Abstract
Objectives
Direct comparative work in morphology and growth on widely dispersed wild primate taxa is rarely accomplished, yet critical to understanding ecogeographic variation, plastic local variation in response to human impacts, and variation in patterns of growth and sexual dimorphism. We investigated population variation in morphology and growth in response to geographic variables (i.e., latitude, altitude), climatic variables (i.e., temperature and rainfall), and human impacts in the vervet monkey (Chlorocebus spp.).
Methods
We trapped over 1600 wild vervets from across Sub-Saharan Africa and the Caribbean, and compared measurements of body mass, body length, and relative thigh, leg, and foot length in four well-represented geographic samples: Ethiopia, Kenya, South Africa, and St. Kitts & Nevis.
Results
We found significant variation in body mass and length consistent with Bergmann’s Rule in adult females, and in adult males when excluding the St. Kitts & Nevis population, which was more sexually dimorphic. Contrary to Rensch’s Rule, although the South African population had the largest average body size, it was the least dimorphic. There was significant, although very small, variation in all limb segments in support for Allen’s Rule. Females in high human impact areas were heavier than those with moderate exposures, while those in low human impact areas were lighter; human impacts had no effect on males.
Conclusions
Vervet monkeys appear to have adapted to local climate as predicted by Bergmann’s and, less consistently, Allen’s Rule, while also responding in predicted ways to human impacts. To better understand deviations from predicted patterns will require further comparative work in vervets.
Keywords: Chlorocebus, vervet, growth, life history, sexual dimorphism
INTRODUCTION
Morphological variation in widespread taxa may arise by a number of mechanisms: natural selection, developmental plasticity in response to local environmental forces, and random processes like genetic drift. Locally differentiable morphological variation in subpopulations of taxa with wide distributions has historically been used to indicate the possible beginnings of speciation (Hill, 1966; Kingdon, 1997). In populations with significant amounts of gene flow, local polytypy and substructuring can result in morphological clines within a single taxon (e.g., Cardini et al. 2007; Roseman & Auerbach, 2015), as well as geographically distinct taxa comprised of multiple environmentally driven phenotypes that may ultimately become fixed by natural selection (Kuzawa and Bragg, 2012; Kuzawa and Thayer, 2011; Lomolino et al. 2006).
Although data from animals living in diverse habitats throughout a taxon’s range are crucial to understanding population differentiation, they are rarely collected in primates. Museum specimens can provide information from a limited number of skins and skeletonized specimens, although the geographic range and number of animals can often be substantial, especially if multiple collections are used (e.g., Fooden, 1979; Groves, 2001; Kingdon, 2013; Albrecht et al., 1990; Cardini et al., 2007; Elton et al. 2010). Data on morphological variation are also often collected from populations of living animals at a single location. Such populations often have limited geographic distributions (e.g., booted macaques: Schillaci and Stallmann, 2005; Gombe chimpanzees: Pusey et al., 2005; ring tailed lemurs: Cuozzo and Sauther, 2006) or are housed in laboratories or semi-naturalistic environments (e.g., rhesus macaques: Tanner et al., 1990; Schwartz and Kemnitz, 1992; Turnquist and Kessler, 1989; mangabeys: Deputte, 1992; baboons: Coelho and Rutenberg, 1989; mandrills: Setchell et al., 2001). Finally, comparisons of morphological variation more broadly within genera may be made across numerous independent study sites, collected by different research groups. Comparison across baboons, for example, may be based on the work of Jolly and Phillips-Conroy (2003) on baboons in the Awash Park, Ethiopia; Altmann and colleagues on baboons in Amboseli National Park, Kenya (Altmann et al., 1981; Altmann et al., 1993); Strum and colleagues on baboons in the Rift Valley area of Kenya (Strum, 1991; Eley et al., 1989) and Johnson (2003) on animals from Botswana. Comparative morphological data are also available for populations of macaques from Singapore (Schillaci et al., 2007), Kojima Island in Japan (Hamada et al., 1986) and Polonnaruwa (Cheverud et al., 1992). Only rarely is information available from free ranging animals living in multiple locations within a taxon’s range and collected by the same researchers using comparable protocols (e.g., macaques: Albrecht, 1980; vervets: Turner et al., 1997; Whitten & Turner, 2004).
Furthermore, in these cases where geographically widespread data are available for adult animals of a particular taxon, it is rare to also have comparable information on multiple life stages. Where comparable data have been collected on immature mammals, the contribution of developmental patterns to adult phenotypes has been critical to understanding adult selective regimes (e.g., Ozgul et al., 2010; Ozgul et al., 2009). Information on animals of various life stages within and across closely related but widely dispersed populations is necessary for a complete understanding of the life history trade-offs that may underlie adult variation in response to the selective challenges of differing environments.
The study reported here relies on morphological measurements obtained from over 1600 free ranging vervet monkeys (the genus Chlorocebus, also called savannah monkeys or African green monkeys; population-specific names include grivets, malbroucks, and others) from multiple locations spanning the range of the genus. Vervets are highly adaptable, semi-terrestrial foragers, able to occupy a variety of tropical and subtropical, seasonal habitats that provide sufficient trees and water, especially in savanna-woodland and open country with riverine forest. Vervets are found throughout sub-Saharan Africa. The genus most likely originated in East/Central Africa, first diverging into West African Ch. sabaeus ~531 kya, followed by the isolation and divergence of Ch. aethiops ~ 446 kya, and a southern expansion from East Africa around ~265 kya (comprised of the ancestral population of Ch. pygerythrus hilgerti, Ch. cynosuros, and Ch. p. pygerythrus, which diverged around ~129 kya), after which populations were introduced to several islands in the Caribbean from West Africa ~350 years ago (Jasinska et al., 2013; Warren et al., 2015; Svardal et al. 2017). Throughout their evolutionary history in Sub-Saharan Africa, these taxa show long histories of gene flow, although they have more recently become genetically isolated (Svardal et al., 2017). They have been observed living in hot and arid areas, as well as more temperate and cooler areas where winter nighttime temperatures may fall below freezing (e.g., Lubbe et al. 2014; Kingdon, 1997). Vervets live in multi-male, multi-female groups, with males migrating between groups in early adulthood, and groups range in size up to 50 animals, although most are considerably smaller (Struhsaker, 1967; Hall & Gartlan, 1965; Fedigan & Fedigan, 1988; Cheney & Seyfarth, 1983; Enstam & Isbell, 2007). Chlorocebus are regarded as pests throughout much of their range since they raid crops and houses for food (Grobler et al. 2006; Brennan et al. 1985; Boulton et al., 1996; Dore, 2013).
It is generally agreed that Chlorocebus may be divided into several diagnosable and geographically defined subspecies or species based on phenotypic characters (Hill, 1966; Groves, 2001; Grubb et al. 2003). Recent genetic work (Turner et al., 2016a; Haus et al., 2013) generally supports these taxonomic groupings, although current taxonomic distinctions below the genus level are disputed (Warren et al., 2015), as well as the status of these groupings as species nested in Chlorocebus or subspecies nested in Chlorocebus aethiops. Svardal et al. (2017) use established taxonomic names for genomically clustered population groups within the samples studied in this paper, with Ch. aethiops describing populations sampled from Ethiopia, Ch. p. hilgerti for populations sampled in Kenya, Ch. p. pygerythrus for populations sampled from South Africa, and Ch. sabaeus for populations sampled in St. Kitts & Nevis with an origin in West Africa. Although Svardal et al. recognize Ch. p. hilgerti and Ch. p. pygerythrus as two genomically distinct populations, with Ch. p. hilgerti being at least as distinguishable from Ch. p. pygerythrus as Ch. cynosuros, current taxonomy subsumes Ch. p. hilgerti and Ch. p. pygerythrus into the Ch. pygerythrus species or subspecies (Groves 2001; Grubb et al. 2003). To avoid further discussion of this taxonomic uncertainty, here we refer to each population by either their country of origin or accepted taxonomic names according to Groves (2001) and Butynski et al. (2013): Ch. aethiops, Ch. p. hilgerti, Ch. p. pygerythrus, and Ch. sabaeus.
The breadth of our sample allows us to examine several predictions suggested by established hypotheses regarding the evolution of body size:
-
(1)Bergmann’s Rule (Bergmann, 1847; Meiri & Dayan, 2003) hypothesizes that animals in colder climates (i.e., geographically at higher latitudes and altitudes and climatically at lower temperatures) will be larger in body size compared to those in warmer climates to stave off the loss of body heat.
- Animals at higher latitudes and altitudes will be larger in body size than those in equatorial latitudes and low altitudes.
- Animals experiencing lower mean annual and winter temperatures will be larger in body size than those experiencing warmer annual and winter temperatures.
-
(2)Allen’s Rule (Allen, 1877) hypothesizes that animals in colder climates (e.g., geographically at higher latitudes and altitudes and climatically at lower temperatures) will have relatively short limbs to preserve body heat.
- Animals at higher latitudes and altitudes will have relatively short limbs compared to those in equatorial latitudes and low altitudes.
- Animals experiencing lower mean annual and winter temperatures will have relatively shorter limbs than those experiencing warmer annual and winter temperatures.
-
(3)
Rensch’s Rule (Rensch, 1950; Clutton-Brock et al., 1977) hypothesizes that sexual dimorphism will be greater in populations that exhibit greater overall body mass.
The animals in our sample come from 61 trapping locations across three countries in Sub-Saharan Africa and two islands in the Caribbean (Fig. 1), representing a wide range and mosaic of ecogeographic, anthropogenic, and climatic variation (Table 1). Our East African sample (Ch. aethiops and Ch. p. hilgerti) comes from mid to high altitudes but with a range of human impacts and climatic variability. Our sample from St. Kitts and Nevis (Ch. sabaeus) come from uniformly low altitudes, high human impacts, and relatively mild and uniformly tropical climate. Our largest sample, from South Africa (Ch. p. pygerythrus), is also the most diverse for all variables, representing high and low altitudes, a range of latitudes, and local climatic variation ranging from wet and temperate to dry and very cold.
Fig. 1.
Chlorocebus sample collection locations in Africa. Individual collection sites in St. Kitts & Nevis were extensive and distributed widely across both islands, and so are not shown here (see Jasinska et al., 2013, for a complete map of trapping sites).
Table 1.
Coordinates and human impact index scores by trapping sites for Chlorocebus. Climate variables are derived from theWorldClim database based on latitude and longitude locations of trapping sites.
| Taxon | Country | Region | Site | Latitude | Longitude | Altitude (m) | Agriculture | Human Food |
Rubbish | Provisioning | Human Activity |
Human Impact Index |
Human Impact Group |
Annual Mean Temp. |
Temp. Seasonality |
Min. Temp. Coldest Month |
Mean Temp. Coldest Quarter |
Annual Precipitation |
Precipitation Seasonality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch. aethiops | Ethiopia | Awash | Awash | 8.9917 | 40.1632 | 925 | 0 | 0 | 0 | 0 | Low | 5 | Low | 25.6 | 2033 | 13.7 | 22.7 | 132 | 291 |
| Ch. aethiops | Ethiopia | Non-Awash | Hawaasa | 7.0504 | 38.4955 | 1712 | 0 | 1 | 1 | 1 | High | 10 | High | 19.2 | 734 | 9.4 | 18.6 | 134 | 394 |
| Ch. aethiops | Ethiopia | Non-Awash | Lake Tana | 12.0266 | 37.0266 | 2102 | 0 | 1 | 1 | 1 | High | 10 | High | 20.1 | 1141 | 10.4 | 19 | 363 | 917 |
|
| |||||||||||||||||||
| Ch. p. hilgerti | Kenya | Kimana | Kimana | −2.8000 | 37.5333 | 1277 | 1 | 1 | 0 | 0 | Moderate | 8 | Moderate | 20.2 | 1500 | 12.7 | 18 | 179 | 374 |
| Ch. p. hilgerti | Kenya | Mosiro | Mosiro | −1.4833 | 36.1000 | 1302 | 0 | 0 | 0 | 0 | Low | 5 | Low | 21.5 | 1088 | 13.2 | 19.8 | 152 | 335 |
| Ch. p. hilgerti | Kenya | Naivasha | Naivasha | −0.7172 | 36.4310 | 1901 | 1 | 1 | 1 | 0 | Moderate | 9 | High | 16.9 | 931 | 8.4 | 15.6 | 133 | 298 |
| Ch. p. hilgerti | Kenya | Samburu | Samburu | 1.2571 | 37.1768 | 1181 | 0 | 0 | 0 | 0 | Low | 5 | Low | 20.1 | 854 | 10.6 | 18.8 | 173 | 337 |
|
| |||||||||||||||||||
