Abstract
Objective:
Studies in mouse models implicate complement activation as a causative factor in adverse pregnancy outcomes (APOs). We investigated whether activation of complement early in pregnancy predicts APOs in women with systemic lupus erythematosus (SLE) and/or antiphospholipid antibodies (aPL).
Methods:
The PROMISSE Study enrolled pregnant women with SLE and/or aPL antibodies (n=487) and pregnant healthy controls (n=204) at <12 weeks gestation and evaluated them monthly. APOs were: fetal/neonatal death, preterm delivery <36 weeks because of placental insufficiency or preeclampsia, and/or growth restriction <5th %ile. Complement activation products were measured on serial blood samples obtained at each monthly visit.
Results:
APO occurred in 20.5% of SLE and/or aPL pregnancies. As early as 12–15 weeks, levels of Bb and sC5b-9 were significantly higher in patients with APOs and remained elevated through 31 weeks compared to those with normal outcomes. Moreover, Bb and sC5b-9 were significantly higher in SLE and/or aPL patients without APOs compared to healthy controls. In logistic regression analyses, Bb and sC5b-9 at 12–15 weeks remained significantly associated with APO (ORadj=1.41 per SD increase; 95% CI: 1.06–1.89; p=0.019 and ORadj=1.37 per SD increase; 95% CI: 1.05–1.80; p=0.022, respectively) after controlling for demographic and clinical risk factors for APOs in PROMISSE. When analyses were restricted to patients with aPL (n=161), associations between Bb at 12–15 weeks and APOs became stronger (ORadj=2.01 per SD increase; 95% CI: 1.16–3.49; p=0.013).
Conclusion:
In pregnant SLE and/or aPL patients, increased Bb and sC5b-9 detectable early in pregnancy is strongly predictive of APOs and supports activation of complement, particularly the alternative pathway, as a contributor to APOs.
Keywords: Systemic Lupus Erythematosus, Antiphospholipid Syndrome, Pregnancy, Complement
INTRODUCTION
Women with systemic lupus erythematosus (SLE) and/or antiphospholipid (aPL) antibodies are at increased risk for adverse pregnancy outcomes (APOs), including preeclampsia, fetal and neonatal death, and fetal growth restriction. Although we have made progress in identifying clinical risk factors and dysregulated circulating anti-angiogenic factors as predictors of APOs, identification of patients destined for complications remains challenging.1,2
Studies in mice implicate inflammation, particularly complement activation and recruitment of neutrophils, as an essential and causative factor in placental insufficiency, fetal loss, and growth restriction.3–5 Activation of complement stimulates infiltrating leukocytes to release TNF-α and soluble fms-like tyrosine kinase-1 (sFlt1), a potent antiangiogenic factor, which are both associated with impaired development of the placenta and preeclampsia.5–7 Complement activation is initiated by classical, alternative and lectin pathways. The convergence of the three pathways on C3 results in generation of common effectors: anaphylatoxins, opsonins, and the membrane attack complex. Mice deficient in alternative and classical pathway complement components (factor B, C4, C3 and C5) and mice treated with inhibitors of complement activation (anti-C5 mAb, anti-factor B mAb, C5a receptor antagonist peptide) are resistant to fetal injury induced by aPL,3,8 indicating that both pathways contribute to damage.
Studies in humans support the role of complement in aPL-associated pregnancy complications,9–11 and in preeclampsia and growth restriction in non-autoimmune women.12,13 Complement fragment C4d, a marker of classical pathway activation, is present in placentae from women with SLE and/or antiphospholipid syndrome (APS) and from women with preeclampsia.12,14–16 Mild hypocomplementemia has also been reported in primary APS in two studies.17,18 The presence of risk variants in complement regulatory proteins in patients with SLE and/or aPL antibodies who develop preeclampsia, and in preeclampsia patients without an associated autoimmune disease, links complement activation to disease pathogenesis.9 Furthermore, in a prospective study of non-autoimmune patients, elevated levels of the alternative pathway complement activation product Bb, was strongly associated with preeclampsia.19 There have been no longitudinal studies examining complement activation in high risk pregnancies.
Accordingly, we investigated whether alterations in plasma levels of complement activation fragments before mid-second trimester predict APOs in women with SLE and/or aPL using data from the PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkers In antiphospholipid antibody Syndrome and Systemic Lupus Erythematosus). PROMISSE is the largest multi-center, multi-ethnic, and multi-racial study to prospectively assess clinical and laboratory predictors of APO in women with aPL and/or SLE with inactive or mild/moderate activity at conception. We hypothesized that complement activation products are elevated early in pregnancy in patients destined for APOs.
METHODS
Patient Population
The PROMISSE Study enrolled pregnant women between September 2003 and August 2013 from 8 U.S. sites and 1 Canadian site. Institutional review boards at each site approved protocols and consent forms; written informed consent was obtained from all subjects. Consecutive pregnant women referred to the study with diagnoses of SLE,20 aPL (defined in Table 1) or both were recruited at <12 weeks’ gestation as previously described.21 Healthy control patients matched for ethnicity/race were also included.
Table 1.
