Abstract
Obese, type 2 diabetics (T2D) are at an increased risk of foot infection with impaired immune function believed to be a critical factor in the infectious process. Here we test the hypothesis that humoral immune defects contribute to exacerbated foot infection in a murine model of obesity/T2D. C57BL/6J mice were rendered obese and diabetic by a high-fat diet for 3 months, and compared to controls receiving a low-fat diet. Following injection of Staphylococcus aureus into the footpad, obese/T2D mice had greater foot swelling and reduced S. aureus clearance than controls. Obese/T2D mice also had impaired humoral immune responses as indicated by lower total IgG levels and lower anti-S. aureus antibody production. Within the draining popliteal lymph nodes (PLN) of obese/T2D mice, germinal center formation was reduced and percentage of germinal center T and B cells was decreased by 40–50%. Activation of both T and B lymphocytes was similarly suppressed in obese/T2D mice. Impaired humoral immunity in obesity/T2D was independent of active S. aureus infection, as a similarly impaired humoral immune response was demonstrated when mice were administered a S. aureus digest. Isolated splenic B cells from obese/T2D mice activated normally, but had markedly suppressed expression of Aicda, with diminished IgG and IgE responses. These results demonstrate impaired humoral immune responses in obesity/T2D including B-cell specific defects in antibody production and class switch recombination. Together, the defects in humoral immunity may contribute to the increased risk of foot infection in obese/diabetic patients.
Introduction
Obesity and type 2 diabetes (T2D) are among the greatest risk factors for infection (1). Obese, T2D patients have a 25% lifetime risk of developing a foot ulcer (2), and approximately 56% of these become infected (3, 4). While infections are common among those with obesity/T2D, the only preventative treatments are reducing body weight to a healthy range (body mass index <30) and avoiding skin ulceration. There is currently no known medical treatment that proactively improves immune responses and lessens infection risk in this population. Importantly, the consequenes of a foot infection are catastrophic, causing substantial physical, emotional, and financial stress. Twenty percent of foot infections result in amputation, the most feared consequence of a foot ulcer (5). Since obese/T2D patients are disproportionally effected by foot infection, new therapies are needed to proactively reduce the risk of infection in this population.
It is generally accepted that diabetes increases the risk of foot ulcers via two primary mechanisms: impaired vascular flow and peripheral neuropathy (2). However, these factors alone do not fully explain the increased risk of infection in this population. Multiple studies have shown obesity to be a risk factor for infection in surgical sites (1, 6). Our studies have shown that obesity, but not hyperglycemia, impairs humoral immune responses to orthopaedic Staphylococcus aureus infection (7). Other studies have demonstrated impaired innate (8, 9) and adaptive immune responses in obesity (7, 10). However, the underlying mechanisms that alter immune function in obesity remain elusive. Much effort has focused on macrophages and neutrophils, demonstrating impaired phagocytosis and bacterial killing despite increased cell recruitment (8, 9, 11). We have previously demonstrated impaired antibody production in obese/T2D mice in a model of orthopaedic implant associated infection, a result that was confirmed in patients infected with S. aureus (7). Recent studies have demonstrated reduced T cell response upon challenge in obese patients despite increased pre-challenge activation (12). Others have demonstrated reduced antibody titers following vaccination in obese individuals (13, 14), an effect that results in increased mortality following infection in mice (15). However, the effect of obesity/T2D on humoral immune responses is largely understudied.
In the absence of infection the immune system of the obese host is in a state of chronic inflammation (16, 17). Importantly, B lymphocytes are known to participate in this chronic inflammatory state. B lymphocytes have been shown to produce IgG auto-antibodies (16) and secrete proinflammatory cytokines (18) within the adipose tissue of high fat-fed mice, propagating inflammation and contributing to insulin resistance. How chronic activation of the immune system in obesity/T2D, particularly involving B cells, impacts normal of B lymphocyte function during an infection is largely unknown.
Together, these previous studies suggest that obesity increases the risk of foot infection in obese/T2D patients by altering immune function. Here, we expand on our previous finding of impaired humoral immunity in obese/T2D, and test the hypothesis that impaired antibody production in obesity is driven, at least in part, by impaired B lymphocyte function.
Materials and Methods
Animals
All handling of mice and associated experimental procedures were reviewed and approved by the University Committee on Animal Resources at the University of Rochester Medical Center. Male C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME) were housed five per cage in one-way housing on a 12-hour light/dark cycle at the University of Rochester. To model obesity and T2D, mice were placed on a high fat (60% kcal, D12492) or low-fat (10% kcal D12450J) diet at five weeks of age (Open Source Diets, Research Diets Inc., New Brunswick, NJ). Prior to infection, fasting blood glucose levels were measured using One Touch glucose meters (Lifescan Inc., Milpitas, CA) after an overnight fast. Glucose tolerance testing was also performed on mice fasted overnight. Lean and HF-fed mice had an average fasting blood glucose of 120mg/dL and 190mg/dL, respectively.
Infection model
Following feeding, chronic foot infections were established. S. aureus (UAMS-1) was cultured overnight, washed, and diluted 1:10 in sterile saline. Mice were given 60mg/kg ketamine and 4mg/kg xylazine as well as a pre-operative dose of buprenorphine. Mice were then injected in the foot with 60μL of the diluted S. aureus. Immediately before and following the injection, the diameter of the footpad was measured using digital calpiers (Mitutoyo, Japan). All feet returned to the original size within 2 hours of injection. Feet were also measured at indicated time points. S. aureus digest: UAMS-1 Δspa was digested at 37°C in the presence of lysozyme and lysostaphin (Sigma) for 3 hours in PBS. The digest was then washed prior to combination with alum (ThermoFisher) per manufacturer’s instructions. Following suspension with alum, 50μL of the S. aureus digest was immediately injected into the footpad of control or obese/T2D mice. Immunogenicity of the digest was confirmed prior to suspension in alum via an ELISA using serum from previously infected mice. Complete S. aureus killing in the digest was confirmed prior to alum suspension by plating on TSB agar plates overnight at 37°C.
Colony Forming Units
S. aureus was isolated and colony forming units quantitated from infected footpads at sacrifice. Necrotic soft tissue surrounding the infection site was placed in 2mL of PBS. Tissues were then homogenized using an IKA T-10 handheld homogenizer (Wilmington, NC). Serial dilutions were then prepared in PBS, and 100uL aliquots were plated on tryptic soy agar. Plates were then placed at 37C for 24 hours before counting colonies.
Histological analysis
Lymph nodes were harvested after sacrifice and fixed overnight in 4% neutral buffered formalin. Samples were then washed, processed, embedded in paraffin, and 5 micron transverse sections were prepared. Immunofluorescent staining: sodium citrate (Dako, Carpinteria CA) was diluted per manufacturer’s instructions and slides were incubated at 95°C - for 40 minutes. Following antigen retrieval, blocking was performed with 10% donkey serum in PBS for 2 hours at RT. Primary antibodies: APC rat anti-mouse-CD45R/B220 (BD Biosciences, San Jose USA), lectin from Arachis hypogaea (PNA) FITC (Sigma, St. Louis USA), and mouse anti-activation induced cytidine deaminase (Thermo Fisher Scientific, Waltham MA) at 1:200 in PBS were incubated overnight at 4 °C. Anti-fluorescein conjugated to Alexafluor 488 (ThermoFisher) or mIgGκ BP-CFL 594 (Santa Cruz, Dallas USA) were used at 1:200 for 1 hour in tris-buffered saline, followed by DAPI staining (ThermoFisher), and fixation using ProLong gold antifade mounting reagent (Life Technologies, Eugene, OR). All slides were visualized using an Olympus VS120 virtual slide scanning microscope with Olympus OlyVIA software. Quantification: Germinal centers were quantitated by a blinded observer by counting the number of PNA+ B220+ areas within the lymph node. Germinal center size and anti-AID staining were determined using Visiopharm software (Brookfield, CO).