| Ch. p. pygerythrus | South Africa | Eastern Cape | Geelhoutbos | −33.7692 | 24.0988 | 369 | 0 | 0 | 0 | 0 | Low | 5 | Low | 15.6 | 2935 | 5.1 | 11.8 | 76 | 220 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Rooiplatt | −33.7692 | 24.0988 | 369 | 1 | 0 | 0 | 0 | Moderate | 7 | Moderate | 15.6 | 2935 | 5.1 | 11.8 | 76 | 220 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Bukela | −33.5722 | 26.1239 | 253 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.4 | 2647 | 6.8 | 13.9 | 58 | 160 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Bushman Sands | −33.2936 | 26.1483 | 313 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.6 | 3048 | 5.6 | 13.5 | 60 | 155 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Intaka Lodge | −33.5708 | 26.6025 | 276 | 0 | 0 | 1 | 0 | Low | 6 | Moderate | 18 | 2248 | 9.2 | 15.2 | 65 | 182 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Leeuwenbosch | −33.5364 | 26.0694 | 269 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 17.7 | 2749 | 6.7 | 14.1 | 54 | 148 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | NMMU | −34.0010 | 25.6715 | 29 | 0 | 1 | 1 | 1 | High | 10 | High | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Pine Lodge | −34.0101 | 25.6887 | 13 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.8 | 2359 | 9.1 | 14.9 | 58 | 172 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Rietfontein | −33.5503 | 26.1247 | 253 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.4 | 2647 | 6.8 | 13.9 | 58 | 160 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Osfontein (Shamwari GR) | −33.4783 | 26.0297 | 245 | 0 | 0 | 1 | 0 | Low | 6 | Moderate | 17.8 | 2872 | 6.4 | 14.1 | 53 | 139 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Bayethe Lodge (Kwantu GR) | −33.3995 | 26.1811 | 442 | 0 | 0 | 1 | 1 | Moderate | 8 | Moderate | 16.9 | 2884 | 5.4 | 13 | 65 | 178 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Harden Lodge (Shamwari GR) | −33.4736 | 26.0422 | 231 | 0 | 0 | 1 | 1 | Moderate | 8 | Moderate | 18 | 2833 | 6.8 | 14.3 | 52 | 138 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Longlee Lodge (Shamwari GR) | −33.4667 | 26.0508 | 228 | 0 | 0 | 1 | 1 | Moderate | 8 | Moderate | 18 | 2833 | 6.8 | 14.3 | 52 | 138 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Ravine (Shamwari GR) | −33.4936 | 26.0067 | 261 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.8 | 2872 | 6.4 | 14.1 | 53 | 139 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Conningly (Shamwari GR) | −33.4089 | 26.1011 | 338 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 17.4 | 2932 | 5.8 | 13.5 | 59 | 157 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Doringkom (Shamwari GR) | −33.2881 | 26.0314 | 400 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 17.8 | 3199 | 5.4 | 13.5 | 57 | 146 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Splitting Image | −33.3975 | 26.2933 | 455 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17 | 2834 | 5.8 | 13.2 | 67 | 184 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Sibuya | −33.6308 | 26.6444 | 15 | 0 | 0 | 0 | 0 | Low | 5 | Low | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Sidbury Club | −33.5025 | 26.1311 | 196 | 0 | 0 | 1 | 1 | Moderate | 8 | Moderate | 18 | 2658 | 7.4 | 14.5 | 53 | 146 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Stellenhof (Addo) | −33.5497 | 26.7083 | 266 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 17.8 | 2255 | 9.2 | 15 | 68 | 190 |
| Ch. p. pygerythrus | South Africa | Eastern Cape | Tslowane | −32.1834 | 26.5020 | 1407 | 0 | 0 | 0 | 0 | Low | 5 | Low | 12.4 | 3625 | 0 | 7.5 | 92 | 261 |
| Ch. p. pygerythrus | South Africa | Free State | Fishery (!Gariep Dam) | −30.6067 | 25.4464 | 1206 | 1 | 1 | 1 | 0 | High | 10 | High | 16.9 | 5380 | 1.7 | 9.6 | 72 | 186 |
| Ch. p. pygerythrus | South Africa | Free State | !Gariep Dam Nature Reserve | −30.6008 | 25.4945 | 1367 | 0 | 0 | 0 | 0 | Low | 5 | Low | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | Free State | Orange River Guest Farm (!Gariep Dam) | −30.6342 | 25.4869 | 1296 | 1 | 0 | 1 | 0 | Moderate | 7 | Moderate | 16.9 | 5309 | 1.9 | 9.7 | 74 | 189 |
| Ch. p. pygerythrus | South Africa | Free State | Parys Country Estate | −26.8939 | 27.4909 | 1429 | 0 | 1 | 1 | 1 | High | 10 | High | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | Free State | Sandveld Nature Reserve 1 | −27.6761 | 25.6817 | 1240 | 0 | 0 | 1 | 0 | Low | 6 | Moderate | 17.8 | 5190 | −0.2 | 10.5 | 82 | 240 |
| Ch. p. pygerythrus | South Africa | Free State | Sandveld Nature Reserve 1 | −27.6811 | 25.7125 | 1240 | 0 | 0 | 1 | 0 | Low | 6 | Moderate | 17.6 | 5184 | −0.2 | 10.3 | 84 | 242 |
| Ch. p. pygerythrus | South Africa | Free State | Sandveld Nature Reserve 1 | −27.6757 | 25.6825 | 1240 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.8 | 5190 | −0.2 | 10.5 | 82 | 240 |
| Ch. p. pygerythrus | South Africa | Free State | Sandveld Nature Reserve 1 | −27.7460 | 25.7686 | 1242 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 17.5 | 5200 | −0.3 | 10.2 | 85 | 241 |
| Ch. p. pygerythrus | South Africa | Free State | Soetdoring Nature Reserve 1 | −28.8219 | 26.0594 | 1262 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 16.7 | 5395 | −1.5 | 9.2 | 79 | 228 |
| Ch. p. pygerythrus | South Africa | Free State | Soetdoring Nature Reserve 2 | −28.8219 | 26.0594 | 1262 | 0 | 0 | 1 | 0 | Low | 6 | Moderate | 16.7 | 5395 | −1.5 | 9.2 | 79 | 228 |
| Ch. p. pygerythrus | South Africa | Free State | Southford Farm (!Gariep Dam) | −30.6067 | 25.4464 | 1206 | 1 | 1 | 1 | 0 | High | 10 | High | 16.9 | 5380 | 1.7 | 9.6 | 72 | 186 |
| Ch. p. pygerythrus | South Africa | Free State | Waschbank (!Gariep Dam) | −30.6167 | 25.4622 | 1206 | 1 | 1 | 1 | 0 | High | 10 | High | 16.6 | 5293 | 1.4 | 9.4 | 74 | 192 |
| Ch. p. pygerythrus | South Africa | Gauteng | Loristo | −25.8116 | 28.3040 | 1424 | 0 | 1 | 1 | 1 | High | 10 | High | 16.7 | 3948 | 1.7 | 11 | 141 | 359 |
| Ch. p. pygerythrus | South Africa | Gauteng | Woodhill | −25.8116 | 28.3040 | 1424 | 0 | 1 | 1 | 1 | High | 10 | High | 16.7 | 3948 | 1.7 | 11 | 141 | 359 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Alize, Blythedale Beach | −29.3747 | 31.3489 | 21 | 0 | 1 | 1 | 0 | High | 9 | High | 21 | 2592 | 11.3 | 17.5 | 128 | 370 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Anerley | −30.6697 | 30.5078 | 14 | 0 | 1 | 1 | 0 | High | 9 | High | 20.2 | 2402 | 12.1 | 17 | 145 | 392 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Futululu | −28.4389 | 32.2823 | 48 | 0 | 0 | 0 | 0 | Low | 5 | Low | 21.8 | 2713 | 12.5 | 18.2 | 131 | 361 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Kwela Lodge | −29.4911 | 31.3600 | 24 | 1 | 1 | 1 | 1 | High | 11 | High | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Maurann Farm | −28.4389 | 32.2823 | 48 | 0 | 1 | 0 | 0 | Low | 6 | Moderate | 21.8 | 2713 | 12.5 | 18.2 | 131 | 361 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Oribi Gorge | −30.6910 | 30.2925 | 427 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 18.2 | 2297 | 9.6 | 15.2 | 141 | 404 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Seula Zimbili | −29.2078 | 31.4242 | 89 | 0 | 1 | 1 | 0 | Moderate | 8 | Moderate | 21.1 | 2631 | 11.2 | 17.5 | 132 | 376 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Thorny Park Estate | −29.1858 | 31.4417 | 40 | 1 | 1 | 1 | 0 | Moderate | 9 | High | 21.1 | 2631 | 11.2 | 17.5 | 132 | 376 |
| Ch. p. pygerythrus | South Africa | KwaZulu-Natal | Zinkwazi Lagoon Lodge | −29.2769 | 31.4397 | 22 | 0 | 1 | 1 | 0 | High | 9 | High | 21.2 | 2595 | 11.4 | 17.7 | 132 | 376 |
| Ch. p. pygerythrus | South Africa | Limpopo | Bird Sanctuary | −23.8962 | 29.4486 | 1257 | 0 | 0 | 0 | 0 | Low | 5 | Low | 17.7 | 3583 | 4.1 | 12.6 | 103 | 292 |
| Ch. p. pygerythrus | South Africa | Limpopo | Lapa Reserve | −23.9006 | 28.3170 | 1176 | 0 | 0 | 0 | 0 | Low | 5 | Low | 18.8 | 3986 | 2.8 | 13 | 114 | 301 |
| Ch. p. pygerythrus | South Africa | Mpumalanga | Blyde Resort | −24.5807 | 30.7723 | 1184 | 0 | 1 | 1 | 0 | Moderate | 8 | Moderate | 17.6 | 2785 | 5.5 | 13.5 | 173 | 504 |
| Ch. p. pygerythrus | South Africa | Mpumalanga | Potholes | −24.5807 | 30.7723 | 1184 | 0 | 1 | 0 | 0 | Moderate | 7 | Moderate | 17.6 | 2785 | 5.5 | 13.5 | 173 | 504 |
| Ch. p. pygerythrus | South Africa | Mpumalanga | Swadini | −24.5170 | 30.8100 | 606 | 0 | 0 | 0 | 0 | Low | 5 | Low | 20.1 | 2983 | 7.6 | 15.8 | 147 | 415 |
| Ch. p. pygerythrus | South Africa | Northern Cape | Benfontein | −28.8245 | 24.8201 | 1177 | 1 | 0 | 0 | 0 | Low | 6 | Moderate | 18.2 | 5189 | 1.7 | 11 | 74 | 207 |
| Ch. p. pygerythrus | South Africa | Northern Cape | Dronfield | −28.7379 | 24.7627 | 1233 | 0 | 0 | 0 | 0 | Low | 5 | Low | − | − | − | − | − | − |
| Ch. p. pygerythrus | South Africa | Northern Cape | Druiswater/Kanoneiland | −28.6552 | 21.0847 | 768 | 0 | 0 | 0 | 0 | Low | 5 | Low | 19.6 | 5336 | 2.8 | 12.4 | 35 | 86 |
|
| |||||||||||||||||||
| Ch. sabaeus | St. Kitts & Nevis | St. Kitts | St. Kitts | 17.3578 | −62.7830 | 155 | 1 | 1 | 1 | 1 | High | 11 | High | 24.1 | 1118 | 19.2 | 22.5 | 193 | 557 |
| Ch. sabaeus | St. Kitts & Nevis | Nevis | Nevis | 17.1554 | −62.5796 | 355 | 1 | 1 | 1 | 1 | High | 11 | High | 25.2 | 1117 | 20.3 | 23.6 | 176 | 509 |
Employing traditional analysis of geographic variation by altitude and latitude, we predict that (a) Ch. p. pygerythrus will be the largest, Ch. sabaeus intermediate, and Ch. p. hilgerti and Ch. aethiops the smallest, and (b) Ch. p. pygerythrus and high-altitude populations in Ch. p. hilgerti should have relatively short limbs. Although recent analyses of Bergmann’s and Allen’s Rules show mixed results regarding the importance of any one climatic variable to the patterns noted in size variation in animals across large geographic areas, including primates (e.g., Meiri & Dyan, 2003; Dunham et al. 2013; Guillaumet et al. 2008), we have also included these in our analysis. We also predict that patterns of dimorphism within Chlorocebus will follow the pattern suggested by Rensch’s Rule. We further suggest that body size and proportion will follow climatic variability as opposed to taxonomic categories. We expect this to be particularly noticeable within the larger Ch. pygerythrus clade, wherein the distance between Kenyan (Ch. p. hilgerti) and South African (Ch. p. pygerythrus) populations is over 5000 km along the eastern side of Africa; this should leave them open to higher environmentally mediated intrapopulational variance in body size and proportion than what we will see within Ch. sabaeus or Ch. aethiops.
Given previous work showing that developmental variation underlies adult patterns in variation, we predict that the rate and pattern of growth will also differ across these populations, such that populations that reach a larger adult size will either be consistently larger throughout development (Smith & Leigh 1998), and/or show a more exaggerated spurt of growth around pubertal age (Shea 1985).
Evolutionary responses to local selection pressures, however, cannot account for all variation in body size. Phenotypic plasticity in body mass is well noted in wild primates, especially in response to environments altered by humans, such as increased caloric availability due to garbage dumps, human crops and food waste, and laboratory diets (Altmann et al., 1993; Alberts & Altmann 2005; Turner et al. 1997; Turner et al. 2016b). As such, we also predict that:
-
(4)
Local, human-mediated resource availability will lead to within-population variability in body mass and length. In particular, populations exposed to environments more highly impacted by humans (e.g., near garbage dumps, crops, or otherwise human-modified areas) will also have larger body mass than those found in environments more isolated from human impacts.
MATERIALS AND METHODS
The data derive from field collections made over many years using a common protocol: Ethiopia in 1973, Kenya in 1978–79; South Africa in 2002–2008, and several African countries and the Caribbean in 2009–2011 in collaboration with the International Vervet Research Consortium (Jasinska et al., 2013). The International Vervet Research Consortium is a multidisciplinary research group that has, in addition to morphological variation, studied variation in patterns of growth and development (Schmitt et al., 2017), genetic/genomic (Jasinska, et al., 2013; Turner et al. 2016a; Warren et al. 2015; Svardal et al., 2017; Schmitt et al., 2017) and transcriptomic (Jasinska et al., 2017) variation, SIV immune response (Ma & Jasinska et al. 2013; Ma & Jasinska et al. 2014; Svardal et al., 2017), hormonal variation (Fourie et al., 2015), C4 isotopes variation in hair (Loudon et al., 2014), gut parasite and disease variation (Gaetano et al. 2014; Senghore et al., 2016), genital morphology and appearance (Cramer et al. 2013; Rodriguez et al. 2015a, 2015b), and other biological parameters within the genus Chlorocebus.
Vervet monkeys were trapped at locations across sub-Saharan Africa, including South Africa, Botswana, Zambia, Ethiopia, The Gambia, Ghana, and on the Caribbean islands of St. Kitts and Nevis (Fig. 1). Trapping in Africa employed individual drop traps as described by Brett et al. (1982) and Grobler and Turner (2010), while trapping in St. Kitts and Nevis was done by local trappers using large group traps (Jasinska et al., 2013). Animals were anesthetized while in the trap and then removed to a processing area. Sex was determined by visual and manual inspection, while age classes were assigned from dental eruption sequences and based on previous observations (Table 2). All animals were weighed with either an electronic or hanging scale, and measured with a tape measure and sliding calipers. Parameters and protocols describing all measurements are available through the Bones and Behavior Working Group (2015; http://www.bonesandbehavior.org/). All animals were released to their social group after sampling and recovery from anesthesia. Observations during trapping allowed us to confirm the animals' social group and local population affiliation.
Table 2.
Dental Age Classes based on dental eruption sequences for Chlorocebus. Age range listed is the lower range for initiation of that age class.
| Dental Age Class | Permanent Dentition Present | Age Range (months) |
|---|---|---|
| 1 | All deciduous | 6 – 115 days |
| 2 | All deciduous, M1 | 12–14 |
| 3 | M1, I1, I2 | 22–27 |
| 4 | M1, I1, I2, M2 | 26–31 |
| 5 | M1, I1, I2, M2, P3, P4 | 32 – 40 |
| 6 | M1, I1, I2, M2, P3, P4, C | 38–41 |
| Adult | Eruption of M3 | > 38 |
For the present study, we chose metrics representative of skeletal size (body length, thigh length, leg length, and foot length) and body mass from a total of 1613 vervets in four geographically and genomically distinct populations: Ch. aethiops in Ethiopia, Ch. p. hilgerti in Kenya, Ch. p. pygerythrus in South Africa, and Ch. sabaeus on the Caribbean islands of St. Kitts and Nevis (Table 3). The Caribbean populations are known to be descended from West African Ch. sabaeus brought to the Caribbean several hundred years ago (Warren et al. 2015). Of the whole sample, 288 females and 460 males were dentally immature. Sexual maturity is typically not reached in vervets until near the time of canine tooth eruption, here denoting the beginning of dental age 6 (Cramer et al., 2013; Rodriguez et al., 2015a); although somatic and skeletal growth often continues beyond the emergence of the third molar, which is here denoted as adult (Bolter & Zihlman, 2003). As is common, dental age and skeletal age are presumed to be similarly correlated across the genus, meaning that comparable dental age implies comparable skeletal developmental age across populations (Šešelj, 2013).
Table 3.
