 | 5.33/0.025* | 2.16/0.20 | 0.067/0.80 | 0.17/0.85 | 13.53/<0.001*** | 2.74/0.10 |
| Y × Rhizobacteria | 3.63/0.034* | 1.69/0.058 | 0.63/0.57 | 0.48/0.71 | 0.59/0.64 | 1.87/0.053 |
| Cv. × CO2 | 19.11/<0.001*** | 6.62/<0.001*** | 2.68/0.008** | 14.11/<0.001*** | 8.26/0.006** | 5.86/0.049* |
| Cv. × Rhizobacteria | 224.53/<0.001*** | 73.67/<0.001*** | 30.41/<0.001*** | 42.06/<0.001** | 26.38/<0.001*** | 195.08/<0.001*** |
| CO2 × Rhizobacteria | 12.81/<0.001*** | 12.22/0.004** | 11.63/0.005** | 14.98/<0.001*** | 4.24/0.02* | 22.02/<0.001*** |
| Y × Cv. × CO2 | 0.01/0.92 | 1.07/0.55 | 0.10/0.75 | 1.16/0.52 | 0.006/0.94 | 4.25/0.085 |
| Y × Cv. × Rhizobacteria | 1.55/0.33 | 1.53/0.17 | 0.064/0.94 | 2.02/0.23 | 0.46/0.68 | 8.87/0.071 |
| Y × CO2 × Rhizobacteria | 0.063/0.69 | 0.51/0.76 | 0.48/0.62 | 1.72/0.45 | 1.78/0.18 | 14.61/0.045* |
| Cv. × CO2 × Rhizobacteria | 8.88/0.006** | 12.61/0.003** | 7.41/0.011* | 5.66/0.005** | 13.55/0.002** | 24.04/0.000*** |
| Y × Cv. × CO2 × Rhizobacteria | 0.19/0.83 | 0.33/0.71 | 0.14/0.87 | 0.32/0.72 | 1.73/0.30 | 0.36/0.70 |
Compared with ambient CO2, elevated CO2 significantly prolonged the larval life-span (+6.21%), pupal duration (+5.56%), and significantly decreased the pupation rate (−18.08%), pupal weight (−8.12%), adult longevity (−6.06%), and fecundity (−22.58%) of M. separata (P < 0.05; Table 6). Also, compared with the CK, rhizobacteria infection with A. brasilense and A. chroococcum both significantly shortened the larval life-span (−5.20% and −5.70%), pupal duration (−3.68% and −3.81%) and fecundity (−10.20% and −9.53%) of M. separata (P < 0.05; Table 6). Moreover, Bt maize significantly prolonged the larval life-span (+13.67%) and pupal duration (+7.54%), shortened the adult longevity (−10.41%), and decreased the pupation rate (−75.55%), pupal weight (−13.54%) and fecundity (−75.46%) of M. separata compared to that for non-Bt maize (P < 0.05; Table 6).
Table 6. The development and reproduction of M. separata larvae fed on Bt maize and non-Bt maize during the heading stage, infected with rhizobacterias under ambient and elevated CO2 in 2016 and 2017.
| Impact factors | Factor levels | Larval life-span (day) | Pupation rate (%) | Pupal weight (g) | Pupal duration (day) | Adult longevity (day) | Fecundity (eggs per female) |
|---|---|---|---|---|---|---|---|
| Cv. | Bt | 24.19 ± 0.16a | 40.98 ± 2.45b | 0.192 ± 0.007b | 10.98 ± 0.18a | 6.63 ± 0.14b | 171.50 ± 13.27b |
| Xy | 21.28 ± 0.17b | 71.94 ± 2.38a | 0.218 ± 0.006a | 10.21 ± 0.19b | 7.32 ± 0.09a | 300.92 ± 28.64a | |
| CO2 | Elevated | 23.42 ± 0.20a | 51.78 ± 2.69b | 0.197 ± 0.007b | 10.78 ± 0.18a | 6.77 ± 0.11b | 212.25 ± 19.13b |
| Ambient | 22.05 ± 0.17b | 61.14 ± 2.68a | 0.213 ± 0.005a | 10.41 ± 0.19b | 7.18 ± 0.13a | 260.17 ± 21.87a | |
| Rhizobacteria infection | AB | 22.53 ± 0.16b | 56.06 ± 2.24 | 0.205 ± 0.004 | 10.54 ± 0.09b | 6.97 ± 0.24 | 228.00 ± 15.27b |
| AC | 22.42 ± 0.15b | 55.53 ± 2.01 | 0.204 ± 0.004 | 10.53 ± 0.08b | 6.96 ± 0.24 | 229.38 ± 17.46b | |
| CK | 23.25 ± 0.16a | 57.79 ± 2.58 | 0.206 ± 0.005 | 10.72 ± 0.13a | 6.99 ± 0.18 | 251.25 ± 23.21a |
Note:
Data in table are average ± SE. Different lowercase letters indicate significant difference between treatments by the Duncan’s test at P < 0.05; the same as in Table 8.
Impacts of CO2 level, transgenic treatment, and rhizobacteria infection on the food utilization of M. separata
There were significant effects of CO2 level, transgenic treatment, and rhizobacteria infection (P < 0.01 or P < 0.001) on food utilization of M. separata fed on both Bt and non-Bt maize infected with A. brasilense and A. chroococcum at both CO2 levels in both years of the study (Table 7).
Table 7. Four-way ANCOVA on the food utilization indices of Mythimna separata fed on Bt maize and non-Bt maize infected with A. brasilense and A. chroococcum under ambient and elevated CO2 in 2016 and 2017.
| Impact factors | The 3rd to 6th instar larvae (n = 828) | ||||
|---|---|---|---|---|---|
| RGR (mg g−1day−1) | RCR (mg g−1day−1) | AD (%) | ECD (%) | ECI (%) | |
| Covariatee | 1.81/0.11 | 3.83/0.067 | 0.781/0.23 | 0.580/0.41 | 0.87/0.32 |
| Ya | 1.19/0.31 | 3.96/0.12 | 4.56/0.061 | 4.82/0.059 | 5.80/0.053 |
| Cv.b | 1545.53/<0.001*** | 302.67/0.<0.001*** | 185.62/0.<0.001*** | 716.17/0.<0.001*** | 1038.95/0.<0.001*** |
| CO2c | 67.09/<0.001*** | 27.98/<0.001*** | 9.69/0.003** | 35.90/<0.001*** | 57.98/<0.001*** |
| Rhizobacteriad | 12.26/<0.001*** | 26.84/<0.001*** | 35.28/<0.001*** | 13.64/<0.001*** | 7.22/0.002** |
| Y × Cv. | 4.27/0.049* | 5.69/0.021* | 1.86/0.18 | 19.21/<0.001*** | 15.64/<0.001*** |
| Y × CO2 | 6.90/0.012* | 4.82/0.033* | 6.73/0.013* | 6.22/0.016* | 3.41/0.071 |
| Y × Rhizobacteria | 5.04/0.010* | 0.17/0.84 | 0.27/0.77 | 0.436/0.65 | 0.25/0.78 |
| Cv. × CO2 | 30.44/<0.001*** | 7.39/0.009** | 1.40/0.043* | 1.80/0.017* | 1.61/0.011* |
| Cv. × Rhizobacteria | 213.46/<0.001*** | 48.31/<0.001*** | 73.42/<0.001*** | 132.82/<0.001*** | 144.91/<0.001*** |
| CO2 × Rhizobacteria | 13.25/<0.001*** | 6.70/0.003** | 9.78/<0.001*** | 13.01/<0.001*** | 12.63/<0.001*** |
| Y × Cv. × CO2 | 0.220/0.64 | 1.83/0.18 | 4.16/0.047* | 0.743/0.39 | 0.56/0.46 |
| Y × Cv. × Rhizobacteria | 3.27/0.047* | 0.55/0.58 | 2.23/0.12 | 4.01/0.025* | 1.41/0.25 |
| Y × CO2 × Rhizobacteria | 0.72/0.49 | 1.88/0.16 | 1.33/0.28 | 2.66/0.080 | 2.47/0.095 |
| Cv. × CO2 × Rhizobacteria | 0.62/0.043* | 13.24/<0.001*** | 9.84/<0.001*** | 9.59/<0.001*** | 9.92/<0.001*** |
| Y × Cv. × CO2 × Rhizobacteria | 0.48/0.62 | 0.087/0.92 | 0.98/0.38 | 0.59/0.56 | 0.06/0.94 |
Notes:
P < 0.05;
P < 0.01;
P < 0.001.
