Abstract
Background
At the point of care, evidence from randomized controlled trials (RCTs) is underutilized in helping clinicians meet their information needs.
Objective
To design interactive visual displays to help clinicians interpret and compare the results of relevant RCTs for the management of a specific patient, and to conduct a formative evaluation with physicians comparing interactive visual versus narrative displays.
Methods
We followed a user-centered and iterative design process succeeded by development of information display prototypes as a Web-based application. We then used a within-subjects design with 20 participants (8 attendings and 12 residents) to evaluate the usability and problem-solving impact of the information displays. We compared subjects’ perceptions of the interactive visual displays versus narrative abstracts.
Results
The resulting interactive visual displays present RCT results side-by-side according to the Population, Intervention, Comparison, and Outcome (PICO) framework. Study participants completed 19 usability tasks in 3 to 11 seconds with a success rate of 78% to 100%. Participants favored the interactive visual displays over narrative abstracts according to perceived efficiency, effectiveness, effort, user experience and preference (all P values <.001).
Conclusions
When interpreting and applying RCT findings to case vignettes, physicians preferred interactive graphical and PICO-framework-based information displays that enable direct comparison of the results from multiple RCTs compared to the traditional narrative and study-centered format. Future studies should investigate the use of interactive visual displays to support clinical decision making in care settings and their effect on clinician and patient outcomes.
Keywords: clinical decision-making, clinician information needs, information display, information foraging theory, information seeking behavior
Introduction
Background
At the point of care, clinicians have many clinical questions that they are unable to answer with the best available evidence [1]. Unanswered questions are missed opportunities to improve patient care decisions and for just-in-time learning [2]. Primary literature resources (eg, PubMed) contain answers to most of these questions [3], but their use at the point of care is still limited due to barriers such as lack of time and significant cognitive effort imposed by the evidence search and interpretation process [4,5].
Abstracts in scientific manuscripts are typically presented according to the well-established “background, introduction, methods, results, discussion, conclusion” structure [6]. However, this study-centered structure may not be optimal to support clinicians’ patient-centered conceptual models. The average time for clinicians to look up clinical questions on PubMed ranges from 5 to 60 minutes [7]. In addition, clinicians report high levels of dissatisfaction with their information seeking experience [8,9]. Ultimately, clinicians’ challenges in consuming evidence from the primary literature may contribute to slowing the translation of scientific evidence [10].
Few studies have examined optimal methods for displaying the results of clinical research reports. Prior work regarding primary literature has focused on displaying systematic reviews and investigating different methods of displaying results across studies, such as short summaries [11-13], tables [14-19], and harvest plots [20]. One recent study examined a novel presentation of clinical trial reports that restructured the visualization into several panels (ie, study purpose, process model and data grid for viewing results, statistical methods, and result interpretations). Using this visualization, translational researchers spent less time understanding and interpreting the clinical trials but maintained the same accuracy [21]. In general, very few of those studies have used any theory to drive their work.
The purpose of this study was to investigate alternative display approaches to present relevant information from randomized controlled trials (RCTs) to support clinical decision-making. Overall, we hypothesized that interactive visual displays would reduce clinicians’ cognitive workload in interpreting RCTs compared with narrative RCT abstracts. Building on Slager et al’s exploratory study on static tabular displays [22], we employed information foraging theory [23] and information visualization techniques to design a high-fidelity prototype with interactive visual displays of RCT results. The information displays were designed to help clinicians rapidly review, synthesize, and compare the results of relevant RCTs for the treatment of a specific patient. In this study, we described the RCT information displays and addressed the following three research questions: (1) Is the interface usable? (2) Is there a difference in perceived efficiency, effort, effectiveness, user experience, and preference between interactive visual displays and narrative abstracts? and (3) Do clinicians’ perceived user experience, efficacy, effort, and effectiveness predict their intention to use interactive visual displays?
Theoretical Framework
The design of the information displays was based upon information foraging theory [23], Shneiderman’s information visualization principles [24], and the Population, Intervention, Comparison, and Outcome (PICO) framework [25]. Information foraging theory was initially proposed for Web designers [26]. Based on an analogy with animals’ foraging, information foraging theory indicates that information seekers use information scent (ie, cues indicating the existence of easily accessible and relevant information) to select information patches to explore maximizing the value (ie, perceived utility of the information) to cost (ie, time and effort required to explore the patch) ratio [23]. Within a certain patch, the concentration of relevant information can be increased through a process called information patch enrichment (eg, use of filters). Information foraging theory is grounded on the Holling Disc Equation, which equals the ratio of the total net amount of valuable information gained to the sum of the total amount of time spent between-patches and within-patches [27]. Shneiderman proposed an information visualization principle according to which information displays should first provide an information overview, with the ability to zoom and filter, and then retrieve details on demand [24].
RCTs are the highest-level evidence in evidence-based medicine [28]. The PICO elements are key components of the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting the quality of RCTs [29]. In addition, the PICO elements have been identified in multiple studies as the critical elements of an RCT [30-32] and are almost ubiquitous within medical journal abstracts [31]. PICO has been reported to be a more effective search input format than the standard PubMed search interface for answering clinical questions [25,33]. More recently, Slager et al found that clinicians favored a PICO-based tabular display over the typical narrative abstracts reported in scientific journals [22].
Methods
Overview
Our study had two phases. The first phase was the process of designing and implementing the information displays. The second was an experimental formative evaluation assessing usability and problem-solving impact. The second phase included three stages: (1) usability test of the interactive visual displays; (2) problem solving for two case vignettes comparing narrative abstracts versus interactive visual displays; and (3) a poststudy questionnaire (Figure 1).
Figure 1.
Visualization of study structure.
Phase One: Design of the Interactive Visual Displays
Design
We followed a user-centered and iterative design process with feedback from informatics students, clinicians, and human factor experts. The study authors (three informatics and human factors specialists and four informatics students; five with clinical backgrounds) designed the first several iterations, followed by feedback from three independent informatics researchers with clinical backgrounds.
We started with low-fidelity prototypes that were designed with user interface mockup software (NinjaMock) [34] and a free website builder (Wix) [35]. After approximately 50 design-feedback iterations, the information displays evolved into a high-fidelity interactive prototype: a Web-based application implemented in HTML, CSS, JavaScript, and an open-source third party graphic library called Highcharts [36].