Summary statistics for vervet morphometric data
| Taxon | Location | Sex | Dental Age | n | Body Weight (kg) | Body Length (cm) | n | Thigh (cm) | Leg (cm) | Foot (cm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | ||||||
| Ch. aethiops (n = 139) | Ethiopia | Male (n = 69) | 1 | 8 | 0.55 | 0.15 | 18.33 | 2.08 | - | - | - | - | - | - | - |
| 2 | 16 | 1.25 | 0.24 | 25.66 | 2.02 | - | - | - | - | - | - | - | |||
| 3 | 4 | 1.61 | 0.11 | 30.20 | 2.72 | - | - | - | - | - | - | - | |||
| 4 | 3 | 1.72 | 0.36 | 29.70 | 2.10 | - | - | - | - | - | - | - | |||
| 5 | 4 | 2.33 | 0.11 | 32.23 | 2.80 | - | - | - | - | - | - | - | |||
| 6 | 5 | 3.54 | 1.06 | 36.32 | 2.48 | - | - | - | - | - | - | - | |||
| Adult | 29 | 4.02 | 0.68 | 38.18 | 2.91 | - | - | - | - | - | - | - | |||
|
| |||||||||||||||
| Female (n =106) | 1 | 2 | 0.24 | 0.13 | 15.45 | 0.64 | - | - | - | - | - | - | - | ||
| 2 | 12 | 1.29 | 0.13 | 25.72 | 2.27 | - | - | - | - | - | - | - | |||
| 3 | 4 | 1.79 | 0.42 | 28.13 | 2.78 | - | - | - | - | - | - | - | |||
| 4 | 4 | 1.84 | 0.42 | 30.15 | 3.99 | - | - | - | - | - | - | - | |||
| 5 | - | - | - | - | - | - | - | - | - | - | - | - | |||
| 6 | 7 | 2.16 | 0.19 | 31.43 | 0.93 | - | - | - | - | - | - | - | |||
| Adult | 41 | 2.70 | 0.51 | 33.28 | 2.02 | - | - | - | - | - | - | - | |||
|
| |||||||||||||||
| Ch. pygerythrus (n = 1065) | Kenya Ch. p. hilgerti (n =352) | Male (n = 183) | 1 | 31 | 0.84 | 0.31 | 21.84 | 5.06 | 32 | 8.95 | 1.47 | 9.20 | 1.57 | 9.05 | 1.22 |
| 2 | 37 | 1.38 | 0.21 | 28.34 | 2.24 | 37 | 10.74 | 1.07 | 11.03 | 1.19 | 10.38 | 1.20 | |||
| 3 | 20 | 1.66 | 0.18 | 30.33 | 2.47 | 20 | 11.58 | 0.92 | 12.13 | 0.90 | 10.93 | 0.69 | |||
| 4 | 22 | 2.01 | 0.29 | 32.64 | 2.11 | 22 | 12.39 | 1.03 | 12.91 | 0.93 | 11.78 | 1.23 | |||
| 5 | 6 | 2.46 | 0.42 | 34.33 | 2.34 | 6 | 14.00 | 0.84 | 14.33 | 0.82 | 13.25 | 0.52 | |||
| 6 | 9 | 3.10 | 0.66 | 37.33 | 1.48 | 9 | 15.67 | 0.79 | 16.11 | 1.05 | 13.39 | 0.99 | |||
| Adult | 58 | 4.34 | 0.64 | 41.60 | 2.98 | 58 | 16.32 | 1.18 | 16.56 | 0.99 | 14.04 | 0.97 | |||
|
| |||||||||||||||
| Female (n = 169) | 1 | 6 | 0.92 | 0.10 | 23.42 | 1.53 | 6 | 9.58 | 0.97 | 10.05 | 0.91 | 9.13 | 0.67 | ||
| 2 | 16 | 1.40 | 0.32 | 28.33 | 2.63 | 16 | 10.81 | 0.98 | 11.38 | 1.18 | 10.47 | 0.85 | |||
| 3 | 9 | 1.63 | 0.11 | 30.61 | 1.96 | 9 | 11.50 | 0.71 | 12.17 | 0.79 | 11.33 | 0.35 | |||
| 4 | 12 | 1.73 | 0.25 | 31.67 | 1.89 | 12 | 11.79 | 1.10 | 12.38 | 1.09 | 11.17 | 0.72 | |||
| 5 | 2 | 2.38 | 0.53 | 35.00 | 0.00 | 2 | 15.00 | 2.12 | 14.50 | 0.71 | 13.25 | 0.35 | |||
| 6 | 31 | 2.41 | 0.33 | 34.47 | 2.28 | 31 | 13.65 | 0.97 | 13.92 | 0.98 | 12.05 | 0.53 | |||
| Adult | 93 | 2.97 | 0.48 | 36.77 | 2.91 | 94 | 14.07 | 1.14 | 14.23 | 1.08 | 12.14 | 0.86 | |||
|
| |||||||||||||||
| South Africa Ch. p. pygerythrus (n =713) | Male (n = 343) | 1 | 27 | 1.37 | 0.42 | 24.46 | 3.52 | 28 | 11.09 | 1.37 | 12.21 | 1.60 | 10.46 | 0.90 | |
| 2 | 78 | 1.86 | 0.45 | 28.51 | 3.07 | 79 | 12.50 | 1.19 | 13.61 | 1.23 | 11.39 | 1.07 | |||
| 3 | 39 | 2.24 | 0.48 | 30.09 | 3.63 | 39 | 13.72 | 1.16 | 15.05 | 1.17 | 12.17 | 1.01 | |||
| 4 | 43 | 2.74 | 0.55 | 33.85 | 3.72 | 43 | 14.71 | 1.21 | 16.21 | 1.16 | 13.13 | 0.98 | |||
| 5 | 21 | 3.15 | 0.53 | 34.71 | 2.94 | 22 | 15.48 | 1.46 | 17.30 | 1.31 | 13.84 | 0.92 | |||
| 6 | 31 | 4.48 | 1.17 | 38.98 | 3.84 | 31 | 17.32 | 1.34 | 18.84 | 1.19 | 14.71 | 1.22 | |||
| Adult | 104 | 5.69 | 0.73 | 40.81 | 3.56 | 104 | 18.50 | 1.24 | 19.95 | 1.08 | 14.92 | 0.80 | |||
|
| |||||||||||||||
| Female (n = 370) | 1 | 27 | 1.27 | 0.33 | 23.81 | 2.41 | 26 | 10.92 | 1.33 | 12.10 | 1.32 | 10.26 | 0.70 | ||
| 2 | 50 | 1.74 | 0.43 | 27.87 | 3.07 | 50 | 12.18 | 1.18 | 13.40 | 1.26 | 11.22 | 0.98 | |||
| 3 | 30 | 2.03 | 0.39 | 28.65 | 3.59 | 30 | 13.25 | 0.94 | 14.42 | 0.86 | 11.53 | 0.72 | |||
| 4 | 37 | 2.50 | 0.51 | 31.97 | 3.04 | 37 | 14.04 | 1.42 | 15.01 | 1.23 | 12.30 | 0.89 | |||
| 5 | 8 | 2.79 | 0.62 | 33.50 | 3.37 | 8 | 15.13 | 1.30 | 16.31 | 1.33 | 12.94 | 0.98 | |||
| 6 | 53 | 3.45 | 0.56 | 35.76 | 2.67 | 53 | 15.42 | 0.89 | 16.65 | 0.67 | 13.02 | 0.72 | |||
| Adult | 165 | 4.09 | 0.66 | 37.18 | 3.30 | 166 | 16.00 | 0.97 | 17.06 | 0.92 | 13.12 | 0.85 | |||
|
| |||||||||||||||
| Ch. sabaeus (n = 409) | St. Kitts & Nevis | Male (n = 212) | 1 | 21 | 0.96 | 0.21 | 22.67 | 5.90 | 22 | 9.50 | 0.83 | 10.93 | 1.82 | 9.59 | 0.81 |
| 2 | 28 | 1.37 | 0.45 | 26.14 | 3.95 | 28 | 11.20 | 1.30 | 12.37 | 2.10 | 10.70 | 1.05 | |||
| 3 | 19 | 2.05 | 0.39 | 29.66 | 2.11 | 19 | 13.11 | 1.17 | 14.53 | 1.18 | 11.84 | 0.80 | |||
| 4 | 15 | 2.36 | 0.46 | 31.40 | 2.85 | 14 | 13.89 | 1.38 | 15.54 | 1.41 | 12.54 | 1.18 | |||
| 5 | 16 | 2.69 | 0.46 | 32.91 | 3.29 | 16 | 14.59 | 1.05 | 16.00 | 1.06 | 13.25 | 1.83 | |||
| 6 | 37 | 4.04 | 0.92 | 36.77 | 3.84 | 38 | 16.84 | 1.47 | 18.58 | 1.39 | 14.45 | 0.85 | |||
| Adult | 90 | 5.67 | 0.74 | 40.41 | 3.08 | 90 | 18.24 | 0.83 | 19.81 | 0.76 | 15.11 | 0.67 | |||
|
| |||||||||||||||
| Female (n = 183) | 1 | 17 | 0.89 | 0.23 | 22.26 | 1.94 | 18 | 9.42 | 0.75 | 10.61 | 0.99 | 9.33 | 0.75 | ||
| 2 | 16 | 1.62 | 0.28 | 26.94 | 2.29 | 17 | 11.62 | 0.82 | 12.79 | 1.06 | 10.68 | 0.79 | |||
| 3 | 9 | 2.28 | 0.49 | 31.78 | 3.36 | 10 | 13.10 | 1.82 | 14.60 | 1.87 | 12.05 | 1.23 | |||
| 4 | 14 | 1.99 | 0.51 | 30.07 | 2.37 | 13 | 13.23 | 1.24 | 14.43 | 1.22 | 11.86 | 0.77 | |||
| 5 | 10 | 2.37 | 0.20 | 31.70 | 2.20 | 10 | 14.00 | 0.78 | 15.56 | 0.73 | 12.20 | 0.59 | |||
| 6 | 53 | 3.41 | 0.68 | 34.59 | 2.99 | 53 | 15.31 | 0.80 | 16.45 | 0.79 | 13.08 | 0.97 | |||
| Adult | 64 | 3.54 | 0.81 | 34.67 | 2.57 | 64 | 15.33 | 0.92 | 16.47 | 0.75 | 12.91 | 0.79 | |||
All measurements were developed by CJJ and TRT and other measurers (CAS and JDC) were trained directly by TRT. During training, repeated measures of the same individual were conducted in tandem with TRT until concordance was reached.
The location of each trapping site is reported in decimal degrees (Table 1), and for most sites measured using hand-held GPS units. For those trapping sites lacking GPS readings, a general latitude and longitude for the trapping area (e.g., game reserve, town) was used. Human impact at each trapping location was assessed according to conditions during the time of trapping using a previously published index developed by Pampush (2010) to study variation in vervet body size, and subsequently used by Loudon et al. (2014) and Fourie et al. (2015) (Table 1). This index includes presence/absence measures of reliable access to 1) agricultural land, 2) human food, 3) rubbish or garbage dumps, and 4) whether animals are regularly provisioned, as well as a three-level scale of human activity within the presumed home range of the group (low, moderate, or high). In the index, point values are assigned to each value, with the lowest tier of human impact each receiving a 1, scaling up by 1 for each level. Added together, these values comprise a human impact group ranging from low (lowest score in each category; index=5), to moderate (index=6–8), to high (index=9–11). These measures take into account only the ecological impact of humans, and do not address local ecological variables (such as native plant productivity) that might also influence body size and growth. As a proxy for these measures, we collected several climatic variables for trapping sites from the WorldClim 2 database, which has a spatial resolution of about 1 km2 (Fick & Hijmans, 2017). Climatic variables considered for inclusion in our models were 1) annual mean temperature (in degrees Celsius), 2) temperature seasonality (measured as the standard deviation of annual mean temperature multiplied by 100), 3) the minimum temperature of the coldest month (in degrees Celsius), 4) the mean temperature of the coldest quarter of the year, 5) annual precipitation (in mm), and 6) precipitation seasonality (measured as the coefficient of variation of monthly precipitation). Climate data were accessed via the R package raster v. 2.6–7 (Hijmans & van Etten, 2012), and assigned to trapping sites based on latitude and longitude.
Statistical analyses
Summary statistics of somatic measures are presented as mean ± standard deviation for each population and age category (Table 3) Due to the uneven sample sizes and differences in variance structure across age/sex/taxon categories, we assessed differences in mean measures using a Welch ANOVA with a Games-Howell Tukey post hoc test implemented using the package userfriendlyscience v. 03-0 (Peters, 2015) in R v. 3.2.0 (R Core Team 2017). We used linear mixed modeling, also implemented in R, to assess the influence of latitude (in decimal degrees), altitude (in meters), human impacts (using the human impact group), and climatic variables on adult body mass and length. We used the absolute value of latitude to allow for negative latitudinal values in the southern hemisphere. We included country of origin as a random effect to control for potentially uncontrolled effects that might be the result of large-scale population differences. With models fit in lme4 v. 1.1–13 (Bates et al., 2015), we used the Akaike Information Criterion with a correction for finite sample sizes (AICc) to compare models with differing fixed effects structures (Burnham & Anderson, 2002) using the package AICcmodavg v. 2.1-1 (Mazerolle, 2017). Final models were assessed for goodness of fit to the data using the R2 for mixed models method reported by Nakagawa and Schielzeth (2013), implemented in the package MuMIn v. 1.15.6 (Barton, 2016). We used MCMC-estimated P values to assess the significance of fixed effects (α = 0.05) for the final models in the package MCMCglmm v. 2.24 (Hadfield, 2010), using flat priors. We allowed the Markov chains a burn-in period of 1,000 iterations, after which we ran 100,000 iterations and sampled every 20th iteration for the posterior distribution.
Relative hind limb lengths were measured as the ratio of each limb segment to body length, respectively (e.g., thigh length/body length). We used the methods above to fit generalized linear mixed models for the hind limb proportions with the exception that models were fit with a beta distribution in the package glmmADMB v. 0.8.3.3 (Fournier et al., 2012) to appropriately model proportions, with country of origin as a random effect. We selected the best model based on comparatively lowest AIC using the package bbmle v. 1.0.20 (Bolker, 2017). To estimate goodness-of-fit, pseudo-R2 values for the best beta regression models were derived from fixed effects only in the package betareg v. 3.1-0 (Cribari-Neto & Zeileis, 2010). To allow for sexual dimorphism, all models were run separately for males and females. Sexual dimorphism for each taxon was measured as the average mass of adult males divided by the average mass of adult females (Smith 1999). We assessed whether differences in sexual dimorphism between populations were significant using the method of Relethford and Hodges (1985).
In our data set, relying on cross-sectional dental eruption means that a range of chronological ages may be encompassed within a single dental age category, as age at particular tooth eruption can vary both within and across taxa, although typically not between sexes (Smith et al., 1994). Turner et al. (1997) and Bolter and Zihlman (2003) have suggested that, in vervets (Ch. p. hilgerti and Ch. tantalus, respectively), these dental age categories represent uneven, approximately 3–12 month periods of age up until adulthood, which contains all adults from the eruption of the third molar onward (Table 2). Here, we interpret significant changes in size between these categories as indicative of patterns of growth. Longitudinal measurement data on wild individuals of known ages will be necessary to validate this assumption. This interpretation of growth is limited, however, to those intervals represented by age categories 6 and prior, after which point the relatively wide chronological age range encompassed by of ‘adult’ age assignment, here after the emergence of the third molar, obscures the pattern of any further growth.
We visualize and compare growth patterns within and across taxa using loess smoothers, or nonparametric locally weighted quadratic least squares regression, implemented in ggplot2 (Wickham, 2009). Localized loess smoothers make no assumptions about the shape of the curve, and are robust to uneven samples (e.g., Leigh 1992; Leigh & Shea 1996). We modeled each trait in each sex within each taxon separately. We attempted this model fitting using estimated chronological age categories from within the range suggested by previous work (Turner et al., 1997; Bolter & Zihlman, 2003). In loess models, estimated chronological age categories were treated as continuous variables, and taxonomic designation and geographic origin were modeled as random effects. For all tests, we considered a P value of less than 0.05 to be significant.
RESULTS
Prediction 1: Population variation in vervet body size will follow Bergmann’s Rule (larger in higher latitudes and altitudes)
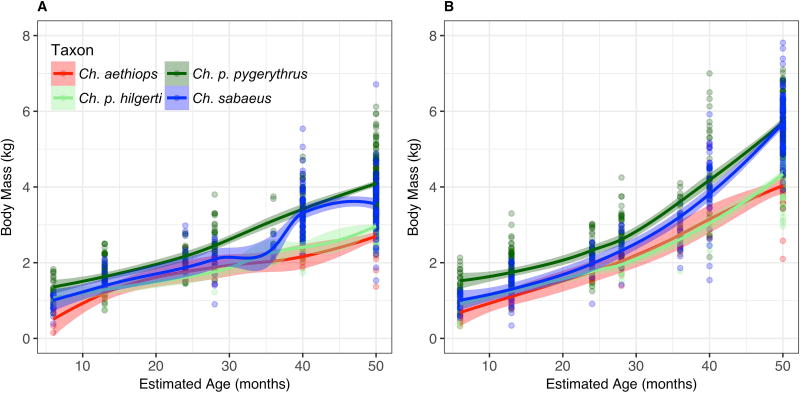
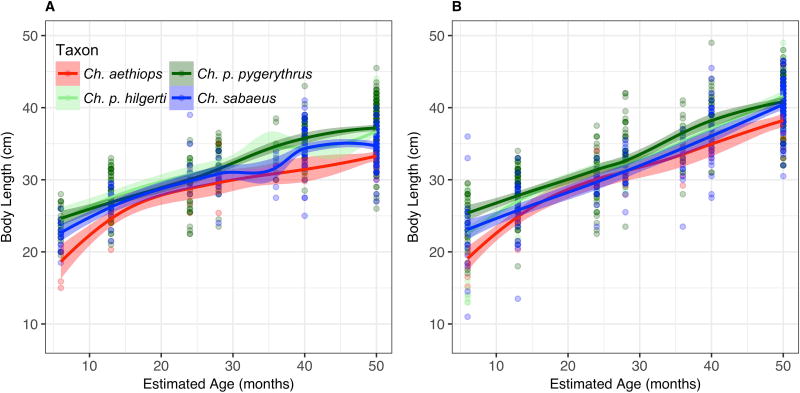
As predicted and consistent with Bergmann’s Rule, our linear model shows a significant positive relationship between body mass and latitude in adult females, with body mass increasing as latitude increases (β= 0.033, p = 0.022; R2GLMM(c) = 0.690; Table 4), but not with altitude (β = 0.000, p = 0.678). Loess curves for adult female body mass suggest that the high latitude Ch. p. pygerythrus population (23° – 34° S) maintains a heavier mass than all other taxa throughout development (Fig. 2a; Table 3), significantly so for dental ages 1 (p < 0.05), 6 (p < 0.001; all populations but Ch. sabaeus), and adults (p < 0.001). Although females of the intermediate latitude Ch. sabaeus population (17° N in St. Kitts; 13° N in ancestral populations in The Gambia) maintain an intermediate mass similar to the sampled Ch. p. hilgerti and Ch. aethiops populations for most of development, between dental ages 5 and 6 they experience a steep increase in mass that nearly catches up in size to Ch. p. pygerythrus females. This rapid growth in Ch. sabaeus females apparently levels off sooner in adulthood than in Ch. p. pygerythrus, as the latter females ultimately reach a significantly higher average adult size. The lower latitude, East African Chlorocebus females, Ch. p. hilgerti (3° S – 1° N) and Ch. aethiops (7° – 12° N), maintain masses similar to each other throughout the life course, with the notable exception that female Ch. aethiops are much smaller than all other taxa at dental age 1, but grow rapidly at this stage to catch up to Ch. p. hilgerti and Ch. sabaeus females by dental age 2. The end result of these growth patterns is a scale of mass in Chlorocebus females that follows Bergmann’s Rule, with the equatorial East African females being the smallest, mid-range Ch. sabaeus females being intermediate, and the high latitude Ch. p. pygerythrus females being the largest.
