Abstract
Background
Breast cancer patients with estrogen receptor (ER)–positive disease have a continuous long-term risk for fatal breast cancer, but the biological factors influencing this risk are unknown. We aimed to determine whether high intratumor heterogeneity of ER predicts an increased long-term risk (25 years) of fatal breast cancer.
Methods
The STO-3 trial enrolled 1780 postmenopausal lymph node–negative breast cancer patients randomly assigned to receive adjuvant tamoxifen vs not. The fraction of cancer cells for each ER intensity level was scored by breast cancer pathologists, and intratumor heterogeneity of ER was calculated using Rao’s quadratic entropy and categorized into high and low heterogeneity using a predefined cutoff at the second tertile (67%). Long-term breast cancer-specific survival analyses by intra-tumor heterogeneity of ER were performed using Kaplan-Meier and multivariable Cox proportional hazard modeling adjusting for patient and tumor characteristics.
Results
A statistically significant difference in long-term survival by high vs low intratumor heterogeneity of ER was seen for all ER-positive patients (P < .001) and for patients with luminal A subtype tumors (P = .01). In multivariable analyses, patients with high intratumor heterogeneity of ER had a twofold increased long-term risk as compared with patients with low intratumor heterogeneity (ER-positive: hazard ratio [HR] = 1.98, 95% confidence interval [CI] = 1.31 to 3.00; luminal A subtype tumors: HR = 2.43, 95% CI = 1.18 to 4.99).
Conclusions
Patients with high intratumor heterogeneity of ER had an increased long-term risk of fatal breast cancer. Interestingly, a similar long-term risk increase was seen in patients with luminal A subtype tumors. Our findings suggest that intratumor heterogeneity of ER is an independent long-term prognosticator with potential to change clinical management, especially for patients with luminal A tumors.
Breast cancer is the most common cancer in women, with an estimated 1 million new cases and more than 400 000 deaths annually worldwide (1). It is a diverse disease, both in the sense of the metastatic potential of the primary tumor and in time for metastasis to occur (2), occasionally spanning more than 20 years between primary tumor diagnosis and metastasis. Endocrine therapy is a cornerstone in the management of estrogen receptor–positive (ER) breast cancer and has improved patient survival considerably (3). However, approximately 50% of patients with ER-positive disease fail to respond to endocrine therapy, and approximately one out of four women with early-stage breast cancer will later develop distant metastatic disease (4,5).
The biological factors influencing the long-term risk of fatal breast cancer are unknown. It is, however, known that patients with ER-positive disease have a continuous long-term risk for fatal breast cancer, in contrast to patients with ER-negative cancer (4). It has been suggested that late fatal disease mechanisms may involve cancer cells staying dormant over a long period of time (6). Given the late onset of fatal disease in ER-positive breast cancer and the lack of clinical studies with long-term follow-up, it is difficult to predict who has a high long-term risk of fatal disease.
We and others have shown that ER expression in primary breast cancer tumors is altered throughout tumor progression, which influences patient survival (7,8). It has further been suggested that breast cancers possess intratumor heterogeneity with varying metastatic capacity (9–11). We hypothesized that patient long-term survival is dependent on the degree of intratumor heterogeneity of ER. Using a large Swedish clinical trial with complete long-term (25 years) follow-up of patients randomly assigned to receive adjuvant tamoxifen vs not, we investigated whether intratumor heterogeneity of ER is associated with an increased long-term risk of fatal breast cancer.
Methods
The Stockholm Tamoxifen Trial
The Stockholm Breast Cancer Study Group conducted randomized trials from 1976 onwards (12,13). The Stockholm Tamoxifen Trial (STO-3) trial enrolled 1780 postmenopausal patients from 1976 until 1990 with lymph node–negative breast cancers with tumors smaller than or equal to 30 mm in diameter, randomly assigned to two years of adjuvant tamoxifen (40 mg daily) vs no adjuvant treatment. In 1983, patients who reconsented and were relapse-free after 2 years of tamoxifen treatment were randomized to 3 additional years of tamoxifen or no further therapy. The STO-3 trial was approved by the ethical committee at Karolinska Institutet, and participants provided oral consent. At the time when the trial was approved and started, written consent was not considered ethically acceptable by the ethical committees in Sweden, but was thought to disturb the trustful patient-doctor relationship; therefore only oral consent was accepted and practiced.
The patient subset with formalin-fixed paraffin-embedded (FFPE) material available is well balanced to the original STO-3 trial cohort with regards to tumor characteristics, such as tumor size (78% vs 81%), ER status (78% vs 80%), and treatment arm assignment (52% vs 50%) (Supplementary Material, available online) (14). All patients included in the STO-3 randomized trial have detailed patient and clinical information.
In Sweden, all residents have a unique national registration number, which enables automatic linkage of various records of personal information from national registers of high validity and essentially complete coverage. Death due to breast cancer was assessed from the Swedish National Cause-of-Death Register with a reported accuracy of more than 96% from January 1, 1961, and onwards (15,16). The information on cause of death is from death certificates filled in by treating physicians. Furthermore, information on contralateral breast cancer was assessed from the Swedish National Cancer Registry. Cancer registration has a legal basis in Sweden, and the Swedish Cancer Registry has breast cancer coverage of more than 96% in validation studies (17). Finally, the Swedish National Total Population Register holds different types of information on individuals including information on emigration from Sweden and has high validity and completeness (18). Thus given the Swedish national registers, patients in the STO-3 trial had complete long-term follow-up until December 31, 2012.
ER, Progesterone Receptor, Human Epidermal Growth Factor Receptor 2, and Ki-67
We first assigned all available FFPE tumor blocks a random order of annotation. In 2014, the FFPE tumors were sectioned at 4 μm and mounted on plus coated glass slides in the Tissue Profiling Facility at the Science for Life Laboratory at Uppsala University. Patient whole-tumor sections were sent to the University of California Davis Medical Center (UCDMC) CLIA laboratory, and immunohistochemistry (IHC) was done for ER, progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 (Supplementary Material, available online). The majority of the IHC-stained slides (approximately 90%) were assessed as being of high quality (with high RNA quality as mentioned below).
Ten pathologists (RB, JB, PC, YC, BD, FH, GK, FL, YZ, and AB) at the University of California with expertise in breast diagnostics, as a part of the ATHENA Breast Health network, scored (on whole-tumor sections with microscopes) the percentage of positive breast cancer cells for each ER intensity level (0, +1, +2, or + 3) compared with established standards, after completing an online training module developed to standardize the distinctions between intensity levels (Supplementary Material, available online) (19). In addition, the pathologists scored the percentage of cancer cells positive for PR, HER2, and Ki-67, where a threshold of 10% or greater was used to define ER and PR receptor positivity, HER2 positivity was defined as intensity 3+ by immunohistochemistry, and the Ki-67 threshold for positivity was 15% or greater. The ER H-Score is defined as the sum of the percentage of ER-positive tumor cells at each intensity level multiplied by an ordinal value corresponding to the intensity level (0 = none, 1 = weak, 2 = moderate, and 3 = strong).
Intratumor Heterogeneity of ER
We used Rao’s quadratic entropy to quantify intratumor heterogeneity of ER within each patient tumor. The Simpson index is one of the most commonly used diversity measurements and has previously been used to assess clonal diversity and genomic intratumor heterogeneity in cancer (20–22). Rao’s quadratic entropy (QE; continuous score) (23,24) uses the Simpson index (22) together with a distance matrix as weights to better quantify intratumor heterogeneity. In our study, the proportions in the Simpson index are defined by the proportion of tumor cells positively stained for ER at each intensity level (+0, +1, +2, or + 3). The distance matrix used as weights reflects the difference in ER intensity. For instance, a difference between ER intensity 0+ and 3+ within a tumor would be weighted as a 3, whereas a difference between ER intensity 1+ and 2+ within a tumor would be weighted as a 1 (Supplementary Material, available online).
The predefined cutoff at the second tertile (67%) for high intratumor heterogeneity was used in order for a smaller proportion of the tumors to be defined with this characteristic (high intratumor heterogeneity), in agreement with other tumor characteristics such as PR status and Ki-67 (25). We refer to these categories as high and low intratumor heterogeneity of ER.
Tumor Grade
Tumor grade according to the Nottingham system was retrospectively assessed by one pathologist (14).
Intrinsic Subtypes (PAM50)
Gene expression data were generated using custom-designed Agilent arrays containing approximately 32.1K probes, representing approximately 21.5K unique genes from FFPE breast cancer tumor tissue. Approximately 90% (or 652 breast cancer tumors) passed the RNA quality check (according to the diagnostic quality model) and were used in the intrinsic subtype analysis. Tumors were assigned to one of five molecular subtypes (luminal A, luminal B, HER2-enriched, basal-like, normal-like) using the PAM50 classifier, as described in Parker et al. (Supplementary Material, available online) (26).
Statistical Methods
Differences in patient and tumor characteristics between high and low intratumor heterogeneity of ER were tested with the Fisher exact test. The intratumor heterogeneity (QE, continuous score) of luminal A and luminal B tumors was contrasted using a Kernel density plot overlaying the distributions.
The outcome of interest was death due to breast cancer, and analyses of long-term breast cancer–specific survival (25 years) by intratumor heterogeneity of ER were performed. We included patients with ER-positive breast cancer because intratumor heterogeneity of ER is not meaningful in ER-negative cancer. Patient follow-up started at the date of primary breast cancer diagnosis and ended at the date of death, contralateral breast cancer diagnosis, emigration from Sweden (only five women emigrated), or end of study follow-up (December 31, 2012).
Kaplan-Meier analyses were performed for high and low intratumor heterogeneity of ER by STO-3 trial arm (four groups: low heterogeneity/treated arm, low heterogeneity/untreated arm, high heterogeneity/treated arm, high heterogeneity/untreated arm) for all patients and for patients with luminal A subtype tumors. Subset analyses for patients with luminal B tumors were not performed because the sample sizes were too small for long-term survival analyses. Statistical significance was assessed using the log-rank test.
Multivariable analysis for high intratumor heterogeneity, with low intratumor heterogeneity as the reference category, was performed using Cox proportional hazard modeling, adjusting for the classical patient and tumor characteristics, that is, all the available standard variables known to be important for breast cancer survival (such as age and calendar period of breast cancer diagnosis, ER-positive stained cells [%], ER H-Score, PR status, HER2 status, Ki-67 status [15% used as positive cutoff], tumor grade, tumor size, and STO-3 trial arm). HER2 status was not adjusted for in the subgroup analysis (tamoxifen-treated, untreated, and luminal A) due to the small number of HER2-positive patients.
The proportional hazard assumption for intratumor heterogeneity was assessed using a time-by-covariate interaction (between intratumor heterogeneity and follow-up time). No statistically significant interaction was noted. An arbitrary level of 5% statistical significance (two-tailed) was used. All data preparation and analysis were done using SAS version 9.4 and R version 3.2.1. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Intratumor Heterogeneity of ER
Patient and tumor characteristics by intratumor heterogeneity of ER are presented in Table 1, and the STO trial consort diagram is presented in Supplementary Figure 1 (available online). Intratumor heterogeneity (low vs high) was well balanced, and no patient or tumor characteristic as tested was statistically significantly associated with intratumor heterogeneity (Table 1). The cutoff value for high intratumor heterogeneity was predefined to be at the second tertile (67%) of the continuous Rao’s quadratic entropy score, which was at 0.855. Thus, the low intratumor heterogeneity category was defined as less than 0.855 (including only positive values), and high intratumor heterogeneity was defined as 0.855 or greater (maximum value was 1.395). In Supplementary Figure 2 (available online), patient tumors according to low/high intratumor heterogeneity of ER and ER H-Score are presented. In tumors with high intratumor heterogeneity of ER, the percentage of ER-positive tumor cells ranged from 20% to 100% (Supplementary Figure 3A, available online). Similarly, tumors with high intratumor heterogeneity of ER had a large span of H-Scores (Supplementary Figure 3B, available online).
