Abstract
It is well known that the electrical signaling in neuronal networks is modulated by chloride (Cl−) fluxes via the inhibitory GABAA and glycine receptors. Here, we discuss the putative contribution of Cl− fluxes and intracellular Cl− to other forms of information transfer in the CNS, namely the bidirectional communication between neurons and astrocytes. The manuscript (i) summarizes the generic functions of Cl− in cellular physiology, (ii) recaps molecular identities and properties of Cl− transporters and channels in neurons and astrocytes, and (iii) analyzes emerging studies implicating Cl− in the modulation of neuroglial communication. The existing literature suggests that neurons can alter astrocytic Cl− levels in a number of ways; via (a) the release of neurotransmitters and activation of glial transporters that have intrinsic Cl− conductance, (b) the metabotropic receptor-driven changes in activity of the electroneutral cation-Cl− cotransporter NKCC1, and (c) transient, activity-dependent changes in glial cell volume which open the volume-regulated Cl−/anion channel VRAC. Reciprocally, astrocytes are thought to alter neuronal [Cl−]i through either (a) VRAC-mediated release of the inhibitory gliotransmitters, GABA and taurine, which open neuronal GABAA and glycine receptor/Cl− channels, or (b) the gliotransmitter-driven stimulation of NKCC1. The most important recent developments in this area are the identification of the molecular composition and functional heterogeneity of brain VRAC channels, and the discovery of a new cytosolic [Cl−] sensor – the Wnk family protein kinases. With new work in the field, our understanding of the role of Cl− in information processing within the CNS is expected to be significantly updated.
Keywords: neuron-astrocyte communication, chloride homeostasis, chloride channels, KCC, NKCC, VRAC, WNK
1. Introduction: the physiological significance of transmembrane Cl− gradients
Chloride (Cl−) is the most abundant physiological anion. Yet, it receives little attention outside of the fields studying excitable cells and acid-base homeostasis. With the exception of kidney tissue and some secretory epithelia, the extracellular Cl− levels are kept in the narrow range of 98–106 mM. In contrast, the intracellular Cl− concentrations can vary from as low as 5 mM in neuronal cells to 65 mM or higher in certain epithelial cells (see for example [1;2]). Intracellular Cl− levels are largely determined by the transmembrane potential and presence of secondary active Cl− transporters. In this review, we propose an important role for [Cl−]i in bidirectional neuron-astrocyte communication. But before focusing on the significance of Cl− in the CNS, it is helpful to briefly recapitulate the general physiological functions of this anion. Fundamentally, Cl− serves as a counterion for movement of the major cations, Na+, K+, Ca2+, and H+ (for comprehensive reviews see [3–5]). The plasmalemmal cation transport in excitable cells and ion-transporting epithelia is assisted by Cl− conductivity pathways, such as voltage-gated Cl− channels. Within the cell, electrogenic proton movement in acidic organelles is facilitated by Cl−/H+ exchangers. Other, equally important Cl− functions include (i) cell volume homeostasis, (ii) regulation of membrane potential, and (iii) cell proliferation and initiation of apoptosis. These are briefly introduced below and summarized in Fig. 1.
Fig. 1. Diverse roles of Cl− in cellular physiology.
A, The presence of membrane-impermeable organic anions (O−) and uncharged molecules (O0) makes cells accumulate positively charged ions, leading to the phenomenon known as Donnan cell swelling. Passive extrusion of Cl− allows cells to maintain electroneutrality across their semi-permeable surface membrane and prevent Donnan swelling. B, When intracellular [Cl−] deviates from its electrochemical equilibrium, activation of the ligand-gated Cl− channels [GABAA and/or glycine (GlyR) receptors] leads to a depolarizing or hyperpolarizing current. C, During apoptosis, cells undergo apoptotic volume decrease as a result of the loss of intracellular K+ and Cl−. D, Cell volume homeostasis relies on the balance of inward and outward ion fluxes, which determine the movement of osmotically obligated water. Left panel, Swollen cells undergo regulatory volume decrease (RVD) driven by loss of KCl and osmotically obligated H2O. KCl is lost due to activation of the functionally coupled K+ channels and volume-regulated anion channels (VRAC), as well as the electroneutral K+,Cl− cotransporters (KCC). Right panel, Shrunken cells engage the process of regulatory volume increase (RVI) that is powered by the accumulation of NaCl. NaCl uptake is mediated by the net activity of the electroneutral Na+,K+,2Cl− cotransporters (NKCC), Na+/H+ exchangers, and Cl−/HCO3− anion exchangers.
1.1 Cl− in the Gibbs-Donnan equilibrium and cell volume regulation
Participation in cellular volume homeostasis represents a very important and evolutionarily conserved role of Cl− in animal cells, which is rarely covered outside of the “specialized” reviews. Cl− contributes to cell volume control via two separate mechanisms.
Under steady-state conditions, low intracellular [Cl−] compensates for the presence of impermeable large-molecular-weight organic molecules, which in the intracellular milieu carry net-negative charge (illustrated in Fig. 1A). Due to the presence of organic anions, cells tend to accumulate positively charged particles in their cytosol and have a propensity to swell, a phenomenon termed Donnan cell swelling [6]. Extrusion of Cl− allows for the maintenance of electroneutrality across semi-permeable cell surface membranes, meaning that the sum of positive and negative charges is the same inside and outside the cell, irrespective of the type of charged particle (Gibbs-Donnan equilibrium). In living cells, colloidal accumulation of water is not completely compensated via this mechanism. Therefore, the Gibbs-Donnan equilibrium is, in reality, a quasi-equilibrium, which is maintained by the constant osmogenic and electrogenic activity of the Na+/K+-ATPase (2 K+ in in exchange of 3 Na+ out, discussed in [7]).
Besides the constant pressure of Donnan swelling, cells experience frequent transient changes in their volume due to a variety of factors, such as (a) changes in extracellular osmolarity, (b) intracellular catabolic processes, which lead to the accumulation of low molecular weight osmolytes, or (c) net accumulation or extrusion of ions and osmolytes due to activation of diverse plasmalemmal transporters and channels. Swollen or shrunken cells engage “emergency” mechanisms, involving a variety of volume-sensitive transporters. These work collectively to extrude or accumulate inorganic ions and/or small organic molecules (see Fig. 1D). The net transfer of ions and other osmolytes drives the movement of osmotically obligated water, and powers cell volume normalization. Swollen cells undergo the process of regulatory volume decrease (RVD), which depends on the cooperative activity of swelling-activated K+ channels, Cl− channels, and/or electroneutral K+-Cl− cotransporters. Shrunken cells restore their volume via regulatory volume increase (RVI) by engaging Na+/K+/Cl− cotransporters and/or the functionally coupled activity of Na+/H+ and Cl−/HCO3− exchangers. The molecular origins and functional properties of the relevant Cl− transporters are summarized in Section 2. This topic can be further explored by reading any of the relevant comprehensive reviews [7–11]. In general terms, RVD is mediated by the net-loss of KCl with the additional contribution of organic osmolytes. RVI is largely the result of net-accumulation of NaCl.
1.2 Regulation of the membrane potential
As the dominant anion in the extracellular milieu, Cl− has a high capacity for modulating the plasma membrane potential. Background (unstimulated) Cl− conductance contributes to setting the resting membrane potential. This aspect of Cl− physiology has been best studied in skeletal muscle cells, in which Cl− currents are responsible for up to 80% of the resting membrane conductance, stabilize the membrane potential, and limit muscle excitability [12]. Accordingly, mutations in ClC-1, the principal voltage-gated Cl− channel in skeletal muscle cells, have been linked to many cases of the autosomal recessive generalized myotonia and the autosomal dominant myotonia congenita, two diseases in which cell hyperexcitability causes skeletal muscle stiffness (see for example [13–15]).
Unlike skeletal muscles, cells in the CNS have comparatively low resting plasmalemmal Cl− conductance and maintain their [Cl−]i away from the levels predicted by the Nernst equilibrium (see Section 2.1). Consequently, neuronal cells utilize the ligand-gated Cl− channels, such as GABAA and glycine receptors, for modulating membrane potential and excitability (see Fig. 1B). Activation of these channels can cause depolarizing or hyperpolarizing currents based on the established transmembrane Cl− gradients (for detailed discussion see Section 2.3). The voltage-gated Cl− channels, e.g. ClC-2, can also regulate intracellular [Cl−] and excitability in neuronal cells and contribute to the buffering of extracellular [Cl−] by astrocytes (for references and discussion see Section 2.4).
1.3 Cell proliferation, migration, and initiation of apoptosis
There is a very extensive literature that links Cl− channels, principally, the volume-sensitive Cl− channels, to proliferation, migration, and apoptosis in numerous cell types (for review see [7;16]). Outside of brain development, the significance of cell proliferation in the CNS is limited. Therefore, we do not discuss the potential role of Cl− in this process. Migration is also not a major feature of normal brain physiology, with the notable exception of the innate immune cells of the CNS, microglia. Under normal physiological conditions microglial cell bodies are immobile, but these cells actively relocate their branched processes in search of pro-inflammatory stimuli and signs of neuronal hyperexcitation. In response to tissue damage or inflammation, microglia retract their processes and move towards the pathological foci, where they engage in phagocytosis and tissue remodeling [17;18]. In microglia, broad spectrum Cl− channel blockers reversibly inhibit the formation of ramified processes and lamellipodia, limit the cytokine-stimulated cell migration, and block proliferation and phagocytosis [19–24]. Outside of the brain, contributions of Cl− channels to cell motility have been demonstrated in many other cell types (for review see [7;25]).
It is also important to acknowledge that Cl− transporters may be important in programmed cell death. One of the hallmarks of apoptosis is a marked cell shrinkage, which has been termed apoptotic volume decrease or AVD [26;27] (illustrated in Fig. 1C). In a number of cell types, AVD depends on the activity of the volume-sensitive channel VRAC (see Section 2.5), is associated with the loss of intracellular Cl−, and can be induced by reductions in extracellular Cl− levels (see for example [26;28–30] and review [7]). In the CNS, activity of VRAC has been directly implicated in the excitotoxic cell death of neurons, and pathological release of excitatory amino acids from astrocytes (see [31–34] and review [35]). Therefore, in the brain, volume-sensitive Cl− channels may contribute to tissue damage, both directly – by promoting apoptosis, and indirectly – via the release of excitotoxic neurotransmitters and downstream activation of cell death pathways.
2. Cl− transporters and channels which govern intracellular Cl− levels
As briefly outlined in the Introduction, plasmalemmal Cl− gradients are established by several secondary active ion transporters, and allow neurons and astrocytes to maintain intracellular Cl− levels either above or below the electrochemical equilibrium for this ion. When [Cl−]i deviates from equilibrium, transient changes in Cl− membrane permeability via voltage-, volume-, and ligand-gated ion channels produce net charge transfer and influence many cellular functions. For neurons, the impact of Cl− currents on cellular excitability is well established and has been covered in several comprehensive reviews (see for example [36–38]). In contrast, the influence of Cl− fluxes on astrocytic properties is less understood, and will be discussed in the present manuscript in the context of bidirectional neuron-astrocyte communication. In this section, we briefly describe the molecular nature of diverse Cl− transporters and ion channels in neural cells, and discuss their differential impact on intracellular Cl− levels (summarized in Fig. 2).