Table 4.
Best model regression results of the influence of latitude, altitude and human impact on adult Chlorocebus a) body size, b) body length, c) thigh/body length, d) leg/body length, and e) foot/body length. Posterior means (parameter estimate or b), 95% credibility intervals, and P values are estimated using MCMC with significant values in bold. Data for insignificant interaction terms are not included in the table. Since random effect estimates did not overlap with zero, both the marginal R2GLMM (assessing the fit of fixed effects) value and conditional R2GLMM (assessing the fit of fixed and random effects) are used to report goodness of fit.
| Adult Females | Adult Males | Adult Males (w/o SKN) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| β | 95% CI | pMCMC | β | 95% CI | pMCMC | β | 95% CI | pMCMC | ||||||||
|
|
|
|||||||||||||||
| lower | upper | lower | upper | lower | upper | |||||||||||
| Body Mass (kg) | Intercept | 2.557 | 1.994 | 3.450 | < 0.001 | 13.222 | −0.964 | 30.115 | 0.061 | 2.302 | 0.956 | 3.921 | 0.003 | |||
| Latitude | 0.033 | 0.006 | 0.058 | 0.022 | −0.358 | −0.784 | 0.188 | 0.231 | 0.083 | 0.035 | 0.136 | 0.006 | ||||
| Altitude | 0.000 | 0.000 | 0.000 | 0.678 | −0.011 | −0.021 | 0.004 | 0.179 | 0.001 | 0.000 | 0.002 | 0.011 | ||||
| Human Index: Low | −0.378 | −0.675 | −0.015 | 0.044 | −15.134 | −31.512 | 1.606 | 0.086 | −0.155 | −0.620 | 0.242 | 0.402 | ||||
| Human Index: High | 0.297 | 0.034 | 0.680 | 0.029 | −10.024 | −24.666 | 7.633 | 0.310 | −0.042 | −0.529 | 0.462 | 0.933 | ||||
| Latitude:Altitude | 0.000 | 0.000 | 0.000 | 0.136 | 0.000 | 0.000 | 0.001 | 0.139 | 0.000 | 0.000 | 0.000 | 0.288 | ||||
| Latitude:HI_Low | - | - | - | - | 0.502 | −0.059 | 0.945 | 0.087 | - | - | - | - | ||||
| Latitude:HI_High | - | - | - | - | 0.196 | −0.256 | 0.722 | 0.340 | - | - | - | - | ||||
| Altitude:HI_Low | - | - | - | - | 0.010 | −0.002 | 0.024 | 0.086 | - | - | - | - | ||||
| Altitude:HI_High | - | - | - | - | 0.009 | −0.005 | 0.020 | 0.244 | - | - | - | - | ||||
| Latitude:Altitude:HI_Low | - | - | - | - | 0.000 | 0.000 | 0.000 | 836.000 | - | - | - | - | ||||
| Latitude:Altitude:HI_High | - | - | - | - | 0.000 | 0.000 | 0.000 | 0.272 | - | - | - | - | ||||
| Country | 0.015 | 0.000 | 0.001 | - | 0.008 | 0.000 | 0.000 | - | 0.032 | 0.000 | 0.000 | - | ||||
|
| ||||||||||||||||
| R2GLMM marginal | 0.627 | 0.581 | 0.588 | |||||||||||||
| R2GLMM conditional | 0.690 | 0.616 | 0.627 | |||||||||||||
|
|
||||||||||||||||
| Body Length (cm) | Intercept | 37.518 | 35.231 | 41.019 | < 0.001 | 56.430 | 37.080 | 75.770 | < 0.001 | 54.810 | 27.510 | 79.500 | 0.008 | |||
| Latitude | −0.094 | −0.166 | −0.023 | 0.008 | −0.643 | −1.137 | −0.149 | 0.009 | −0.616 | −1.136 | −0.118 | 0.021 | ||||
| Altitude | 0.001 | 0.001 | 0.001 | < 0.001 | −0.005 | −0.019 | 0.010 | 0.508 | 0.003 | −0.019 | 0.010 | 0.669 | ||||
| Human Index: Low | −4.181 | −4.795 | −3.572 | < 0.001 | −29.460 | −47.770 | −12.360 | 0.002 | −29.510 | −47.050 | −10.570 | 0.001 | ||||
| Human Index: High | −0.898 | −1.503 | −0.158 | 0.012 | −10.240 | −29.420 | 11.110 | 0.322 | −16.550 | −43.590 | 9.744 | 0.232 | ||||
| Latitude:Altitude | - | - | - | - | 0.000 | 0.000 | 0.000 | 0.708 | 0.000 | 0.000 | 0.001 | 0.891 | ||||
| Latitude:HI_Low | 0.152 | 0.125 | 0.176 | < 0.001 | 0.873 | 0.347 | 1.430 | 0.002 | 0.873 | 0.313 | 1.422 | 0.001 | ||||
| Latitude:HI_High | 0.011 | −0.020 | 0.036 | 0.570 | 0.260 | −0.398 | 0.872 | 0.425 | 0.475 | −0.409 | 1.328 | 0.286 | ||||
| Altitude:HI_Low | - | - | - | - | 0.022 | 0.008 | 0.036 | 0.002 | 0.022 | 0.007 | 0.036 | 0.002 | ||||
| Altitude:HI_High | - | - | - | - | 0.007 | −0.008 | 0.021 | 0.354 | 0.010 | −0.006 | 0.028 | 0.257 | ||||
| Latitude:Altitude:HI_Low | - | - | - | - | −0.001 | −0.001 | 0.000 | 0.004 | −0.001 | −0.001 | 0.000 | 0.006 | ||||
| Latitude:Altitude:HI_High | - | - | - | - | 0.000 | −0.001 | 0.000 | 0.518 | 0.000 | −0.001 | 0.000 | 0.370 | ||||
| Country | 8.547 | 0.273 | 25.460 | - | 104.300 | 3.440 | 294.100 | - | 472.700 | 5.160 | 1197.000 | - | ||||
|
| ||||||||||||||||
| R2GLMM marginal | 0.373 | 0.257 | 0.363 | |||||||||||||
| R2GLMM conditional | 0.375 | 0.259 | 0.365 | |||||||||||||
|
|
||||||||||||||||
Fig. 2.
Loess models for changes in body mass (kg) by estimated chronological age in Chlorocebus a) females, and b) males with 95% confidence sheaths. Each cluster of points represents the midpoint of the interval of a single dental age category, from 1 to 6. Category 7, or ‘Adult’, includes adults in whom at least one third molar has erupted, and whose mean age is unknown. Their data points are therefore situated on the plot at 50 months, the start of this age interval, when the animal is at or close to adult size.
The scaling of Bergmann’s Rule, however, does not adequately describe the variation seen in males, as there is no significant relationship between body size and latitude (β = −0.358, p = 0.231) or altitude (β = −0.011, p = 0.179; R2GLMM(c) = 0.616; Table 4). Although the size relationship between the larger Ch. p. pygerythrus and smaller East African taxa holds, the relatively rapid growth of Ch. sabaeus males from Dental Age 2 onward to eventually match the mass of Ch. p. pygerythrus adult males is unexpected within the context of Bergmann’s Rule (Fig. 2b; Table 4). Indeed, when the Ch. sabaeus males are removed from the regression equation, Bergmann’s Rule is supported, with significant positive relationships between body weight and both latitude (β = 0.083, p = 0.006) and altitude (β = 0.001, p = 0.011; Table 4). Both Ch. p. hilgerti and Ch. aethiops males, like the females, remain matched in size, and significantly smaller than both Ch. p. pygerythrus and Ch. sabaeus for much of their development.
Models including climatic variables in models for body mass show no effects of latitude and altitude, but also no consistent evidence of climatic effects on body mass variation. In females, the best model indicated no significant effects on body mass of any covariate considered (Table 5). In males, there were significant but negligibly small effects of temperature seasonality and annual precipitation, and, consistent with Bergmann’s Rule, slightly larger negative effects on body mass of the minimum temperature of the coldest month (β = −0.055, p = 0.046). A small but negative relationship between body mass and precipitation seasonality (β = −0.017, p < 0.001; Table 5) suggests that male body size may decrease in areas with highly seasonal rainfall, which may also be consistent with Bergmann’s Rule. For the sake of consistency, we also ran the global model with climatic variables on males excluding Ch. sabaeus, and found the same significant but small effects of temperature seasonality and annual precipitation, as well as a larger negative relationship between male body mass and annual mean temperature, consistent with Bergmann’s Rule.
Table 5.
Best global model regression results of the influence of latitude, altitude and human impact with climatic variables on adult Chlorocebus a) body size, b) body length. Posterior means (parameter estimate or b), 95% credibility intervals, and P values are estimated using MCMC with significant values in bold. Data for insignificant interaction terms are not included in the table. Since random effect estimates did not overlap with zero, both the marginal R2GLMM (assessing the fit of fixed effects) value and conditional R2GLMM (assessing the fit of fixed and random effects) are used to report goodness of fit.
| Adult Females | Adult Males | Adult Males (w/o SKN) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| β | 95% Cl | pMCMC | β | 95% Cl | pMCMC | β | 95% Cl | pMCMC | ||||||||
|
|
|
|||||||||||||||
| lower | upper | lower | upper | lower | upper | |||||||||||
| Body Mass (kg) | Intercept | 2.924 | 0.637 | 5.021 | 0.011 | 4.081 | 2.936 | 5.262 | < 0.001 | 5.455 | 2.831 | 8.063 | 0.002 | |||
| Latitude | 0.031 | −0.008 | 0.069 | 0.131 | - | - | - | - | - | - | - | - | ||||
| Altitude | 0.000 | 0.000 | 0.001 | 0.240 | 0.000 | 0.000 | 0.001 | 0.097 | 0.000 | 0.000 | 0.001 | 0.658 | ||||
| Human Index: Low | −0.272 | −0.700 | 0.174 | 0.230 | - | - | - | - | - | - | - | - | ||||
| Human Index: Moderate | −0.248 | −0.585 | 0.102 | 0.173 | - | - | - | - | - | - | - | - | ||||
| Annual Mean Temp. | - | - | - | - | - | - | - | - | −0.109 | −0.201 | 0.016 | 0.019 | ||||
| Temp. Seasonality | 0.000 | 0.000 | 0.000 | 0.081 | 0.000 | 0.000 | 0.001 | 0.003 | 0.000 | 0.000 | 0.001 | 0.014 | ||||
| Min. Temp. Coldest Month | 0.068 | −0.063 | 0.191 | 0.291 | −0.055 | −0.109 | −0.001 | 0.046 | - | - | - | - | ||||
| Mean Temp. Coldest Quarter | −0.099 | −0.233 | 0.039 | 0.164 | - | - | - | - | - | - | - | - | ||||
| Annual Precipitation | 0.001 | 0.000 | 0.001 | 0.096 | 0.002 | 0.001 | 0.002 | < 0.001 | 0.001 | 0.000 | 0.002 | 0.011 | ||||
| Precipitation Seasonality | - | - | - | - | −0.017 | −0.025 | −0.009 | < 0.001 | −0.004 | −0.012 | 0.003 | 0.263 | ||||
| Country | 0.016 | 0.000 | 0.000 | - | 0.000 | 0.000 | 0.000 | - | 2.387 | 0.000 | 7.249 | - | ||||
|
| ||||||||||||||||
| R2GLMM marginal | 0.636 | 0.547 | 0.492 | |||||||||||||
| R2GLMM conditional | 0.700 | 0.580 | 0.512 | |||||||||||||
|
|
||||||||||||||||
| Body Length (cm) | Intercept | 39.243 | 35.172 | 43.387 | < 0.001 | 51.972 | 38.193 | 64.674 | < 0.001 | 46.511 | 45.444 | 47.543 | < 0.001 | |||
| Latitude | - | - | - | - | −0.507 | −0.660 | −0.350 | < 0.001 | - | - | - | - | ||||
| Altitude | 0.003 | 0.002 | 0.004 | < 0.001 | 0.002 | 0.001 | 0.003 | < 0.001 | 0.001 | 0.001 | 0.001 | < 0.001 | ||||
| Human Index: Low | - | - | - | - | −1.342 | −1.863 | −0.814 | < 0.001 | - | - | - | - | ||||
| Human Index: Moderate | - | - | - | - | 0.141 | −0.416 | 0.681 | 0.620 | - | - | - | - | ||||
| Annual Mean Temp. | - | - | - | - | −0.487 | −0.690 | −0.294 | < 0.001 | - | - | - | - | ||||
| Temp. Seasonality | - | - | - | - | - | - | - | - | - | - | - | - | ||||
| Min. Temp. Coldest Month | 0.726 | 0.618 | 0.842 | < 0.001 | 0.534 | 0.397 | 0.662 | < 0.001 | 0.563 | 0.478 | 0.640 | < 0.001 | ||||
| Mean Temp. Coldest Quarter | −0.773 | −0.949 | −0.593 | < 0.001 | - | - | - | - | −0.949 | −1.036 | −0.862 | < 0.001 | ||||
| Annual Precipitation | - | - | - | - | - | - | - | - | 0.003 | 0.002 | 0.003 | < 0.001 | ||||
| Precipitation Seasonality | - | - | - | - | - | - | - | - | ||||||||
| Country | 12.680 | 0.420 | 34.290 | - | 173.000 | 10.050 | 518.700 | - | 0.017 | 0.000 | 0.013 | - | ||||
|
| ||||||||||||||||
| R2GLMM marginal | 0.333 | 0.203 | 0.235 | |||||||||||||
| R2GLMM conditional | 0.335 | 0.205 | 0.236 | |||||||||||||
|
|
||||||||||||||||
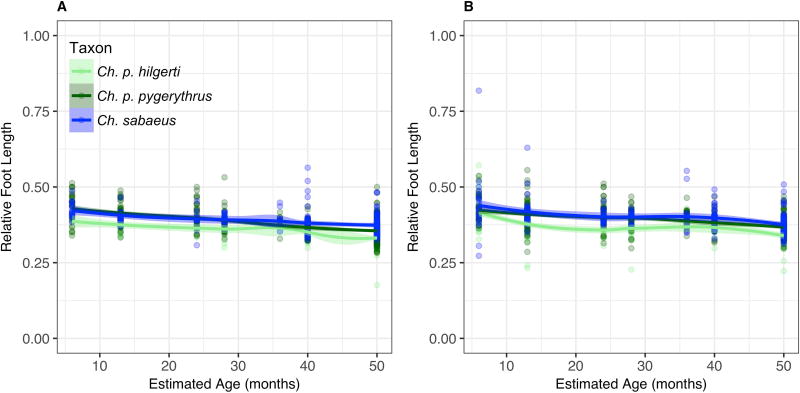
Prediction 2: Population variation in vervet limb length will follow Allen’s Rule (shorter in higher latitudes and altitudes)
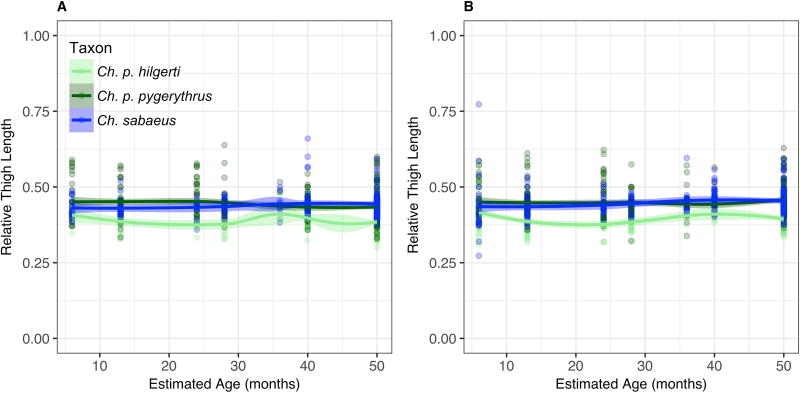
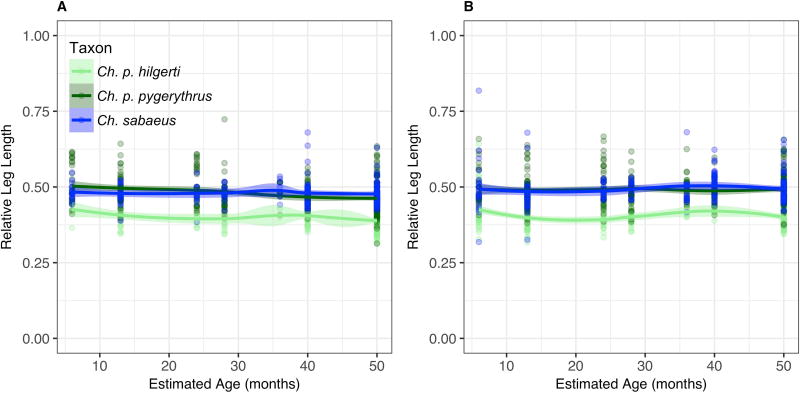
Contrary to expectations according to Allen’s Rule, both thigh and leg lengths for the adult equatorial Ch. p. hilgerti population are absolutely and relatively (compared to body length) shorter than those in Ch. p. pygerythrus, at almost every age, or Ch. sabaeus from dental age 6 onward (Fig. 4 and Fig. 5; Table 6). Our models that only take into account latitude, altitude, and human impacts do suggest that relative limb length is significantly related to altitude, latitude, and their interaction effects, supporting Allen’s Rule in both sexes for both the thigh and the leg. However, the magnitudes of the effects of these predictors are so small in each trait that they do not appear to have a large influence on trait variation (Table 6). Differences among taxa in relative thigh and leg length are already apparent by dental age 1, and growth in all taxa appears to be uniform across age categories. The one exception is Ch. p. hilgerti, in which a small sample size and some large individuals trapped at dental age 5 in Mosiro result in outliers having a profound effect on loess models at that age, although they do not appear to have influenced the Allen’s Rule model.
