Abstract
Administration of mesenchymal stem cells (MSCs) to diseased hearts improves cardiac function and reduces scar size. These effects occur via the stimulation of endogenous repair mechanisms, including regulation of immune responses, tissue perfusion, inhibition of fibrosis, and proliferation of resident cardiac cells, although rare events of transdifferentiation into cardiomyocytes and vascular components are also described in animal models. While these improvements demonstrate the potential of stem cell therapy, the goal of full cardiac recovery has yet to be realized in either preclinical or clinical studies. To reach this goal, novel cell-based therapeutic approaches are needed. Ongoing studies include cell combinations, incorporation of MSCs into biomaterials, or pre-conditioning or genetic manipulation of MSCs to boost their release of paracrine factors, such as exosomes, growth factors, microRNAs, etc. All of these approaches can augment therapeutic efficacy. Further study of the optimal route of administration, the correct dose, the best cell population(s), and timing for treatment are parameters that still need to be addressed in order to achieve the goal of complete cardiac regeneration. Despite significant progress, many challenges remain.
Keywords: mesenchymal stem cell, cardiovascular disease, regenerative medicine
Graphical Abstract
Administering mesenchymal stem cells (MSCs) to the injured heart improves cardiac function and reduces scar size, primarily by stimulating endogenous repair mechanisms. We review the progress, challenges, and potential of MSC-based therapy, and we propose that combining MSCs with other cell type(s) improves therapeutic efficacy.
Main Text
The last two decades have witnessed the development of novel regenerative medicine approaches for cardiovascular diseases. However, the ultimate goal of complete repair of the heart in response to damage has yet to be realized. Among the biggest hurdles to this goal is the inability of the adult heart to promote sufficient de novo cardiomyogenesis to replace cells lost to disease due to its (1) intractable genetic and epigenetic state, (2) limited cellular plasticity, and (3) proclivity to succumb to pro-inflammatory and pro-fibrotic immune pathways. To this end, gene and stem cell therapies are among the most promising regenerative approaches, and several designs are currently being tested, both alone and in combination.
Some of the most promising gene transfer therapies are designed to up-1, 2, 3, 4, 5, 6 or downregulate7, 8 the expression of cardiac, vascular, or immune system genes, which are abnormally expressed in the pathologic heart. Other approaches ectopically express oncogenes to force proliferation of adult cardiomyocytes.9 More recently, the transfer of lineage reprogramming gene cocktails into myocardial scars for converting non-cardiomyocytes into beating cardiomyocyte-like cells has been gaining support.10 Finally, recent advances in the development of high-precision genome-engineering tools, such as the CRISPR/Cas system,11 have introduced the possibility of using gene therapy to permanently edit and correct disease-causing mutations in the genome of adult cardiomyocytes.12, 13 However, despite the great promise, most preclinical and clinical studies have illustrated important limitations in the translation of gene therapy toward the clinical setting. For example, recent gene transfer clinical trials in patients with heart disease did not succeed in reaching the anticipated levels of efficacy seen in preclinical animal models.14, 15, 16 Similarly, a major caveat in using gene transfer for lineage reprogramming or cell proliferation-based cardiac therapies is the high risk of such interventions introducing ectopic cardiomyocytic or neoplastic formation if the recipient cell(s) and the activity of the transferred gene(s) are not well controlled. Finally, preclinical proof-of-concept experiments highlight important limitations in the applicability of the CRISPR/Cas system for cardiac regenerative medicine, such as the need for in situ genome editing of billions of cardiomyocytes at the single-cell level, without disrupting their function or introducing unwanted off-target mutations,12 as well as the genetic complexity of heart diseases, which are generally of polygenic or unknown genetic origin.
Cardiac cell-based therapies aim to overcome the limitations of gene therapy via the adoptive transfer of healthy cells, rather than isolated genes. Cells are thought to operate either directly, by replacing the unhealthy cells in the damaged tissue, or indirectly, via the secretion of molecules and microvesicles that stimulate endogenous mechanisms of immune regulation and cardiac regeneration. The cell grafts are derived from adult tissues, such as bone marrow,17, 18 skeletal muscle,19 and the heart itself20, 21 or from pluripotent stem cells22, 23, and they may be autologous17 or allogeneic18, 24 in origin. However, as with gene therapy, cell therapy faces many challenges toward clinical translation. For example, contrary to the original hypothesis that transplanted cells would directly remuscularize and regenerate the damaged myocardium, most of the adult cell types show limited cardiomyocyte differentiation capacity, while their long-term engraftment is minimal regardless of histocompatibility, due to immune clearance from the host myocardium.25 Similarly, strategies with bona fide cardiomyogenic cells, such as pluripotent stem cell-derived cardiac precursors and cardiomyocytes,26 or reprogrammed cells27 suffer from poor engraftment, regardless of histocompatibility, and, more importantly, they may become a source of neoplasia28 or arrhythmogenesis24, 29 until cleared from the host myocardium by the immune system. Nonetheless, the current consensus is that, despite the lack of evidence for durable, direct tissue replacement, numerous cell therapy regimens for cardiac dysfunction exert unequivocal beneficial effects, in part via incompletely understood paracrine mechanisms, which stimulate endogenous repair30, 31, 32 by, among other mechanisms, stimulating endogenous cardiac precursors and cardiomyocyte proliferation,32, 33, 34, 35, 36 secreting proangiogenic and prosurvival molecules and microvesicles,37, 38, 39, 40 and modulating the immune system41, 42, 43, 44 (Table 1).
Table 1.
Comparison of Different Regenerative Approaches for Cardiovascular Disease
| MSCs | CSCs | iPSCs | Gene Therapy | |
|---|---|---|---|---|
| Accessibility | readily accessible in large quantities from bone marrow55 and adipose tissue,61 also from placenta, umbilical cord, etc.;25 ideal for clinical use as allogeneic (MHC-mismatched), off-the-shelf cellular therapy due to immunoprivileged phenotype25 | requires access to adult cardiac tissue;20, 21 most likely clinical use as autologous cell therapy; allogeneic use may be possible;20, 21, 41, 48, 164, 165 the on-going CAREMI trial is testing the safety, feasibility, and efficacy of allogeneic CSCs in patients with acute myocardial infarction;165 unclear if immunosuppression necessary | requires reprogramming of somatic cells followed by directed differentiation into MSCs, CPCs, coronary vascular cells or CMs;35, 166 development of patient-specific, autologous cells clinically challenging due to manufacturing (costs and time);167 most likely clinical use as allogeneic (MHC matched) therapy24 | multiple techniques (e.g., TALENs, CRISPR/Cas); require development of live viral vectors for delivery in patient168 |
| Clinical and preclinical efficacy | reduction of fibrosis in scarred tissues;151 improved LVEF and endothelial function42, 60 | reduction in infarct size; improved LVEF20 | improved LVEF24, 169 | improved LVEF90, 170, 171 |
| Safety | no evidence for tumorigenic or arrhythmogenic risk after extensive preclinical and clinical testing both as autologous or allogeneic therapy17, 18, 42, 60 | no evidence for tumorigenic or arrhythmogenic risk after preclinical and clinical testing both as autologous or allogeneic (CDC) therapy20, 21, 41, 48 | no clinical data; preclinical studies indicate increased risk for arrhythmogenesis24, 172 and possibly tumor formation;173, 174, 175 high risk for immune rejection if histocompatibility mismatch | minimal tumorigenic, immunogenic, or arrhythmogenic risk from current clinical studies;176, 177 risk for mosaic gene disruption and introduction of novel off-target mutations following CRISPR/Cas gene therapy;12, 13 safety of direct cardiac cell reprogramming or cell proliferation strategies currently undetermined |
| Mechanism of action | anti-inflammatory and antifibrotic effects; stimulate endogenous repair by paracrine signaling (growth factors and microvesicles);32, 87, 88 limited capacity for direct tissue replacement78, 79, 80 | stimulate endogenous repair by paracrine signaling (growth factors and microvesicles); limited capacity for direct tissue replacement41, 48, 178 | unclear; evidence for direct remuscularization24, 172 or no evidence of remuscularization;112 evidence for paracrine effects37, 112, 169 | modulates gene expression |
Combinatorial approaches may prove to be the most efficacious. Combining cell and gene therapy offers the advantage that cells can be genetically engineered ex vivo prior to transplantation, offering a safer alternative and more precise control of gene expression compared to gene therapy alone.27, 38, 45, 46, 47 Another such approach involves the ex vivo engineering of cell grafts with mixtures of physiologically relevant cell types, such as cardiomyocytes; cardiac precursors; and vascular, neuronal, immune, and mesenchymal cell types,35, 41, 48, 49, 50, 51, 52, 53, 54 which may offer a more comprehensive regenerative strategy compared to each cell type alone, given that both cardiomyogenic and non-cardiomyogenic cells are necessary for heart function and regeneration.
Here we focus on the role of one particular cell type, the mesenchymal stem cell (MSC), in cell-based therapies for heart disease. We review the progress and challenges of MSC-based therapy in cardiac regeneration, emphasizing recent works and controversies, and we discuss future perspectives for MSCs in cardiovascular regeneration.