Demographic and Clinical Variables by Outcome Status at Baseline among Patients with aPL and/or SLE
| Variable | APO (N = 100) | No APO (N = 387) | P-value* |
|---|---|---|---|
| Demographic characteristics | |||
| Race/ethnicity, N (%) | 0.135 | ||
| Non-Hispanic white | 52 (52) | 223 (58) | |
| Hispanic white | 5 (5) | 30 (8) | |
| African American | 21 (21) | 57 (15) | |
| Asian | 10 (10) | 42 (11) | |
| Other | 2 (2) | 17 (4) | |
| Do not know | 10 (10) | 18 (5) | |
| BMI, N (%) | 0.0009 | ||
| <25 | 43 (45) | 228 (63) | |
| 25-30 | 25 (26) | 83(23) | |
| >30 | 28 (29) | 52(14) | |
| Missing | 4 | 24 | |
| Maternal age, Mean (SD) | 30.49 (5.17) | 31.55 (4.79) | 0.054 |
| Clinical history | |||
| Parity, N (%) | 0.20 | ||
| Nulliparous | 53 (53) | 231 (60) | |
| Parous without history of PE | 36 (36) | 132 (34) | |
| Parous with history of PE | 11 (10) | 24 (6) | |
| APL**/SLE status, N (%) | 0.0004 | ||
| APL+/SLE− | 28 (28) | 73 (19) | |
| APL+/SLE+ | 21 (21) | 39 (10) | |
| APL−/SLE+ | 51 (51) | 275 (71) | |
| Lupus nephritis ever, N (%) | 0.28 | ||
| No | 71 (71) | 295 (76) | |
| Yes | 29 (29) | 92 (24) | |
| Thrombosis, N (%) | <0.0001 | ||
| No | 74 (74) | 352 (91) | |
| Yes | 26 (26) | 35 (9) | |
| Systolic, Mean (SD) | 116.63 (13.05) | 110.32 (11.42) | <0.0001 |
| Diastolic, Mean (SD) | 71.72 (10.01) | 66.42 (8.77) | <0.0001 |
| Smoking, N (%) | 0.48 | ||
| Never | 71 (71) | 290 (75) | |
| Ever | 24 (24) | 84 (22) | |
| Current | 5 (5) | 11 (3) | |
| Laboratory values | |||
| LAC, N (%) | <.0001 | ||
| No | 59 (59) | 334 (86) | |
| Yes | 41 (41) | 53 (14) | |
| Proteinuria (500-1000 mg/day), N (%) | 0.122 | ||
| No | 89 (89) | 362 (94) | |
| Yes | 11 (11) | 25 (6) | |
| Disease activity (SLE only, n=386) | |||
| SLEPDAI, Mean (SD) | 3.81 (3.31) | 2.52(2.76) | 0.003 |
| SLEPDAI >4 | 0.0002 | ||
| No | 47 (67%) | 264 (86%) | |
| Yes | 23 (33%) | 44 (14%) | |
| PGA, Mean (SD) | 0.57 (0.63) | 0.34 (0.49) | 0.006 |
| PGA >1 | 0.002 | ||
| No | 51 (80%) | 278 (92%) | |
| Yes | 13 (20%) | 23 (8%) | |
| Current Medications | |||
| Aspirin N (%) | 0.37 | ||
| No | 50 (50) | 174 (45) | |
| Yes | 50 (50) | 213 (55) | |
| Antihypertensive, N (%) | <0.0001 | ||
| No | 82 (82) | 366 (95) | |
| Yes | 18 (18) | 21 (5) | |
| Heparin, N (%) | 0.0002 | ||
| No | 46 (46) | 256 (66) | |
| Yes | 54 (54) | 131 (34) | |
| Glucocorticoids, N (%) | 0.33 | ||
| Current | 35 (35%) | 119 (31%) | |
| Prior | 23 (23%) | 118 (30%) | |
| Never | 42 (42%) | 150 (39%) | |
| Daily dose (mg, current users), Mean (SD) | 9.13 (7.48) | 7.66 (5.68) | 0.29 |
By Pearson’s chi-square tests for the categorical variables or two-sample t-tests for the continuous variables.
APL positive is defined as anticardiolipin antibody [aCL: IgG ≥40 GPL units; IgM ≥40 MPL units] and/or positive lupus anticoagulant [RVVT, kaolin, dilute TTI or PTT LA] and/or anti-β2 glycoprotein I [antiβ2GPI: IgG ≥40 GPL units; IgM ≥ 40 MPL units] at least twice between 6 weeks and 5 years apart of which one must be during the PROMISSE pregnancy at a core laboratory, as previously described)21.
Inclusion criteria were: live singleton intrauterine pregnancy confirmed by ultrasound; age 18–45 years; hematocrit >26%. Exclusion criteria, to minimize confounding by known causes of APOs not specifically associated with SLE and/or aPL included: prednisone >20 mg/day; urine protein (mg)/creatinine (gram) ratio ≥1000 on random sampling or 24 hour collection; erythrocyte casts on urinalysis; serum creatinine >1.2 mg/dL; type I or II diabetes mellitus; blood pressure >140/90 mmHg.