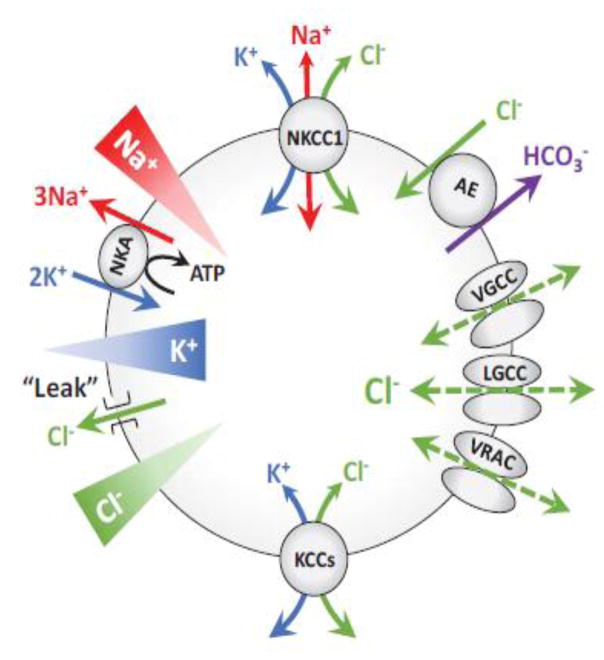
Fig. 2. Overview of ion transporters and channels, which are responsible for setting intracellular Cl− levels in neural cells.
The transmembrane gradients of Na+ and K+ are established by the Na+/K+-ATPases (NKA). The negative charge on the plasmalemmal membrane passively drives Cl− outside of the cell via a plethora of mechanisms, which are collectively termed Cl− “leak”. However, in neurons and astrocytes [Cl−]i deviates from the levels predicted by the electrochemical equilibrium due to the activity of the Na+,K+,2Cl− cotransporter (NKCC1) and/or the K+,Cl− cotransporters (KCC1-4). Opening of voltage-gated Cl− channels (VGCC), ligand-gated Cl− channels (LGCC), or volume-regulated anion channels (VRAC) moves Cl− in or out of the cell, depending on the electrochemical driving force. Additionally, Cl− movement also occurs via the Cl−/HCO3− anion exchangers (AE3 and others).
2.1 Na+, K+, 2Cl− cotransporters (NKCC)
NKCCs are two closely related proteins – NKCC1 (SLC12A2) and NKCC2 (SLC12A1), which belong to the large SLC12A superfamily of cation-chloride cotransporters. NKKC1 is ubiquitous, and can be found at various levels in all types of brain cells (reviewed in [39]). NKCC2 is the kidney-specific isoform [40] and, therefore, is not discussed in this manuscript. Both NKCCs mediate the bidirectional, electroneutral symport of one Na+, one K+, and two Cl− in or out of the cell. Because the combined electrochemical gradients for Na+, K+, and Cl− favor inward transport, NKCCs facilitate Cl− accumulation above the level of its electrochemical equilibrium [39].
In neuronal physiology, the ability of NKCC1 to drive net-accumulation of intracellular Cl− is functionally significant. Early in development, neural precursors and immature neurons express high levels of NKCC1 and consequently have elevated intracellular [Cl−] [36]. In these cells, GABA and glycine act as excitatory neurotransmitters [41]. Throughout the maturation process, neurons downregulate NKCC1 and replace it with electroneutral KCC transporters (see Section 2.2). Such a change leads to the precipitous drop in intracellular [Cl−], and this transition converts the actions of GABA and glycine from excitatory to inhibitory (reviewed in [36;38]). Based on the data derived from gramicidin-perforated patch recordings in brain slices, resting [Cl−]i drops from 37 mM in E16 neural precursors to 12 mM in mature cortical neurons at P16 [42]. In the adult brain, [Cl−]i was measured as low as 5 mM in certain neuronal populations [1].
Unlike neurons, astrocytes preserve high activity of NKCC1 throughout development and, therefore, have higher intracellular [Cl−]. This aspect of astroglial physiology has been thoroughly explored in cell cultures. In cultured astrocytes, the intracellular [Cl−] has been estimated at 20 to 50 mM. These estimates are derived using various experimental techniques, including steady-state isotope distribution (31–50 mM, [43]), sharp electrodes (20–40 mM [44]), and electrophysiological recordings with the K+ ionophore gramicidin (29 mM [45]). The data on astrocytic Cl− levels in vivo and in situ are scarce. An early in vivo study used 36Cl− distribution to estimate glial (largely astrocytic) [Cl−] to be 46 mM in the cortex and 36 mM in the cerebellum [46]. More recently, Untiet et al. measured [Cl−]i of 52 and 35 mM in immature and mature cerebellar Bergman glial cells, respectively, in brain slices using a chloride-sensitive MQAE FLIM signal [47]. Consistently, a number of studies in brain slices collected indirect evidence for high astrocytic [Cl−]i, including the hyperpolarizing effects of GABA receptor agonists and the depolarizing effects of Cl− channel blockers [48;49].
While discussing the impact of NKCC1 activity on cellular functions, it is important to remember that this transporter is potently stimulated by protein phosphorylation, low [Cl−]i, and cell shrinkage (reviewed in [37;38]). Early studies in non-neural cells found that NKCC1 is activated by numerous agonists linked to the cAMP-dependent PKA, Ca2+/DAG-dependent PKCs, c-Jun N-terminal kinase (JNK), Erk1/2, and others (e.g. [50–54]). Yet, NKCC1 stimulation by various protein kinases is cell type-specific; in some cases, the same signaling cascades have been reported to produce opposite functional effects (discussed in [39;55]). For example, in the majority of cell types PKCs activate NKCC1, while in the kidney MDCK cell line it is completely inhibited by the PKC agonist PMA [56]. Based on this information and due to the lack of relevant phosphorylation motifs in the protein stricture, it has been concluded that many protein kinases modulate NKCC1 indirectly [39;55]. Recently, the critical role for the WNT/SPAK/OSR1 signaling cascade in the regulation of NKCCs and other cation-chloride cotransporters has been established (reviewed in [57–59], and discussion in Section 3.2). This signaling axis may be the actual target for indirect effects of the previously implicated protein kinases (see for example [60]).
2.2 K+, Cl− cotransporters (KCC)
The electroneutral KCC cotransporters encompass four additional members of the SLC12A family and include KCC1 (SLC12A4), KCC2 (SLC12A5), KCC3 (SLC12A6), KCC4 (SLC12A7). All KCCs mediate the bidirectional transfer of one K+ and one Cl− in or out of the cell (reviewed in [61;62]). In the context of [Cl−]i homeostasis, KCCs are the functional opposites of NKCCs because they thermodynamically favor the efflux of intracellular Cl−, and drive [Cl−]i below the level of its electrochemical equilibrium. KCC1 is expressed in a nearly ubiquitous manner, while KCC2 is neuron specific [62–64]. KCC3 and KCC4 are abundant in the brain, but present at diverse levels in various neuronal and astroglial populations [65–67].
In the CNS, the neuron-specific KCC2 has been particularly well-studied. Functional upregulation of KCC2 during neuronal maturation is responsible for the switch in the actions of GABA and glycine receptors from excitatory to inhibitory [38;41;68]. This isoform differs from other KCCs because it is constitutively active. Unlike KCC2, the “classical” KCC isoforms (1, 3, and 4) are largely silent under isoosmotic conditions, but become strongly activated in response to cell swelling [38;61]. Deletions and mutations in KCC3 and KCC4 produce severe central and peripheral phenotypes, and the relevant changes have been considered in the context of cell volume regulation in either neuronal or glial cells [69;70].
Although the essential role of protein phosphorylation/dephosphorylation in the regulation of KCCs has been known for many years, the underlying molecular mechanisms have been elucidated only recently. All KCCs are inhibited by direct phosphorylation by SPAK and OSR1, two closely related kinases which belong to the WNK signaling cascade [71;72] (see Section 3.2 for detailed description of signaling pathway). In contrast, dephosphorylation of KCCs by the serine-threonine phosphatases (PP) type 1 and 2A leads to their activation. This process was first explored for KCC1 in red blood cells [61;73–75]. It appears that the membrane-bound PP1 responds to changes in ionic strength, while the membrane-bound PP2A responds to cell swelling [75]. More recent model studies further elaborated on the mechanisms for phosphatase actions, and established both direct interactions with KCC proteins and involvement of the scaffolding protein, apoptosis-associated tyrosine kinase AATYK1 [76;77].
2.3 The ligand-gated GABA and glycine receptors-channels
The most recognized Cl− permeability pathways in the brain are the ionotropic receptors for two inhibitory amino acid neurotransmitters, γ-aminobutyric acid (GABA) and glycine. These two families of receptors are structurally related and belong to the superfamily of Cys-loop receptor proteins [78;79].
GABAA channels are pentameric ligand-gated Cl− channels assembled from nineteen diverse members of the GABAA family (GABR genes, for comprehensive reviews see [80;81]). In mature neurons, GABAA channels are located in either the postsynaptic membrane, where they mediate fast neuronal inhibition, or at extrasynaptic sites, where they respond to ambient extracellular GABA levels and produce long-term inhibition. As already mentioned, the functional impact of neuronal GABAA receptors is determined by chloride-cation cotransporters, mainly by the opposing work of NKCC1 and KCC2 (see Sections 2.1 and 2.2).
Astrocytes also express GABAA receptors [48;82;83], although the receptor arrangement in these cells is less clear. Unlike their actions in mature neurons, opening of GABAA channels in astrocytes causes outward Cl− movement and membrane depolarization (see for example [48;84]). The functional role of GABAA currents in astroglia may be questioned because these cells have a highly clamped membrane potential, which is stabilized by the electrical connectivity within the astrocytic syncytium [85]. Yet, electrophysiological experiments in primary astrocyte cultures suggest that GABAA receptors are physiologically relevant. In hippocampal slices GABAA-mediated depolarization activates astrocytic voltage-gated Ca2+ channels and causes cytosolic [Ca2+] transients [83]. Another brain slice study reported modulation of the activity of astrocytic K+ channels by GABAA agonists [45].
The structurally related glycine receptor (GlyR) family has five distinct isoforms, GlyRα1-4 (GLRA1-4) and GlyRβ (GLRB), which assemble in either homo- or heteropentameric channels, all of which are activated by glycine [86;87]. Various GlyR subunits are expressed throughout the CNS, with the highest abundance in the spinal cord, brainstem nuclei, and retina [87]. In neurons, GlyRs play a role, which is similar to GABAA, and in the adult brain mediate hyperpolarization due to Cl− movement into the cell [86;87]. Very little information is available on GlyRs in astrocytes. To the best of our knowledge, only one study has identified functional astroglial GlyR Cl− currents in situ, in spinal cord astrocytes [88]. Based on available expression data, it seems unlikely that GlyRs play a dominant role in astroglia.