Year (2016 vs. 2017).
Transgenic treatment (Bt maize vs. non-Bt maize).
CO2 levels (elevated CO2 vs. ambient CO2).
Rhizobacteria infection (A. brasilense and A. chroococcum vs. the control buffer), the same as in Tables 4 and 5.
Initial weight as a covariate for RGR and RCR, and food consumption as a covariate for AD and ECI, and food assimilated as a covariate for ECD.
Compared with ambient CO2, elevated CO2 significantly reduced the RGR (−9.95%), ECD (−16.05%), and ECI (−17.95%), and significantly enhanced the RCR (+10.44%) and AD (+5.59%) of M. separata (P < 0.05; Table 8). Compared with the CK, rhizobacteria infection with A. brasilense and A. chroococcum both significantly decreased the ECD (−9.28% and −7.48%) and ECI (−9.22% and −7.91%), and significantly increased the RGR (+4.75% and +5.56%), RCR (+6.78% and +7.53%) and AD (+5.28% and +4.93%) in M. separata (P < 0.01; Table 8). Moreover, significant decreases in RGR (−13.85%), ECD (−41.25%) and ECI (−31.97%), and significant increases in RCR (+16.60%) and AD (+7.88%) were found when M. separata fed on Bt maize compared to that on non-Bt maize (P < 0.05; Table 8).
Table 8. The food utilization of M. separata larvae fed on Bt maize and non-Bt maize during the heading stage, infected with rhizobacterias under ambient and elevated CO2 in 2016 and 2017.
| Impact factors | Factor levels | RGR (mg g−1day−1) | RCR (mg g−1day−1) | AD (%) | ECD (%) | ECI (%) |
|---|---|---|---|---|---|---|
| Cv. | Bt | 82.79 ± 6.43b | 1636.31 ± 13.23a | 56.13 ± 0.72a | 9.26 ± 0.98b | 5.13 ± 0.57b |
| Xy | 94.26 ± 7.16a | 1403.36 ± 14.80b | 52.03 ± 0.69b | 13.08 ± 1.23a | 6.77 ± 0.66a | |
| CO2 | Elevated | 84.33 ± 8.67b | 1595.24 ± 16.14a | 55.55 ± 0.87a | 10.34 ± 1.52b | 5.46 ± 0.76b |
| Ambient | 92.72 ± 6.59a | 1444.43 ± 15.01b | 52.61 ± 0.81b | 12.00 ± 1.21a | 6.44 ± 0.63a | |
| Rhizobacteria infection | AB | 89.64 ± 5.52a | 1549.07 ± 9.32a | 55.06 ± 0.85a | 10.78 ± 1.35b | 5.75 ± 0.67b |
| AC | 90.34 ± 5.52a | 1559.90 ± 9.32a | 54.88 ± 0.85a | 10.96 ± 1.35b | 5.82 ± 0.67b | |
| CK | 85.58 ± 6.14b | 1450.73 ± 9.54b | 52.30 ± 0.62b | 11.78 ± 1.13a | 6.28 ± 0.66a |
Interactive influence of CO2 level, transgenic treatment, and rhizobacteria infection on growth, development and reproduction of M. separata
In addition to the significant main effects of CO2 level, transgenic treatment, and rhizobacteria infection, there were significant two-way and three-way interaction of these three main effects on larval life-span, pupation rate, pupal weight and duration, adult longevity, and fecundity of M. separata fed on Bt and non-Bt maize infected with A. brasilense and A. chroococcum under both CO2 levels in both years of the study (P < 0.05, P < 0.01 or P < 0.001; Table 5).
Transgenic treatment × CO2
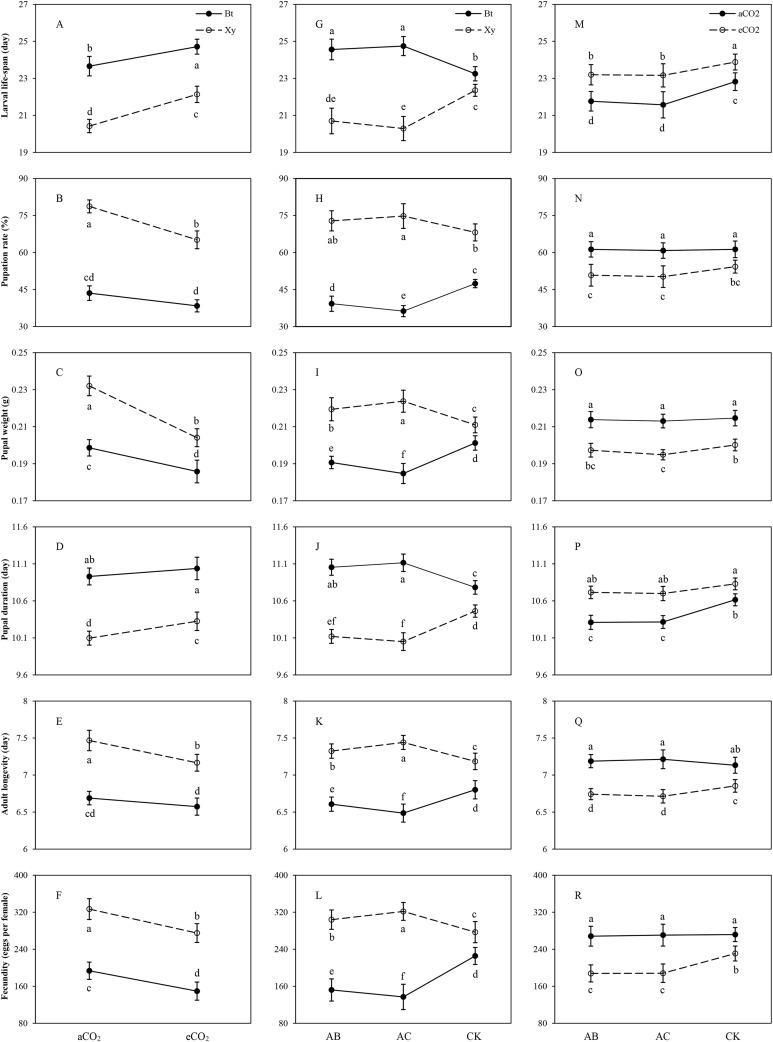
Similar trends were found in the measured growth, development and reproduction indexes of M. separata fed on both Bt and non-Bt maize cultivars grown under elevated CO2 in contrast to ambient CO2, infected with A. brasilense (AB) and A. chroococcum (AC) as well as the CK in 2016 and 2017 (Figs. 1A–1F). Compared with ambient CO2, elevated CO2 significantly prolonged the larval life-span (Bt maize: +6.44%; non-Bt maize: +8.39%) and pupal duration (non-Bt maize: +7.27%) and shortened the adult longevity (non-Bt maize: −6.19%), and significantly decreased the pupation rate (non-Bt maize: −20.81%), pupal weight (Bt maize: −7.03%; non-Bt maize: −13.73%) and fecundity (Bt maize: −29.43%; non-Bt maize: −18.85%) when M. separata fed on Bt maize and non-Bt maize (P < 0.05; Figs. 1A–1F).
Figure 1. Effects of bi-interactions between transgenic treatment and CO2, between transgenic treatment and rhizobacteria and between CO2 and rhizobacteria on development and reproduction of Mythimna separata.
Larval life-span–(A), (G), (M); Pupation rate–(B), (H), (N); Pupal weight–(C), (I), (O); Pupal duration–(D), (J), (P); Adult longevity–(E), (K), (Q); Fecundity–(F), (L), (R); Each value represents the average (±SE). Different lowercase letters indicate significant differences treatments by the Duncan test at P < 0.05.
