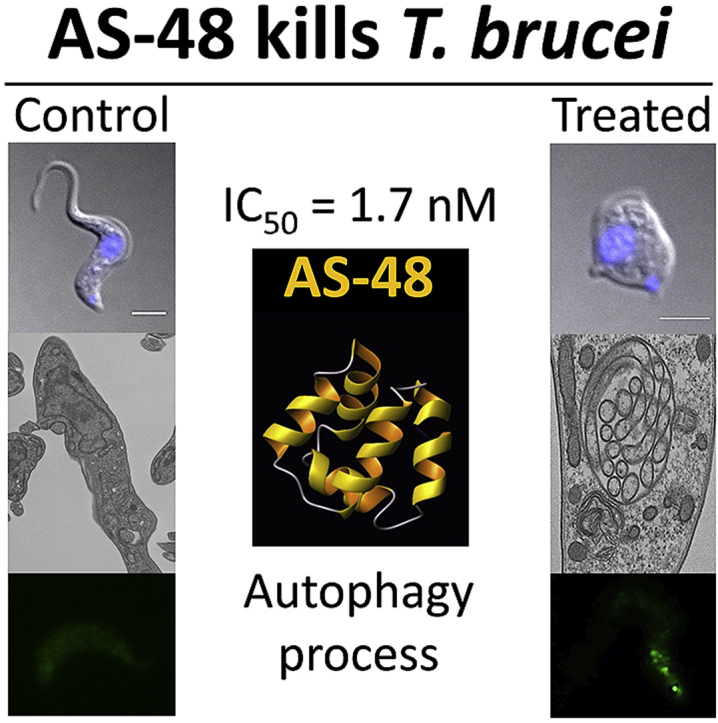
Abstract
The parasitic protozoan Trypanosoma brucei is the causative agent of human African trypanosomiasis (sleeping sickness) and nagana. Current drug therapies have limited efficacy, high toxicity and/or are continually hampered by the appearance of resistance. Antimicrobial peptides have recently attracted attention as potential parasiticidal compounds. Here, we explore circular bacteriocin AS-48's ability to kill clinically relevant bloodstream forms of T. brucei gambiense, T. brucei rhodesiense and T. brucei brucei. AS-48 exhibited excellent anti-trypanosomal activity in vitro (EC50 = 1–3 nM) against the three T. brucei subspecies, but it was innocuous to human cells at 104-fold higher concentrations. In contrast to its antibacterial action, AS-48 does not kill the parasite through plasma membrane permeabilization but by targeting intracellular compartments. This was evidenced by the fact that vital dye internalization-prohibiting concentrations of AS-48 could kill the parasite at 37 °C but not at 4 °C. Furthermore, AS-48 interacted with the surface of the parasite, at least in part via VSG, its uptake was temperature-dependent and clathrin-depleted cells were less permissive to the action of AS-48. The bacteriocin also caused the appearance of myelin-like structures and double-membrane autophagic vacuoles. These changes in the parasite's ultrastructure were confirmed by fluorescence microscopy as AS-48 induced the production of EGFP-ATG8.2-labeled autophagosomes. Collectively, these results indicate AS-48 kills the parasite through a mechanism involving clathrin-mediated endocytosis of VSG-bound AS-48 and the induction of autophagic-like cell death. As AS-48 has greater in vitro activity than the drugs currently used to treat T. brucei infection and does not present any signs of toxicity in mammalian cells, it could be an attractive lead compound for the treatment of sleeping sickness and nagana.
Keywords: Trypanosoma brucei, Antimicrobial peptides, AS-48, Autophagy, Sleeping sickness, Trypanocidal drugs
Graphical abstract
Highlights
-
•
AS-48 kills Trypanosoma brucei efficiently and is innocuous in mammalian cells.
-
•
It has greater in vitro activity than drugs currently in use.
-
•
AS-48 must be internalized by the parasite in order to exert its trypanocidal effect.
-
•
AS-48 uptake involves VSG binding and clathrin-mediated endocytosis.
-
•
AS-48 induces an autophagic-related cell death.
1. Introduction
African trypanosomes are protozoan parasites responsible for neglected tropical diseases that affect both humans and livestock with devastating consequences. They threaten the lives of nearly 70 million people across more than 36 sub-Saharan African countries (Vreysen et al., 2013; Welburn et al., 2009). In humans, Trypanosoma brucei gambiense and T. brucei rhodesiense produce sleeping sickness or Human African Trypanosomiasis (HAT), whereas T. brucei brucei causes nagana in cattle, one of the main causes of poverty in sub-Saharan Africa (Reinhardt, 2008). These parasites have a digenetic life cycle that includes procyclic forms (PCF), which reside in the insect vector (the tsetse fly), and bloodstream forms (BSF), which replicate in the mammalian host and cause the disease. There are several differences between the two forms. For example, BSF express the variant surface glycoprotein (VSG) that forms a densely packed surface coat (David Barry and McCulloch, 2001; Natesan et al., 2007) which is lost during differentiation to PCF. In addition, BSF endocytosis is clathrin dependent and 10-fold faster than in PCF (Morgan et al., 2001; Pal et al., 2002). Drugs currently used to treat HAT have limited efficacy, they are toxic and/or are hampered by the continual appearance of resistance and, therefore, there is an urgent need for new medications (Cullen and Mocerino, 2017). Ideally, to avoid cross-resistance phenomena, any new drugs must have a different uptake mechanism and/or action than those in use today.
Antimicrobial peptides (AMPs) have recently attracted attention as potential parasiticidal compounds. They constitute a family of small polypeptides, with diverse spectra, modes of action, molecular weights, genetic origins, and biochemical properties (Riley and Wertz, 2002). A few AMPs from mammalian (Gonzalez-Rey et al., 2006; McGwire et al., 2003), insect (Hu and Aksoy, 2006) or synthetic (Haines et al., 2003) origins has been assayed against Trypanosoma sp., with a positive effect in the low micromolar range, highlighting their potential in HAT treatment (McGwire et al., 2003). Bacteriocins are a family of ribosomally synthesized AMPs secreted by bacteria that inhibit the growth of closely related species (narrow spectrum) or across genera (broad spectrum). They are biotechnologically very relevant since they show low eukaryotic toxicity and so they can be used as natural preservatives in the food industry (Ramu et al., 2015). Furthermore, some bacteriocins have a remarkable therapeutic potential in terms of local and systemic infections, highlighting the potential value of the these natural molecules as alternatives to antibiotics and other drugs (Montalban-Lopez et al., 2011). They are of even greater interest since the increasing incidence of multidrug-resistant microorganisms has become a growing risk to global public health. One of the best characterized bacteriocin is AS-48, a 70-residue circular peptide produced by some strains of Enterococcus faecalis (Maqueda et al., 2004). AS-48 exerts a bactericidal/lytic action on sensitive cells (most Gram-positive and some Gram-negative bacteria). Its primary target is the bacterial membrane, in which it forms pores, leading to dissipation of the proton motive force and cell death; a similar mechanism to the one proposed for defensins and other cationic antibacterial peptides. This feature, together with its remarkable stability and solubility over a wide pH range, suggests that this bacteriocin could be a good candidate for clinical and veterinary applications (Montalban-Lopez et al., 2011). Although AS-48 has no activity against the majority of eukaryotes, some protozoa with anionic phospholipids exposed on the parasite surface allow the interaction with this type of strongly cationic peptides. Indeed, the leishmanicidal activity of AS-48 at low micromolar concentrations has recently been demonstrated by inducing both plasma membrane permeabilization and a fast bioenergetic collapse leading to apoptotic parasite death (Abengózar et al., 2017).
The current study explores the efficacy of the circular bacteriocin AS-48 against the complex T. brucei causative agent of HAT and nagana. The results show AS-48 efficiently kills in vitro BSF of T. brucei in the low nanomolar range. It is taken up by clathrin-mediated endocytosis and induces an autophagic-related cell death. These results suggest AS-48 is a promising new trypanocidal agent.