In the context of receptor pharmacology and physiology, it is important to note that both GABAA and glycine receptors can be activated by the atypical aminosulfonic acid taurine, which in the brain serves as the endogenous agonist for these receptor-channels (reviewed in [89]).
2.4 Voltage-gated Cl− channels (ClC family)
The voltage-gated Cl− channels belong to the evolutionarily conserved ClC family (encoded by the CLCN1 through CLCN7, CLCNKA and CLCNKB genes). These have been discovered based on their homology with ClC-0 channels from the electric organ of ray Torpedo marmorata [90]. In mammals, there are nine diverse ClC proteins, four of which (ClC-1, ClC-2, ClC-Ka and ClC-Kb) form plasmalemmal Cl− channels, while five others (ClC-3 through ClC-7) function as intracellular Cl−/H+ exchangers (reviewed in [3]). As already briefly mentioned in Section 1.2, voltage-gated Cl− channels have a high capacity for modulating resting membrane potential and membrane conductance. Because neurons utilize ligand-gated Cl− fluxes to control their excitability, these cells tend to have low expression of voltage-gated Cl− channels. The same applies to astrocytes, whose membrane permeability is dominated by K+ currents. Yet, even the limited expression of voltage-gated Cl− channels in neural cells has a strong impact on their physiology.
ClC-2 (CLCN2) has been detected in both neurons and astrocytes, but the expression of this channel strongly varies depending on the brain region and cell subtype [91;92]. It seems that ClC-2 conductance counteracts GABAA function, because overexpression of CLC-2 in dorsal root ganglia neurons blunted GABA effects on neuronal excitability [93]. In hippocampal slices prepared from ClC-2-null animals, pyramidal neurons had lowered resting Cl− conductance and associated increases in their excitability [94]. The effect of ClC-2-deletion on electrical activity in neuronal networks was more complex, producing a net inhibition, which has been explained by the elevated excitability and activity of inhibitory neurons [94]. That being said, the overall impact of ClC-2 on neuronal excitability is far from clear. Mutations in ClC-2 have been proposed as a susceptibility factor in idiopathic generalized epilepsy, although the direct link between ClC-2 and epilepsy remains tenuous [95]. ClC-2 knock-out mice have no changes in seizure thresholds [96].
In astrocytes, immunoreactivity of ClC-2 is region-specific and highly polarized, with a high abundance reported next to GABAergic neurons [91]. The latter findings led to the suggestion that ClC-2 may mediate Cl− delivery to the neuronal populations with intense GABAergic activity [91]. Whether ClC-2 function significantly impacts membrane potential and electrical properties of glial cells is still under debate. Deletion of ClC-2 does not dramatically change [Cl−]i levels in Bergman glial cells, suggesting its minimal contribution to resting membrane conductance [47]. Nonetheless, the ClC-2 knockout animals have severe glial phenotypes, particularly in their white matter. ClC-2−/− mice develop age-dependent leukodystrophy, manifesting as widespread myelin vacuolation in the brain and spinal cord [96]. In humans, similar leukodystrophy was subsequently identified in three adult and three pediatric patients carrying ClC-2 mutations [97]. It is unknown in which cell type, astrocytes or oligodendrocytes, the loss ClC-2 causes myelin deficiencies. Nevertheless, the growing consensus in the field is that glial ClC-2 is critical for ion and water homeostasis in the brain. ClC-2 functions are likely additionally modulated by two auxiliary proteins, GlialCAM and MLC1, mutations of which cause leukodystrophies with phenotypes resembling those observed upon ClC-2 deletion (see [98–101]).
The widely distributed ClC-3 (CLCN3) is an intracellular Cl−/H+ exchanger that was initially cloned from the brain [3;102]. There, it is expressed in acidic intracellular organelles, particularly in synaptic vesicles [103]. Deletion of ClC-3 limits synaptic vesicle acidification and neurotransmitter uptake, and causes postnatal degeneration of the hippocampus and retina [103]. A number of publications expressed an alternative view that ClC-3, or some of its splice variants, can also mediate plasmalemmal Cl− conductance (e.g. [104–106]). Nevertheless, the bulk of the ClC-3 work and the recent papers, which extensively characterized the heteroexpressed ClC-3 variants, all strongly indicate that the previously reported plasmalemmal “ClC-3” conductances have likely been mediated by other types of endogenous anion channels [3;107;108]. Other intracellular Cl−/H+ exchangers, ClC-4 through ClC-7, have also been detected in neurons and astrocytes, with ClC-6 showing a neuron-specific pattern of expression [109–112].
2.5 Volume-regulated anion channels (LRRC8 family)
There is one Cl− channel that can be detected in virtually every type of mammalian cell: namely, the volume-regulated anion channel (VRAC). Initially, VRACs were identified only on a functional level, as the robust Cl− currents induced by cellular swelling upon exposure to hypoosmotic media. These volume-sensitive Cl− currents displayed very similar biophysical properties across many cell types, and thus, were ascribed to be mediated by the same channel or group of highly related channels (reviewed in [16;113;114]). Based on their unique biophysical profile, VRAC channel has been alternatively referred to as volume-sensitive outwardly rectifying Cl− channel (VSOR), or volume-sensitive organic osmolyte-anion channel (VSOAC) [16;113;114]. The VSOAC name highlights the channel permeability to both inorganic anions (such as Cl− and HCO3−) and a variety of small, organic osmolytes, either charged or uncharged. This latter feature of VRAC is highly important for understanding its roles in the brain.
The molecular nature of VRAC remained elusive for several decades. Many candidate proteins were proposed to mediate swelling-activated Cl− conductance, but all of them were eventually rejected (discussed in [115;116]). Recently, two laboratories used genome-wide siRNA screens to independently identify VRAC as the heteromeric product of proteins belonging to the leucine repeat-rich containing family 8 (LRRC8) [117;118]). The LRRC8 family includes five related proteins, LRRC8A-E. One of these, LRRC8A, is indispensable for ion conductance, but must heteromerize with at least one additional subunit to produce functional VRAC channels [117;119;120]. RNAseq data and quantitative PCR indicate that all five LRRC8 proteins are expressed in brain cells, including neurons and astrocytes, however the expression levels of LRRC8E are very low, at least 10-fold lower compared to other members of the LRRC8 family [121;122]. The precise assembly of the partnering subunits determines the cell-type specific biophysical properties of VRAC [117;119;120], including its signature channel inactivation at positive potentials [117;123], and the selectivity for organic osmolytes passing through the VRAC pore [124–126].
The main function of VRAC is in cell volume regulation (see Section 1.1). VRAC activity drives regulatory cell volume decrease via two mechanisms: (i) it provides a route for Cl− and HCO3− release, and in such a way assists in the electroneutrality of swelling-activated fluxes of K+ via separate but functionally coupled K+ channels, (ii) it creates a pathway for the release of small organic osmolytes. Altogether, VRAC directly or indirectly facilitates the movement of numerous osmolytes, and in such a way drives the efflux of osmotically obligated water (reviewed in [7–9]). Physiological stimuli that lead to VRAC activation are not limited to cell swelling. Stimulation of Gq-coupled GPCRs or oxidative stress have been found to produce limited VRAC activation and act synergistically even with small degrees of cellular swelling in various cell types, including glial cells [127–131]. The underlying mechanisms for GPCR actions are incompletely understood, but likely involve multiple intracellular signaling cascades. The Ca2+-dependent PKC isoforms, PKCα and β, have been most frequently implicated in the receptor-stimulated VRAC opening, however many other protein kinases may also contribute (see [132–135] and reviews [116;136;137]). This information is helpful for understanding VRAC behavior under physiological conditions and in pathological states.
Once activated, VRAC acts much like the ligand-gated Cl− channels and moves Cl− toward its electrochemical equilibrium, with the consequences that have been already discussed in Section 2.4. What makes VRAC unique among other Cl− channels is its ability to facilitate the efflux of various neuroactive substances, including the excitatory amino acids glutamate and aspartate, the inhibitory neurotransmitters GABA and taurine, and perhaps others [119;122;125]. The latter aspect of VRAC physiology is highly important for bidirectional neuron-astrocyte signaling as reviewed by one of us in [35], and further discussed in Section 4.2. Recent findings suggest that certain cells, including brain astrocytes, express several distinct LRRC8-containing VRAC heteromers; one of which is preferentially responsible for the movement of Cl− and anionic amino acids, while the other favors small uncharged molecules, including GABA, taurine, and glutamine [124–126].
Because of their recent discovery, the cell type-specific functions of the LRRC8 proteins, including LRRC8A, are yet to be explored using the powerful tools of molecular genetics. Global deletion of LRRC8A produces a severe phenotype, with significant embryonic and postnatal lethality, and defects in numerous organs and tissues [138]. The tissue-specific LRRC8A knockouts are now being developed, but none have been characterized thus far.
2.6 Anion exchangers
In addition to Cl− transporters and permeability pathways, which are discussed in the prior sections, brain cells express a variety of Cl−/bicarbonate transporters belonging to the large SLC4 transporter family [139]. These proteins are important for the exchange of products of cellular metabolism (CO2) and regulation of pHi; however, they also deserve brief mentioning due to their direct or indirect impact on [Cl−]i.
Two members of the Cl−-transporting SLC4 family, which are most abundant in the CNS, include the electroneutral Cl−/HCO3− anion exchanger 3 (AE3, encoded by SLC4A3), and the Na+-activated Cl−/HCO3− exchanger, (NDCBE, product of SLC4A8) (reviewed in [139]). These two exchangers perform functionally opposite roles in the context of Cl− transport and pH regulation. AE3 is stimulated by alkalosis and exports metabolically produced bicarbonate in exchange for extracellular Cl−, thus serving as an acid and Cl− loader. In contrast, the NDCBE exchanger responds to cytosolic acidification by taking up one Na+ and 2 HCO3− ions in exchange for one Cl−, and thus works as an acid and Cl− extruder [140]. As mentioned in Section 1.1, the coordinated work of AE anion exchangers and Na+/H+ exchangers allows for regulatory volume increase in shrunken cells, as their functionally coupled work accumulates cytosolic NaCl (for comprehensive review see [141]).
3. Cl− as an intracellular signaling ion
As discussed in the previous two sections, the impact of Cl− on cellular functions is attributed to the charge transfer across plasmalemmal or intracellular membranes, without considering the potential intracellular signaling properties for this anion (but see review [142]). Yet, older reports and recently accumulated evidence suggest that this notion may be overly simplistic, and that alternative mechanisms of Cl− actions within the cell also exist.