Transgenic treatment × Rhizobacteria
An inverse trend was found in the measured growth, development and reproduction indexes of M. separata fed on Bt maize and non-Bt maize, which were infected with A. brasilense (AB) and A. chroococcum (AC) under ambient and elevated CO2 in 2016 and 2017 (Figs. 1G–1L). Compared with the CK, rhizobacteria infection significantly prolonged the larval life-span (AB: +7.63%; AC: +8.45%), pupal duration (AB: +4.53%; AC: +5.08%) and shortened the adult longevity (AB: −4.88%; AC: −6.94%), and decreased pupation rate (AB: −20.83%; AC: −30.81%), pupal weight (AB: −7.24%; AC: −10.65%) and fecundity (AB: −48.36%; AC: −64.60%) when M. separata larvae fed on Bt maize (P < 0.01; Figs. 1G–1L); and rhizobacteria infection significantly shortened the larval life-span (AB: −7.97%; AC: −10.15%), pupal duration (AB: −5.36%; AC: −6.08%) and prolonged the adult longevity (AB: +3.95%; AC: +5.62%), and increased pupation rate (AC: +9.73%), pupal weight (AB: +6.27%; AC: +8.16%) and fecundity (AB: +9.75%; AC: +16.16%) when M. separata larvae fed on non-Bt maize (P < 0.01; Figs. 1G–1L).
CO2 × Rhizobacteria
Similar trends were found in the larval life-span, pupation rate, pupal weight, and pupal duration, while inverse trends were observed in adult longevity and fecundity of M. separata under ambient and elevated CO2, which fed on Bt maize vs. non-Bt maize infected with A. brasilense (AB) and A. chroococcum (AC) as well as the CK in 2016 and 2017 (Figs. 1M–1R). Compared with the CK, rhizobacteria infection significantly shortened the larval life-span (AB: −6.92%; AC: −7.84%) and pupal duration (AB: −5.01%; AC: −5.01%) of M. separata under ambient CO2, and significantly shortened the larval life-span (AB: −4.98%; AC: −5.11%) and decreased the pupal weight (AC: −4.77%) under elevated CO2 (P < 0.05; Figs. 1M–1P); and rhizobacteria infection significantly decreased the adult longevity (AB: −4.63%; AC: −5.09%) and fecundity (AB: −22.90%; AC: −22.58%) of M. separata under elevated CO2 (P < 0.05; Figs. 1Q and 1R).
Transgenic treatment × CO2 × Rhizobacteria
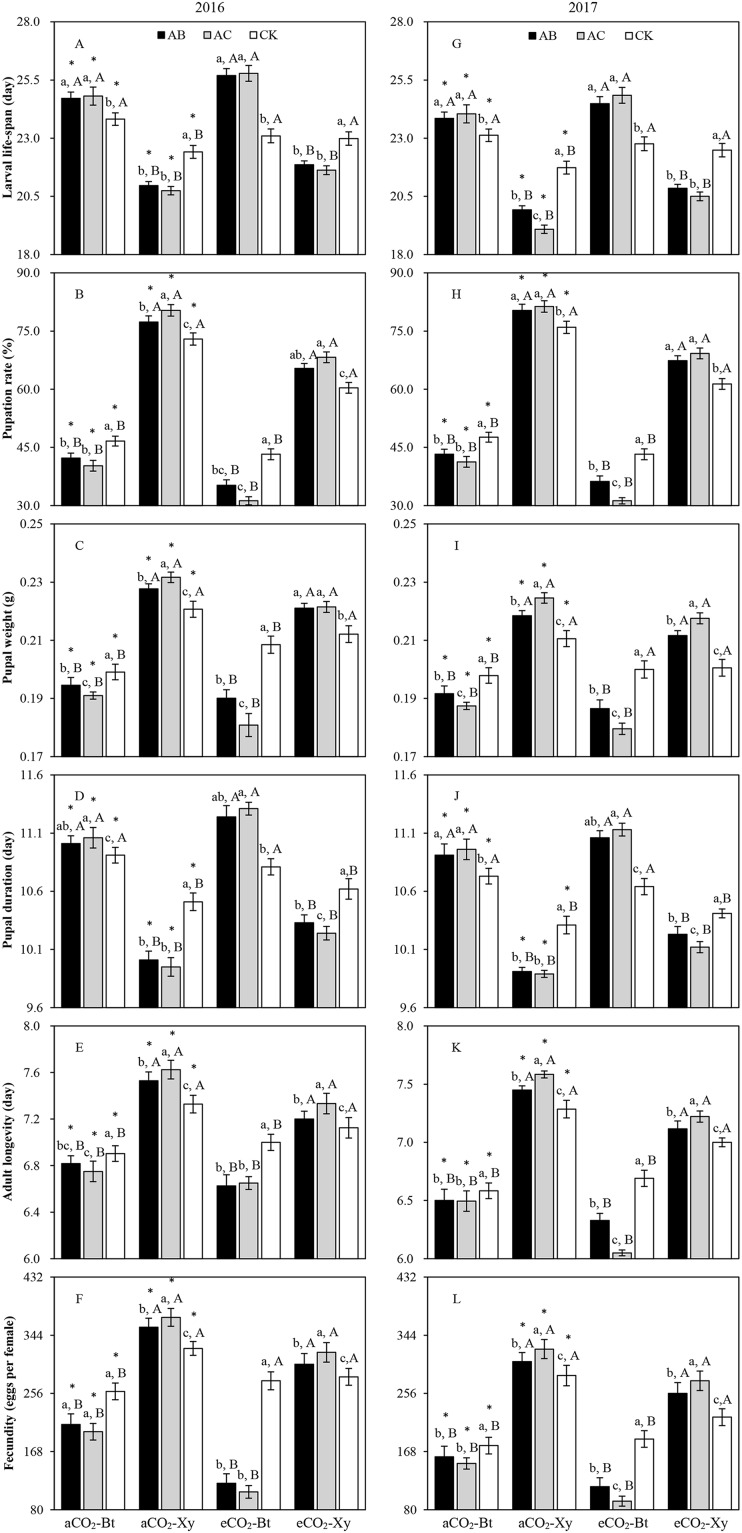
There were opposite trends in the measured growth, development and reproduction indexes of M. separata fed on Bt maize and non-Bt maize infected with A. brasilense (AB) and A. chroococcum (AC) compared with the CK in 2016 and 2017 regardless of CO2 level (Fig. 2). In comparison with the CK, rhizobacteria infection with A. brasilense and A. chroococcum both significantly prolonged the larval life-span and pupal duration of M. separata fed on Bt maize, and significantly shortened the larval life-span and pupal duration of M. separata fed on non-Bt maize under the same CO2 level; and rhizobacteria infection with A. brasilense and A. chroococcum both significantly reduced the pupation rate, pupal weight, adult longevity and fecundity of M. separata fed on Bt maize, and significantly enhanced the pupation rate, pupal weight, adult longevity and fecundity of M. separata fed on non-Bt maize under the same CO2 level. Moreover, compared with ambient CO2, there were opposite trends in the larval life-span, pupal weight, pupal duration, adult longevity and fecundity of M. separata fed on Bt maize infected with A. brasilense and A. chroococcum compared with the CK under elevated CO2 in both years; compared with ambient CO2, elevated CO2 significantly decreased the pupation rate of M. separata fed on Bt maize, and decreased the pupation rate, pupal weight, adult longevity and fecundity of M. separata fed on non-Bt maize, and prolonged the larval life-span and pupal duration of M. separata fed on non-Bt maize infected with A. brasilense and A. chroococcum compared with the CK in both years.
Figure 2. Impacts of the tri-interactions among CO2 level, transgenic treatment, and rhizobacteria infection on the growth, development and reproduction of M. separata in 2016 (A–F) and 2017 (G–L).
Each value represents the average (+SE). Different lowercase and uppercase letters, and *indicated significant difference among three types of rhizobacteria infection for same type of maize under same CO2 level, between Bt maize and non-Bt maize for same type of rhizobacteria infection under same CO2 level, and between ambient and elevated CO2 for same type of maize and rhizobacteria infection by the Duncan test at P < 0.05 respectively.
Interactive effects of CO2 level, transgenic treatment, and rhizobacteria infection on food utilization of M. separata
In addition to significant main effects of CO2 level, transgenic treatment, and rhizobacteria infection, two- and three-way interactions of these factors influenced the RGR, RCR, AD, ECD, and ECI of M. separata larvae fed on Bt maize and non-Bt maize infected with A. brasilense and A. chroococcum under ambient and elevated CO2 in both years (P < 0.05, P < 0.01 or P < 0.001; Table 7).