Detailed medical and obstetrical information and serial blood samples were obtained at screening and monthly from 12 weeks’ gestation until the end of pregnancy and rheumatology assessments at each trimester.1,2
Adverse Pregnancy Outcomes
APOs defined for PROMISSE included one or more of the following: 1) fetal death after 12 weeks’ gestation unexplained by chromosomal abnormalities, anatomical malformation, or congenital infection; 2) neonatal death before hospital discharge due to complications of prematurity and/or placental insufficiency; 3) indicated preterm delivery at less than 36 weeks due to gestational hypertension, preeclampsia, or placental insufficiency; and 4) small-for gestational-age (SGA) neonate (<5th percentile).
Laboratory assays
Complement levels were measured in a blinded manner by MicroVue™ EIA kits for Bb Plus Fragment, sC5b-9 Plus Fragment, Ba Fragment, C3a Plus Fragment, C4d Fragment, iC3b Fragment and C5a. Duplicate samples were run in two plates and averaged to obtain final results. Angiogenic factor (sFlt1 and PlGF) levels were measured as described.2
Statistical methods
Categorical and continuous variables were compared between groups using Fisher’s exact and T-tests, respectively. Correlations were estimated using Pearson’s correlation. Odds ratios for the associations between APO status and complement levels were estimated using logistic regression models including the following covariates selected a priori based on clinical considerations and prior studies: race/ethnicity, SLE status, lupus anti-coagulant (LAC) status, history of thrombosis, screening diastolic pressure, aspirin use, anti-hypertensive use, and BMI. Model performance was evaluated using the Hosmer-Lemeshow (HL) goodness-of-fit test and area under the ROC curve (AUC). To address the potential for over-fitting, leave-one-out cross-validation was performed. Complement levels measured at the time of or after an APO were excluded. Missing data were handled with both listwise deletion and multiple imputation (MI) using the Markov chain Monte Carlo approach. Missing data rates were 0%-6% for baseline variables and 29.5%-31.1% for 12–15 and 16–19 week complement variables. Given similarity in parameter estimates across missing data methods, MI results are provided in supplementary Table S1. Change in complement levels over time were analyzed by linear mixed effects models. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc.).
RESULTS
Study Population and Pregnancy Outcomes
Of 770 pregnant women with SLE and/or aPL screened for PROMISSE, 487 who met study inclusion/exclusion criteria, had documented outcomes, and had at least one blood sample evaluated for complement levels during pregnancy were included: 326 (66.9%) with SLE and without aPL, 60 (12.3%) with SLE and aPL, and 101 (20.7%) with only aPL. APOs occurred in 100 cases (20.5%): fetal death in 27 (5.5%), neonatal death in 5 (1.0%), indicated preterm delivery for placental insufficiency or gestational hypertensive disease in 49 (10.1%), and SGA in 47 (9.7%). Also included were 204 healthy controls, 7 of whom had APOs (3.4%).
Baseline Demographic and Clinical Risk Factors
Women with SLE and/or aPL who had subsequent APOs were more likely to be positive for aPL and LAC, have higher systolic and diastolic blood pressures, higher BMI, history of thrombosis, and exposure to heparin and anti-hypertensive medications, compared to women without APOs (Table 1). Race/ethnicity, age, parity, history of lupus nephritis, proteinuria, aspirin use, and smoking status at enrollment were not significantly associated with pregnancy outcome in bivariate analyses.
Complement activation products: Bb and sC5b-9
We initially focused on Bb and sC5b-9, the complement measures which varied the least among the healthy controls (i.e., smallest coefficients of variation at all visits). As early as 12–15 weeks, patients with APOs demonstrated significant elevations in Bb and sC5b-9 compared to patients without APO (Figure 1A, 1B). These differences tended to increase through week 31. SLE and/or aPL patients without APO had consistently higher levels of Bb and sC5b-9 compared to the reference group, the healthy controls without APO (N=197). Bb levels were elevated at 12–15 weeks even in the subset of 138 SLE patients with SLEPDAI ≤2 at baseline, compared to the reference group (p<0.001). In all groups, Bb decreased as pregnancy progressed, but the rate of decrease was smaller in patients with APOs compared to patients without APOs (p=0.06) and compared to the reference group (p=0.002), suggesting increased complement activation and cleavage of Bb. Patients with APO also showed less decline in sC5b-9 compared to patients without APO (p=0.02) and the reference group (p=0.002). Correlations between Bb and sC5b-9 in diseased patients ranged from 0.49 (p<0.001) at 12–15 weeks to 0.68 (p<0.001) at 36–39 weeks.
Figure 1.