3.1 Direct regulation of ion channels and transporters by [Cl−]i
The intracellular Cl− levels can directly modulate activities of some ion channels and transporters. In the simplest case, [Cl−]i regulates biophysical properties of the voltage-gated Cl− channels, ClC-0 and ClC-2, by acting as the gating particle within the channel pore [143;144]. More intriguingly, intracellular Cl− can allosterically modify activities of other ion channels, which do not conduct this anion. In neural and muscle cells of C. elegans, increases in [Cl−]i and [Ca2+]i additively activate the high conductance K+ channel cSLO-2 [145]. Binding of these two regulatory ions occurs at adjacent C-terminal sites [145]. In mammals, the cSLO-2 orthologues, mSLO2.1 (also known as Slick, the product of KCNT2 gene) and mSLO2.2 (Slack, encoded by KCNT1) produce K+ channels, which are activated by increases in [Cl−]i and [Na+]i, and inhibited by physiological levels of ATPi [146;147]. Together, this unusual pattern of regulation makes SLO2 channels unique polymodal sensors for metabolic stress and possibly hypoxia. Regulation by [Cl−]i is not restricted to K+ channels. The nonselective cation channel TRPM7 (Transient Receptor Potential subfamily M member 7) is inhibited by high intracellular Cl− via anion biding to a poorly characterized domain within the protein [148].
Besides ion channels, direct sensitivity to [Cl−]i was established for two splice variants of the electrogenic Na+/HCO3− cotransporter, NBCe1 (SLC4A4) [149]. NBCe1 and other related HCO3− transporters from the SLC4 family were initially discovered in the renal tissue [150], but soon found elsewhere, including in astrocytes and neurons [151;152]. The activity of the NBCe1-B and NBCe1-C isoforms is strongly inhibited in the physiological range of [Cl−]i (5–60 mM) via interaction with one or more Cl−-binding GXXXP motifs [149]. The Cl−-dependent changes in transporter activity modulate acid-base homeostasis, and, in such a way, alter a variety of cellular functions. There are data indicating that [Cl−]i can also modulate the activity of the Na+/H+ exchanger NHE-1, in a manner dependent on the C-terminal domain of the protein [153].
3.2 Cl−i is a modulator of intracellular signaling cascades
A number of early experimental observations in 1980s and early 1990s, led to the hypothesis that Cl− can regulate intracellular signaling cascades. Thus, in purified enzyme assays, activity of Gαi/o was strongly inhibited by Cl− and Br− with the half-maximal effects at 3–20 mM, while other anions showed no effect [154]. In permeabilized neutrophils, lowering [Cl−]i elicited robust protein phosphorylation and an oxidative burst, likely via the activation of small GTP-binding proteins [155]. In salivary epithelial cells, a [Cl−]i- and pertussis toxin-sensitive G-protein has been found to regulate the activity of Na+ channels [156]. Along the same lines, in the airway epithelium, [Cl−]i regulates protein phosphorylation via changes in the activity of nucleoside diphosphate kinase [157]. Surprisingly, these and other early findings gained little traction. More recently the idea of [Cl−]i sensing has been firmly linked to serine/threonine protein kinases belonging to the WNK (With No lysine [K]) family [59;158;159].
WNK refers to the unique catalytic site structure of the four kinases in this family, which lack one of the conserved lysine residues responsible for the coordination of ATP within their active center [158]. After their initial cloning, WNK1 and WNK4 genes were soon linked to familial cases of hypertension, hyperkalemia, and hyperchloremia [160], and later to pathological changes in activity of the thiazide-sensitive Na+,Cl− cotransporter (NCC) and the bumetanide-sensitive NKCC2 (reviewed in [59]). Subsequent work established that WNKs do not phosphorylate these transporters directly, but rather act via two closely related downstream protein kinases: SPAK (Sterile20-related Proline-Alanine-rich Kinase) and OSR1 (Oxidative Stress Responsive kinase 1) [161–164]. In addition to the activation of NKCC1/2, WNK-SPAK/OSR1 signaling cascade reciprocally regulates (inhibits) the activity of all four KCC transporters, thus providing coordinated control over the critical Cl− influx and efflux pathways [71;72] Among WNK family members, WNK3 appears to be the most abundant and functionally significant enzyme in the CNS, but other WNK proteins are also expressed [165;166].
The idea that one or more WNK kinases serve as intracellular Cl− sensors had been around for a while and was indirectly supported by findings of [Cl−]i-sensitivity of cation-chloride cotransporters (for early discussion see [167]). However, the direct experimental support for this hypothesis has been collected in very recent studies. Several groups identified the structural basis for direct modulation of WNK1, WNK3, and WNK4 by [Cl−]i [168;169]. Cl− (or Br−) binds to the DLG motif in the kinase domain and N-terminal activation loop of the WNK enzymes. This anion binding inhibits protein autophosphorylation, and prevents enzyme activation [168]. For WNK1 and WNK3, the inhibitory actions of Cl− develop in the concentration range of 5–20 mM. WNK4 is apparently more sensitive to [Cl−]i and is potently inhibited in the concentration range of 5–10 mM [169]. The proposed mechanism of WNK-mediated [Cl−]i sensing and the downstream effects on cation-Cl− cotransporters are depicted in a simplified form in Fig. 3.
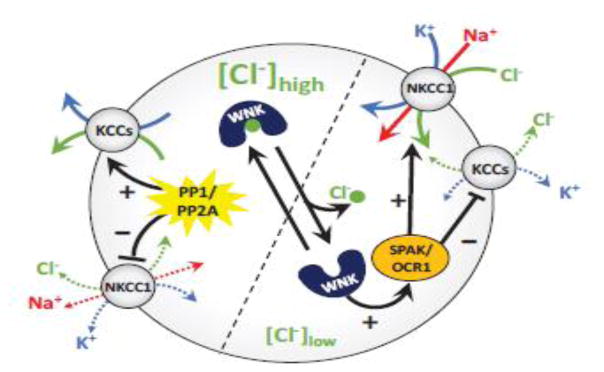
Fig. 3. Cl− as an intracellular signaling ion.
Recent findings identified the With No lysine [K] (WNK) protein kinases as intracellular [Cl−] sensors. Binding of Cl−, which occurs in the N-terminal activation loop of WNK1-4, inhibits autophosphorylation and activity of these enzymes. Reductions in [Cl−]i promote WNK1-4 autophosphorylation and activation. Active WNKs phosphorylate and stimulate two closely related protein kinases SPAK/OSR1, which in turn phosphorylate NKKC1 and KCC1-4. The functional effects of phosphorylation on cation-Cl− cotransporters are opposite: activation of NKCC1 and inhibition of KCC1-4. When WNKs are inactive, NKCC1 and KCC1-4 are dephosphorylated by the serine/threonine protein phosphatases PP1 and PP2A. See text for additional details.
Although the cell volume- and Cl−-sensitive changes in activity of the WNK cascade have been mainly considered in the context of regulation of SLC12A transporters, the relevant kinases have multiple targets, both membrane and intracellular. For example, in C. elegans the WNK signaling axis, specifically the SPAK-like kinase, inhibits the activity of the Cl− channels belonging to the ClC family [170;171]. There were also reports about direct interactions between WNKs and serum- and glucocorticoid-induced protein kinase 1 (SGK1) [172], WNK1-dependent regulation of the Erk cascade proteins MEKK2/3 [173], and WNK-dependent phosphorylation of claudins [174], etc.
In the context of astroglial physiology, it may be relevant to mention the putative role of [Cl−]i in regulating exocytosis. Astrocytes are the secretory cells of the CNS, which, among other mechanisms, utilize vesicular secretion for release of bioactive molecules [175]. In the past, intra-cellular Cl− has been shown to be important, or even obligatory for sustaining exocytosis in neurohypophysial nerve endings, cultured brain pituitary cells, and beta cells of the pancreas (e.g. [176–178]). This is likely related to the function of intracellular Cl−/H+ exchangers from the ClC family, which support vesicular acidification [178], but may also involve activation of small GTPases [179]. Interestingly, the effects of [Cl−]i on exocytosis can be bell-shaped, with non-physiologically high Cl−i suppressing this process [176]. It remains to be explored if the newly discovered [Cl−]i sensors WNK kinases are involved in these phenomena.
4. Potential role of Cl− in the crosstalk between neurons and astrocytes
Over the last two decades, the field of astroglial physiology has undergone a dramatic transformation. Rapidly accumulating evidence supports the existence of extensive and reciprocal signaling between neurons and astrocytes, which is largely mediated by neuro-transmitters and gliotransmitters (see [180–183]). Activity-dependent fluctuations in extracellular ion levels, such as K+ and Na+, and intracellular transients in [Ca2+] and [Na+], represent important elements of intercellular information transfer [181;184;185]. In this section, we make an argument that Cl− fluxes also play a role in neuron-astrocyte signaling (see Fig. 4).
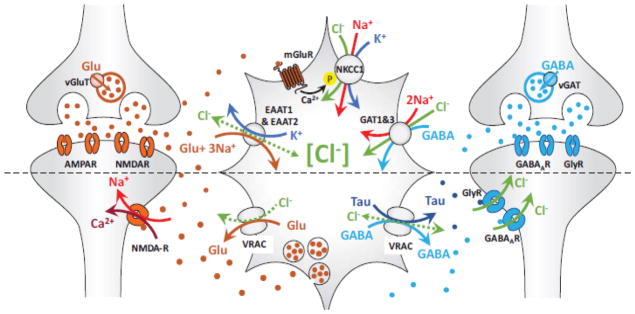
Fig. 4. The role of Cl− in the bidirectional astrocyte-neuron communication.
The transfer of information from neurons to astrocytes, and the reverse process, are schematically separated by a dashed line. Top, Neurons alter astrocytic [Cl−]i through the release of both excitatory and inhibitory neurotransmitters. Glutamate uptake through the Na+/K+-dependent excitatory amino acid transporters EAAT1 and EAAT2, gates the Cl− permeability pore. Additionally, glutamate, ATP and several other signaling molecules activate GPCR pathways (such as mGluR receptors) and stimulate Cl− uptake via NKCC1. Uptake of the inhibitory transmitter GABA through the GABA transporters, GAT-1 or GAT-3, is stoichiometrically associated with the symport of 2 Na+ and one Cl−. Bottom, Astrocytes directly modify neuronal [Cl−]i via release of the gliotransmitters, GABA and taurine, which subsequently activate Cl− fluxes through the neuronal GABAA and glycine receptor (GlyR) channels. The main pathway for astrocytic GABA and taurine release is thought to be the volume-regulated anion channel (VRAC), but other release mechanisms also exist. See text for additional details.
4.1 Do neurons modify astrocytic Cl− and is it of functional consequence?
A well-characterized role of astroglia is maintenance of an optimal environment for neuronal activity, which is accomplished via uptake of neurotransmitters, export of metabolites, and regulation of ionic composition of the extracellular milieu, particularly extracellular K+ levels (reviewed in [186–188]). These processes are predominantly concentrated in astrocytic endfeet engulfing neuronal synapses and brain microvessels. Many of the relevant astrocytic transporters directly or indirectly impact the transmembrane Cl− fluxes.