Fig. 4.
Loess models for changes in relative thigh length (cm) by estimated chronological age in Chlorocebus a) females, and b) males with 95% confidence sheaths. Each cluster of points represents the midpoint of the interval of a single dental age category, from 1 to 6. Category 7, or ‘Adult’, includes adults in whom at least one third molar has erupted, and whose mean age is unknown. Their data points are therefore situated on the plot at 50 months, the start of this age interval, when the animal is at or close to adult size.
Fig. 5.
Loess models for changes in relative leg length (cm) by estimated chronological age in Chlorocebus a) females, and b) males with 95% confidence sheaths. Each cluster of points represents the midpoint of the interval of a single dental age category, from 1 to 6. Category 7, or ‘Adult’, includes adults in whom at least one third molar has erupted, and whose mean age is unknown. Their data points are therefore situated on the plot at 50 months, the start of this age interval, when the animal is at or close to adult size.
Table 6.
Best global model beta regression results of the influence of latitude, altitude and human impact for adult Chlorocebus a) ratio of thigh to body length, b) ratio of leg to body length, and c) ratio of foot to body length, using Country as a random effect. Pseudo-R2 value to assess goodness-of-fit is only for fixed-effects, and does not include the random effect of country.
| Adult Females | Adult Males | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| β | Std. Error | p-value | β | Std. Error | p-value | ||
| Thigh to BL Ratio | Intercept | −0.039 | 0.086 | 0.649 | 0.051 | 0.075 | 0.497 |
| Latitude | −0.011 | 0.004 | 0.007 | −0.014 | 0.004 | < 0.001 | |
| Altitude | 0.000 | 0.000 | < 0.001 | 0.000 | 0.000 | < 0.001 | |
| Human Index: Low | 0.395 | 0.303 | 0.192 | −0.221 | 0.457 | 0.628 | |
| Human Index: Moderate | −0.431 | 0.473 | 0.362 | −0.628 | 1.150 | 0.585 | |
| Latitude:Altitude | 0.000 | 0.000 | < 0.001 | 0.000 | 0.000 | < 0.001 | |
| Latitude:HI_Low | −0.013 | 0.010 | 0.198 | 0.012 | 0.015 | 0.429 | |
| Latitude:HI_Moderate | 0.014 | 0.015 | 0.346 | 0.022 | 0.035 | 0.534 | |
| Altitude:HI_Low | 0.000 | 0.000 | 0.090 | 0.000 | 0.000 | 0.796 | |
| Altitude:HI_Moderate | 0.000 | 0.000 | 0.803 | 0.000 | 0.001 | 0.752 | |
| Latitude:Altitude:HI_Low | 0.000 | 0.000 | 0.078 | 0.000 | 0.000 | 0.635 | |
| Latitude:Altitude:HI_Moderate | 0.000 | 0.000 | 0.820 | 0.000 | 0.000 | 0.723 | |
|
| |||||||
| Pseudo-R2 | 0.305 | 0.362 | |||||
|
|
|||||||
| Leg to BL Ratio | Intercept | 0.059 | 0.086 | 0.491 | 0.232 | 0.078 | 0.003 |
| Latitude | −0.007 | 0.004 | 0.058 | −0.014 | 0.004 | 0.001 | |
| Altitude | 0.000 | 0.000 | < 0.001 | 0.000 | 0.000 | < 0.001 | |
| Human Index: Low | −0.077 | 0.305 | 0.802 | −0.678 | 0.482 | 0.160 | |
| Human Index: Moderate | −0.726 | 0.475 | 0.126 | 0.085 | 1.200 | 0.943 | |
| Latitude:Altitude | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | < 0.001 | |
| Latitude:HI_Low | −0.001 | 0.010 | 0.922 | 0.024 | 0.015 | 0.115 | |
| Latitude:HI_Moderate | 0.021 | 0.015 | 0.176 | 0.000 | 0.036 | 0.989 | |
| Altitude:HI_Low | 0.000 | 0.000 | 0.770 | 0.000 | 0.000 | 0.317 | |
| Altitude:HI_Moderate | 0.000 | 0.000 | 0.379 | 0.000 | 0.001 | 0.752 | |
| Latitude:Altitude:HI_Low | 0.000 | 0.000 | 0.451 | 0.000 | 0.000 | 0.218 | |
| Latitude:Altitude:HI_Moderate | 0.000 | 0.000 | 0.542 | 0.000 | 0.000 | 0.776 | |
|
| |||||||
| Pseudo-R2 | 0.474 | 0.541 | |||||
|
|
|||||||
| Foot to BL Ratio | Intercept | −0.314 | 0.078 | < 0.001 | −0.260 | 0.069 | < 0.001 |
| Latitude | −0.012 | 0.004 | 0.001 | −0.013 | 0.004 | < 0.001 | |
| Altitude | 0.000 | 0.000 | < 0.001 | 0.000 | 0.000 | < 0.001 | |
| Human Index: Low | −0.281 | 0.276 | 0.309 | −0.961 | 0.422 | 0.023 | |
| Human Index: Moderate | −0.646 | 0.434 | 0.137 | −0.611 | 1.057 | 0.563 | |
| Latitude:Altitude | 0.000 | 0.000 | < 0.001 | 0.000 | 0.000 | < 0.001 | |
| Latitude:HI_Low | 0.008 | 0.009 | 0.359 | 0.032 | 0.014 | 0.017 | |
| Latitude:HI_Moderate | 0.021 | 0.014 | 0.137 | 0.021 | 0.032 | 0.506 | |
| Altitude:HI_Low | 0.000 | 0.000 | 0.418 | 0.001 | 0.000 | 0.030 | |
| Altitude:HI_Moderate | 0.000 | 0.000 | 0.326 | 0.000 | 0.001 | 0.685 | |
| Latitude:Altitude:HI_Low | 0.000 | 0.000 | 0.555 | 0.000 | 0.000 | 0.028 | |
| Latitude:Altitude:HI_Moderate | 0.000 | 0.000 | 0.335 | 0.000 | 0.000 | 0.649 | |
|
| |||||||
| Pseudo-R2 | 0.239 | 0.288 | |||||
|
|
|||||||
Foot size in Chlorocebus shows the same steady growth pattern across age classes as thigh and leg (Fig. 6; Table 6). As in the rest of the lower limb, and consistent with Allen’s Rule, there is a significant relationship between foot length and latitude, altitude, and their interaction effect for both sexes (Table 6). Additionally, in males there was a significant effect of human impacts, with males in low impact sites having a significantly smaller ratio of foot length to body length (β = −0.961, p = 0.023), or relatively small feet. The estimated effects of these predictors are near zero, however, and for relative foot length goodness-of-fit of the models are not as high as for the rest of the lower limb (Table 6). In dental age 1, Ch. p. pygerythrus individuals of both sexes have significantly larger feet than in Ch. sabaeus and Ch. p. hilgerti, but a relatively high rate of growth leads to adults in the Ch. sabaeus population having feet comparable in size to Ch. p. pygerythrus adults and significantly larger than Ch. p. hilgerti adults (p < 0.001).
Fig. 6.
Loess models for changes in relative foot length (cm) by estimated chronological age in Chlorocebus a) females, and b) males with 95% confidence sheaths. Each cluster of points represents the midpoint of the interval of a single dental age category, from 1 to 6. Category 7, or ‘Adult’, includes adults in whom at least one third molar has erupted, and whose mean age is unknown. Their data points are therefore situated on the plot at 50 months, the start of this age interval, when the animal is at or close to adult size.
Models including climatic variables, for the most part, show latitude and altitude to be insignificant to morphological variation, with the exception of relative leg length in females, which increased slightly with latitude (β = 0.006, p = 0.013; Table 7). There were consistent effects of two individual climatic variables across the lower limb in both sexes: a slight increase in relative thigh, leg, and foot length with increased annual mean temperature, and increases of similar magnitude in relation to decreased minimum temperature of the coldest month (Table 7); the former is consistent with Allen’s Rule, while the latter is not. Unlike females, males show a consistent effect of human impacts (longer relative limb segment lengths in low impact areas compared to high), annual precipitation (minimal but positive), and precipitation seasonality (small but negative) across all sections of the lower limb. Models for thigh length in females also suggest a positive relationship with temperature seasonality, while leg length in females show small but significant effects of annual precipitation and precipitation seasonality similar to those seen in males. Finally, there is also a positive relationship between mean temperature of the coldest quarter of the year and relative foot length in females, which is consistent with Allen’s Rule.
Table 7.
Best global model regression results of the influence of latitude, altitude and human impact with climatic variables on adult Chlorocebus a) ratio of thigh to body length, b) ratio of leg to body length, and c) ratio of foot to body length, using Country as a random effect. Pseudo-R2 value to assess goodness-of-fit is only for fixed-effects, and does not include the random effect of country.
| Adult Females | Adult Males | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| β | Std. Error | p-value | β | Std. Error | p-value | ||
| Thigh to BL Ratio | Intercept | −0.532 | 0.161 | 0.001 | −0.904 | 0.138 | < 0.001 |
| Latitude | - | - | - | - | - | - | |
| Altitude | - | - | - | - | - | - | |
| Human Index: Low | - | - | - | 0.127 | 0.038 | 0.001 | |
| Human Index: Moderate | - | - | - | −0.005 | 0.033 | 0.891 | |
| Annual Mean Temp. | 0.033 | 0.011 | 0.003 | 0.042 | 0.009 | < 0.001 | |
| Temp. Seasonality | 0.000 | 0.000 | 0.003 | - | - | - | |
| Min. Temp. Coldest Month | −0.030 | 0.010 | < 0.001 | −0.032 | 0.005 | < 0.001 | |
| Mean Temp. Coldest Quarter | - | - | - | - | - | - | |
| Annual Precipitation | - | - | - | 0.000 | 0.000 | < 0.001 | |
| Precipitation Seasonality | - | - | - | −0.003 | 0.001 | < 0.001 | |
|
| |||||||
| Pseudo-R2 | 0.124 | 0.328 | |||||
|
|
|||||||
| Leg to BL Ratio | Intercept | −1.190 | 0.147 | < 0.001 | −1.090 | 0.141 | < 0.001 |
| Latitude | 0.006 | 0.002 | 0.013 | - | - | - | |
| Altitude | - | - | - | - | - | - | |
| Human Index: Low | −0.021 | 0.039 | 0.594 | 0.100 | 0.039 | 0.010 | |
| Human Index: Moderate | −0.063 | 0.028 | 0.025 | 0.026 | 0.034 | 0.442 | |
| Annual Mean Temp. | 0.065 | 0.015 | < 0.001 | 0.068 | 0.009 | < 0.001 | |
| Temp. Seasonality | 0.000 | 0.000 | 0.137 | - | - | - | |
| Min. Temp. Coldest Month | −0.045 | 0.011 | < 0.001 | −0.047 | 0.005 | < 0.001 | |
| Mean Temp. Coldest Quarter | - | - | - | - | - | - | |
| Annual Precipitation | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | < 0.001 | |
| Precipitation Seasonality | −0.002 | 0.001 | 0.012 | −0.005 | 0.001 | < 0.001 | |
|
| |||||||
| Pseudo-R2 | 0.454 | 0.532 | |||||
|
|
|||||||
| Foot to BL Ratio | Intercept | −0.876 | 0.133 | < 0.001 | −1.100 | 0.127 | < 0.001 |
| Latitude | - | - | - | - | - | - | |
| Altitude | - | - | - | - | - | - | |
| Human Index: Low | - | - | - | 0.117 | 0.035 | 0.001 | |
| Human Index: Moderate | - | - | - | 0.015 | 0.031 | 0.635 | |
| Annual Mean Temp. | - | - | - | 0.034 | 0.008 | < 0.001 | |
| Temp. Seasonality | - | - | - | - | - | - | |
| Min. Temp. Coldest Month | −0.027 | 0.008 | < 0.001 | −0.023 | 0.005 | < 0.001 | |
| Mean Temp. Coldest Quarter | 0.033 | 0.011 | 0.003 | - | - | - | |
| Annual Precipitation | - | - | - | 0.000 | 0.000 | 0.003 | |
| Precipitation Seasonality | - | - | - | −0.002 | 0.001 | < 0.001 | |
|
| |||||||
| Pseudo-R2 | 0.001 | 0.239 | |||||
|
|
|||||||
Prediction 3: Sexual dimorphism in vervet populations will follow Rensch’s Rule, with large taxa also having larger dimorphism
Considerable sexual dimorphism in adult body mass and length was found in each population (Table 8). In Ch. aethiops, Ch. p. hilgerti, and Ch. sabaeus males were not significantly larger than same-aged females in age classes 1–6, for either trait, while in Ch. p. pygerythrus there was significant sexual dimorphism in mass from dental age 6 (p < 0.05; Figs. 2 and 3; Tables 3 and 8). Contrary to Rensch’s Rule and our predictions, the sexual dimorphism in Ch. p. pygerythrus (the population with the largest overall body mass) was the least pronounced, although it did not differ significantly from the dimorphism seen in Ch. p. hilgerti and Ch. aethiops. The most pronounced sexual dimorphism was found in the Ch. sabaeus population, which was significantly more dimorphic than Ch. aethiops (t = −3.848, df = 220, p < 0.001), Ch. p. hilgerti (t = 4.86, df = 301, p < 0.001), and Ch. p. pygerythrus (t = 3.59, df = 419, p < 0.001) populations.
Table 8.
Body size dimorphism in adult Chlorocebus
| Taxon | Sex | n | Weight (kg) | Dimorphism | Body Length (cm) | Dimorphism | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mean | S.D. | Mean | S.D. | |||||||
| Ch. aethiops | F | 41 | 2.70 | 0.51 | 1.49 | p < 0.001 | 33.28 | 2.02 | 1.15 | p < 0.001 |
| M | 29 | 4.02 | 0.68 | 38.18 | 2.91 | |||||
|
| ||||||||||
| Ch. p. hilgerti | F | 93 | 2.97 | 0.48 | 1.46 | p < 0.001 | 36.77 | 2.91 | 1.13 | p < 0.001 |
| M | 58 | 4.34 | 0.64 | 41.60 | 2.98 | |||||
|
| ||||||||||
| Ch. p. pygerythrus | F | 165 | 4.09 | 0.66 | 1.39 | p < 0.001 | 37.18 | 3.30 | 1.10 | p < 0.001 |
| M | 104 | 5.69 | 0.73 | 40.81 | 3.56 | |||||
|
| ||||||||||
| Ch. sabaeus | F | 64 | 3.54 | 0.81 | 1.60 | p < 0.001 | 34.67 | 2.57 | 1.17 | p < 0.001 |
| M | 90 | 5.67 | 0.74 | 40.41 | 3.08 | |||||
Fig. 3.
Loess models for changes in body length (cm) by estimated chronological age in Chlorocebus a) females, and b) males with 95% confidence sheaths. Each cluster of points represents the midpoint of the interval of a single dental age category, from 1 to 6. Category 7, or ‘Adult’, includes adults in whom at least one third molar has erupted, and whose mean age is unknown. Their data points are therefore situated on the plot at 50 months, the start of this age interval, when the animal is at or close to adult size.
Prediction 4: Regional variation in size will scale with human-mediated resources
Body Mass
In keeping with our predictions, results from our linear models without climate covariates indicate that human impacts have a large and significant effect on body mass in female Chlorocebus. In adult females, this impact is scaled in accordance with the level of impact, with body mass being significantly lower in low human impact sites (β = −0.378, p = 0.044; Table 4) and significantly higher with high human impacts (β = 0.297, p = 0.029) compared to sites with moderate impacts. In males, both including and excluding Ch. sabaeus males, there was no discernible effect of human impacts on body size (Table 4). With climatic variables included, human impacts appear to only affect adult males, where lower human impacts correspond to shorter body length (Table 5) and longer relative limb lengths (Table 7).
Due to small regional sample sizes and subsequently large confidence intervals surrounding growth curves for regional populations, regional differences in body mass are difficult to differentiate in our loess models, although there were some clear outliers in most taxa. In keeping with a previously published report on these data (Turner et al., 1997), the Naivasha population was significantly heavier than the other three Ch. p. hilgerti populations, with this difference being most extreme in males; females in Kimana were also heavier than those from either Mosiro or Samburu (Suppl. Fig. 1a).
Within Ch. p. pygerythrus, access to human resources may also mediate body size. The largest-bodied Ch. p. pygerythrus population, for both males and females, comes from Gauteng, South Africa, and was trapped at or near Woodhill Golf Estate in the southeast of the city of Pretoria (Suppl. Fig. 1b). There is also a steep, early increase in size in male Ch. p. pygerythrus from the Northern Cape. This early gain in size is lost by adulthood, at which point Northern Cape males fall within the range of all other Ch. p. pygerythrus populations.
Within Ch. sabaeus, males from the two islands of St. Kitts and Nevis cannot be differentiated, while females do show some differences across islands in body mass (Suppl. Fig. 1c). Females from St. Kitts appear to be larger at almost every dental age, with the difference being particularly pronounced in dental ages 5 and 6. By adulthood this pattern has reversed, with females from Nevis growing past the size of females from St. Kitts. Due to the large age range binned within dental age 7, however, it is unclear whether this is the result of rapid or sustained post-pubertal growth, or from oversampling of older individuals in the adult category on Nevis compared to St. Kitts.
Within Ch. aethiops, very few individuals were weighed in non-Awash trapped populations. While females trapped in the Awash largely mirror the general pattern also seen in Ch. p. hilgerti to grow slowly to a relatively small adult size, males and females trapped in non-Awash areas including near the Lake Tana textile mill (n = 2) and the tourist area of Hawaasa (n = 7) appear to be of a size comparable to Ch. p. pygerythrus and Ch. sabaeus (Suppl. Fig. 1d).
Body Length
In both adult males (β = −29.460, p = 0.002; Table 4) and females (β = −4.181, p = 0.008) body length is significantly smaller in areas with low human impacts compared to those with moderate or high human impacts. Also in both sexes, there appears to be a significant positive interaction between latitude and human impacts, such that both males (β = 0.873, p = 0.002) and females (β = 0.152, p < 0.001) have significantly shorter body lengths at lower latitudes when human impacts are low compared to moderate or high. Although the best model for females did not include an interaction between altitude and human impacts, in males this same effect is seen with altitude (β = 0.022, p = 0.002). These results suggest that areas with moderate to high human impacts may buffer body length from the effects responsible for Bergmann’s Rule in this trait.
As in regional comparisons of body mass, body lengths in Ch. p. hilgerti females from Naivasha and Kimana appear to be longer than other populations, although Naivasha males at dental age 1 are significantly smaller than in other populations (Suppl. Fig. 2a). Similar patterns to those seen in body mass can also be seen in Ch. p. pygerythrus, with longer body lengths in Gauteng vervets, as well as an early increase in length in the Northern Cape (Suppl. Fig. 2b). A comparably large, but slightly later at dental age 4, early increase in body length can be seen in those vervets sampled from Mpumalanga.
Within Ch. sabaeus, growth in body length is slightly different than in body mass. While males again appear largely undifferentiated across islands, females from Nevis appear significantly larger both as juveniles and adults, although infants (dental age 1) and subadults (dental age 6) appear similar in size (Suppl. Fig. 2c).
As with body mass, average body length in Ch. aethiops individuals sampled outside the Awash region appears to be large compared to that seen in the Awash (Suppl. Fig. 2d). However, small sample sizes from outside the Awash urge caution in interpreting these differences.
Hind Limb Length
Models for relative hind limb length show no significant effect of human impacts nor any interaction effect between latitude and human impacts. Regional variation in thigh and leg lengths appear largely similar within taxa, with the exception of generally high variation at dental age 1 (Suppl. Fig. 3 and Suppl. Fig. 4). Potential exceptions include apparent divergences in females younger than ~30 months, in which we see relatively long thighs and legs in females and, to a lesser extent, males in Ch. p. pygerythrus from Limpopo in South Africa (Suppl. Fig. 3b and 4b). There are no significant regional differences in foot length (Suppl. Fig. 5).
DISCUSSION
Ecogeographic Variation and Climate
Information on body size and shape from individuals representing multiple taxa in a widely distributed group of closely related organisms allows for an assessment of the ways in which form may be modulated by local environmental constraints and selective pressures. Both excluding and including climatic variables, the patterns in mass and relative limb length noted in this study differ from those found in previous assessments of pan-African variation in vervets based on cranial size and shape (Cardini et al., 2007; Elton et al., 2010), wherein clinal variation in cranial size was consistently larger in populations nearer the Congo basin rather than in higher latitudes and, according to the authors, most probably associated with levels of rainfall. Differences among taxa in adult phenotypes and sexual dimorphism suggest some unique adaptive interpretations both within and across taxa as well as plastic responses to human impact, while variation in developmental patterns appears to underlie these differences. Our results, taken into consideration with those of Cardini et al. (2007), seem to also suggest that the cranium may be responding to different ecogeographic pressures than the body, and that size variation across populations in these two sets of traits may not be tightly correlated.
In our global models not incorporating climatic variables, geographic variation like latitude and altitude, along with human impacts in the case of body mass, consistently come out as significant covariates consistent with the patterns predicted by Bergmann’s and Allen’s Rules. With the climatic variables included, the geographic variables and human impacts become unimportant to variation in these traits, although there are no universal climatic effects across all measures, and those measures that do come out as significant are not always in accord with Bergmann’s and Allen’s Rules. This variation suggests that different aspects of physiology and parts of the body must respond to climatic variation in unique ways.
The hind limb shows some surprising patterns in Chlorocebus. Sexual dimorphism in the thigh begins at dental age 5, which is earlier than either body mass or body length. The thigh length of juvenile Ch. p. hilgerti vervets is also significantly less than that of the Ch. p. pygerythrus populations, despite the fact that Ch. p. hilgerti are similar in body length at this age. That Ch. p. hilgerti should have such relatively short hind limbs conflicts with an ecogeographic interpretation of relative limb size differences between these two closely-related taxa, and calls for a more systematic investigation of the factors affecting limb morphology that might take into account other potential intervening factors, such as differences in substrate use and relative biomechanical advantage of these relative limb differences between taxa both in adults (e.g., Weinstein, 2011), and on an ontogenetic scale that takes allometry into account (e.g., Shea, 1992; Young, 2005; Bezanson, 2009). Systematic deviations across taxa are often presumed to carry some signal of selection, as independent morphological changes in the foot and hind limb can be adaptations to species- and age-specific needs during locomotor ontogeny (Carrier, 1996; Lawler 2006). A closer investigation of how tightly the development of the hind limb is integrated with the rest of the body, taking into account ontogenetic allometry, along with comparative data on locomotor behavior, would be necessary to accurately characterize this relationship and to determine whether it is adaptively linked to functional constraints (e.g., Lawler, 2006, 2008).
Anthropogenic Impacts and Local Variation
In addition to supporting larger adaptive patterns of ecogeographic variation in body size represented by Bergmann’s Rule, both our linear and growth models suggest an equally significant role of plastic responses to human impacts on vervet body size and relative limb length. In Kenyan Ch. p. hilgerti, according to Turner et al. (1997), the divergent adult body mass and length and their associated growth patterns in Naivasha and Kimana are more likely due to access to human foods in intensely cultivated areas than to any evolutionary responses to either altitude or climate.
Similar human impacts can be seen within South African populations. The very large body sizes seen in Gauteng Ch. p. pygerythrus are largely due to sampling in the southeast of urban Pretoria – in such an urban setting, access to human food could lead to a higher calorie diet and increased body size, as seen in other primate species (Altmann et al. 1993; Altmann & Alberts 2005). The notable early increase in size in males from the Northern Cape is more difficult to attribute to a particular source. Further sampling and investigation may be needed to ascertain whether these differences are due to sampling error or local ecological factors selecting for an earlier increase in size in these populations. Contrary to published reports that primates in captivity or sanctuaries are often larger than wild populations due to the increased caloric value of human-provisioned diets (i.e., as found by Turner et al., 2016b, on St. Kitts), vervets of both sexes from the well-sampled Riverside Wildlife Rehabilitation Centre in Limpopo do not appear to be significantly heavier than wild-trapped populations in South Africa, although they were considerably smaller in body length at almost every age while also appearing to have comparatively long thigh and leg lengths relative to other populations sampled in South Africa. This differently proportioned body may reflect the poor nutrition or early life trauma that are characteristic of pets or rescued wildlife (e.g., Jones-Engel et al., 2001), as stunting based on poor nutrition and systemic disease has been noted to affect body length disproportionately to limb length, resulting in relatively longer limbs (e.g., Krishna et al., 1996; van den Berg et al., 2016). The divergent growth patterns in body length of vervets sampled in Mpumalanga may be due to their being largely sampled at or near tourist resorts at Swadini, Londolozi, and Blyde River Canyon. Vervets in these areas may also have had access to human provisioning, but why there is a more apparent increase in body length than body mass in these groups is unclear.
Differences in Dimorphism
Bergmann’s rule appears to hold across taxa in the genus Chlorocebus, with the notable exception of Ch. sabaeus males from St. Kitts & Nevis. Among primates, sexual dimorphism is attained via a number of developmental routes, and can vary markedly even between closely related taxa (Leigh 1992; Leigh and Shea, 1996; Turner et al. 1997). As in previous studies (Bolter and Zihlman, 2003; Turner et al. 1997), within each Chlorocebus population there is some evidence of modular differences in both rate of growth and overall patterns of growth. While growth patterns in Ch. sabaeus appear generally different from those seen in the South and East African taxa, the male pattern, with comparatively rapid growth in all traits during late development (age classes 4–6), leads to a particularly large adult male size relative to females.
Pronounced adult sexual dimorphism, as seen in Ch. sabaeus, is often attributed to sexual selection based on increased male-male competition rather than sex-specific responses to environmental stress (e.g., Gaulin and Sailer, 1984; Leutenegger, 1978). Although such selective pressures may be operational in all vervets, which show at least moderate sexual dimorphism, these results suggest it may be extreme in Ch. sabaeus. As directly comparative behavioral data are currently lacking across Chlorocebus populations, it is unclear whether Ch. sabaeus males face more extreme intrasexual competition than their congeners. Another physiological trait that separates Ch. sabaeus from other taxa of Chlorocebus is their remarkably blanched scrota; while most Chlorocebus males have vivid blue scrota, scrota in Ch. sabaeus blanch during development to become pale or white in adults (Cramer et al., 2013). Previous work seeking to understand the social role of this secondary sexual coloration has found it to mediate dominance status among males (Gerald, 2001), and that it may be a reliable signal of male health or quality (Gartlan & Brain, 1968; Isbell, 1995), although color itself does not appear to influence female mate choice (Gerald, 2010). Whether the extreme body size dimorphism found in Ch. sabaeus represents an adaptive response to (or perhaps cause of) this lack of color – representing a shift in signals most associated with reproductive success for males (e.g., Badyaev et al., 2002), be it through intrasexual assessment or female choice – remains a question for future study. Comparative behavioral studies on the various vervet populations could help answer these questions by examining male-male competition and female availability in different taxa. Given that scrotal coloration varies during ontogeny, and that the developmental pattern of Ch. sabaeus appears to differ from the rest of the genus, developmental studies could shed a particularly interesting light on this question. All we can say, at the moment, is that Ch. sabaeus is a compelling case suggesting that both ecogeographic factors and differing levels of sexual selection may be operating across populations to produce the pattern seen, and that more direct, comparative studies combining behavioral and morphological data between these taxa could yield interesting results.
That South African vervets are the heaviest population and yet are also the least dimorphic contradicts larger phylogenetic patterns that support Rensch’s Rule in primates (Clutton-Brock et al., 1977; Leutenegger and Cheverud, 1982; Leutenegger, 1978), although some previous intrageneric comparisons within primates have similarly shown a lack of support (Gordon, 2006). There are several potential explanations for this unexpected pattern, all of which remain speculative: intralocus sexual conflict in South African but not in other vervet populations, a phenomenon by which body size evolution towards a particular sex-specific optimum (e.g., larger body size for males) forces mass and size in the sexes to converge (e.g., Tarka et al., 2014; Lande, 1980), could explain this pattern; some unique fitness benefit for females in South Africa to become comparatively large independent of selection acting on males (e.g., fecundity advantage models: Darwin, 1874; Shine, 1988); sexual selection for larger females via either male choice or to combat male coercion (although the literature shows varying accounts of male coercion and choice in vervets: Isbell et al., 2002; Fairbanks & McGuire, 1987; Keddy, 1986; Young et al., 2017); or, to return to Bergmann’s Rule, the increase in mass overall in the South African population could be due to sexually unbiased selective pressures acting on both sexes to increase size. South African vervets live in areas that are seasonally very cold, and are exposed to particularly low nighttime temperatures that can drop well below freezing, which has been noted to cause large seasonal fluctuations in core body temperature (Lubbe et al., 2014). These have been offset by both larger body size, which stabilizes core body temperature in Ch. p. pygerythrus (Henzi et al., 2017), and also the adoption of behavioral (Danzy et al., 2012) and social (McFarland et al., 2015; Henzi et al., 2017) strategies to mitigate the loss of body heat that have not yet been noted in other Chlorocebus taxa. That larger body size occurs in both males and females in South Africa also suggests a common etiology within the population, which need not be genetic in origin: recent work in humans, for example, suggest that such clinal variation in body mass as seen in Chlorocebus may also be reached by adaptive shifts in gut microbiota across populations (Suzuki and Worobey, 2014). Exactly how this clinal variation is achieved in response to temperature, and how it informs sexual dimorphism, will require further genomic and physiological comparisons between vervet taxa, with perhaps the most fruitful being in the latitudinally wide-spread clade consisting of Ch. p. hilgerti, Ch. cynosuros, and Ch. p. pygerythrus.
CONCLUSION
Our results suggest that the taxonomic divisions often recognized between populations of the genus Chlorocebus do show some significant differences in both adult phenotype and developmental patterns, but also that widely geographically separated populations within taxa also carry with them significant differences in morphology and growth. These population differences may reflect unique responses in each to ecogeographic, socio-sexual, or biomechanical selective pressures acting on adult phenotypes via natural selection or during ontogeny via developmental plasticity; further, more targeted work across populations is needed to adequately address the variation seen in this sample. This work generates many more questions than answers. Why does the Ch. pygerythrus clade show divergent ecogeographic signals with respect to Allen’s rule? Is the exaggerated sexual dimorphism of the St. Kitts & Nevis Ch. sabaeus due to more intense male-male competition than other vervet populations, and does it represent a trade-off in sexual signaling? What are the genetic systems that underlie these traits, and can they give us an idea of how these growth and adult phenotypes function and are related? Finally, exactly how much of this variation is truly adaptive, how much due to plastic responses to local conditions and random effects related to species-specific demographic and population history? To truly tease apart the pressures and processes that underlie these phenotypic differences will require much more extensive comparative work in behavioral ecology and developmental morphology within and across these populations.
Supplementary Material
Supp. Fig. 1. Loess models for changes in body mass (kg) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 2. Loess models for changes in body length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 3. Loess models for changes in thigh length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 4. Loess models for changes in leg length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 5. Loess models for changes in foot length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Acknowledgments
We are deeply grateful to the many people who have worked with us over the years to obtain these data. We would like to acknowledge assistance in the field by Fred Brett in Ethiopia; Nicholas Dracopoli, James Else and The Institute for Primate Research in Kenya; Anna Jasinska, Oliver “Pess” Morton, Yoon Jung, Stephanie Groman, David Jentsch, Eugene Redmond and the St. Kitts Biomedical Research Foundation in St. Kitts; numerous field assistants in South Africa including especially Gabriel Coetzer, Magali Jacquier, Helene DeNys, JD Pampush, Elzet Answegen, Tegan Gaetano, Dewald DuPlessis, Micah Beller, David Beller and Pess Morton; the University of Limpopo and the University of the Free State; and Martin Antonio and the Medical Research Council in the Gambia. We gratefully acknowledge the IUCN Red List for the use of distribution maps of all Chlorocebus taxa. The map of trapping sites was produced by the UWM Cartography and GIS Center under the direction of Donna Genzmer. All techniques for trapping, sedation and sampling have been approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee and the Animal Research Committee at the University of California Los Angeles. Collections were conducted under permits and permissions issued by the following: Ethiopian Wildlife Conservation Authority; Gambia Department of Parks and Wildlife Management; Ministry of Tourism and Wildlife, Kenya; in South Africa the Department of Economic Development and Environmental Affairs, Eastern Cape; Department of Tourism, Environmental and Economic Affairs, Free State Province; Ezemvelo KZN Wildlife; Department of Economic Development, Environment and Tourism, Limpopo; the Department of Agriculture, Conservation and Environment, Mpumalanga and the Department of Environment and Nature Conservation, Northern Cape, as well as the South African national department of Environmental Affairs. We would also like to acknowledge our appreciation to all the veterinarians who worked with us to safely obtain samples, especially Lizanne Meiring and Murray Stokoe. This manuscript was greatly improved by comments from an associate editor and two reviewers, for which we are grateful. This work was funded by The Fulbright Foundation, NSF (SOC 74-24166, BNS 770-3322, BCS 0938969) NIH (R01RR0163009), the University of Wisconsin-Milwaukee, USA, The University of the Free State, South Africa, the University of Limpopo, South Africa and Coriell Institute, United States.
References
- Albrecht GH. Latitudinal, taxonomic, sexual, and insular determinants of size variation in pigtail macaques, Macaca nemestrina. Am J Primatol. 1980;1:141–152. [Google Scholar]
- Albrecht GH, Jenkins PD, Godfrey LR. Ecogeographic size variation among the living and subfossil prosimians of Madagascar. Am J Primatol. 1990;22:1–50. doi: 10.1002/ajp.1350220102. [DOI] [PubMed] [Google Scholar]
- Allen JA. The influence of physical conditions in the genesis of species. Radic Rev. 1877;1:108–140. [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57:490–501. [Google Scholar]
- Altmann J, Altmann S, Hausfater G. Physical maturation and age estimates of yellow baboons, Papio cynocephalus, in Amboseli National Park, Kenya. Am J Primatol. 1981;1:389–399. doi: 10.1002/ajp.1350010404. [DOI] [PubMed] [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Badyaev AV, Hill GE, Weckworth BV. Species divergence in sexually selected traits: increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution. 2002;56:412–419. doi: 10.1111/j.0014-3820.2002.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Barton K. MuMIn: multi-model inference, R package version 1.15.6. 2016 http://CRAN.R-project.org/package=MuMIn.
- Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- Bergmann C. Über die verhältnisse der wärmeökonomieder thierezuihrer grösse. Göttinger Studien Pt. 1847;1:595–708. [Google Scholar]
- Bezanson M. Life history and locomotion in Cebus capucinus and Alouatta palliata. Am J Phys Anthropol. 2009;140:508–517. doi: 10.1002/ajpa.21099. [DOI] [PubMed] [Google Scholar]
- Bolker B. bbmle: Tools for general maximum likelihood estimation. R package version 1.0.20. 2017 https://CRAN.R-project.org/package=bbmle.
- Bolter DR, Zihlman AL. Morphometric analysis of growth and development in wild-collected vervet monkeys (Cercopithecus aethiops), with implications for growth patterns in Old World monkeys, apes and humans. J Zool. 2003;260:99–110. [Google Scholar]
- [Accessed 14 Jan 2016];Bones and Behavior Working Group. 2015 http://www.bonesandbe havior.org.
- Boulton AM, Horrocks JA, Baulu J. The Barbados vervet monkey (Cercopithecus aethiops sabaeus): Changes in population size and crop damage, 1980–1994. Int J Primatol. 1996;17:831–844. [Google Scholar]
- Brett FL, Turner TR, Jolly CJ, Cauble R. Trapping baboons and vervet monkeys from wild, free-ranging populations. J Wildl Manag. 1982;46:164–174. [Google Scholar]
- Brennan EJ, Else JG, Altmann J. Ecology and behavior of a pest primate: vervet monkeys in a tourist-lodge habitat. Afr J Ecol. 1985;23:35–55. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multi-model inference: a practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Butynski TM, Kingdon J, Kalina J, editors. Mammals of Africa: Primates. London: Bloomsbury; 2013. [Google Scholar]
- Cardini A, Jansson A-U, Elton S. A geometric morphometric approach to the study of ecogeographical and clinal variation in vervet monkeys. J Biogeogr. 2007;34:1663–1678. [Google Scholar]
- Carrier DR. Ontogenetic limits on locomotor performance. Physiol Zool. 1996;69:467–488. [Google Scholar]
- Cheney DL, Seyfarth RM. Nonrandom dispersal in free-ranging vervet monkeys: social and genetic consequences. Am Natural. 1983;122:392–412. [Google Scholar]
- Cheverud JM, Wilson P, Dittus WPJ. Primate population studies at Polonnaruwa. III. Somatometric growth in a natural population of toque macaques (Macaca sinica) J Hum Evol. 1992;23:51–77. doi: 10.1002/ajp.1350270209. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Harvey PH, Rudder B. Sexual dimorphism, socionomic sex ratio and body mass in primates. Nature. 1977;269:797–800. doi: 10.1038/269797a0. [DOI] [PubMed] [Google Scholar]
- Cramer JD, Gaetano T, Gray JP, Grobler JP, Lorenz JG, Freimer BN, Schmitt CA, Turner TR. Variation in scrotal color among widely distributed vervet monkey populations (Chlorocebus aethiops pygerythrus and Chlorocebus aethiops sabaeus) Am J Primatol. 2013;75:752–762. doi: 10.1002/ajp.22156. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Rutenberg GW. Neonatal nutrition and longitudinal growth in baboons: adiposity measured by skinfold thickness. Am J Hum Biol. 1989;1:429–442. doi: 10.1002/ajhb.1310010405. [DOI] [PubMed] [Google Scholar]
- Cribari-Neito F, Zeileis A. Beta regression in R. J Stat Softw. 2010;34(2):1–24. URL: www.jstatsoft.org/article/view/v034i02/v34i02.pdf. [Google Scholar]
- Cuozzo FP, Sauther ML. Severe wear and tooth loss in wild ring-tailed lemurs (Lemur catta): a function of feeding ecology, dental structure, and individual life history. J Hum Evol. 2006;51:490–505. doi: 10.1016/j.jhevol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Danzy J, Grobler JP, Freimer N, Turner TR. Sunbathing: a behavioral response to seasonal climatic change among South African vervet monkeys (Chlorocebus aethiops) Afr Prim. 2012;7:230–237. [Google Scholar]
- Darwin CR. The descent of man, and selection in relation to sex. 2. New York: Appleton; 1874. [Google Scholar]
- Deputte BL. Life history of captive grey-cheeked mangabeys: physical and sexual development. Int J Primatol. 1992;13:509–531. [Google Scholar]
- Dore KM. Doctoral dissertation. The University of Wisconsin - Milwaukee; 2013. An anthropological investigation of the dynamic human/vervet monkey (Chlorocebus aethiops sabaeus) interface in St. Kitts, West Indies. [Google Scholar]
- Dunham AE, Maitner BS, Razafindratsima OH, Simmons MC, Roy CL. Body size and sexual dimorphism in primates: influence of climate and net primary productivity. J Evol Biol. 2013;26:2312–2320. doi: 10.1111/jeb.12239. [DOI] [PubMed] [Google Scholar]
- Eley RM, Strum SC, Muschemi G, Reid GDF. Nutrition, body composition, activity patterns, and parasitism of free-ranging troops of olive baboons (Papio anubis) in Kenya. Am J Primatol. 1989;18:209–219. doi: 10.1002/ajp.1350180304. [DOI] [PubMed] [Google Scholar]
- Elton S, Dunn J, Cardini A. Size variation facilitates population divergence but does not explain it all: an example study from a widespread African monkey. Biol J Linn Soc. 2010;101:823–843. [Google Scholar]
- Enstam KL, Isbell LA. The guenons (genus Cercopithecus) and their allies: behavioral ecology of polyspecific associations. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in perspective. Oxford: Oxford University Press; 2007. pp. 252–274. [Google Scholar]
- Fairbanks LA, McGuire MT. Mother-infant relationships in vervet monkeys: response to new adult males. Int J Primatol. 1987;8:351–366. [Google Scholar]
- Fedigan L, Fedigan LM. Cercopithecus aethiops: a review of field studies. In: Gautier-Hion A, Bourlière F, Gautier JP, Kingdon J, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge (UK): Cambridge University Press; 1988. pp. 389–411. [Google Scholar]
- Fick SE, Hijmans RJ. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017 doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- Fooden J. Taxonomy and evolution of the sinica group of macaques: I. Species and subspecies accounts of Macaca sinica. Primates. 1979;20:109–140. [Google Scholar]
- Fourie NH, Turner TR, Brown JL, Pampush JD, Lorenz JG, Bernstein RM. Variation in vervet (Chlorocebus aethiops) hair cortisol concentrations reflects ecological disturbance by humans. Primates. 2015;56:365–373. doi: 10.1007/s10329-015-0486-y. [DOI] [PubMed] [Google Scholar]
- Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, Sibert J. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw. 2012;27:233–249. [Google Scholar]
- Gaetano TJ, Danzy J, Mtshali MS, Theron N, Schmitt CA, Grobler JP, Freimer N, Turner TR. Mapping correlates of parasitism in wild South African vervet monkeys (Chlorocebus aethiops) S African J Wildlife Res. 2014;44(1):56–70. [Google Scholar]
- Gaulin SJC, Sailer LD. Sexual dimorphism in weight among the primates: the relative impact of allometry and sexual selection. Int J Primatol. 1984;5:515–535. [Google Scholar]
- Gartlan JS, Brain CK. Ecology and social variability in Cercopithecus aethiops and C. mitis. In: Jay J, editor. Primates. New York: Holt, Rinehart and Winston; 1968. pp. 253–292. [Google Scholar]
- Gerald MS. Primate colour reveals social status and predicts aggressive outcome. Anim Behav. 2001;61:559–566. [Google Scholar]
- Gerald MS, Ayala J, Ruíz-Lambides A, Waitt C, Weiss A. Do females pay attention to secondary sexual coloration in vervet monkeys (Chlorocebus aethiops)? Naturwissenschaften. 2010;97:89–96. doi: 10.1007/s00114-009-0619-5. [DOI] [PubMed] [Google Scholar]
- Gordon AD. Scaling of size and dimorphism in primates II macroevolution. Int J Primatol. 27:63–105. [Google Scholar]
- Grobler JP, Turner TR. A novel trap design for the capture and sedation of vervet monkeys (Chlorocebus aethiops) S Afr J Wildl Res. 2010;40:163–168. [Google Scholar]
- Grobler P, Jacquier M, de Nys H, Blair M, Whitten PL, Turner TR. Primate sanctuaries, taxonomy, and survival: a case study from South Africa. Ecol Environmental Anthropol. 2006;2:12–16. [Google Scholar]
- Groves CP. Primate Taxonomy. Washington, DC: Smithsonian Books; 2001. [Google Scholar]
- Grubb P, Butynski TM, Oates JF, Bearder SK, Disotell TR, Groves CP, Struhsaker TT. Assessment of the diversity of African primates. Int J Primatol. 2003;24:1301–1357. [Google Scholar]
- Guillaumet A, Ferdy J-B, Desmarais E, Godelle B, Crochet P-A. Testing Bergmann’s rule in the presence of potentially confounding factors: a case study with three species of Galerida larks in Morocco. J Biogeography. 2008;35:579–591. [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J Stat Softw. 2010;33:1–22. [Google Scholar]
- Hall KRL, Gartlan JS. Ecology and behavior of the vervet monkey, Cercopithecus aethiops, Lolui Island, Lake Victoria. J Zool. 1965;145:37–56. [Google Scholar]
- Hamada Y, Iwamoto M, Watanabe T. Somatometrical features of Japanese monkeys in the Koshina islet: in viewpoint of somatometry, growth, and sexual maturation. Primates. 1986;27:471–484. [Google Scholar]
- Haus T, Akom E, Agwanda B, Hofreiter M, Roos C, Zinner D. Mitochondrial diversity and distribution of African green monkeys (Chlorocebus Gray, 1870) Am J Primatol. 2013;75:350–360. doi: 10.1002/ajp.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzi SP, Hetem R, Fuller A, Maloney S, Young C, Mitchell D, Barrett L, McFarland R. Consequences of sex-specific sociability for thermoregulation in male vervet monkeys during winter. J Zool. 2017 doi: 10.1111/jzo.12448. [DOI] [Google Scholar]
- Hijmans RJ, van Etten J. raster: geographic analysis and modeling with raster data. R package version 2.6–7. 2012 http:llCRAN.R-project.org/package=raster.