2. Materials and methods
2.1. Strains and culture conditions
‘Single marker’ (S16) T. brucei (Lister 427, antigenic type MiTat 1.2, clone 221a) BSF and T. brucei 449 PCF were cultured as previously described (Wirtz et al., 1999; Cabello-Donayre et al., 2016). T. brucei rhodesiense (EATRO3 ETat1.2 TREU164) and T. brucei gambiense (ELIANE strain) BSF were grown at 37 °C, 5% CO2 in HMI-9 medium supplemented with 20% heat-inactivated fetal bovine serum (hiFBS, Invitrogen). To generate clathrin depleted cell lines, the plasmid p2T7TiCLH was transfected into the T. b. brucei S16 cell line (Allen et al., 2003). The human MRC-5 cell line (fibroblasts derived from lung tissue) was cultured in DMEM (Invitrogen) medium plus 10% hiFBS. The producer AS-48 Enterococcus faecalis UGRA-10 strain (Cebrián et al., 2012) was growth in Brain Hearth Infusion (BHI) or Brain Hearth Agar (BHA) (Merck) media at 37 °C without aeration. For AS-48 production, UGRA-10 strain was cultured at 28 °C in Esprion 300 (E−300, DMV Int., Veghel, Netherland) plus 1% glucose (E−300-G) following the conditions established by Ananou et al. (2008).
2.2. Drug susceptibility assay
AS-48 susceptibility was performed as previously described (Carvalho et al., 2015). Briefly, T. b. rhodesiense, T. b. gambiense and T. b. brucei S16 BSF (104 cells) were incubated in 96-well plates with increasing concentrations of AS-48 (0.1–50 nM) for 72 h at 37 °C, 5% CO2 in culture medium. Cell proliferation was determined using the alamarBlue® assay (Räz et al., 1997). T. b. brucei PCF (106 cells) and MRC-5 cells (2 × 103 cells) were incubated in 96-well plates with AS-48 (0.01–12.5 μM) for 72 h at 28 °C or 37 °C, respectively. Cell proliferation was determined using the MTT colorimetric assay as previously described (Pérez-Victoria et al., 2006).
2.3. Bacteriocins purification and FITC labelling
AS-48 was purified to homogeneity from cultures of enterococcal producer strains in the conditions established previously (Ananou et al., 2008) and lyophilized until its use, when it was dissolved in PBS. Fluorescein-labeled AS-48 (FITC-AS-48) was obtained as described (Abengózar et al., 2017).
2.4. Determination of plasma membrane permeabilization
Plasma membrane integrity was tested using SYTOX® green dye as described elsewhere (Carvalho et al., 2015). Briefly, T. b. brucei BSF (1 × 107 mL−1) were incubated with 0.05, 0.1 or 0.5 μM AS-48 during 10, 30 or 60 min at 4 or 37 °C. Then, 5 nM SYTOX® green was add for 10 min at 4 °C. Washed parasites were analyzed in a FACScan flow cytometer (Becton Dickinson, CA, USA). Triton™ X-100 (0.1%) (Sigma) was used as control of permeabilization.
2.5. Determination of AS-48 uptake
T. b. brucei BSF (1 × 107 mL−1) were incubated at 4 or 37 °C with FITC-AS-48 (0.2 μM) for 10, 30, 45 and 60 min and analyzed by flow cytometry as described above.
2.6. TbClathrin RNAi
p2T7TiCLH transfected cells were selected with 2.5 μg mL−1 G418 and 5 μg mL−1 Hygromycin. Clones were induced with 5 μg mL−1 doxycycline and selected for growth defect and human transferrin (HT) uptake decrease (Bart et al., 2015). The effect of AS-48 on the growth was performed in triplicate with cells induced for 4 h.
2.7. Analysis of the interaction between VSG and AS-48
VSG from T. b. brucei Lister 427, antigenic type MiTat 1.2, clone 221a was purified as described (Cross, 1984; Navarro and Cross, 1996). The interaction between AS-48 and VSG was determined by ELISA as previously described in (Gonzalez-Rey et al., 2007) but with some modifications. Briefly, 96-well ELISA plate was coated with 100 μl/well of purified VSG (10 μgmL−1 in phosphate buffer 0.1 M pH 9.0) and incubated overnight at 4 °C. Wells were washed four times with 200 μl of washing buffer (PBS containing 0.01% Tween-20), then blocked with fetal bovine serum at room temperature during 2 h. After four further rinses with washing buffer, 100 μl AS-48 (10 μgmL−1 in PBS pH 7.4) were added and incubated 4 h at 37 °C (control wells received only PBS). Wells were washed four times as above described, and 100 μl anti-AS-48 (rabbit antibodies) (Maqueda et al., 1993) was added to each well (diluted 1/100) and incubated for 2 h at 37 °C and then, the plate was washed again four times and 100 μL/well of horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibodies (Abcam) was added during 2 h at 37 °C. After eight further rinses with washing buffer, color was developed using ABTS (2,2′-azino-di-[3-ethyl-benzothiazoline-6 sulfonic acid] diammonium salt) as substrate of HRP and the absorbance was measured at 450 nm.
2.8. Fluorescence microscopy
T. b. brucei BSF (2 × 106 cells) were harvested and washed with TDB-glucose, resuspended in 1 mL in the same buffer and incubated for 10 min at 37 °C. Then, 1 μM of FITC-AS-48 were added and immediatley cells were fixed for one hour at 4 °C in 2% paraformaldehyde (PFA) diluted in cold PBS. Parasites were finally washed twice with PBS and labeled cells were analyzed in microscope system (Cell R IX81; Olympus).
2.9. Wide field microscopy
BSF parasites (2 × 106 cells) were fixed (2% PFA for one hour), washed and stained with DAPI (4,6-diamidino-2-phenylindole) (Sigma) to label DNA. Samples were visualized under differential interference contrast (DIC) optics. Images acquisition were performed with a motorized microscope system (Cell R IX81; Olympus) with a 100 × NA 1.4 oil objective (Olympus), a MT20 illumination system, and a charge-coupled device camera (Orca; Hamamatsu Photonics). Images displaying maximum intensity projections (Z stack = 0.2 μm) from 3D image data sets were processed using an ImageJ software v. 1.43 (National Institutes of Health). Time-lapse microscopy video showing BSF trypanosomes was recorded under control conditions (37 °C and 5% CO2). Frames collected using a 63 × NA 1.4 oil objective are displayed at a rate of 10 frames/s.
2.10. Transmission electron microscopy (TEM)
BSF parasites (2 × 106 mL−1) were incubated with 0.1 μM AS-48 at 37 °C for 30 min. Parasites were then fixed and treated as described elsewhere (Pérez-Victoria et al., 2006). Ultrathin sections of 500 Å were cut on Ultracent S Leica microtome, counterstained with uranyl acetate and lead citrate. Samples were imaged in a Zeiss 902 transmission electron microscope.
2.11. Determination of autophagy
To generate the EGFP-ATG8.2 (Tb927.7.5910) fused protein, a PCR fragment was amplified using the oligonucleotides AAGCTTATGAGTAAAAAAGATAGCAAGTAC and GGATCCTTAGCATCCAAATGTCGCCTC. The vector, pLew100 (kindly provided by Dr Duszenko, University of Tübingen, Germany) was digested with HindIII and BamHI, and the ATG8.2 was cloned using the same restriction sites. The HindIII site was used for fusing the EGFP at the N-terminal. For integration, the plasmid was linearized with NotI and transfected as described (Cabello-Donayre et al., 2016). Cloned transgenic cells lines were selected on 5 μg mL−1phleomycin and the cells were induced with 5 μg mL−1 of doxycycline. EGFP-ATG8.2 BSF parasites (concentrated to 107 mL−1) were treated with 100 nM AS-48 during 30 and 60 min at 37 °C, washed and fixed as above described. Autophagy was monitored by fluorescence microscopy after counting the number of autophagosomes labeled with EGFP-ATG8.2 per cell (more than 200 cells were observed in each condition).
2.12. Statistical analysis
Experiments were performed three times in duplicate. All data are presented as mean and standard deviation (S.D.). Statistical significance was determined by Student's t-test. Significance was considered as p < 0.05.