Astrocytes express two main Na+/K+-dependent excitatory amino acid transporters; namely, EAAT1 (encoded by SLC1A3) and EAAT2 (SLC1A2) in humans, or GLAST and GLT-1, respectively, in rodents (reviewed in [189;190]). These transporters concentrate glutamate inside the cell using the energy of established gradients for monovalent cations. Each electrogenic transport cycle takes one glutamate molecule inside the cell together with 3 Na+ and 1 H+, in exchange for 1 K+ (reviewed in [191]). What is important for the present discussion – the activity of plasmalemmal glutamate transporters is also associated with Cl− currents [192–195]. EAATs’ Cl− permeability is uncoupled from the neurotransmitter transport, and, therefore, Cl− movement is governed by its electrochemical gradient. Accordingly, when gated by glutamate, EAATs behave like small-conductance ligand-gated Cl− channels (e.g. [195] and review [196]). In astrocytes, stimulation of the EAATs would be expected to decrease intracellular [Cl−]. In fact, this is exactly what has been confirmed in a recent study by the Fahlke laboratory. In slice recordings in Bergman glia, they found that developmental upregulation in the activity of GLAST and GLT-1 is associated with a drop in the intracellular [Cl−] from 52 to 35 mM [47]. As would be predicted, EAAT inhibitors increased the intracellular [Cl−] in Bergman glial by more than 15 mM [47]. Remarkably, the related neuronal EAAT4 and EAAT5 seem to function predominantly as ligand-gated inhibitory Cl− channels, rather than glutamate transporters per se (discussed in [190;196]).
The inhibitory amino acid transmitter GABA is taken inside the cells by three Na+- and Cl−-dependent GAT transporters, GAT-1 (SLC6A1), GAT-2 (SLC6A13), and GAT-3 (SLC6A11), and the betaine-GABA transporter BGT-1 (SLC6A12) (reviewed in [190;197]). Among these, BGT1 is probably of minor importance because BGT-1-null mice have normal development and no high seizure susceptibility phenotype, indicating normal GABA signaling [198]. GAT-1 is found in both neurons and astroglia, GAT-2 levels are very low to undetectable in the brain, and the expression of GAT-3 appears to be restricted to astrocytes [190]. Activity of all GABA transporters depends on the gradients of Na+ and Cl−. Although the transport stoichiometry of these transporters is not fixed, normally each working cycle transfers 2 Na+ and 1 Cl− with one neurotransmitter molecule [199;200]. While GABA uptake is dependent on and associated with Cl− movement, much like for the EAATs, there is some evidence that GABA transporters also act as Cl−/anion channels [201]. The last caveat notwithstanding, GABA uptake by astrocytes is expected to lead to elevation in [Cl−]i. Besides the possible modulation by neurotransmitter transporters, shifts in [Cl−]i may also occur as a result of activation of GABAA receptors, which has been already discussed in Section 2.3. An overview of astrocytic GABA receptors and GABA responses can be found in the comprehensive review by Porter and McCarthy [202].
Another significant mechanism through which neuronal activity can be coupled to changes in astrocytic [Cl−]i is activation of NKCC1, which can be driven by metabotropic neurotransmitter receptors. The majority of such receptors on astrocytes are linked to either PLC-dependent increases in the intracellular [Ca2+] or cAMP signaling (reviewed in [202;203]). As outlined in Sections 2.1 and 3.2, NKCC1 activity is potently modulated by numerous Ca2+ and cAMP-dependent protein kinases, most likely via their downstream effects on the WNK signaling cascade. The physiological consequences of NKCC1 activation would be two-fold: (i) elevation of intracellular [Cl−]i and (ii) cellular swelling, with the latter leading to opening of the swelling-activated channel, VRAC. In addition, metabotropic receptors for glutamate, ATP, and adenosine, and few other signaling molecules have been found to lead to limited, Ca2+-dependent VRAC opening, even in the absence of cell swelling (see for example [128;135;204]). Regardless of the stimuli, VRAC opening will reduce [Cl−]i.
Altogether, the impact of neuronal activity on astrocytic [Cl−]i is not uniform, and likely context-dependent. Activation of membrane Cl− conductance during uptake of the excitatory neurotransmitters glutamate and aspartate, opening of GABAA receptor-channels, opening of VRAC, or hyperpolarization-induced ClC-2 activity, are all likely to reduce intracellular [Cl−]. On the other hand, the activity of the Na+,Cl−-dependent transporters for GABA and several other neurotransmitters, or activation of NKCC1 should lead to elevations in [Cl−]i. As discussed in the preceding text, we do not think that Cl− movement causes significant shifts in the astrocytic membrane potential. Instead, [Cl−]i may modulate astrocytic functions via changes in the activity of the Cl−-sensitive WNK protein kinases.
4.2 How astrocytes modify neuronal Cl− and why is it functionally significant?
As a part of a “tripartite synapse”, astrocytes respond to and modulate neuronal synaptic activity via a Ca2+-dependent release of gliotransmitters, most notably glutamate and ATP, but also a variety of other substances [180–183]. Although the idea of vesicular gliotransmitter release dominates the field, several alternative mechanisms have also been considered, including the activation of VRAC, and others [128;205;206]. In this subsection, we discuss only the processes which rely on Cl− signaling within neuronal cells.
The most obvious way in which astrocytes can modulate neuronal [Cl−]i is the activation of neuronal GABA and glycine receptor-channels. It has been proposed that tonic GABA release in the healthy and pathological brain is largely of astrocytic origin, and mediated by the Bestrophin-1 Cl−/anion channels [207–209]. We speculate that the LRRC8-containing VRACs may also be involved in this process, because they are permeable to GABA, and, much like Bestrophins, can be activated in a Ca2+-dependent fashion [125;128]. In support of potential VRAC involvement, it has been known for years that activity of magnocellular neurons in supraoptic and paraventricular nuclei of the hypothalamus is modulated by gliotransmitter release by a volume-sensitive anion channel, likely VRAC. Hypothalamic astrocytes and specialized pituicytes in the neurohypophysis contain high levels of the atypical aminosulfonic acid taurine, and release it to the extracellular space both tonically, and in an osmolarity-dependent manner [210;211]. Taurine strongly modulates the secretion of vasopressin and oxytocin by the supraoptic magnocellular neurons, by acting as an agonist at their glycine receptors and modulating their membrane potential via Cl− fluxes (see [212;213] and review [214]).
It is important to recall that neuronal [Cl−]i and the polarity of GABA actions are determined by the electroneutral cation-Cl− cotransporters. In neurons, the net activity of NKCC1 can be regulated at the expression level or via transporter phosphorylation. In cellular models, activation of neuronal NMDA, AMPA, or metabotropic mGluR1 and mGluR5 receptors leads to the Ca2+-dependent increase in NKCC1 activity [215;216]. Such activation may be driven at least in part by the release of excitatory gliotransmitters. The role of NKCC1 in setting normal neuronal [Cl−]i and determining GABA actions during development has been already discussed (see Section 2.1). In a pathological context, NKCC1 can promote excitation and tissue damage, for example in epileptogenesis and traumatic brain injury [217;218]. Whether astrocytes can modify the activity of the functionally opposite neuronal KCCs is less clear, yet, any potential effects cannot be overlooked. Deletion of the neuron-specific KCC2 or neuron- and astrocyte expressed KCC3 and KCC4 lead to early postnatal mortality or severe central and peripheral phenotypes (reviewed in [38;70]). Finally, modulation of neuronal [Cl−]i, by astrocytes or otherwise, can regulate the activity of WNK kinases, and in such a way provides regulatory feedback to KCCs and NKCC1, but also mediates other effects via phosphorylation of alternative targets (see Section 3.2).
5. Perspectives and challenges
The regulatory role of Cl− conductance in neuronal signaling is well recognized. Therefore, it is very intuitive that astrocytic release of the inhibitory neuro/gliotransmitters, GABA and taurine, can provide a mechanism for the regulation of neuronal excitation. Substantial evidence in support of this notion already exists, at least for select brain areas (see Section 4.2). What remains uncertain is how the inhibitory gliotransmitters are being released from astrocytes. A vesicular release mechanism has been proposed for a number of gliotransmitters, and is still very much a part of the discussion. However, astrocytes express low-to undetectable levels of the vesicular GABA transporter vGAT (SLC32A1) [121]. Consistently, unlike synaptic vesicles in neurons, astrocytic vesicles do not contain measurable levels of GABA or glycine [219]. We are not aware of any vesicular transporters, which would accommodate taurine. Based on these considerations, alternative mechanisms for glial GABA and taurine release are being pursued, including plasmalemmal amino acid transporters, as well as VRAC and Best1 anion channels (see Sections 2.5 and 4.2). This area is still very much in flux.
Unlike the clarity of the role of Cl− in regulation of neural function, we have a long way to go toward elucidating whether this anion plays signaling functions in astrocytes. The fact that astrocytic intracellular [Cl−] is kept above the level of its electrochemical equilibrium, indirectly implies the significance of Cl− in astroglial physiology. However, modulation of membrane potential by Cl−fluxes is likely less important in astrocytes as compared to neurons. Activation of Cl− permeability is expected to produce a moderate hyperpolarization of the already highly negative cell that is electrically coupled to its neighbors via an astrocytic syncytium. Perhaps the recent discovery that the WNK kinase family members can act as [Cl−]i sensors and modulate the activity of other Cl− permeability pathways points to new, unexpected signaling properties for this anion. Additionally, there are a few reports that intracellular Cl− levels may impact exocytosis and vesicular release processes. Because astrocytes are considered to be the secretory cells of the CNS (see Section 3.2), exploring the putative connection between [Cl−]i and gliotransmitter release may yield new, unexpected information.
The field faces a number of significant technical and conceptual challenges in testing specific and distinct roles for Cl− fluxes and intracellular Cl− levels in neuron-astrocyte communication. The biggest obstacle is in separating the impact of intracellular Cl− signaling from the effects of changes in the membrane potential, because it is difficult to clamp intracellular [Cl−] without changing membrane polarization. Perhaps, new, specifically devised electrophysiological approaches will be of further assistance. Other barriers in the field are represented by the lack of Cl− sensors with a good dynamic range, which would match physiological Cl− levels, and the deficit of selective tools for manipulation of [Cl−]i in vivo. The latter two obstacles appear to be surmountable with the recent advances in the development of protein-based Cl− sensors and optogenetics tools. We think that introducing the Cl−-conducting channelrhodopsins into glial cells may be as instructive as recent studies in neurons. The relevant experiments will shed a light on already expected and, perhaps, as-yet-unanticipated Cl− functions in glial physiology, and strengthen the idea of Cl− as a signaling ion between neurons and astrocytes.
Highlights.
The transmembrane chloride fluxes via GABAA and glycine receptors are well-known to regulate excitability and communication within neuronal networks. Here we make an argument that chloride signaling is an important element in bidirectional neuron-astrocyte communication.
Neurons modulate intracellular chloride levels in astrocytes as a result of (i) chloride fluxes associated with neurotransmitter uptake, (ii) modulation of astrocytic cation-chloride cotransporters, particularly NKCC1, (iii) activity-dependent astrocytic swelling and opening of chloride/anion channel VRAC.