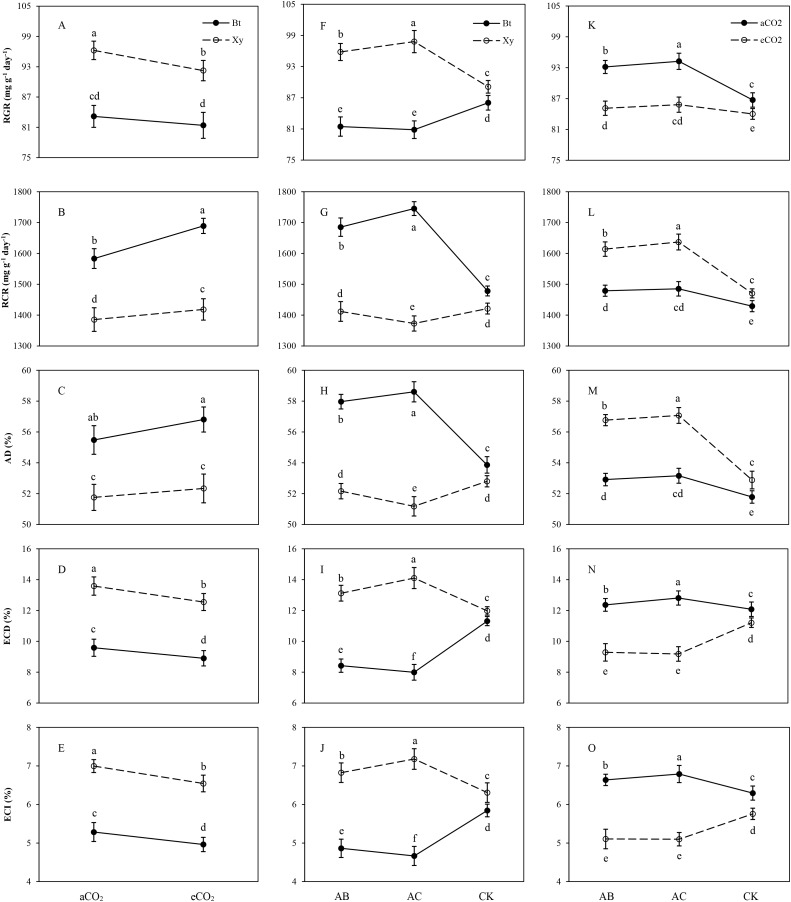
Transgenic treatment × CO2
Similar trends were found in the measured food utilization indexes of M. separata fed on Bt maize (Bt) and non-Bt maize (Xy) grown under elevated CO2 in contrast to ambient CO2, infected with A. brasilense (AB) and A. chroococcum (AC) as well as the CK in 2016 and 2017 (Figs. 3A–3E). Compared with ambient CO2, elevated CO2 significantly decreased the RGR (non-Bt maize: −7.34%), ECD (Bt maize: −9.67%; non-Bt maize: −10.25%) and ECI (Bt maize: −8.53%; non-Bt maize: −8.89%), and significantly increased the RCR (Bt maize: +9.69%; non-Bt maize: +6.37%) when M. separata larvae fed on Bt maize and non-Bt maize (P < 0.05; Figs. 3A–3E).
Figure 3. Effects of bi-interactions between transgenic treatment and CO2, between transgenic treatment and rhizobacteria and between CO2 and rhizobacteria on food utilization of Mythimna separata larvae.
RGR–(A), (F), (K); RCR–(B), (G), (L); AD–(C), (H), (M); ECD–(D), (I), (N); ECI–(E), (J), (O); Each value represents the average (±SE). Different lowercase letters indicate significant differences treatments by the Duncan test at P < 0.05.
Transgenic treatment × Rhizobacteria
Inverse trend was found in the measured food utilization indexes of M. separata fed on Bt maize and non-Bt maize, which were infected with A. brasilense (AB) and A. chroococcum (AC) under ambient and elevated CO2 in 2016 and 2017 (Figs. 3F–3J). Compared with the CK, rhizobacteria infection significantly enhanced the RGR (AB: +9.53%; AC: +11.78%), ECD (AB: +11.61%; AC: +19.79%) and ECI (AB: +10.08%; AC: +15.79%), and significantly decreased the RCR (AC: −6.52%) and AD (AC: −6.19%) when M. separata larvae fed on non-Bt maize (P < 0.001; Figs. 3F–3J); and rhizobacteria infection significantly decreased the RGR (AB: −9.62%; AC: −10.41%), ECD (AB: −34.32%; AC: −41.55%) and ECI (AB: −20.16%; AC: −25.28%), and significantly increased the RCR (AB: +14.99%; AC: +19.06%) and AD (AB: +9.60%; AC: +10.79%) when M. separata larvae fed on Bt maize (P < 0.001; Figs. 3F–3J).
CO2 × Rhizobacteria
Similar trends were observed in RGR, RCR, and AD, while inverse trends were shown in ECD and ECI of M. separata under ambient and elevated CO2, which fed on Bt maize and non-Bt maize infected with A. brasilense (AB) and A. chroococcum (AC) vs. CK (Figs. 3K–3O). Compared with the CK, rhizobacteria infection significantly decreased ECD (AB: −20.71%; AC: −22.07%) and ECI (AB: −12.77%; AC: −12.89%) of M. separata larvae under elevated CO2, and significantly increased ECD (AB: +5.35%; AC: +8.04%) and ECI (AB: +7.43%; AC: +9.85%) of M. separata larvae under ambient CO2 (P < 0.05; Fig. 3); and rhizobacteria infection significantly enhanced RGR (AB: +3.32% and +7.40%; AC: +5.14% and +8.67%), RCR (AB: +9.78% and +5.29%,; AC: +11.32% and +5.93%) and AD (AB: +7.34% and +4.18%; AC: +7.92% and +4.66%) under elevated and ambient CO2, respectively (P < 0.01; Figs. 3K–3O).
Transgenic treatment × CO2 × Rhizobacteria
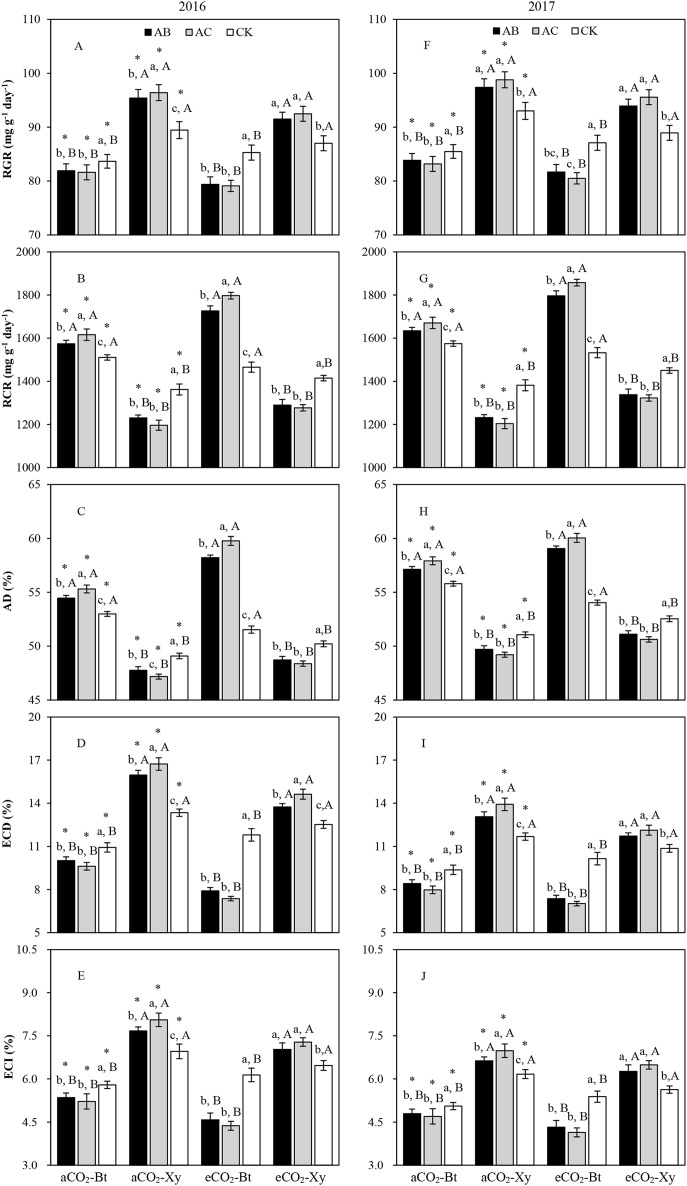
There were opposite trends in the measured food utilization indexes of M. separata larvae fed on Bt maize (Bt) and non-Bt maize infected with A. brasilense (AB) and A. chroococcum (AC) compared with the CK in both years regardless of CO2 level (Fig. 4). In comparison with the CK, rhizobacteria infection with A. brasilense and A. chroococcum both significantly decreased RGR, ECD, and ECI of M. separata fed on Bt maize, and significantly increased RGR, ECD, and ECI of M. separata fed on non-Bt maize under the same CO2 level; and rhizobacteria infection with A. brasilense and A. chroococcum both significantly enhanced RCR and AD of M. separata fed on Bt maize, and significantly reduced RCR and AD of M. separata larvae fed on non-Bt maize under the same CO2 level. Moreover, compared with ambient CO2, elevated CO2 significantly increased RCR and AD, and significantly decreased RGR, ECD, and ECI of M. separata larvae fed on same type of maize cultivar infected with A. brasilense and A. chroococcum in both years (P < 0.05; Fig. 4). Furthermore, there were significant decreases in RGR, ECD, and ECI, and significant increases in RCR and AD of M. separata larvae fed on Bt maize in contrast to non-Bt maize infected with same type of rhizobacteria species within the same CO2 level in both years.