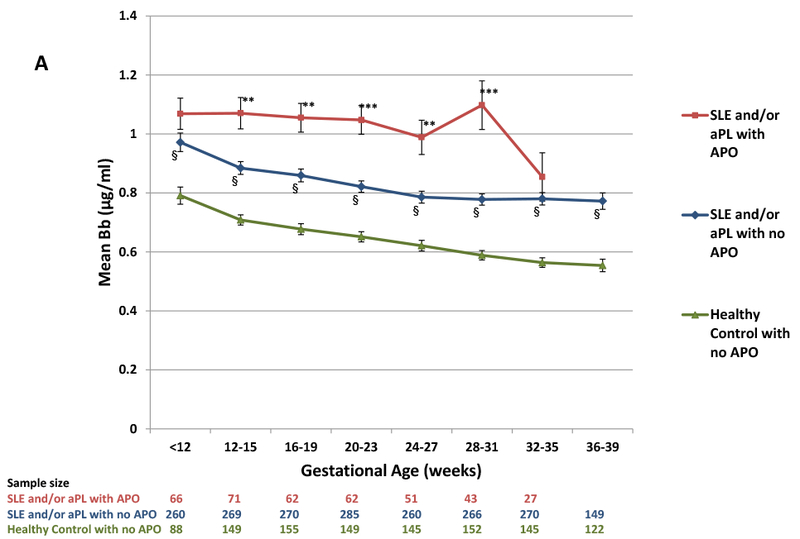
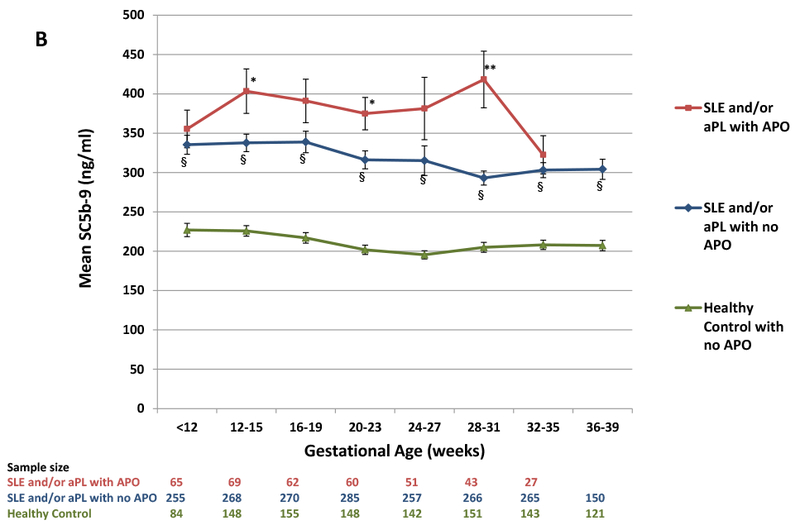
(A) Levels of Bb and (B) levels of sC5b-9 during pregnancy for patients with SLE and/or aPL with and without APO and healthy control women (no SLE, no APL) without APO. Values represent means and standard errors. Sample size is the number of specimens available from individual patients from each group at each time point.
P-values by two sample t-tests:
SLE and/or aPL with APO vs. SLE and/or aPL with no APO: * = <0.05, **= <0.01, *** = <0.0001;
SLE and/or aPL with no APO vs Healthy Control: § = <0.0001
Early elevations in complement tended to persist throughout pregnancy: correlations between 12–15 and 36–39 week levels were 0.62 for Bb (p < 0.001) and 0.44 for sC5b-9 (p< 0.001). In ROC analysis of the 12–15 week measures, the optimal cut-points for discriminating APO from non-APO in diseased patients when sensitivity (SE) and specificity (SP) are equally weighted were >1.04 μg/ml for Bb (SE=46%, SP=75%, PPV=33%, NPV=84%) and >347 ng/ml for sC5b-9 (SE=52%, SP=68%, PPV=29%, NPV=85%).
Multivariable Analyses of Complement Levels
Bb at 12–15 weeks remained significantly associated with APOs after controlling for potential confounders (Table 2; ORadj=1.41 per SD increase; 95% CI: 1.06–1.89; p=0.019). SC5b-9 at 12–15 weeks was also significantly associated with APO in logistic regression analyses (ORadj=1.37 per SD increase; 95% CI: 1.05–1.80; p=0.022). At 16–19 weeks, odds ratios were ORadj=1.59 per SD increase for Bb (95% CI: 1.16–2.17; p=0.004) and ORadj=1.26 per SD increase of sC5b-9 (95% CI: 0.94–1.68; p=0.12). Between 20–31 weeks, Bb was significantly associated with APO at all visits; sC5b-9 levels at 28–31 weeks and not earlier were significant [results not shown].
Table 2. Adjusted odds ratios for complement factors measured at 12-15 and 16-19 weeks.