3. Results and discussion
3.1. AS-48 efficiently kills BSF T. brucei in the low nanomolar range
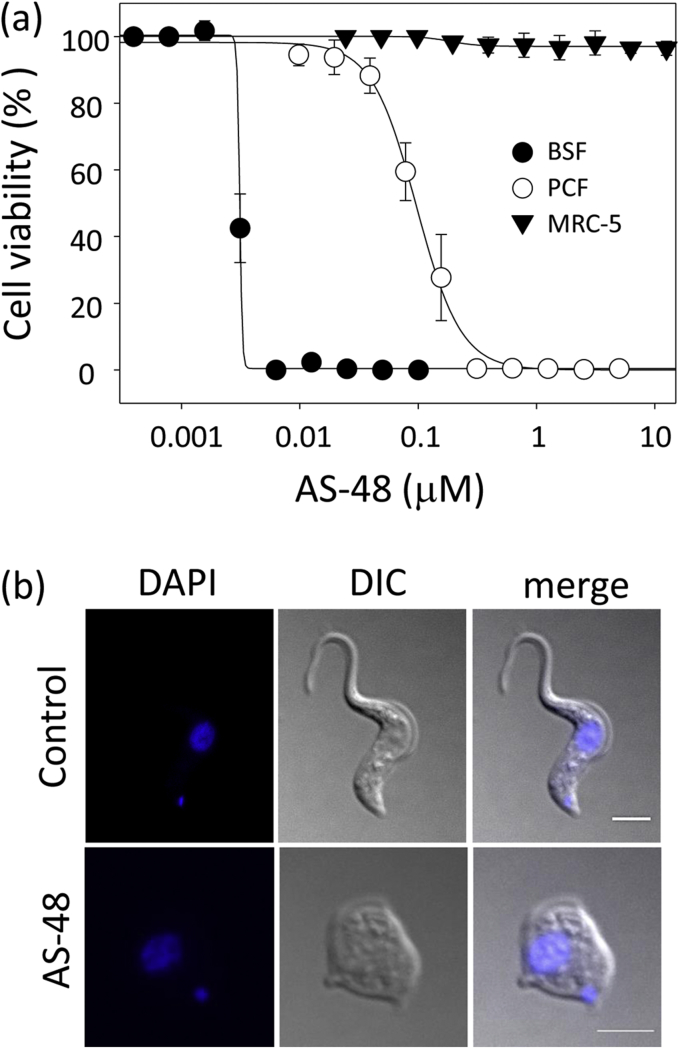
We first investigated bacteriocin AS-48's ability to kill the mammalian infective stage (BSF) of three subspecies of T. brucei that cause HAT and/or nagana. AS-48 exhibited a high capacity to inhibit the in vitro proliferation of BSF at low nanomolar range concentrations (Fig. 1 and Table 1). Thus, the EC50 (50% efficacy concentration) ranged from 1.70 ± 0.19 nM for T. brucei rhodesiense, 2.61 ± 0.08 nM for T. brucei gambiense to 3.12 ± 0.15 nM for T. brucei brucei S16. By contrast, we did not observe any cytotoxic effect against MRC-5 (control), a non-tumoral human cell line extensively used as a control of drug toxicity (De Rycker et al., 2013; Sánchez-Fernández et al., 2015; Belmonte-Reche et al., 2016; Pham et al., 2017), at 12.5 μM AS-48, whereas only 22% growth inhibition was described at 50 μM AS-48 in the murine monocytic cell line Raw 264.7 (Abengózar et al., 2017). The selectivity index of AS-48 was therefore higher than 1.6-3 × 104 fold. Our EC50 values in BSF were even lower than those described for trypanocidal agents currently in use, such as suramin, pentamidine or melarsoprol (Bakunova et al., 2009). Indeed, the AS-48 trypanocidal effect was stronger than the cidal effect described against bacteria: the most susceptible being Listeria monocytogenes (140 nM) (Mendoza et al., 1999) and Enterococcus (500 nM) (Maqueda et al., 2004). In general, the EC50 against T. brucei were several orders of magnitude lower than those described for the majority of the AMPs actives against these parasites, such as pleurocidin (3.7 μM), CP-26 (1.7 μM), attacin (0.3 μM), stomoxyn (37 μM), indolicin (5.2 μM) or some human antimicrobial compounds like the neuropeptide VIP (2.8 μM), adrenomedullin (1.8 μM) or LL-37 (1.7 μM) (Harrington, 2011). These EC50 values were even lower than those described for peptide leucinostatin A and B (6–7 nM), which were more effective against T. b. brucei than T. b. rhodesiense and with selectivity indexes 80-fold lower than AS-48 (Ishiyama et al., 2009; Harrington, 2011). In addition, the AS-48 effect on BSF occurred after just a short incubation period (either in the presence (Fig. 1b and Table 2) or absence (data not shown) of FBS), suggesting a trypanocidal effect on the parasites. This feature is an additional advantage of AS-48, since fast-acting trypanocidal drugs that can eliminate the parasite in as few doses as possible are more preferable than cytostatic compounds (Rycker et al., 2012).
Fig. 1.
AS-48 kills BSF of T. brucei (a) AS-48 inhibits the proliferation of T. brucei. PCF (open circles) or BSF (filled circles) of T. brucei and control MRC-5 human cells (filled triangles) were incubated with increasing concentrations of AS-48 at 37 °C and for 72 h. Cell viability was determined using an alamarBlue®-based assay and expressed as the percentage of untreated control samples. The results show the mean ± SD for one of the three independent experiments performed in triplicate. (b) Cytocidal effect of AS-48 on BSF of T. brucei. BSF of T. brucei were incubated with 100 nM AS-48 for 60 min at 37 °C and the cells were fixed and stained with DAPI (left panel). The middle panel corresponds to Nomarsky (DIC) images and the right panel shows the images once merged. Scale bar: 2 μm.
Table 1.
AS-48 activity on HAT and nagana-causing T. brucei subspecies. The results are the mean ± SD of three independent experiments performed in triplicate. EC50: 50% inhibitory concentration of AS-48.
| EC50 (nM) | ||||
|---|---|---|---|---|
| BSF |
PCF |
Human cells |
||
| T. brucei rhodesiense | T. brucei gambiense | T. brucei brucei | T. brucei brucei | MRC-5 |
| 1.7 ± 0.2 | 2.6 ± 0.1 | 3.1 ± 0.2 | 140.0 ± 57.0 | No growth inhibition at 12,500 |
Table 2.
Trypanocidal concentrations of AS-48 do not permeabilize the parasite plasma membrane. The effect of AS-48 on plasma membrane permeability was determined by flow cytometry using SYTOX® Green as described in Materials and Methods. Intracellular fluorescence after 0.1% Triton™ X-100 treatment was used as a control of 100% permeabilization. Parasite death under the same conditions was monitored using optical microscopy to calculate the percentage of non-motile parasites.
| AS-48 (μM) | Time (min) | 4 °C |
37 °C |
||
|---|---|---|---|---|---|
| Intracellular fluorescence (% of control) | Cell death (%) | Intracellular fluorescence (% of control) | Cell death (%) | ||
| 0.05 | 10 | <2 | <10 | <2 | 12.0 ± 3.4 |
| 30 | <2 | <10 | <2 | 26.2 ± 20.8 | |
| 60 | <2 | <10 | <2 | 34.6 ± 4.8 | |
| 0.1 | 10 | <2 | <10 | <2 | 9.4 ± 3.4 |
| 30 | <2 | <10 | <2 | 37.6 ± 26.0 | |
| 60 | <2 | <10 | <2 | 63.4 ± 12.5 | |
| 0.5 | 10 | <2 | <10 | <2 | 26.6 ± 13.9 |
| 30 | <2 | <10 | 3.3 ± 3.1 | 86.0 ± 12.4 | |
| 60 | <2 | <10 | 7.1 ± 0.5 | 95.5 ± 2.5 | |
Remarkably, AS-48 was also active against PCF of T. b. brucei (EC50 = 140 ± 57 nM) (Fig. 1 and Table 1). Although this EC50 is 45 times higher than the one for BSF, it is much lower than those reported for other AMPs, which do not usually present any activity or only in the high micromolar range (Harrington, 2011), with attacin being the exception (Hu and Aksoy, 2005).
Additionally, several studies have shown that AS-48 has no activity against the majority of eukaryotic cells tested, including yeast and parasites such as Naegleria fowleri or Acanthamoeba (Grande Burgos et al., 2014; Maqueda et al., 2004), in agreement with the absence of toxicity against the human cell line we assayed in this study (Fig. 1). However, AS-48's lethality against Leishmania promastigotes and intracellular amastigotes, with scarce cytotoxicity on macrophages, has been reported recently, although the EC50 values were 103–104-fold higher (1.3–10.2 μM) (Abengózar et al., 2017) than the ones studied here.
3.2. AS-48 has a plasma membrane pore-formation-independent killing effect against T. brucei
According to the bactericidal mode of action described for AS-48, wherein its primary target is the bacterial membrane (Sánchez-Barrena et al., 2003), its effect on trypanosome may be a result of the peptide binding to the parasite surface and subsequently forming pores in the plasma membrane. To analyze this hypothesis, we investigated the effect of AS-48 on T. brucei plasma membrane integrity by using flow cytometry to monitor the uptake of the vital dye SYTOX® Green (Carvalho et al., 2015). This stain does not penetrate intact cells and becomes fluorescent when it binds to the DNA of permeabilized cells. The viability of the treated trypanosomes under these conditions was checked with an optical microscope (Luque-Ortega et al., 2012; Greene and Hajduk, 2016; Castillo-Acosta et al., 2013).