Flow cytometry
Lymph nodes were harvested and passed through a 70μM nylon strainer (Corning Inc, Corning NY USA) in ice cold PBS with 1% fetal bovine serum. Cells were then pelleted and stained with fixable viability stain v450 (BD) for 40 minutes. Following washing, Fc block (rat anti-mouse CD16/CD32) was performed for 5 minutes prior to staining. The following antibodies were used for phenotyping of cells: BD Biosciences (San Jose, CA) - BV711 rat anti-mouse IL-10, BV650 rat anti-mouse CD8a, BV605 rat anti-mouse IL-4, PerCP-Cy 5.5 rat anti-mouse IFN-gamma, APC-Cy 7 rat anti-mouse CD19, FITC hamster anti-mouse CD69, PerCP-Cy5.5 rat anti-mouse Ly-6G and Ly-6C, PE rat anti-mouse GL-1, Alexa fluor 700 rat anti-mouse CD3, Per-CP Cy5.5 hamster anti-mouse CD69, BV711 rat anti-mouse F4/80, BB515 rat anti-mouse GL7, Alexa Fluor 700 rat anti-mouse CD4, and Alexa fluor 647 mouse anti-mouse RoR gamma T. E Bioscience (San Diego, CA) - PE-Cy7 anti-mouse CD11c and PE anti-mouse CD11b.
Super folding variant-GFP UAMS-1 S. aureus was used for cytometric analyses on infected feet. Intracellular staining was performed using a cell fixation/permeabilization kit (BD Biosciences). All analysis was performed on a BD LSR II 18-color flow cytometer (BD Biosciences). Compensation was completed using anti-mouse or anti-rat/hamster compensation control beads (BD). Fluorescent minus one (FMO) controls were used to determine positive gating for all antibodies except for CD19, CD3, CD4, and CD11b. All FMO controls were performed on pooled samples from both control and obese/T2D mice. Gating strategies are outlined in Supplemental Figure 1.
Serum analysis
Total IgG and IgM assays: 2μg/mL goat anti-mouse IgG and IgM (Southern Biotech, Birmingham, Al) were coated onto 96 well plates (Nunc 442404). After blocking in 3% BSA for 1hr, serum at 1:10,000 was added to wells (100μL) and incubated at 4°C for 1 hr. After 5 washes with PBST, secondary antibody (anti-IgG or anti-IgM; Southern Biotech) conjugated to HRP was added at a dilution of 1:4000 and incubated for 1 hour at 4°C. KPL Sureblue peroxidase was used as a substrate (52-00-01, Gaithersburg, MD). For measuring in vitro antibody production, the same method was used with 100μL of undiluted conditioned media after 48h of stimulation.
Anti- S. aureus ELISA assay
S. aureus extracts were prepared by incubating 1.0mL of an overnight culture of ΔSpa UAMS-1 S. aureus with 20mg of lysozyme (Sigma) and 1mg lysostaphin (Sigma) in sterile water. Extract was then used as a coating antigen in an ELISA at 1:2000 in PBS. After blocking and washing in PBST, serum was added at 1:10 followed by IgG or IgM secondary conjugated to HRP, followed by incubation with KPL Sureblue. Absorbance was measured on a plate reader at 450nm. Results are reported as arbitrary units by dividing the absorbance of each sample by the mean absorbance of lean mice.
RNA extraction and sequencing
RNA sequencing was performed on naive lymph nodes. Following sacrifice, lymph nodes were harvested, immediately flash frozen, and stored at −80°C. Prior to extraction of RNA, lymph nodes were pulverized at liquid nitrogen temperatures in Lysing Matrix B tubes (MP Biomedicals). RNA was then extracted by mechanical lysis in ice-cold acid phenol using the Fast-Prep-24 instrument (MP Biomedicals) at a setting of 5.5 for 40s. Extracted RNA was purified using RNeasy mini-columns (Life Technologies). Contaminating genomic DNA was removed using TurboDNase (Ambion). Illumina library construction for RNAseq: Libraries were prepared from rRNA-depleted RNA (Ribo-Zero; Epicenter) using the ScriptSeq v2 RNAseq library preparation kit (Epicentre) according to the manufacturer’s instructions. Libraries were sequenced on a Illumina HiSeq2500 platform with 4–5 libraries multiplexed per lane and ~50 million 100nt single-direction reads for each sample. Initial sequence data from the HiSeq2500 was evaluated for quality. The low quality sequence reads were removed prior to final analysis using Seqclean (http://sourceforge.net/projects/seqclean/). The remaining high quality processed reads were then mapped or aligned to reference mus musculus genomes with SHRiMP version 2.2.3 (19) to match sequence reads to specific mouse genes. Differential expression levels of all genes were determined using Cufflinks (cuffDiff2) version 2.0.2 (20) and a false discovery rate (FDR) of 0.05, which calculates gene expression as the number of sequence reads that map to each gene. A greater than log2-fold increase or decrease in expression level was used to identify genes that were significantly different between control and obese/T2D mice. Three mice were used per group for RNAsequencing experiments. All RNA-Seq data (raw sequence reads and primary analysis) have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series Accession number GEO113075 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113075). Enrichment analysis was performed using Igenuity Pathway Analysis software (Qiagen, Redwood City CA) and ensemble annotation software (21).
RT-qPCR
RNA samples were DNase treated (Invitrogen, Carlsbad CA) and 15μL of RNA was then added to iScript cDNA synthesis per manufacturer’s instructions (BIO-RAD, Hercules CA). 1.5μL of cDNA was then added to 7μL water, 10μL of SYBR green (Quanta Biosciencesm, Gaithersburg MD) and 1.5μL of forward and reverse primer mix. PCR was carried out on a Rotor-Gene Q (Qiagen, Hilden Germany) RT-PCR machine. Primers: Aicda forward: AAGAATGCACGATCGCCTCT, reverse: CTTTGGATCTGGGCGCTTTG. BCl6 forward: CACACCCGTCCATCATTGAA, BCL6 reverse: TGTCCTCACGGTGCCTTTTT Rpl32 forward: AAGCGAAACTGGCGGAAAC reverse: TCAGGATCTGGCCCTTGAACC, Actin forward GCTACAGCTTCACCACCACA reverse: GGGGTGTTGAAGGTCTCAAA
Splenic B cell isolation and stimulation
Following 12 weeks on diet, spleens were harvested and passed through a 70μM nylon filter in sterile, ice cold phosphate buffered saline. B lymphyctes were isolated using an EasySep negative selection mouse B cell enrichment kit (Stem Cell Technologies, Vancouver, CA) per the manufacturer’s instructions. B lymphocytes were then plated at 1x106 cells/mL in 24 well plates and cultured with RPMI 1640 (life Technologies, Grand Island USA), supplemented with gentamycin and 10% FBS. Cells were then stimulated with 20ng/mL S. aureus peptidoglycan (Sigma-Adlrich), 1μg/mL CD40ligand + 20ng/mL IL-4 + anti-HA, or anti-mouse IgM.
Statistics
Multiple analyte comparisons were measured using two-way ANOVA and Bonferroni’s post test. Unpaired t-test was used when two groups were compared, including area under the curve measurements. Mann-Whitney U test was used for non-normally distributed data. All statistics were analyzed using Graphpad Prism.
Results
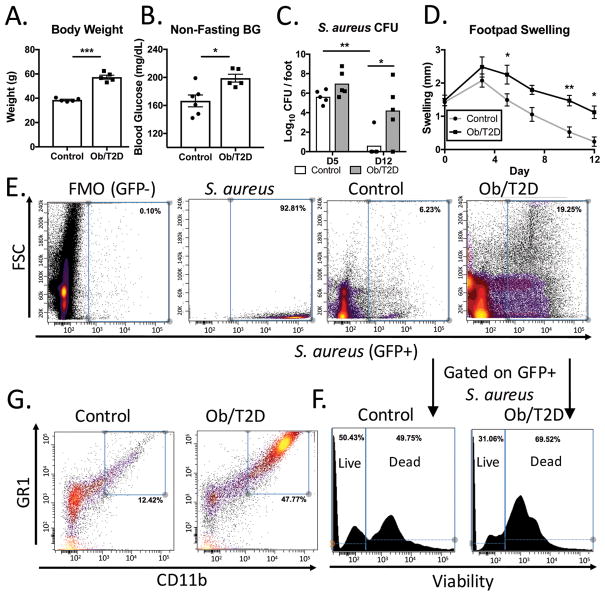
Mice fed a high fat-diet for three months developed the hallmarks of obesity and T2D (Figure 1A, B) (7, 22). A foot infection was then initiated in the obese mice and lean controls by injecting S. aureus into the mouse footpad. At day 5 post infection, there was a trend towards impaired clearance of S. aureus from the footpads of obese/T2D mice (Figure 1C). By day 12, infections persisted in the footpads of obese/T2D mice, but were cleared in all but one control mouse (Figure 1C). Footpad swelling was measured throughout the infection as a marker of inflammation and immune cell migration to the infection site. The high fat-fed obese mice had increased footpad swelling as early as day 5 post-infection (Figure 1D). By day 12, significant foot swelling remained in obese/T2D mice, but swelling had resolved in control mice (Figure 1D). These results are similar to a study in a genetic model of obesity that reported increased immune cell infiltration into infected footpads of obese mice and impaired bacterial clearance (11).
Figure 1. Impaired S. aureus clearance and increased inflammatory cell infiltration in obesity/T2D.
C57BL6/J mice were fed a diet consisting of 60% Kcal from fat or 10% Kcal from fat for 3 months. Mice where then A. weighed and B. non-fasting blood glucose was measured prior to footpad infection with S. aureus. C. Infected mice were harvested for CFU quantitation at day 5 and day 12 (n=5 for each group). D. Footpad swelling was measured as a readout for immune cell infiltration. Control and obese/T2D mice were infected with SF-GFP S. aureus, harvested at day 7, and pooled for flow cytometry. E. Gaiting on GFP+ S. aureus indicated an increased proportion of S. aureus associated with host cells in infected footpads of obese/T2D mice F. Further gaiting on the GFP+ S. aureus population with a mammalian specific viability dye revealed an increased proportion of S. aureus in dead cells of obese/T2D mice. G. Proportion of non-infected live cells (Gated on FSC, SSC and viability) that were granulocytes. *p<.05, ** p<.01 *** p <.001. A–C unpaired student T test. D. Two way ANOVA and Bonferroni post-hoc analysis. Each point represents one mouse, error bars represent SEM. E–G are pooled specimens from 6 mice and are representative of 2 independent experiments.
To track the fate of S. aureus in the obese and lean mice, footpads were infected with SF-GFP UAMS-1 S. aureus, a GFP-expressing isolate. At day 7 post-infection, footpad tissue was harvested, pooled, stained, and analyzed by flow cytometry. Consistent with increased CFU, there was a considerably greater proportion of fluorescence associated with GFP+ S. aureus in samples from obese/T2D mice (19%) compared to lean-fed controls (6%) (Figure 1E). Interestingly, a substantial portion of GFP+ S. aureus were bound or internalized by host cells in obesity/T2D, as indicated by association with high FSC (cell size). Further analysis of the GFP+ S. aureus population in obese/T2D mice revealed that the large majority of bacteria were observed in non-viable cells (69%). In contrast, only half of remaining GFP+ S. aureus were within non-viable cells of lean mice (Figure 1F). Despite the greater S. aureus burden in obese/T2D mice, the largest proportion (47% of total cells) of non-infected live cells were CD11b+ Gr-1+ granulocytes (Figure 1G). In contrast, only 12% of the live cell fraction in control mice was composed of granulocytes (Figure 1G). Together, the data suggest that obesity/T2D is associated with impaired innate immune responses to S. aureus footpad infection.
Impaired humoral immune response and germinal center formation in obesity/T2D
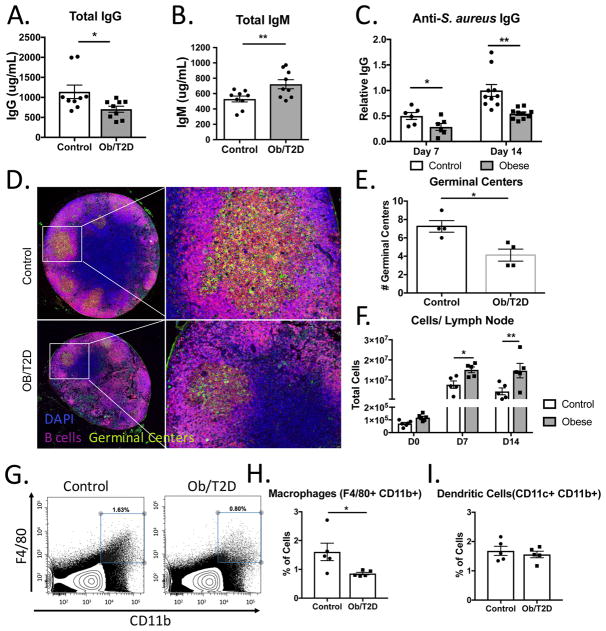
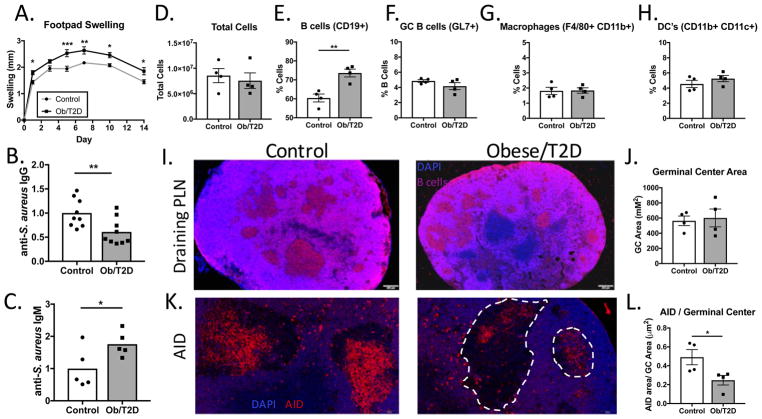
We previously demonstrated an impaired humoral immune response in obese/T2D mice to an S. aureus orthopaedic implant infection (7). In the footpad infection model, obese/T2D mice again displayed impaired total IgG, increased total IgM, and reduced anti-S. aureus IgG levels (Figure 2A–C). Consistent with reduced anti-S. aureus IgG levels, immunofluorescent staining (B220+, peanut agglutinin+) revealed reduced germinal center formation in popliteal lymph nodes (PLN) from obese/T2D mice at D14 post-infection (Figure 2D, E). Diminished germinal center formation in obesity/T2D led to further examination of cell populations within the draining PLN by flow cytometry. Approximately 100-fold more cells were isolated from the draining PLN of both control and obese mice at days 7 and 14 post-infection compared to non-infected mice (Figure 2F) with marked increases in B and T lymphocytes (Figures 3B, 4B). However, PLN from obese/T2D mice consistently yielded larger cell numbers than controls. Thus, the reduction in antibody production and germinal center formation in PLN from infected obese/T2D mice was associated with increased cellularity. Despite increased total cell numbers, the proportion of macrophages (F4/80+ CD11b+) in the lymph nodes was lower in obese/T2D mice (Figure 2G, H). There was no difference in the percentage of neutrophils (not shown) or dendritic cells within the draining PLN in obese/T2D mice compared to controls (Figure 2I). Thus, obesity/T2D was associated with impaired germinal center formation and antibody production, a phenotype that is linked with increased lymph node cellularity and impaired macrophage infiltration.
Figure 2. Impaired humoral immune responses and germinal center formation in obese/T2D mice.
Mice (n=6–10) were infected in the footpad with S. aureus. At 14d, A. total IgG and B. total IgM, and C. anti-S. aureus IgG were measured by ELISA. Mice (n=4) were sacrificed at d14 and lymph nodes were isolated for histological staining. D. Representative fluorescent micrographs obtained at 2x and 20x magnification of medial sections of lymph nodes from control and obese/T2D mice (n=4) stained with DAPI (blue, cells), B220 (pink, B cells), and peanut agglutinin (green, PNA; germinal centers). E. is quantitation of germinal center numbers per lymph node. F. Popliteal lymph nodes (n=4–5 mice) were isolated for flow cytometry, and total cells were quantitated prior to infection, 7d and 14d post-infection. G. Representative surface staining for macrophages in the draining popliteal lymph node 7d post-infection. H. Quantitation of G. I. Quantitation of total dendritic cells in draining popliteal lymph node. *p<.05, ** p<.01. unpaired student T test except for F., Two way ANOVA and Bonferroni post-hoc analysis. Quantification in E. is from lymph nodes from 4 mice. Each data point represents an average of 3 sections per node, all sections separated by 100–150um.
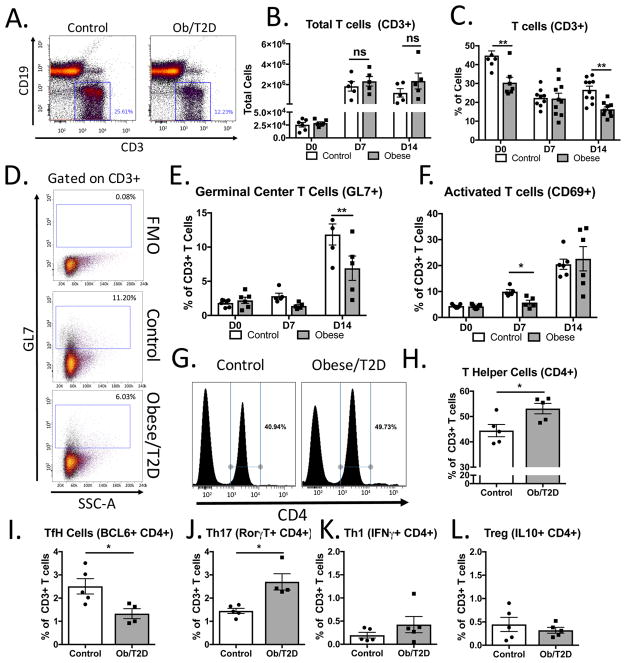
Figure 3. Impaired T cell activation and polarization in obese/T2D mice.
Mice (n=4–7 per group) were infected in the footpad with S. aureus. Draining popliteal lymph nodes were harvested, stained, and analyzed by flow cytometry. A. Representative plots 14d post-infection of CD19+ B cells and CD3+ T cells. B. Quantitation of CD3+ T cells from A. C. % of total T cells from draining PLN in A. D. Representative images of gating strategy for germinal center T cells (CD3+, CD4+, GL7+). E. Quantitation of germinal centers before, 7 and 14d post-infection. F. Quantitation of CD69+ activated T cells. G. Representative surface staining for CD4 demonstrating increased CD4+ T cells in obese/T2D mice. H. Quantitation of G. Intracellular cytokine and transcription factor staining of T cells was quantitated from poplitaeal lymph nodes of infected mice in G. I. BCL6+ staining of TfH cells, J. Th17 cells, K. T regulatory cells, and L. Th1 cells (all gates on CD3+ CD4+ cells). *p<.05, ** p<.01. unpaired student T test (H–L). Others were analyzed by two way ANOVA and Bonferroni post-hoc analysis.
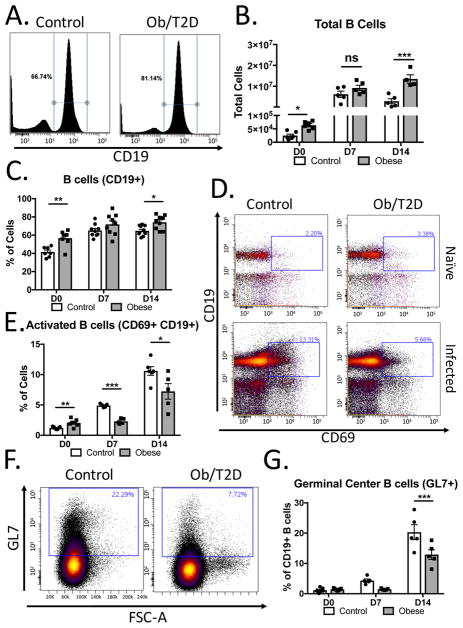
Figure 4. Impaired B cell activation and reduced germinal center B cells in obese/T2D mice.
Mice (n=5–7 per group) were infected in the footpad with S. aureus. Draining popliteal lymph nodes were harvested, stained, and analyzed by flow cytometry. A. Representative plots 14d post-infection of B cells (CD19+). B. Quantitation of B cells in A. C. % of total B cells from A. D. Representative images of gating strategy for activated B cells(CD19+, GL7+). E. Quantitation of activated B cells from D. F. Representative plots of germinal center (Gl7+) B cells (gated on CD19). G. Quantitation of F. on uninfected, d7 infected and d14 infected.*p<.05, ** p<.01. Two way ANOVA and Bonferroni post-hoc analysis.
Obesity/T2D is associated with impaired T cell activation, germinal center T cell recruitment, and T cell polarization
Since obesity was associated with reduced germinal center formation, we next sought to determine the effect of obesity/T2D on T lymphocytes within the draining PLN. As with total cells (Figure 2F), there was a robust increase in CD3+ T cells in the draining PLN from day 0 to day 7, persisting to day 14 post-infection (Figure 3A, B) in both control and obese/T2D mice. In contrast to the difference in total cellularity, no differences in total T cells were noted between obese/T2D and control mice at any of the time points (Figure 3B). Moreover, the number of T cells persisted from D7 to D14 in both cohorts. Interestingly, despite the same number of total T cells, obese/T2D mice had a reduced proportion of CD3+ T cells at day 0 and day 14 post-infection (Figure 3C) compared to control mice. 25% of the total cells were composed of T lymphocytes at day 14 in lean controls, as opposed to only 12% in the obese/T2D. This, coupled with the same number of total T cells in each group, indicates increased infiltration or proliferation of other cell types into the PLN in obese/T2D mice.
A reduction in germinal centers in obesity/T2D (Figure 2D, E) led us to speculate that germinal center T cells would also be reduced in this population. Consistent with this reasoning, PLN from obese/T2D mice had 50% fewer CD4+ GL7+ germinal center T cells compared to controls 14 days post-infection (Figure 3D, E). We also noted a trend towards decreased germinal center T cells at day 7 post-infection in obese/T2D mice (Figure 3E). We next sought to establish if the reduction in germinal center T cells in obesity/T2D was a result of impaired T cell activation and antigen presentation. Consistent with this, obese/T2D mice had a 50% decrease in activated T cells compared to lean-fed controls by day 7 post-infection (Figure 3F). This effect was transient however, as the percentage of activated T cells in obese/T2D mice normalized to that of the lean control by day 14. Together, these results associate impaired T cell activation with reduced germinal center T cells in the PLN of obese/T2D mice following footpad S. aureus infection.
Previous studies have established enhanced T cell polarization and recruitment to the adipose tissue in obesity. Adipose tissue inflammation and insulin resistance have been implicated with Th1 and Th17 cell polarization and infiltration (23, 24). Therefore, we speculated that distorted T cell polarization due to a high fat-diet and obesity would be further propagated following S. aureus infection. To this end, we further examined T helper cell subsets within the draining PLN 14 days post-infection. Interestingly, obesity/T2D was associated with an increase in CD4+ T helper cells compared to control mice (Figure 3G, H). Having established reduced germinal center T cells in obesity/T2D, we next examined T follicular helper (TfH) cell subsets. There was a 50% reduction in BCL6+ TfH cells in obese/T2D mice compared to controls (Figure 3I), a result consistent with impaired germinal center formation in this population. Conversely, obesity/T2D was associated with an increase in Th17 cells in the draining PLN compared to lean-fed mice (Figure 3J). T regulatory cell populations and Th1 populations were also present but showed no difference between obese/T2D mice and control mice (Figure 3K, L). Negligible numbers of TH2 cells were found in either group (<.01% cells, data not shown). Together, we conclude that obesity/T2D is associated with impaired T cell activation, reduced recruitment of TfH cells to germinal centers, and alterations in T cell polarization.
Obesity/T2D is associated with reduced GC B cells and aberrant B cell activation
Due to reduced germinal centers and impaired antibody production in obesity/T2D, B cells were assessed in the draining PLN. Interestingly, significantly more B cells were located in the PLN from obese/T2D mice than control mice prior to infection (Figure 4A, B). At day 7 post-infection there was a greater than 100-fold increase in the total number of B cells in both control and obese/T2D mice compared to naïve lymph nodes. By day 14, the absolute number of B lymphocytes was reduced in lean-fed control mice (Figure 4B). This was in marked contrast to the number of total B cells from obese/T2D mice which remained elevated and was significantly greater than the number of B lymphocytes in lean-fed control PLNs (Figure 4B). Moreover, B lymphocytes were an increased percentage of the total cell fraction in obese/T2D mice compared to controls (Figure 4C). These data implicate B lymphocytes as the primary driver of the increased cell infiltration/proliferation (Figure 2F) in the lymph nodes of obese/T2D mice following S. aureus infection.
Consistent with impaired antigen presentation (25, 26), the percentage of activated B cells was reduced in obesity/T2D at day 7 and day 14 following infection (Figure 4D, E). Moreover, the 50% reduction in activated B cells in obesity/T2D correlated with a similar reduction in germinal center B cells in this population by day 14 post-infection (Figure 4F, G). We also noted a trend towards reduced germinal center B cells 7 days post-infection in obese/T2D mice. These results associate obesity/T2D with reduced B cell activation and reduced germinal center B cells.
Impaired antibody response in obesity/T2D independent of bacterial killing
To determine if reductions in antibody production, germinal centers, and germinal center B cells reflected a decreased ability of obese/T2D mice to kill live S. aureus as part of antigen presentation, mice were challenged with S. aureus that was heat killed, treated with lysozyme and lysostaphin, and prepared in alum. Interestingly, obese/T2D mice displayed an increase in footpad swelling over the duration of the 14 days compared to controls, indicating that impaired bacterial killing was not necessary for elevated inflammation in the diabetic foot (Figure 5A). Consistent with results in mice infected with live S. aureus, obese/T2D mice had impaired anti-S. aureus IgG production (Figure 5B). Moreover, there was an increase in anti-S. aureus IgM production in obese/T2D mice (Figure 5C). This is consistent with our previous results in bone infection that indicated impaired class switch recombination in obesity/T2D (7). Quantitation by flow cytometry of the draining PLN revealed no difference in the total number of cells when comparing control and obese/T2D mice at day 14 post-injection (Figure 5D). Similar to obese/T2D mice infected with live S. aureus, we noted an increased proportion of B lymphocytes in this population (Figure 5E). Despite reduced anti-S. aureus IgG levels in obesity/T2D, the total proportion of GC B cells, macrophages, and dendritic cells, were equivalent to that of controls 14d post-challenge (Figure 5F–H). Moreover, histology revealed no differences in germinal centers or total germinal center area in obesity/T2D compared to lean mice 14 days post-injection. (Figure 5I, J).
Figure 5. Impaired germinal center reactions and humoral immunity in obese/T2D mice occur in the presence of normal numbers of antigen presenting cells.
Mice (n=8–9) were challenged in the footpad with enzymatically digested S. aureus mixed in alum. A. Footpad swelling was determined prior to harvest. B. Anti-S. aureus IgG antibody production and C. anti-S. aureus IgM were measured in serum 14d post-challenge. Draining popliteal lymph nodes were isolated for flow cytometry and D. total cells, E. proportion of CD19+ B cells, F. GC B cells (GL7+ CD19+), G. macrophages (F480+ CD11b+), and H. dendritic cells (CD11b+ CD11c+) were quantitated. I. Immunofluorescent staining was performed on popliteal lymph nodes 14d post infection to determine germinal centers (DAPI-blue, B cells-pink). J. Quantitation of germinal center area in I. K. Immunofluorescent staining for activation induced cytidine deaminase (AID). Dashed white lines outline germinal centers. L. Quantitation of K. *p<.05, ** p<.01. unpaired student T test except A, two way ANOVA and Bonferroni post-hoc analysis. Each point represents one mouse, error bars indicated SEM. For quantitation in J. and L., each point is the average of 3 sections, 100um apart, from one lymph node.
The surprising observation of impaired antibody production despite normal germinal center formation led us to hypothesize that class-switch recombination (CSR) is impaired in obese/T2D mice independent of antigen presentation. To examine this possibility, immunofluorescent staining for activation induced cytidine deaminase (AID) was performed on PLN of mice challenged for 14 days with killed S. aureus. AID is an enzyme that is crucial for CSR and is upregulated in germinal center B cells (27, 28). Consistent with the indication of impaired CSR in obesity/T2D, there was a significant reduction in AID+ immunostaining in germinal centers in this population compared to lean-fed controls (Figure 5K). This finding translated statistically, as obese/T2D mice had reduced total AID+ staining within germinal centers compared to controls despite normal germinal center formation (Figure 5L). Together, these data imply that diminished antibody production in obesity/T2D is due in part to impaired CSR and reduced AID in germinal centers.
Reduced antibody production and Aicda expression are associated with increased inflammation in obesity/T2D
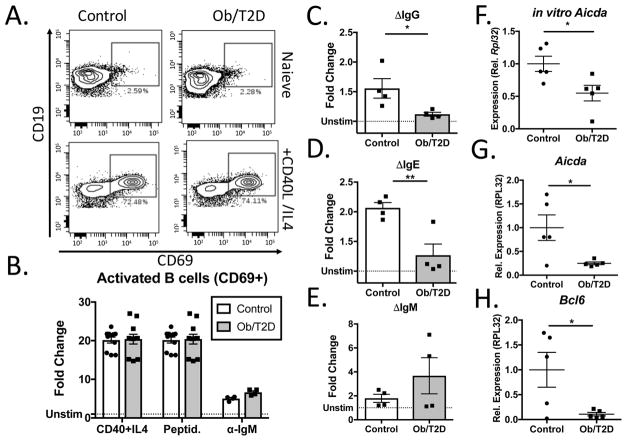
To directly examine the influence of obesity/T2D on B cell activation and class switch recombination, naïve B cells were isolated from the spleen of obese/T2D mice and control mice. Following stimulation with CD40 ligand and IL4 for 48 hours, B cell activation was assessed by quantitating CD69+ cells by flow cytometry. No differences were noted in the proportion of activated B cells in obese/T2D mice compared to controls (Figure 6A, B). Comparable results were attained when B cells were stimulated with peptidoglycan and anti-mouse IgM for 48 hours (Figure 6B). Consistent with this, CFSE staining to measure proliferation revealed no differences between obese/T2D mice and controls 2d post-stimulation (Data not shown). Together, these results reveal no defect in B lymphocyte activation in obesity/T2D.
Figure 6. Obesity/T2D is associated with impaired class switch recombination in vitro and in vivo.
B lymphocytes isolated from the spleens of control or obese/T2D mice (n≥4) were cultured with vehicle control or CD40 ligand + IL-4, followed by immunostaining for activated B cells (CD69+) A. Representative panels demonstrating in vitro activation 48h post-stimulation. B. Quantitation of activated activated B cells from A. or from stimulation with peptidoglycan or anti-mouse IgM. Fold change in C. IgG, D. IgE, and E. IgM produced by splenic B cells activated with CD40L + IL-4 after 4 days. F. Aicda expression was measured 4d post-stimulation of splenic B cells with CD40L + IL-4. G. Aicda and H. Bcl6 expression in popliteal lymph nodes of mice infected for 7d with S. aureus. *p<.05, ** p<.01. unpaired student T test. B. is pooled data from three separate experiments performed in triplicate. C–F is pooled data from four independent experiments, each performed in triplicate.
Isolated B lymphocytes were subsequently stimulated for 4 days with CD40L + IL4 to induce CSR and to observe antibody production. Consistent with our in vivo findings, B lymphocytes from obese/T2D mice produced markedly less IgG and IgE in vitro compared to controls (Figure 6C, D). No difference was noted in IgM production (Figure 6E). The finding of impaired CSR in obesity/T2D despite normal activation led us to further interrogate AID. Consistent with in vivo staining for AID, Aicda expression was significantly reduced in B cells from obese/T2D mice stimulated in vitro for 2d (Figure 6F). To validate these results, a second cohort of mice were infected for 7 days and lymph nodes harvested for RNA. Again, a marked reduction in Aicda expression was observed in obese/T2D mice (Figure 6G). Additionally, expression of Bcl6, a gene that is crucial for CSR in germinal centers, was almost completely suppressed in lymph nodes from obese/T2D mice compared to control mice (Figure 6H). Together, these results reveal impaired CSR in B lymphocytes of obese, type 2 diabetic mice.
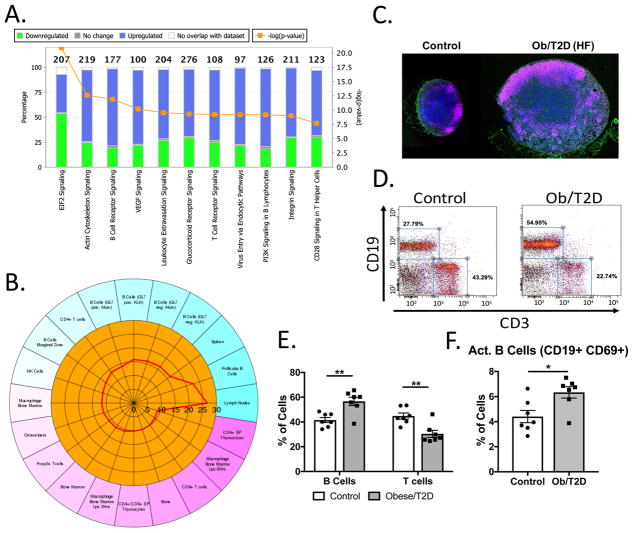
It is logical to assume that high fat-diet induced obesity/T2D elicited changes in naïve lymph nodes and B lymphocytes prior to S. aureus infection, which contributed to the subsequent impaired humoral immune response to infection. In support of this, whole transcriptome sequencing of naïve lymph nodes from obese/T2D and control mice revealed 1802 differentially expressed genes: 1512 were upregulated in obesity and 290 were downregulated (Figure 7A, B). While many genes were differentially expressed, their differences were relatively small, as only 2 genes were 10-fold increased in obese/T2D PLN compared to controls. Notably, Il17f was 16-fold more highly expressed and Mki67 was 9-fold higher in obese/T2D mice compared to controls (Suppl. Table 1). Pathway analysis revealed an enrichment in terms associated with B cell receptor signaling, T cell receptor signaling, cell migration, chemokine signaling and transcription in obese/T2D mice (Figure 7A). Moreover, cell type enrichment revealed enrichment in B cell subsets, particularly follicular B cells in obese/T2D mice compared to controls (Figure 7B). These results indicate increased B cell infiltration and accumulation in the naïve PLN of obese/T2D mice.
Figure 7. Increased activation and inflammation in naïve lymph nodes from obese/T2D mice.
Popliteal lymph nodes were isolated from naïve obese/T2D and control mice (n=3), and whole transcriptome analysis performed. A. Gene ontology terms that were significantly enriched in obese/T2D mice using Ingenuity pathway analysis are shown. B. Cell type enrichment was performed using ENSEMBLE demonstrating increased follicular B cells in obesity/T2D. C. Representative histological images demonstrating increased follicular B cells in obesity/T2D (DAPI-blue, B cells-pink, peanut agglutinin GC-green). Flow cytometry was performed on naïve lymph nodes from control and obese/T2D mice. D. Representative flow cytometry panels demonstrating B cell and T cell populations in naïve popliteal lymph nodes of obese/T2D mice and control mice. E. Proportion of CD19+ B cells or CD3+ T cells in naïve PLN. F. Quantification of activated B cells (CD69+) within the naive PLN. *p<.05, ** p<.01. unpaired student T test. Each point represents one mouse or biological replicate. N>6 for C–F. Error bars indicate SEM.
Histological analysis of naïve PLN revealed increased lymph node size and B cell infiltration/proliferation in obese/T2D mice (Figure 7C), confirming RNAseq results. No generation of germinal centers was noted in obesity/T2D despite an increase in B cells (Figure 7C). Flow cytometry on naïve PLN from uninfected, obese/T2D mice has demonstrated increased total cellularity (Figure 2F) and increased total B cells compared to lean-fed controls (Figure 4C). Further analysis demonstrated reciprocal changes in B cell and T cell percentages (Figure 7D, E) in obese/T2D mice, a result that was primarily driven by an increase in the absolute number of B lymphocytes in obesity/T2D. Further analysis of naïve PLN revealed an increase in activated B cells in obesity/T2D (Figure 7F). Together, these data indicate that the naïve PLN from obese/T2D mice have an influx of B lymphocytes, and that these cells are in a proinflammatory, activated state. These changes likely predispose the host to defective humoral immune response to infection.
Discussion
In this study, we demonstrate impaired S. aureus clearance by obese/T2D mice in a model of foot infection. Importantly, this result was associated with increased cellular infiltration at the infection site, impaired innate immune function, and reduced humoral immune responses. With regards to infection, peripheral vascular disease and neuropathy associated with T2D account for much of the increased risk of foot ulcers in diabetic patients. However, these factors alone do not fully account for the impaired immune response that appears to be at least partially attributable to obesity. In support of this, multiple studies have demonstrated an association between obesity and increased infection susceptibility following surgery (1, 6, 29). Moreover, obese patients are also at increased risk of developing community associated infections such as influenza (30), periodontitis (8), and skin infections (31). Consistent with other studies (32), we demonstrate that obese/T2D mice have an increase in inflammatory cell infiltration to the infection site and impaired bacterial killing (Figure 1) (8, 9, 33). Specifically, the primary source of inflammatory cells within the footpad of obese/T2D mice 7 days post- infection were CD11b+ GR1+ granulocytes. Despite increased phagocytes in obesity/T2D, we noted reduced clearance of S. aureus. Moreover, an increased proportion of S. aureus was observed to be extracellular or residing within dead host cells in the obese population. To our knowledge, this is the first study linking impaired immune function with reduced bacterial clearance in a model of diet-induced obesity/T2D and foot infection. These results were similar to a footpad infection in genetically modified leptin deficient mice, which also demonstrated impaired S. aureus killing in obesity (11). Additionally, our results reveal an impaired humoral immunity that may be a major driver of increased infection risk in the obese/T2D population. Moreover, we have previously demonstrated in a model of bone infection that obesity predisposes mice and patients to infection, a result that was independent of hyperglycemia (7). Therefore, we conclude that to reduce the incidence of foot infection, it is likely insufficient to only treat the underlying hyperglycemia in obesity/T2D. Rather effective treatment to restore immune function and reduce infection risk may require addressing the other elements of the obesity phenotype. To date, the most effective approach remains reducing body mass index.
Given the impaired innate immune function of obese/T2D in the foot infection model, it is not surprising that defects were also noted within the draining PLN. To our knowledge, the effect of impaired innate immunity and reduced bacterial killing in obesity/T2D on humoral immune function has not been established. In this report, the humoral immune response in obese/T2D mice infected with live S. aureus was characterized by impaired antibody production, reduced germinal center formation, impaired B and T cell activation, altered T cell polarization, and reduced germinal center B and T cells. Moreover, at day 7 post-infection this was associated with reduced numbers of macrophages within the draining PLN. Interestingly, when enzymatically digested S. aureus was administered, we noted a rescue in germinal center formation and macrophage number in the lymph nodes of obese/T2D mice. This argues that impaired S. aureus killing in the obese/T2D foot instigates impaired antigen presentation and lymphocyte activation in the draining PLN. It is also worth noting that the kinetics of inflammation within the PLN matched that of inflammation in the footpad. In both control and obese/T2D mice, there was a robust increase in cells within the footpad by day 7, and a subsequent increase in total cells in the PLN. By day 14 most lean mice resolved the infection, and there was a subsequent decrease in total lymphocytes within the draining PLN. In contrast, in obese/T2D mice, inflammation persisted in the foot concurrently with enhanced inflammation and cell recruitment/proliferation in the draining PLN. Taken together, we demonstrate here a clear association in obesity/T2D between impaired innate immune killing of S. aureus, increased lymph node cellularity, aberrant lymphocyte activation, and diminished humoral immunity. Further studies must be completed to conclusively show the cellular source and mechanism of impaired antigen presentation in the lymph nodes in obesity/T2D.
We also demonstrate impaired B lymphocyte function in obesity/T2D independent of S. aureus killing. Supporting this conclusion, obese/T2D mice challenged with killed S. aureus had impaired anti-S. aureus antibody production, similar to those infected with live S. aureus. Moreover, reduced anti-S. aureus IgG in obese/T2D was established despite correcting for antigen presenting cells, lymphocyte activation, and germinal center formation when challenged with killed S. aureus. This implies that impaired humoral immune responses in obesity/T2D are due, at least in part, to compromised B lymphocyte function. This is consistent with studies demonstrating altered B cell responses in ex vivo stimulated B cells from obese mice (34). Consistent with this finding, we noted impaired in vitro IgG and IgE production by previously naïve B lymphocytes in obese/T2D mice stimulated with CD40L+IL-4 to induce CSR. Moreover, this phenomenon was associated with a reduction in Aicda (AID) gene expression in both PLN of infected obese/T2D mice in vivo and stimulated B cells from obese/T2D mice in vitro. AID is a critical enzyme in CSR, producing double stranded breaks in the DNA of B cells, and ultimately instigating the production of class-switched, highly specific antibodies (27, 35, 36). Our data argue that reduced AID expression and impaired CSR in B lymphocytes from obese/T2D mice is a source of dampened immunoglobulin production in this population. Consistent with these findings, clinical studies have shown that obesity is associated with impaired humoral immunity to influenza vaccination (13) and reduced titers to the tetanus vaccine in obese children (14). This further implicates impaired humoral immune function with obesity and argues that the defect is not specific to S. aureus. However, further studies must be completed to conclusively determine the role of impaired class-switching and antibody production in obesity/T2D in other infectious diseases.
It is tempting to link increased inflammation in the naïve obese/T2D lymph node with impaired humoral immune responses and antibody production upon immune challenge. It is generally accepted that diet-induced obesity is associated with increased systemic inflammation (17, 37). Consistent with this, B lymphocytes are instrumental in the progression of diabetes, driving inflammation and ultimately insulin resistance (16, 18). How obesity-induced inflammation and activation of B cells affect responses to infection has not been established. It was recently revealed that elevated TNF-α production by B cells in aged mice limits B cell responses, impairs class switch recombination, and reduces AID production following LPS stimulation (38). Importantly, diabetes and obesity are driven, at least in part, by elevated proinflammatory cytokines (37, 38). Moreover, we have previously shown that TNF-Tg mice accumulate CD23+CD21highCD1dhigh B cells in inflamed lymph nodes (Bin), which are non-activated polyclonal antigen capturing cells (25, 26). Thus, accumulation of these bystander cells from obese/T2D chronic inflammation could also contribute to the increase in B cells, and inefficient germinal center reactions, we observed. It is also possible that reduced immunoglobulin production by obese/T2D mice is a result of altered T cell polarization. In support of this, TfH cells were decreased and Th17 cells were increased in lymph nodes of infected obese/T2D mice (Figure 3). Additionally, IL17f was highly expressed in the non-infected obese lymph node (Supplementary Table 1). IL17A and IL17F are implicated in the inflammation of obesity (39, 40), and Th17 cells have a known role in B cell class switching to isotypes IgG2a and IgG3 (41). We did not observe any differences in immunoglobulin isotype production (data not shown), and ex vivo B cells from obese mice had reduced CSR in the absence of Th17 stimulus. Nonetheless, it is possible that altered T cell polarization negatively affects immunoglobulin production in obese/T2D mice.
In this study, we demonstrate inflammation within the naïve lymph nodes in obese/T2D mice by whole transcriptome sequencing, and further validated this by flow cytometry, demonstrating increased activation of lymph node B cells in this population. One side effect of enhanced inflammatory cytokine production in aging and obesity is an increase in microRNA-155 (42, 43). Importantly, miR-155 is a known regulator AID and CSR (28, 44). Therefore, chronic low grade inflammation associated with obesity may mediate down regulation of B cell responses via an AID and miR-155 dependent mechanism, ultimately decreasing B lymphocyte antibody production. The source of inflammation in B lymphocytes is currently unclear. Exogenous cytokine production and inflammation are well known comorbidities of obesity (17). However, altered metabolism in B lymphocytes as a result of changes in substrate (ie. increases in saturated fat) may also play a role in impaired B cell responses and inflammation. Consistent with this, the humoral immune response is known to be tightly regulated by metabolism (45) and studies have shown that B cell function in obesity can be modulated by fatty acid supplementation (34, 46). Nonetheless, further studies must be completed to fully elucidate the mechanism of impaired B lymphocyte class-switching in obesity and type 2 diabetes.
Together, these results demonstrate impaired immunity in obesity/T2D that contributes to impaired clearance of S. aureus in a model of foot infection. Moreover, we demonstrate impaired humoral and innate immune function in obesity/T2D, each instrumental in reduced antibody production. The defects in humoral immunity likely contribute to the increased risk of infection in obese/diabetic patients and warrants further study.
Supplementary Material
Acknowledgments
The authors wish to thank Ann Gill, Sarah Mack, and Kathy Maltby for their very capable technical skills. The support of the Histology, Biochemistry, and Molecular Imaging Core in the Center for Musculoskeletal Research at the University of Rochester is greatly appreciated. RNA-Seq and transcriptome analysis described in this study were completed by the University of Rochester Genomics Research Center (URGRC).
Footnotes
Author contributions: All authors contributed to the study design, acquisition of data and data analysis. CWF, AB, ES, SRG, MJZ, RAM were responsible for drafting and editing the manuscript. All authors read and approved the submitted version. This work was supported by AOTrauma Research (CPP Bone Infection), NIH P30 AR069655, NIH P50 AR072000, NIH UL1 TR000042, and NIH T32 AR053459.