| Predictor variable | Bb (12-15 weeks) N=323 subjects, 70 events | Bb (16-19 weeks) N= 317 subjects, 60 events | sC5b-9 (12-15 weeks) N= 320 subjects, 68 events | sC5b-9 (16-19 weeks) N= 317 subjects, 60 events | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
|
| ||||||||
| Non-Hispanic White (yes vs no) | 0.74 (0.39-1.40) | 0.35 | 0.64(0.32-1.27) | 0.20 | 0.70 (0.37-1.33) | 0.27 | 0.55 (0.28-1.09) | 0.086 |
| SLE status (positive vs negative) | 1.27 (0.51-3.22) | 0.61 | 0.82 (0.31-2.18) | 0.69 | 1.07 (0.43-2.71) | 0.88 | 0.72 (0.27-1.95) | 0.52 |
| LAC status (positive vs negative) | 5.81 (2.55-13.25) | <0.0001 | 2.41 (0.94-6.15) | 0.067 | 6.58 (2.89-14.98) | <.0001 | 2.85 (1.12-7.26) | 0.028 |
| History of thrombosis (yes vs no) | 2.51 (1.11-5.65) | 0.027 | 2.85 (1.26-6.46) | 0.012 | 2.35 (1.04-5.29) | 0.040 | 2.73 (1.23-6.08) | 0.014 |
| Diastolic pressure > 80 mm Hg (yes vs no) | 3.13 (1.42-6.91) | 0.005 | 4.46 (2.01-9.88) | 0.0002 | 2.82 (1.29-6.20) | 0.010 | 4.27 (1.97-9.26) | 0.0002 |
| Aspirin Use (yes vs no) | 0.57 (0.29-1.11) | 0.095 | 0.58 (0.28-1.17) | 0.13 | 0.57 (0.29-1.12) | 0.104 | 0.65 (0.32-1.31) | 0.22 |
| Current anti-hypertensive (yes vs no) | 3.82(1.44-10.10) | 0.007 | 4.37(1.60-11.95) | 0.004 | 4.61 (1.75-12.14) | 0.002 | 4.37 (1.64-11.63) | 0.003 |
| BMI | 0.43 | 0.48 | 0.27 | 0.18 | ||||
| < 25 | 1 | 1 | 1 | 1 | ||||
| 25-30 | 0.78 (0.38-1.59) | 0.82 (0.36-1.82) | 0.70(0.34-1.45) | 0.68(0.31-1.51) | ||||
| > 30 | 1.28 (0.54-3.02) | 1.33 (0.53-3.36) | 1.29(0.55-3.05) | 1.42 (0.57-3.51) | ||||
| Bb per SD increase* | 1.41 (1.06-1.89) | 0.019 | 1.59 (1.16-2.17) | 0.004 | ----- | ---- | ||
| sC5b-9 per SD increase * | ----- | ----- | 1.37 (1.05-1.80) | 0.022 | 1.26 (0.94-1.68) | 0.12 | ||
|
| ||||||||
| Hosmer-Lemeshow Goodness-of-fit test | 0.85 | 0.32 | 0.30 | 0.22 | ||||
| AUC | 0.80 | 0.80 | 0.80 | 0.80 | ||||
| Cross-validated AUC | 0.76 | 0.75 | 0.76 | 0.74 | ||||
SD for Bb = 0.38 μg/ml at 12-15 weeks and 0.37 μg/ml at 16-19 weeks; SD for sC5b9 = 196 ng/ml at 12-15 weeks and 223ng/ml at 16-19 weeks.
Results above were based on diseased patients. Among all healthy subjects, Bb, but not sC5b-9, was significantly elevated at 12–15 weeks in the 7 who had APO compared to non-APO (Bb: 0.91 vs 0.71 μg/ml, p = 0.03; sC5b-9: 241.5 vs 226.5 ng/ml; p=0.66 ). To assess the independent effects of disease status and complement on APO, a separate logistic regression was fit with main effects for Bb at 12–15 weeks and disease (yes/no), based on all diseased and healthy subjects. The adjusted odds ratio for APO was 4.82 (95% CI: 2.01–11.55; p<0.001) comparing diseased and healthy subjects, and for Bb was 1.61 (95% CI: 1.26–2.06; p<0.001) per SD increase. The odds ratio for sC5b-9 at 12–15 weeks after adjusting for disease status was 1.34 per SD increase (95 CI: 1.06–1.70; p=0.01). Adjustment for additional covariates was not feasible due limited APOs in the healthy group.
Relationship with angiogenic factors
Given prior evidence that complement activation stimulated release of sFlt1 by leukocytes22 and that angiogenic dysregulation early in pregnancy is associated with APOs,2 we examined the relationship between levels of complement activation products and angiogenic factors. Correlations between Bb and sC5b-9 measured at or before 12–15 weeks with sFlt1, PlGF, and sFlt1/PlGF measured at the same and subsequent visits were modest and did not exceed 0.25. Adjustment for sFlt1/PlGF in multivariable analyses did not affect the association between APOs and Bb measured at 12–15 weeks (ORadj=1.39; 95% CI: 1.03–1.88; p=0.034) or at 16–19 weeks (ORadj=1.60; 95% CI: 1.14–2.2; p=0.006). SC5b-9 results were also minimally affected after adjusting for sFlt1/PlGF at 12–15 weeks (ORadj=1.34; 95% CI: 1.00–1.78; p=0.047) and 16–19 weeks (ORadj =1.30; 95% CI: 0.95–1.76; p=0.10).