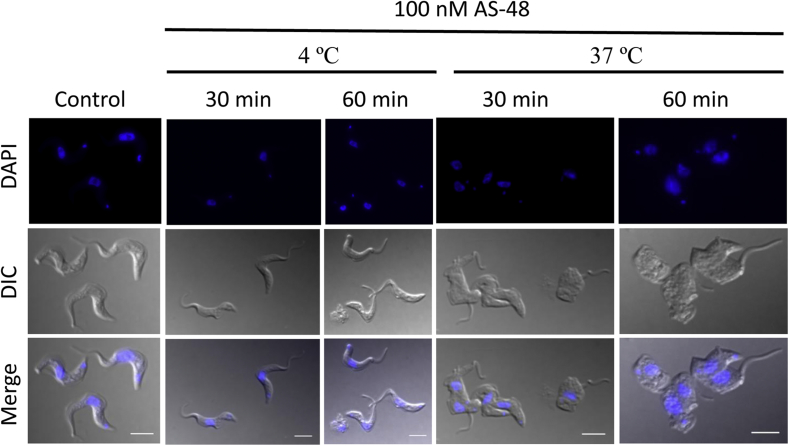
BSF parasites were treated with different concentrations of AS-48 (50, 100 and 500 nM) for 10, 30 and 60 min at 37 °C (Table 2). We used AS-48 concentrations that were higher than the EC50 obtained (Table 1) because the incubation times were shorter (10–60 min vs 72 h) and a lot more cells were used (107 cells mL−1 vs 104 cells mL−1) in this experiment. As shown in Table 2 and Fig. 2, AS-48 concentrations in the order of 100 nM, that were significantly trypanocidal at 37 °C (around 40–65% cell death in 30–60 min), only slightly increased SYTOX® Green accumulation (<2%) compared with the control for maximal cell permeabilization, obtained using 0.1% Triton™ X-100. Furthermore, this trypanocidal effect was temperature dependent as neither parasite permeabilization nor death was detected at 4 °C, even at the higher concentrations and periods tested (500 nM for 60 min) (Table 2 and Fig. 2).
Fig. 2.
The trypanocidal effect of AS-48 is temperature-dependent. BSF of T. brucei were incubated with 100 nM AS-48 for 30 and 60 min at 4 °C and 37 °C, respectively. Parasites were then fixed and stained with DAPI (upper panel). The middle panel shows the DIC images and the lower panel shows the merged images. Scale bar: 2 μm. These images are representative of four independent experiments performed in duplicate.
Collectively these data indicate that AS-48 does not permeate the parasite plasma membrane and therefore promote the rapid influx and efflux of small molecules, as occurs in bacteria and even in Leishmania (Abengózar et al., 2017; Sánchez-Hidalgo et al., 2011). T. brucei must undergo a cell death mechanism that differs from the bactericidal mechanism, which usually involves ion leakage through pores formed once AS-48 has interacted with the cell membrane.
3.3. Role of clathrin-mediated endocytosis in AS-48 internalization
As T. brucei endocytosis is mostly blocked at 4 °C (Yeaman et al., 2001), the absence of a trypanocidal effect at this temperature could suggest that AS-48 needs to be internalized to exert its activity, a requisite already proposed for neuropeptides (Delgado et al., 2008). Although T. brucei endocytosis is limited to the flagellar pocket, a small specialized plasma membrane invagination surrounding the base of the flagellum, this process is extremely efficient in BSF of the parasites (Allen et al., 2003; Field and Carrington, 2009).
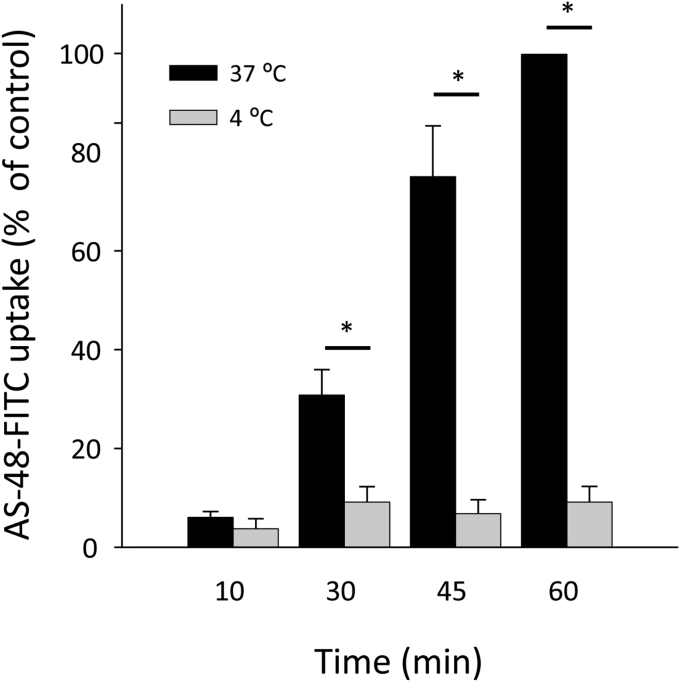
To explore the relationship between endocytosis and AS-48 activity, we first studied the uptake of labeled AS-48 by BSF T. brucei. After validating that FITC-AS-48 killed the parasites with the same potency as the unlabeled peptide (data not shown), they were incubated with 0.2 μM FITC-AS-48 for 10, 30, 45 and 60 min at physiological temperature (37 °C) and also at 4 °C to inhibit endocytosis. The results (Fig. 3) show that while FITC-AS-48 accumulation increased with time at 37 °C, the peptide was not internalized at 4 °C. Intracellular FITC-AS-48 accumulation at 37 °C could be even higher than the showed in Fig. 3 as FITC fluorescence could be diminished in acidic compartments of the endocytic pathway (Lanz et al., 1997). This suggests endocytosis indeed plays an essential role in AS-48 uptake, and internalization is necessary for this bacteriocin to become trypanocidally active. That could explain the higher activity of AS-48 in BSF compared to PCF, as the later has a reduced rate of endocitosys (Morgan et al., 2001; Pal et al., 2002). Unfortunately, due to its high trypanocidal activity and the low sensitivity of FITC labeling, we were unable to analyze the intracellular presence of AS-48 by fluorescence microscopy.
Fig. 3.
The uptake of fluorescent AS-48 by BSF of T. brucei is temperature-dependent. BSF were incubated at 4 °C or 37 °C with 0.2 μM of fluorescent FITC-AS-48 for 10, 30, 45 and 60 min. AS-48 accumulation was analyzed by flow cytometry, as described in Material and Methods. The results are the mean ± SD of three experiments. Significant differences were determined using Student's t-test (*, p < 0.05 versus 4 °C).
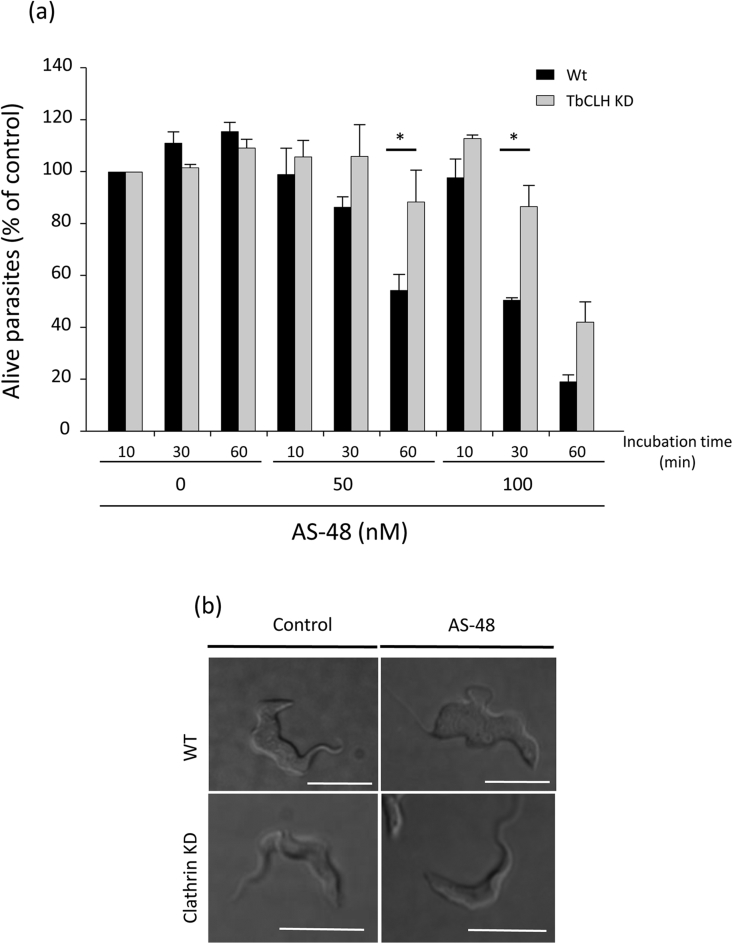
In a further attempt to definitively determine the role of endocytosis in AS-48 trypanocidal action, we decided to specifically inhibit endocytosis by reducing clathrin heavy chain (TbCLH) expression through RNA interference (RNAi), since endocytosis is an exclusively clathrin-dependent mechanism in T. brucei (Allen et al., 2003). After confirming that human transferrin uptake by endocytosis decreased to 59% within 4 h of RNAi induction and without affecting cell growth (data not shown), TbCLH knockdown (KD) cells were incubated at 50 or 100 nM AS-48 and then cell survival assessed after 10, 30 and 60 min. A statistically significant increase in cell survival (90% vs. 55%) was observed for the TbCLH KD cells after 30 min (100 nM AS-48) and 60 min (50 nM AS-48) (Fig. 4 and supplemental video), indicating that clathrin-depleted cells were less permissive to the action of AS-48.