Astrocytes can modify neuronal chloride levels and excitability via the release of GABA and taurine, two endogenous agonists of inhibitory GABAA and glycine receptors. Astrocytic release of inhibitory gliotransmitters involves VRAC and perhaps other release pathways.
Finally, the recent discovery that the WNK family protein kinases are regulated by chloride opens the possibility that changes in cytosolic levels of this anion may act as an intracellular signal, both in neurons and astrocytes.
Acknowledgments
The work in Authors’ laboratory was supported by NIH grant R01 NS61953 (to A.A.M).
Major Abbreviations
- AE
anion exchanger
- AVD
apoptotic volume decrease
- EAAT
excitatory amino acid transporter
- GABA
γ-aminobutyric acid
- KCC
K+-Cl− cotransporter
- NKCC
Na+-K+-2Cl− cotransporter
- RVD
regulatory volume decrease
- RVI
regulatory volume increase
- VRAC
volume-regulated anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Glykys J, Dzhala V, Egawa K, Balena T, Saponjian Y, Kuchibhotla KV, Bacskai BJ, Kahle KT, Zeuthen T, Staley KJ. Local impermeant anions establish the neuronal chloride concentration. Science. 2014;343:670–675. doi: 10.1126/science.1245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H, Muallem S. Na+, K+, and Cl− transport in resting pancreatic acinar cells. J Gen Physiol. 1995;106:1225–1242. doi: 10.1085/jgp.106.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 4.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 5.Stauber T, Jentsch TJ. Chloride in vesicular trafficking and function. Annu Rev Physiol. 2013;75:453–477. doi: 10.1146/annurev-physiol-030212-183702. [DOI] [PubMed] [Google Scholar]
- 6.Donnan FG. Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Ein Beitrag zur physikalisch-chemischen Physiologie (The theory of membrane equilibrium and membrane potential in the presence of a non-dialyzable electrolyte A contribution to physical-chemical physiology) 1911:572–581. [Google Scholar]
- 7.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 9.Mongin AA, Orlov SN. Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology. 2001;8:77–88. doi: 10.1016/s0928-4680(01)00074-8. [DOI] [PubMed] [Google Scholar]
- 10.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RKH. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 11.Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- 12.Bretag AH. Muscle chloride channels. Physiol Rev. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- 13.Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- 14.George AL, Jr , Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen’s disease (autosomal dominant myotonia congenita) Nat Genet. 1993;3:305–310. doi: 10.1038/ng0493-305. [DOI] [PubMed] [Google Scholar]
- 15.Kubisch C, Schmidt-Rose T, Fontaine B, Bretag AH, Jentsch TJ. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum Mol Genet. 1998;7:1753–1760. doi: 10.1093/hmg/7.11.1753. [DOI] [PubMed] [Google Scholar]
- 16.Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 18.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 19.Eder C, Klee R, Heinemann U. Involvement of stretch-activated Cl− channels in ramification of murine microglia. J Neurosci. 1998;18:7127–7137. doi: 10.1523/JNEUROSCI.18-18-07127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zierler S, Frei E, Grissmer S, Kerschbaum HH. Chloride influx provokes lamellipodium formation in microglial cells. Cell Physiol Biochem. 2008;21:55–62. doi: 10.1159/000113747. [DOI] [PubMed] [Google Scholar]
- 21.Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HW, Kettenmann H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl− current and chemotaxis in murine microglia. J Immunol. 2002;168:3221–3226. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- 22.Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl− channels and their involvement in proliferation of rat microglial cells. Glia. 1996;17:225–236. doi: 10.1002/(SICI)1098-1136(199607)17:3<225::AID-GLIA5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Ducharme G, Newell EW, Pinto C, Schlichter LC. Small-conductance Cl(−) channels contribute to volume regulation and phagocytosis in microglia. Eur J Neurosci. 2007;26:2119–2130. doi: 10.1111/j.1460-9568.2007.05802.x. [DOI] [PubMed] [Google Scholar]
- 24.Furtner T, Zierler S, Kerschbaum HH. Blockade of chloride channels suppresses engulfment of microspheres in the microglial cell line, BV-2. Brain Res. 2007;1184:1–9. doi: 10.1016/j.brainres.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 25.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C. Cells move when ions and water flow. Pflugers Arch. 2007;453:421–432. doi: 10.1007/s00424-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 26.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci U S A. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeno E, Shimizu T, Okada Y. Normotonic cell shrinkage induces apoptosis under extracellular low Cl conditions in human lymphoid and epithelial cells. Acta Physiol (Oxf) 2006;187:217–222. doi: 10.1111/j.1748-1716.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 29.Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK. Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol. 2010;298:C14–C25. doi: 10.1152/ajpcell.00654.2008. [DOI] [PubMed] [Google Scholar]
- 30.Dezaki K, Maeno E, Sato K, Akita T, Okada Y. Early-phase occurrence of K+ and Cl− efflux in addition to Ca2+ mobilization is a prerequisite to apoptosis in HeLa cells. Apoptosis. 2012;17:821–831. doi: 10.1007/s10495-012-0716-3. [DOI] [PubMed] [Google Scholar]
- 31.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- 33.Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue H, Okada Y. Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J Neurosci. 2007;27:1445–1455. doi: 10.1523/JNEUROSCI.4694-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mongin AA. Volume-regulated anion channel--a frenemy within the brain. Pflugers Arch. 2016;468:421–441. doi: 10.1007/s00424-015-1765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- 37.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 38.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 40.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 41.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 42.Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimelberg HK. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim Biophys Acta. 1981;646:179–184. doi: 10.1016/0005-2736(81)90285-6. [DOI] [PubMed] [Google Scholar]
- 44.Kettenmann H, Backus KH, Schachner M. gamma-Aminobutyric acid opens Cl−channels in cultured astrocytes. Brain Res. 1987;404:1–9. doi: 10.1016/0006-8993(87)91349-7. [DOI] [PubMed] [Google Scholar]
- 45.Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia. 2002;39:207–216. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- 46.Smith QR, Johanson CE, Woodbury DM. Uptake of 36Cl and 22Na by the brain-cerebrospinal fluid system: comparison of the permeability of the blood-brain and blood-cerebrospinal fluid barriers. J Neurochem. 1981;37:117–124. doi: 10.1111/j.1471-4159.1981.tb05298.x. [DOI] [PubMed] [Google Scholar]
- 47.Untiet V, Kovermann P, Gerkau NJ, Gensch T, Rose CR, Fahlke C. Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation. Glia. 2017;65:388–400. doi: 10.1002/glia.23098. [DOI] [PubMed] [Google Scholar]
- 48.MacVicar BA, Tse FW, Crichton SA, Kettenmann H. GABA-activated Cl− channels in astrocytes of hippocampal slices. J Neurosci. 1989;9:3577–3583. doi: 10.1523/JNEUROSCI.09-10-03577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walz W, Wuttke WA. Independent mechanisms of potassium clearance by astrocytes in gliotic tissue. J Neurosci Res. 1999;56:595–603. doi: 10.1002/(SICI)1097-4547(19990615)56:6<595::AID-JNR5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Lytle C, Forbush B., III The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992;267:25438–25443. [PubMed] [Google Scholar]
- 51.Torchia J, Lytle C, Pon DJ, Forbush B, III, Sen AK. The Na-K-Cl cotransporter of avian salt gland. Phosphorylation in response to cAMP-dependent and calcium-dependent secretogogues. J Biol Chem. 1992;267:25444–25450. [PubMed] [Google Scholar]
- 52.Liedtke CM. The role of protein kinase C in alpha-adrenergic regulation of NaCl(K) cotransport in human airway epithelial cells. Am J Physiol. 1995;268:L414–L423. doi: 10.1152/ajplung.1995.268.3.L414. [DOI] [PubMed] [Google Scholar]
- 53.Andersen GO, Enger M, Thoresen GH, Skomedal T, Osnes JB. Alpha1-adrenergic activation of myocardial Na-K-2Cl cotransport involving mitogen-activated protein kinase. Am J Physiol. 1998;275:H641–H652. doi: 10.1152/ajpheart.1998.275.2.H641. [DOI] [PubMed] [Google Scholar]
- 54.Klein JD, Lamitina ST, O’Neill WC. JNK is a volume-sensitive kinase that phosphorylates the Na-K-2Cl cotransporter in vitro. Am J Physiol. 1999;277:C425–C431. doi: 10.1152/ajpcell.1999.277.3.C425. [DOI] [PubMed] [Google Scholar]
- 55.Haas M, Forbush B., III The Na-K-Cl cotransporters. J Bioenerg Biomembr. 1998;30:161–172. doi: 10.1023/a:1020521308985. [DOI] [PubMed] [Google Scholar]
- 56.Gagnon F, Orlov SN, Tremblay J, Hamet P. Complete inhibition of Na+, K+, Cl− cotransport in Madin-Darby canine kidney cells by PMA-sensitive protein kinase. Biochim Biophys Acta. 1998;1369:233–239. doi: 10.1016/s0005-2736(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 57.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 58.Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014;7:re3. doi: 10.1126/scisignal.2005365. [DOI] [PubMed] [Google Scholar]
- 59.Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 60.Smith L, Smallwood N, Altman A, Liedtke CM. PKCdelta acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem. 2008;283:22147–22156. doi: 10.1074/jbc.M801752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem. 2000;10:341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- 62.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 63.Gillen CM, Brill S, Payne JA, Forbush B., III Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J Biol Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 64.Arroyo JP, Kahle KT, Gamba G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Aspects Med. 2013;34:288–298. doi: 10.1016/j.mam.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Pearson MM, Lu J, Mount DB, Delpire E. Localization of the K(+)-Cl(−) cotransporter, KCC3, in the central and peripheral nervous systems: expression in the choroid plexus, large neurons and white matter tracts. Neuroscience. 2001;103:481–491. doi: 10.1016/s0306-4522(00)00567-4. [DOI] [PubMed] [Google Scholar]