Subgroup analyses
Because studies in mouse models of aPL-associated APO strongly implicate complement activation and reveal complement deposition on trophoblasts,8 we analyzed complement analytes in the subgroup of aPL-positive patients with or without SLE (n=161). Associations between Bb and APO increased for both the 12–15 week measures (ORadj=2.01 per SD increase; 95% CI: 1.16–3.49; p=0.013) and 16–19 week measures (ORadj=2.46 per SD increase; 95% CI: 1.27–4.76; p=0.008) after adjusting for the same variables in Table 2; Bb results remained highly significant after further adjustment for heparin (p=0.01 and p=0.009, respectively). Odds ratios for sC5b-9 in aPL positive subjects were similar to those based on the overall study population using the 12–15 week levels (ORadj=1.35 per SD increase; 95% CI: 0.86–2.12; p=0.19) and 16–19 week levels (ORadj=1.16 per SD increase; 95% CI: 0.69–1.94; p=0.57).
Among SLE patients without aPL antibodies (n=326), associations between Bb with APO were weaker (12–15 weeks: ORadj =1.28 per SD increase; 95% CI: 0.87–1.86; p=0.21; 16–19 weeks: ORadj =1.45 per SD increase; 95% CI: 0.99–2.14; p=0.06) than corresponding results in the aPL positive group. In contrast, associations of sC5b-9 with APOs in the SLE only patients were comparable to those in the aPL positive group (12–15 weeks: ORadj=1.39 per SD increase; 95% CI: 0.98–2.01; p=0.07; 16–19 weeks: ORadj=1.33 per SD increase; 95% CI: 0.93–1.90; p=0.12). Baseline SLEPDAI scores were significantly correlated with Bb and sC5b-9 at both 12–15 and 16–19 weeks (ρ=0.22–0.30; p<0.001 for all correlations). In addition, among all SLE patients, mean Bb was significantly elevated at both visits in those who were LAC-positive versus negative (12–15 weeks: 1.21 μg/ml vs. 0.87 μg/ml; p<0.001; 16–19 weeks: 1.19 μg/ml vs. 0.85 μg/ml; p<0.001); mean sC5b-9 was non-significantly elevated (12–15 weeks: 399.5 ng/ml vs 356.3 ng/ml, respectively; p=0.28; 16–19 weeks: 433.3 ng/ml vs 358.5 ng/ml; p=0.19).
Other complement factors
C5a, C4d, and iC3b levels at 12–15 weeks and 16–19 weeks were not significantly associated with APOs (Supplementary Figures). C3a was significantly associated with APO in bivariate analyses, but not when added to the multivariable models that included Bb or sC5b-9, whereas Bb remained significant (12–15 weeks: p=0.057; 16–19 weeks: p=0.004). Limited data were available for Ba (>40% had missing values), precluding rigorous evaluation of this analyte. However, consistent with the contribution of alternative pathway activation, Ba appeared to be a stronger predictor of APOs than sC5b-9 at both 12–15 weeks (ORadj=1.74 per SD increase; 95% CI: 1.24–2.43; p=0.001) and 16–19 weeks (ORadj=1.47 per SD increase; 95% CI: 1.06–2.05; p=0.02), when added to the models in Table 2. It was also a stronger predictor of APOs than Bb at 12–15 weeks (ORadj=1.59 per SD increase; 95% CI: 1.10–2.30; p=0.01) but not at 16–19 weeks (ORadj=1.25 per SD increase; 95% CI: 0.89–1.75; p=0.20).
DISCUSSION
In our study that included nearly 500 pregnant patients with SLE and/or aPL, increased levels of Bb and sC5b-9 early in pregnancy were significantly associated with APOs. This supports the concept that complement activation, particularly the alternative pathway, contributes to abnormal placental development that leads to pregnancy complications. That the association of alternative complement activation with APO was greatest in the patients with aPL is consistent with our experiments showing that mice deficient in factor B or treated with an inhibitor of factor B activation were protected from aPL-induced fetal resorptions and growth restriction.3,23
Bb levels fell as pregnancy progressed in uncomplicated pregnancies in both diseased and healthy subjects. However, in those destined for APOs, Bb levels were significantly higher throughout pregnancy, and the rate of decrease during pregnancy was lower. Lynch et al. similarly showed that Bb levels decreased with gestational age in normotensive women, while dysregulation of Bb activation occurred between 10–20 weeks gestation in those who developed preeclampsia.24 Others have shown that elevated Bb is present in amniotic fluid of women with severe preeclampsia, supporting the concept that complement is activated at the maternal-fetal interface.25,26
We also found a significant association between elevated sC5b-9 levels and APOs, but this was not the case in a study of non-autoimmune women who developed preeclampsia.27 Nonetheless, a role for terminal pathway complement activation, as a cause or consequence of preeclampsia, is supported by reports of markedly increased urinary sC5b-9 excretion and correlations between urinary sC5b-9 and angiogenic dysregulation.28,29
Although antibodies are essential to the pathogenesis of SLE and aPL, the alternative pathway fragment Bb, rather than classical pathway components, was the strongest predictor of APO. The correlation between Bb and sC5b-9 further supports that the alternative pathway is a key driver of complement activation in pregnancy complications. Whether the complement cascade is triggered by the classical, lectin, or alternative pathway, activation is amplified by the alternative pathway. Classical pathway activation depends on affinity and subclass of multiplexed antibodies, and aPL are skewed towards IgG2 subclass, relatively ineffective classical pathway activators.30 Excess deposition of non-classical pathway activating antibodies can inhibit membrane-bound complement regulators and allow unabated activation of the alternative pathway. Furthermore, cleavage of complement components by neutrophil- and platelet-derived proteases can by-pass the classical pathway.31
In normal pregnancy, the human placenta is subjected to complement-mediated immune attack at the maternal-fetal interface.32,33 Complement activation is controlled in successful pregnancies by inhibitory proteins on trophoblast cells.34–36 In patients with APL and/or SLE, the excessive complement activation, evident in the circulation and in the placenta,12,14–16 overwhelms regulatory pathways and places the fetus at risk.