Fig. 4.
AS-48 is internalized by BSF of T. brucei through clathrin-dependent endocytosis. (a) Clathrin-depleted cells were incubated with 0 (control), 0.05, 0.1 and 0.5 μM AS-48 for 10, 30 and 60 min and the number of live cells counted. The data are represented as cell survival percentages with respect to the control (cells incubated for 10 min in the absence of AS-48). Experiments were performed in triplicate upon 4 h of TbCLH RNAi induction. The results are shown as the mean ± SD of three experiments (*, p < 0.05 versus non depleted controls). (b) Representative DIC pictures extracted from videos displayed in Supplementary data (See Supp. Data). T. b. brucei WT (upper panel) vs. TbCLT KD (lower panel), with (right) or without (left) AS-48.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.03.002.
The following is the supplementary data related to this article:
Wt
WT + 30 min 0.1μM AS48.
Clathrin KD 4h.
Clathrin KD 4h + 30 min 0.1μM AS48.
These results strongly imply that clathrin-mediated endocytosis is involved in AS-48 internalization within the parasite. This mode of action targeting the trypanosome's interior is considered to be an attractive drug development strategy (Alsford et al., 2013; Barrett et al., 2007). Indeed endocytosis also mediates the targeting of a natural trypanocide such as ApoL1 (Vanhollebeke et al., 2008), and trypanocidal drugs, e.g., suramin (Alsford et al., 2012), into trypanosomes.
3.4. AS-48 interact with VSG at the surface of the parasite
Although we were yet to determine which kind of endocytosis was responsible for the uptake of the cationic bacteriocin AS-48, it was tempting to think that it could take place once it had interacted with anionic residues on the outer surface of the parasite (Souto-Padrón et al., 1990), as suggested for the neuropeptide VIP, another cationic trypanocidal peptide believed to bind to negatively charged glycoproteins such as VSG (Gonzalez-Rey et al., 2006).
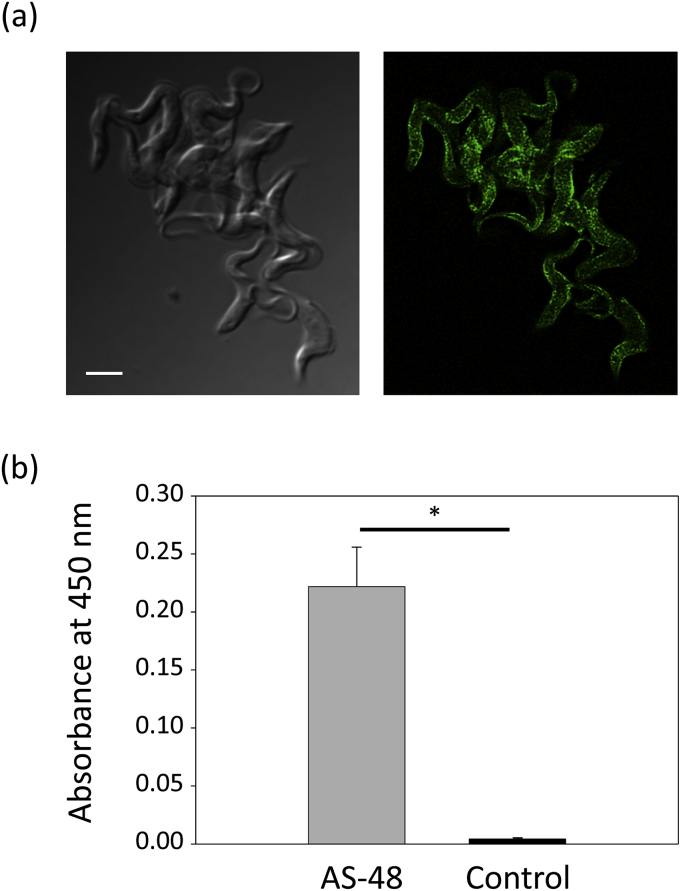
To explore this possibility, first we analyzed whether labeled AS-48 interacted with the surface of the parasites. Fig. 5a shows that after adding high concentration (1 μM) of FITC-AS-48 to the parasites and proceed to their immediate fixation, the peptide was mainly located at the plasma membrane in most cells, evidencing a direct interaction with the surface of the parasite. Then, we monitoryze the ability of AS-48 to interact with purified VSG using an ELISA-like assay. To asses that, VSG was first affixed to a ELISA plate through passive adsorption and then, AS-48 was applied (except in controls wells) so that it could bind to VSG. After washing the plate to eliminate free peptide, AS-48 was detected using polyclonal antibodies against AS-48 and a secondary antibody covalently linked to HRP, that produces a detectable absorption signal in the presence of a HRP substrate. Fig. 5b shows the significative absorbance at 450 nm detected only when AS-48 was added to the VSG-containing wells, indicating a specific interaction between VSG and AS-48.
Fig. 5.
AS-48 binds VSG at the surface of BSF of T. brucei (a) FITC-AS48 binds to the surface of the parasites. 1 μM FITC-AS-48 was added to BSF parasites and cells were immediately fixed before being observed by fluorescence microscopy. The representative image shows how FITC-AS-48 label the plasma membrane in most cells. Scale bar: 5 μm (b) AS-48 interact with isolated VSG. 96-well ELISA plate coated with purified VSG were incubated with AS-48 or PBS (control), and the peptide bound to VSG was detected with anti-AS-48 (rabbit), HRP-conjugated anti-rabbit IgG antibodies and the HRP substrate ABTS. The results shows ELISA well absorbance at 450 nm and are the mean ± SD of two independent experiments performed in triplicate. Significant differences were determined using Student's t-test (* p < 10−5 versus control).
These results strongly suggest that AS-48 is endocytosed after its interaction with VSG. The high number of VSG copies on the parasitic surface (107 molecules per cell) and its extremely rapid internalization and recycling by endocytosis (the VSG coat is completely internalized in 12.5 min) (Engstler et al., 2004) could explain the AS-48's efficiency and specificity against T. brucei. Interestingly, this mechanism could also help prevent resistance developing through reduced drug uptake (Alsford et al., 2013) because endocytosis is essential for the parasite (Field and Carrington, 2009) and possibly also explain our inability to generate AS-48-resistant parasites (MMG et al., unpublished results).