- 66.Karadsheh MF, Byun N, Mount DB, Delpire E. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience. 2004;123:381–391. doi: 10.1016/j.neuroscience.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Le Rouzic P, Ivanov TR, Stanley PJ, Baudoin FM, Chan F, Pinteaux E, Brown PD, Luckman SM. KCC3 and KCC4 expression in rat adult forebrain. Brain Res. 2006;1110:39–45. doi: 10.1016/j.brainres.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 68.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 69.Gagnon KB, Delpire E. Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts. Am J Physiol Cell Physiol. 2013;304:C693–C714. doi: 10.1152/ajpcell.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kahle KT, Khanna AR, Alper SL, Adragna NC, Lauf PK, Sun D, Delpire E. K-Cl cotransporters, cell volume homeostasis, and neurological disease. Trends Mol Med. 2015;21:513–523. doi: 10.1016/j.molmed.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de los Heros P, Alessi DR, Gourlay R, Campbell DG, Deak M, Macartney TJ, Kahle KT, Zhang J. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl− co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mercado A, de Los HP, Melo Z, Chavez-Canales M, Murillo-de-Ozores AR, Moreno E, Bazua-Valenti S, Vazquez N, Hadchouel J, Gamba G. With no lysine L-WNK1 isoforms are negative regulators of the K+-Cl− cotransporters. Am J Physiol Cell Physiol. 2016;311:C54–C66. doi: 10.1152/ajpcell.00193.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jennings ML, al-Rohil N. Kinetics of activation and inactivation of swelling-stimulated K+/Cl− transport. The volume-sensitive parameter is the rate constant for inactivation. J Gen Physiol. 1990;95:1021–1040. doi: 10.1085/jgp.95.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jennings ML, Schulz RK. Okadaic acid inhibition of KCl cotransport. Evidence that protein dephosphorylation is necessary for activation of transport by either cell swelling or N-ethylmaleimide. J Gen Physiol. 1991;97:799–817. doi: 10.1085/jgp.97.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bize I, Guvenc B, Robb A, Buchbinder G, Brugnara C. Serine/threonine protein phosphatases and regulation of K-Cl cotransport in human erythrocytes. Am J Physiol. 1999;277:C926–C936. doi: 10.1152/ajpcell.1999.277.5.C926. [DOI] [PubMed] [Google Scholar]
- 76.Gagnon KB, England R, Diehl L, Delpire E. Apoptosis-associated tyrosine kinase scaffolding of protein phosphatase 1 and SPAK reveals a novel pathway for Na-K-2C1 cotransporter regulation. Am J Physiol Cell Physiol. 2007;292:C1809–C1815. doi: 10.1152/ajpcell.00580.2006. [DOI] [PubMed] [Google Scholar]
- 77.Gagnon KB, Delpire E. Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl cotransporter (NKCC1) function: the N-terminal tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) AND PP1. J Biol Chem. 2010;285:14115–14121. doi: 10.1074/jbc.M110.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Q Rev Biophys. 2010;43:449–499. doi: 10.1017/S0033583510000168. [DOI] [PubMed] [Google Scholar]
- 79.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 80.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem. 2012;287:40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bormann J, Kettenmann H. Patch-clamp study of gamma-aminobutyric acid receptor Cl− channels in cultured astrocytes. Proc Natl Acad Sci U S A. 1988;85:9336–9340. doi: 10.1073/pnas.85.23.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- 84.Kettenmann H, Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci. 1985;5:3295–3301. doi: 10.1523/JNEUROSCI.05-12-03295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W, McTigue DM, Enyeart JJ, Terman D, Zhou M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia. 2016;64:214–226. doi: 10.1002/glia.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- 87.Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 88.Pastor A, Chvatal A, Sykova E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci. 1995;7:1188–1198. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 89.Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- 90.Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514. doi: 10.1038/348510a0. [DOI] [PubMed] [Google Scholar]
- 91.Sik A, Smith RL, Freund TF. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neuroscience. 2000;101:51–65. doi: 10.1016/s0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 92.Smith RL, Clayton GH, Wilcox CL, Escudero KW, Staley KJ. Differential expression of an inwardly rectifying chloride conductance in rat brain neurons: a potential mechanism for cell-specific modulation of postsynaptic inhibition. J Neurosci. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 94.Rinke I, Artmann J, Stein V. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. J Neurosci. 2010;30:4776–4786. doi: 10.1523/JNEUROSCI.6299-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleefuss-Lie A, Friedl W, Cichon S, Haug K, Warnstedt M, Alekov A, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H. CLCN2 variants in idiopathic generalized epilepsy. Nat Genet. 2009;41:954–955. doi: 10.1038/ng0909-954. [DOI] [PubMed] [Google Scholar]
- 96.Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hubner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci. 2007;27:6581–6589. doi: 10.1523/JNEUROSCI.0338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Depienne C, Bugiani M, Dupuits C, Galanaud D, Touitou V, Postma N, van BC, Polder E, Tollard E, Darios F, Brice A, de Die-Smulders CE, Vles JS, Vanderver A, Uziel G, Yalcinkaya C, Frints SG, Kalscheuer VM, Klooster J, Kamermans M, Abbink TE, Wolf NI, Sedel F, van der Knaap MS. Brain white matter oedema due to ClC-2 chloride channel deficiency: an observational analytical study. Lancet Neurol. 2013;12:659–668. doi: 10.1016/S1474-4422(13)70053-X. [DOI] [PubMed] [Google Scholar]
- 98.Leegwater PA, Yuan BQ, van der Steen J, Mulders J, Konst AA, Boor PK, Mejaski-Bosnjak V, van der Maarel SM, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS. Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet. 2001;68:831–838. doi: 10.1086/319519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van der Knaap MS, Boor I, Estevez R. Megalencephalic leukoencephalopathy with subcortical cysts: chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet Neurol. 2012;11:973–985. doi: 10.1016/S1474-4422(12)70192-8. [DOI] [PubMed] [Google Scholar]
- 100.Jeworutzki E, Lopez-Hernandez T, Capdevila-Nortes X, Sirisi S, Bengtsson L, Montolio M, Zifarelli G, Arnedo T, Muller CS, Schulte U, Nunes V, Martinez A, Jentsch TJ, Gasull X, Pusch M, Estevez R. GlialCAM, a protein defective in a leukodystrophy, serves as a ClC-2 Cl(−) channel auxiliary subunit. Neuron. 2012;73:951–961. doi: 10.1016/j.neuron.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoegg-Beiler MB, Sirisi S, Orozco IJ, Ferrer I, Hohensee S, Auberson M, Godde K, Vilches C, de Heredia ML, Nunes V, Estevez R, Jentsch TJ. Disrupting MLC1 and GlialCAM and ClC-2 interactions in leukodystrophy entails glial chloride channel dysfunction. Nat Commun. 2014;5:3475. doi: 10.1038/ncomms4475. [DOI] [PubMed] [Google Scholar]
- 102.Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 103.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, Jahn R, Jentsch TJ. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 104.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 105.Shimada K, Li X, Xu G, Nowak DE, Showalter LA, Weinman SA. Expression and canalicular localization of two isoforms of the ClC-3 chloride channel from rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2000;279:G268–G276. doi: 10.1152/ajpgi.2000.279.2.G268. [DOI] [PubMed] [Google Scholar]
- 106.Robinson NC, Huang P, Kaetzel MA, Lamb FS, Nelson DJ. Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current. J Physiol. 2004;556:353–368. doi: 10.1113/jphysiol.2003.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guzman RE, Grieschat M, Fahlke C, Alekov AK. ClC-3 is an intracellular chloride/proton exchanger with large voltage-dependent nonlinear capacitance. ACS Chem Neurosci. 2013;4:994–1003. doi: 10.1021/cn400032z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guzman RE, Miranda-Laferte E, Franzen A, Fahlke C. Neuronal ClC-3 Splice Variants Differ in Subcellular Localizations, but Mediate Identical Transport Functions. J Biol Chem. 2015;290:25851–25862. doi: 10.1074/jbc.M115.668186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brandt S, Jentsch TJ. ClC-6 and ClC-7 are two novel broadly expressed members of the CLC chloride channel family. FEBS Lett. 1995;377:15–20. doi: 10.1016/0014-5793(95)01298-2. [DOI] [PubMed] [Google Scholar]
- 110.Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch TJ. Cloning and functional expression of rat CLC-5, a chloride channel related to kidney disease. J Biol Chem. 1995;270:31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- 111.Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poet M, Kornak U, Schweizer M, Zdebik AA, Scheel O, Hoelter S, Wurst W, Schmitt A, Fuhrmann JC, Planells-Cases R, Mole SE, Hubner CA, Jentsch TJ. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc Natl Acad Sci U S A. 2006;103:13854–13859. doi: 10.1073/pnas.0606137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 114.Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 115.Stauber T. The volume-regulated anion channel is formed by LRRC8 heteromers - molecular identification and roles in membrane transport and physiology. Biol Chem. 2015;396:975–990. doi: 10.1515/hsz-2015-0127. [DOI] [PubMed] [Google Scholar]
- 116.Pedersen SF, Okada Y, Nilius B. Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR) Pflugers Arch. 2016;468:371–383. doi: 10.1007/s00424-015-1781-6. [DOI] [PubMed] [Google Scholar]
- 117.Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science. 2014;344:634–638. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 118.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell. 2014;157:447–458. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gaitan-Penas H, Gradogna A, Laparra-Cuervo L, Solsona C, Fernandez-Duenas V, Barrallo-Gimeno A, Ciruela F, Lakadamyali M, Pusch M, Estevez R. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys J. 2016;111:1429–1443. doi: 10.1016/j.bpj.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M, Patapoutian A. LRRC8 proteins form volume-regulated anion channels that sense ionic strength. Cell. 2016;164:499–511. doi: 10.1016/j.cell.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyzinski-Garcia MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol. 2014;592:4855–4862. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ullrich F, Reincke SM, Voss FK, Stauber T, Jentsch TJ. Inactivation and anion selectivity of volume-regulated anion channels (VRACs) depend on C-terminal residues of the first extracellular loop. J Biol Chem. 2016;291:17040–17048. doi: 10.1074/jbc.M116.739342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA, Kucukosmanoglu A, Xu G, Voss FK, Reincke SM, Stauber T, Blomen VA, Vis DJ, Wessels LF, Brummelkamp TR, Borst P, Rottenberg S, Jentsch TJ. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015;34:2993–3008. doi: 10.15252/embj.201592409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lutter D, Ullrich F, Lueck JC, Kempa S, Jentsch TJ. Selective transport of neurotransmitters and modulators by distinct volume-regulated LRRC8 anion channels. J Cell Sci. 2017;130:1122–1133. doi: 10.1242/jcs.196253. [DOI] [PubMed] [Google Scholar]
- 126.Schober AL, Wilson CS, Mongin AA. Molecular composition and heterogeneity of the LRRC8-containing swelling-activated osmolyte channels in primary rat astrocytes. J Physiol. 2017;595:6939–6951. doi: 10.1113/JP275053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol. 2002;283:C569–C578. doi: 10.1152/ajpcell.00438.2001. [DOI] [PubMed] [Google Scholar]
- 129.Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem. 2004;279:13301–13304. doi: 10.1074/jbc.C400020200. [DOI] [PubMed] [Google Scholar]
- 130.Haskew-Layton RE, Mongin AA, Kimelberg HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- 131.Harrigan TJ, Abdullaev IF, Jourd’heuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nilius B, Prenen J, Walsh MP, Carton I, Bollen M, Droogmans G, Eggermont J. Myosin light chain phosphorylation-dependent modulation of volume- regulated anion channels in macrovascular endothelium. FEBS Lett. 2000;466:346–350. doi: 10.1016/s0014-5793(00)01097-8. [DOI] [PubMed] [Google Scholar]
- 133.Hermoso M, Olivero P, Torres R, Riveros A, Quest AFG, Stutzin A. Cell volume regulation in response to hypotonicity is impaired in HeLa cells expressing a protein kinase C alpha mutant lacking kinase activity. J Biol Chem. 2004;279:17681–17689. doi: 10.1074/jbc.M304506200. [DOI] [PubMed] [Google Scholar]
- 134.Rudkouskaya A, Chernoguz A, Haskew-Layton RE, Mongin AA. Two conventional protein kinase C isoforms, alpha and betaI, are involved in the ATP-induced activation of volume-regulated anion channel and glutamate release in cultured astrocytes. J Neurochem. 2008;105:2260–2270. doi: 10.1111/j.1471-4159.2008.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akita T, Okada Y. Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol. 2011;589:3909–3927. doi: 10.1113/jphysiol.2011.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fisher SK, Cheema TA, Foster DJ, Heacock AM. Volume-dependent osmolyte efflux from neural tissues: regulation by G-protein-coupled receptors. J Neurochem. 2008;106:1998–2014. doi: 10.1111/j.1471-4159.2008.05510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Franco R, Panayiotidis MI, de La Paz LD. Autocrine signaling involved in cell volume regulation: the role of released transmitters and plasma membrane receptors. J Cell Physiol. 2008;216:14–28. doi: 10.1002/jcp.21406. [DOI] [PubMed] [Google Scholar]
- 138.Kumar L, Chou J, Yee CS, Borzutzky A, Vollmann EH, von Andrian UH, Park SY, Hollander G, Manis JP, Poliani PL, Geha RS. Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function. J Exp Med. 2014;211:929–942. doi: 10.1084/jem.20131379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO(3)(−)) transporters. Mol Aspects Med. 2013;34:159–182. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwiening CJ, Boron WF. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(−)-HCO3− exchange. J Physiol. 1994;475:59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 142.Orlov SN, Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol. 2006;210:161–172. doi: 10.1007/s00232-006-0857-9. [DOI] [PubMed] [Google Scholar]
- 143.Chen TY, Miller C. Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J Gen Physiol. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pusch M, Jordt SE, Stein V, Jentsch TJ. Chloride dependence of hyperpolarization-activated chloride channel gates. J Physiol. 1999;515:341–353. doi: 10.1111/j.1469-7793.1999.341ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yuan A, Dourado M, Butler A, Walton N, Wei A, Salkoff L. SLO-2, a K+ channel with an unusual Cl− dependence. Nat Neurosci. 2000;3:771–779. doi: 10.1038/77670. [DOI] [PubMed] [Google Scholar]
- 146.Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 147.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu H, Zhang Z, Lis A, Penner R, Fleig A. TRPM7 is regulated by halides through its kinase domain. Cell Mol Life Sci. 2013;70:2757–2771. doi: 10.1007/s00018-013-1284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. Intracellular Cl− as a signaling ion that potently regulates Na+/HCO3− transporters. Proc Natl Acad Sci U S A. 2015;112:E329–E337. doi: 10.1073/pnas.1415673112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3− transporte. J Gen Physiol. 1983;81:53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.O’Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J Neurophysiol. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- 152.Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci. 2000;20:6839–6848. doi: 10.1523/JNEUROSCI.20-18-06839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aharonovitz O, Kapus A, Szaszi K, Coady-Osberg N, Jancelewicz T, Orlowski J, Grinstein S. Modulation of Na+/H+ exchange activity by Cl−. Am J Physiol Cell Physiol. 2001;281:C133–C141. doi: 10.1152/ajpcell.2001.281.1.C133. [DOI] [PubMed] [Google Scholar]
- 154.Higashijima T, Ferguson KM, Sternweis PC. Regulation of hormone-sensitive GTP-dependent regulatory proteins by chloride. J Biol Chem. 1987;262:3597–3602. [PubMed] [Google Scholar]
- 155.Grinstein S, Furuya W, Downey GP. Activation of permeabilized neutrophils: role of anions. Am J Physiol. 1992;263:C78–C85. doi: 10.1152/ajpcell.1992.263.1.C78. [DOI] [PubMed] [Google Scholar]
- 156.Dinudom A, Komwatana P, Young JA, Cook DI. Control of the amiloride-sensitive Na+ current in mouse salivary ducts by intracellular anions is mediated by a G protein. J Physiol. 1995;487:549–555. doi: 10.1113/jphysiol.1995.sp020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Muimo R, Banner SJ, Marshall LJ, Mehta A. Nucleoside diphosphate kinase and Cl(−)-sensitive protein phosphorylation in apical membranes from ovine airway epithelium. Am J Respir Cell Mol Biol. 1998;18:270–278. doi: 10.1165/ajrcmb.18.2.2850. [DOI] [PubMed] [Google Scholar]
- 158.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 159.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 161.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 162.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 163.Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, Cobb MH. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 165.Kahle KT, Rinehart J, de Los HP, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci U S A. 2005;102:16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Begum G, Yuan H, Kahle KT, Li L, Wang S, Shi Y, Shmukler BE, Yang SS, Lin SH, Alper SL, Sun D. Inhibition of WNK3 Kinase Signaling Reduces Brain Damage and Accelerates Neurological Recovery After Stroke. Stroke. 2015;46:1956–1965. doi: 10.1161/STROKEAHA.115.008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda) 2006;21:326–335. doi: 10.1152/physiol.00015.2006. [DOI] [PubMed] [Google Scholar]
- 168.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7:ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep. 2008;9:70–75. doi: 10.1038/sj.embor.7401128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Falin RA, Morrison R, Ham AJ, Strange K. Identification of regulatory phosphorylation sites in a cell volume- and Ste20 kinase-dependent ClC anion channel. J Gen Physiol. 2009;133:29–42. doi: 10.1085/jgp.200810080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285:25161–25167. doi: 10.1074/jbc.M110.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu BE, Stippec S, Lenertz L, Lee BH, Zhang W, Lee YK, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem. 2004;279:7826–7831. doi: 10.1074/jbc.M313465200. [DOI] [PubMed] [Google Scholar]
- 174.Le Moellic C, Boulkroun S, Gonzalez-Nunez D, Dublineau I, Cluzeaud F, Fay M, Blot-Chabaud M, Farman N. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol. 2005;289:C1513–C1521. doi: 10.1152/ajpcell.00314.2005. [DOI] [PubMed] [Google Scholar]
- 175.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 2016;35:239–257. doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dayanithi G, Nordmann JJ. Chloride and magnesium dependence of vasopressin release from rat permeabilized neurohypophysial nerve endings. Neurosci Lett. 1989;106:305–309. doi: 10.1016/0304-3940(89)90181-x. [DOI] [PubMed] [Google Scholar]
- 177.Rupnik M, Zorec R. Cytosolic chloride ions stimulate Ca(2+)-induced exocytosis in melanotrophs. FEBS Lett. 1992;303:221–223. doi: 10.1016/0014-5793(92)80524-k. [DOI] [PubMed] [Google Scholar]
- 178.Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, Thevenod F, Renstrom E. Priming of insulin granules for exocytosis by granular Cl(−) uptake and acidification. J Cell Sci. 2001;114:2145–2154. doi: 10.1242/jcs.114.11.2145. [DOI] [PubMed] [Google Scholar]
- 179.Rupnik M, Zorec R. Intracellular Cl− modulates Ca2+-induced exocytosis from rat melanotrophs through GTP-binding proteins. Pflugers Arch. 1995;431:76–83. doi: 10.1007/BF00374379. [DOI] [PubMed] [Google Scholar]
- 180.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 181.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 183.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 184.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 185.Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia. 2016;64:1611–1627. doi: 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- 186.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr , Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 190.Zhou Y, Danbolt NC. GABA and Glutamate Transporters in Brain. Front Endocrinol (Lausanne) 2013;4:165. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 192.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 193.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 194.Ryan RM, Mindell JA. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2007;14:365–371. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 195.Machtens JP, Kortzak D, Lansche C, Leinenweber A, Kilian P, Begemann B, Zachariae U, Ewers D, de Groot BL, Briones R, Fahlke C. Mechanisms of anion conduction by coupled glutamate transporters. Cell. 2015;160:542–553. doi: 10.1016/j.cell.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 196.Fahlke C, Kortzak D, Machtens JP. Molecular physiology of EAAT anion channels. Pflugers Arch. 2016;468:491–502. doi: 10.1007/s00424-015-1768-3. [DOI] [PubMed] [Google Scholar]
- 197.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 198.Lehre AC, Rowley NM, Zhou Y, Holmseth S, Guo C, Holen T, Hua R, Laake P, Olofsson AM, Poblete-Naredo I, Rusakov DA, Madsen KK, Clausen RP, Schousboe A, White HS, Danbolt NC. Deletion of the betaine-GABA transporter (BGT1; slc6a12) gene does not affect seizure thresholds of adult mice. Epilepsy Res. 2011;95:70–81. doi: 10.1016/j.eplepsyres.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Keynan S, Kanner BI. gamma-Aminobutyric acid transport in reconstituted preparations from rat brain: coupled sodium and chloride fluxes. Biochemistry. 1988;27:12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- 200.Clark JA, Amara SG. Stable expression of a neuronal gamma-aminobutyric acid transporter, GAT-3, in mammalian cells demonstrates unique pharmacological properties and ion dependence. Mol Pharmacol. 1994;46:550–557. [PubMed] [Google Scholar]
- 201.Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]
- 202.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 203.Gundersen V, Storm-Mathisen J, Bergersen LH. Neuroglial transmission. Physiol Rev. 2015;95:695–726. doi: 10.1152/physrev.00024.2014. [DOI] [PubMed] [Google Scholar]
- 204.Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- 205.Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 207.Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 208.Yoon BE, Jo S, Woo J, Lee JH, Kim T, Kim D, Lee CJ. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol Brain. 2011;4:42. doi: 10.1186/1756-6606-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rosso L, Peteri-Brunback B, Poujeol P, Hussy N, Mienville JM. Vasopressin-induced taurine efflux from rat pituicytes: a potential negative feedback for hormone secretion. J Physiol. 2004;554:731–742. doi: 10.1113/jphysiol.2003.056267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci. 2012;32:12518–12527. doi: 10.1523/JNEUROSCI.1380-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 215.Sun D, Murali SG. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am J Physiol. 1998;275:C772–C779. doi: 10.1152/ajpcell.1998.275.3.C772. [DOI] [PubMed] [Google Scholar]
- 216.Schomberg SL, Su G, Haworth RA, Sun D. Stimulation of Na-K-2Cl cotransporter in neurons by activation of Non- NMDA ionotropic receptor and group-I mGluRs. J Neurophysiol. 2001;85:2563–2575. doi: 10.1152/jn.2001.85.6.2563. [DOI] [PubMed] [Google Scholar]
- 217.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 218.Wang F, Wang X, Shapiro LA, Cotrina ML, Liu W, Wang EW, Gu S, Wang W, He X, Nedergaard M, Huang JH. NKCC1 up-regulation contributes to early post-traumatic seizures and increased post-traumatic seizure susceptibility. Brain Struct Funct. 2017;222:1543–1556. doi: 10.1007/s00429-016-1292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP. Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci. 2013;33:3413–3423. doi: 10.1523/JNEUROSCI.3497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]