Figure 4. Impacts of the tri-interactions among CO2, transgenic treatment, and rhizobacteria infection on the food utilization of M. separata from the third to the sixth instar larvae in 2016 (A–E) and 2017 (F–J).
Each value represents the average (+SE). Different lowercase and uppercase letters, and *indicated significant difference among three types of rhizobacteria infection for same type of maize under same CO2 level, between Bt maize and non-Bt maize for same type of rhizobacteria infection under same CO2 level, and between ambient and elevated CO2 for same type of maize and rhizobacteria infection by the Duncan test at P < 0.05 respectively.
Discussion
Insects are sensitive to environmental variations, and environmental stresses can cause changes on their growth, development, fecundity, food utilization and the occurrence and distribution of populations as a result of metabolic rate fluctuation (Bloom et al., 2010). In this study, elevated CO2 significantly prolonged larval and pupal duration and decreased pupation rate and pupal weight of M. separata compared to ambient CO2. Elevated CO2 negatively affected the larval survival, weight, duration, pupation, and adult emergence of cotton bollworm, H. armigera (Akbar et al., 2016), and reduced the egg laying by Cactus moth Cactoblastis cactorum (Stange, 1997) and Achaea Janata (Rao et al., 2013). In this study, elevated CO2 significantly increased the RCR (+10.44%) and the AD (+5.59%) (i.e., AD), and significantly reduced the RGR (−9.95%), ECD (−16.05%) and ECI (−17.95%) of M. separata larvae compared with ambient CO2. RGRs of Gypsy moth (Lymantria dispar) were reported to be reduced by 30% in larvae fed on Quercus petraea exposed to elevated CO2 (Hattenschwiler & Schafellner, 2004). RCR was significantly higher for H. armigera larva fed maize grown at 375 and 750 ppm CO2 in contrast to ambient CO2 condition, and elevated CO2 significantly decreased the ECI food, the ECD food, and the RGR of H. armigera larvae compared with ambient CO2 (Yin et al., 2010). According to the “Nutrition compensation hypothesis,” elevated CO2 can affect the development fitness of herbivores by changing the nutritional components, above and below-ground biomass, and photosynthetic rate of host plants indirectly (Ainsworth & Rogers, 2007; Jackson et al., 2009; Zavala, Nabity & Delucia, 2013), including increased C/N ratio and decreased nitrogen content etc. Declined growth rate, reproduction, and survival rate were found in the chewing mouthparts insects (e.g., H. armigera, Spodoptera exigua, M. separata), and the food consumption of which increased so that they could obtain necessary nutrition to survive (Bottomley, Rogers & Prior, 1993; Rogers et al., 2006). Yin et al. (2010) reported that elevated CO2 increased the food consumption and prolonged the development time of H. armigera, which due to the reduced nutritional quality of maize leaves, as a result of reduced nitrogen content and increased C/N ratio. Elevated CO2 significantly reduced the food conversion rate and enhanced the food ingestion of H. armigera, which attribute to reduced nitrogen content of the cotton, Simian-3 (Chen, Ge & Parajulee, 2005a; Chen et al., 2005b). Thus, Chen, Ge & Parajulee (2005a) and Chen et al. (2005b) inferred that elevated CO2 might be unfavorable to H. armigera. Our results in maize system appear to be similar to the study by Chen, Ge & Parajulee (2005a) and Chen et al. (2005b) in a cotton system.
Although the transgenic corn, Zea mays L., hybrids expressing the Cry insecticidal protein from Bacillus thuringiensis (Bt) were developed to control H. zea, O. nubilalis, S. frugiperda, and M. separata (Koziel et al., 1993; Armstrong et al., 1995; Jouanin et al., 1998; Lynch, Plaisted & Warnick, 1999), few studies focused on the defense responses of transgenic cry1Ie maize to corn armyworm under elevated CO2, especially on the growth, development and food utilization of the pest insects. Prutz & Dettner (2005) reported that the transgenic Bacillus thuringiensis-maize could result in decreased growth rate and increased mortality, which might attribute to the termination of larval metamorphosis. Most studies showed that adverse effects on life-table parameters of different herbivores were direct by the Cry protein (Lawo, Wäckers & Romeis, 2010), which might be due to the interaction of feeding inhibitors and growth inhibitors (e.g., secondary plant substances) (Smith & Fischer, 1983). Effects of elevated CO2 on the plant nutrition, metabolism and secondary defense metabolism might adverse for the growth, development and nutrition utilization of herbivores (Akbar et al., 2016). The insects possessed more nutrients to meet their growth needs and prolong the food digestion time in the midgut so that the RCR and AD increased (Reynolds, Nottingham & Stephens, 1985). In this study, we found that some negative effects of transgenic cry1Ie maize (Bt) and Xianyu 335 (Xy) grown in elevated CO2 on the food utilization indices (including RGR, ECD, and ECI) of M. separata larvae and some positive effects on the RCR and AD, which indicated that the resistance responses of Bt maize might persist under elevated CO2, and M. separata might ingest more food to get enough nutrition for surviving in limited developmental time under elevated CO2. Meanwhile the Bt maize and its parental line (Xianyu 335) prolonged their larval life-span and pupal duration, decreased growth rate and increased mortality that might result in lowering of pests’ occurrence. According to the “carbon nutrition balance hypothesis” (Gebauer, Strain & Reynolds, 1997), elevated CO2 would increase the fixed organic matter in plant while increase C-based secondary metabolites and decrease N-based secondary metabolites, thus affecting the insects resistance of plants. Robinson, Ryan & Newman (2012) indicated that elevated CO2 increased 19% phenols, 22% condensed tannins, and 27% flavonoids, while the terpenoids and NBSC decreased by 13% and 16% respectively. Coviella, Stipanovic & Trumble (2002) anticipated that the primary CO2 effect on Bt toxin production would be due to differences in N concentration within the plant. In a meta-analytical review of 33 studies that simultaneously increased CO2 conditions compared to ambient conditions, Zvereva & Kozlov (2006) showed that nitrogen concentration in plants was reduced under elevated CO2, and this decrease was stronger for woody compared to herbaceous plants. If conditions of increased carbon (e.g., elevated CO2) allow plants to allocate significantly more resources to condensed tannins and gossypol, then the enzyme composition in the insect herbivore is expected to also change. Similarly, if Bt toxin production changes due to elevated CO2, then the insect herbivore’s body enzymes should also be changed in this circumstance.