Data Structure
First, we searched a set of RCTs in PubMed related to three clinical case vignettes that were used in the evaluation phase. Next, we manually extracted data, including PICO elements, from each of the selected RCTs. The following data were extracted from each RCT on a spreadsheet: PMID, journal, publication date, study title, study acronym, population inclusion criteria, population age, study sample size, study country, aim, and main conclusion. For study arms, we extracted the study arm intervention and number of participants. For each study arm, we extracted name and results of all major outcome measures, overall adverse event rate, and most common adverse events. Last, we designed an Extensible Markup Language (XML) schema to represent the RCT data, created XML instances for each RCT, and transformed the XML instances into Java Script Object Notation format for consumption by the application. The RCTs used in the prototype were manually searched and selected, as the purpose of the study was to compare the information displays as opposed to search engines. RCT data were manually extracted since the purpose of the study was not to investigate automated methods to extract PICO elements.
Phase Two: Formative Evaluation Comparing Narrative Abstracts Versus Interactive Visual Displays
For formative evaluation, we used a within-subjects experimental design. The within-subjects design has advantages over a randomized, between-subjects design: it has higher statistical power, requiring a smaller sample size; and it enables participants to directly compare two designs. We tested the usability of the interactive visual displays and compared subjects' perceptions about narrative abstract displays versus interactive visual displays for clinical problem solving using case vignettes.
Study Setting
Formative evaluation sessions were conducted via online meetings using a Web meeting software (WebEx). Participants accessed an instance of the interactive visual displays hosted at the University of Utah Center for High Performance Computing [37]. The Uniform Resource Locator (URL) was shared with participants at the beginning of the evaluation sessions.
Participant Recruitment
We recruited 20 participants (8 attending physicians and 12 residents) who had not previously been exposed to the interactive visual displays (see Multimedia Appendix 1, Table A1). Participants were recruited from the Departments of Family Medicine at the University of Utah and Partners Healthcare via announcements that were sent to departmental email lists. We also employed the snowball sampling technique which asked study participants to promote the study among their colleagues. All participants received a US $100 incentive to participate in the study sessions. A previous study with a similar design [22] demonstrated that 20 participants were enough to detect a moderate difference between interactive visual displays and narrative displays with a power of 0.80.
Information Displays Evaluation Procedure
We randomized participants to the order of presentation of the two tools and to the vignette-tool assignment. Each session began with a brief introduction about the study and a short one-page PDF tutorial explaining how to use the interactive visual displays. To ensure consistency, verbal instructions were read from a predefined script for each session. Each formative evaluation session included three stages: (1) usability test of the interactive visual displays; (2) problem solving for two case vignettes comparing narrative abstracts versus interactive visual displays; and (3) a poststudy questionnaire assessing the participant’s perception of the efficiency, effort, effectiveness, user experience, and preference of interactive visual displays versus narrative abstracts. In the first two stages, the participants were asked to share their screens via WebEx, and their screen interactions were recorded for data analysis. In the third stage, participants were asked to stop screen-sharing while answering the poststudy questionnaire to ensure anonymity and minimize the Hawthorne effect. A waiver of written consent was approved by the Institutional Review Board of the University of Utah. Participants provided verbal consent before the study session.
Case Vignettes and RCTs
We prepared three case vignettes (Table 1), which presented challenges related to patient treatment. The vignettes were obtained from the literature and adapted by clinicians in our team. For each case vignette, we searched for potentially relevant RCTs using PubMed’s Clinical Query treatment filter and a Medical Subject Heading (MeSH) term that matched the main disease of the case.
Table 1.
Case vignettes used in the formative evaluation.
| Case vignette | Stage Used | Complexitya | Designer | Number of studies |
| Acute coronary syndrome | Usability | Easy | Article author (PUb) | 2 |
| Rheumatoid arthritis | Problem solving | Complex | Adapted from Medscape [38] | 10 |
| Diabetes mellitus | Problem solving | Complex | Adapted from Hirsch et al [39] | 10 |
aThe complexity level of each vignette was determined by the number of factors involved in each treatment case.
bPU refers to co-author Prasad Unni.
We manually screened the retrieved articles for RCTs on the diseases of interest and presented the same RCTs in the same order, both within PubMed and the interactive visual displays. The case vignettes and selected RCTs are available in Multimedia Appendix 1 (case vignettes).
Stage One: Usability of Interactive Visual Displays
We developed 19 tasks to test the usability of the key information and features provided by interactive visual displays. Most of the tasks required participants to perform an action (eg, highlight, access, switch). We read the tasks aloud one-by-one to each participant and let them complete the tasks independently without assistance. We measured the time spent and success on each task. We also tape recorded the session and transcribed participants’ comments.
Stage Two: Problem Solving
Participants were told that the rheumatoid arthritis and diabetes mellitus cases are relatively complex and that there are multiple reasonable treatment options for each case. We asked each participant to complete this stage in no more than 10 minutes in order to simulate the time pressure of a real patient visit [1,40]. We notified participants when there were 3 minutes and 1 minute left to finish the session. Within each case vignette session, participants could go back to the vignette description at any time.
For PubMed, participants were given a hyperlink that gave access to a search results page with the 10 RCTs in PubMed’s default search results display format (see Multimedia Appendix 1, Figure A1). No washout time was provided between the two case vignettes. At the end of the problem-solving stage, we asked each participant to provide a summary of the evidence they found and their decision about the treatment for the patient.
Stage Three: Participant Information-Seeking Experience Questionnaire
In this stage, we asked participants to complete an online REDCap [41] questionnaire regarding their information-seeking experiences with the tools. The questions (see Multimedia Appendix 1, “Post Evaluation Survey”) were adapted from the System Usability Scale [42], the National Aeronautics and Space Administration (NASA) Task Load Index (NASA-TLX) tool [43], and from Slager et al [22]. Two versions of the questionnaire were used, depending on which tool the participant was randomly assigned to use first. Each participant only needed to complete one survey.
The questionnaire started with items about participants’ demographics, experience with cases in the domain of the vignettes, and experience with literature searching. Next, using 17 Likert scale items, participants were asked to rate interactive visual displays versus narrative abstracts according to perceived efficiency, effectiveness, effort, user experience, and preference. The anchors for each question juxtaposed narrative abstracts in PubMed on one end and interactive visual displays on the other with the direction of the anchor randomized. For example, the hypothesis that there would be a difference in perceived effectiveness for users between interactive visual displays and narrative abstracts was assessed by four survey items: (1) comprehend the meaning of the information presented well, (2) identify relevant information to understand the study, (3) effectively identify relevant RCTs from the search results, and (4) accomplish tasks with minimal frustration. The results were constructed so that there were separate ratings for narrative abstracts and interactive visual displays for each question by centering the scores for both displays separately. Participants were then asked to rate the intention to use and learnability of using interactive visual displays on a 1 (“Strongly Disagree”) to 9 (“Strongly Agree”) scale. Scales were created for each of the constructs as the sum of the ratings given to each of the items in the construct. We reported internal reliability for the scales using Cronbach alpha (Table 2). Last, participants were asked to provide suggestions for improving interactive visual displays.
Table 2.
Construct items and Cronbach alpha.
| Construct and items | Cronbach alpha | |
| Experience with cases in the domain of the vignettes | .793 | |
|
|
Dealing with patients in the same clinical domain of the narrative abstracts case vignette |
|
|
|
Dealing with cases with similar clinical complexity as in the case presented in the narrative abstracts vignette | |
|
|
Dealing with patients in the clinical domain of the interactive visual display case vignette | |
|
|
Dealing with cases with similar clinical complexity as in the case presented in the interactive visual display vignette | |
| Experience with literature searching | .870 | |
|
|
Experience in using computers for work activities |
|
|
|
Experience in using medical literature search tools in general (eg, PubMed, UpToDate) | |
|
|
Experience in using PubMed for medical literature search | |
| Efficiency | .877 | |
|
|
Scan the information quickly |
|
|
|
Quickly obtain the gist of the study findings | |
|
|
Locate information rapidly | |
|
|
Interpret individual RCTa results quickly | |
|
|
Quickly compare the results of multiple RCTs | |
|
|
Quickly determine study relevance for the case vignette | |
| Effectiveness | .921 | |
|
|
Comprehend the meaning of the information presented well |
|
|
|
Identify relevant information to understand the study | |
|
|
Effectively identify relevant RCTs from the search results | |
|
|
Accomplish tasks with minimal frustration | |
| Effort | .823 | |
|
|
Spend the least degree of mental effort |
|
|
|
Accomplish task effortlessly | |
| User experience | .921 | |
|
|
Be satisfied with the presentation (ie, format of the display) of the information |
|
|
|
Easily use the user interface | |
|
|
Enjoy exploring information | |
|
|
Have fun seeking information to find answers | |
| Intention to use | .971 | |
|
|
Help me with clinical decisions for specific patients |
|
|
|
Find evidence during patient consultations | |
|
|
Find evidence after patient consultations | |
|
|
Prepare for patient appointments | |
|
|
Prepare for patient rounds | |
|
|
Prepare for teaching | |
aRCT: randomized controlled trial.
Data Analysis
We analyzed the usability results and the Likert scale items in problem solving to address the following questions below. We performed all statistical analyses using IBM SPSS Statistics Premium 24 [44].
Is the Interface Usable?
We conducted both qualitative and quantitative analyses to answer this question. We employed a qualitative analysis software (ATLAS.ti [45]) to code, categorize, and analyze users’ verbalizations in the usability stage. To establish reliability for success and time measures, two authors (JB and DB) developed and tested coding protocols and employed the Cohen kappa and Pearson’s correlation coefficient (PCC) to measure the interrater agreements. When both measurement metrics reached 0.80, we split the remaining sessions between coders in order to reduce the workload. For each task, we reported the mean, standard deviation, median, and range for the time spent and reported the success rate. We analyzed the correlation between the experience with literature searching and success rate of the usability tasks, and also categorized the open-ended comments and reported the descriptive statistics.
Is There a Difference in Perceived Efficiency, Effort, Effectiveness, User Experience and Preference Between Interactive Visual Displays and Narrative Abstracts?
We employed the paired t-test to assess differences in ratings for each variable. We also assessed if there was a difference between interactive visual displays and narrative abstracts after controlling for years of expertise, tool presentation order, clinical role, experience with literature searching, and experience with cases in the domain of the vignettes.
Do Clinicians' Perceived User Experience, Efficacy, Effort, and Effectiveness Predict Their Intention to Use Interactive Visual Displays?
To answer this question, we regressed intention to use on user experience, efficacy, effort, and effectiveness.
Results
Interactive Visual Displays
Following Shneiderman's principles, the interactive visual displays provided information overviews and filters with the option to retrieve details on-demand. These principles guided the design of each of the features in Table 3. These features are operationalized in one of five information displays, which are article list, text summary, comparison table, efficacy graph, and side effects graph. The displays can be launched for each case vignette by clicking on the “i” icons at our website [37]. Figures 2-4 depict the features listed in Table 3. A drop-down menu is used for switching between five information displays. Article list and text summary information displays aim to help users judge the relevance of an RCT based on the study patient characteristics and the interventions under investigation. The comparison table, efficacy graph, and side effects graph information displays allow users to compare the results of relevant RCTs side-by-side. To avoid visual cluttering, we limited the maximum number of studies to four that can be displayed in the comparison table, efficacy graph, and side effects graph information displays.
Table 3.
Design principles that inspired each feature in the interactive visual displays.
| Information display | Feature | Design principle |
| Article list | Information about study population and interventions | Information scent |
| Article list | Hyperlink to full abstract within PubMed | Details on demand |
| Article list | Ability to select specific, most relevant studies for further visualization | Information patch enrichment, filter and zoom |
| Comparison table | Population, Intervention, Comparison, and Outcome (PICO) table structure | Information scent |
| Comparison table | Hyperlink to full abstract within PubMed and hyperlink to efficacy and side effect graph | Details on demand and zoom |
| Efficacy graph/side effects graph | Ability to choose different outcome measures or side effects | Information scent and zoom |
Figure 2.
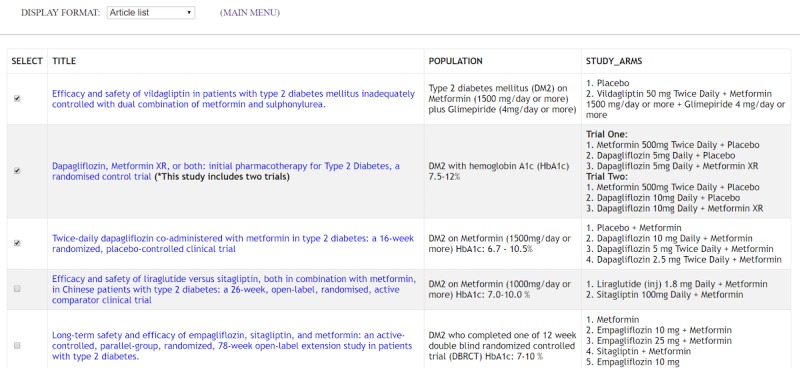
Article list table with trials on various treatments for diabetes mellitus. This display is the landing page of the information displays. It provides a table with the title, patient population, and study arms of each study. The goal is to allow clinicians to quickly scan each study and select relevant ones for further review.
Figure 4.
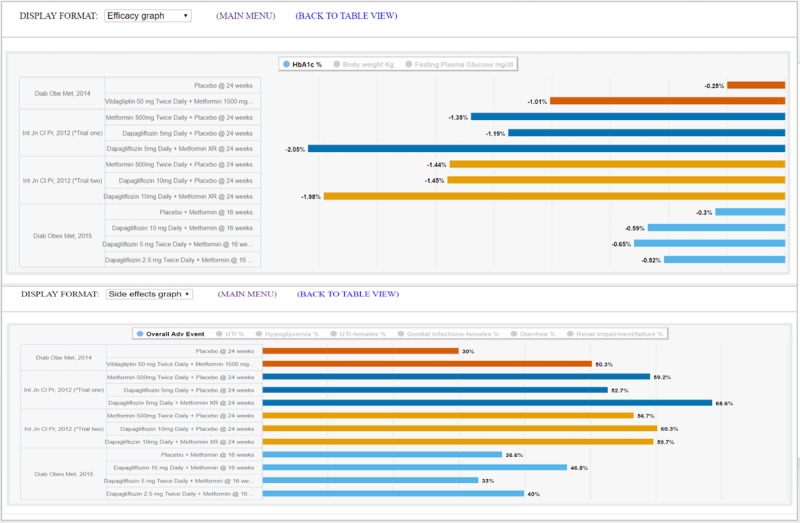
Efficacy graph display (top) and side effects graph display (bottom) with four trials on various treatments for diabetes mellitus. These two displays provide graphical comparisons of study primary outcomes and adverse effects respectively. Users can choose to set the bar graph for a specific outcome measure, overall adverse effects, or the most common adverse effect across all the arms of the selected studies.
Formative Evaluation
The formative evaluation results are structured according to the research questions.
The Interface is Usable
After two WebEx recording sessions (one resident and one attending) were coded and analyzed by DB and JB independently, the interrater agreement Cohen kappa (1.00) and PCC (0.92) were higher than the threshold established a priori, so we split the coding of the remaining recording sessions between two coders. Overall, the participants were able to solve each of the 19 usability tasks within a median time of 3 to 11 seconds, and a success rate of 78% to 100% (Table 4).
Table 4.
Time to completion (in seconds) and completion success rate for 19 usability tasks.
| Usability task | Average time, seconds (SD) | Median time, seconds (range) | Success rate (%) | |
| On the Article list format |
|
|
|
|
|
|
Highlight the study arms of the first study | 7 (5) | 5 (3-18) | 83 |
|
|
Highlight the population of the second study | 7 (14) | 3 (1-59) | 89 |
|
|
Access the PubMed abstract of the first study | 7 (6) | 4 (3-21) | 82 |
| This tool provides a textual summary of RCTsa. Please find out how to switch to the text summary of the two listed studies. | 4 (1) | 4 (2-6) | 83 | |
| On the Text summary format |
|
|
|
|
|
|
What is the RCT publication journal and year of the first study? | 7 (6) | 5 (2-26) | 100 |
|
|
Highlight the aim and conclusion of the second study | 3 (1) | 4 (1-6) | 100 |
|
|
This tool also provides comparison views for multiple RCTs. Please switch to the comparison view for the two listed studies. | 4 (3) | 3 (1-13) | 94 |
| On the Comparison table format |
|
|
|
|
|
|
Highlight the study population of the first study | 6 (2) | 6 (2-10) | 83 |
|
|
Highlight the study with the largest sample size | 4 (3) | 4 (1-14) | 100 |
|
|
Highlight the research arms of the second study | 5 (2) | 5 (2-9) | 94 |
|
|
Identify one of the study endpoints reported in both studies | 8 (5) | 7 (3-20) | 83 |
|
|
Within trial 1, which drug therapy resulted in greatest total cholesterol reduction? | 11 (10) | 9 (3-44) | 78 |
|
|
Which drug therapy across the two trials showed the best response in terms of total cholesterol reduction? | 6 (4) | 5 (2-17) | 78 |
|
|
Which drug therapy across the two trials showed the best response in terms of high density lipoprotein increase? | 12 (7) | 11 (4-25) | 100 |
|
|
Highlight the conclusion of the first trial | 4 (5) | 3 (1-23) | 100 |
|
|
This tool also provides graphical visualization of RCTs. Please switch to the graphical view. | 5 (3) | 5 (2-12) | 89 |
| On the Efficacy graph format |
|
|
|
|
|
|
Set the graph to show LDLb outcomes | 4 (2) | 3 (1-8) | 83 |
|
|
Which drug regimen across the two trials showed the greatest reduction in LDL? | 4 (2) | 3 (1-10) | 100 |
|
|
Switch back to the main menu | 3 (1) | 3 (1-6) | 100 |
aRCT: randomized controlled trial.
bLDL: low density lipoprotein.
Figure 3.
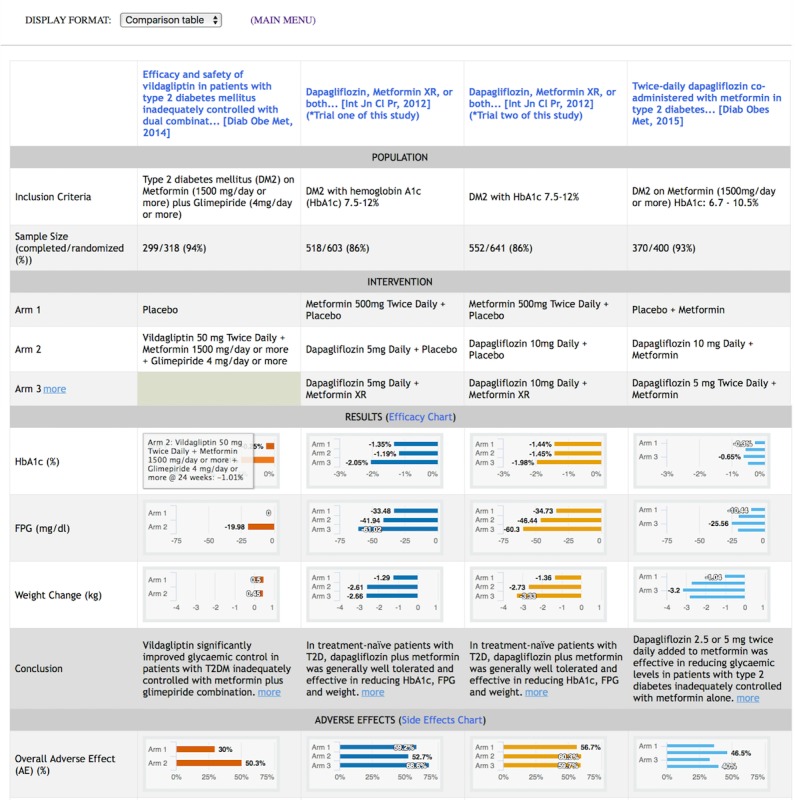
Comparison table display with four trials on various treatments for diabetes mellitus. This display contains key elements of selected studies in a tabular format according to the Population, Intervention, Comparison, Outcomes (PICO) framework [29-32]. Studies are displayed in columns, and attributes of studies are displayed in rows. Study results for primary outcomes and adverse events are represented in bar graphs [46]. Hovering over a bar brings up a callout with details on the intervention of the selected study arm. The scale of each measure is normalized across all studies to enable direct visual comparison. An illustration of the comparison table display for randomized controlled trials on rheumatoid arthritis is available in Multimedia Appendix 1, Figure A2.
Experience with literature searching was modestly correlated with success for the usability tasks (r=0.417, P=.10). A total of 14 out of 20 participants responded to the open-ended comments section, which we analyzed into categories. “Great tool” was the most frequent comment category (6 out of 14 participants), followed by “allow more than 4 studies for comparison” (5 out of 14 participants). Other less frequent comment categories included: request for more features, request for more information, request for clearer display, and prefer narrative abstracts in PubMed (see Multimedia Appendix 1, Table A2).
Clinicians Favored Interactive Visual Displays Over Narrative Abstracts on Perceived Efficiency, Effectiveness, Effort, User Experience, and Preference
The paired t-test results showed that clinicians favored interactive visual displays over narrative abstracts on all of the variables: efficiency t(18)=10.43 (mean 7.86 vs 2.14, respectively), effectiveness t(19)=6.90 (mean 7.36 vs 2.64), effort t(19)=8.24 (mean 7.50 vs 2.50), user experience t(19)=7.94 (mean 7.51 vs 2.49), and preference t(19)=8.62 (mean 8.00 vs 2.00). All differences were significant (P<.001). Figure 5 displays the comparison results.
Figure 5.
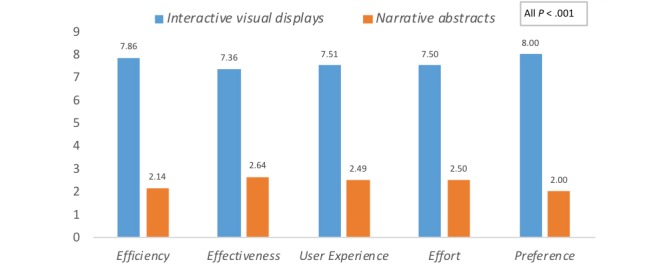
Mean differences for participants’ perceived efficiency, effectiveness, effort, user experience and preference of interactive visual displays versus narrative abstracts.
In addition, participants’ years of expertise, tool presentation order, clinical role, experience with literature searching, and experience with cases in the domain of the vignettes were not correlated with any of the participants’ perception variables (all P values >.05; Multimedia Appendix 1, Table A3), which indicates that there is no need to control for these factors when comparing the difference between the two tools.
Do Clinicians' Perceived User Experience, Efficacy, Effort, and Effectiveness Predict Their Intention to Use Interactive Visual Displays?
A scale for intention to use was created from six variables and had a Cronbach alpha of .971 (Table 2). We regressed intention to use on user experience, efficacy, effort, and effectiveness. The stepwise linear regression, which removes the variable with the highest beta weight sequentially, showed that efficiency was the only item that entered the prediction model (R2(16)=0.661, t=5.59, F(16)=31.26 and P<.001) after controlling for all others.
Discussion
Summary
The goal of this work was to design a novel information display to help clinicians interpret, compare, and apply evidence from RCTs in clinical settings. We previously investigated a static structured PICO table for representing clinical trial reports and found that clinicians preferred a tabular PICO display over PubMed’s default search results display [22]. We choose PubMed’s default search results display as a baseline based on two reasons: (1) PubMed is the most widely used resource for browsing the biomedical literature, including RCT publications; and (2) PubMed is representative of resources in the same category (eg, Ovid, EBSCO, Scopus) since biomedical literature databases rely on the same narrative abstracts provided by biomedical journals. In this current study, we added graphical and interactive features to the previous static structured display and conducted a formative evaluation with 20 physicians in a simulation setting with case vignettes. Our results showed that when interpreting and applying research findings to patient care, physicians strongly preferred interactive visual displays that enable direct comparison of the results from multiple RCTs over narrative abstracts.
Information Processing Issues
Our findings suggest that the cognitive tasks involved in reviewing the literature are perhaps more complex than we had previously been aware. The tasks may involve a compilation of information processing goals (epistemic goals) that vary according to the clinical situation. Human information processing is essentially goal-oriented, so tailoring information to address specific goals is important [47]. Prior work in this area has found that task problem-solving is the most common information need in this context [48]. Our work suggests that displays that show adverse events, results by specific outcomes, and population descriptions by experimental arm match the information processing goals of clinicians seeking research information for medication decision-making.
In addition to exploring information processing goals, our results also suggest the need for further exploration of risky decision-making in work settings. One area that might be particularly fruitful is the well-established and robust findings from research in the “description-experience gap” [49]. This body of research has found large differences in decision-making between choices based on experience versus choices based on the provision of descriptive information. In general, physicians may weigh the probability of a loss (adverse events) and gain (treatment effectiveness) differently when being presented evidence rather than from their experience. Examining how displays can improve the accuracy of decision-making probability estimates is also an area of further research [49].
Information Foraging and information visualization principles (listed in Table 3) guided this study. Participants’ preference for interactive visual displays can be attributed to the following reasons. First, interactive displays, as the central piece of visual analytics [50], provide clinicians with multiple advantages [51,52]. For example, with the interactive functions, only relevant information is presented up-front, and further details can be provided on demand. Second, the use of graphics reduces clinicians’ cognitive effort when interpreting the results of multiple clinical trials [53]. In our displays, users can make direct comparisons both between and within clinical trials on the same display, thereby minimizing working memory overload [54]. Third, the PICO framework has been recommended to clinicians when formulating evidence-based clinical questions [25]. Therefore, our PICO tabular displays provide a consistent structure that is compatible with clinicians’ mental models, facilitating their understanding of the gist of the evidence presented in RCTs [55].
Technology Adoption
According to the technology acceptance model proposed by Davis [56] and expanded on by the unified theory of acceptance and use of technology [57], perceived ease-of-use (PEOU), performance beliefs (ie, how well does it help me do my task), perceived effort, and social norms predict the actual use of a new technology. Findings from our usability study (PEOU) suggest that the prototype is easy to use. Most participants completed the usability task correctly within a short period of time, with minimal training. In the clinical problem-solving session (performance beliefs and effort), participants’ preference of the interactive visual displays was significantly higher than the narrative displays according to several perceived ability measurements. The performance of perceived ability measurements was not correlated with any of the clinicians’ characteristics, suggesting that our finding is generalizable to a different range of users. The within-subjects design with randomized vignette assignment and tool presentation order minimized the impact of the participant’s individual differences. We did not measure social norms. In sum, the interactive visual displays have the potential to ease clinicians' effort to interpret evidence from the primary literature at the point of care.
The stepwise regression analysis of the clinical problem-solving stage showed that efficiency was the only factor that predicted intention to use. Multicollinearity analysis also showed that only one dimension exists, which means that all predicting factors are correlated with each other. It is likely that perceived efficiency or effectiveness is the most general latent variable. It is possible that all of the poststudy questions measure the participants’ general attitude towards the tool with little distinction among factors.
Implications for the Reporting of RCTs
Our study findings add to the growing evidence supporting alternative information display formats to convey the gist of clinical studies [11-22], suggesting that the standard format of scientific reporting, especially for article abstracts, is worth reconsidering. The ideal abstract display format should match clinicians’ mental models to reduce cognitive workload in interpreting clinical study results. Much progress has been made with the increased adoption of structured abstracts, which are more readable, easier to search, preferred by readers, and easier to peer review than traditional unstructured abstracts [58,59]. Our findings suggest that interactive visual displays could further improve the presentation of summaries of clinical studies.
One important challenge in enabling interactive visual displays of clinical studies is the lack of a widely adopted standard data model for reporting study methods and results in a computable format. National clinical trial registries such as ClinicalTrials.gov have taken an important step towards the implementation of structured reporting. However, several challenges still exist, such as automatically extracting key study data from clinical trial registries [60], incomplete linkage between clinical trial publications and clinical trial registration [61], and time delay between clinical trial publication and reporting of results in clinical trial registries [60]. Increasing requirements for structured reporting of clinical trials could be a possible solution. For example, core clinical journals could adopt and require structured reporting of clinical trial results using a common computable data model.
Limitations
This study has several limitations. First, we have not analyzed how much time participants spent looking at each component or piece of information in the information displays. Methods based on eye-tracking devices can be used in future studies to provide deeper insight into how users process the information presented on the screen. Second, the case vignettes did not have a single right or wrong answer, so it was impossible to measure the effect of the interactive visual displays on the accuracy of clinical decisions. Nevertheless, the vignettes were purposefully complex to stimulate a challenging information-seeking experience. Third, in this simulation study, we limited the information displays to 10 studies per case vignette. In real search sessions, the number of studies in a search result can be much higher.
Future Work
The RCT data under the interactive visual displays were manually extracted from a limited set of hand-selected RCTs. Future work is needed to automate the RCT data extraction process, leveraging resources such as ClinicalTrials.gov or RCT data extraction algorithms [60,62-64]. This work is underway, with a prototype currently available. Future studies should also implement the interactive visual displays in clinical settings and investigate their effect on clinicians’ patient care decisions and clinical outcomes.
Conclusion
This study shows that when interpreting and applying research findings to patient care, physicians preferred graphical, interactive, and PICO-framework-based information displays that enable direct comparison of the results from multiple RCTs compared to the traditional narrative format of article abstracts. Future studies should investigate the use of these displays in clinical care settings and their effect on improving clinicians’ patient care decisions and clinical outcomes.
Acknowledgments
This project was supported by National Library of Medicine grants 1R01LM011416-01 and T15LM007124, and National Cancer Institute Grant U24CA204800. We would also like to thank the Richard A Fay and Carol M Fay Endowed Graduate Fellowship for funding support.
Abbreviations
- CONSORT
Consolidated Standards of Reporting Trials
- LDL
low density lipoprotein
- MeSH
medical subject heading
- NASA
National Aeronautics and Space Administration
- PCC
Pearson’s correlation coefficient
- PEOU
perceived ease-of-use
- PICO
Population, Intervention, Comparison, and Outcome
- RCT
randomized controlled trial
- URL
Uniform Resource Locator
- XML
Extensible Markup Language
Supplementary tables and figures, case vignettes, and post evaluation survey.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Del Fiol G, Workman T, Gorman P. Clinical questions raised by clinicians at the point of care: a systematic review. JAMA Intern Med. 2014 May;174(5):710–8. doi: 10.1001/jamainternmed.2014.368.1846630 [DOI] [PubMed] [Google Scholar]
- 2.Leape L, Bates D, Cullen D, Cooper J, Demonaco H, Gallivan T, Hallisey R, Ives J, Laird N, Laffel G. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995 Jul 05;274(1):35–43. [PubMed] [Google Scholar]
- 3.Maviglia S, Yoon C, Bates D, Kuperman G. KnowledgeLink: impact of context-sensitive information retrieval on clinicians' information needs. J Am Med Inform Assoc. 2006;13(1):67–73. doi: 10.1197/jamia.M1861. http://europepmc.org/abstract/MED/16221942 .M1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis J. Why most published research findings are false. PLoS Med. 2005 Aug;2(8):e124. doi: 10.1371/journal.pmed.0020124. http://dx.plos.org/10.1371/journal.pmed.0020124 .04-PLME-E-0321R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook D, Sorensen K, Hersh W, Berger R, Wilkinson J. Features of effective medical knowledge resources to support point of care learning: a focus group study. PLoS One. 2013;8(11):e80318. doi: 10.1371/journal.pone.0080318. http://dx.plos.org/10.1371/journal.pone.0080318 .PONE-D-13-33090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rennels G, Shortliffe E, Stockdale F, Miller P. A computational model of reasoning from the clinical literature. Comput Methods Programs Biomed. 1987 Apr;24(2):139–49. doi: 10.1016/0169-2607(87)90025-3.0169-2607(87)90025-3 [DOI] [PubMed] [Google Scholar]
- 7.Coumou H, Meijman F. How do primary care physicians seek answers to clinical questions? A literature review. J Med Libr Assoc. 2006 Jan;94(1):55–60. http://europepmc.org/abstract/MED/16404470 . [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogendam A, Stalenhoef A, Robbé PF, Overbeke A. Answers to questions posed during daily patient care are more likely to be answered by UpToDate than PubMed. J Med Internet Res. 2008 Oct 03;10(4):e29. doi: 10.2196/jmir.1012. http://www.jmir.org/2008/4/e29/ v10i4e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele RH, Poiro NC, Scalzo DC, Nemergut EC. Speed, accuracy, and confidence in Google, Ovid, PubMed, and UpToDate: results of a randomised trial. Postgrad Med J. 2010 Aug;86(1018):459–65. doi: 10.1136/pgmj.2010.098053.86/1018/459 [DOI] [PubMed] [Google Scholar]
- 10.Balas E, Boren S. Managing clinical knowledge for health care improvement. Yearb Med Inform. 2000;(1):65–70.me00010065 [PubMed] [Google Scholar]
- 11.Perrier L, Persaud N, Thorpe K, Straus S. Using a systematic review in clinical decision making: a pilot parallel, randomized controlled trial. Implement Sci. 2015 Aug 15;10:118. doi: 10.1186/s13012-015-0303-4. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-015-0303-4 .10.1186/s13012-015-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrier L, Kealey M, Straus S. An iterative evaluation of two shortened systematic review formats for clinicians: a focus group study. J Am Med Inform Assoc. 2014 Oct;21(e2):e341–6. doi: 10.1136/amiajnl-2014-002660. http://europepmc.org/abstract/MED/24786378 .amiajnl-2014-002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrier L, Kealey M, Straus S. A usability study of two formats of a shortened systematic review for clinicians. BMJ Open. 2014 Dec 23;4(12):e005919. doi: 10.1136/bmjopen-2014-005919. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=25537782 .bmjopen-2014-005919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G, Oxman A, Akl E, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011 Apr;64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026.S0895-4356(10)00330-6 [DOI] [PubMed] [Google Scholar]
- 15.Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. http://europepmc.org/abstract/MED/18436948 .336/7650/924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa R, Mbuagbaw L, Ikobaltzeta I, De Stio C, McCullagh L, Alonso-Coello P, Meerpohl J, Vandvik P, Brozek J, Akl E, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Guyatt G, Schünemann H. Comparison between the standard and a new alternative format of the summary-of-findings tables in Cochrane review users: study protocol for a randomized controlled trial. Trials. 2015 Apr 16;16:164. doi: 10.1186/s13063-015-0649-6. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-015-0649-6 .10.1186/s13063-015-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langendam M, Akl E, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Syst Rev. 2013 Sep 23;2:81. doi: 10.1186/2046-4053-2-81. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/2046-4053-2-81 .2046-4053-2-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustafa R, Wiercioch W, Santesso N, Cheung A, Prediger B, Baldeh T, Carrasco-Labra A, Brignardello-Petersen R, Neumann I, Bossuyt P, Garg A, Lelgemann M, Bühler D, Brozek J, Schünemann HJ. Decision-making about healthcare related tests and diagnostic strategies: user testing of GRADE evidence tables. PLoS One. 2015;10(10):e0134553. doi: 10.1371/journal.pone.0134553. http://dx.plos.org/10.1371/journal.pone.0134553 .PONE-D-15-03801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch V, Petticrew M, Petkovic J, Moher D, Waters E, White H, Tugwell P, PRISMA-Equity Bellagio group Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): explanation and elaboration. J Clin Epidemiol. 2016 Feb;70:68–89. doi: 10.1016/j.jclinepi.2015.09.001. https://linkinghub.elsevier.com/retrieve/pii/S0895-4356(15)00420-5 .S0895-4356(15)00420-5 [DOI] [PubMed] [Google Scholar]
- 20.Crick K, Wingert A, Williams K, Fernandes R, Thomson D, Hartling L. An evaluation of harvest plots to display results of meta-analyses in overviews of reviews: a cross-sectional study. BMC Med Res Methodol. 2015 Oct 26;15:91. doi: 10.1186/s12874-015-0084-0. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-015-0084-0 .10.1186/s12874-015-0084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong M, Hsu W, Taira R. Evaluating a novel summary visualization for clinical trial reports: a usability study. AMIA Annu Symp Proc. 2016;2016:2007–2015. http://europepmc.org/abstract/MED/28269960 . [PMC free article] [PubMed] [Google Scholar]
- 22.Slager S, Weir C, Kim H, Mostafa J, Del Fiol G. Physicians' perception of alternative displays of clinical research evidence for clinical decision support - a study with case vignettes. J Biomed Inform. 2017 Jul;71S:S53–S59. doi: 10.1016/j.jbi.2017.01.007.S1532-0464(17)30007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirolli P, Card S. Information foraging. Psychological Review. 1999;106(4):643–675. doi: 10.1037/0033-295X.106.4.643. [DOI] [Google Scholar]
- 24.Shneiderman B. The eyes have it: a task by data type taxonomy for information visualizations. Proceedings 1996 IEEE Symposium on Visual Languages; 3-6 Sept. 1996; Boulder, CO, USA. IEEE Comput Soc Press; 1996. Sep 03, pp. 336–343. [DOI] [Google Scholar]
- 25.Schardt C, Adams M, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007 Jun 15;7:16. doi: 10.1186/1472-6947-7-16. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-7-16 .1472-6947-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huberman BA, Pirolli PL, Pitkow JE, Lukose RM. Strong regularities in world wide web surfing. Science. 1998 Apr 03;280(5360):95–7. doi: 10.1126/science.280.5360.95. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=9525865 . [DOI] [PubMed] [Google Scholar]
- 27.Holling C. Some characteristics of simple types of predation and parasitism. Can Entomol. 2012 May 31;91(07):385–398. doi: 10.4039/ent91385-7. [DOI] [Google Scholar]
- 28.Burns P, Rohrich R, Chung K. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011 Jul;128(1):305–10. doi: 10.1097/PRS.0b013e318219c171. http://europepmc.org/abstract/MED/21701348 .00006534-201107000-00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Schulz K, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001 Apr 14;357(9263):1191–4.S0140673600043373 [PubMed] [Google Scholar]
- 30.Flaherty R. A simple method for evaluating the clinical literature. Fam Pract Manag. 2004 May;11(5):47–52. http://www.aafp.org/link_out?pmid=15162611 . [PubMed] [Google Scholar]
- 31.Dawes M, Pluye P, Shea L, Grad R, Greenberg A, Nie JY. The identification of clinically important elements within medical journal abstracts: Patient-Population-Problem, Exposure-Intervention, Comparison, Outcome, Duration and Results (PECODR) Inform Prim Care. 2007;15(1):9–16. http://hijournal.bcs.org/index.php/jhi/article/view/640 .640 [PubMed] [Google Scholar]
- 32.Kim SN, Martinez D, Cavedon L, Yencken L. Automatic classification of sentences to support evidence based medicine. BMC Bioinformatics. 2011 Mar 29;12 Suppl 2:S5. doi: 10.1186/1471-2105-12-S2-S5. https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-12-S2-S5 .1471-2105-12-S2-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MEDLINE/PubMed via PICO. 2018. [2013-02-15]. http://pubmedhh.nlm.nih.gov/nlmd/pico/piconew.php .
- 34.NinjaMock. 2018. [2018-03-21]. https://ninjamock.com/s/XCTXS .
- 35.Wix Interactive visual displays in Wix. 2018. [2018-03-21]. http://drprasad30.wixsite.com/ksviz .