3.5. AS-48 kills Trypanosoma brucei by inducing autophagy
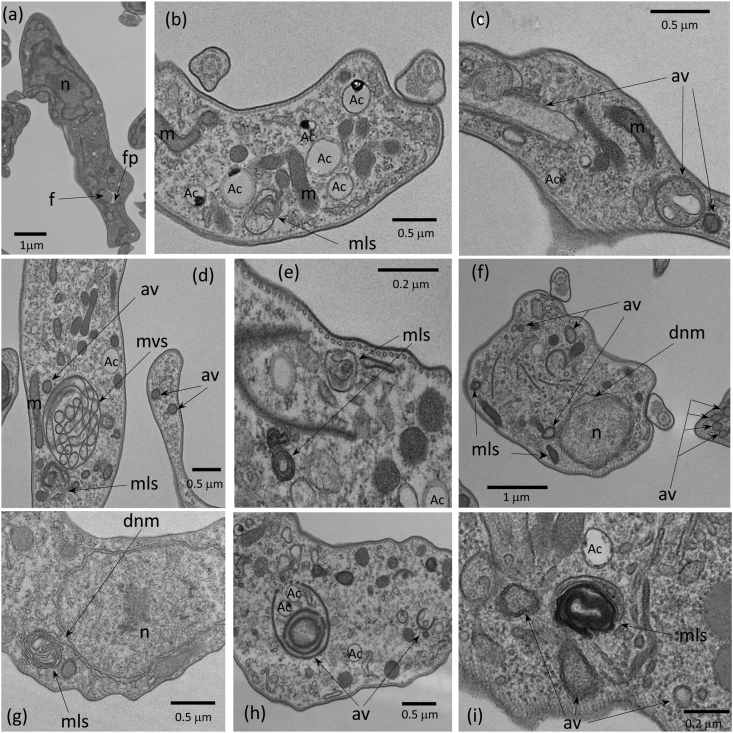
In any case, once AS-48 has combined and reacted with the surface, then it is internalized and subsequently interacts with the intracellular components. To further explore its intracellular effects, AS-48-treated parasites were examined by transmission electron microscopy (TEM), during which we observed profound ultrastructural alterations (Fig. 6). Incubation with 100 nM AS-48 for 30 min did not affect plasma membrane integrity but rather induced an extensive increase in the number of vesicular structures within the cytosol, including the presence of double-membrane vesicles resembling autophagic vacuoles (Fig. 6c, f, h and i), myelin-like structures (Fig. 6b, d, g and i), multivesicular structures (Fig. 6d) and even alterations of the nuclear envelope with regions of the outer nuclear membrane separated from the inner nuclear membrane (Fig. 6f and g). The high frequency and wide variety of autophagic-like vacuoles and related structures observed in parasites after treatment with AS-48 strongly indicate that treated cells die primarily by autophagy. Indeed, similar ultrastructural alterations were observed after treatment with compounds that induce autophagic cell death in Trypanosoma brucei (at much higher concentrations) such as dihydroxyacetone (DHA) (at 3 mM) (Uzcátegui et al., 2007) and the neuropeptide VIP (at 40 μM) (Gonzalez-Rey et al., 2006).
Fig. 6.
Ultrastructural alterations on BSF cells caused by AS-48. Representative transmission micrographs of ultrathin resin sections of BSF parasites before (a) or after (b–i) treatment with 100 nM AS-48 for 60 min; note the morphological features of autophagic cell death. Autophagic-like vacuoles (av), myelin-like structures (mls), multivesicular structures (mvs) and dilated nuclear membrane (dnm) are shown with black arrows. f, flagellum; fp, flagellar pocket; m, mitochondrion; n, nucleus; Ac, acidocalcisome. Scale bars are indicated on the images.
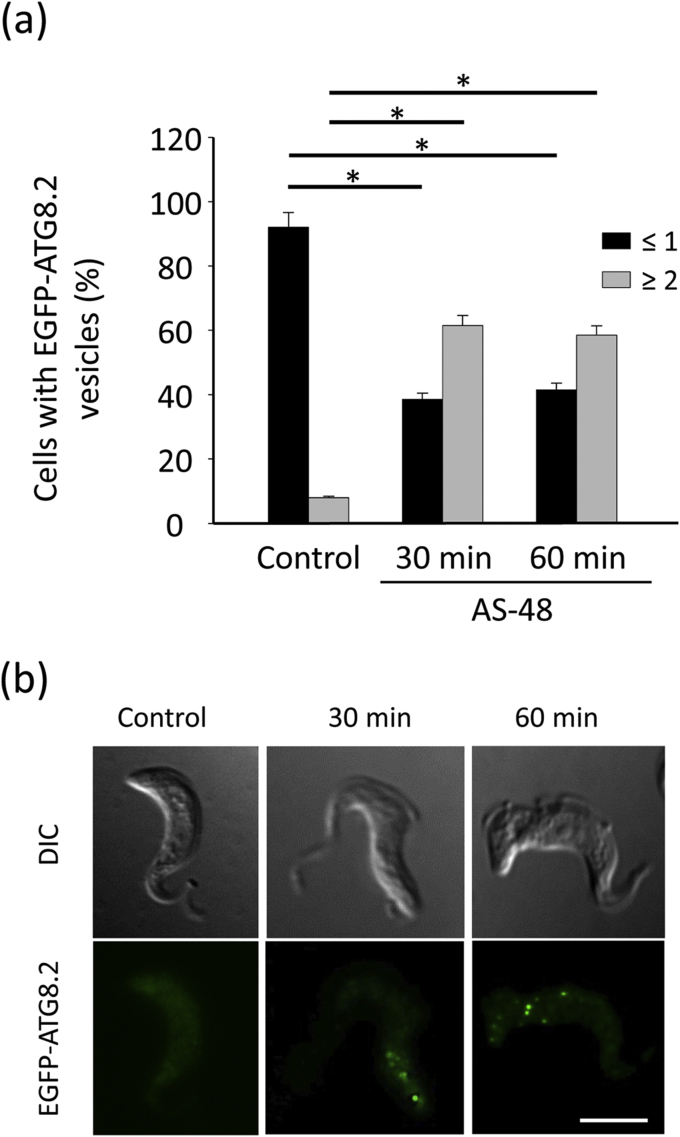
To definitively confirm the role of autophagy in the AS-48-induced cell death mechanism, we assessed autophagy occurring in AS-48-treated parasites by monitoring the formation of ATG8.2-containing autophagosomes. ATG8.2 is a ubiquitin-like protein required for autophagosome membrane formation (Proto et al., 2014). A soluble form is present in the cytosol of cells. However, in conditions that favor autophagy, ATG8.2 is recruited into the membrane of autophagosomes, and their number can be monitored by fluorescence microscopy after labeling ATG8.2 with a fluorescent tag (Proto et al., 2014). So we first cloned ATG8.2 with an EGFP tag at the N-terminal of the gene. Then, EGFP-ATG8.2 transfected BSF parasites were incubated with 100 nM AS-48 for 30 and 60 min, and the number of labeled autophagosomes (EGFP-ATG8.2 puncta) per cell was quantified by fluorescence microscopy. In most control cells (99.5%), EGFP-ATG8.2 was mainly distributed throughout the cytoplasm (63%) or in a single punctate structure (36%), whereas almost no parasite (0.5%) contained more than one autophagosome (Fig. 7). Contrastingly, AS-48 treated parasites showed a significant increase in the number of autophagosomes per cell, with around 60% of the parasites containing two or more autophagosomes, whereas only a minority of cells presented no EGFP-ATG8.2 puncta (15%) (Fig. 7). In fact the mean number of autophagosomes per cell increased from 0.38 to 2.01 after AS-48 treatment. These findings help shed light on the bacteriocin AS-48-induced autophagy that occurs in BSF of T. brucei.
Fig. 7.
AS-48 increases the number of autophagosomes on BSF of T. brucei. (a) BSF parasites transfected with EGFP-ATG8.2 were incubated in the presence of 100 nM AS-48 for 0, 30 and 60 min at 37 °C. The parasites were then fixed and autophagy was monitored by counting the number of EGFP-ATG8.2-labeled autophagosomes per cell and represented as the percentage of cells containing more or less than one autophagosome for each set of conditions. The results are the mean ± SD of two independent experiments performed in duplicate. Significant differences were determined using Student's t-test (*, p < 0.05 versus control) (b). A representative image showing the fluorescent autophagosomes at the same time as those described above. Scale bar: 5 μm.
Notably, this lethal mechanism is different from the one recently put forward to explain the leishmanicidal effect of AS-48 (Abengózar et al., 2017). In Leishmania, AS-48 produced partial plasma membrane permeabilization and mitochondrial dysfunction leading to apoptotic cell death. In T. brucei, AS-48 does not form pores at the plasma membrane but it is taken up through clathrin-mediated endocytosis after its interaction with VSG at the parasite surface, generating AS-48 containing phagosomes. The bacteriocin could then alter the normal structure of these membranous vesicles from the endocytic pathway, as AS-48 is a membrane-interacting peptide that, once accumulated in the membrane, changes to a different dimeric conformation (from DF-I to DF-II) which produces membrane-damaging pores (Jenssen et al., 2006; Sánchez-Barrena et al., 2003). Indeed the myelin-like structures, autophagic vacuoles and multivesicular structures observed after AS-48 treatment could develop after these endocytic membranes damaged by AS-48 are degraded by autophagy (Dezfuli et al., 2006; Uzcátegui et al., 2007). This is probably indicative of a reparative cell response to the damage AS-48 causes to membrane structure and function, as already suggested for the case of the trypanocidal agent DHA (Uzcátegui et al., 2007). AS-48 could eventually lead to an exacerbated increase in autophagy resulting in parasite death, as also proposed in the case of DHA (Uzcátegui et al., 2007). Regardless, as the causal-effect relationship of autophagy and cell death is not well understood in T. brucei, specially in BSF, we can not rule out that while autophagy can be triggered to rescue cell death caused by AS-48, this pathway of autophagy itself may not lead to cell death (Li et al., 2012).