Most of the nitrogen, however, is found in the form of N2 which approximately amounts to 78% in the atmosphere. As plants cannot use this form of nitrogen directly, some microbes can change the N2 into ammonia. Most free living microbes in soil which can fix nitrogen and whose activities in enhancing the growth of plants are bacteria namely Azotobacter sp. and Azospirillum sp. These two bacteria are particularly important in maize production system due to their greater nitrogen fixing ability. Azospirillum acquires carbohydrate directly from sieve tube as a resource of carbon which promotes its growth (Olivera et al., 2004). Azospirillum can be used to promote the growth of sprouts under normal and arid conditions (Alejandra et al., 2009). Azospirillum also provides more flexibility to cell wall which enhances the growth (Pereyra et al., 2010) and increases products of wheat in waterless plot of land (Martin, 2009). Furthermore, azospirillum had the highest efficiency in nitrogen fixation at the root of sweet corn and it would reach the highest point of nitrogen fixation in the week 4 amounting to 0.20 mgNhr−1m−2 (Toopakuntho, 2010). Azospirillum can also create auxin, a substance promoting growth of maize, of 53.57 mg/ml (Phookkasem, 2011). Therefore, we used techniques of rhizobacteria (A. brasilense and A. chroococcum) inoculation of maize seeds to stimulate plant N uptake to increase in biomass N relative to C under elevated CO2, increase Bt toxin production for transgenic cry1Ie maize and create a substance promoting maize plant growth. In this study, we found that elevated CO2 significantly enhanced the rhizosphere soil densities both A. brasilense and A. chroococcum at the maize harvest, but there was no significant difference of the rhizosphere soil densities both A. brasilense and A. chroococcum between elevated and ambient CO2 at the maize seedling after 14 days. We hypothesize that the elevated CO2 increased the maize root bifurcation and soil nutrition (e.g., carbohydrates, amino acids and multi-trace elements) for rhizobacteria to provide the living space and nutrition with a long-time environmental effect. Other researchers have also shown positive effects of elevated CO2 on the bacterial community in the rhizosphere of maize (Chen et al., 2012). Moreover, significant adverse effects on the growth, development, reproduction, and food utilization of M. separata were observed when the host substrate maize was exposed to rhizobacteria treatments, which might be attributed to rhizobacteria stimulating plant N uptake to increase Bt toxin production for transgenic cry1Ie maize and promoting growth of its parental line (Xianyu 335) (Olivera et al., 2004; Stitt & Krapp, 1999).
There was no significant year-to-year variation in our field research data. Therefore, the overall results clearly indicate that increasing CO2 had negative effects on M. separata. Resistance performance of transgenic cry1Ie maize decreased under elevated CO2 as shown by decreased RGR, ECD, and ECI. The rhizobacteria treatments (A. brasilense and A. chroococcum) had positive effects on improving the effectiveness of Bt maize on target Lepidoptera pest management via decreased RGR, ECD, and ECI of M. separata that fed on transgenic cry1Ie maize and promoting growth of Xianyu 335 via increased RGR, ECD, and ECI of M. separata. Under future predicted climate changes (e.g., elevated CO2), it is particularly important to understand the field insect resistance traits of resistant crops to target pests. In an environment of accelerated greenhouse effect, Bt maize may have decreased resistance performance in the field with inhibiting effect on the development and food utilization of insects. Therefore, we used techniques of rhizobacteria (A. brasilense & A. chroococcum) inoculation of maize seeds to stimulate plant N uptake to increase in biomass N relative to C under elevated CO2, increase Bt toxin production for transgenic cry1Ie maize, and create a substance promoting maize growth.
Conclusion
Overall, our results indicated that elevated CO2 and Bt maize were negative against development and food utilization of M. separata. Rhizobacteria infection significantly increased the larval life-span, pupal duration, RCR and AD of M. separata, and significantly decreased RGR, ECD and ECI of M. separata fed on Bt maize; there were opposite trends in development and food utilization of M. separata fed on non-Bt maize infected with rhizobacterias compared with the CK in 2016 and 2017 regardless of CO2 level. This study demonstrates that the use of rhizobacteria (e.g., A. brasilense and A. chroococcum) as pest control enhancer especially under elevated CO2 is significantly more beneficial in transgenic Bt maize system compared to that in non-transgenic system. Rhizobacteria (A. brasilense & A. chroococcum), as being one potential biological regulator to enhance nitrogen utilization efficiency of crops, could make the Bt maize facing lower field hazards from the target pest of M. separate, and finally improve the sustainability and resistance of Bt maize against target lepidoptera pests, especially under future CO2 raising.
Supplemental Information
Funding Statement
This research was funded by the National Nature Science Foundations of China (NSFC) (31272051), the National Key Research and Development Program of China (2017YFD0200400), the Special Program for New Transgenic Variety Breeding of the Ministry of Science and Technology, China (2016ZX08012005), and the Research Grant from the Innovation Project for Graduate Student of Jiangsu Province (KYLX16_1063). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Zhuo Li performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Megha N. Parajulee conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Fajun Chen conceived and designed the experiments, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Ainsworth & Rogers (2007).Ainsworth EA, Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment. 2007;30(3):258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth et al. (2007).Ainsworth EA, Rogers A, Leakey ADB, Heady LE, Gibon Y, Stitt M, Schurr U. Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? Journal of Experimental Botany. 2007;58(3):579–591. doi: 10.1093/jxb/erl233. [DOI] [PubMed] [Google Scholar]
- Akbar et al. (2016).Akbar SM, Pavani T, Nagaraja T, Sharma HC. Influence of CO2 and temperature on metabolism and development of Helicoverpa armigera (Noctuidae: Lepidoptera) Environmental Entomology. 2016;45(1):229–236. doi: 10.1093/ee/nvv144. [DOI] [PubMed] [Google Scholar]
- Alejandra et al. (2009).Alejandra PM, Ballesteros FM, Creus CM, Sueldo RJ, Barassi CA. Seedling growth promotion by Azospirillum brasilense under normal and drought conditions remains unaltered in Tebuconazole-treated wheat seeds. European Journal of Soil Biology. 2009;45(1):20–27. doi: 10.1016/j.ejsobi.2008.09.015. [DOI] [Google Scholar]
- Ali et al. (2016).Ali A, Rashid MA, Huang QY, Lei CL. Effect of UV-A radiation as an environmental stress on the development, longevity, and reproduction of the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) Environmental Science & Pollution Research. 2016;23(17):17002–17007. doi: 10.1007/s11356-016-6865-0. [DOI] [PubMed] [Google Scholar]
- Armstrong et al. (1995).Armstrong CL, Parker GB, Pershing JC, Brown SM, Sanders PR, Duncan DR, Stone T, Dean DA, DeBoer DL, Hart J, Howe AR, Morrish FM, Pajeau ME, Petersen WL, Reich BJ, Rodriguez R, Santino CG, Sato SJ, Schuler W, Sims SR, Stehling S, Tarochione LJ, Fromm ME. Field evaluation of European corn borer control in progeny of 173 transgenic corn events expressing an insecticidal protein from Bacillus thuringiensis. Crop Science. 1995;35(2):550–557. doi: 10.2135/cropsci1995.0011183X003500020045x. [DOI] [Google Scholar]
- Biari, Gholami & Rahmani (2008).Biari A, Gholami A, Rahmani HA. Growth promotion and enhanced nutrient uptake of maize (Zea mays L.) by application of plant growth promoting rhizobacteria in arid region of Iran. Journal of Biological Sciences. 2008;8(6):1015–1020. doi: 10.3923/jbs.2008.1015.1020. [DOI] [Google Scholar]
- Bi (1981).Bi FC. A new artificial diet of Mythimna separate. Acta Entomologica Sinica. 1981;24(4):379–383. doi: 10.16380/j.kcxb.1981.04.006. [DOI] [Google Scholar]
- Bloom et al. (2010).Bloom AJ, Burger M, Asensio JSR, Cousins AB. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science. 2010;328(5980):899–903. doi: 10.1126/science.1186440. [DOI] [PubMed] [Google Scholar]
- Bottomley, Rogers & Prior (1993).Bottomley PA, Rogers HH, Prior SA. NMR imaging of root water distribution in intact Vicia faba L plants in elevated atmospheric CO2. Plant, Cell and Environment. 1993;16(3):335–338. doi: 10.1111/j.1365-3040.1993.tb00878.x. [DOI] [Google Scholar]
- Carrière, Crowder & Tabashnik (2010).Carrière Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evolutionary Applications. 2010;3(5–6):561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassán et al. (2009).Cassán F, Perrig D, Sgroy V, Masciarellia O, Pennab C, Lunaa V. Azospirillum brasilense Az39 and Bradyrhizobium japonicum E109, inoculated singly or in combination, promote seed germination and early seedling growth in corn (Zea mays L.) and soybean (Glycine max L.) European Journal of Soil Biology. 2009;45(1):28–35. doi: 10.1016/j.ejsobi.2008.08.005. [DOI] [Google Scholar]
- Cattaneo et al. (2006).Cattaneo MG, Yafuso C, Schmidt C, Huang CY, Rahman M, Olson C, Ellers-Kirk C, Orr BJ, Marsh SE, Antilla L, Dutilleul P, Carrière Y. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(20):7571–7576. doi: 10.1073/pnas.0508312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Ge & Parajulee (2005a).Chen FJ, Ge F, Parajulee MN. Impact of elevated CO2 on tri-trophic interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environmental Entomology. 2005a;34(1):37–46. doi: 10.1603/0046-225X-34.1.37. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2012).Chen SN, Gu J, Fu QX, Sun W, Qian X, Gao H, Qing QJ. Effects of inoculating azotobacter on soil enzyme activities and bacterial community functional diversity in the rhizosphere of maize (Zea mays L.) Journal of Plant Nutrition and Fertilizer. 2012;18(2):444–450. [Google Scholar]
- Chen, Wu & Ge (2004).Chen FJ, Wu G, Ge F. Growth, development and reproduction of the cotton bollworm, Helicoverpa armigera (Hübner) reared on milky grains of wheat grown in elevated CO2 concentration. Acta Entomologica Sinica. 2004;47(6):774–779. doi: 10.16380/j.kcxb.2004.06.014. [DOI] [Google Scholar]
- Chen et al. (2011).Chen FJ, Wu G, Ge F, Parajulee MN. Relationships between exogenous-toxin quantity and increased biomass of transgenic Bt crops under elevated carbon dioxide. Ecotoxicology and Environmental Safety. 2011;74(4):1074–1080. doi: 10.1016/j.ecoenv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2005b).Chen FJ, Wu G, Ge F, Parajulee MN, Shrestha RB. Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance, and feeding of an insect herbivore, the cotton bollworm. Entomologia Experimentalis et Applicata. 2005b;115(2):341–350. doi: 10.1111/j.1570-7458.2005.00258.x. [DOI] [Google Scholar]
- Cornelissen (2011).Cornelissen T. Climate change and its effects on terrestrial insects and herbivory patterns. Neotropical Entomology. 2011;40(2):155–163. doi: 10.1590/S1519-566X2011000200001. [DOI] [PubMed] [Google Scholar]
- Coviella, Stipanovic & Trumble (2002).Coviella CE, Stipanovic RD, Trumble JT. Plant allocation to defensive compounds: interactions between elevated CO2 and nitrogen in transgenic cotton plants. Journal of Experimental Botany. 2002;53(367):323–331. doi: 10.1093/jexbot/53.367.323. [DOI] [PubMed] [Google Scholar]
- Ciais et al. (2013).Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Quéré CL, Myneni RB, Piao SL, Thornton P. Carbon and other biogeochemical cycles. Climate change 2013. The physical science basis, working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. 2013. pp. 465–570.
- Gao et al. (2009).Gao HJ, Xiao NW, Li JS, Chen FJ, Zhai BP. Effects of double atmospheric CO2 concentration on nitrogen metabolism of transgenic Bt cotton under different nitrogen fertilization levels. Chinese Journal of Ecology. 2009;28(11):2213–2219. doi: 10.13292/j.1000-4890.2009.0378. [DOI] [Google Scholar]
- Gebauer, Strain & Reynolds (1997).Gebauer RLE, Strain BR, Reynolds JF. The effect of elevated CO2 and N availability on tissue concentrations and whole plant pools of carbon-based secondary compounds in loblolly pine (Pinus taeda) Oecologia. 1997;113(1):29–36. doi: 10.1007/s004420050350. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2016).Guo JF, He KL, Hellmich RL, Bai SX, Zhang TT, Liu YJ, Ahmed T, Wang ZY. Field trials to evaluate the effects of transgenic cry1Ie maize on the community characteristics of arthropod natural enemies. Scientific Reports. 2016;6(1):22102. doi: 10.1038/srep22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägele & Rowell-Rahier (1999).Hägele BF, Rowell-Rahier M. Dietary mixing in three generalist herbivores: nutrient complementation or toxin dilution? Oecologia. 1999;119(4):521–533. doi: 10.1007/s004420050815. [DOI] [PubMed] [Google Scholar]
- Hattenschwiler & Schafellner (2004).Hattenschwiler S, Schafellner C. Gypsy moth feeding in the canopy of a CO2-enriched mature forest. Global Change Climate. 2004;10(11):1899–1908. doi: 10.1111/j.1365-2486.2004.00856.x. [DOI] [Google Scholar]
- Huang et al. (2005).Huang J, Hu R, Rozelle S, Pray C. Insect-resistant GM rice in farmers’ fields: assessing productivity and health effects in China. Science. 2005;308(5722):688–690. doi: 10.1126/science.1108972. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2014).Huang FN, Qureshi JA, Meagher JRL, Reisig DD, Head GP, Andow DA, Ni XZ, Kerns D, Buntin GD, Niu Y, Yang F, Dangal V. Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLOS ONE. 2014;9(11):e112958. doi: 10.1371/journal.pone.0112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison et al. (2010).Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, Abrahamson M, Hamilton KL, Steffey KL, Gray ME, Hellmich RL, Kaster LV, Hunt TE, Wright RJ, Pecinovsky K, Rabaey TL, Flood BR, Raun1 ES. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science. 2010;330(6001):222–225. doi: 10.1126/science.1190242. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) (2014).Intergovernmental Panel on Climate Change (IPCC) Climate Change 2014: Impacts, Adaptation, and Vulnerability. 2014. [Google Scholar]
- Jackson et al. (2009).Jackson RB, Cook CW, Pippen JS, Palmer SM. Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology. 2009;90(12):3352–3366. doi: 10.1890/08-1609.1. [DOI] [PubMed] [Google Scholar]
- Jia et al. (2016).Jia HR, Geng LL, Li YH, Wang Q, Diao QY, Zhou T, Dai PL. The effects of Bt Cry1Ie toxin on bacterial diversity in the midgut of Apis mellifera ligustica (Hymenoptera: Apidae) Scientific Reports. 2016;6(1):24664. doi: 10.1038/srep24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2017).Jiang SL, Lu YQ, Dai Y, Qian L, Muhammad AB, Li T, Wan GJ, Parajulee MN, Chen FJ. Impacts of elevated CO2 on exogenous Bacillus thuringiensis toxins and transgene expression in transgenic rice under different levels of nitrogen. Scientific Reports. 2017;7(1):14716. doi: 10.1038/s41598-017-15321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2016).Jiang XF, Zhang L, Yang HX, Sappington TW, Cheng YX, Luo LZ. Biocontrol of the oriental armyworm, Mythimna separata, by the tachinid fly Exorista civilis is synergized by Cry1Ab protoxin. Scientific Reports. 2016;6(1):26873. doi: 10.1038/srep26873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanin et al. (1998).Jouanin L, Bonade-Bottino M, Girard C, Morrot G, Giband M. Transgenic plants for insect resistance. Plant Science. 1998;131(1):1–11. doi: 10.1016/S0168-9452(97)00239-2. [DOI] [Google Scholar]
- Koziel et al. (1993).Koziel MG, Beland GL, Bowman C, Carozzi NB, Crenshaw R, Crossland L, Dawson J, Desai N, Hill M, Kadwell S, Launis K, Lewis K, Maddox D, McPherson K, Meghji MR, Merlin E, Rhodes R, Warren GW, Wright M, Evola SV. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Nature Biotechnology. 1993;11(2):194–200. doi: 10.1038/nbt0293-194. [DOI] [Google Scholar]