- 36.Highcharts API. 2018. [2012-11-05]. http://www.highcharts.com/
- 37.Interactive visual displays website. 2018. [2018-03-21]. http://lite.bmi.utah.edu/rctcomp/index.html .
- 38.Medscape. 2016. Options for patients with newly diagnosed rheumatoid arthritis who do not adequately respond to methotrexate http://www.medscape.org/viewarticle/574579 .
- 39.Hirsch I, Molitch M. Clinical decisions. Glycemic management in a patient with type 2 diabetes. N Engl J Med. 2013 Oct 03;369(14):1370–2. doi: 10.1056/NEJMclde1311497. [DOI] [PubMed] [Google Scholar]
- 40.Ely J, Osheroff J, Ebell M, Bergus G, Levy B, Chambliss M, Evans E. Analysis of questions asked by family doctors regarding patient care. BMJ. 1999 Aug 07;319(7206):358–61. doi: 10.1136/bmj.319.7206.358. http://europepmc.org/abstract/MED/10435959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. https://linkinghub.elsevier.com/retrieve/pii/S1532-0464(08)00122-6 .S1532-0464(08)00122-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooke J. SUS - a quick and dirty usability scale usability and context. 1996. [2018-03-21]. https://hell.meiert.org/core/pdf/sus.pdf .
- 43.Hart S, Staveland L. Development of NASA-TLX (Task Load Index): results of empirical and theoretical research. In: Hancock P, Meshkati N, editors. Human Mental Workload. Amsterdam, Netherlands: Elsevier; 1988. pp. 139–183. [DOI] [Google Scholar]
- 44.IBM Corp. 2018. [2018-04-23]. IBM SPSS Statistics for Windows, Version 24 http://www-01.ibm.com/support/docview.wss?uid=swg24041224 .
- 45.ATLAS.ti. 2018. [2018-06-06]. https://atlasti.com/
- 46.Spence I, Lewandowsky S. Displaying proportions and percentages. Appl Cognit Psychol. 1991 Jan;5(1):61–77. doi: 10.1002/acp.2350050106. [DOI] [Google Scholar]
- 47.Xie I. Dimensions of tasks: influences on information‐seeking and retrieving process. J Doc. 2009 Apr 24;65(3):339–366. doi: 10.1108/00220410910952384. [DOI] [Google Scholar]
- 48.Agarwal N. Synthesis Lectures on Information Concepts, Retrieval, and Services. San Rafael, CA: Morgan & Claypool Publishers; 2017. Dec 08, Exploring context in information behavior: seeker, situation, surroundings, and shared identities; pp. i–163. [DOI] [Google Scholar]
- 49.Lejarraga T, Pachur T, Frey R, Hertwig R. Decisions from experience: from monetary to medical gambles. J Behav Dec Making. 2015 Apr 17;29(1):67–77. doi: 10.1002/bdm.1877. [DOI] [Google Scholar]
- 50.Thomas J, Cook K, editors. Illuminating the Path: The Research and Development Agenda for Visual Analytics. Richland, WA: National Visualization and Analytics Center; 2005. [Google Scholar]
- 51.Card S, Mackinlay J, Shneiderman B. Readings in Information Visualization: Using Vision to Think. San Francisco, CA: Morgan Kaufmann Publishers; 1999. Jan, [Google Scholar]
- 52.Robertson G, Czerwinski M, Fisher D, Lee B. Selected human factors issues in information visualization. Rev Hum Factors Ergon. 2009 Sep;5(1):41–81. doi: 10.1518/155723409X448017. [DOI] [Google Scholar]
- 53.Vekiri I. What is the value of graphical displays in learning? Educ Psychol Rev. 2002;14(3):261–312. doi: 10.1023/A:1016064429161. [DOI] [Google Scholar]
- 54.Mignini L, Champaneria R, Mishanina E, Khan K, EBM-CONNECT Collaboration Graphical displays for effective reporting of evidence quality tables in research syntheses. Reprod Health. 2016 Mar 09;13:21. doi: 10.1186/s12978-016-0130-3. https://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-016-0130-3 .10.1186/s12978-016-0130-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pirolli P. Information Foraging Theory: Adaptive Interaction with Information. Oxford, United Kingdom: Oxford University Press, Inc; 2007. [DOI] [Google Scholar]
- 56.Davis FDD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly. 1989 Sep;13(3):319. doi: 10.2307/249008. [DOI] [Google Scholar]
- 57.Venkatesh V, Morris M, Davis G, Davis F. User acceptance of information technology: toward a unified view. MIS Quarterly. 2003;27(3):425. doi: 10.2307/30036540. [DOI] [Google Scholar]
- 58.Hartley J. Current findings from research on structured abstracts. J Med Libr Assoc. 2004 Jul;92(3):368–71. http://europepmc.org/abstract/MED/15243644 . [PMC free article] [PubMed] [Google Scholar]
- 59.Hartley J. Current findings from research on structured abstracts: an update. J Med Libr Assoc. 2014 Jul;102(3):146–8. doi: 10.3163/1536-5050.102.3.002. http://europepmc.org/abstract/MED/25031553 .JMLA-D-13-00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim H, Bian J, Mostafa J, Jonnalagadda S, Del Fiol G. Feasibility of extracting key elements from ClinicalTrials.gov to support clinicians' patient care decisions. AMIA Annu Symp Proc. 2016;2016:705–714. http://europepmc.org/abstract/MED/28269867 . [PMC free article] [PubMed] [Google Scholar]
- 61.Huser V, Cimino JJ. Linking ClinicalTrials.gov and PubMed to track results of interventional human clinical trials. PLoS One. 2013;8(7):e68409. doi: 10.1371/journal.pone.0068409. http://dx.plos.org/10.1371/journal.pone.0068409 .PONE-D-13-17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu W, Speier W, Taira RK. Automated extraction of reported statistical analyses: towards a logical representation of clinical trial literature. AMIA Annu Symp Proc. 2012;2012:350–9. http://europepmc.org/abstract/MED/23304305 . [PMC free article] [PubMed] [Google Scholar]
- 63.Summerscales R, Argamon S, Bai S, Hupert J, Schwartz A. Automatic summarization of results from clinical trials. IEEE Int Conf Bioinforma Biomed Internet IEEE; 2011; Atlanta, GA, USA. IEEE; 2011. Nov 12, pp. 372–377. [DOI] [Google Scholar]
- 64.Kang T, Zhang S, Tang Y, Hruby GW, Rusanov A, Elhadad N, Weng C. EliIE: An open-source information extraction system for clinical trial eligibility criteria. J Am Med Inform Assoc. 2017 Nov 01;24(6):1062–1071. doi: 10.1093/jamia/ocx019.3098256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures, case vignettes, and post evaluation survey.