4. Conclusions
AS-48, the first bacteriocin reported to have activity against T. brucei, kills the BSF of different subspecies of this parasite at lower concentrations than those required for drugs currently used to treat HAT. AS-48 is also innocuous to human cells, presenting a selectivity index of more than 104-fold. Unlike its bactericidal mechanism of action, AS-48 does not kill the parasite through permeabilization of its plasma membrane. In contrast, it is taken up by clathrin-mediated endocytosis afer its interaction with VSG at the surface of the parasite and induces autophagic cell death. As AS-48 is a very stable circular peptide that exhibits high resistance to exopeptidases and very low immunogenicity, our results suggest that this bacteriocin could represent an attractive lead compound against T. brucei.
Acknowledgments
We wish to thank Dr. Michael Duszenko (Interfaculty Institute for Biochemistry, University of Tübingen, Germany), and Prof. Mark Field (University of Dundee, UK) who kindly provided the plasmids pLew100 and p2T7TiCL, respectively.
This work was supported by Spanish grants SAF2011-28215 (JMPV), SAF2016-80228-R (JMPV) and SAF2013-48971-C2-1-R (MM) from the Ministerio de Economía y Competitividad, and BIO1786 (JMPV) from the Junta de Andalucía and by FEDER funds from the EU to JMPV. MMG was recipient of a FPI fellowship from the Spanish Ministerio de Economía y Competitividad: SAF2011-28215, SAF2016-80228-R and SAF2013-48971-C2-1-R. MMG was a student of the Biochemistry and Molecular Biology Ph.D. program of the University of Granada (Spain). JMB is funded by “Fondo de Investigación Sanitaria” (FIS) TRPY 1283/15. MN is granted by ISCIII -Subdirección General de Redes y Centros de Investigación Cooperativa (RICET) RD12/0018/0015.
Contributor Information
Rubén Cebrián, Email: rcebrian@ugr.es.
José M. Pérez-Victoria, Email: josepv@ipb.csic.es.
References
- Abengózar M.Á., Cebrián R., Saugar J.M., Gárate T., Valdivia E., Martínez-Bueno M., Maqueda M., Rivas L. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02288-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C.L., Goulding D., Field M.C. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 2003;22:4991–5002. doi: 10.1093/emboj/cdg481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F., Turner D.J., Field M.C., Berriman M., Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsford S., Field M.C., Horn D. Receptor-mediated endocytosis for drug delivery in African trypanosomes: fulfilling Paul Ehrlich's vision of chemotherapy. Trends Parasitol. 2013;29:207–212. doi: 10.1016/j.pt.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ananou S., Muñoz A., Gálvez A., Martínez-Bueno M., Maqueda M., Valdivia E. Optimization of enterocin AS-48 production on a whey-based substrate. Int. Dairy J. 2008;18:923–927. doi: 10.1016/j.idairyj.2008.02.001. [DOI] [Google Scholar]
- Bakunova S.M., Bakunov S.A., Patrick D.A., Kumar E.V.K.S., Ohemeng K.A., Bridges A.S., Wenzler T., Barszcz T., Jones S.K., Werbovetz K.A., Brun R., Tidwell R.R. Structure-activity study of pentamidine analogues as antiprotozoal agents. J. Med. Chem. 2009;52:2016–2035. doi: 10.1021/jm801547t. [DOI] [PubMed] [Google Scholar]
- Barrett M.P., Boykin D.W., Brun R., Tidwell R.R. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 2007;152:1155–1171. doi: 10.1038/sj.bjp.0707354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart J.-M., Cordon-Obras C., Vidal I., Reed J., Perez-Pastrana E., Cuevas L., Field M.C., Carrington M., Navarro M. Localization of serum resistance-associated protein in Trypanosoma brucei rhodesiense and transgenic Trypanosoma brucei brucei. Cell Microbiol. 2015;17:1523–1535. doi: 10.1111/cmi.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte-Reche E., Martínez-García M., Peñalver P., Gómez-Pérez V., Lucas R., Gamarro F., Pérez-Victoria J.M., Morales J.C. Tyrosol and hydroxytyrosol derivatives as antitrypanosomal and antileishmanial agents. Eur. J. Med. Chem. 2016;119:132–140. doi: 10.1016/j.ejmech.2016.04.047. [DOI] [PubMed] [Google Scholar]
- Cabello-Donayre M., Malagarie-Cazenave S., Campos-Salinas J., Gálvez F.J., Rodríguez-Martínez A., Pineda-Molina E., Orrego L.M., Martínez-García M., Sánchez-Cañete M.P., Estévez A.M., Pérez-Victoria J.M. Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters. Mol. Microbiol. 2016;101:895–908. doi: 10.1111/mmi.13430. [DOI] [PubMed] [Google Scholar]
- Carvalho L., Martínez-García M., Pérez-Victoria I., Manzano J.I., Yardley V., Gamarro F., Pérez-Victoria J.M. The oral antimalarial drug Tafenoquine shows activity against Trypanosoma brucei. Antimicrob. Agents Chemother. 2015;59:6151–6160. doi: 10.1128/AAC.00879-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Acosta V.M., Vidal A.E., Ruiz-Pérez L.M., Van Damme E.J.M., Igarashi Y., Balzarini J., González-Pacanowska D. Carbohydrate-binding agents act as potent trypanocidals that elicit modifications in VSG glycosylation and reduced virulence in Trypanosoma brucei. Mol. Microbiol. 2013;90:665–679. doi: 10.1111/mmi.12359. [DOI] [PubMed] [Google Scholar]
- Cebrián R., Baños A., Valdivia E., Pérez-Pulido R., Martínez-Bueno M., Maqueda M. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain. Food Microbiol. 2012;30:59–67. doi: 10.1016/j.fm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Cross G.A. Release and purification of Trypanosoma brucei variant surface glycoprotein. J. Cell. Biochem. 1984;24:79–90. doi: 10.1002/jcb.240240107. [DOI] [PubMed] [Google Scholar]
- Cullen D.R., Mocerino M. A brief review of drug discovery research for Human African Trypanosomiasis. Curr. Med. Chem. 2017 doi: 10.2174/0929867324666170120160034. [DOI] [PubMed] [Google Scholar]
- David Barry J., McCulloch R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 2001;49:1–70. doi: 10.1016/S0065-308X(01)49037-3. [DOI] [PubMed] [Google Scholar]
- De Rycker M., Hallyburton I., Thomas J., Campbell L., Wyllie S., Joshi D., Cameron S., Gilbert I.H., Wyatt P.G., Frearson J.A., Fairlamb A.H., Gray D.W. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrob. Agents Chemother. 2013;57:2913–2922. doi: 10.1128/AAC.02398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M., Anderson P., Garcia-Salcedo J.A., Caro M., Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2008;16:406–416. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]
- Dezfuli B.S., Simoni E., Giari L., Manera M. Effects of experimental terbuthylazine exposure on the cells of Dicentrarchus labrax (L.) Chemosphere. 2006;64:1684–1694. doi: 10.1016/j.chemosphere.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Engstler M., Thilo L., Weise F., Grünfelder C.G., Schwarz H., Boshart M., Overath P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- Field M.C., Carrington M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E., Chorny A., Delgado M. VIP: an agent with license to kill infective parasites. Ann. N. Y. Acad. Sci. 2006;1070:303–308. doi: 10.1196/annals.1317.032. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E., Chorny A., Varela N., O'Valle F., Delgado M. Therapeutic effect of urocortin on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of regulatory T cells. Arthritis Rheum. 2007;56:531–543. doi: 10.1002/art.22394. [DOI] [PubMed] [Google Scholar]