Infiltrating leukocytes recruited and activated by complement are a source of the excess anti-angiogenic factor sFlt1 that impairs early placental development. Subsequent hypoperfusion of the intervillous space stimulates trophoblasts to synthesize large amounts of sFlt-1 as pregnancy progresses. Because the latter is not directly driven by complement, the modest relationship between sFlt1 levels and complement activation fragments was not surprising.
Although PROMISSE patients had quiescent, stable, or mildly-active disease, even those without APO had significantly higher levels of Bb and sC5b-9 at baseline (6–11 weeks) compared to healthy controls. Prolonged activation of complement, also described in non-pregnant patients with inactive SLE and those with primary APS, may lower the threshold for exaggerated, uncontrolled activation that recruits leukocytes and unleashes potent inflammatory and anti-angiogenic mediators associated with placental insufficiency.17,37,38
Interestingly, baseline SLEPDAI scores correlated significantly with early measures of Bb and sC5b-9. A small study also reported that elevations of Bb and sC5b-9 accompanied disease activity in pregnant SLE patients.39 Interpretation of complement is confounded in pregnancy because circulating complement reflects both synthesis (enhanced by estrogen) and consumption.40 Our findings of early increased complement activation products in women destined for APOs, and our previous report that less increase in C3 levels from baseline to second trimester was predictive of APO, argue that excess complement activation is the driver rather than consequence of APOs. The presence of classical pathway complement fragment C4d deposition on placentae from women with SLE and/or APS and women with preeclampsia, and in kidneys of women with preeclampsia,12–16 provides further evidence for local activation of classical or lectin pathways by aPL or necrotic fetoplacental debris. Such activation, regardless of the source, is then augmented through the alternative pathway. Importantly, circulating levels of complement fragments may not reflect the extent of activation in the placenta.
Our study had limitations. We did not measure pre-pregnancy levels of complement activation products so could not differentiate whether increased complement consumption was due to SLE or placental inflammation. Sample sizes did not allow for analyses stratified by APO type. Whether patient aPL antibodies were complement-fixing was not determined, and not possible for LAC, the aPL associated with greatest risk.
In conclusion, we demonstrate that complement pathway activation is associated with APOs in patients with SLE and/or APL. Aberrant complement activation, whether initiated by immune complexes in SLE or by aPL, may trigger or amplify inflammation at the maternal fetal-interface and thereby contribute to the pathogenesis of APOs.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number RO1 AR49772. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are grateful to Mary S. Stephenson, Alan Peaceman and Munther Khamashta for contributing patients to the study. We thank Quidel Corporation for their guidance with the complement split product measurements and S. Ananth Karumanchi for thoughtful discussions about angiogenic factors.
Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award number RO1 Ar49772 (PROMISSE Study, MYK, MMG, EK, CAL, MP, DWB, MDL, LRS, JTM, TFP, AS, JPB, JES), Mary Kirkland Center for Lupus Research (JES, MDL) and NIH AR43727, AR69572 (MP).
Footnotes
ClinicalTrials.gov identifier: NCT00198068
Preliminary data included in this publication were reported in an abstract at the American College of Rheumatology (ACR) Meeting in 2015.
Contributorship: All authors made substantial contributions to study design or acquisition, analysis or interpretation of data. MYK, JES, JPB wrote the manuscript and all authors contributed to revising it critically for important intellectual content. All authors have read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Competing Interests:
DWB reports serving on the UCB pharmaceuticals Advisory Board; JES has received an investigator-initiated grant from UCB pharmaceuticals and consulting fees from Alnylam and Alexion.