- Hill WCO. Primates. Comparative Anatomy and Taxonomy. VI. Catarrhini, Cercopithecoidea, Cercopithecinae. Edinburgh, UK: Edinburgh University Press; 1966. [Google Scholar]
- Isbell LA. Season and social correlated of changes in hair, skin, and scrotal condition in vervet monkeys (Cercopithecus aethiops) of Amboseli National Park, Kenya. Am J Primatol. 1995;36:61–70. doi: 10.1002/ajp.1350360105. [DOI] [PubMed] [Google Scholar]
- Isbell LA, Cheney DL, Seyfarth RM. Why vervet monkeys (Chlorocebus aethiops) live in multimale groups. In: Glenn ME, Cords M, editors. The guenons: diversity and adaptation in African monkeys. New York: Kluwer Academic Publishers; 2002. pp. 173–187. [Google Scholar]
- Jasinska AJ, Schmitt CA, Service SK, Cantor RM, Dewar K, Jentsch DJ, Kaplan JR, Turner TR, Warren WC, Weinstock GM, Woods RP, Freimer NB. Systems biology of the vervet monkey. ILAR / Natl Res Council. 2013;54:122–143. doi: 10.1093/ilar/ilt049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Zelaya I, Service SK, Peterson C, Cantor RM, Choi O-W, DeYoung J, Eskin E, Fairbanks LA, Fears S, Furterer A, Huang YS, Ramensky V, Schmitt CA, Svardal H, Jorgensen MJ, Kaplan JR, Villar D, Aken BL, Flicek P, Nag R, Wong ES, Blangero J, Dyer TD, Bogomolov M, Benjamini Y, Weinstock GM, Dewar K, Sabatti C, Wilson RK, Jentsch JD, Warren W, Coppola G, Woods RP, Freimer NB. Genetic variation and gene expression across multiple tissues and developmental stages in a non-human primate. Nature Genetics. 2017;49:1714–1721. doi: 10.1038/ng.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol. 2003;120:83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Jolly CJ, Phillips-Conroy JE. Testicular size, mating system, and maturation schedules in wild anubis and hamadryas baboons. Int J Primatol. 2003;24:125–142. [Google Scholar]
- Jones-Engel L, Schillaci MA, Engel G, Paputungan U, Froehlich J. Characterizing primate pet ownership in Sulawesi: implications for disease transmission. In: Patterson J, Wallis J, editors. Commensalism and conflict: the human primate interface. Norman, OK: American Society of Primatology; 2005. pp. 197–221. [Google Scholar]
- Keddy AC. Female mate choice in vervet monkeys (Cercopithecus aethiops sabaeus) Am J Primatol. 1986;10:125–134. doi: 10.1002/ajp.1350100204. [DOI] [PubMed] [Google Scholar]
- Kingdon J. The Kingdon field guide to African mammals. San Diego: Academic Press; 1997. [Google Scholar]
- Kingdon J, Happold D, Butynski T, Hoffmann M, Happold M, Kalina J. Mammals of Africa. Volume II: Primates. London: Bloomsbury Publishing; 2013. [Google Scholar]
- Krishna M, Upadhyay D. Increased limb lengths in patients with shortened spines due to tuberculosis in early childhood. Spine. 1996;21:1045–1047. doi: 10.1097/00007632-199605010-00010. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Bragg JM. Plasticity in human life history strategy: implications for contemporary human variation and the evolution of genus Homo. Curr Anthropol. 2012;53(suppl. 6):S369–S382. [Google Scholar]
- Kuzawa CW, Thayer ZM. Timescales of human adaptation: the role of epigenetic processes. Epigenomics. 2011;3:221–234. doi: 10.2217/epi.11.11. [DOI] [PubMed] [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Lawler RR. Sifaka positional behavior: ontogenetic and quantitative genetic approaches. Am J Phys Anthropol. 2006;131:261–271. doi: 10.1002/ajpa.20430. [DOI] [PubMed] [Google Scholar]
- Lawler RR. Morphological integration and natural selection in the postcranium of wild Verreaux’s sifaka (Propithecus verreauxi verreauxi) Am J Phys Anthropol. 2008;136:204–213. doi: 10.1002/ajpa.20795. [DOI] [PubMed] [Google Scholar]
- Leigh SR. Patterns of variation in the ontogeny of primate body size dimorphism. J Hum Evol. 1992;23:27–50. [Google Scholar]
- Leigh SR, Shea BT. Ontogeny of body size variation in African apes. Am J Phys Anthropol. 1996;99:43–65. doi: 10.1002/(SICI)1096-8644(199601)99:1<43::AID-AJPA3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Leutenegger W. Scaling of sexual dimorphism in body size and breeding system in primates. Nature. 1978;272:610–611. doi: 10.1038/272610a0. [DOI] [PubMed] [Google Scholar]
- Leutenegger W, Cheverud J. Correlates of sexual dimorphism in primates: ecological and size variables. Int J Primatol. 1982;3:387–402. [Google Scholar]
- Lomolino MV, Sax DF, Riddle BR, Brown JH. The island rule and a research agenda for studying ecogeographical patterns. J Biogeography. 2006;33:1503–1510. [Google Scholar]
- Loudon JE, Grobler JP, Sponheimer M, Moyer K, Lorenz JG, Turner TR. Using the stable carbon and nitrogen isotope compositions of vervet monkeys (Chlorocebus pygerythrus) to examine questions in ethnoprimatology. PLoS One. 2014;9(7):e100758. doi: 10.1371/journal.pone.0100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbe A, Hetem RS, McFarland R, Barrett L, Henzi PS, Mitchell D, Meyer LCR, Maloney SK, Fuller A. Thermoregulatory plasticity in free-ranging vervet monkeys, Chlorocebus pygerythrus. J Comp Physiol B. 2014;184:799–809. doi: 10.1007/s00360-014-0835-y. [DOI] [PubMed] [Google Scholar]
- Ma D, Jasinska AJ, Feyertag F, Wijewardana V, Kristoff J, He Tianyu, Raehtz K, Schmitt CA, Jung Y, Dione M, Antonio M, Tracy R, Turner T, Robertson DL, Pandrea I, Freimer NB, Apetrei C. Factors associated with SIV transmission in a natural African nonhuman primate host in the wild. J Virol. 2014;88:6778–6792. doi: 10.1128/JVI.03606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jasinska A, Kristoff J, Grobler P, Turner T, Jung Y, Schmitt C, Raehtz K, Martinez N, Wijewardana V, Tracy R, Pandrea I, Freimer N, Apetrei C. SIVagm infection in wild African green monkeys from South Africa: epidemiology, natural history, and evolutionary considerations. PLoS Pathogens. 2013;9:1–18. doi: 10.1371/journal.ppat.1003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle MJ. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c) R package version 2.1-1. 2017 https://cran.r-project.org/package=AICcmodavg.
- McFarland R, Fuller A, Hetem RS, Mitchell D, Maloney SK, Henzi SP, Barrett L. Social integration confers thermal benefits in a gregarious primate. J Anim Ecol. 2015;84:871–878. doi: 10.1111/1365-2656.12329. [DOI] [PubMed] [Google Scholar]
- Meiri S, Dayan T. On the validity of Bergmann’s Rule. J Biogeogr. 2003;30:331–351. [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- Ozgul A, Tulijapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science. 2009;325:464–467. doi: 10.1126/science.1173668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. Coupled dynamics of body mass and population growth in response to environmental change. Nature. 2010;466:482–485. doi: 10.1038/nature09210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampush JD. Masters thesis. University of Wisconsin–Milwaukee; Milwaukee WI: 2010. Human food access and its effects on South African vervet body mass. [Google Scholar]
- Peters G. userfriendlyscience: Quantitative analysis made accessible. R package version 0.3-0. 2015 http://CRAN.R-project.org/package=userfriendlyscience.
- Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol. 2005;26:3–31. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2017 https://www.R-project.org.
- Relethford JH, Hodges DC. A statistical test for differences in sexual dimorphism between populations. Am J Phys Anthropol. 1985;66:55–61. doi: 10.1002/ajpa.1330660105. [DOI] [PubMed] [Google Scholar]
- Rensch B. Die abhangigkeit der relativen sexualdifferenz von der körpergroße. Bonner Zoologische Beiträge. 1950;1:58–69. [Google Scholar]
- Rodriguez RL, Cramer JD, Schmitt CA, Gaetano TJ, Grobler JP, Freimer NB, Turner TR. Adult age confounds estimates of static allometric slopes in a vertebrate. Ethol Ecol Evol. 2015a;27:412–421. doi: 10.1080/03949370.2014.986767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RL, Cramer JD, Schmitt CA, Gaetano TJ, Grobler JP, Freimer NB, Turner TR. The static allometry of sexual and non-sexual traits in vervet monkeys. Biol J Linnean Soc. 2015b;114:527–537. doi: 10.1111/bij.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman CC, Auerbach BM. Ecogeography, genetics, and the evolution of human body form. J Hum Evol. 2015;78:80–90. doi: 10.1016/j.jhevol.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Schillaci MA, Jones-Engel L, Lee BPY-H, Fuentes A, Aggimarangsee N, Engel GA, Sutthipat T. Morphology and somatometric growth of long-tailed macaques (Macaca fascicularis fascicularis) in Singapore. Biol J Linn Soc. 2007;92:675–694. [Google Scholar]
- Schillaci MA, Stallmann RR. Ontogeny and sexual dimorphism in booted macaques (Macaca ochreata) J Zool. 2005;267:19–29. [Google Scholar]
- Schmitt CA, Service S, Cantor RM, Jasinska AJ, Jorgensen MJ, Kaplan JR, Freimer NB. High heritability of obesity and obesogenic growth are both highly heritable and modified by diet in a nonhuman primate model, the African green monkey (Chlorocebus aethiops sabaeus) Int J Obesity. 2017 doi: 10.1038/ijo.2017.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SM, Kemnitz JW. Age- and gender-related changes in body size, adiposity, and endocrine and metabolic parameters in free-ranging rhesus macaques. Am J Phys Anthropol. 1992;89:109–121. doi: 10.1002/ajpa.1330890110. [DOI] [PubMed] [Google Scholar]
- Senghore M, Bayliss S, Kwambana-Adams B, Foster-Nyarko E, Manneh J, Dione M, Badji H, Ebruke C, Dought E, Thorpe H, Jasinska A, Schmitt C, Cramer J, Turner T, Weinstock G, Freimer N, Pallen M, Feil E, Antonio M. Whole-genome sequencing reveals transmission of Staphylococcus aureus from humans to green monkeys in The Gambia. Appl Env Microbiol. 82:5910–5917. doi: 10.1128/AEM.01496-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šešelj M. Relationship between dental development and skeletal growth in modern humans and its implications for interpreting ontogeny in fossil hominins. Am J Phys Anthropol. 2013;150:38–47. doi: 10.1002/ajpa.22209. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx) Am J Phys Anthropol. 2001;115:349–360. doi: 10.1002/ajpa.1091. [DOI] [PubMed] [Google Scholar]
- Shea BT. The ontogeny of sexual dimorphism in the African apes. Am J Primatol. 1985;8:183–188. doi: 10.1002/ajp.1350080208. [DOI] [PubMed] [Google Scholar]
- Shea BT. Ontogenetic scaling of skeletal proportions in the talapoin monkey. J Hum Evol. 1992;23:283–307. [Google Scholar]
- Shine R. The evolution of large body size in females: a critique of Darwin’s “fecundity advantage” model. Am Nat. 1988;131:124–131. [Google Scholar]
- Smith BH, Crummett TL, Brandt KL. Ages of eruption of primate teeth: a compendium for aging individuals and comparing life histories. Yrbk Phys Anthropol. 1994;37:177–231. [Google Scholar]
- Smith RJ. Statistics of sexual size dimorphism. J Hum Evol. 1999;36:423–459. doi: 10.1006/jhev.1998.0281. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Leigh SR. Sexual dimorphism in primate neonatal body mass. J Hum Evol. 1998;34:173–201. doi: 10.1006/jhev.1997.0190. [DOI] [PubMed] [Google Scholar]
- Struhsaker TT. Social structure among vervet monkeys (Cercopithecus aethiops) Behaviour. 1967;29:83–121. [PubMed] [Google Scholar]
- Strum SC. Weight and age in wild olive baboons. Am J Primatol. 1991;25:219–237. doi: 10.1002/ajp.1350250403. [DOI] [PubMed] [Google Scholar]
- Suzuki TA, Worobey M. Geographical variation of human microbial composition. Biol Lett. 2014;10:20131037. doi: 10.1098/rsbl.2013.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svardal H, Jasinska AJ, Apetrei C, Coppola G, Huang Y, Schmitt CA, Jacquelin B, Müller-Trutwin M, Weinstock G, Grobler JP, Wilson RK, Turner TR, Warren WC, Freimer NB, Nordborg M. Ancient hybridization and strong adaptation to viruses across African vervet monkey populations. Nature Genetics. 49:1705–1713. doi: 10.1038/ng.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarka M, Åkesson M, Hasselquist D, Hansson B. Intralocus sexual conflict over wing length in a wild migratory bird. Am Nat. 2014;183:62–73. doi: 10.1086/674072. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Wilson ME, Rudman CG. Pubertal growth spurt in the female rhesus monkey: relation to menarche and skeletal maturation. Am J Hum Biol. 1990;2:101–106. doi: 10.1002/ajhb.1310020202. [DOI] [PubMed] [Google Scholar]
- Turner TR, Anapol F, Jolly CJ. Growth, development, and sexual dimorphism in vervet monkeys (Cercopithecus aethiops) at four sites in Kenya. Am J Phys Anthropol. 1997;103:19–35. doi: 10.1002/(SICI)1096-8644(199705)103:1<19::AID-AJPA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Turner TR, Coetzeer WG, Schmitt CA, Lorenz J, Freimer NB, Grobler JP. Localized population divergence of vervet monkeys (Chlorocebus spp.) in South Africa: evidence from mtDNA. Am J Phys Anthropol. 2016a;159:17–30. doi: 10.1002/ajpa.22825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TR, Cramer JD, Nisbett A, Gray JP. A comparison of adult body size between captive and wild vervet monkeys (Chlorocebus aethiops sabaeus) on the island of St. Kitts. Primates. 2016b;57:211–220. doi: 10.1007/s10329-015-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist JE, Kessler MJ. Free-ranging Cayo Santiago rhesus monkeys (Macaca mulatta): I. Body size, proportion, and allometry. Am J Primatol. 1989;19:1–13. doi: 10.1002/ajp.1350190102. [DOI] [PubMed] [Google Scholar]
- van den Berg L, Nel M, Brand D, Bosch J, Human W, Lawson S, Walsh C. Agreement between measured height, and height predicted from ulna length, in adult patients in Bloemfontein, South Africa. S Afr J Clin Nutr. 2016;29:127–132. [Google Scholar]
- Warren WC, Jasinska AJ, Garcia-Perez R, Svardel H, Tomlinson C, Rocchi M, Archidiacono N, Capozzi O, Minx P, Montague MJ, Kyung K, Hillier LW, Kremitzki M, Graves T, Chiang C, Hughes J, Tran N, Wang Y, Ramensky V, Choi O, Jung YJ, Schmitt CA, Juretic N, Wasserscheid J, Turner TR, Wiseman RW, Tuscher JJ, Karl JA, Schmitz JE, Zahn R, O’Connor DH, Redmond E, Nisbett A, Jacquelin B, Müller-Trutwin MC, Brenchley JM, Dione M, Antonio A, Schroth GP, Kaplan JR, Jorgensen MJ, Thomas GWC, Hahn MW, Raney B, Aken B, Schmitz J, Churakov G, Noll A, Stanyon R, Webb D, Thibaud-Nissen F, Nordborg M, Marques-Bonet T, Dewar K, Weinstock GM, Wilson RK, Freimer NB. The genome of the vervet (Chlorocebus aethiops sabaeus) Genome Research. 2015;25:1921–1933. doi: 10.1101/gr.192922.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein K. Climatic and altitudinal influences on variation in Macaca limb morphology. Anat Res Int. 2011;2011:714624. doi: 10.1155/2011/714624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten PL, Turner TR. Endocrine mechanisms of primate life history trade-offs: Growth and reproductive maturation in vervet monkeys. Am J Hum Biol. 2009;21:754–761. doi: 10.1002/ajhb.20939. [DOI] [PubMed] [Google Scholar]
- Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- Young C, McFarland R, Barrett L, Henzi SP. Formidable females and the power trajectories of socially integrated male vervet monkeys. Anim Behav. 2017;125:61–67. [Google Scholar]
- Young JW. Ontogeny of muscle mechanical advantage in capuchin monkeys (Cebus albifrons and Cebus apella) J Zool. 2005;267:351–362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Fig. 1. Loess models for changes in body mass (kg) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 2. Loess models for changes in body length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 3. Loess models for changes in thigh length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 4. Loess models for changes in leg length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.
Supp. Fig. 5. Loess models for changes in foot length (cm) by estimated chronological age in sampled regional populations of a) Ch. p. hilgerti, b) Ch. p. pygerythrus, c) Ch. sabaeus, and d) Ch. aethiops. Regional populations are denoted in the legend.