Characteristics of MSCs
MSCs are stromal cells found in most fully developed organs, including bone marrow, adipose tissue, placenta, umbilical cord, heart, and peripheral blood.25, 54, 55, 56, 57 They are multipotent stem cells, likely derived from diverse embryonic lineages, that self-renew and differentiate into several cell types, both mesoderm and neuroectoderm derived.58 Currently, the precise definition of MSCs remains unclear; but, to be classified as MSCs, cells must meet the following minimal characterization criteria, as defined by the International Society for Cellular Therapy: express a certain set of cluster of differentiation (CD) markers (CD105, CD73, and CD90); lack expression of hematopoietic lineage CD markers (CD45, CD34, CD14, CD19, and HLA-DR); be plastic adherent when maintained under standard culture conditions; and exhibit the ability to form into colony-forming unit fibroblasts (CFU-Fs) and differentiate into osteoblasts, adipocytes, and chondroblasts in vitro.59
MSCs are considered an attractive option for cell-based therapy because of their ease of isolation and secretion of bioactive molecules and microvesicles that activate tissue repair processes and modulate immune and inflammatory cells.25 Immune rejection is an important concern with allogeneic (allo) cell-based therapy, but the lack of cell surface histocompatibility complex (HLA) class II molecules and T cell costimulatory molecules and their paracrine-mediated immunomodulatory activity25, 42 provide MSCs an immunoprivileged status that abrogates the need for pharmacological immunosuppression and evades their destruction by the host immune system. Hence, MSCs are safely used as allografts and are well tolerated in cardiomyopathy patients, similar to autologous (auto) MSCs.60
Most studies have examined MSCs derived from bone marrow (BM) and adipose tissue (AT). MSCs from these tissues are easily isolated in large quantities, retain their immunomodulatory characteristics,55 and can produce high amounts of extracellular matrix components. However, these MSCs are not identical. Adipose tissue-derived MSCs (AT-MSCs) produce collagen (I, II, and III), while bone marrow-derived MSCs (BM-MSCs) exhibit high proangiogenic activity61 and have a greater immunosuppressive effect compared to AT-MSCs.62, 63, 64 BM-MSCs are the most competent cell type for the repression of induced T cell proliferation, and, via a paracrine mechanism, they showed a high anti-proliferative effect on phytohemagglutinin-primed T cells.64
Other potentially important sources of MSCs include the umbilical cord matrix, amniotic fluid, peripheral blood, and the heart itself, and each appears to have characteristic properties. For example, while MSCs isolated from the umbilical cord matrix (UCM-MSCs) have similar effects on blocking T cell activation as BM- and AT-MSCs, they have no apparent effect on B cells, unlike BM- and AT-MSCs, both of which inhibit B cell activation.65 Amniotic fluid-derived MSCs (AF-MSCs) have multipotent differentiation capability,66, 67 and, compared to BM-MSCs, they may also express the core pluripotency transcription factor OCT4.68, 69 Similarly, a type of circulating MSC present in the peripheral blood70 may also express pluripotency markers, such as SSEA-4, Oct-3/4, and TRA-1-81.71 However, the role of these markers remains unclear, since these circulating cells lack pluripotent differentiation capacity.72
CD105+/CD90+/CD45– MSC/MSC-like cells have been isolated from adult human,54 mouse,56, 57 and swine57 hearts. The human adult resident cardiac MSCs express cardiac lineage markers such as GATA-4 and smooth muscle actin. Compared to BM-MSCs, cardiac MSCs express comparable levels of major histocompatibility complex (MHC) classes I and II, but they exhibit slow growth in vitro.54 Importantly, they also express the costimulatory molecule CD40. Therefore, it is unclear if they exhibit the same immunoprivileged characteristic as BM-MSCs.54, 73 Murine cardiac mesenchymal cells (CMCs) can be separated into two groups based on the time for plastic adherence.57 The rapidly and slowly adhering CMCs were administered intramyocardially 3 days post-myocardial infarction (MI); 30 days later, the slowly adhering CMCs exhibited greater therapeutic efficacy than the rapidly adhering cells. Despite the therapeutic potential of these and other cardiac-derived stem cells, isolating these cells requires a cardiac biopsy, a further limitation.
MSCs derived from induced pluripotent stem cells (iPSC-MSCs) exhibit a similar phenotype as BM-MSCs; they differentiate into multiple cell types, exhibit tissue repair potential in ischemic disease,74, 75 and display important anti-inflammatory actions.76 iPSC-MSCs exhibit enhanced proliferative capacity compared to BM-MSCs, and they can be expanded for up to 40 passages while maintaining a normal diploid karyotype and a stable gene expression and surface antigen profile.74 With these inherent advantages, iPSC-MSCs represent a potential alternative cell source for tissue repair in ischemic disease. However, prior to clinical applications, systematic in vivo preclinical studies must be conducted to assess safety and tumor formation.75, 77
MSC Therapy: Mechanism of Action in Cardiac Regeneration
Under the proper conditions, MSCs are capable of transdifferentiating into functional cardiomyocytes in vivo and in vitro.78, 79, 80 For example, transplantation of human MSCs, expressing a β-galactosidase reporter gene, into immunodeficient mice with experimental myocardial damage resulted in β-galactosidase+ cardiomyocytes within the host myocardium, suggestive of cardiomyocyte differentiation of the xenografted MSCs.81 Similarly, sex-mismatched cell therapy experiments, in porcine models of acute32 and chronic MI,82 provide evidence that engrafted male porcine MSCs differentiated into cardiomyocytes within the female host myocardium, as ascertained by co-localization of the Y chromosome with cardiac transcription factors GATA-4, Nkx2.5, and the structural cardiac protein α-sarcomeric actin. Importantly, differentiated myocytes showed evidence of coupling to host myocardium via gap junctions composed of connexin-43.82 More recently, Cre-loxP-based genetic fate-mapping studies in mice delineated a pool of adult resident cardiac MSC-like epicardial cells, which could be culture expanded, and they retained full capacity to differentiate into cardiomyocytes in vitro and in vivo.56 However, the majority of these studies demonstrate that the level of direct MSC contribution to cardiomyocyte replacement is low and, therefore, unlikely to represent a therapeutically meaningful MSC mechanism of action.17, 32, 60, 83, 84
Many studies indicate that beneficial effects of MSC therapy can occur through paracrine signaling. The MSC secretome modulates several key cell processes that contribute to cardiovascular protection and/or repair under different pathological conditions.32, 33, 82, 85, 86, 87, 88, 89, 90 While MSCs from different sources share a substantial degree of similarity, there are variations in marker expression profile and secretomes.55, 91, 92 For example, AT-MSCs secrete higher amounts of angiogenic and anti-apoptotic growth factors, such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), as well as interleukin-6 (IL-6) in vitro, whereas BM-MSCs secrete higher amounts of the cell migration-related chemokine stromal cell-derived factor (SDF)-1α.92 UCM- (Wharton’s jelly) MSCs secrete higher levels of immune-signaling molecules and neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), compared to BM-MSCs, suggesting a greater beneficial role for Wharton jelly-MSCs in neurodegenerative diseases.93 Similarly, MSCs derived from embryonic stem cells (ESC-MSCs), which, like iPSC-MSCs, appear to represent an alternative source for stem cell therapy, may also better support neurogenic-related processes compared with BM-MSCs, which enhance angiogenesis in vitro94, 95 and in the damaged myocardium.85, 96, 97 Furthermore, a recent clinical study by Premer et al.86 shows that allogeneic MSC therapy improves endothelial function in patients with heart disease. Allogeneic MSCs secrete higher levels of nitric oxide than autologous MSCs, and patients receiving allogeneic MSCs had reduced levels of circulating VEGF compared to autologous MSCs.86 These differences in the MSC secretome suggest the potential use of differentially sourced MSCs to address specific medical conditions.98
MSCs promote endogenous cardiomyocyte regeneration. For example, MSC therapy stimulates survival and proliferation of adult cardiomyocytes via Akt-mediated pathways.32, 87, 88 In addition, SDF-1 secretion by MSCs increases the migration, proliferation, and cardiomyocyte differentiation of endogenous c-kit+ cardiac stem and/or progenitors.32, 33, 89 Notably, a recent study suggested that, despite its beneficial chemotactic effects, antagonism of SDF-1 in mobilized cardiac precursor cells enhanced their differentiation into cardiomyocytes, highlighting a potentially important mechanism of cardiomyogenesis involving transient up- and downregulation of the SDF-1/CXCR-4-signaling pathway. Based on these observations, it is intriguing to speculate that persistent activation of SDF-1 with gene therapy may be less preferable than transient, cell-based approaches for the treatment of heart failure.90
MSCs also exert beneficial effects on extracellular matrix remodeling. For example, MSCs modulate the phenotype of cardiac fibroblasts and their ability to degrade extracellular matrix, and, consequently, they promote the reduction of fibrosis in scarred tissues.99 They also exert a local antifibrotic effect following transplantation. Exposure of MSCs to type I collagen present in fibrotic tissue promotes dysregulation of myocyte regeneration and repair, and it leads to a downregulation of growth and inflammatory gene expression and a resultant decrease in MSC-induced myoblast proliferation.100, 101 The secretion of unique paracrine compounds, such as HGF, amplifies cardioprotection after MSC transplantation into the injured myocardium.102
Perhaps the most potent cardioprotective effects of MSC administration are elicited through immunomodulatory and anti-inflammatory actions. MSCs attenuate the cytotoxic activity of splenic lymphocytes, inhibit nuclear factor κB (NF-κB), reduce tumor necrosis factor alpha (TNF-α) and IL-6 protein expression, and stimulate IL-10 expression in the peri-infarct myocardium.25, 61, 103 TNF-α is an important activator of inflammation and TNF-α levels are increased in heart disease.104 In the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON)-dilated cardiomyopathy (DCM) clinical trial (ClinicalTrials.gov: NCT01392625), patients with non-ischemic dilated cardiomyopathy (NIDCM) had elevated levels of TNF-α at baseline, but these levels were significantly reduced 6 months following the administration of either allo- or auto-BM-MSCs, although allo-MSCs exhibited superior efficacy. This reduction correlated with improvements in cardiac function.42
As mentioned above, the lack of HLA class II surface markers and their immunomodulatory properties provide MSCs with the ability to exert their effects without being recognized by the host immune system. Paradoxically, pretreating MSCs with the IL-6 family member cardiotrophin-1, prior to intramyocardial injection into infarcted mice, improved engraftment and infarct size reduction.105 This result suggests that the host immune system itself helps to maintain MSC immunoprotection. This interplay was further illustrated by ixmyelocel-T cells, a mixed population of bone marrow mononuclear cells enriched for CD90+ MSCs (50-fold) and CD45+CD14+ M2 macrophages (∼200-fold). This mixture of cells improved angiogenesis and endothelial protection in a rat hindlimb ischemia model.44 Open-label phase IIA trials of ixmyelocel-T (ClinicalTrials.gov: NCT00765518 and NCT01020968) demonstrated that intramyocardial delivery of this cell combination improves clinical, functional, symptomatic, and quality-of-life outcomes in patients with heart failure due to ischemic dilated cardiomyopathy.106 A phase IIB clinical trial (ClinicalTrials.gov: NCT01670981) that has completed recruitment is designed to test the efficacy, safety, and tolerability of ixmyelocel-T compared to placebo (vehicle control) when administered via transendocardial, catheter-based injections to patients with end-stage heart failure due to ischemic dilated cardiomyopathy. Results up to 12 months post-injection suggest that the treatment significantly reduces clinical cardiac events, thereby improving patient outcomes.49, 107
Several modes of intercellular communications play a role in MSC mechanism of action. In addition to the release of paracrine factors, MSCs actually form tunneling nanotubes with adjacent cells capable of transferring organelles, such as mitochondria, and they also exchange microvesicles, macromolecular complexes, and exosomes40, 108, 109 between cells. The regulation of intercellular mitochondrial transport from MSCs to other cells has been attributed to an intrinsic expression of MIRO1, a mitochondrial Rho GTPase1, in the MSC.110 Moreover, the efficiency of this transfer is enhanced by the formation of tunneling nanotubes (TNTs) via activation of the TNT-α/NF-κB-signaling pathway. iPSC-MSCs overexpress MIRO1 and also have greater sensitivity to the pro-inflammatory cytokine TNF-α compared to BM-MSCs, boosting mitochondrial transfer potency.111 Simultaneously, the vesicles, macromolecular complexes, and exosomes release a wide range of functional proteins, mRNAs, and microRNAs (miRNAs) capable of protecting cardiac tissue from ischemic injury by promoting blood vessel formation and enhancing cell proliferation, thereby reducing infarct size and preserving cardiac systolic and diastolic performance.40, 108, 109, 112
Extracellular vesicles (EVs), released by a variety of cells, are thought to exert a wide variety of effects on target tissues. These small EVs, also known as exosomes, are 30–100 nm in diameter, and they contain large quantities of bioactive compounds, such as functional proteins, mRNAs, and miRNAs.108 EVs released by MSCs exposed to hypoxia and/or starvation produce positive effects on neoangiogenesis and functional recovery in the infarcted heart. MSCs release a large quantity of EVs upon exposure to hypoxic conditions in vitro. These EVs appear to protect the cells from undergoing apoptosis.109 In an acute MI rat model, the intramyocardial injection of MSC-EVs markedly enhanced blood flow recovery, reduced infarct size, and preserved cardiac systolic and diastolic performance.109 MSC-derived exosomes (MSC-Exos) boost the effect of cardiac stem cells (CSCs) in an MI model. Zhang et al.40 pre-treated CSCs with MSC-Exos in vitro and observed an incremental increase in the proliferation, migration, and angiogenic potency of the CSCs. This in vivo study showed that the treatment of CSCs with MSC-Exos enhanced engraftment and capillary density, reduced cardiac fibrosis, and improved cardiac outcome in a rat acute MI model.40 A recent novel study by Mayourian et al.113 utilized a mathematical model associated with a three-dimensional human engineered cardiac tissue (hECT) to elucidate the paracrine signaling and heterocellular coupling (connexins forming gap junctional channels between different cell types) of MSCs on human cardiac contractility and arrhythmogenicity. Their results confirmed that MSC-Exos are taken up by the cardiomyocytes and fibroblasts that constitute hECT and this paracrine signaling is responsible for significantly increasing the contractility of the hECT, also producing an anti-arrhythmic effect.113
Another poorly understood modulator is the effect of aging on MSC function. This understanding is important so as to optimize the use of autologous cells in the elderly. MSC function and therapeutic efficacy appear to decrease with donor age. Older individuals are typically afflicted by cardiovascular and other diseases. Disease and the cumulative exposure to other stressors, including reactive oxygen species, telomere attrition, and a decline in the fidelity of DNA repair and protein turnover systems, compromises the self-renewal potential and differentiation capacity of stem cells.114, 115 This age-dependent decline in stem cell number and function, including multilineage differentiation, homing, immune modulation, and wound healing,116 can shift the secretome profile of human MSCs toward the expression of senescence-associated factors.117 MSCs from patients with atherosclerosis secrete higher levels of cytokines, including interferon (IFN)-γ, IL12p70, IL-13, IL-2, and IL-4, key factors of the senescence-associated secretome.118 These changes suggest that it is of primary importance to monitor and prevent the appearance of a senescent phenotype in ex vivo expanded human MSCs (hMSCs).119 Not only does MSC senescence in vitro correlate with diminished function, but it may also explain why MSCs from older donors are less effective for in vitro immunopotency assays.120 A possible candidate to reverse MSC age-related senescence is SIRT3. SIRT3 expression is reduced upon MSC expansion. Increasing SIRT3 expression reverses this decline and improves MSC longevity and differentiation capacity.120
Does the age of the recipient limit the response to cell therapy?83, 121, 122 In the Transendocardial Autologous Cells in Ischemic Heart Failure Trial (TAC-HFT) and POSEIDON trial, older patients with chronic ischemic cardiomyopathy (ICM) were administered allogeneic or autologous MSCs via a transendocardial route. These trials demonstrate that older patients (≥60 years old) respond similarly to younger patients (<60 years old) with respect to reduction in scar size, improvement in 6-min walk distance test (6MWD), and the Minnesota living with heart failure questionnaire (MLHFQ) total score.83 This result suggests that recipient age does not reduce the effects of MSC therapy in patients with ICM.
MSCs for Cardiac Repair
Preclinical Studies
The cardiac regenerative capacity of MSCs has been explored in several small81, 123, 124, 125 and large animal32, 126, 127, 128, 129, 130, 131, 132, 133, 134 models of heart disease, including acute and chronic ischemic cardiomyopathies. Autologous,127, 129 allogeneic,32, 82, 128 or xenogeneic135, 136 MSCs have been studied, using different routes of administration, including intracoronary,129, 131, 132, 133, 134, 137 transendocardial,32, 128, 135 systemic,124 and intravenous.43, 125, 132 Most of these approaches support that MSC therapy, regardless of source, is effective at reducing scar size and improving left ventricular ejection fraction (LVEF). Specifically, a meta-analysis of 52 preclinical animal studies of cell therapy for ischemic heart disease138 suggested that MSC therapy is safe and associated with moderate (∼7.5%) but significant improvements in LVEF. However, the effect of cell therapy on LVEF decreased slightly after 8-week follow-up. Animals treated with BM-MSCs also exhibited a better outcome compared to those treated with mononuclear cells.138 While BM-MSCs have been most frequently utilized as a treatment of diseased hearts in a large animal model,32, 41, 48, 139, 140 other sources, such as obtained from adipose tissue132, 133, 134 and umbilical cord blood,131 have also produced significant improvement in LV function, perfusion, and remodeling in a large animal model of MI.
The inability of a single cell type to completely reverse the effects of MI prompted the examination of cell combination therapy (CCT; Figure 1). In swine models of MI, BM-MSCs and CSCs from xenogeneic (human),140 autologous,48 or allogeneic41 sources were co-injected into the border zone. In all three studies, CCT has greater therapeutic efficacy than a single cell type, augmenting cardiac regeneration and LV functional recovery without adverse immunologic reaction.41, 48, 140 The success of the combinatorial approach formed the basis for the CONCERT-HF (Combination of Mesenchymal and C-kit+ Cardiac Stem Cells as Regenerative Therapy for Heart Failure; ClinicalTrials.gov: NCT02501811) clinical trial. CONCERT-HF is a double-blind, placebo-controlled trial in which patients receive 150 × 106 autologous MSCs and/or 5 × 106 autologous CSCs. MSCs have also been tested in combination with ESCs and produced significant functional benefit in murine studies. The transplantation of hMSCs into an immunocompetent rat model of MI created a suppressive local microenvironment that mitigated the expected rejection of the co-injected hESCs, thus favorably affecting cell engraftment and functional recovery.50 These and other studies,141, 142, 143, 144 demonstrating that CCT is a more effective treatment for MI than a single cell type, represent just one example of the next generation of approaches designed to optimize cell-based therapy.
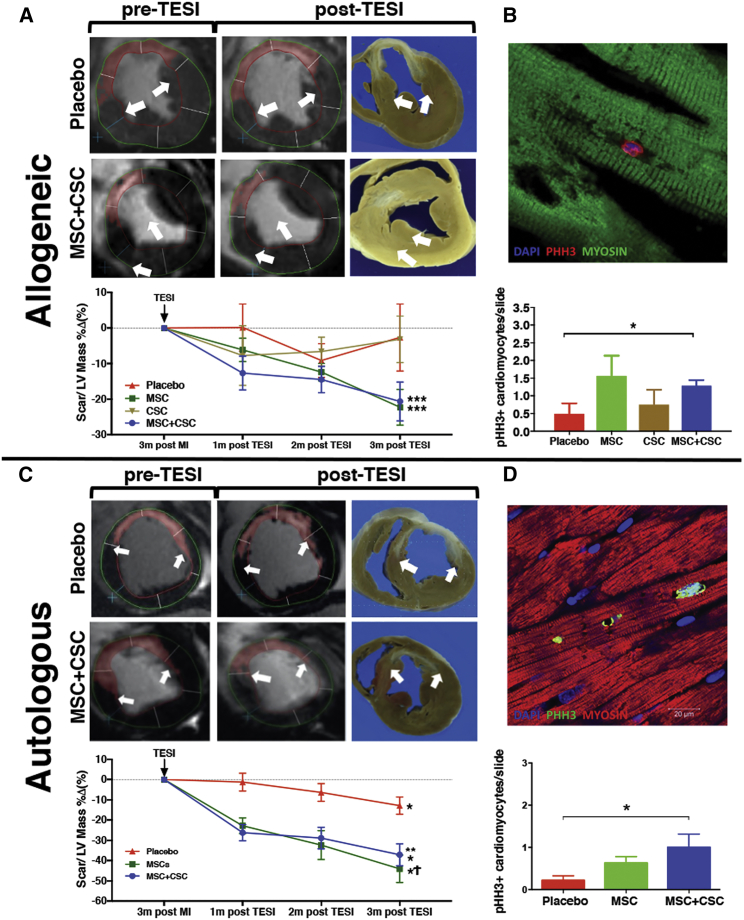
Figure 1.
Cell Combination Therapy (MSC + CSC) Reduces Scar Size and Promotes Mitosis of Endogenous Cells in a Swine Chronic Myocardial Infarction Model
Delayed enhancement cardiac magnetic resonance short-axis representative images showing and quantifying the chronologic change of scar size in placebo and combination cell treatment groups pre- and post-transendocardial stem cell injection (TESI), from both allogeneic (A) and autologous (C) studies in swine MI. Graphs show that change in scar mass, as a percentage of left ventricular (LV) mass, decreased significantly following TESI of allogenic and autologous cell combination and mesenchymal stem cell therapies compared to placebo or cardiac stem cell therapy alone. *p < 0.05 within group, **p < 0.001 MSC + CSC versus placebo at all time points post-TESI, ***p < 0.0001 MSC + CSC or MSC versus placebo at all time points post-TESI, Ϯp < 0.05 MSC versus placebo at all time points post-TESI. MSC, mesenchymal stem cell; CSC, ckit+ cardiac stem cell. (B and D) Representative confocal microscopy images showing mitosis of cardiomyocytes in response to allogeneic (B) or autologous (D) combination stem cell treatment, using the mitosis-specific marker phospho-histone H3 (PHH3). Graphs represent PHH3 cardiomyocytes/slide in the remote (allogeneic) and border (autologous) zones. *p < 0.05 MSC + CSC versus placebo. Adapted from Natsumeda et al.41 (allogeneic cell study) and Karantalis et al.48 (autologous cell study).
Another promising MSC-based strategy is combining cells with biomaterials designed to improve retention. One of the first attempts consisted of a patch comprising a fibrin matrix seeded with autologous MSCs labeled with β-galactosidase in a porcine MI model.127 This approach significantly increased LV systolic wall thickness in the infarct zone, and transplanted cells differentiated into cells with myocyte-like characteristics. A robust increase of neovascularization, as indicated by von Willebrand factor-positive angioblasts and capillaries in transplanted hearts, was also observed.127 This approach has since seen significant improvements. In small animals, a variety of studies show that biomaterials comprising collagen145, 146 or natural extracellular matrix (ECM) scaffold, containing decellularized bovine pericardial tissue scaffold147 and decellularized human myocardium148 and combined with a cellular component (MSCs), restores mechanical function to the heart.145, 146, 147, 148 Likewise, studies in large animals are also being performed associating MSCs with a polymeric cardiac patch material containing poly(glycerol sebacate) (PGS), fibrinogen, and VEGF scaffold149 or natural ECM scaffolds with decellularized porcine myocardium150 and urinary bladder matrix.151 The ideal properties of these biomaterials include, but are not limited to, high porosity, high surface area-to-cell volume ratio, microenvironment similar to natural ECM, good mechanical properties, biodegradability, and biocompatibility.152 Despite the tremendous advances in engineered cardiac tissue, the complete restoration of myocardial function remains elusive, and work needs to be done in order to potentiate the biological activity of these biomaterials to offer biophysical support to the damaged heart.
Determining the route(s) of administration that maximizes the therapeutic benefits of MSCs has been difficult. Despite the numerous preclinical and clinical studies, there is no overall consensus as to the preferable route. However, a recent meta-analysis suggests that, for acute MI, the transendocardial route produces superior reduction in scar size and improvement in LVEF in both swine and human studies.153 Further studies directly comparing the route of injection are needed to confirm this conclusion and to determine the most effective route of injection in other cardiovascular diseases in order to optimize the design of new clinical studies.154
Clinical Trials
The encouraging outcomes of preclinical studies using MSCs as a treatment for diseased myocardium have set the scene for worldwide clinical trials, many of which have used BM-MSCs.17, 42, 60, 106, 155, 156 In the POSEIDON study, administration of allo- and auto-hMSCs to patients with ICM was equally safe, and there was no increase in serious adverse events or immunologic reactions with allo-hMSC therapy.60 Similarly, safety and clinical efficacy was revealed following allo-MSC or auto-MSC transplantation in patients with NIDCM.42 In both studies, allo-MSCs, unlike auto-MSCs, improved endothelial function.86 In the recent TRIDENT (Transendocardial Stem Cell Injection Delivery Effects on Neomyogenesis Study) study,155 no evidence of immune reaction was seen in ICM patients who received either 20 or 100 million allogeneic BM-MSCs. Only one patient had a calculated panel reactive antibody (cPRA) response incidence following cell transplantation, corroborating previous hMSC allograft studies,42, 60 but the patient did not experience immunologic rejection. Treatment with human BM-MSCs also reduced the serum levels of TNF-α,155 a marker of heart disease progression.157 Functionally, both doses reduced infarct scar size, but only the group that received 100 million hBM-MSCs demonstrated improved LVEF, suggesting that the higher dose provides a greater benefit to cardiac function.155 Similar to the TRIDENT study, allo mesenchymal precursor cells (MPCs), delivered by transendocardial injection to patients with heart failure, improved cardiac structure and function without clinically symptomatic immune responses. Three different cell doses were tested (25, 75, and 150 million). The highest dose produced a reduction in major adverse cardiac events and provided some beneficial effects on left ventricular remodeling.158
In the MSC-HF (Autologous Mesenchymal Stromal Cell Therapy in Heart Failure) trial (Clinicaltrials.gov: NCT00644410), patients with severe ICM were administered a mean dose of 77.5 + 67.9 × 106 auto-BM-MSCs, depending on their MSC proliferation. Cell treatment improved numerous parameters of cardiac function, but only left ventricular end systolic volume (LVESV) exhibited a dose-dependent effect. These patients had significant reductions in hospitalization for angina, suggesting that autologous MSC transplantation can be beneficial for severe heart failure treatment.156
In CHART-1 (Safety and Efficacy of Autologous Cardiopoietic Cells for Treatment of Ischemic Heart Failure) (Clinicaltrials.gov: NCT01768702), the therapeutic potential of BM-MSCs exposed to a cardiopoietic treatment (cardiopoietic stem cells) was studied. Similar to the MSC-HF study, the number of both intramyocardial injections (<16 injections, 16–19 injections, and >20 injections) and cells injected (≤600 million autologous cardiopoietic stem cells) was dependent on ex vivo proliferation. The major findings in this study suggest that fewer injections (≤20, directly correlated with a lower dose received per patient) improve indices of remodeling and function better than higher doses of cells, consistent with the idea that there is an upper limit to cell dosing.159
Comparable to the positive cardiac effect promoted by the transplantation of BM-MSCs, AT-MSCs have shown a potential benefit in heart failure. Ventricular function, myocardial perfusion, and exercise capacity were preserved in the PRECISE (A Randomized Clinical Trial of Adipose-Derived Stem & Regenerative Cells In the Treatment of Patients With Non Revacularizable Ischemic Myocardium) clinical trial, the first randomized, placebo-controlled, double-blind trial to examine the safety and feasibility of transendocardial injections of autologous adipose-derived regenerative cells (ADRCs) into patients with ICM.160 In another study, the efficacy and safety of cryopreserved allogeneic AT-MSCs (called CSCC-ASC; Allogeneic Adipose Tissue-Derived Stromal/Stem Cell Therapy in Patients With Ischemic Heart Disease and Heart Failure - a Safety Study) from healthy donors delivered by intramyocardial injection into 10 patients with ischemic heart disease and ischemic heart failure (IHF) was tested. Approximately 15 injections of 0.3 mL CSCC-ASC (total of 100 million cells) were delivered via transendocardial stem cell injection into viable myocardium into the border zone of infarcted tissue. As a primary endpoint result (major immunologic reaction of allogeneic CSCC-ASC therapy), four of ten patients developed donor-specific de novo HLA class I antibodies, and only two patients had donor-specific antibodies at baseline.161 The study is currently in phase II (ClinicalTrials.gov: NCT02387723) to assess the safety and efficacy of CSCC-ASC in a larger placebo-controlled trial in IHF patients.162 Another approach is to pre-treat autologous AT-MSCs prior to administration into ICM patients. In the MyStromalCell trial (Mesenchymal Stromal Cell Therapy in Patients With Chronic Myocardial Ischemia; ClinicalTrials.gov: NCT01449032), AT-MSCs were pretreated with VEGF-A165. Patients receiving these cells significantly improved their exercise capacity whereas the placebo group did not.163 This small example of the many recent clinical trials illustrates the variety of approaches and sources of MSCs being studied as a therapeutic approach to heart disease.
Future Perspectives
The first stage of cardiac regenerative medicine with MSCs has been successfully completed with a number of high-quality, independently conducted clinical studies supporting its safety and efficacy in patients with heart disease, while highlighting important limitations that need to be addressed as the field continues to move forward.
First, although the original idea of cell-based regenerative medicine strategies envisioned the development of autologous cell grafts for direct tissue replacement, the majority of preclinical and clinical trials, regardless of whether the cells are of somatic or PSC origin, indicates that the success of the field will likely require the development of allogeneic, off-the-shelf products with the capacity to promote endogenous mechanisms of regeneration. To this end, MSCs from young or perinatal healthy tissues, or derived from PSCs, are likely to continue dominating the field, since, compared to other somatic and PSC-based cell types, they can be easily manufactured under a relatively low regulatory and financial burden, and they can be readily available either as autologous or allogeneic grafts without compromising safety and efficacy.17, 42, 60
Second, there is an increasing need for improving our understanding of the biology and role of the different types of MSCs, in order to refine their manufacturing and maximize their capacity to promote repair. It will be particularly important to compare the different types of MSCs from different tissue sources (e.g., bone marrow, adipose, perinatal, and cranial and/or dental tissues) in terms of immunologic, antifibrotic, and cardiac regenerative properties. What are the normal functions of each cell type within the host tissue? Does the sex of the donor affect cell function? To what extent are these cell types affected by aging and/or disease?
To this end, focusing on developmental biology-guided basic research approaches could facilitate the identification of MSC lineages that are particularly relevant to cardiac development, growth, and/or regeneration, as well as provide key mechanistic insights regarding the molecular pathways underlying their self-renewal and therapeutic properties. For example, preconditioning and genetic manipulation can reprogram MSCs to secrete appropriate stimulatory or inhibitory molecules, and they can be combined with the above approaches. These approaches can also be directed toward increasing survival and engraftment of MSCs post-administration or for the production of therapeutic exosomes and/or EVs (Figure 2).
Figure 2.
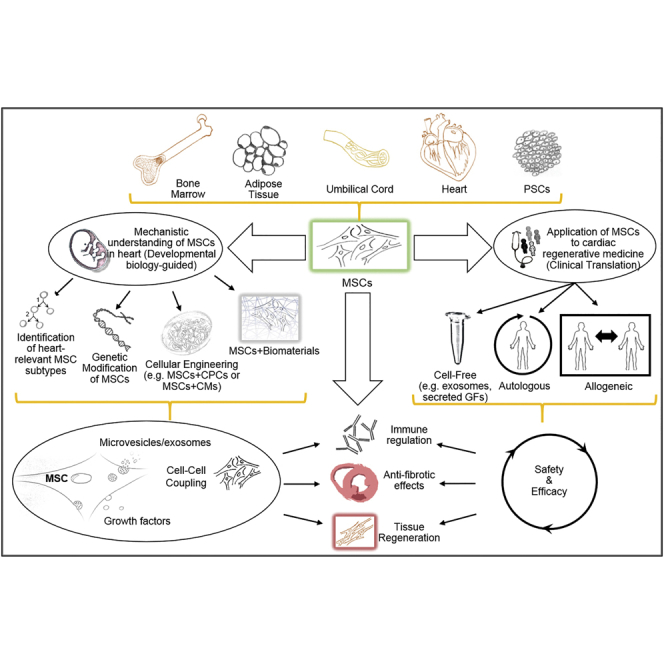
MSCs Are a Particularly Attractive Cellular Platform for Cell-Based Cardiac Regenerative Medicine
MSCs can be isolated from virtually all tissues, including bone marrow, adipose tissue, umbilical cord, and the heart itself, or they can be generated from pluripotent stem cells. Ongoing research, examining the relationship of MSCs to heart development and repair, will guide the development of more refined strategies, such as isolating bona fide cardiac MSC lineages, generating genetically engineered MSCs with better defined growth factor and exosome secretomes, and optimizing combination strategies of MSCs with cardiac progenitor cells and/or biomaterials more relevant to cardiac organogenesis. Translation of this research should address the most important clinical concerns, including feasibility, safety, and efficacy of utilizing MSCs as the basis for cell-based and novel cell-free, autologous and/or allogeneic regenerative medicine approaches. These approaches are designed to safely and effectively augment MSC repair of the damaged heart by delivering microvesicles/exosomes and growth factors and by cell-cell coupling that together activate anti-inflammatory and antifibrotic pathways and stimulate the proliferation of endogenous cardiac precursors, cardiomyocytes, and coronary vascular cells.
While continuing to improve MSCs, the next (interrelated) direction is to optimize cell administration. The most effective route of cell administration must be determined. The transendocardial route (transendocardial stem cell injection [TESI]) appears to be the most efficacious compared to delivering cells through the circulation.153 However, cell-laced bioengineered patches are also effective, albeit requiring more invasive surgical procedures. Unique delivery systems may be optimal for distinct types of heart disease.
A final direction is to continue exploring the synergy of combining MSCs with another cell type that complements the properties of MSCs (e.g., CSCs41, 48, 140, 141 or macrophages49) to improve cardiac function more than with MSCs alone. Combinations of more than two cell types (sub-populations) and/or combining cell and pharmaceutical approaches are also potential approaches. Over the past ∼20 years, stem cell therapy for heart disease has progressed from being virtually non-existent to being a feasible, safe, and encouraging clinical alternative. The challenge is to maintain this momentum by continuously optimizing approaches so as achieve complete cardiac repair.
Conflicts of Interest
K.E.H. reports having a patent for cardiac cell-based therapy and holding equity in Vestion Inc. J.M.H. reports having a patent for cardiac cell-based therapy and holding equity in Vestion Inc. J.M.H. maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc. did not play a role in the design, conduct, or funding of the study. J.M.H. is the Chief Scientific Officer, a compensated consultant, and advisory board member for Longeveron and holds equity in Longeveron. J.M.H. is also the co-inventor of intellectual property licensed to Longeveron. Longeveron did not play a role in the design, conduct, or funding of the study. L.B. and W.B. have no conflicts of interest.
Acknowledgments
This work was funded by NIH grant R01 HL107110 to J.M.H. J.M.H. is also funded by NIH grants R01 HL137355 5UM and R01 HL113460, the Starr Foundation, and the Soffer Family Foundation.
References
- 1.Becker C., Lacchini S., Muotri A.R., da Silva G.J., Castelli J.B., Vassallo P.F., Menck C.F., Krieger J.E. Skeletal muscle cells expressing VEGF induce capillary formation and reduce cardiac injury in rats. Int. J. Cardiol. 2006;113:348–354. doi: 10.1016/j.ijcard.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 2.Post M.J., Sato K., Murakami M., Bao J., Tirziu D., Pearlman J.D., Simons M. Adenoviral PR39 improves blood flow and myocardial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R494–R500. doi: 10.1152/ajpregu.00460.2005. [DOI] [PubMed] [Google Scholar]
- 3.Hedman M., Hartikainen J., Syvänne M., Stjernvall J., Hedman A., Kivelä A., Vanninen E., Mussalo H., Kauppila E., Simula S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 4.Henry T.D., Annex B.H., McKendall G.R., Azrin M.A., Lopez J.J., Giordano F.J., Shah P.K., Willerson J.T., Benza R.L., Berman D.S., VIVA Investigators The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 5.Byrne M.J., Power J.M., Preovolos A., Mariani J.A., Hajjar R.J., Kaye D.M. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 6.Zsebo K., Yaroshinsky A., Rudy J.J., Wagner K., Greenberg B., Jessup M., Hajjar R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 7.Wahlquist C., Jeong D., Rojas-Muñoz A., Kho C., Lee A., Mitsuyama S., van Mil A., Park W.J., Sluijter J.P., Doevendans P.A. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King K.R., Aguirre A.D., Ye Y.X., Sun Y., Roh J.D., Ng R.P., Jr., Kohler R.H., Arlauckas S.P., Iwamoto Y., Savol A. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 2017;23:1481–1487. doi: 10.1038/nm.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulalio A., Mano M., Dal Ferro M., Zentilin L., Sinagra G., Zacchigna S., Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 10.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G., Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansen A.K., Molenaar B., Versteeg D., Leitoguinho A.R., Demkes C., Spanjaard B., de Ruiter H., Akbari Moqadam F., Kooijman L., Zentilin L. Postnatal Cardiac Gene Editing Using CRISPR/Cas9 With AAV9-Mediated Delivery of Short Guide RNAs Results in Mosaic Gene Disruption. Circ. Res. 2017;121:1168–1181. doi: 10.1161/CIRCRESAHA.116.310370. [DOI] [PubMed] [Google Scholar]
- 13.Ma H., Marti-Gutierrez N., Park S.W., Wu J., Lee Y., Suzuki K., Koski A., Ji D., Hayama T., Ahmed R. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–419. doi: 10.1038/nature23305. [DOI] [PubMed] [Google Scholar]
- 14.Kastrup J., Jørgensen E., Rück A., Tägil K., Glogar D., Ruzyllo W., Bøtker H.E., Dudek D., Drvota V., Hesse B., Euroinject One Group Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J. Am. Coll. Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 15.Stewart D.J., Kutryk M.J., Fitchett D., Freeman M., Camack N., Su Y., Della Siega A., Bilodeau L., Burton J.R., Proulx G., Radhakrishnan S., NORTHERN Trial Investigators VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol. Ther. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Desai A.S., Barnard D., Bouchard A., Jaski B., Lyon A.R. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 17.Heldman A.W., DiFede D.L., Fishman J.E., Zambrano J.P., Trachtenberg B.H., Karantalis V., Mushtaq M., Williams A.R., Suncion V.Y., McNiece I.K. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suncion V.Y., Ghersin E., Fishman J.E., Zambrano J.P., Karantalis V., Mandel N., Nelson K.H., Gerstenblith G., DiFede Velazquez D.L., Breton E. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ. Res. 2014;114:1292–1301. doi: 10.1161/CIRCRESAHA.114.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawa Y., Yoshikawa Y., Toda K., Fukushima S., Yamazaki K., Ono M., Sakata Y., Hagiwara N., Kinugawa K., Miyagawa S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ. J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 20.Chugh A.R., Beache G.M., Loughran J.H., Mewton N., Elmore J.B., Kajstura J., Pappas P., Tatooles A., Stoddard M.F., Lima J.A. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11, Suppl 1):S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malliaras K., Makkar R.R., Smith R.R., Cheng K., Wu E., Bonow R.O., Marbán L., Mendizabal A., Cingolani E., Johnston P.V. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J. Am. Coll. Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 23.Menasché P., Vanneaux V., Fabreguettes J.R., Bel A., Tosca L., Garcia S., Bellamy V., Farouz Y., Pouly J., Damour O. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: a translational experience. Eur. Heart J. 2015;36:743–750. doi: 10.1093/eurheartj/ehu192. [DOI] [PubMed] [Google Scholar]
- 24.Shiba Y., Gomibuchi T., Seto T., Wada Y., Ichimura H., Tanaka Y., Ogasawara T., Okada K., Shiba N., Sakamoto K. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538:388–391. doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 25.Golpanian S., Wolf A., Hatzistergos K.E., Hare J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016;96:1127–1168. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes S., Chong J.J.H., Paige S.L., Iwata M., Torok-Storb B., Keller G., Reinecke H., Murry C.E. Comparison of Human Embryonic Stem Cell-Derived Cardiomyocytes, Cardiovascular Progenitors, and Bone Marrow Mononuclear Cells for Cardiac Repair. Stem Cell Reports. 2015;5:753–762. doi: 10.1016/j.stemcr.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalit P.A., Salick M.R., Nelson D.O., Squirrell J.M., Shafer C.M., Patel N.G., Saeed I., Schmuck E.G., Markandeya Y.S., Wong R. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell. 2016;18:354–367. doi: 10.1016/j.stem.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamura A., Miyagawa S., Fukushima S., Kawamura T., Kashiyama N., Ito E., Watabe T., Masuda S., Toda K., Hatazawa J. Teratocarcinomas Arising from Allogeneic Induced Pluripotent Stem Cell-Derived Cardiac Tissue Constructs Provoked Host Immune Rejection in Mice. Sci. Rep. 2016;6:19464. doi: 10.1038/srep19464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong J.J., Yang X., Don C.W., Minami E., Liu Y.W., Weyers J.J., Mahoney W.M., Van Biber B., Cook S.M., Palpant N.J. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eschenhagen T., Bolli R., Braun T., Field L.J., Fleischmann B.K., Frisén J., Giacca M., Hare J.M., Houser S., Lee R.T. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow A., Stuckey D.J., Kidher E., Rocco M., Jabbour R.J., Mansfield C.A., Darzi A., Harding S.E., Stevens M.M., Athanasiou T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Reports. 2017;9:1415–1422. doi: 10.1016/j.stemcr.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzistergos K.E., Quevedo H., Oskouei B.N., Hu Q., Feigenbaum G.S., Margitich I.S., Mazhari R., Boyle A.J., Zambrano J.P., Rodriguez J.E. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatzistergos K.E., Saur D., Seidler B., Balkan W., Breton M., Valasaki K., Takeuchi L.M., Landin A.M., Khan A., Hare J.M. Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circ. Res. 2016;119:921–930. doi: 10.1161/CIRCRESAHA.116.309281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loffredo F.S., Steinhauser M.L., Gannon J., Lee R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye L., Chang Y.H., Xiong Q., Zhang P., Zhang L., Somasundaram P., Lepley M., Swingen C., Su L., Wendel J.S. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong S.G., Huber B.C., Lee W.H., Kodo K., Ebert A.D., Ma Y., Nguyen P.K., Diecke S., Chen W.Y., Wu J.C. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation. 2015;132:762–771. doi: 10.1161/CIRCULATIONAHA.114.015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangi A.A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J.S., Dzau V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 39.Duran J.M., Makarewich C.A., Sharp T.E., Starosta T., Zhu F., Hoffman N.E., Chiba Y., Madesh M., Berretta R.M., Kubo H., Houser S.R. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ. Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Yang J., Yan W., Li Y., Shen Z., Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J. Am. Heart Assoc. 2016;5:e002856. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natsumeda M., Florea V., Rieger A.C., Tompkins B.A., Banerjee M.N., Golpanian S., Fritsch J., Landin A.M., Kashikar N.D., Karantalis V. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J. Am. Coll. Cardiol. 2017;70:2504–2515. doi: 10.1016/j.jacc.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hare J.M., DiFede D.L., Rieger A.C., Florea V., Landin A.M., El-Khorazaty J., Khan A., Mushtaq M., Lowery M.H., Byrnes J.J. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartolucci J., Verdugo F.J., González P.L., Larrea R.E., Abarzua E., Goset C., Rojo P., Palma I., Lamich R., Pedreros P.A. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]) Circ. Res. 2017;121:1192–1204. doi: 10.1161/CIRCRESAHA.117.310712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledford K.J., Murphy N., Zeigler F., Bartel R.L., Tubo R. Therapeutic potential of ixmyelocel-T, an expanded autologous multicellular therapy for treatment of ischemic cardiovascular diseases. Stem Cell Res. Ther. 2015;6:25. doi: 10.1186/s13287-015-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulandavelu S., Karantalis V., Fritsch J., Hatzistergos K.E., Loescher V.Y., McCall F., Wang B., Bagno L., Golpanian S., Wolf A. Pim1 Kinase Overexpression Enhances ckit+ Cardiac Stem Cell Cardiac Repair Following Myocardial Infarction in Swine. J. Am. Coll. Cardiol. 2016;68:2454–2464. doi: 10.1016/j.jacc.2016.09.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu W., Zhao M., Mattapally S., Chen S., Zhang J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ. Res. 2018;122:88–96. doi: 10.1161/CIRCRESAHA.117.311504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haider H.Kh., Jiang S., Idris N.M., Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 48.Karantalis V., Suncion-Loescher V.Y., Bagno L., Golpanian S., Wolf A., Sanina C., Premer C., Kanelidis A.J., McCall F., Wang B. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2015;66:1990–1999. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel A.N., Henry T.D., Quyyumi A.A., Schaer G.L., Anderson R.D., Toma C., East C., Remmers A.E., Goodrich J., Desai A.S., ixCELL-DCM Investigators Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412–2421. doi: 10.1016/S0140-6736(16)30137-4. [DOI] [PubMed] [Google Scholar]
- 50.Puymirat E., Geha R., Tomescot A., Bellamy V., Larghero J., Trinquart L., Bruneval P., Desnos M., Hagège A., Pucéat M., Menasché P. Can mesenchymal stem cells induce tolerance to cotransplanted human embryonic stem cells? Mol. Ther. 2009;17:176–182. doi: 10.1038/mt.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh Y., Cho G.S., Li Z., Hong I., Zhu R., Kim M.J., Kim Y.J., Tampakakis E., Tung L., Huganir R. Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons. Cell Stem Cell. 2016;19:95–106. doi: 10.1016/j.stem.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubach M., Adelmann R., Haustein M., Drey F., Pfannkuche K., Xiao B., Koester A., Udink ten Cate F.E., Choi Y.H., Neef K. Mesenchymal stem cells and their conditioned medium improve integration of purified induced pluripotent stem cell-derived cardiomyocyte clusters into myocardial tissue. Stem Cells Dev. 2014;23:643–653. doi: 10.1089/scd.2013.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brodarac A., Šarić T., Oberwallner B., Mahmoodzadeh S., Neef K., Albrecht J., Burkert K., Oliverio M., Nguemo F., Choi Y.H. Susceptibility of murine induced pluripotent stem cell-derived cardiomyocytes to hypoxia and nutrient deprivation. Stem Cell Res. Ther. 2015;6:83. doi: 10.1186/s13287-015-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monsanto M.M., White K.S., Kim T., Wang B.J., Fisher K., Ilves K., Khalafalla F.G., Casillas A., Broughton K., Mohsin S. Concurrent Isolation of 3 Distinct Cardiac Stem Cell Populations From a Single Human Heart Biopsy. Circ. Res. 2017;121:113–124. doi: 10.1161/CIRCRESAHA.116.310494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chong J.J., Chandrakanthan V., Xaymardan M., Asli N.S., Li J., Ahmed I., Heffernan C., Menon M.K., Scarlett C.J., Rashidianfar A. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wysoczynski M., Guo Y., Moore J.B., 4th, Muthusamy S., Li Q., Nasr M., Li H., Nong Y., Wu W., Tomlin A.A. Myocardial Reparative Properties of Cardiac Mesenchymal Cells Isolated on the Basis of Adherence. J. Am. Coll. Cardiol. 2017;69:1824–1838. doi: 10.1016/j.jacc.2017.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 59.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 60.Hare J.M., Fishman J.E., Gerstenblith G., DiFede Velazquez D.L., Zambrano J.P., Suncion V.Y., Tracy M., Ghersin E., Johnston P.V., Brinker J.A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amable P.R., Teixeira M.V., Carias R.B., Granjeiro J.M., Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014;5:53. doi: 10.1186/scrt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fazzina R., Iudicone P., Fioravanti D., Bonanno G., Totta P., Zizzari I.G., Pierelli L. Potency testing of mesenchymal stromal cell growth expanded in human platelet lysate from different human tissues. Stem Cell Res. Ther. 2016;7:122. doi: 10.1186/s13287-016-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayatollahi M., Talaei-Khozani T., Razmkhah M. Growth suppression effect of human mesenchymal stem cells from bone marrow, adipose tissue, and Wharton’s jelly of umbilical cord on PBMCs. Iran. J. Basic Med. Sci. 2016;19:145–153. [PMC free article] [PubMed] [Google Scholar]
- 64.Karaöz E., Çetinalp Demircan P., Erman G., Güngörürler E., Eker Sarıboyacı A. Comparative Analyses of Immunosuppressive Characteristics of Bone-Marrow, Wharton’s Jelly, and Adipose Tissue-Derived Human Mesenchymal Stem Cells. Turk. J. Haematol. 2017;34:213–225. doi: 10.4274/tjh.2016.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ribeiro A., Laranjeira P., Mendes S., Velada I., Leite C., Andrade P., Santos F., Henriques A., Grãos M., Cardoso C.M. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 2013;4:125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perin L., Sedrakyan S., Da Sacco S., De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008;86:85–99. doi: 10.1016/S0091-679X(08)00005-8. [DOI] [PubMed] [Google Scholar]
- 67.Savickiene J., Treigyte G., Baronaite S., Valiuliene G., Kaupinis A., Valius M., Arlauskiene A., Navakauskiene R. Human Amniotic Fluid Mesenchymal Stem Cells from Second- and Third-Trimester Amniocentesis: Differentiation Potential, Molecular Signature, and Proteome Analysis. Stem Cells Int. 2015;2015:319238. doi: 10.1155/2015/319238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roubelakis M.G., Pappa K.I., Bitsika V., Zagoura D., Vlahou A., Papadaki H.A., Antsaklis A., Anagnou N.P. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 69.You Q., Tong X., Guan Y., Zhang D., Huang M., Zhang Y., Zheng J. The biological characteristics of human third trimester amniotic fluid stem cells. J. Int. Med. Res. 2009;37:105–112. doi: 10.1177/147323000903700112. [DOI] [PubMed] [Google Scholar]
- 70.Zvaifler N.J., Marinova-Mutafchieva L., Adams G., Edwards C.J., Moss J., Burger J.A., Maini R.N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petrini M., Pacini S., Trombi L., Fazzi R., Montali M., Ikehara S., Abraham N.G. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 2009;18:857–866. doi: 10.1089/scd.2008.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babaie Y., Herwig R., Greber B., Brink T.C., Wruck W., Groth D., Lehrach H., Burdon T., Adjaye J. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–510. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 73.Hatzistergos K.E., Vedenko A. Cardiac Cell Therapy 3.0: The Beginning of the End or the End of the Beginning? Circ. Res. 2017;121:95–97. doi: 10.1161/CIRCRESAHA.117.311293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lian Q., Zhang Y., Zhang J., Zhang H.K., Wu X., Zhang Y., Lam F.F., Kang S., Xia J.C., Lai W.H. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–1123. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 75.Jung Y., Bauer G., Nolta J.A. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun Y.Q., Zhang Y., Li X., Deng M.X., Gao W.X., Yao Y., Chiu S.M., Liang X., Gao F., Chan C.W. Insensitivity of Human iPS Cells-Derived Mesenchymal Stem Cells to Interferon-γ-induced HLA Expression Potentiates Repair Efficiency of Hind Limb Ischemia in Immune Humanized NOD Scid Gamma Mice. Stem Cells. 2015;33:3452–3467. doi: 10.1002/stem.2094. [DOI] [PubMed] [Google Scholar]
- 77.Fukuta M., Nakai Y., Kirino K., Nakagawa M., Sekiguchi K., Nagata S., Matsumoto Y., Yamamoto T., Umeda K., Heike T. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS ONE. 2014;9:e112291. doi: 10.1371/journal.pone.0112291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szaraz P., Gratch Y.S., Iqbal F., Librach C.L. J Vis Exp; 2017. In Vitro Differentiation of Human Mesenchymal Stem Cells into Functional Cardiomyocyte-like Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makino S., Fukuda K., Miyoshi S., Konishi F., Kodama H., Pan J., Sano M., Takahashi T., Hori S., Abe H. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pei Z., Zeng J., Song Y., Gao Y., Wu R., Chen Y., Li F., Li W., Zhou H., Yang Y. In vivo imaging to monitor differentiation and therapeutic effects of transplanted mesenchymal stem cells in myocardial infarction. Sci. Rep. 2017;7:6296. doi: 10.1038/s41598-017-06571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toma C., Pittenger M.F., Cahill K.S., Byrne B.J., Kessler P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 82.Quevedo H.C., Hatzistergos K.E., Oskouei B.N., Feigenbaum G.S., Rodriguez J.E., Valdes D., Pattany P.M., Zambrano J.P., Hu Q., McNiece I. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golpanian S., El-Khorazaty J., Mendizabal A., DiFede D.L., Suncion V.Y., Karantalis V., Fishman J.E., Ghersin E., Balkan W., Hare J.M. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J. Am. Coll. Cardiol. 2015;65:125–132. doi: 10.1016/j.jacc.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karantalis V., DiFede D.L., Gerstenblith G., Pham S., Symes J., Zambrano J.P., Fishman J., Pattany P., McNiece I., Conte J. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cao Y., Sun Z., Liao L., Meng Y., Han Q., Zhao R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 86.Premer C., Blum A., Bellio M.A., Schulman I.H., Hurwitz B.E., Parker M., Dermarkarian C.R., DiFede D.L., Balkan W., Khan A., Hare J.M. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beigi F., Schmeckpeper J., Pow-Anpongkul P., Payne J.A., Zhang L., Zhang Z., Huang J., Mirotsou M., Dzau V.J. C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the PI3K-AKT-CDK7 pathway. Circ. Res. 2013;113:372–380. doi: 10.1161/CIRCRESAHA.113.301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mirotsou M., Zhang Z., Deb A., Zhang L., Gnecchi M., Noiseux N., Mu H., Pachori A., Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc. Natl. Acad. Sci. USA. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki G., Iyer V., Lee T.C., Canty J.M., Jr. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ. Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 90.Chung E.S., Miller L., Patel A.N., Anderson R.D., Mendelsohn F.O., Traverse J., Silver K.H., Shin J., Ewald G., Farr M.J. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur. Heart J. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Struys T., Moreels M., Martens W., Donders R., Wolfs E., Lambrichts I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs (Print) 2011;193:366–378. doi: 10.1159/000321400. [DOI] [PubMed] [Google Scholar]
- 92.Nakanishi C., Nagaya N., Ohnishi S., Yamahara K., Takabatake S., Konno T., Hayashi K., Kawashiri M.A., Tsubokawa T., Yamagishi M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ. J. 2011;75:2260–2268. doi: 10.1253/circj.cj-11-0246. [DOI] [PubMed] [Google Scholar]
- 93.Donders R., Bogie J.F.J., Ravanidis S., Gervois P., Vanheusden M., Marée R., Schrynemackers M., Smeets H.J.M., Pinxteren J., Gijbels K. Human Wharton’s Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev. 2018;27:65–84. doi: 10.1089/scd.2017.0029. [DOI] [PubMed] [Google Scholar]
- 94.Chen J., Liu Z., Hong M.M., Zhang H., Chen C., Xiao M., Wang J., Yao F., Ba M., Liu J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS ONE. 2014;9:e115316. doi: 10.1371/journal.pone.0115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gomes S.A., Rangel E.B., Premer C., Dulce R.A., Cao Y., Florea V., Balkan W., Rodrigues C.O., Schally A.V., Hare J.M. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Markel T.A., Wang Y., Herrmann J.L., Crisostomo P.R., Wang M., Novotny N.M., Herring C.M., Tan J., Lahm T., Meldrum D.R. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2308–H2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Billing A.M., Ben Hamidane H., Dib S.S., Cotton R.J., Bhagwat A.M., Kumar P., Hayat S., Yousri N.A., Goswami N., Suhre K. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci. Rep. 2016;6:21507. doi: 10.1038/srep21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mias C., Lairez O., Trouche E., Roncalli J., Calise D., Seguelas M.H., Ordener C., Piercecchi-Marti M.D., Auge N., Salvayre A.N. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 100.De Lisio M., Jensen T., Sukiennik R.A., Huntsman H.D., Boppart M.D. Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res. Ther. 2014;5:74. doi: 10.1186/scrt463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexakis C., Partridge T., Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 102.Shabbir A., Zisa D., Lin H., Mastri M., Roloff G., Suzuki G., Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1428–H1438. doi: 10.1152/ajpheart.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uccelli A., Pistoia V., Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 104.Prabhu S.D., Frangogiannis N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bortolotti F., Ruozi G., Falcione A., Doimo S., Dal Ferro M., Lesizza P., Zentilin L., Banks L., Zacchigna S., Giacca M. In Vivo Functional Selection Identifies Cardiotrophin-1 as a Cardiac Engraftment Factor for Mesenchymal Stromal Cells. Circulation. 2017;136:1509–1524. doi: 10.1161/CIRCULATIONAHA.117.029003. [DOI] [PubMed] [Google Scholar]
- 106.Henry T.D., Traverse J.H., Hammon B.L., East C.A., Bruckner B., Remmers A.E., Recker D., Bull D.A., Patel A.N. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circ. Res. 2014;115:730–737. doi: 10.1161/CIRCRESAHA.115.304554. [DOI] [PubMed] [Google Scholar]
- 107.Henry T.D., Schaer G.L., DeMaria A., Recker D., Remmers A.E., Goodrich J., Patel A.N. The ixCELL-DCM Trial: Rationale and Design. Cell Transplant. 2016;25:1689–1699. doi: 10.3727/096368916X691295. [DOI] [PubMed] [Google Scholar]
- 108.Cervio E., Barile L., Moccetti T., Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:482171. doi: 10.1155/2015/482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bian S., Zhang L., Duan L., Wang X., Min Y., Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. (Berl.) 2014;92:387–397. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 110.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B.K., Jha K.A., Barhanpurkar A.P. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Y., Yu Z., Jiang D., Liang X., Liao S., Zhang Z., Yue W., Li X., Chiu S.M., Chai Y.H. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Reports. 2016;7:749–763. doi: 10.1016/j.stemcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu K., Wu Q., Ni C., Zhang P., Zhong Z., Wu Y., Wang Y., Xu Y., Kong M., Cheng H. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ. Res. 2018;122:958–969. doi: 10.1161/CIRCRESAHA.117.311578. [DOI] [PubMed] [Google Scholar]
- 113.Mayourian J., Cashman T.J., Ceholski D.K., Johnson B.V., Sachs D., Kaji D.A., Sahoo S., Hare J.M., Hajjar R.J., Sobie E.A., Costa K.D. Experimental and Computational Insight Into Human Mesenchymal Stem Cell Paracrine Signaling and Heterocellular Coupling Effects on Cardiac Contractility and Arrhythmogenicity. Circ. Res. 2017;121:411–423. doi: 10.1161/CIRCRESAHA.117.310796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Denu R.A., Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell. Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R., Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp. Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 116.Yu K.R., Kang K.S. Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology. 2013;59:557–563. doi: 10.1159/000353857. [DOI] [PubMed] [Google Scholar]
- 117.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.Y., Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kizilay Mancini Ö., Lora M., Shum-Tim D., Nadeau S., Rodier F., Colmegna I. A Proinflammatory Secretome Mediates the Impaired Immunopotency of Human Mesenchymal Stromal Cells in Elderly Patients with Atherosclerosis. Stem Cells Transl. Med. 2017;6:1132–1140. doi: 10.1002/sctm.16-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Turinetto V., Vitale E., Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016;17:E1164. doi: 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Denu R.A. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxid. Med. Cell. Longev. 2017;2017:5841716. doi: 10.1155/2017/5841716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kinkaid H.Y., Huang X.P., Li R.K., Weisel R.D. What’s new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J. Card. Surg. 2010;25:359–366. doi: 10.1111/j.1540-8191.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 122.Perin E.C., Willerson J.T., Pepine C.J., Henry T.D., Ellis S.G., Zhao D.X., Silva G.V., Lai D., Thomas J.D., Kronenberg M.W., Cardiovascular Cell Therapy Research Network (CCTRN) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann. N Y Acad. Sci. 2003;996:152–157. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 124.Barbash I.M., Chouraqui P., Baron J., Feinberg M.S., Etzion S., Tessone A., Miller L., Guetta E., Zipori D., Kedes L.H. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 125.Nagaya N., Fujii T., Iwase T., Ohgushi H., Itoh T., Uematsu M., Yamagishi M., Mori H., Kangawa K., Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 126.Vulliet P.R., Greeley M., Halloran S.M., MacDonald K.A., Kittleson M.D. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 127.Liu J., Hu Q., Wang Z., Xu C., Wang X., Gong G., Mansoor A., Lee J., Hou M., Zeng L. Autologous stem cell transplantation for myocardial repair. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H501–H511. doi: 10.1152/ajpheart.00019.2004. [DOI] [PubMed] [Google Scholar]
- 128.Amado L.C., Saliaris A.P., Schuleri K.H., St John M., Xie J.S., Cattaneo S., Durand D.J., Fitton T., Kuang J.Q., Stewart G. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yin C.Q., Chen J.L., Wang Y.F., Cao F., Cheng R., Pei X.T. [Autologus bone marrow-derived mesenchymal stem cells intracoronary delivery after acute myocardial infarction in miniature pig] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005;27:696–699. [PubMed] [Google Scholar]
- 130.Schuleri K.H., Feigenbaum G.S., Centola M., Weiss E.S., Zimmet J.M., Turney J., Kellner J., Zviman M.M., Hatzistergos K.E., Detrick B. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu C.B., Huang H., Sun P., Ma S.Z., Liu A.H., Xue J., Fu J.H., Liang Y.Q., Liu B., Wu D.Y. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016;5:1004–1013. doi: 10.5966/sctm.2015-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vilahur G., Oñate B., Cubedo J., Béjar M.T., Arderiu G., Peña E., Casaní L., Gutiérrez M., Capdevila A., Pons-Lladó G. Allogenic adipose-derived stem cell therapy overcomes ischemia-induced microvessel rarefaction in the myocardium: systems biology study. Stem Cell Res. Ther. 2017;8:52. doi: 10.1186/s13287-017-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hong S.J., Hou D., Brinton T.J., Johnstone B., Feng D., Rogers P., Fearon W.F., Yock P., March K.L. Intracoronary and retrograde coronary venous myocardial delivery of adipose-derived stem cells in swine infarction lead to transient myocardial trapping with predominant pulmonary redistribution. Catheter. Cardiovasc. Interv. 2014;83:E17–E25. doi: 10.1002/ccd.24659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee H.W., Lee H.C., Park J.H., Kim B.W., Ahn J., Kim J.H., Park J.S., Oh J.H., Choi J.H., Cha K.S. Effects of Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells on Acute Myocardial Infarction in a Porcine Model. Yonsei Med. J. 2015;56:1522–1529. doi: 10.3349/ymj.2015.56.6.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Berry M.F., Engler A.J., Woo Y.J., Pirolli T.J., Bish L.T., Jayasankar V., Morine K.J., Gardner T.J., Discher D.E., Sweeney H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 136.Grinnemo K.H., Månsson A., Dellgren G., Klingberg D., Wardell E., Drvota V., Tammik C., Holgersson J., Ringdén O., Sylvén C., Le Blanc K. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J. Thorac. Cardiovasc. Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 137.Ly H.Q., Hoshino K., Pomerantseva I., Kawase Y., Yoneyama R., Takewa Y., Fortier A., Gibbs-Strauss S.L., Vooght C., Frangioni J.V., Hajjar R.J. In vivo myocardial distribution of multipotent progenitor cells following intracoronary delivery in a swine model of myocardial infarction. Eur. Heart J. 2009;30:2861–2868. doi: 10.1093/eurheartj/ehp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van der Spoel T.I., Jansen of Lorkeers S.J., Agostoni P., van Belle E., Gyöngyösi M., Sluijter J.P., Cramer M.J., Doevendans P.A., Chamuleau S.A. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 139.Cai M., Shen R., Song L., Lu M., Wang J., Zhao S., Tang Y., Meng X., Li Z., He Z.X. Bone Marrow Mesenchymal Stem Cells (BM-MSCs) Improve Heart Function in Swine Myocardial Infarction Model through Paracrine Effects. Sci. Rep. 2016;6:28250. doi: 10.1038/srep28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams A.R., Hatzistergos K.E., Addicott B., McCall F., Carvalho D., Suncion V., Morales A.R., Da Silva J., Sussman M.A., Heldman A.W., Hare J.M. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quijada P., Salunga H.T., Hariharan N., Cubillo J.D., El-Sayed F.G., Moshref M., Bala K.M., Emathinger J.M., De La Torre A., Ormachea L. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ Res. 2015;117:695–706. doi: 10.1161/CIRCRESAHA.115.306838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y., Zhang G., Hou Y., Chen J., Wang J., Zou C., Li D., Li H., Zhang Q., Wang A., Fan Q. Transplantation of microencapsulated Schwann cells and mesenchymal stem cells augment angiogenesis and improve heart function. Mol. Cell. Biochem. 2012;366:139–147. doi: 10.1007/s11010-012-1291-1. [DOI] [PubMed] [Google Scholar]
- 143.Zhang G., Hu Q., Braunlin E.A., Suggs L.J., Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng. Part A. 2008;14:1025–1036. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 144.Zhou Y., Singh A.K., Hoyt R.F., Jr., Wang S., Yu Z., Hunt T., Kindzelski B., Corcoran P.C., Mohiuddin M.M., Horvath K.A. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2014;148:1131–1137. doi: 10.1016/j.jtcvs.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Simpson D., Liu H., Fan T.H., Nerem R., Dudley S.C., Jr. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maureira P., Marie P.Y., Yu F., Poussier S., Liu Y., Groubatch F., Falanga A., Tran N. Repairing chronic myocardial infarction with autologous mesenchymal stem cells engineered tissue in rat promotes angiogenesis and limits ventricular remodeling. J. Biomed. Sci. 2012;19:93. doi: 10.1186/1423-0127-19-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wei H.J., Chen C.H., Lee W.Y., Chiu I., Hwang S.M., Lin W.W., Huang C.C., Yeh Y.C., Chang Y., Sung H.W. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 148.Godier-Furnémont A.F., Martens T.P., Koeckert M.S., Wan L., Parks J., Arai K., Zhang G., Hudson B., Homma S., Vunjak-Novakovic G. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc. Natl. Acad. Sci. USA. 2011;108:7974–7979. doi: 10.1073/pnas.1104619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ravichandran R., Venugopal J.R., Mukherjee S., Sundarrajan S., Ramakrishna S. Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Eng. Part A. 2015;21:1288–1298. doi: 10.1089/ten.TEA.2014.0265. [DOI] [PubMed] [Google Scholar]
- 150.Wang B., Borazjani A., Tahai M., Curry A.L., Simionescu D.T., Guan J., To F., Elder S.H., Liao J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J. Biomed. Mater. Res. A. 2010;94:1100–1110. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Potapova I.A., Doronin S.V., Kelly D.J., Rosen A.B., Schuldt A.J., Lu Z., Kochupura P.V., Robinson R.B., Rosen M.R., Brink P.R. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Domenech M., Polo-Corrales L., Ramirez-Vick J.E., Freytes D.O. Tissue Engineering Strategies for Myocardial Regeneration: Acellular Versus Cellular Scaffolds? Tissue Eng. Part B Rev. 2016;22:438–458. doi: 10.1089/ten.teb.2015.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kanelidis A.J., Premer C., Lopez J., Balkan W., Hare J.M. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circ. Res. 2017;120:1139–1150. doi: 10.1161/CIRCRESAHA.116.309819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Golpanian S., Schulman I.H., Ebert R.F., Heldman A.W., DiFede D.L., Yang P.C., Wu J.C., Bolli R., Perin E.C., Moyé L., Cardiovascular Cell Therapy Research Network Concise Review: Review and Perspective of Cell Dosage and Routes of Administration From Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl. Med. 2016;5:186–191. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Florea V., Rieger A.C., DiFede D.L., El-Khorazaty J., Natsumeda M., Banerjee M.N., Tompkins B.A., Khan A., Schulman I.H., Landin A.M. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study) Circ. Res. 2017;121:1279–1290. doi: 10.1161/CIRCRESAHA.117.311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mathiasen A.B., Qayyum A.A., Jørgensen E., Helqvist S., Fischer-Nielsen A., Kofoed K.F., Haack-Sørensen M., Ekblond A., Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) Eur. Heart J. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 157.Haudek S.B., Taffet G.E., Schneider M.D., Mann D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 2007;117:2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perin E.C., Borow K.M., Silva G.V., DeMaria A.N., Marroquin O.C., Huang P.P., Traverse J.H., Krum H., Skerrett D., Zheng Y. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ. Res. 2015;117:576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 159.Teerlink J.R., Metra M., Filippatos G.S., Davison B.A., Bartunek J., Terzic A., Gersh B.J., Povsic T.J., Henry T.D., Alexandre B., CHART Investigators Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur. J. Heart Fail. 2017;19:1520–1529. doi: 10.1002/ejhf.898. [DOI] [PubMed] [Google Scholar]
- 160.Perin E.C., Sanz-Ruiz R., Sánchez P.L., Lasso J., Pérez-Cano R., Alonso-Farto J.C., Pérez-David E., Fernández-Santos M.E., Serruys P.W., Duckers H.J. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 161.Kastrup J., Haack-Sørensen M., Juhl M., Harary Søndergaard R., Follin B., Drozd Lund L., Mønsted Johansen E., Ali Qayyum A., Bruun Mathiasen A., Jørgensen E. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl. Med. 2017;6:1963–1971. doi: 10.1002/sctm.17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kastrup J., Schou M., Gustafsson I., Nielsen O.W., Møgelvang R., Kofoed K.F., Kragelund C., Hove J.D., Fabricius-Bjerre A., Heitman M. Rationale and Design of the First Double-Blind, Placebo-Controlled Trial with Allogeneic Adipose Tissue-Derived Stromal Cell Therapy in Patients with Ischemic Heart Failure: A Phase II Danish Multicentre Study. Stem Cells Int. 2017;2017:8506370. doi: 10.1155/2017/8506370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qayyum A.A., Mathiasen A.B., Mygind N.D., Kühl J.T., Jørgensen E., Helqvist S., Elberg J.J., Kofoed K.F., Vejlstrup N.G., Fischer-Nielsen A. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017;2017:5237063. doi: 10.1155/2017/5237063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hensley M.T., Tang J., Woodruff K., Defrancesco T., Tou S., Williams C.M., Breen M., Meurs K., Keene B., Cheng K. Intracoronary allogeneic cardiosphere-derived stem cells are safe for use in dogs with dilated cardiomyopathy. J. Cell. Mol. Med. 2017;21:1503–1512. doi: 10.1111/jcmm.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sanz-Ruiz R., Casado Plasencia A., Borlado L.R., Fernández-Santos M.E., Al-Daccak R., Claus P., Palacios I., Sádaba R., Charron D., Bogaert J. Rationale and Design of a Clinical Trial to Evaluate the Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With Acute Myocardial Infarction and Left Ventricular Dysfunction: The Randomized Multicenter Double-Blind Controlled CAREMI Trial (Cardiac Stem Cells in Patients With Acute Myocardial Infarction) Circ. Res. 2017;121:71–80. doi: 10.1161/CIRCRESAHA.117.310651. [DOI] [PubMed] [Google Scholar]
- 166.Zhao T., Zhang Z.N., Rong Z., Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 167.Yoshida Y., Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ. Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- 168.Hulot J.S., Ishikawa K., Hajjar R.J. Gene therapy for the treatment of heart failure: promise postponed. Eur. Heart J. 2016;37:1651–1658. doi: 10.1093/eurheartj/ehw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tachibana A., Santoso M.R., Mahmoudi M., Shukla P., Wang L., Bennett M., Goldstone A.B., Wang M., Fukushi M., Ebert A.D. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ. Res. 2017;121:e22–e36. doi: 10.1161/CIRCRESAHA.117.310803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Watanabe S., Ishikawa K., Fish K., Oh J.G., Motloch L.J., Kohlbrenner E., Lee P., Xie C., Lee A., Liang L. Protein Phosphatase Inhibitor-1 Gene Therapy in a Swine Model of Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2017;70:1744–1756. doi: 10.1016/j.jacc.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fish K.M., Ladage D., Kawase Y., Karakikes I., Jeong D., Ly H., Ishikawa K., Hadri L., Tilemann L., Muller-Ehmsen J. AAV9.I-1c delivered via direct coronary infusion in a porcine model of heart failure improves contractility and mitigates adverse remodeling. Circ Heart Fail. 2013;6:310–317. doi: 10.1161/CIRCHEARTFAILURE.112.971325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shiba Y., Fernandes S., Zhu W.Z., Filice D., Muskheli V., Kim J., Palpant N.J., Gantz J., Moyes K.W., Reinecke H. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ahmed R.P., Ashraf M., Buccini S., Shujia J., Haider H.Kh. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011;6:171–178. doi: 10.2217/rme.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee A.S., Tang C., Hong W.X., Park S., Bazalova-Carter M., Nelson G., Sanchez-Freire V., Bakerman I., Zhang W., Neofytou E. Brief Report: External Beam Radiation Therapy for the Treatment of Human Pluripotent Stem Cell-Derived Teratomas. Stem Cells. 2017;35:1994–2000. doi: 10.1002/stem.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Riegler J., Ebert A., Qin X., Shen Q., Wang M., Ameen M., Kodo K., Ong S.G., Lee W.H., Lee G. Comparison of Magnetic Resonance Imaging and Serum Biomarkers for Detection of Human Pluripotent Stem Cell-Derived Teratomas. Stem Cell Reports. 2016;6:176–187. doi: 10.1016/j.stemcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ylä-Herttuala S., Baker A.H. Cardiovascular Gene Therapy: Past, Present, and Future. Mol. Ther. 2017;25:1095–1106. doi: 10.1016/j.ymthe.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karvinen H., Pasanen E., Rissanen T.T., Korpisalo P., Vähäkangas E., Jazwa A., Giacca M., Ylä-Herttuala S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011;18:1166–1172. doi: 10.1038/gt.2011.66. [DOI] [PubMed] [Google Scholar]
- 178.Gallet R., Dawkins J., Valle J., Simsolo E., de Couto G., Middleton R., Tseliou E., Luthringer D., Kreke M., Smith R.R. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]