Abstract
Viral respiratory tract infections (VRTI) remain a leading cause of morbidity and mortality among infants and young children. In mice, optimal protection to VRTI is mediated by recruitment of effector T cells to the lungs and respiratory tract, and subsequent establishment of tissue resident memory T cells (Trm) which provide longterm protection. These critical processes of T cell recruitment to the respiratory tract, their role in disease pathogenesis, and establishment of local protective immunity remain undefined in pediatric VRTI. Here we investigated T cell responses in the upper and lower respiratory tract (URT and LRT, respectively) of infants and young children with VRTI, revealing developmental regulation of T cell differentiation and Trm generation in situ. We show a direct concurrence between T cell responses in the URT and LRT, including a preponderance of effector CD8+T cells that was associated with disease severity. During infant VRTI, there was an accumulation of terminally differentiated effector cells (Temra) in the URT and LRT with reduced Trm in the early neonatal period, with decreased Temra and increased Trm formation with age during the early years of childhood. Moreover, human infant T cells exhibit increased expression of the transcription factor T-bet compared to adult T cells, suggesting a mechanism for preferential generation of effector over Trm cells. The developmental regulation of respiratory T cell responses as revealed here is important for diagnosing, monitoring and treating VRTI in the critical early life stages.
Keywords: Infant Immunity, RSV, T-bet, Differentiation, Lung Injury
INTRODUCTION
Viral respiratory tract infections (VRTI) are a major cause of morbidity and mortality in children, especially those under 5 years (1). VRTI in infants and young children can result in clinically significant lung injury with the timing, inciting pathogen and resultant disease severity associated to alterations in pulmonary function in later life (2, 3). The mechanisms underlying increased susceptibility to VRTI in infants, in particular, remains incompletely understood with both viral and host factors likely contributing to disease severity. It has generally been considered that overwhelming disease in infants is related to impaired T cell responses, which are required to mediate viral clearance and establish long-term protective immunological memory (1). However, other evidence points to distinct, but not necessarily impaired responses of infant T cells (4–6). A greater understanding of the developing immune system during active VRTI and its relationship to disease severity is crucial to improving preventative and therapeutic modalities.
Viral clearance requires effective and coordinated responses from subsets of T cells in the barrier and mucosal surfaces during active infection. T cell differentiation to effector cells following naïve T cell activation is influenced by the local cytokine environment, and expression of various transcription factors (7). In particular, the transcription factor T-bet is required for differentiation to Th1-effector cells producing IFN-γ, important for anti-viral immunity (8, 9). T-bet also determines effector and memory T cell fate, with high T-bet levels promoting terminal effector generation and lower levels enabling memory T cell generation (6, 10, 11). Subsets of memory T cells include central memory (Tcm) and effector memory (Tem) subsets which circulate and enter lymphoid and peripheral tissues, respectively while resident memory (Trm) cells are maintained within diverse tissue sites but do not circulate (12–14). Mouse models of VRTI have demonstrated that Trm are generated in the lung and upper respiratory tract and can mediate efficacious protective immunity to viral challenge (15–19). Human Trm have been identified in lungs and multiple tissues sites and share phenotypic and transcriptional features with mouse Trm (20–23); however the role and establishment of human Trm during VRTI has not been well studied.
Infants produce virus specific CD8+T cells in response to respiratory infection (24), however, mouse models have shown that pathogen specific T cells generated in infancy do not persist in a manner similar to those generated by adults (6, 11). Developmental differences in early life T cells favor rapid proliferation and terminal differentiation over the generation of long lived memory responses (6). We previously showed that infant mice show reduced establishment and persistence of lung Trm following respiratory infection or intranasal vaccination compared to adult mice (11). Importantly, decreased Trm generation was intrinsic to infant T cells in mice, due to elevated expression of T-bet (11). Whether similar developmental regulation of Trm generation occurs in human T cells has not previously been assessed.
Here, we sampled nasopharynx and endotracheal aspirates representing the upper and lower respiratory tract (URT, LRT), along with blood of infants and children with VRTI to investigate the developmental and spatial aspects of the pediatric T cell response to VRTI. We show that local immune responses in both the URT and LRT are distinct from those in blood; URT and LRT contain predominant Tem cells and terminal effector T cell populations while blood mostly contains naïve T cells. Disease severity is likewise correlated to T cell subset composition in the URT and LRT, which is further influenced by the viral pathogen. We identify developmental differences in T cell subset differentiation during the early years of childhood, favoring formation of Temra cells during infancy and Trm generation increasing with age, correlating with increased expression of the transcription factor T-bet in pediatric T cells. Our results reveal important insights into the dynamics of pulmonary T cell responses to VRTI during early life, their association to disease pathogenesis, and intrinsic factors which control developmental regulation of fate determination.
MATERIALS AND METHODS
Study Design
Infants and children (< 10 years of age) admitted to the Pediatric Intensive Care Unit (PICU) at Morgan Stanley Children’s Hospital/New York Presbyterian Hospital (New York, NY) with acute respiratory failure, defined as treatment with noninvasive (either continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP)), or invasive (endotracheal tube or tracheostomy) mechanical ventilation were recruited to the study from April 2012 to December 2017 (Table 1). Testing for viral pathogens was performed by multiplex PCR by FilmArray (BioFire Diagnostics, Salt Lake City, UT) and included; Adenovirus, Coronavirus (229E, HKU1, NL63, OC43), Human Metapneumovirus (HMPV), Human Rhino/Enterovirus, Influenza A, Influenza B, Parainfluenza (subtypes 1-4), and Respiratory Syncytial Virus (RSV). Exclusion criteria included primary immunodeficiency, trisomy 21, or patients receiving chemotherapy or immunotherapy.
Table 1.
Demographic Data of enrolled Subjects with VRTI.
| Invasive Ventilation | Non-Invasive Ventilation | ||
|---|---|---|---|
| N | 28 | N | 34 |
| Sex (male) | 19 (68%) | Sex (male) | 21 (62%) |
| Median Age; Years (range) | 0.75 (0.03-10) | Median Age; Years (range) | 0.5 (0.06-3) |
| Viral Infection (Co-infection)1 | Viral Infection (Co-infection) | ||
| RSV | 12 (1) | RSV | 25 (3) |
| Human Metapneumovirus | 5 | Human Metapneumovirus | 3 (1) |
| Coronavirus | 4 (1) | Coronavirus | 2 (2) |
| Rhino/Enterovirus | 4 | Rhino/Enterovirus | 6 (4) |
| Parainfluenza | 2 | Parainfluenza | 3 (1) |
| Influenza | 1 | Adenovirus | 1 (1) |
| Adenovirus | 1 | ||
| PARDS/At Risk PARDS | 14 | PARDS/At Risk PARDS | 2 |
Viral etiology diagnosed by PCR (See methods). Subjects who had more than one virus present on viral testing denoted by “co-infection” status.
An additional cohort of healthy control subjects were enrolled (young children < 5 years of age and adults (aged 25-35)). Children having phlebotomy for routine pediatric screening purposes were consented to donate an additional aliquot of blood for research purposes and healthy adults were recruited to provide a blood sample (5mL) to serve as an additional control group. All protocols were approved by the institutional review board of Columbia University Medical Center (New York, NY) and informed consent was obtained from parents of subjects prior to enrollment in study.
Sample Collection and Preparation
Nasopharyngeal washes of the URT and/or endotracheal tube or tracheostomy aspirates of the LRT were collected daily until resolution of acute respiratory failure or 2 weeks, whichever came first. Blood samples were obtained (1 mL for subjects up to 3kg; then 1ml/kg up to a maximum of 5mL), when permitted on the first day of study enrollment and 72 hours later or at resolution of acute respiratory failure. URT and LRT samples were obtained during routine care performed by the PICU nurse or respiratory therapist. Samples were suctioned into a sterile sputum trap after instillation of 0.9% saline and passage of catheter into nares or artificial airway and suctioned into sterile sputum trap. Airway and blood samples (including flow cytometry and cytokine assays) were processed as previously described (25).
Clinical Data
Demographic and clinical data were extracted from electronic medical records, and included hospital length of stay, PICU length of stay, duration of mechanical ventilation, and mortality. Significant lung injury was determined to be present if subjects met the criteria for pediatric acute respiratory distress syndrome (PARDS) (26). PARDS is a consensus definition for diagnosing the presence of lung injury in children. In contrast to the adult criteria for acute respiratory distress syndrome (27, 28), PARDS does not necessitate arterial blood sampling, which can be more difficult to obtain in children. The PARDS criteria allow application of oxygen saturation (universally available) in place of blood sampling and also provides the ability to identify PARDS in subjects receiving noninvasive mechanical ventilation in addition to those receiving invasive mechanical ventilation.
Flow cytometry
The following Abs were used for myeloid cell and lymphocyte surface and intracellular staining and flow cytometric analysis: CD3 (OKT3), CD4 (SK3), CD11c (3.9), CD14 (M5E2), CD16 (3G8), CD24 (ML5), CD45 (HI30), CD45RA (HI100), CD56 (HCD56), CD123 (6H6), CD169 (7-239), CD206 (15-2), purchased from Biolegend (San Diego, CA); CD45RO (UCHL1), T-bet (eBio4B10), HLA-DR (LN3), purchased from eBioscience-ThermoFisher (San Jose, CA); CD8 (SK1), CD69 (FN50), CD103 (Ber-ACT8), purchased from BD Biosciences (San Jose, CA). Chemokine quantification was performed using a cytometric bead array, human chemokine kit (BD Biosciences). Stained cellular samples were acquired using an LSRII flow cytometer (BD Biosciences); chemokine samples were acquired using a LSR Fortessa (BD Biosciences) and data were analyzed using FCS express v6 (De Novo Software, Glendale, CA).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Version 7.03 (GraphPad, San Diego, CA). Mann-Whitney, Fisher’s exact test, or Wilcoxon matched pairs signed rank test were used to determine significance between groups. Linear regression testing was used to perform correlation analysis. All hypothesis testing was conducted at the 0.05 level of statistical significance.
RESULTS
A total of 62 pediatric subjects with respiratory failure due to VRTI were recruited to the study. The majority (34/62) of subjects enrolled received only noninvasive ventilation; however, a large number (28/62) also received invasive mechanical ventilation (Table 1, S1). The majority of subjects were infants under 12 months of age, with the next largest group between the ages of 1-4 years, with a few subjects older than 4 years old. RSV was the most commonly detected virus followed by rhino/enterovirus and HMPV. Most subjects were infected with only one virus but several had co-infection with multiple viruses (Table 1 and S1). The presence of clinically significant acute lung injury, as defined by PARDS(26) (see methods), was diagnosed in 25% (16/62) of enrolled subjects. We analyzed a total of 113 URT samples obtained from nasopharyngeal aspirates, 82 LRT samples obtained from endotracheal tube or tracheostomy aspirates as previously described (25), and 21 blood samples. These samples include 43 paired URT and LRT samples from 15 individuals.
Immune cell composition of the URT
We analyzed the immune cell composition in the URT based on a gating strategy that enabled quantitation of the major innate and adaptive immune cell types (29) (Fig. S1). Neutrophils represented the predominant cell type among CD45+ cells (Table 2), similar to the composition we previously identified in LRT samples (29). Significant proportions of eosinophils and monocytes, along with lower frequency populations of mast cells, T cells, B cells, dendritic cells, and NK cells were also present in the URT (Table 2). We also assessed chemokine content in the URT to determine correlations with cell recruitment. We detected high concentrations of IL-8, a neutrophil-homing chemokine, with significant levels of T cell chemoattractants CXCL9 and CXCL10 in some samples and negligible MCP-1 and CCL5 (Fig. S2). These results suggest that elevated levels of specific chemokines promote recruitment of neutrophils and lymphocytes to the URT during VRTI.
Table 2.
Cellular Composition of URT Samples.
| URT Cellular Composition (n=7) | Median % (Range) |
|---|---|
| Neutrophils | 86.61 (75.31-95.45) |
| Eosinophils | 8.16 (1.51-15.21) |
| Monocytes | 3.68 (1.57-6.88) |
| Mast Cells | 0.51 (0.11-2.51) |
| T Cells | 0.51 (0.24-1.27) |
| B Cells | 0.23 (0.06-4.04) |
| Dendritic Cells | 0.14 (0.01-0.18) |
| NK Cells | 0.13 (0.07-0.39) |
Median value with ranges of CD45+ cells present in URT samples.
T cell subset composition is similar between URT and LRT and distinct from blood
We compared the composition of T cells from the URT to those in the LRT and blood among subjects of varying ages receiving mechanical ventilation (Fig. 1). T cells from pediatric blood were predominantly CD4+ with a median CD8:CD4 ratio of 0.34 (range; 0.09-1.04) (Fig. 1B and 2A), which is lower than for adult blood (30). By contrast, T cells within the URT and LRT (98 of 195) contained increased frequencies of CD8+ T cells, with an increased ratio of CD8:CD4 T cells, often with CD8+T cells predominating over CD4+T cells (Fig. 1B and 2A). These results show altered T cell composition in the upper and lower respiratory compartments compared to blood in infants with VRTI, suggesting a localized response.
Figure 1. T cell composition in pediatric respiratory and blood sampls.
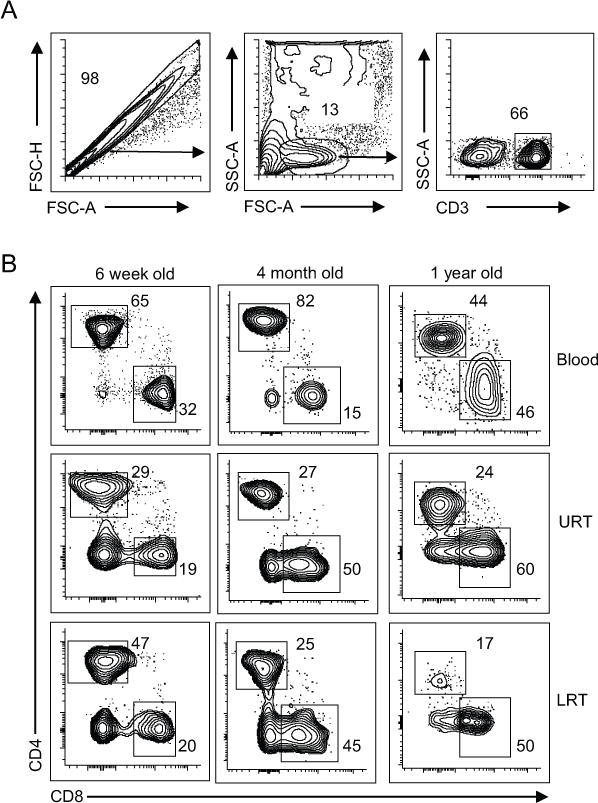
Representative flow cytometry plots of T cell populations from matched blood (top row), upper respiratory tract (URT, middle row), and lower respiratory tract (LRT, bottom row) samples of representative VRTI subjects aged 6 weeks (left), 4 months (middle), and 1 year (right). (A) Gating strategy for analysis of T cells with single cells selected based on forward scatter properties, then lymphocytes based on forward and side scatter, followed by cells expressing CD3. (B) CD4+ and CD8+T cell composition of CD3+ T cells in each site.
Figure 2. Inverse predominance of CD4+ and CD8+ T cells in the blood and respiratory tract of VRTI subjects.
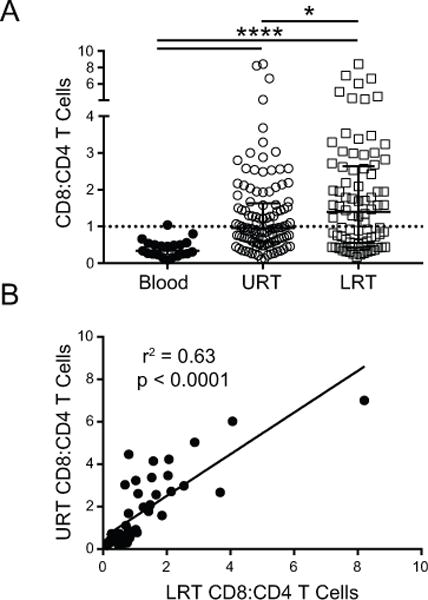
(A) Plots shows CD8:CD4 T cell content of all samples obtained for Blood (n=21), URT (n=113) and LRT (n=82). Median CD8:CD4 T cell ratio for Blood was 0.34 (range; 0.09-1.04), URT was 0.96 (range; 0.12-8.42) and LRT was 1.40 (range; 0.17-8.42). Individual data points depicted with median and interquartile range. Dashed line represents a CD8:CD4 ratio of 1. (* = p=0.02, ****= p<0.0001). (B) Correlation analysis of the CD8:CD4 T cell ratio for all paired URT and LRT samples. P values obtained by Wilcoxon matched-pairs signed rank test and R2 values obtained by linear regression analysis.
In the results above, the T cell composition in the URT more closely resembled that of the LRT compared to blood (Fig. 2A). To directly compare T cell composition in the URT and LRT, we analyzed paired nasopharyngeal and endotracheal tube aspirates obtained from the same patient for each sample day. Linear regression analysis demonstrated a strong correlation between the CD8:CD4 T cell ratio in the URT and LRT for all paired samples (r2 = 0.63; p<0.0001) (Fig, 2B). This strong correlation in T cell composition between these two respiratory sites in children suggests that sampling the URT can provide a representative indication of pulmonary T cell responses.
T cell composition and disease severity
Lung injury is a severe complication of pediatric VRTI which we previously found occurred in a significant frequency of infants requiring mechanical ventilation (25). In order to assess whether T cell composition in the URT or LRT associated with disease severity, we applied the new pediatric specific criteria for acute lung injury, PARDS (26). Pediatric subjects with VRTI who developed PARDS had significantly increased peak CD8:CD4 T cell ratios in both the URT and LRT compared to subjects who did not develop PARDS (Fig. 3A). These results show that T cell responses in both the URT and LRT are indicative of disease severity.
Figure 3. Respiratory CD8:CD4 T cell ratio associated with disease severity during VRTI.
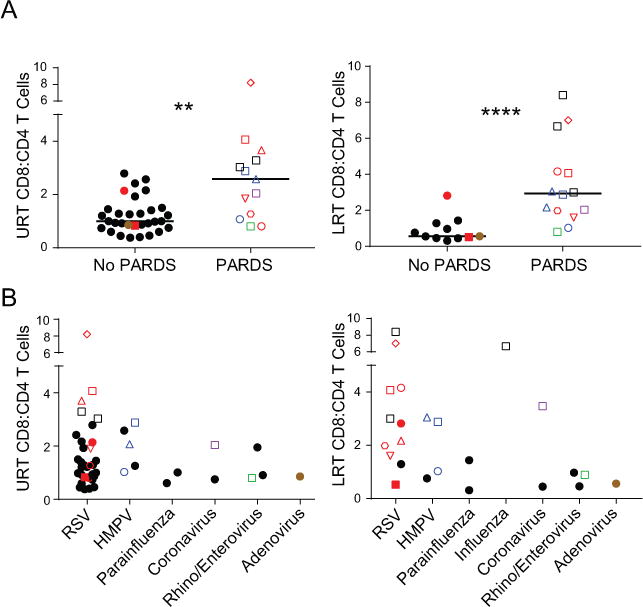
(A) Peak CD8:CD4 T cell ratio within an individual during the course of disease from the URT (left, n=31) and LRT (right, n=13) stratified by subjects diagnosed as having PARDS or not have PARDS. (LRT; PARDS median 2.94; range 0.8 – 8.4 vs no PARDS median 0.56; range 0.31 – 2.82, p < 0.0001; URT; PARDS median 2.58; range 0.8-8.21 vs no PARDS median 1; range 0.38 to 2.79, p = 0.002) (B) Peak CD8:CD4 T cell ratio in URT (left) and LRT (right) as in (A) stratified by viral pathogen. Bars in graphs represent medians. Filled data points represent subjects without PARDS while open points depict those with PARDS. Subjects who contributed both URT and LRT samples are denoted by unique color/shape combinations; as an example, the open red square is a subject with RSV who had samples analyzed from both the URT and LRT. Black data points represent individual subjects who contributed samples from only one site (URT or LRT). P values obtained by Mann-Whitney testing.
We further investigated whether T cell content and/or disease severity was associated with specific viral infections. Increased frequencies of CD8+ T cells in the URT and LRT of subjects who developed PARDS occurred mostly in subjects infected with RSV and HMPV (Fig. 3B), which constituted the majority of subjects enrolled (Table 1). However, subjects with influenza and coronavirus who developed PARDS also exhibited local accumulation of CD8+ T cells (Fig. 3B). This increased CD8+T cell content in the URT and LRT of children with PARDS from VRTI across multiple viral pathogens supports a role for the local immune response in the development of lung injury during VRTI.
Developmental changes in respiratory T cell subset composition
We further investigated the T cell subset composition in blood, URT and LRT based on coordinate expression of CD45RA and CCR7, delineating naïve T cells (CCR7+/CD45RA+), central memory T cells (Tcm; CCR7+/CD45RA−), effector memory T cells (Tem; CCR7−/CD45RA−), and terminal effector T cells (Temra; CCR7−/CD45RA+) (Fig. 4A). In the blood, both CD4+ and CD8+T cells were predominantly naïve (70->90%) with much lower proportions of Tcm, Temra and Tem cells (1-15%). In stark contrast, the predominant subset in both the URT and LRT was Tem for both CD4+ and CD8+T cells, with very low frequencies of naïve T cells (1-31%) (Fig 4A, B). For CD4+T cells, smaller but significant proportions of Tcm cells were found in both respiratory tract compartments, while Temra cells were found in very low frequencies in most subjects with some variability (Fig. 4A,B). Moreover, for CD8+Tcells, significant populations of Temra cells were observed in both URT and LRT, over a broad range of frequencies (7-81%) (Fig. 4A, B). Notably, for both CD4+ and CD8+ T cells subsets, there were no statistically significant differences in composition between the URT and LRT when comparing paired samples from the same subject (Fig. 4C). These results demonstrate biased localization of Tem and Temra subsets in the respiratory tract during pediatric VRTI.
Figure 4. Developmental differences in T cell subset composition in the URT and LRT.
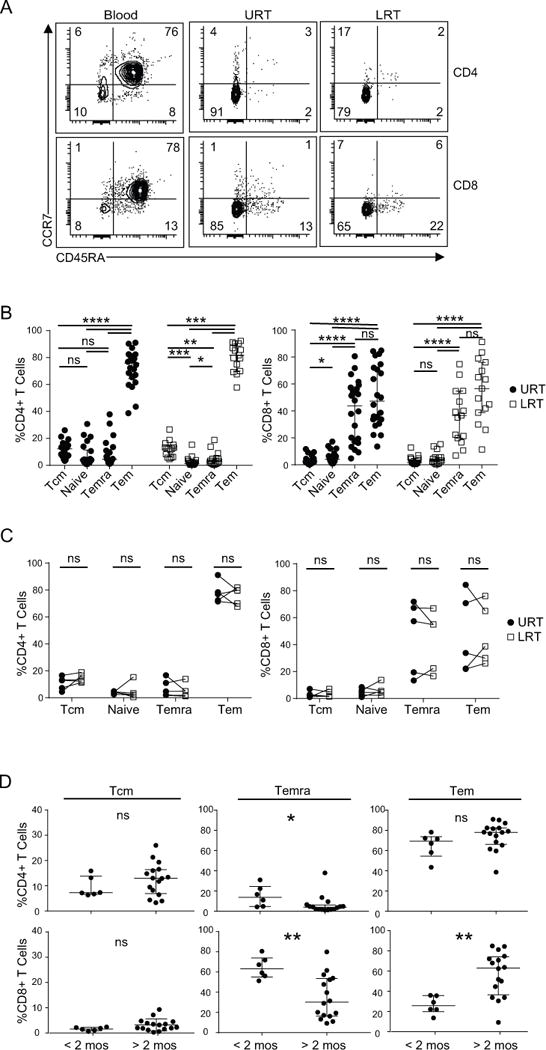
(A) Representative flow cytometry plots from a single subject infected with RSV showing CCR7 and CD45RA expression by CD4+ T cells (top) and CD8+ T cells (bottom) in the indicated sites. T cell subsets are identified as; naïve (CCR7+/CD45RA+), Temra (CCR7−/CD45RA+), Tcm (CCR7+/CD45RA−), and Tem (CCR7−/CD45RA−). (B) Compiled data showing T cell CD4+ T cells (left) or CD8+ T cells (right) in URT (filled circles, n=22) and LRT (open squares, n=15). For URT CD4+ T cell subsets; Tcm median 12.32% (range; 3.31-26%), naïve median 4.02% (range; 0.89-30.71%), Temra median 4.59% (range; 1.13-30.97%) and Tem median 75.77% (range; 43.53-91.05%) and CD8+ subsets; Tcm median 2.18% (range; 0.24-9.29%), naïve median 4.34% (range; 0.96-17.32%), Temra median 49.61% (range; 9.33-80.62%) and Tem median 47.32% (range; 13.6-84.84%). For LRT CD4+T cell subsets; Tcm median 12.29% (range; 4.61-26.63%), naïve median 1.3% (range; 0.37-15.28%), Temra median 2.91% (range; 0.29-18.77%) and Tem median 81.81% (range; 57.79-92.37%), and CD8+ subsets; Tcm median 1.72% (range; 0.57-12.9%), naïve median 3.29% (range; 0.43-15.38%), Temra median 36.66% (range; 6.94-74.77%) and Tem median 56.64% (range; 11.37-91.32%). (C) Correlation of CD4+ T cell (left) or CD8+ T cell (right) subset frequency in paired URT (filled circles) and LRT (open square) samples from five subjects. (D) Stratification of CD4+ T cell (top row) and CD8+ T cell (bottom row) subset data obtained from URT samples based on age (<2 months; n =6 and >2 months; n=16). P values obtained by Mann-Whitney t-test and represented by *; (* = p<0.05, ** = p<0.01, ***= p<0.001, ****= p<0.0001).
We asked whether the wide variability we found in subset composition, particularly among CD8+ Tem and Temra subsets, was associated with age over the early postnatal period. Plotting T cell subset frequency with age revealed a cluster of higher Temra frequencies during early infancy which exhibited a steep decline after 2 months of age, suggesting this response does not wane but rather changes abruptly after the early neonatal period (Fig. S3A). Compiling subset data of URT samples from subjects grouped by early neonatal (<2 months) to later (>2 months) reveals no significant difference in proportions of CD4+Tcm, or Tem cells (Fig. 4D). However, for CD8+T cells, there was a marked increase in Temra cells (and compensatory decrease in Tem cells) in infants <2 months of age, compared to those >2 months old (Fig. 4D). A similar trend toward increased Temra cells was observed for CD4+T cells from young infants, although the overall frequency was still low (<30%) (Fig. 4D). The inverse relation of Temra and Tem cells was correlated for CD8+ T cells (Fig. S3B), but not for other CD8+ or CD4+T cell subsets.
We analyzed the relationship of local T cell subset responses and the development of PARDS. There were no significant differences in local T cell subset composition (CD4+ Tem/Tcm/Temra or CD8+ Tem/Temra) between subjects who developed PARDS and those who did not (not shown). These results suggest that T cell differentiation in response to respiratory viral infection in early life is skewed to terminal effector-like (Temra) over memory T cell fate, consistent with results in mouse neonatal T cells (11); however, these early life T cell subset variations are not necessarily related to increased disease severity.
Establishment of Trm in the respiratory tract in early life
Mouse models have shown that Trm in the airways and lungs mediate optimal protective immunity to respiratory viruses (15–17, 31). We assessed whether Trm cells were present in the URT and LRT of infants and young children with VRTI, based on expression of cell surface markers known to be associated with human lung Trm including, CD69 and CD103 (32, 33). Expression of CD69 with or without CD103 was found on the majority of Tem-phenotype CD4+ and CD8+T cells from the URT and LRT (not shown) samples in subjects as young as 6 weeks of age, but not on T cells from paired blood samples which were uniformly CD69− and CD103− (Fig. 5A). CD69 was expressed by the majority of both CD4+ and CD8+Tem cells in the URT and LRT and this fraction did not change significantly with age during the early childhood years (Fig. 5B). By contrast, CD103 was expressed by a higher frequency of CD69+CD8+Tem cells compared to CD69+CD4+Tem cells in the URT and LRT, and age-related effects were observed with CD103 expression (Fig. 5C). Importantly, co-expression of CD69 and CD103, thought to represent the most mature Trm cells (20, 34) increased with age during the early years of life for both CD4+ and CD8+T cells in the URT and LRT (Fig 5C). These results indicate that CD69+ memory T cells are present in the respiratory tract during pediatric VRTI throughout the early years of life, but that the frequency of fully differentiated CD103-expressing Trm cells increases with age during this period.
Figure 5. Trm phenotype cells in respiratory samples and variation with age.
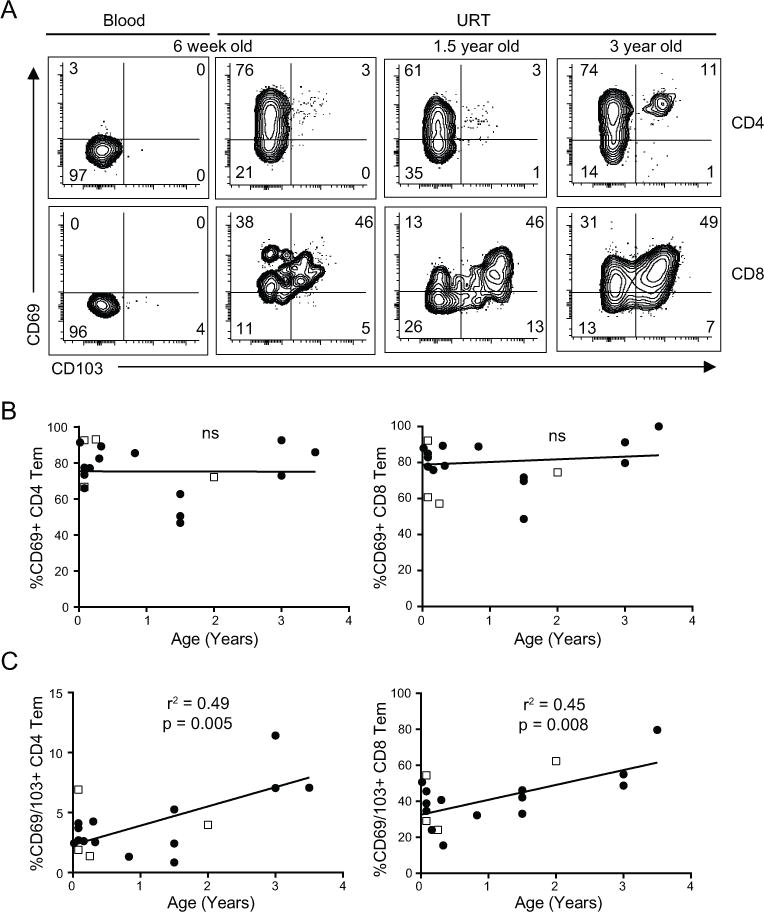
(A) Representative flow cytometry plots showing CD69 and CD103 expression by CD4+ (top row) and CD8+ (bottom row) memory T cells (Tem) in blood and URT samples from infected subjects of indicated ages, including paired blood and URT from the 6 week old subject. (B) CD69 expression by CD4+ (left) and CD8+ (right) T cells in the URT (closed circles, n=14) and LRT (open squares, n=4) as a function of age. Statistical analysis performed only on URT samples for consistency. (C) CD69/CD103 co-expression on CD4+ (left) and CD8+ (right) memory T cells in the URT and LRT with age as in (B). R and p values obtained by linear regression.
Differences in expression of transcription factor T-bet in early life
Our findings that infants infected with VRTI in the early months of life exhibited both increased Temra accumulation and decreased Trm formation in the respiratory tract suggested an altered differentiation pathway in early life T cell responses. We previously showed in an infant mouse model of influenza infection that infant T cells exhibited increased upregulation of the transcription factor T-bet, which inhibited Trm formation (11). We therefore investigated T-bet expression by human infant T cell subsets in blood or airways, to assess whether a similar increased T-bet expression is observed in human early life T cells. In T cells obtained from healthy adult blood donors (Table S1), the highest level of T-bet expression was found in CD8+ Temra followed by Tem cells, with low T-bet levels in naïve T cells (Fig. 6A) as previously described (8, 9). In pediatric blood, T-bet expression was also higher in Temra and Tem cells, compared to naïve T cells, and was further increased in Tem compared to Temra cells (Fig. 6A). These differences in relative T-bet expression in pediatric versus adult T cell subsets are significant as shown in paired analysis of multiple donors (Fig. 6B, left), and in compiled data showing a higher ration of T-bet expression in Temra/Tem cells in adults compared to pediatric samples (right).
Figure 6. Increased T-bet expression by Tem cells in early life and locally during VRTI.
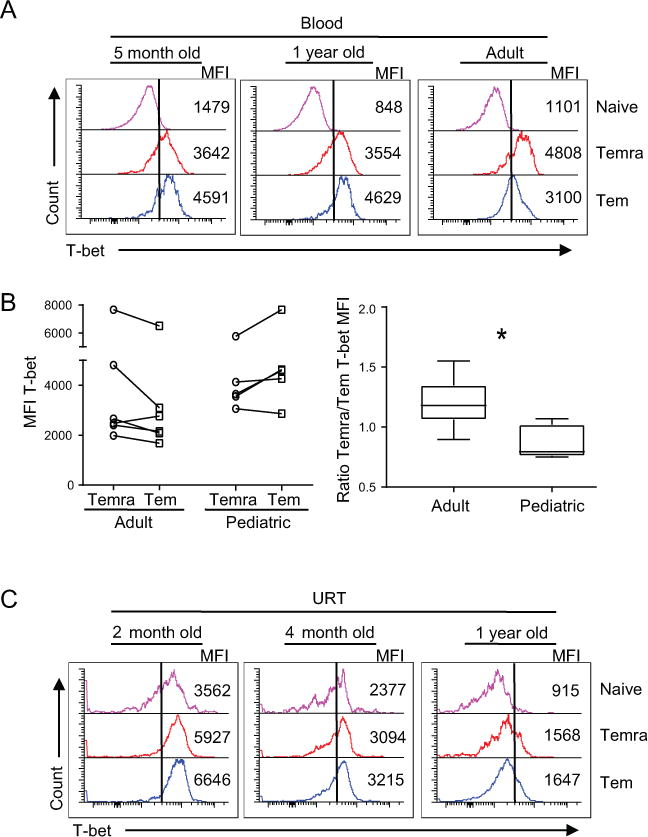
(A) T-bet expression by CD8+ T cell subsets obtained from infant and adult peripheral blood shown as representative flow cytometry plots showing naïve (top), Temra (middle), and Tem (bottom) with the MFI indicated. (B) Relative differences in T-bet expression by CD8+ Temra and Tem cells in adult (n=6) and pediatric (n=5) blood samples. Left: T-bet expression (MFI) by Temra and Tem cells with line connecting paired samples from individual subjects. Right: Ratio of T-bet expression on Temra to Tem cells (right) from individual samples shown on left; adult (median 1.18; range 0.89-1.55) and pediatric (median 0.79; range 0.75-1.07). P value obtained by Mann-Whitney (* = p=0.02). (C) Flow cytometry plots depict T-bet (x axis) expression by indicated T cell subsets from URT samples of three subjects with VRTI. Samples are arranged as stated in (A).
We further investigated T-bet expression in airway samples, identifying similar trends of increased expression of T-bet by pediatric Tem compared to Temra cells in blood, URT and LRT samples from an individual subject (Fig, S3C). Pediatric URT T cells from infected infants of different ages exhibited higher T-bet expression on Tem compared to Temra cells, with some overall decrease in T-bet expression with age (Fig 6C). These data suggest that increased T-bet expression is associated with T cell differentiation by pediatric T cells during early life.
DISCUSSION
Infants are exposed to a multitude of viral pathogens with varying virulence factors during their formative years (35–37), with viral clearance ultimately relying on T cell activation and effector differentiation (38). Virus specific T cells are generated in early life (24) but whether these responses give rise to long lasting memory or prevent tissue injury remains unknown. Here, we show that infants and young children mount a robust T cell response in both the URT and LRT, with increased CD8+T cell infiltration associated with lung injury and certain types of viral infections. We demonstrate that during active VRTI in infants and young children, local responses are predominated by Tem cells while those in circulation remain overtly naïve. In the airways, Temra cells predominated in the early neonatal period and decreased thereafter, while development of mature Trm cells occurred later after the first year of life. Importantly, this altered T cell differentiation was associated with increased T-bet expression in respiratory tract T cells during early infancy, and in circulating Tem cells in the blood. Our results identify developmental regulation of local respiratory T cell responses by subset and localization, with intrinsic alterations in transcriptional regulation of human T cells in early life.
Studies in mice and humans have demonstrated the importance of respiratory Trm in mediating protection from VRTI (15, 39). The establishment of local memory responses in the URT is critical to prevention of infection in the LRT (16) and intranasal vaccination has been shown to induce generation of virus specific pulmonary Trm, capable of providing protection in mice (31, 40). We found that Trm-phenotype cells expressing CD69 are detectable in the respiratory tract in the youngest subjects—as early at 6 weeks of age. However, CD103 expression by these CD69+ memory T cells, which is a feature of mucosal CD8+Trm, increases with age in the first few years of life, consistent with our previous findings that CD69+memory T cells in infant mucosal tissues had reduced CD103 expression compared to mucosal tissues from older children or adults (32). These results suggest that full Trm maturation requires sustained exposure to antigens, multiple prior infections, or prolonged exposure to signals from the tissue environment. Lung-specific factors such as those derived from commensal microbial species, cytokines and chemokines may be differentially present in infant lungs compared to those of older children, with resulting effects on Trm maturation.
Concomitant with reduced Trm cells in infant URT samples was an increased in Temra cell frequency, indicating a preponderance of terminal effector cell generation. We likewise show elevated T-bet expression in infant compared to adult T cells in the blood, and elevated T-bet expression in infant URT suggesting intrinsic properties of infant T cells may impact their differentiation fate. Notably, our results with human pediatric respiratory samples are similar to our previous studies in mice where we found that increased T-bet expression in infant T cells promoted effector T cell generation and recruitment to the lung but inhibited Trm formation during early life infection and intranasal vaccination (11). We propose that targeting T-bet during early life infection or vaccination may be advantageous for promoting establishment of protective, long-lived T cell populations in the respiratory environment.
Studying cellular immune responses in children is limited due to difficulties in obtaining samples from the local site of infection. Mouse models have shown that following viral respiratory infection, T cells in the URT are clonally related to those in the LRT (41). We have demonstrated a correlation between T cell responses in the URT and LRT in both magnitude and subset diversity during VRTI in children. Additionally, the T cell response in these compartments is associated with the development of PARDS in the setting of multiple inciting viral agents. While we did not quantify viral load in the respiratory tract of enrolled subjects, multiple studies have shown considerable overlap in viral titers and disease severity (42–45), suggesting that host responses play a critical role in determining disease course.
In conclusion, our results provide novel evidence for an association of local immunologic responses to disease severity in pediatric VRTI. These findings have important implications for disease treatment and development of protective responses, including potential non-invasive avenues which could be used for diagnostic, prognostic, or mechanistic investigations. Our results lend further evidence to the need to study localized responses to further the understanding of immune mediated protection during active viral infection.
Supplementary Material
Acknowledgments
We wish to thank the families who participated in this study. We would like to thank the PICU nurses and respiratory therapists for their help in the collection of samples. We would like to thank Filip Cvetkovski for critical reading.
Abbreviations
- VRTI
Viral Respiratory Tract Infection
- PARDS
Pediatric Acute Respiratory Distress Syndrome
- URT
Upper Respiratory Tract
- LRT
Lower Respiratory Tract
- Trm
Resident Memory T Cell
- Tem
Effector Memory T Cell
- Tcm
Central Memory T cell
- Temra
Effector Memory RA+ T cell
- RSV
respiratory syncytial virus
- HMPV
human Metapneumovirus
- PICU
Pediatric Intensive Care Unit
- CPAP
continuous positive airway pressure
- BiPAP
bilevel positive airway pressure
Footnotes
Supported by NIH AI100119 awarded to D.L.F., and Louis V. Gerstner, Jr. Scholar award to T.J.C. Research performed in the CCTI Flow Cytometry Core, supported in part by National Institutes of Health awards P30CA013696, S10RR027050 and 5P30DK063608. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: T.J.C, D.L.F, K.D.Z., J.S.B., T.M.R., conceived the presented work. T.J.C., K.D.Z., M.C.Y., S.H., designed and performed the experiments. T.J.C., J.S.B, K.P, and T.M.R contributed to sample preparation. D.L.F supervised the project. All authors provided critical feedback and helped shape the analysis of data and preparation of manuscript.
References
- 1.Meissner HC. Viral Bronchiolitis in Children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 3.Raedler D, Schaub B. Immune mechanisms and development of childhood asthma. Lancet Respir Med. 2014;2:647–656. doi: 10.1016/S2213-2600(14)70129-8. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med. 2014;20:1206–1210. doi: 10.1038/nm.3670. [DOI] [PubMed] [Google Scholar]
- 5.Zens KD, Connors T, Farber DL. Tissue compartmentalization of T cell responses during early life. Semin Immunopathol. 2017 doi: 10.1007/s00281-017-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, Rudd BD. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol. 2014;193:177–184. doi: 10.4049/jimmunol.1400553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hough KP, Chisolm DA, Weinmann AS. Transcriptional regulation of T cell metabolism. Mol Immunol. 2015;68:520–526. doi: 10.1016/j.molimm.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zens KD, Chen JK, Guyer RS, Wu FL, Cvetkovski F, Miron M, Farber DL. Reduced generation of lung tissue-resident memory T cells during infancy. J Exp Med. 2017;214:2915–2932. doi: 10.1084/jem.20170521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 15.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, Reading PC, Wakim LM. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam6970. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, Brasser G, Jongejan A, Jonkers RE, Nota B, Basak O, Clevers HC, Moerland PD, Amsen D, van Lier RA. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol. 2016;17:1467–1478. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 21.Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. 2015;36:428–435. doi: 10.1016/j.it.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thome JJC, Yudanin NA, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial Map of Human T Cell Compartmentalization and Maintenance over Decades of Life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, Farber DL. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Reports. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidema J, Lukens MV, van Maren WW, van Dijk ME, Otten HG, van Vught AJ, van der Werff DB, van Gestel SJ, Semple MG, Smyth RL, Kimpen JL, van Bleek GM. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J Immunol. 2007;179:8410–8417. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 25.Connors TJ, Ravindranath TM, Bickham KL, Gordon CL, Zhang F, Levin B, Baird JS, Farber DL. Airway CD8(+) T Cells Are Associated with Lung Injury during Infant Viral Respiratory Tract Infection. Am J Respir Cell Mol Biol. 2016;54:822–830. doi: 10.1165/rcmb.2015-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric G, Acute Lung Injury Consensus Conference Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 27.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 28.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, Kraft M, Hollingsworth JW, Gunn MD, Tighe RM. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am J Respir Cell Mol Biol. 2016;54:13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bofill M, Janossy G, Lee CA, MacDonald-Burns D, Phillips AN, Sabin C, Timms A, Johnson MA, Kernoff PB. Laboratory control values for CD4 and CD8 T lymphocytes. Implications for HIV-1 diagnosis. Clin Exp Immunol. 1992;88:243–252. doi: 10.1111/j.1365-2249.1992.tb03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zens KD, Chen JK, Farber DL. Vaccine-Generated Lung Tissue-Resident Memory T cells Provide Heterosubtypic Protection to Influenza Infection. J Clin Invest Insight. 2016;1:e85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T, Farber DL. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, Farber DL. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol. 2015;16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin ET, Fairchok MP, Stednick ZJ, Kuypers J, Englund JA. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207:982–989. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain S, Finelli L, C.E.S. Team Community-acquired pneumonia among U.S. children. N Engl J Med. 2015;372:2167–2168. doi: 10.1056/NEJMc1504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remakus S, Sigal LJ. Memory CD8(+) T cell protection. Adv Exp Med Biol. 2013;785:77–86. doi: 10.1007/978-1-4614-6217-0_9. [DOI] [PubMed] [Google Scholar]
- 39.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, Maybeno M, Del Rosario J, Trujillo-Torralbo MB, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, Chiu C. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545–554. doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surman SL, Rudraraju R, Woodland DL, Dash P, Thomas PG, Hurwitz JL. Clonally related CD8+ T cells responsible for rapid population of both diffuse nasal-associated lymphoid tissue and lung after respiratory virus infection. J Immunol. 2011;187:835–841. doi: 10.4049/jimmunol.1100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen RR, Schinkel J, Dek I, Koekkoek SM, Visser CE, de Jong MD, Molenkamp R, Pajkrt D. Quantitation of respiratory viruses in relation to clinical course in children with acute respiratory tract infections. Pediatr Infect Dis J. 2010;29:82–84. doi: 10.1097/INF.0b013e3181b6de8a. [DOI] [PubMed] [Google Scholar]
- 43.De Vincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 44.Utokaparch S, Marchant D, Gosselink JV, McDonough JE, Thomas EE, Hogg JC, Hegele RG. The relationship between respiratory viral loads and diagnosis in children presenting to a pediatric hospital emergency department. Pediatr Infect Dis J. 2011;30:e18–23. doi: 10.1097/INF.0b013e3181ff2fac. [DOI] [PubMed] [Google Scholar]
- 45.Feikin DR, Fu W, Park DE, Shi Q, Higdon MM, Baggett HC, Brooks WA, Knoll M Deloria, Hammitt LL, Howie SRC, Kotloff KL, Levine OS, Madhi SA, Scott JAG, Thea DM, Adrian PV, Antonio M, Awori JO, Baillie VL, DeLuca AN, Driscoll AJ, Ebruke BE, Goswami D, Karron RA, Li M, Morpeth SC, Mwaba J, Mwansa J, Prosperi C, Sawatwong P, Sow SO, Tapia MD, Whistler T, Zaman K, Zeger SL, KL OB, Murdoch DR, P. S. Group Is Higher Viral Load in the Upper Respiratory Tract Associated With Severe Pneumonia? Findings From the PERCH Study. Clin Infect Dis. 2017;64:S337–S346. doi: 10.1093/cid/cix148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