- Grande Burgos M.J., Pérez Pulido R., López Aguayo M., del C., Gálvez A., Lucas R. The cyclic antibacterial peptide enterocin AS-48: isolation, mode of action, and possible food applications. Int. J. Mol. Sci. 2014;15:22706–22727. doi: 10.3390/ijms151222706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene A.S., Hajduk S.L. Trypanosome lytic Factor-1 initiates oxidation-stimulated osmotic lysis of Trypanosoma brucei brucei. J. Biol. Chem. 2016;291:3063–3075. doi: 10.1074/jbc.M115.680371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines L.R., Hancock R.E.W., Pearson T.W. Cationic antimicrobial peptide killing of african Trypanosomes and Sodalis glossinidius, a bacterial symbiont of the insect vector of sleeping sickness. Vector Borne Zoonotic Dis. 2003;3:175–186. doi: 10.1089/153036603322662165. [DOI] [PubMed] [Google Scholar]
- Harrington J.M. Antimicrobial peptide killing of african Trypanosomes. Parasite Immunol. 2011;33:461–469. doi: 10.1111/j.1365-3024.2011.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C., Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol. Microbiol. 2006;60:1194–1204. doi: 10.1111/j.1365-2958.2006.05180.x. [DOI] [PubMed] [Google Scholar]
- Hu Y., Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem. Mol. Biol. 2005;35:105–115. doi: 10.1016/j.ibmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ishiyama A., Otoguro K., Iwatsuki M., Namatame M., Nishihara A., Nonaka K., Kinoshita Y., Takahashi Y., Masuma R., Shiomi K., Yamada H., Ōmura S. In vitro and in vivo antitrypanosomal activities of three peptide antibiotics: leucinostatin A and B, alamethicin I and tsushimycin. J. Antibiot. (Tokyo) 2009;62:303–308. doi: 10.1038/ja.2009.32. [DOI] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz E., Gregor M., Slavķk J., Kotyk A. Use of FITC as a fluorescent probe for intracellular pH measurement. J. Fluoresc. 1997;7:317–319. doi: 10.1023/A:1022586127784. [DOI] [Google Scholar]
- Li F.-J., Shen Q., Wang C., Sun Y., Yuan A.Y., He C.Y. A role of autophagy in Trypanosoma brucei cell death. Cell Microbiol. 2012;14:1242–1256. doi: 10.1111/j.1462-5822.2012.01795.x. [DOI] [PubMed] [Google Scholar]
- Luque-Ortega J.R., de la Torre B.G., Hornillos V., Bart J.-M., Rueda C., Navarro M., Amat-Guerri F., Acuña A.U., Andreu D., Rivas L. Defeating Leishmania resistance to miltefosine (hexadecylphosphocholine) by peptide-mediated drug smuggling: a proof of mechanism for trypanosomatid chemotherapy. J. Control. Release Off. J. Control. Release Soc. 2012;161:835–842. doi: 10.1016/j.jconrel.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Maqueda M., Gálvez A., Bueno M.M., Sanchez-Barrena M.J., González C., Albert A., Rico M., Valdivia E. Peptide AS-48: prototype of a new class of cyclic bacteriocins. Curr. Protein Pept. Sci. 2004;5:399–416. doi: 10.2174/1389203043379567. [DOI] [PubMed] [Google Scholar]
- Maqueda M., Gálvez A., Martínez-Bueno M., Guerra I., Valdivia E. Neutralizing antibodies against the peptide antibiotic AS-48: immunocytological studies. Antimicrob. Agents Chemother. 1993;37:148–151. doi: 10.1128/aac.37.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwire B.S., Olson C.L., Tack B.F., Engman D.M. Killing of african Trypanosomes by antimicrobial peptides. J. Infect. Dis. 2003;188:146–152. doi: 10.1086/375747. [DOI] [PubMed] [Google Scholar]
- Mendoza F., Maqueda M., Gálvez A., Martínez-Bueno M., Valdivia E. Antilisterial activity of peptide AS-48 and study of changes induced in the cell envelope properties of an AS-48-adapted strain of Listeria monocytogenes. Appl. Environ. Microbiol. 1999;65:618–625. doi: 10.1128/aem.65.2.618-625.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban-Lopez M., Sanchez-Hidalgo M., Valdivia E., Martinez-Bueno M., Maqueda M. Are bacteriocins Underexploited? NOVEL applications for OLD antimicrobials. Curr. Pharm. Biotechnol. 2011;12:1205–1220. doi: 10.2174/138920111796117364. [DOI] [PubMed] [Google Scholar]
- Morgan G.W., Allen C.L., Jeffries T.R., Hollinshead M., Field M.C. Developmental and morphological regulation of clathrin-mediated endocytosis in Trypanosoma brucei. J. Cell Sci. 2001;114:2605–2615. doi: 10.1242/jcs.114.14.2605. [DOI] [PubMed] [Google Scholar]
- Natesan S.K.A., Peacock L., Matthews K., Gibson W., Field M.C. Activation of endocytosis as an adaptation to the mammalian host by Trypanosomes. Eukaryot. Cell. 2007;6:2029–2037. doi: 10.1128/EC.00213-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M., Cross G.A. DNA rearrangements associated with multiple consecutive directed antigenic switches in Trypanosoma brucei. Mol. Cell Biol. 1996;16:3615–3625. doi: 10.1128/mcb.16.7.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Hall B.S., Nesbeth D.N., Field H.I., Field M.C. Differential endocytic functions of Trypanosoma brucei Rab5 isoforms reveal a glycosylphosphatidylinositol-specific endosomal pathway. J. Biol. Chem. 2002;277:9529–9539. doi: 10.1074/jbc.M110055200. [DOI] [PubMed] [Google Scholar]
- Pérez-Victoria J.M., Cortés-Selva F., Parodi-Talice A., Bavchvarov B.I., Pérez-Victoria F.J., Muñoz-Martínez F., Maitrejean M., Costi M.P., Barron D., Di Pietro A., Castanys S., Gamarro F. Combination of suboptimal doses of inhibitors Targeting different domains of LtrMDR1 efficiently overcomes resistance of Leishmania spp. to miltefosine by inhibiting drug efflux. Antimicrob. Agents Chemother. 2006;50:3102–3110. doi: 10.1128/AAC.00423-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T., Walden M., Butler C., Diaz-Gonzalez R., Pérez-Moreno G., Ceballos-Pérez G., Gomez-Pérez V., García-Hernández R., Zecca H., Krakoff E., Kopec B., Ichire O., Mackenzie C., Pitot M., Ruiz L.M., Gamarro F., González-Pacanowska D., Navarro M., Dounay A.B. Novel 1,2-dihydroquinazolin-2-ones: design, synthesis, and biological evaluation against Trypanosoma brucei. Bioorg. Med. Chem. Lett. 2017;27:3629–3635. doi: 10.1016/j.bmcl.2017.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto W.R., Jones N.G., Coombs G.H., Mottram J.C. Tracking autophagy during proliferation and differentiation of Trypanosoma brucei. Microb. Cell Graz Austria. 2014;1:9–20. doi: 10.15698/mic2014.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu R., Shirahatti P.S., Devi A.T., Prasad A., J K., M S L.F., Z B.L., MN N.P D. Bacteriocins and Their applications in food preservation. Crit. Rev. Food Sci. Nutr. 2015;0 doi: 10.1080/10408398.2015.1020918. [DOI] [Google Scholar]
- Räz B., Iten M., Grether-Bühler Y., Kaminsky R., Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997;68:139–147. doi: 10.1016/s0001-706x(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Reinhardt . 2008. Chronique ONU | Travailler ensemble: La mouche tsé-tsé et la pauvreté rurale.http://www.un.org/french/pubs/chronique/2002/numero2/0202p17_la_mouche_tsetse.html [WWW Document] (Accessed 10 19 2016) [Google Scholar]
- Riley M.A., Wertz J.E. Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- Rycker M.D., O'Neill S., Joshi D., Campbell L., Gray D.W., Fairlamb A.H. A static-cidal assay for Trypanosoma brucei to aid hit prioritisation for progression into drug discovery programmes. PLoS Neglected Trop. Dis. 2012;6:e1932. doi: 10.1371/journal.pntd.0001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Barrena M.J., Martínez-Ripoll M., Gálvez A., Valdivia E., Maqueda M., Cruz V., Albert A. Structure of bacteriocin AS-48: from soluble state to membrane bound state. J. Mol. Biol. 2003;334:541–549. doi: 10.1016/j.jmb.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández E.M., Gómez-Pérez V., García-Hernández R., Fernández J.M.G., Plata G.B., Padrón J.M., Mellet C.O., Castanys S., Gamarro F. Antileishmanial activity of sp2-iminosugar derivatives. RSC Adv. 2015;5:21812–21822. doi: 10.1039/C5RA02627J. [DOI] [Google Scholar]
- Sánchez-Hidalgo M., Montalbán-López M., Cebrián R., