Abstract
Despite strong indications that increased consumption of added sugars correlates with greater risks of developing cardiometabolic syndrome (CMS) and cardiovascular disease (CVD), independent of the caloric intake, the worldwide sugar consumption remains high. In considering the negative health impact of overconsumption of dietary sugars, increased attention is recently being given to the role of the fructose component of high-sugar foods in driving CMS. The primary organs capable of metabolizing fructose include liver, small intestine, and kidneys. In these organs, fructose metabolism is initiated by ketohexokinase (KHK) isoform C of the central fructose-metabolizing enzyme KHK. Emerging data suggest that this tissue restriction of fructose metabolism can be rescinded in oxygen-deprived environments. In this review, we highlight recent progress in understanding how fructose metabolism contributes to the development of major systemic pathologies that cooperatively promote CMS and CVD, reference recent insights into microenvironmental control of fructose metabolism under stress conditions and discuss how this understanding is shaping preventive actions and therapeutic approaches.
Keywords: Fructose, Cardiometabolic syndrome, Cardiac hypertrophy, Atherosclerosis, Nonalcoholic fatty liver disease, Diabetes type 2, Fructolysis, Ketohexokinase, Hypertension, Alternative splicing, SF3B1, HIF, Hypoxia
Introduction
Diet-related cardiometabolic syndrome (CMS) is a major burden of both industrialized and developing countries, largely in response to sedentary lifestyle-driven imbalance between caloric intake and consumption.1 Food patterns have a major impact on numerous cardiometabolic risk factors, all hallmarks of the CMS, including glucose intolerance and insulin resistance, hypertension, dyslipidaemia, endothelial dysfunction, obesity, inflammation, and adipocyte dysfunction.2 While the total fat intake has decreased during the last decades in Europe and the USA, the amount of added sugar, consumed mainly as sucrose (containing 50% glucose and 50% fructose) and high fructose corn syrup (HFCS; containing 42–55% fructose) remains high.3 High fructose corn syrup was first introduced in the USA in 1972 as an inexpensive way to sweeten soft drinks and processed food.4 Since then the intake of HFCS has increased dramatically, up to 27.5 kg per capita in 2007.4
Fructose has been considered an ideal replacement for glucose in the diet of diabetic patients as its ingestion is not coupled to increased secretion of insulin.5 However, already in 1954 high fructose consumption has been linked to the development of insulin resistance.6 In fact, the increase in prevalence of diabetes, obesity, and coronary artery disease (CAD) in the past few decades correlate with an exponential rise in HFCS consumption.7,8
In this review, we will first discuss how changes in global sugar availability has impacted the development of CMS and CAD, outline the biochemical basis of fructose metabolism and highlight key differences in the regulation of fructolysis and glycolysis in the context of the liver. Next, we discuss recent progress in understanding the regulation of fructose metabolism by oxygen-sensitive signalling and the pathologic consequences in the heart. Finally, we will discuss translational strategies to prevent and/or treat fructose related-CMS pathologies.
Cardiometabolic syndrome: a major global burden
The dramatic worldwide increase of obesity is leading to a concomitant rise of CMS, with major impact on cardiovascular, renal, cerebrovascular, and immunological health. It is important to note that there is no clear consensus on the definition of CMS, also known as ‘metabolic syndrome’ or ‘syndrome X’, which complicates comparisons across studies. The two most common definitions are provided by the World Health Organization (WHO) and the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III).9 The WHO criteria require the presence of diabetes and insulin resistance and two risk factors [obesity, systemic arterial hypertension, dyslipidaemia with high triglycerides or reduced high-density lipoprotein cholesterol (HDL-C) levels, or micro-albuminuria]. The NCEP-ATP III criteria focus more on early disease detection and necessitate any three of the following clinical abnormalities: hyperglycaemia, central obesity, arterial hypertension, dyslipidaemia [e.g. elevated triglyceride levels, high apolipoprotein (Apo) B, and reduced HDL].9 More recently, the presence of non-alcoholic fatty liver disease (NAFLD) has been shown to predict even more precisely the presence of insulin resistance than do ATP III criteria.10 Alanine aminotransferase (ALT) levels in serum, and intrahepatic lipid accumulation, are also diagnostically informative.11–13 The Japanese government has therefore included a threshold level for ALT as part of their CMS definition.10
Global obesity has more than doubled since 1980. In 2014, more than 1.9 billion adults were overweight and 600 millions of them obese.14 The trend is similar for type 2 diabetes. Since 1980, the number of diabetic people nearly quadrupled from 108 million to 422 million in 2014.15 Today, some form of dyslipidaemia affects more than 50% of Americans and Germans.16,17 The advent of statins has reduced severe dyslipidaemia, and new treatment options with inhibitors targeting proprotein convertase subtilisin/kexin type (PCSK)9 may lead to a further decrease of this condition.18,19 However, over 50% of people with dyslipidaemia remain undiagnosed, excluding them from secondary prevention and pharmacological treatments.20 Hypertension is similarly on the rise, with an incidence of around 15% in the 1930 s (USA) to around 30% nowadays or even 45–55% in many European countries. The increased prevalence for diabetes and hypertension closely parallels increased rates of CAD and heart failure, stroke, and renal insufficiency and failure.21 Cardiovascular disease (CVD), for a long time most prevalent in the western world, has become the number one cause of death worldwide, as obesity, type 2 diabetes, and dyslipidaemia spread globally.22
The role of fat, added sugar, and dietary patterns in cardiometabolic syndrome and cardiovascular disease
Whereas there is little doubt about over-nutrition and inactivity being main causative factors for CMS and CVD, there remains ongoing debate about which nutrient class is a main culprit. In the last six decades, this debate was dominated by the diet-heart hypothesis that focuses on cholesterol and fats. The hypothesis goes back to discoveries of Anitschkow and Chaltow23 in 1913, who induced atherosclerosis in rabbits by feeding them a cholesterol-rich diet, but the idea was popularized most notably by Ancel Keys24 in the 1950’s. Keys argued that elevated mean cholesterol levels and cardiovascular mortality positively correlated with the amount of saturated fats and cholesterol in the diet, suggesting (though not proving) a causal relationship.25 In response, the American Heart Association (AHA) recommended in 1961 to reduce daily cholesterol consumption below 300 milligrams.26 The Multiple Risk-factor Intervention Trial (MR-FIT) as well as the Lipid Research Clinics Coronary Primary Prevention Trial (CPPT) supported the diet-heart hypothesis and the dietary guidelines by the AHA in principle. The influence of a recommended intake of mono- and polyunsaturated fatty acids (e.g. olive oil rich in alpha linoleic acid and oily fish), instead of saturated fats on blood cholesterol levels and cardiovascular outcome during a 10-year observational period was however small when cleared for participants who died of heart attack or during the study period.27,28 These results already suggested that blood cholesterol levels are only marginally influenced by low-cholesterol diets. Moreover, no randomized controlled trial has shown that the exclusive replacement of saturated fat with linoleic acid significantly reduces CAD events or deaths.29 In the light of this absence of evidence, the AHA and the Dietary Guidelines Advisory Board in the USA removed dietary cholesterol as a nutrient of concern in 2015.30
Of note, the historical AHA guidelines on the reduction of cholesterol intake are an oversimplification of the ‘good Mediterranean diet’ proposed by Keys, which is mainly vegetarian and low in saturated fats, which are replaced with mono- and polyunsaturated fats.31 The Mediterranean diet likely remains a quite powerful and cost-effective method to reduce cardiovascular risk and the prevalence of CMS.32 However, this is rather due to a mixture of fruits, vegetables, nuts, complex carbohydrates, and moderate alcohol consumption, in combination with low intake of added sugars and meats. Indeed, the Lyon Heart Study showed a 50–70% lower risk of recurrent heart disease, independent of body mass index (BMI), in the Mediterranean diet group supplemented with alpha linoleic acid, compared to the control group receiving a prudent western diet.33 The PREDIMED trial demonstrated most prominently a reduction in stroke in response to Mediterranean diets enriched with extra-virgin olive oil or mixed nuts, compared to a control group advised only to limit fat intake.28 Thus, controlling the amount and type of ingested fat alone is insufficient to positively affect the cardiovascular outcome.
John Yudkin34 introduced in 1959 the concept that sugar rather than fat drives obesity and cardiovascular mortality.35 Numerous clinical studies support the hypothesis of Yudkin that there is no link between the consumption of saturated fatty acids and cardiovascular death.36 In contrast, randomized clinical trials and epidemiologic studies in adults and children have demonstrated that an increased intake of added sugar, in particular through sweetened beverages, leads to more weight gain37 and higher risk of (visceral) obesity,38 hypertension, type 2 diabetes,39 dyslipidaemia,40,41 and CVD.8,42,43 Convincing evidence of a causal relation between intake of added sugars and cardiovascular risk factors other than body weight are often denied. However, a systematic review and meta-analyses of 39 randomized controlled trials suggest a causal relationship between sugar consumption and elevated blood pressure and lipids.44 In a prospective cohort of nationally representative US adults, the association between added sugar intake and CVD mortality remained significant even when adjusted for conventional cardiovascular risk factors such as blood pressure or serum cholesterol.42 Moreover, the detected relationship was consistent across BMI, physical activity levels, age, sex, ethnicity (except non-Hispanic blacks), and diet quality.42
Whereas the consumption of saturated fatty acids has decreased in the previous six decades, the intake of free sugars (added sugars and natural sugar sources, e.g. from honey and fruit juices) in the USA has steadily increased since the beginning of the 19th century, from 2.9 kg/year/person in 1822 to 48.9 kg/year/person in 1999.10 Free sugar consumption in Europe has shown signs of a decrease since 2000, but it remains still 35 kg/year/person nowadays, which equals to about 20% of total energy content, far more than the WHO recommendation of 9.1 kg per year, i.e., 5% of total energy content.45 People who derive 10–25% of their caloric intake from sugar have a 30% higher risk for cardiovascular mortality. Those deriving more than 25% calories from sugar, which is roughly on par with average sugar consumption in the USA and Germany, the relative risk of cardiovascular mortality is nearly tripled.42 Remarkably, nearly 50% of added sugars are ingested through sugar-sweetened beverages (e.g. soda, tea, fruit drinks).46 In consequence, the current European guidelines in CVD prevention highly discourage the intake of sweetened beverages.32 Another significant contribution of added sugar comes from the consumption of processed food like bakery products and snacks.46 In industrially produced food, sugar is often used for both to enhance flavour and attenuate suppression of appetite.47 This is especially true for fructose, which affects ghrelin production in the gastrointestinal tract and leptin secretion from adipocytes.48 The anorexigenic hormone leptin was found in higher postprandial levels after glucose than after fructose ingestion, whereas the orexigenic ghrelin was reduced.49 Interestingly, leptin-responsive neurons can also activate pathways in the periphery that are critical for stimulating energy expenditure and fat oxidation.50 People with high-fructose intake may thus have decreased energy expenditure compared to people with equal caloric intake of diets rich in glucose or starch, and thus gain more weight. This conclusion is supported by several epidemiological studies indicating a significant relationship between fructose-sweetened beverage consumption and BMI, even after adjusting for total energy intake.51–55 In addition to the production of adipocytokines also endocannabinoid release is altered in response to increased fructose intake. The induced hypothalamic endocannabinoid synthesis is linked with increased appetite56 whereas elevated endocannabinoids from the adipose tissue lead to coronary circulatory dysfunction.57
Fructose uptake and metabolism
Dietary sugars are largely ingested either as sucrose, which is cleaved by the brush-border hydrolase sucrase into glucose and fructose,58 or free glucose and fructose in form of HFCS (Figure 1). The gut transporters sodium-glucose transporter 1 (SGLT1) and glucose-transporter (GLUT) 5 take up glucose and fructose, respectively, into enterocytes.59 The presence of fructose increases the expression of GLUT5 mRNA and protein in rodents and humans.60 Multiple proteins contribute to this process including Ras-related protein RAB11a, that is crucial for endosomal protein trafficking of GLUT5.60 Fructose uptake by the gut is limited after one-time consumption.61 Fructose absorption can be increased however, when given in form of sucrose or in combination with glucose,61 with the highest uptake when glucose and fructose are provided in equal amounts.61 Moreover, fructose uptake can be increased by chronic fructose intake.60 Certain amino acids (e.g. alanine, proline, and glutamine) can also facilitate fructose absorption, most likely by solvent drag and passive diffusion.62
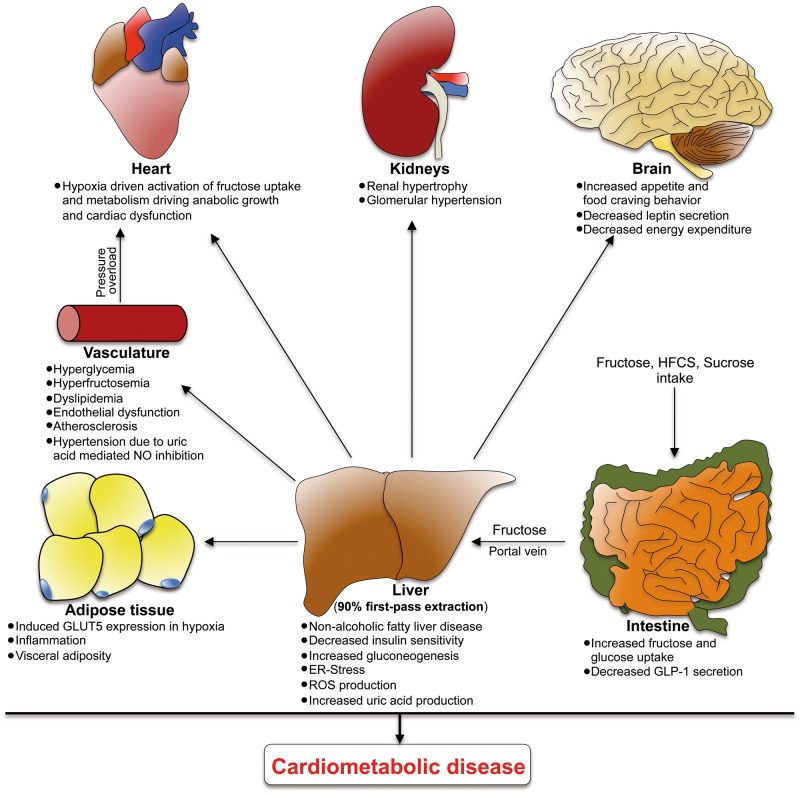
Figure 1.
Systemic effects of increased fructose uptake. Consequences of fructose ingestion are not limited to the intestine or liver, but also affect multiple organs including adipose tissue, the vasculature, heart, and kidney, as well as the satiety regulation regions in the brain. HFCS, high fructose corn syrup; NO, nitric oxide; GLUT5, glucose transporter 5.
After gut absorption, fructose enters the portal circulation, reaches the liver, is taken up by hepatocytes through a GLUT2-mediated process and immediately converted by KHK-C to fructose-1-phopsphate (F-1-P) (Figures 1 and 2). KHK-C is the protein product of one of two alternatively spliced isoforms of the KHK gene. It differs from the KHK-A isoform by a mutually exclusive exon. KHK-C displays superior affinity for fructose and is also prominently expressed in the kidney and small intestine, whereas all other tissues express KHK-A, which lacks high affinity to fructose.63 A main difference between hepatic fructose and glucose metabolism is that KHK-C-driven phosphorylation of fructose and its degradation to the triosephosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G-3-P) is unrestricted while glycolysis is tightly regulated according to the cellular energy state at the level of phosphofructokinase (PFK). Increased fructose uptake and metabolism can thereby induce a decline in hepatocellular ATP levels resulting in a facilitated high glycolytic flux due to absent PFK inhibition. Moreover, postprandial levels of F-1-P can also allosterically activate glucokinase-regulatory protein which increases glucose uptake by inducing the shuttling of glucokinase from nucleus into the cytoplasm leading to increased phosphorylation to glucose-6-phosphate (G-6-P), by nearly three-fold compared with the absence of fructose.64 In addition, experiments with fructose-treated rabbits revealed increased intracellular levels of fructose-2, 6-biphosphate, a potent activator of phosphofructokinase. Finally, increased levels of F-1-P have also shown to induce the liver-type pyruvate kinase.65 Fructose thus appears to be able to stimulate both hepatic glucose uptake and glycolysis in the liver, bypassing hormonal regulation.
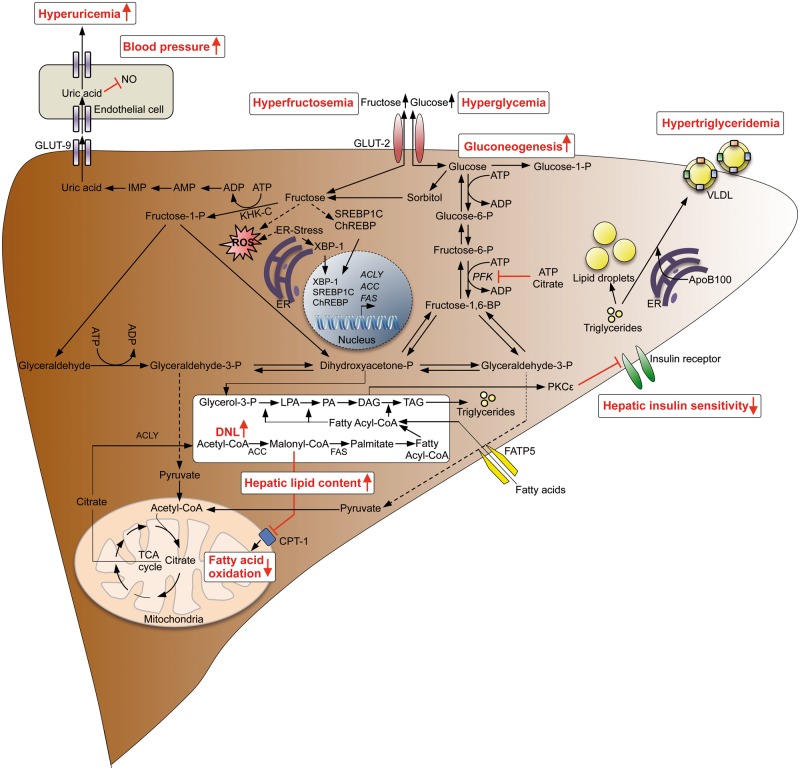
Figure 2.
Consequences of hepatic fructose metabolism. Increased sugar consumption results in increased uptake of fructose, resulting in ATP depletion that may cause increased uric acid production and hyperuricemia leading to arterial hypertension. Fructose is further metabolized to glyceraldehyde 3-phosphate and dihydroxyacetonephosphate inducing triacylglycerol synthesis and DNL, leading to hepatic steatosis, whereas fatty acid oxidation is repressed. Diacylglycerol activates PKCepsilon, resulting in decreased hepatic insulin sensitivity and induction of gluconeogenesis. Fructose produced by the polyol pathway may also be transported back to the blood stream leading to hyperfructosemia. ACC, acetyl-coa carboxylase; ACLY, ATP citrate lyase; CPT1, carnitine-palmitoyltransferase 1; ChREBP, carbohydrate-responsive element-binding protein; DAG, diacylglycerole; FAS, fatty acid synthase; FATP5, fatty acid transport protein 5; GLUT2, glucose transporter 2; GLUT9, glucose transporter 9; LPA, lysophosphatidic acid; NO, nitric oxide; PFK, phosphofructokinase; PA, phosphatidic acid; PKCε, protein kinase C epsilon type; SREBP1, sterol regulatory element binding transcription factor 1; TAG, triacylglycerole; XBP-1, X-box binding protein 1.
Fructose metabolism and liver pathologies
The mechanisms underlying the association between an increased sugar consumption and cardiometabolic risk are still far from being understood. However, there is growing evidence that in particular the fructose component in sucrose plays an important role in the pathophysiologic consequences of elevated sugar intake.
Once degraded to the two triosephosphates fructose carbons then in principle be used for gluconeogenesis, lactate production, or production of Acetyl-CoA to be oxidized in mitochondria or, most importantly, used for de novo lipogenesis (DNL). Dihydroxyacetone phosphate is also an important precursor for synthesis of the glycerol backbone in the formation of neutral lipids. Thus, the unregulated delivery of excess carbons to the glycolytic pathway in the liver can overwhelm normal regulatory steps, forcing enhanced DNL and triglyceride synthesis, which explains in part the lipogenic phenotype that is related to excessive fructose intake.10 This behaviour provides a potential explanation for a key role of KHK-C in the development of specific features of CMS, a notion underscored by the fact that wild-type mice getting 32 or 45% of their total energy intake as fructose develop CMS,66 KHK-deficient mice do not.66 KHK-deficient mice are also protected from the adverse effects of excess glucose consumption as the liver converts excess glucose into fructose via the polyol pathway.67 These observations are consistent with a broader role of KHK-signalling and fructose metabolism in supporting multiple key aspects of the CMS.
The typical and most common hepatic manifestation of CMS is NAFLD, defined as excessive hepatic fat accumulation in individuals who have no apparent liver disease and whose alcohol intake is less than 30 g per day for men and 20 g per day for woman.68 It affects already one billion people worldwide and correlates well with other manifestations of CMS such as insulin resistance, hypertriglyceridaemia, and CVD.69 Individuals with NAFLD typically have elevated circulating levels of triglycerides and Low-density lipoprotein cholesterol (LDL-C), and low levels of HDL-C.70 The primary cause of death in individuals with NAFLD is coronary heart disease and up to 80% of people with chronic heart failure have hepatic dysfunction.71–73 Increased carotid intima media thickness is already present in children with NAFLD, indicating that adverse systemic consequences of NAFLD start early.3 In the past, increased fat and caloric intake combined with decreased physical activity was regarded as the main cause of NAFLD. More recently, population studies have revealed a close association between specifically overconsumption of refined sugar and the development of NAFLD in both adults and children.10,74,75 Moreover, adults with biopsy-proven NAFLD have a two- to three-fold higher fructose consumption, compared to healthy controls.76,77 In addition, sugar-driven NAFLD has a higher risk of hepatic inflammation and fibrosis, and fructose restriction improves NAFLD.78–81 Isotope analyses have revealed that, normally, dietary fatty acids have only a minor contribution to liver triglycerides, in the range of 5–15%, while fatty acids derived from lipolysis in adipose tissue label up to 60% of liver triglycerides.82,83 Strikingly, the contribution of DNL in the liver becomes more prominent in the presence of NAFLD, rising from 10% up to 26%.82,83
Although both increased fat and sugar intake can induce NAFLD, enzymes of DNL are particularly upregulated in fructose-rich diets.68 Fructose readily enters the portal vein after ingestion to be directly delivered to the liver. In contrast, long-chain fatty acids absorbed in the intestine first enter the lymphatic system as chylomicrons and are delivered to the systemic circulation. The liver exposure to dietary fat is thus not different compared to other tissues, which is in contrast to fructose.68 Moreover, high-carbohydrate diets cause the cytosolic production of Acetyl-CoA from citrate.84 Cytosolic Acetyl-CoA is a mandatory carbon donor in the de novo synthesis of fatty acids, the first step of which is acetyl-CoA carboxylase-mediated synthesis of malonyl-CoA.84 Malonyl-CoA directly inhibits fatty acid oxidation by inhibiting carnitine-palmitoyltransferase 1 (CPT1)85 (Figure 2). Furthermore, in addition to fructose, cytosolic Acetyl-CoA activates lipogenic transcription factors like sterol regulatory element-binding transcription factor 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP), stimulating every step of DNL.68 ChREBP was also shown to induce hepatic fibroblast growth factor 21 (FGF-21) expression upon acute fructose intake, leading to elevated circulating FGF-21.86 Plasma levels of FGF-21 faithfully reflect intrahepatic lipid accumulation, suggesting FGF-21 as a promising biomarker for fructose-induced NAFLD.87
Elevated fructose intake does not only drive DNL but also hepatic insulin resistance. When the capacity to convert diacylglycerol (DAG) to triacylglycerol is exceeded, protein kinase C epsilon becomes activated, leading to inhibition of insulin receptor kinase- mediated tyrosine phosphorylation of insulin receptor substrate-1 and -2 and ultimately to reduced phosphoinositol 3-kinase and AKT Serine/Threonine Kinase 2 (AKT2) activation.88 Reduced AKT2 activation diminishes glycogen production by glycogen synthase and releases the suppression by insulin of gluconeogenesis and GLUT2 mediated glucose release.88 Consequently, even though fructose consumption results in only a minimal rise of blood glucose levels, a chronic fructose-rich diet may lead to hepatic insulin resistance and glucose intolerance. Insulin also can activate SREBP1c, and thereby hepatic uptake of free fatty acids and DNL.89 If insulin sensitivity is impaired, the suppression of hepatic gluconeogenesis is altered, but paradoxically the effect on DNL is unaffected.68 Thus, obese patients with impaired glucose tolerance or diabetes and NFLD have induced DNL.
The main product of hepatic DNL is palmitic acid.90 Palmitic acid has been demonstrated to be a major driver of atherosclerosis and CAD by increasing the lectin-type oxidized LDL receptor 1 (LOX-1) expression and uptake of oxidized LDL in macrophages.91,92 Moreover cholesterol efflux from macrophages is inhibited thereby eliciting inflammation.93 In two studies in which normal and overweight subjects consumed 25% of energy in form of either HFCS, fructose or glucose for 14 days, post-prandial triglycerides and fasting and post-prandial levels of LDL, non-HDL-C, apoB, and apoB to apoA ratio were significantly increased in the HFCS and fructose but not glucose group.94 LDL particle size was reduced in fructose containing diets and a more atherogenic LDL-subclass distribution was seen.95 A study under similar conditions but conducted over 10 weeks resulted in a similar outcome.96 Yet another study performed by the same group demonstrated that consumption of beverages providing 10, 17.5, or 25% of energy requirements (Ereq) from HFCS leads to a dose-dependent increases of several risk factors for CVD within 2 weeks in young male and female volunteers. For example, the concentrations of non–HDL-C, LDL-C, apoB, uric acid, post-prandial apoCIII, and post-prandial triglyceride increase in proportion with the intake dose of HFCS.97 The same amount of fructose intake results in increased hepatic DNL and hepatic lipid content, compared with a complex carbon diet for 9 days.98 Also acute post-prandial levels of total cholesterol, LDL, and HDL-C were increased after ingestion of 50 g fructose compared with the same amount of glucose or sucrose.99 In another double-blind, randomized, cross-over trial, nine healthy, normal weight males consumed each four different sugar-sweetened beverages (medium fructose at 40 g/day; high fructose, high glucose, or high sucrose all at 80 g/day) for three consecutive weeks.100 Energy intake was controlled by food-records. LDL- and total cholesterol were significantly higher after medium fructose, high fructose and high sucrose.100 Moreover, clamp experiments revealed that hepatic suppression of glucose production by insulin was much lower after high fructose than after high glucose diet.100 In a similar trial, male or female volunteers were fed with a diet containing just 17% fructose or glucose for 6 weeks. Dietary fructose intake was associated with increased fasting and postprandial plasma triacylglycerol concentrations only in men. No effects were observed regarding total-, HDL, or LDL-C in either men or women.101 These studies differ from results of other meta-analyses where no association between fructose consumption and uric acid, LDL-C or postprandial triglyceride levels have been obvious.102–104 Possible explanations for the contrasting results include lack of a proper control group, since fructose ingestion was compared to sucrose-diets,105,106 a noticeable energy restriction that prevents uric acid formation,107,108 the use of milk as a vehicle for the sugars, leading to milk overconsumption compared to normal population,97,109 the statistical analyses employed,97,109 and lack of an objective measure of compliance, e.g. by addition of riboflavin in the beverage.96,97
Another important meta-analysis focused on the link between fructose and NAFLD.110 The authors argued that the apparent link between added sugar/HFCS consumption and NAFLD is confounded by excessive energy intake and recommended that the evidence is not sufficiently robust to draw a conclusion on a possible association between added sugar/HFCS intake and NAFLD.110 This conclusion was reached since only the hypercaloric-fructose and not weight-maintenance diets were leading to increased liver fat.110 However, the follow-up in the iso-caloric fructose diets study was only 4 weeks.110 Intervention studies with a duration of 6 months clearly showed an increase in liver fat after consumption of 1 L/d cola compared with the same amount of milk, diet cola, or water consuming an iso-caloric diet,38 and a decrease in liver fat in adults and children with diagnosed NAFLD when fructose intake was limited.111,112
In sum, several reasons account for increased postprandial triglyceride levels and NAFLD following consumption of fructose-containing sugars increases post-prandial triglyceride levels and NAFLD for several reasons: KHK-C is not regulated by hepatic energy status and results in unregulated hepatic fructose uptake. The excess substrate leads to increased DNL while inhibiting fatty acid oxidation resulting in decreased hepatic insulin sensitivity. Increased intrahepatic lipid content promotes very low density lipoprotein (VLDL) production and excretion leading to increased postprandial triglycerides (Figure 1). Moreover, the unrestricted KHK-C activity results in ATP depletion and uric acid production via the purine degradation pathway. Postprandial hypertriglyceridaemia and increased uric acid levels are both risk factors for CVD113–115
Regulation of fructose metabolism by environmental cues: the hypoxia connection
Oxygen fuels the generation of energy in form of ATP through oxidative phosphorylation, which is designed to maximize ATP yield. Anaerobic energy production through glycolysis is inefficient in generating ATP. Thus, oxygen is a central component of cellular metabolism and harnessing it is critical for maintaining cell and tissue function. Accordingly, disrupted oxygen homeostasis is associated with multiple disease states ranging from obesity and diabetes to cancer, ischaemia, and heart disease.
Physiologic or pathophysiologic conditions that lead to a reduction in the amount of O2 available to a tissue, activate the transcription factor hypoxia-inducible factor (HIF). Hypoxia-inducible factor plays a central role in the transcriptional response to changes in oxygen availability. It is composed of an O2-labile α-subunit (HIF1α, HIF2α, HIF3α) and a stable β-subunit (HIF1β).116 Together, these subunits bind hypoxia-responsive elements and induce the transcriptional activation of target genes whose protein products contribute to various cellular processes including cell survival, angiogenesis, and metabolic reprogramming (Figure 3).
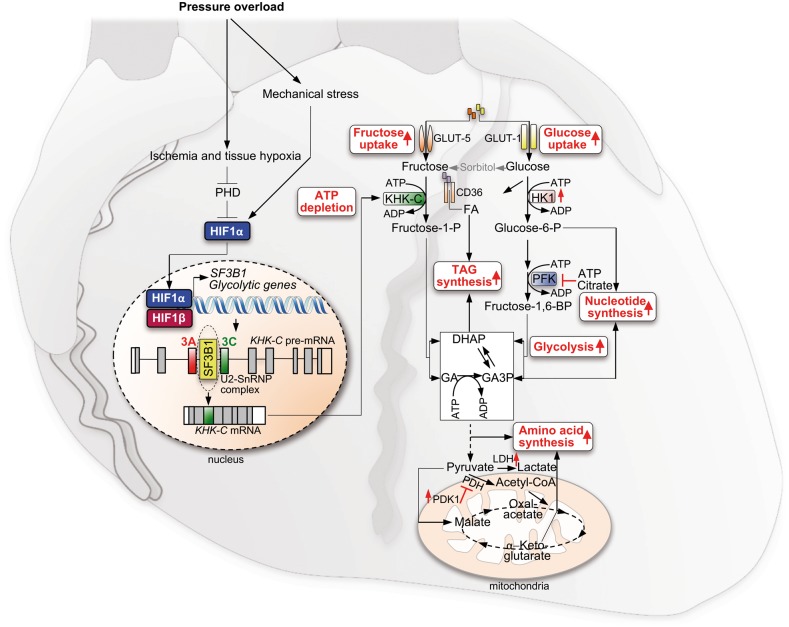
Figure 3.
Fructose-driven cardiac hypertrophy and ATP depletion mediated by the HIF-SF3B1-KHK-C Axis. Pressure overload-induced left ventricular wall stress activates a hypoxic response, mediated by activation and accumulation of HIF1, which drives the expression of the splice factor SF3B1. Increased levels of SF3B1 change splicing of KHK pre-mRNA due to different branch point recognition, resulting in KHK-C expression. In parallel with HIF-induced glycolysis, fructose uptake and metabolism drive anabolic cell growth, by induction of TAG, amino acid and nucleotide biosynthesis. GLUT1, glucose transporter 1; GLUT5, glucose transporter 5; GA, glyceraldehyde; GA3P, glyceraldehyde 3-phosphate; HK1, hexokinase 1; HIF1α, hypoxia-inducible factor 1α, KHK-C, ketohexokinase C; LDH, lactate-dehydrogenase; PDH, pyruvate-dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; SF3B1, splice factor 3 subunit B1.
While HIF activation is well known to mediate a shift from oxidative phosphorylation to glycolysis, recent evidence suggests that HIF also impacts fructose metabolism in the context of cardiac pathologic stress-induced hypertrophic growth.117 The underlying molecular mechanism involves functional interactions between HIF and components of the core splicing machinery resulting in KHK isoform switching. This process can be triggered by increased left ventricular wall stress and tissue hypoxia that promote accumulation of HIF1α, which induces the transcriptional activation of splice factor 3 subunit B1 (SF3B1) (Figure 3). SF3B1 is a core component of the U2 (small nuclear ribonucleoprotein (snRNP) complex of the spliceosome, a ribonucleoprotein complex central for pre-mRNA splicing. It targets and binds to the branch point, a distinct region on the intronic pre-mRNA, upstream of the next exon to be included.118 In the healthy heart, the U2snRNP complex targets primarily the branchpoint region upstream of exon 3 of the KHK pre-mRNA. Consequently, regular cardiomyocytes express KHK-A isoform, which is characterized by a low affinity towards fructose, avoiding fructose uptake and metabolism.117 Increased levels of SF3B1 in response to hypoxia drive the use of an alternative branch-point upstream of the mutually exclusive exon 4 of the KHK pre-mRNA, leading to expression of KHK-C117 (Figure 3). KHK-C expression activates fructose metabolism and uptake, while genetic deletion of Khk in the mouse or the expression of KHK-A diminishes fructose metabolism.66,117 Increased fructose uptake has been linked to cardiac, renal, and adipose tissue hypertrophy.117,119–121 However, it is so far unclear if this link is a direct effect of increased fructose uptake or rather due to secondary effects induced by increased DNL, insulin resistance, inflammation, or reactive oxygen species production.122
Chronic stabilization of HIF-1α, triggered for example by myocardial ischaemia, causes pathologic hypertrophic growth and contractile dysfunction (Figure 3). The role of HIF in driving the SF3B1-KHK-C axis suggests a mechanistic link between hypoxia-mediated activation of anabolic cellular growth and fructose metabolism. Activated cardiac fructolysis ensures a high glycolytic turnover, achieved by the unrestricted phosphorylation of incoming fructose by KHK-C resulting in increased ATP utilization, in line with the observed relative decline of ATP in diseased hearts of hypertrophic cardiomyopathy and aortic stenosis patients where the HIF1α-SF3B1-KHK-C axis is active.117,123,124 Consequently, the ATP depletion prevents allosteric inhibition of PFK1 by too high levels of ATP or citrate, allowing the maintenance of a high glycolytic flux and sufficient ATP generation, critical for anabolic growth (Figure 3). Similarly as observed in the diseased heart, increased fructose uptake and metabolism was recently identified as being highly activated in naked-mole rats under extreme hypoxia, preventing inhibition of glycolysis by phosphofructokinase and thus, ensuring survival even in prolonged anoxia.125 This observation implicates a therapeutic strategy, that activation of cardiac fructose metabolism in response to myocardial infarction might improve the short-term ischaemia tolerance of the heart.
Glycolysis is the starting point of various biosynthetic pathways within the cell. Hypoxia-inducible factor stabilization in cardiomyocytes can thus result in increased amino acid biosynthesis, induction of both the non-oxidative and oxidative pentose phosphate pathways, and nucleotide and triacylglycerol biosynthesis. Redox balance can also be significantly affected, due to extensive use of reducing equivalents for macromolecular biosynthesis. In addition, fructolysis may support anabolic growth also by channelling additional carbons into DHAP, which are further metabolized into triacylglycerol, nucleotide, and amino acid biosynthesis (Figure 3). Moreover, KHK-C expression is accompanied by transcriptional induction of GLUT5, as has also been noted in hypoxic adipocytes.126 Consistent with these findings, metabolomic experiments revealed that the anabolic state of the cell during HIF1 stabilization depends in large part on KHK-C expression, whereas depletion of KHK-A had no effect.117
Finally, recent work suggests that excessive hepatic glucose which is converted to fructose via the polyol pathway and is not completely metabolized to F-1-P can also be secreted systemically,67 likely explaining why diabetic patients have increased plasma fructose levels.127 Increased circulating fructose drives fructose and glucose uptake in cardiomyocytes, leading to cardiac hypertrophy.117,119 Consequently, the HIF1-SF3B1-KHK-C axis might explain in part why diabetic patients are at higher risk for heart disease.
Dietary restrictions and molecular therapies
Cardiovascular disease is the primary cause of death in patients with CMS. Primary prevention is thus imperative. This should include weight management, consumption of a heart-healthy diet, restriction of sugar as close as possible to 25 g daily as recommended by the WHO, increased physical activity, smoking cessation, and reduction of mental stress.32 However, many studies have revealed the difficulty of successful lifestyle interventions,128,129 with only moderate effects on reduction of major risk factors of CAD, including hypertension, dyslipidaemia, and insulin resistance. Drug therapies targeting blood pressure, lipid, and sugar metabolism and insulin sensitivity are thus widely used.130–132
In light of the consequences of induced hepatic and cardiac fructose metabolism discussed here, inhibitors that target specifically KHK-C could be promising new therapeutic agents. In experiments with Khk null mice, fructose-induced NAFLD and NASH as well as left ventricular hypertrophy caused by pressure-overload were prevented.66,117 Thus, KHK-C specific inhibitors might offer a powerful therapeutic option, without significant systemic side effects. Early screening results are promising,133,134 but experimental data in models of NAFLD or cardiac hypertrophy have not yet been reported.
Conclusions
Ample clinical and basic biological evidence indicates that consumption of excess sugar promotes the development of CMS, CVD, and type 2 diabetes. This occurs both directly and indirectly. In the liver, GLUT2 and KHK-C mediated unrestricted hepatic fructose uptake and metabolism causes intrahepatic lipid accumulation, dyslipidaemia, decreased insulin sensitivity, and hyperuricemia. In the heart, HIF1α-driven activation of fructose uptake and metabolism by an SF3B1-mediated splice shift resulting in expression of KHK-C instead of KHK-A supports anabolic cardiac growth and systolic and diastolic dysfunction in response to cardiac stress. Epidemiological data and interventional studies corroborate experimental data obtained in cells and animal models and indicate increased cardiovascular mortality in people with elevated fructose intake. Moreover, controlled diet intervention studies in humans revealed increased rate of cardiovascular risk factors, especially increased levels of circulating lipids and decreased insulin sensitivity, in response to fructose intake. An important lesson taken from the diet-heart hypothesis is that a healthy diet and prevention of excessive caloric intake is likely the most effective nutritional strategy to prevent CMS and CVD.
Funding
Grants from the Swiss Heart Foundation and SNF Sinergia to W.K.; and the NIH (NIDDK DK107667) to Z.A.
Conflict of interest: none declared.
References
- 1. Popkin BM, Adair LS, Ng SW.. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC.. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 3. Madan SA, John F, Pyrsopoulos N, Pitchumoni CS.. Nonalcoholic fatty liver disease and carotid artery atherosclerosis in children and adults: a meta-analysis. Eur J Gastroenterol Hepatol 2015;27:1237–1248. [DOI] [PubMed] [Google Scholar]
- 4. Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang D-H, Gersch MS, Benner S, Sánchez-Lozada LG.. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 5. Bohannon NV, Karam JH, Forsham PH.. Endocrine responses to sugar ingestion in man. Advantages of fructose over sucrose and glucose. J Am Diet Assoc 1980;76:555–560. [PubMed] [Google Scholar]
- 6. Hill R, Baker N, Chaikoff IL.. Altered metabolic patterns induced in the normal rat by feeding an adequate diet containing fructose as sole carbohydrate. J Biol Chem 1954;209:705–716. [PubMed] [Google Scholar]
- 7. Bray GA, Nielsen SJ, Popkin BM.. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–543. [DOI] [PubMed] [Google Scholar]
- 8. Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB.. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Després J-P, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P.. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039–1049. [DOI] [PubMed] [Google Scholar]
- 10. Lim JS, Mietus-Snyder M, Valente A, Schwarz J-M, Lustig RH.. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7:251–264. [DOI] [PubMed] [Google Scholar]
- 11. Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CDA, Heine RJ, Diamant M.. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 2007;191:391–396. [DOI] [PubMed] [Google Scholar]
- 12. Goessling W, Massaro JM, Vasan RS, D'agostino RB, Ellison RC, Fox CS.. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology 2008;135:1935–1944, 1944.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kunutsor SK, Seddoh D, Zirlik A.. Alanine aminotransferase and risk of the metabolic syndrome: a linear dose-response relationship. PLoS One 2014;9:e96068.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collaboration NRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016;387:1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheidt-Nave C, Du Y, Knopf H, Schienkiewitz A, Ziese T, Nowossadeck E, Gößwald A, Busch MA.. Verbreitung von Fettstoffwechselstörungen bei Erwachsenen in Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:661–667. [DOI] [PubMed] [Google Scholar]
- 17. Cohen JD, Cziraky MJ, Cai Q, Wallace A, Wasser T, Crouse JR, Jacobson TA.. 30-Year trends in serum lipids among United States adults: results from the National Health and Nutrition Examination Surveys II, III, and 1999–2006. Am J Cardiol 2010;106:969–975. [DOI] [PubMed] [Google Scholar]
- 18. Eldor R, Raz I.. American Diabetes Association indications for statins in diabetes. Diabetes Care 2009;32:S384–S391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R, Wasserman SM, Raal FJ.. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 20. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH.. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Circulation 2006;113:898–918. [DOI] [PubMed] [Google Scholar]
- 21. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome. Diabetes Care 2005;28:1769–1778. [DOI] [PubMed] [Google Scholar]
- 22. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J-P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P-H, Yip P, Zabetian A, Zheng Z-J, Lopez AD, Murray CJL.. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anitschkow NCS. Ueber experimentelle Choles teria steatose und ihre Bedeutung fur die Entstehung einiger pathologischer Prozesse. Zentralbl Allg Pathol Pathol Anat 1913;24:1–9. [Google Scholar]
- 24. Keys A. Prediction and possible prevention of coronary disease. Am J Public Heal Nations Heal 1953;43:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keys A, Mienotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H.. The diet and 15-year death rate in the seven countries study. Am J Epidemiol 1986;124:903–915. [DOI] [PubMed] [Google Scholar]
- 26. Association CC for M and CP of the AH. DIetary fat and its relation to heart attacks and strokes. JAMA 1961;175:389–391. [PubMed] [Google Scholar]
- 27. The multiple risk factor intervention trial (mrfit): A national study of primary prevention of coronary heart disease. JAMA 1976; 235: 825–827. https://www.ncbi.nlm.nih.gov/pubmed/946311. [PubMed] [Google Scholar]
- 28. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA.. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 29. Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR.. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016;353:i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Scientific Report of the 2015 Dietary Guidelines Advisory Commitee. 2015.
- 31. Keys A. Mediterranean diet and public health: personal reflections. Am J Clin Nutr 1995;61:1321S–1323S. [DOI] [PubMed] [Google Scholar]
- 32. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, Worp HB. V D, Dis I. V, Verschuren WMM, Binno S, Backer GD, Roffi M, Aboyans V.. 2016 European Guidelines on cardiovascular disease prevention in clinical practiceThe Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorgeril M. D, Salen P, Martin J-L, Monjaud I, Delaye J, Mamelle N.. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction. Circulation 1999;99:779 LP–785. [DOI] [PubMed] [Google Scholar]
- 34. Yudkin J. The causes and cure of obesity. Lancet 1959; 2: 1135–1138. [DOI] [PubMed] [Google Scholar]
- 35. Yudkin J. Patterns and trends in carbohydrate consumption and their relation to disease. Proc Nutr Soc 1964;23:149–162. [DOI] [PubMed] [Google Scholar]
- 36. Souza RJ. D, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS.. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015; 351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS.. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B.. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–289. [DOI] [PubMed] [Google Scholar]
- 39. Siegel KR, Echouffo-Tcheugui JB, Ali MK, Mehta NK, Narayan KM, Chetty V.. Societal correlates of diabetes prevalence: an analysis across 94 countries. Diabetes Res Clin Pract 2012;96:76–83. [DOI] [PubMed] [Google Scholar]
- 40. Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB.. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welsh JA, Sharma A, Cunningham SA, Vos MB.. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011;123:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB.. ADded sugar intake and cardiovascular diseases mortality among us adults. JAMA Intern Med 2014;174:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koning L, de Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB.. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation 2012;125:1735–1741, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morenga LA, Te Howatson AJ, Jones RM, Mann J.. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014;100:65–79. [DOI] [PubMed] [Google Scholar]
- 45. World Health Organization. Sugars intake for adults and children: Guideline. 2015. http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed 15 July 2017). [PubMed]
- 46. Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J.. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–1020. [DOI] [PubMed] [Google Scholar]
- 47. Gearhardt N, Davis A, Kuschner C, Rachel D, Brownell K.. The addiction potential of hyperpalatable foods. Curr Drug Abuse Rev 2011;4:140–145. [DOI] [PubMed] [Google Scholar]
- 48. Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ.. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–2972. [DOI] [PubMed] [Google Scholar]
- 49. Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 2016;53:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS.. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013;309:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bremer AA, Byrd RS, Auinger P.. Differences in male and female adolescents from various racial groups in the relationship between insulin resistance-associated parameters with sugar-sweetened beverage intake and physical activity levels. Clin Pediatr (Phila) 2010;49:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rhee JJ, Mattei J, Campos H.. Association between commercial and traditional sugar-sweetened beverages and measures of adiposity in Costa Rica. Public Health Nutr 2012;15:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balcells E, Delgado-Noguera M, Pardo-Lozano R, Roig-González T, Renom A, González-Zobl G, Muñoz-Ortego J, Valiente-Hernández S, Pou-Chaubron M, Schröder H.. Soft drinks consumption, diet quality and BMI in a Mediterranean population. Public Health Nutr 2011;14:778–784. [DOI] [PubMed] [Google Scholar]
- 54. Bermudez OI, Gao X.. Greater consumption of sweetened beverages and added sugars is associated with obesity among US young adults. Ann Nutr Metab 2010;57:211–218. [DOI] [PubMed] [Google Scholar]
- 55. Ludwig DS, Peterson KE, Gortmaker SL.. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001;357:505–508. [DOI] [PubMed] [Google Scholar]
- 56. Lowette K, Roosen L, Tack J, Vanden BP. Effects of high-fructose diets on central appetite signaling and cognitive function. Front Nutr 2015;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quercioli A, Pataky Z, Vincenti G, Makoundou V, Marzo V, Di Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, Mach F, Schindler TH.. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J 2011;32:1369–1378. [DOI] [PubMed] [Google Scholar]
- 58. Holmes R, Lobley RW.. Intestinal brush border revisited. Gut 1989;30:1667–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Drozdowski LA, Thomson ABR.. Intestinal sugar transport. World J Gastroenterol 2006;12:1657–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel C, Douard V, Yu S, Gao N, Ferraris RP.. Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J 2015;29:4046–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rumessen JJ, Gudmand-Hoyer E.. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 1986;27:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Skoog SM, Bharucha AE.. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 2004;99:2046–2050. [DOI] [PubMed] [Google Scholar]
- 63. Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT.. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem 2009;57:763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Choi JM, Seo M-H, Kyeong H-H, Kim E, Kim H-S.. Molecular basis for the role of glucokinase regulatory protein as the allosteric switch for glucokinase. Proc Natl Acad Sci USA 2013;110:10171–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eggleston LV, Woods HF.. Activation of liver pyruvate kinase by fructose-1-phosphate. FEBS Lett 1970;6:43–45. [DOI] [PubMed] [Google Scholar]
- 66. Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, MacLean PS, Jackman MR, Asipu A, Roncal-Jimenez CA, Kosugi T, Rivard CJ, Maruyama S, Rodriguez-Iturbe B, Sanchez-Lozada LG, Bonthron DT, Sautin YY, Johnson RJ.. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 2012;109:4320–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzicky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sánchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Lanaspa MA, Johnson RJ.. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Softic S, Cohen DE, Kahn CR.. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci 2016;61:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Loomba R, Sanyal AJ.. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 70. Chatrath H, Vuppalanchi R, Chalasani N.. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis 2012;32:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Targher G, Day CP, Bonora E.. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 72. Alvarez AM, Mukherjee D.. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol 2011;20:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cohen DE, Fisher EA.. Lipoprotein metabolism, dyslipidemia, and nonalcoholic fatty liver disease. Semin Liver Dis 2013;33:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M.. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Papandreou D, Karabouta Z, Pantoleon A, Rousso I.. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite 2012;59:939–944. [DOI] [PubMed] [Google Scholar]
- 76. Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R.. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 2007;47:711–717. [DOI] [PubMed] [Google Scholar]
- 77. Ouyang X, Cirillo P, Sautin Y, Mccall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF.. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T.. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci 2004;49:1578–1583. [DOI] [PubMed] [Google Scholar]
- 79. Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM.. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jin R, Welsh AJ, Le N-A, Holzberg J, Sharma P, Martin RD, Vos BM.. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 2014;6:3187–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mager DR, Iñiguez IR, Gilmour S, Yap J.. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). J Parenter Enter Nutr 2015;39:73–84. [DOI] [PubMed] [Google Scholar]
- 82. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ.. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ.. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Strable MS, Ntambi JM.. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 2010;45:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saggerson D. Malonyl-CoA, a key signaling molecule in mammalian cells. Annu Rev Nutr 2008;28:253–272. [DOI] [PubMed] [Google Scholar]
- 86. Fisher Ffolliott M, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, Hellerstein MK, Hudgins LC, Maratos-Flier E, Herman MA.. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab 2017;6:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W.. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010;53:934–940. [DOI] [PubMed] [Google Scholar]
- 88. Jornayvaz FR, Shulman GI.. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab 2012;15:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Owen JL, Zhang Y, Bae S-H, Farooqi MS, Liang G, Hammer RE, Goldstein JL, Brown MS.. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci USA 2012;109:16184–16189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ma W, Wu JHY, Wang Q, Lemaitre RN, Mukamal KJ, Djousse L, King IB, Song X, Biggs ML, Delaney JA, Kizer JR, Siscovick DS, Mozaffarian D.. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J Clin Nutr 2015;101:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ishiyama J, Taguchi R, Yamamoto A, Murakami K.. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis 2010;209:118–124. [DOI] [PubMed] [Google Scholar]
- 92. Praagman J, Jonge EAL. D, Kiefte-de Jong JC, Beulens JWJ, Sluijs I, Schoufour JD, Hofman A, Schouw YT. V D, Franco OH.. Dietary saturated fatty acids and coronary heart disease risk in a Dutch middle-aged and elderly population. Arterioscler Thromb Vasc Biol 2016;36:2011–2018. [DOI] [PubMed] [Google Scholar]
- 93. Afonso MS, Lavrador MSF, Koike MK, Cintra DE, Ferreira FD, Nunes VS, Castilho G, Gioielli LA, Paula Bombo R, Catanozi S, Caldini EG, Damaceno-Rodrigues NR, Passarelli M, Nakandakare ER, Lottenberg AM.. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J Nutr Biochem 2016;32:91–100. [DOI] [PubMed] [Google Scholar]
- 94. Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, Chen G, Fong TH, Lee V, Menorca RI, Keim NL, Havel PJ.. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011;96:E1596–E1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K.. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–485. [DOI] [PubMed] [Google Scholar]
- 96. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, Mcgahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ.. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ.. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015;101:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schwarz J-M, Noworolski SM, Wen MJ, Dyachenko A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, Bergeron N, Bersot TP, Rao MN, Schambelan M, Mulligan K.. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 2015;100:2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jameel F, Phang M, Wood LG, Garg ML.. Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation. Lipids Health Dis 2014;13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K.. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bantle JP, Raatz SK, Thomas W, Georgopoulos A.. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 2000;72:1128–1134. [DOI] [PubMed] [Google Scholar]
- 102. David Wang D, Sievenpiper JL, Souza RJ, de Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Buono M, Di Jenkins AL, Leiter LA, Wolever TMS, Beyene J, Kendall CWC, Jenkins DJA.. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125–133. [DOI] [PubMed] [Google Scholar]
- 103. Buul VJ, van Tappy L, Brouns FJPH.. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr Res Rev 2014;27:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J.. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–688. [DOI] [PubMed] [Google Scholar]
- 105. Huttunen JK, Makinen KK, Scheinin A.. Turku sugar studies XI. Effects of sucrose, fructose and xylitol diets on glucose, lipid and urate metabolism. Acta Odontol Scand 1976;34:345–351. [DOI] [PubMed] [Google Scholar]
- 106. Crapo PA, Kolterman OG.. The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr 1984;39:525–534. [DOI] [PubMed] [Google Scholar]
- 107. Forster H, Heller G.. [Studies on the significance of carbohydrates in a fully synthetic fat-free diet]. Dtsch Med Wochenschr 1973;98:1156–1163. [DOI] [PubMed] [Google Scholar]
- 108. Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Perez-Mendez O, Vazquez A, Ruiz A, Lanaspa MA, Jimenez CR, Johnson RJ, Lozada L-GS.. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011;60:1551–1559. [DOI] [PubMed] [Google Scholar]
- 109. Yu Z, Lowndes J, Rippe J.. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res 2013;33:1043–1052. [DOI] [PubMed] [Google Scholar]
- 110. Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH.. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Volynets V, Machann J, Kuper MA, Maier IB, Spruss A, Konigsrainer A, Bischoff SC, Bergheim I.. A moderate weight reduction through dietary intervention decreases hepatic fat content in patients with non-alcoholic fatty liver disease (NAFLD): a pilot study. Eur J Nutr 2013;52:527–535. [DOI] [PubMed] [Google Scholar]
- 112. Vos MB, Weber MB, Welsh J, Khatoon F, Jones DP, Whitington PF, McClain CJ.. Fructose and oxidized ldl in pediatric nonalcoholic fatty liver disease: a pilot study. Arch Pediatr Adolesc Med 2009;163:674–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. O’Keefe JH, Bell DSH.. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol 2007;100:899–904. [DOI] [PubMed] [Google Scholar]
- 114. Boren J, Matikainen N, Adiels M, Taskinen M-R.. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 2014;431:131–142. [DOI] [PubMed] [Google Scholar]
- 115. Soltani Z, Rasheed K, Kapusta DR, Reisin E.. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep 2013;15:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mirtschink P, Krek W.. Hypoxia-driven glycolytic and fructolytic metabolic programs: pivotal to hypertrophic heart disease. Biochim Biophys Acta 2016;1863:1822–1828. [DOI] [PubMed] [Google Scholar]
- 117. Mirtschink P, Krishnan J, Grimm F, Sarre A, Hörl M, Kayikci M, Fankhauser N, Christinat Y, Cortijo C, Feehan O, Vukolic A, Sossalla S, Stehr SN, Ule J, Zamboni N, Pedrazzini T, Krek W.. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015;522:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bonnal S, Vigevani L, Valcárcel J.. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov 2012;11:847–859. [DOI] [PubMed] [Google Scholar]
- 119. Johnson RJ, Sanchez-Lozada LG, Nakagawa T.. The effect of fructose on renal biology and disease. J Am Soc Nephrol 2010;21:2036–2039. [DOI] [PubMed] [Google Scholar]
- 120. Zubiria MG, Farina JP, Moreno G, Gagliardino JJ, Spinedi E, Giovambattista A.. Excess fructose intake-induced hypertrophic visceral adipose tissue results from unbalanced precursor cell adipogenic signals. FEBS J 2013;280:5864–5874. [DOI] [PubMed] [Google Scholar]
- 121. Du L, Heaney AP.. Regulation of adipose differentiation by fructose and GluT5. Mol Endocrinol 2012;26:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Charrez B, Qiao L, Hebbard L.. The role of fructose in metabolism and cancer. Horm Mol Biol Clin Investig 2015;22:79–89. [DOI] [PubMed] [Google Scholar]
- 123. Mahmod M, Francis JM, Pal N, Lewis A, Dass S, Silva R, De Petrou M, Sayeed R, Westaby S, Robson MD, Ashrafian H, Neubauer S, Karamitsos TD.. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson 2014;16:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ashrafian H, Redwood C, Blair E, Watkins H.. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet 2003;19:263–268. [DOI] [PubMed] [Google Scholar]
- 125. Park TJ, Reznick J, Peterson BL, Blass G, Omerbašić D, Bennett NC, Kuich PHJL, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH, Kosten T, Lutermann H, Gavaghan V, Eigenbrod O, Bégay V, Amoroso VG, Govind V, Minshall RD, Smith ESJ, Larson J, Gotthardt M, Kempa S, Lewin GR.. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017;356:307 LP–311. [DOI] [PubMed] [Google Scholar]
- 126. Stuart Wood I, Wang B, Lorente-Cebrián S, Trayhurn P.. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-d-glucose uptake in human adipocytes. Biochem Biophys Res Commun 2007;361:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kawasaki T, Akanuma H, Yamanouchi T.. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care 2002;25:353–357. [DOI] [PubMed] [Google Scholar]
- 128. Yamaoka K, Tango T.. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med 2012;10:138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang X, Devlin HM, Smith B, Imperatore G, Thomas W, Lobelo F, Ali MK, Norris K, Gruss S, Bardenheier B, Cho P, Garcia de Quevedo I, Mudaliar U, Jones CD, Durthaler JM, Saaddine J, Geiss LS, Gregg EW.. Effect of lifestyle interventions on cardiovascular risk factors among adults without impaired glucose tolerance or diabetes: a systematic review and meta-analysis PLoS One 2017;12:e0176436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H-P, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen M-R, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C.. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboratio. Eur Heart J 2013;34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 131. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Mario C, Di FJR, Gersh BJ, Gitt AK, Hulot J-S, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, Wall EE, van der VCJM, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V.. 2013 ESC guidelines on the management of stable coronary artery disease The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 132. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Meer P, van der FG, McMurray JJV, Aboyans V, Achenbach S, Agewall S, Al AN, Atherton JJ, Bauersachs J, John CA.. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureThe Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 133. Le MT, Lanaspa MA, Cicerchi CM, Rana J, Scholten JD, Hunter BL, Rivard CJ, Randolph RK, Johnson RJ.. Bioactivity-guided identification of botanical inhibitors of ketohexokinase. PLoS One 2016;11:e0157458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Maryanoff BE, O'neill JC, Mccomsey DF, Yabut SC, Luci DK, Jordan AD, Masucci JA, Jones WJ, Abad MC, Gibbs AC, Petrounia I.. Inhibitors of ketohexokinase: discovery of pyrimidinopyrimidines with specific substitution that complements the ATP-binding site. ACS Med Chem Lett 2011;2:110428133546010.. [DOI] [PMC free article] [PubMed] [Google Scholar]