References
- 1.Dowsey MM, Choong PF. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467:1577–1581. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. Jama. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Smith DM, Weinberger M, Katz BP. A controlled trial to increase office visits and reduce hospitalizations of diabetic patients. Journal of general internal medicine. 1987;2:232–238. doi: 10.1007/BF02596446. [DOI] [PubMed] [Google Scholar]
- 4.Block P. The diabetic foot ulcer: a complex problem with a simple treatment approach. Military medicine. 1981;146:644–646. [PubMed] [Google Scholar]
- 5.Levin ME. Management of the diabetic foot: preventing amputation. Southern medical journal. 2002;95:10–20. [PubMed] [Google Scholar]
- 6.Winfield RD, Reese S, Bochicchio K, Mazuski JE, Bochicchio GV. Obesity and the Risk for Surgical Site Infection in Abdominal Surgery. The American surgeon. 2016;82:331–336. [PubMed] [Google Scholar]
- 7.Farnsworth CW, Shehatou CT, Maynard R, Nishitani K, Kates SL, Zuscik MJ, Schwarz EM, Daiss JL, Mooney RA. A humoral immune defect distinguishes the response to Staphylococcus aureus infections in mice with obesity and type 2 diabetes from that in mice with type 1 diabetes. Infect Immun. 2015;83:2264–2274. doi: 10.1128/IAI.03074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amar S, Zhou Q, Shaik-Dasthagirisaheb Y, Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci U S A. 2007;104:20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A. 2009;106:10740–10745. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infect Immun. 2009;77:1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard C, Wadowski M, Goruk S, Cameron L, Sharma AM, Field CJ. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ open diabetes research & care. 2017;5:e000379. doi: 10.1136/bmjdrc-2016-000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Holland LA, Weir S, Noah TL, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. International journal of obesity (2005) 2012;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39:137–141. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlsson EA, Meliopoulos VA, van de Velde NC, van de Velde LA, Mann B, Gao G, Rosch J, Tuomanen E, McCullers J, Vogel P, Schultz-Cherry S. A Perfect Storm: Increased Colonization and Failure of Vaccination Leads to Severe Secondary Bacterial Infection in Influenza Virus-Infected Obese Mice. mBio. 2017:8. doi: 10.1128/mBio.00889-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 18.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David M, Dzamba M, Lister D, Ilie L, Brudno M. SHRiMP2: sensitive yet practical SHort Read Mapping. Bioinformatics (Oxford, England) 2011;27:1011–1012. doi: 10.1093/bioinformatics/btr046. [DOI] [PubMed] [Google Scholar]
- 20.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birney E, Andrews TD, Bevan P, Caccamo M, Chen Y, Clarke L, Coates G, Cuff J, Curwen V, Cutts T, Down T, Eyras E, Fernandez-Suarez XM, Gane P, Gibbins B, Gilbert J, Hammond M, Hotz HR, Iyer V, Jekosch K, Kahari A, Kasprzyk A, Keefe D, Keenan S, Lehvaslaiho H, McVicker G, Melsopp C, Meidl P, Mongin E, Pettett R, Potter S, Proctor G, Rae M, Searle S, Slater G, Smedley D, Smith J, Spooner W, Stabenau A, Stalker J, Storey R, Ureta-Vidal A, Woodwark KC, Cameron G, Durbin R, Cox A, Hubbard T, Clamp M. An overview of Ensembl. Genome research. 2004;14:925–928. doi: 10.1101/gr.1860604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farnsworth CW, Schott EM, Jensen SE, Zukoski J, Benvie AM, Refaai MA, Kates SL, Schwarz EM, Zuscik MJ, Gill SR, Mooney RA. Adaptive upregulation of Clumping Factor A (ClfA) by S. aureus in the obese, type 2 diabetic host mediates increased virulence. Infection and immunity. 2017 doi: 10.1128/IAI.01005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. The Journal of clinical investigation. 2017;127:5–13. doi: 10.1172/JCI88876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csóka B, Pacher P, Bai P, Haskó G. New Piece in the Jigsaw Puzzle: Adipose Tissue–Derived Stem Cells From Obese Subjects Drive Th17 Polarization. Diabetes. 2015;64:2341–2343. doi: 10.2337/db15-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Kuzin I, Moshkani S, Proulx ST, Xing L, Skrombolas D, Dunn R, Sanz I, Schwarz EM, Bottaro A. Expanded CD23(+)/CD21(hi) B cells in inflamed lymph nodes are associated with the onset of inflammatory-erosive arthritis in TNF-transgenic mice and are targets of anti-CD20 therapy. J Immunol. 2010;184:6142–6150. doi: 10.4049/jimmunol.0903489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moshkani S, Kuzin, Adewale F, Jansson J, Sanz I, Schwarz EM, Bottaro A. CD23+ CD21(high) CD1d(high) B cells in inflamed lymph nodes are a locally differentiated population with increased antigen capture and activation potential. J Immunol. 2012;188:5944–5953. doi: 10.4049/jimmunol.1103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasqualucci L, Bhagat G, Jankovic M, Compagno M, Smith P, Muramatsu M, Honjo T, Morse HC, 3rd, Nussenzweig MC, Dalla-Favera R. AID is required for germinal center-derived lymphomagenesis. Nature genetics. 2008;40:108–112. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 28.Zan H, Casali P. Epigenetics of Peripheral B-Cell Differentiation and the Antibody Response. Frontiers in immunology. 2015;6:631. doi: 10.3389/fimmu.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94:e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- 30.Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, Fadel SA, Tran D, Fernandez E, Bhatnagar N, Loeb M. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ (Clinical research ed) 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. International journal of obesity (2005) 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 32.Brotfain E, Hadad N, Shapira Y, Avinoah E, Zlotnik A, Raichel L, Levy R. Neutrophil functions in morbidly obese subjects. Clinical and experimental immunology. 2015;181:156–163. doi: 10.1111/cei.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano H, Kinoshita M, Fujino K, Nakashima M, Yamamoto Y, Miyazaki H, Hamada K, Ono S, Iwaya K, Saitoh D, Seki S, Tanaka Y. Insulin treatment directly restores neutrophil phagocytosis and bactericidal activity in diabetic mice and thereby improves surgical site Staphylococcus aureus infection. Infect Immun. 2012;80:4409–4416. doi: 10.1128/IAI.00787-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. Journal of immunology (Baltimore, Md : 1950) 2017;198:4738–4752. doi: 10.4049/jimmunol.1601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature reviews Immunology. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mesin L, Ersching J, Victora GD. Germinal Center B Cell Dynamics. Immunity. 2016;45:471–482. doi: 10.1016/j.immuni.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frasca D, Romero M, Diaz A, Alter-Wolf S, Ratliff M, Landin AM, Riley RL, Blomberg BB. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, Kuchibhatla R, McDonnell ME, Xiao Q, Kepler TB, Apovian CM, Lauffenburger DA, Nikolajczyk BS. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity (Silver Spring) 2016;24:102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eljaafari A, Robert M, Chehimi M, Chanon S, Durand C, Vial G, Bendridi N, Madec AM, Disse E, Laville M, Rieusset J, Lefai E, Vidal H, Pirola L. Adipose Tissue-Derived Stem Cells From Obese Subjects Contribute to Inflammation and Reduced Insulin Response in Adipocytes Through Differential Regulation of the Th1/Th17 Balance and Monocyte Activation. Diabetes. 2015;64:2477–2488. doi: 10.2337/db15-0162. [DOI] [PubMed] [Google Scholar]
- 41.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karkeni E, Astier J, Tourniaire F, El Abed M, Romier B, Gouranton E, Wan L, Borel P, Salles J, Walrand S, Ye J, Landrier JF. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. The Journal of clinical endocrinology and metabolism. 2016;101:1615–1626. doi: 10.1210/jc.2015-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frasca D, Diaz A, Romero M, Ferracci F, Blomberg BB. MicroRNAs miR-155 and miR-16 Decrease AID and E47 in B Cells from Elderly Individuals. Journal of immunology (Baltimore, Md : 1950) 2015;195:2134–2140. doi: 10.4049/jimmunol.1500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R, Papavasiliou FN. MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity. 2008;28:621–629. doi: 10.1016/j.immuni.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boothby M, Rickert RC. Metabolic Regulation of the Immune Humoral Response. Immunity. 2017;46:743–755. doi: 10.1016/j.immuni.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whelan J, Gowdy KM, Shaikh SR. N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections. European journal of pharmacology. 2016;785:10–17. doi: 10.1016/j.ejphar.2015.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.