- Lawo, Wäckers & Romeis (2010).Lawo NC, Wäckers FL, Romeis J. Characterizing indirect prey-quality mediated effects of a Bt crop on predatory larvae of the green lacewing, Chrysoperla carnea. Journal of Insect Physiology. 2010;56(11):1702–1710. doi: 10.1016/j.jinsphys.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Lindroth et al. (2001).Lindroth RL, Koppera BJ, Parsonsa WFJ, Bockheimb JG, Karnoskyc DF, Hendreyd GR, Pregitzerc KS, Isebrandse JG, Sober J. Consequences of elevated carbon dioxide and ozone for foliar chemical composition and dynamics in trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera) Environmental Pollution. 2001;115(3):395–404. doi: 10.1016/S0269-7491(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Ling (2010).Ling J. Promotion research of new maize varieties Xianyu 335 in shandong province. Agricultural Technology Service. 2010;27(7):943–944. [Google Scholar]
- Liu et al. (2017).Liu Y, Fu X, Mao L, Xing Z, Wu K. Identification of host plant use of adults of a long-distance migratory insect, Mythimna separata. PLOS ONE. 2017;12(9):e0184116. doi: 10.1371/journal.pone.0184116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2012).Lu YH, Wu KM, Jiang YY, Guo YY, Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487(7407):362–365. doi: 10.1038/nature11153. [DOI] [PubMed] [Google Scholar]
- Lynch, Plaisted & Warnick (1999).Lynch RE, Plaisted WD, Warnick D. Evaluation of transgenic sweet corn hybrids expressing CrylA(b) toxin for resistance to corn earworm and fall armyworm(Lepidoptera: Noctuidae) Journal of Economic Entomology. 1999;92(1):246–252. doi: 10.1093/jee/92.1.246. [DOI] [PubMed] [Google Scholar]
- Martin (2009).Martin DZ. Field performance of a liquid formulation of Azospirillum brasilense on dryland wheat productivity. European Journal of Soil Biology. 2009;45(1):3–11. doi: 10.1016/j.ejsobi.2008.07.001. [DOI] [Google Scholar]
- Massad & Dyer (2010).Massad TJ, Dyer LA. A meta-analysis of the effects of global environmental change on plant-herbivore interactions. Arthropod-Plant Interactions. 2010;4(3):181–188. doi: 10.1007/s11829-010-9102-7. [DOI] [Google Scholar]
- Olivera et al. (2004).Olivera M, Tejera N, Iribarna C, Ocana A, Luch CL. Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Physiologia Plantarum. 2004;121(3):498–505. doi: 10.1111/j.0031-9317.2004.00355.x. [DOI] [Google Scholar]
- O’Neill et al. (2010).O’Neill BF, Zangerl AR, Dermody O, Bilgin DD, Casteel CL, Zavala JA, DeLucia EH, Berenbaum MR. Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus) Journal of Chemical Ecology. 2010;36(1):35–45. doi: 10.1007/s10886-009-9727-0. [DOI] [PubMed] [Google Scholar]
- Pang et al. (2005).Pang J, Zhu JG, Xie ZB, Chen GP, Liu G, Zhang YL. Effects of elevated CO2 on nutrient uptake by rice and nutrient contents in rice grain. Chinese Journal of Rice. 2005;19(4):350–354. [Google Scholar]
- Percy et al. (2002).Percy KE, Awmack CS, Lindroth RL, Kubiske ME, Kopper BJ, Isebrands JG, Pregitzerk KS, Hendrey GR, Dickson RE, Zak DR, Oksanenq E, Soberk J, Harrington R, Karnoskyk DF. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature. 2002;420(6913):403–407. doi: 10.1038/nature01028. [DOI] [PubMed] [Google Scholar]
- Pereyra et al. (2010).Pereyra CM, Ramella NA, Pereyra MA, Barassi CA, Creus CM. Changes in cucumber hypocotyl cell wall dynamics caused by Azospirillum brasilense inoculation. Plant Physiology and Biochemistry. 2010;48(1):62–69. doi: 10.1016/j.plaphy.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Phookkasem (2011).Phookkasem C. Kasetsart University; 2011. Effects of Azospirillum on nitrogen fixation and growth enhancement of maize. Master’s thesis. [Google Scholar]
- Prutz & Dettner (2005).Prutz G, Dettner K. Effects of transgenic Bacillus thuringiensis-maize on larval food consumption, utilization and growth in the grass-moth species Chilo partellus under laboratory conditions (Lepidoptera: Crambidae) Entomologia Generalis. 2005;28(3):161–172. doi: 10.1127/entom.gen/28/2005/161. [DOI] [Google Scholar]
- Rao et al. (2013).Rao MS, Srinivas K, Vanaja M, Manimanjari D, Rao CAR, Venkateswarlu B. Response of multiple generations of semilooper, Archaea janata feeding on castor to elevated CO2. Journal of Environmental Biology. 2013;34(5):877–883. [PubMed] [Google Scholar]
- Raubenheimer & Simpson (1992).Raubenheimer D, Simpson SJ. Analysis of covariance: an alternative to nutritional indices. Entomologia Experimentalis Et Applicata. 1992;62(3):221–231. doi: 10.1111/j.1570-7458.1992.tb00662.x. [DOI] [Google Scholar]
- Reynolds, Nottingham & Stephens (1985).Reynolds SE, Nottingham SF, Stephens AE. Food and water economy and its relation to growth in fifth instar larvae of tobacco hornworm, Manduca sexta. Journal of Insect Physiology. 1985;31(2):119–127. doi: 10.1016/0022-1910(85)90016-2. [DOI] [Google Scholar]
- Richardson et al. (2009).Richardson AE, Barea JM, Mcneill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil. 2009;321(1–2):305–339. doi: 10.1007/s11104-009-9895-2. [DOI] [Google Scholar]
- Robinson, Ryan & Newman (2012).Robinson EA, Ryan GD, Newman JA. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytologist. 2012;194(2):321–336. doi: 10.1111/j.1469-8137.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- Rogers et al. (2006).Rogers A, Gibon V, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP. Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant, Cell and Environment. 2006;29(8):1651–1658. doi: 10.1111/j.1365-3040.2006.01549.x. [DOI] [PubMed] [Google Scholar]
- Smith & Fischer (1983).Smith CM, Fischer NH. Chemical factors of an insect resistant soybean genotype affecting growth and survival of the soybean looper. Entomologia Experimentalis Et Applicata. 1983;33(3):343–345. doi: 10.1111/j.1570-7458.1983.tb03278.x. [DOI] [Google Scholar]
- Stange (1997).Stange G. Effects of changes in atmospheric carbon dioxide on the location of hosts by the moth, Cactoblastis cactorum. Oecologia. 1997;110(4):539–545. doi: 10.1007/s004420050192. [DOI] [PubMed] [Google Scholar]
- Stitt & Krapp (1999).Stitt M, Krapp A. The interactions between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell & Environment. 1999;22(6):583–621. doi: 10.1046/j.1365-3040.1999.00386.x. [DOI] [Google Scholar]
- Toopakuntho (2010).Toopakuntho S. Kasetsart University; 2010. Nitrogen fixation of free living microorganism in no tillage sweet corn cultivation system. M. Sci. thesis. [Google Scholar]
- Trębicki et al. (2017).Trębicki P, Dáder B, Vassiliadis S, Fereres A. Insect-plant-pathogen interactions as shaped by future climate: effects on biology, distribution and implications for agriculture. Insect Science. 2017;24(6):975–989. doi: 10.1111/1744-7917.12531. [DOI] [PubMed] [Google Scholar]
- Walters et al. (2010).Walters FS, Fontes CM, Hart H, Warren GW, Chen JS. Lepidopteran-active variable-region sequence imparts Coleopteran activity in eCry3.1Ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Applied and Environmental Microbiology. 2010;76(10):3082–3088. doi: 10.1128/AEM.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamprai, Mala & Sinma (2014).Yamprai A, Mala T, Sinma K. The study on the fixed nitrogen and nitrogenase activity in the day-round of Azotobacter and Azosprillum grown with maize in KamphaengSaen soil series. Modern Applied Science. 2014;8(6):27–36. doi: 10.5539/mas.v8n6p27. [DOI] [Google Scholar]
- Yin et al. (2010).Yin J, Sun Y, Wu G, Ge F. Effects of elevated CO2 associated with maize on multiple generations of the cotton bollworm, Helicoverpa armigera. Entomologia Experimentalis Et Applicata. 2010;136(1):12–20. doi: 10.1111/j.1570-7458.2010.00998.x. [DOI] [Google Scholar]
- Zavala, Nabity & Delucia (2013).Zavala JA, Nabity PD, Delucia EH. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annual Review of Entomology. 2013;58(1):79–97. doi: 10.1146/annurev-ento-120811-153544. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang YW, Liu YJ, Ren Y, Liu Y, Liang GM, Song FP, Bai SX, Wang JH, Wang GY. Overexpression of a novel Cry1Ie, gene confers resistance to Cry1Ac-resistant cotton bollworm in transgenic lines of maize. Plant Cell, Tissue and Organ Culture. 2013;115(2):151–158. doi: 10.1007/s11240-013-0348-5. [DOI] [Google Scholar]
- Zvereva & Kozlov (2006).Zvereva EL, Kozlov MV. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a meta-analysis. Global Change Biology. 2006;12(1):27–41. doi: 10.1111/j.1365-2486.2005.01086.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.