REFERENCES
- 1.Buyon JP, Kim MY, Guerra MM, et al. Predictors of Pregnancy Outcomes in Patients With Lupus: A Cohort Study. Annals of internal medicine 2015;163:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MY, Buyon JP, Guerra MM, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol 2016;214:108 e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardi G, Berman J, Redecha P, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. The Journal of clinical investigation 2003;112:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qing X, Redecha PB, Burmeister MA, et al. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney international 2011;79:331–9. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Irani RA, Zhang Y, et al. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension 2012;60:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. Journal of immunology 2005;174:485–90. [DOI] [PubMed] [Google Scholar]
- 7.Gelber SE, Brent E, Redecha P, et al. Prevention of Defective Placentation and Pregnancy Loss by Blocking Innate Immune Pathways in a Syngeneic Model of Placental Insufficiency. Journal of immunology 2015;195:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holers VM, Girardi G, Mo L, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. The Journal of experimental medicine 2002;195:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med 2011;8:e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reggia R, Ziglioli T, Andreoli L, et al. Primary anti-phospholipid syndrome: any role for serum complement levels in predicting pregnancy complications? Rheumatology (Oxford) 2012;51:2186–90. [DOI] [PubMed] [Google Scholar]
- 11.De Carolis S, Botta A, Santucci S, et al. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus 2012;21:776–8. [DOI] [PubMed] [Google Scholar]
- 12.Buurma A, Cohen D, Veraar K, et al. Preeclampsia is characterized by placental complement dysregulation. Hypertension 2012;60:1332–7. [DOI] [PubMed] [Google Scholar]
- 13.Penning M, Chua JS, van Kooten C, et al. Classical Complement Pathway Activation in the Kidneys of Women With Preeclampsia. Hypertension 2015;66:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. American journal of obstetrics and gynecology 2007;196:167 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen D, Buurma A, Goemaere NN, et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol 2011;225:502–11. [DOI] [PubMed] [Google Scholar]
- 16.Viall CA, Chamley LW. Histopathology in the placentae of women with antiphospholipid antibodies: A systematic review of the literature. Autoimmun Rev 2015;14:446–71. [DOI] [PubMed] [Google Scholar]
- 17.Oku K, Atsumi T, Bohgaki M, et al. Complement activation in patients with primary antiphospholipid syndrome. Ann Rheum Dis 2009;68:1030–5. [DOI] [PubMed] [Google Scholar]
- 18.Ziglioli T, Andreoli L, M M. Low complement levels during pregnancy are associated with obstetric complications in patients with primary antiphospholipid syndrome. Ann Rheum Dis 2009;68:213–4. [Google Scholar]
- 19.Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta 2010;31:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 21.Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis and rheumatism 2012;64:2311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. The Journal of experimental medicine 2006;203:2165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 2005;42:87–97. [DOI] [PubMed] [Google Scholar]
- 24.Lynch AM, Wagner BD, Giclas PC, West NA, Gibbs RS, Holers VM. The Relationship of Longitudinal Levels of Complement Bb During Pregnancy with Preeclampsia. Am J Reprod Immunol 2016;75:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banadakoppa M, Vidaeff AC, Yallampalli U, Ramin SM, Belfort MA, Yallampalli C. Complement Split Products in Amniotic Fluid in Pregnancies Subsequently Developing Early-Onset Preeclampsia. Dis Markers 2015;2015:263109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman MC, Rumer KK, Kramer A, Lynch AM, Winn VD. Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am J Reprod Immunol 2014;71:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch AM, Murphy JR, Gibbs RS, et al. The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG 2010;117:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension 2013;62:1040–5. [DOI] [PubMed] [Google Scholar]
- 29.Guseh SH, Feinberg BB, Dawood HY, Yamamoto HS, Fichorova RN, Burwick RM. Urinary excretion of C5b-9 is associated with the anti-angiogenic state in severe preeclampsia. Am J Reprod Immunol 2015;73:437–44. [DOI] [PubMed] [Google Scholar]
- 30.Sammaritano LR, Ng S, Sobel R, et al. Anticardiolipin IgG subclasses: association of IgG2 with arterial and/or venous thrombosis. Arthritis Rheum 1997;40:1998–2006. [DOI] [PubMed] [Google Scholar]
- 31.Kolev M, Le Friec G, Kemper C. Complement--tapping into new sites and effector systems. Nat Rev Immunol 2014;14:811–20. [DOI] [PubMed] [Google Scholar]
- 32.Wells M, Bennett J, Bulmer JN, Jackson P, Holgate CS. Complement component deposition in uteroplacental (spiral) arteries in normal human pregnancy. J Reprod Immunol 1987;12:125–35. [DOI] [PubMed] [Google Scholar]
- 33.Morgan BP, Holmes CH. Immunology of reproduction: protecting the placenta. Curr Biol 2000;10:R381–3. [DOI] [PubMed] [Google Scholar]
- 34.Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. Journal of immunology 1993;151:1562–70. [PubMed] [Google Scholar]
- 35.Holmes CH, Simpson KL, Wainwright SD, et al. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. Journal of immunology 1990;144:3099–105. [PubMed] [Google Scholar]
- 36.Cunningham DS, Tichenor JR, Jr. Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clin Immunol Immunopathol 1995;74:156–61. [DOI] [PubMed] [Google Scholar]
- 37.Putterman C, Furie R, Ramsey-Goldman R, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med 2014;1:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breen KA, Seed P, Parmar K, Moore GW, Stuart-Smith SE, Hunt BJ. Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thrombosis and haemostasis 2012;107:423–9. [DOI] [PubMed] [Google Scholar]
- 39.Buyon JP, Tamerius J, Ordorica S, Young B, Abramson SB. Activation of the alternative complement pathway accompanies disease flares in systemic lupus erythematosus during pregnancy. Arthritis Rheum 1992;35:55–61. [DOI] [PubMed] [Google Scholar]
- 40.Abramson SB, Buyon JP. Activation of the complement pathway: comparison of normal pregnancy, preeclampsia, and systemic lupus erythematosus during pregnancy. Am J Reprod Immunol 1992;28:183–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