Table 1.
Patient and tumor characteristics at primary breast cancer diagnosis by intratumor heterogeneity of ER
| STO-3 trial |
||||
|---|---|---|---|---|
| Low heterogeneity | High heterogeneity | P* | Total No. of patients | |
| No. (%) | No. (%) | |||
| STO-3 trial arm | .93 | |||
| Tamoxifen-treated arm | 205 (51.6) | 102 (52.0) | 307 | |
| Untreated arm | 192 (48.4) | 94 (48.0) | 286 | |
| Patient characteristics | ||||
| Calendar period of primary diagnosis | .80 | |||
| 1976–1979 | 59 (14.9) | 33 (16.8) | 92 | |
| 1980–1984 | 146 (36.8) | 69 (35.2) | 215 | |
| 1985–1990 | 192 (48.3) | 94 (48.0) | 286 | |
| Age at primary diagnosis, y | .71 | |||
| 45–54 | 43 (10.8) | 17 (8.7) | 60 | |
| 55–64 | 199 (50.1) | 98 (50.0) | 297 | |
| 65–74 | 155 (39.1) | 81 (41.3) | 236 | |
| Primary tumor characteristics | ||||
| Type of surgery | .53 | |||
| Breast-conserving surgery and RT | 93 (23.4) | 41 (20.9) | 134 | |
| Mastectomy | 304 (76.6) | 155 (79.1) | 459 | |
| Progesterone receptor status | .92 | |||
| Positive | 266 (68.4) | 131 (67.9) | 397 | |
| Negative | 123 (31.6) | 62 (32.1) | 185 | |
| Unknown | 8 | 3 | 11 | |
| HER2 status† | 1.00 | |||
| Positive | 17 (4.3) | 8 (4.1) | 25 | |
| Negative | 378 (95.7) | 187 (95.9) | 565 | |
| Unknown | 2 | 1 | 3 | |
| Ki-67 status‡ | .06 | |||
| Positive | 90 (23.8) | 30 (16.6) | 120 | |
| Negative | 288 (76.2) | 151 (83.4) | 439 | |
| Unknown | 19 | 15 | 34 | |
| Tumor grade | .52 | |||
| 1 | 86 (21.9) | 44 (22.9) | 130 | |
| 2 | 248 (63.1) | 126 (65.6) | 374 | |
| 3 | 59 (15.0) | 22 (11.5) | 81 | |
| Unknown | 4 | 4 | 8 | |
| Tumor size, mm | .36 | |||
| pT < 20 | 319 (81.2) | 164 (84.5) | 483 | |
| pT ≥ 20 | 74 (18.8) | 30 (15.5) | 104 | |
| Unknown | 4 | 2 | 6 | |
| Intrinsic subtypes (PAM50) | .21 | |||
| Basal | 6 (1.6) | 1 (0.6) | 7 | |
| HER2-enriched | 17 (4.7) | 4 (2.3) | 21 | |
| Luminal A | 233 (64.0) | 103 (59.2) | 336 | |
| Luminal B | 80 (22.0) | 46 (26.4) | 126 | |
| Normal-like | 28 (7.7) | 20 (11.5) | 48 | |
| Unknown | 33 | 22 | 55 | |
Two-sided Fisher exact test. ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; RT = radiotherapy; STO-3 = Stockholm Tamoxifen trial 3.
HER2 positive defined as 3+ by immunohistochemistry.
Ki-67 cutoff for positivity at 15%.
We investigated whether high intratumor heterogeneity of ER was more predominant in the luminal B subtype, which is generally considered an aggressive characteristic as compared with luminal A, using a Kernel density plot overlaying the distributions (QE, continuous score). However, a very similar distribution of intratumor heterogeneity of ER was observed (Figure 1). Indeed, approximately one-third of the tumors were of high intratumor heterogeneity of ER regardless of luminal A or luminal B subtype (Table 1), and no statistically significant association was observed between luminal A and luminal B tumor subtype, and high vs low intratumor heterogeneity (Fisher exact test, P = .26).
Figure 1.
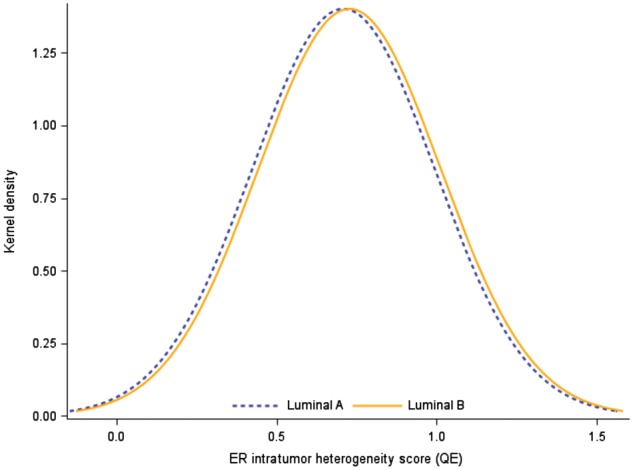
Kernel density plot of the intratumor heterogeneity of the estrogen receptor (quadratic entropy QE, continuous score) for luminal A vs luminal B subtype tumors (PAM50). ER = estrogen receptor.
Long-term Breast Cancer–Specific Survival Analysis
Univariate Survival Analysis
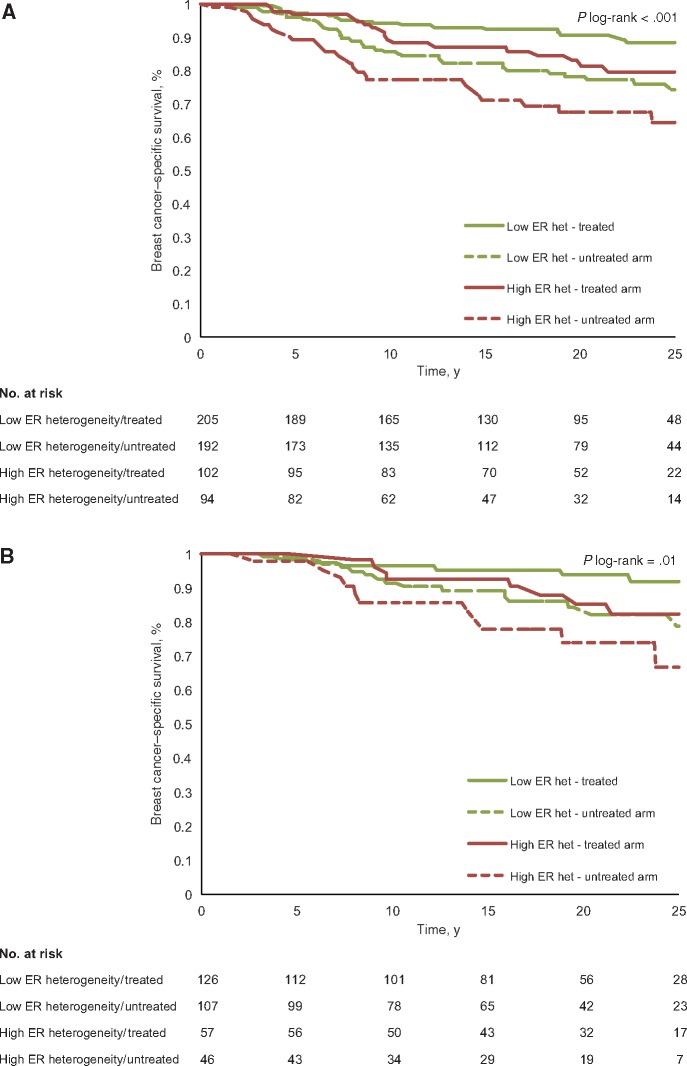
We performed Kaplan-Meier analysis by intratumor heterogeneity of ER and trial arm (four groups: low heterogeneity/treated arm, low heterogeneity/untreated arm, high heterogeneity/treated arm, high heterogeneity/untreated arm). A statistically significant difference in long-term survival was seen (log-rank, P < .001) (Figure 2A). Patients with low intratumor heterogeneity of ER in the tamoxifen-treated arm had excellent survival (25-year breast cancer–specific survival, 88.3%), followed by treated patients with high intratumor heterogeneity (79.6%), untreated patients with low intratumor heterogeneity (74.3%), and lastly untreated patients with high intratumor heterogeneity (64.3%). In addition, Kaplan-Meier analysis including patients with luminal A subtype tumors (by intratumor heterogeneity of ER and trial arm; four groups) was performed. A statistically significant difference in long-term survival by intratumor heterogeneity and trial arm was also seen for luminal A patients, together with a similar survival graph pattern by intratumor heterogeneity and trial arm, as described above (log-rank, P = .01) (Figure 2B).
Figure 2.
Kaplan-Meier analyses by intratumor heterogeneity of estrogen receptor (ER) and trial arm (four groups: low intratumor heterogeneity of ER/treated arm, low heterogeneity/untreated arm, high heterogeneity/treated arm, high heterogeneity/untreated arm). The P value is based on a two-sided log-rank test; numbers at risk are shown underneath each graph.
Multivariable Survival Analysis
Multivariable analysis of breast cancer–specific survival by intratumor heterogeneity of ER was performed using Cox proportional hazard modeling, adjusting for classical patient and tumor characteristics (such as age and calendar period of breast cancer diagnosis, ER-positive stained cells [%], ER H-Score, PR status, HER2 status, Ki-67 status, tumor grade, tumor size, and STO trial arm), as well as the crude estimates (only adjusting for age and period at breast cancer diagnosis). Patients with high intratumor heterogeneity of ER had an approximately twofold increased long-term risk of fatal breast cancer disease as compared with patients with low heterogeneity (hazard ratio [HR] = 1.98, 95% confidence interval [CI] = 1.31 to 3.00) (Table 2). The hazard ratio effects for all covariates in the model (crude and adjusted) are presented in Supplementary Table 1 (available online). Competing risk analysis using Fine-Gray regression modeling yielded very similar results (data not shown).
Table 2.
Risk of long-term breast cancer–specific death (25 years) by intratumor heterogeneity of ER in ER-positive breast cancer
| 25-y breast cancer–specific survival |
|||||
|---|---|---|---|---|---|
| STO-3 trial |
Intratumor heterogeneity of ER |
25-y breast cancer–specific deaths | Crude estimates adjusted for age and period of diagnosis | Adjusted estimates for patient and tumor characteristics | |
| Patients included | Category | No. | No. | HR (95% CI) | HR (95% CI) |
| All patients | High | 196 | 44 | 1.64 (1.11 to 2.44) | 1.98 (1.31 to 3.00)* |
| Low | 397 | 56 | 1.00 (ref.) | 1.00 (ref.) | |
| Tamoxifen-treated arm | High | 102 | 17 | 1.94 (0.99 to 3.80) | 2.15 (1.07 to 4.34)† |
| Low | 205 | 18 | 1.00 (ref.) | 1.00 (ref.) | |
| Untreated arm | High | 94 | 27 | 1.52 (0.93 to 2.50) | 1.91 (1.12 to 3.27)† |
| Low | 192 | 38 | 1.00 (ref.) | 1.00 (ref.) | |
| Luminal A tumor subtype | High | 103 | 19 | 1.83 (0.99 to 3.39) | 2.43 (1.18 to 4.99)† |
| Low | 233 | 22 | 1.00 (ref.) | 1.00 (ref.) | |
Modeled by multivariable proportional hazard (Cox) analyses adjusted for treatment arm, age and calendar period of diagnosis, ER-positive stained cells, ER H-Score, progesterone receptor (PR) status, HER2 status, Ki-67 status, tumor grade, and tumor size. CI = confidence interval; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; HR = hazard ratio; STO-3 = Stockholm Tamoxifen trial 3; ref. = referent.
Modeled by multivariable proportional hazard (Cox) analyses adjusted for age and calendar period of diagnosis, ER-positive stained cells, ER H-Score, progesterone receptor (PR) status, Ki-67 status, tumor grade, and tumor size. The luminal A analysis was additionally adjusted for treatment arm.
Furthermore, analysis in each trial arm revealed a 2.2-fold and 1.9-fold increased risk of fatal breast cancer in the tamoxifen-treated and untreated arms, respectively, for patients with high intratumor heterogeneity of ER compared with patients with low heterogeneity (tamoxifen-treated arm: HR = 2.15, 95% CI = 1.07 to 4.34, and untreated arm: HR = 1.91, 95% CI = 1.12 to 3.27) (Table 2). Finally, in patients with luminal A subtype tumors, a more than twofold risk of fatal breast cancer was seen for patients with high intratumor heterogeneity of ER compared with patients with low heterogeneity (HR = 2.43, 95% CI = 1.18 to 4.99) (Table 2).
Discussion
In this study, we investigated whether high intratumor heterogeneity of ER is associated with an increased long-term risk of fatal breast cancer. Our findings indicate that intratumor heterogeneity of ER is an independent prognosticator for long-term breast cancer–specific survival. Notably, intratumor heterogeneity of ER was also associated with long-term fatal breast cancer risk in patients with luminal A subtype, as patients with high intratumor heterogeneity of ER had a statistically significant and marked increased risk of fatal breast cancer.
It is widely recognized that primary breast cancer tumors possess intratumor heterogeneity that may give rise to cells with varying metastatic capacity (9,10). More recently, we and others have shown that the clinically used breast cancer markers alter their expression throughout tumor progression (8), and genetic studies have shown that metastases may arise from a minority of cells within the primary tumor (11,27,28). Indeed, our findings that high intratumor heterogeneity of ER is associated with poor breast cancer–specific survival are consistent with the notion that tumors with high intratumor heterogeneity may harbor cells with the capacity to metastasize because of the cells’ capacity to adapt over time and under therapeutic pressure.
Patients with ER-positive disease, including those with low-risk node-negative disease, have a limited early risk but a continuous long-term risk for fatal breast cancer (3,4,29–31). It is clear that classical tumor characteristics such as lymph node status and tumor size can predict early risk, but it remains controversial how to identify the patients at later risk. Lymph node positivity, grade, and high expression of genes involved in the ER-signaling pathways have, however, been suggested to be important for late breast cancer disease (32–34). Moreover, the observation that patients with high intratumor heterogeneity of ER had a continuous risk for fatal breast cancer up to 25 years, at end of follow-up, in our study suggests that intratumor heterogeneity of ER may be useful as a long-term breast cancer–specific survival predictor.
The continuous long-term risk for patients with ER-positive disease has been suggested to involve cancer cells staying dormant over long periods of time. Even for patients with low-stage disease, micrometastases in the bone marrow are not uncommon (35), and circulating breast cancer tumor cells have been found even 20 years after diagnosis in patients who are clinically disease free (36). From these and other observations, it is clear that more knowledge about tumor dormancy and how to maintain the balance between tumor cell growth and tumor cell death is needed. Indeed, given that ER-positive disease is associated with an increased long-term risk of fatal disease, one may hypothesize that having dormant tumor cells with different ER expression levels, as compared with more homogeneous ER expression, may influence patient survival.
We retrospectively analyzed the STO-3 trial, a trial of tamoxifen vs no taxomifen from 1976 until 1990, enabling long-term analysis of breast cancer survival (12,13). Because the clinical management and treatment has changed over time, our study shares the same limitations with other long-term follow-up studies (4). The STO-3 trial was performed before aromatase inhibitors were developed; the duration of tamoxifen therapy was shorter than currently recommended, and we lack information on treatment at relapse. Approximately half of the patients from the original trial cohort had tumor blocks available for molecular analysis and were thus included in our study. We confirmed, however, that patient and tumor characteristics in our study were equally distributed between the arms and well balanced to the original STO-3 cohort with regards to characteristics such as tumor size, ER status, and treatment arm assignment (14). Finally, assessments of IHC markers are known to vary; however, in our study, ER (together with PR, HER2, and Ki-67) was recently, in 2014, stained at a single medical center laboratory and assessed by experienced breast cancer pathologists who had been harmonized with regard to the scoring of IHC markers, including ER.
In conclusion, patients with high intratumor heterogeneity of ER have statistically significantly worse long-term survival as compared with patients with low intratumor heterogeneity. Interestingly, intratumor heterogeneity of ER also identified patients at increased long-term risk for fatal breast cancer in patients with luminal A subtype tumors, generally considered to be a low-risk group. Intratumor heterogeneity of ER should be easy to translate into clinical routine given that ER is assessed for every newly diagnosed breast cancer patient. Importantly, routine clinical assessment of intratumor heterogeneity of ER may identify ER-positive breast cancer patients at high long-term risk for fatal breast cancer, which could change clinical management, especially for patients with luminal A subtype tumors. Finally, future validation of our findings in prospective studies with long-term follow-up and ER-stained slides available is warranted and planned.
Funding
This work was supported by the Swedish Research Council (grant No. 521-2014-2057 to LSL and grant No. 2014-2271 to KC), FORTE (grant No. 2014-1962 to LSL and grant No. 2016-00081 to KC), Swedish Cancer Society (grant No. CAN2016/684 to KC), Stiftelsen Gösta Miltons Donationsfond (The Gösta Milton Donation Fund to LSL), California Breast Cancer Research Program BCRP award (grant No. 180B-0065, Predicting Breast Cancer Recurrence To Improve Care to LJE), and Cancerföreningen i Stockholm (Stockholm Cancer Society).
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
LJV is a cofounder, stockholder, and part time employee of Agendia. The other authors have declared no potential conflicts of interest.
We thank Neil E. Hubbard, Olanu Aina, Judith Walls, and Herlina Sugandha of UC Davis for help with slide staining and organization for the reader scoring. We also thank the Tissue Profiling Facility at the Science for Life Laboratory, Uppsala University, for excellent technical assistance.
Conference presentations: Selected for Oral presentation at the European Cancer Congress (ECCO/ESMO) in Vienna, Austria, 2015, and at the IMPAKT Breast Cancer Conference in Brussels, Belgium, 2017.
Supplementary Material
References
- 1. Kamangar F, Dores GM, Anderson WF.. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;2414:2137–2150. 10.1200/JCO.2005.05.2308 [DOI] [PubMed] [Google Scholar]
- 2. Pichon MF, Broet P, Magdelenat H et al. , . Prognostic value of steroid receptors after long-term follow-up of 2257 operable breast cancers. Br J Cancer. 1996;7312:1545–1551. 10.1038/bjc.1996.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J et al. , . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;3789793:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colleoni M, Sun Z, Price KN et al. , . Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the International Breast Cancer Study Group trials I to V. J Clin Oncol. 2016;349:927–935. 10.1200/JCO.2015.62.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheppard VB, Faul LA, Luta G et al. , . Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;3222:2318–2327. 10.1200/JCO.2013.51.7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang XH, Giuliano M, Trivedi MV et al. , . Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res. 2013;1923:6389–6397. 10.1158/1078-0432.CCR-13-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amir E, Miller N, Geddie W et al. , . Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;306:587–592. 10.1200/JCO.2010.33.5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindstrom LS, Karlsson E, Wilking UM et al. , . Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;3021:2601–2608. 10.1200/JCO.2011.37.2482 [DOI] [PubMed] [Google Scholar]
- 9. Fidler IJ, Kripke ML.. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;1974306:893–895. 10.1126/science.887927 [DOI] [PubMed] [Google Scholar]
- 10. Poste G, Fidler IJ.. The pathogenesis of cancer metastasis. Nature. 1980;2835743:139–146. 10.1038/283139a0 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Waters J, Leung ML et al. , . Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;5127513:155–160. 10.1038/nature13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fornander T, Rutqvist LE, Cedermark B et al. , . Adjuvant tamoxifen in early breast cancer: Occurrence of new primary cancers. Lancet. 1989;18630:117–120. [DOI] [PubMed] [Google Scholar]
- 13. Rutqvist LE, Johansson H, Stockholm Breast Cancer Study Group. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007;462:133–145. 10.1080/02841860601034834 [DOI] [PubMed] [Google Scholar]
- 14. Jerevall PL, Ma XJ, Li H et al. , . Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer. 2011;10411:1762–1769. 10.1038/bjc.2011.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larsson LG, Nystrom L, Wall S et al. , . The Swedish randomised mammography screening trials: Analysis of their effect on the breast cancer related excess mortality. J Med Screen. 1996;33:129–132. 10.1177/096914139600300305 [DOI] [PubMed] [Google Scholar]
- 16. Rutqvist LE. Validity of certified causes of death in breast carcinoma patients. Acta Radiol Oncol. 1985;245:385–390. 10.3109/02841868509134405 [DOI] [PubMed] [Google Scholar]
- 17. Barlow L, Westergren K, Holmberg L et al. , . The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol. 2009;481:27–33. 10.1080/02841860802247664 [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Almqvist C, Bonamy AK et al. , . Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;312:125–136. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 19. Engelberg JA, Retallack H, Balassanian R et al. , . “Score the Core” web-based pathologist training tool improves the accuracy of breast cancer IHC4 scoring. Hum Pathol. 2015;4611:1694–1704. [DOI] [PubMed] [Google Scholar]
- 20. Martins FC, De S, Almendro V et al. , . Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2012;26:503–511. 10.1158/2159-8290.CD-11-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SY, Gonen M, Kim HJ et al. , . Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J Clin Invest. 2010;1202:636–644. 10.1172/JCI40724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson EH. Measurement of diversity. Nature. 1949;163:688. 10.1038/163688a0 [DOI] [Google Scholar]
- 23. Potts SJ, Krueger JS, Landis ND et al. , . Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab Invest. 2012;929:1342–1357. 10.1038/labinvest.2012.91 [DOI] [PubMed] [Google Scholar]
- 24. Rao C. Diversity and dissimilarity coefficients: A unified approach. Theoret Pop Biol. 1982;21:24–43. 10.1016/0040-5809(82)90004-1 [DOI] [Google Scholar]
- 25. Tobin NP, Harrell JC, Lovrot J et al. , . Molecular subtype and tumor characteristics of breast cancer metastases as assessed by gene expression significantly influence patient post-relapse survival. Ann Oncol. 2015;261:81–88. 10.1093/annonc/mdu498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parker JS, Mullins M, Cheang MC et al. , . Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;278:1160–1167. 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding L, Ellis MJ, Li S et al. , . Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;4647291:999–1005. 10.1038/nature08989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao R, Davis A, McDonald TO et al. , . Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat Genet. 2016;4810:1119–1130. 10.1038/ng.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pagani O, Price KN, Gelber RD et al. , . Patterns of recurrence of early breast cancer according to estrogen receptor status: A therapeutic target for a quarter of a century. Breast Cancer Res Treat. 2009;1172:319–324. 10.1007/s10549-008-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metzger-Filho O, Sun Z, Viale G et al. , . Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from International Breast Cancer Study Group trials VIII and IX. J Clin Oncol. 2013;3125:3083–90. 10.1200/JCO.2012.46.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sestak I, Dowsett M, Zabaglo L et al. , . Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;10519:1504–1511. 10.1093/jnci/djt244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dubsky P, Brase JC, Jakesz R et al. , . The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;10912:2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esserman LJ, Moore DH, Tsing PJ et al. , . Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;1292:607–616. 10.1007/s10549-011-1564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kennecke HF, Olivotto IA, Speers C et al. , . Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol. 2007;181:45–51. [DOI] [PubMed] [Google Scholar]
- 35. Mansi JL, Gogas H, Bliss JM et al. , . Outcome of primary-breast-cancer patients with micrometastases: A long-term follow-up study. Lancet. 1999;3549174:197–202. 10.1016/S0140-6736(98)10175-7 [DOI] [PubMed] [Google Scholar]
- 36. Meng S, Tripathy D, Frenkel EP et al. , . Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;1024:8152–8162. 10.1158/1078-0432.CCR-04-1110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.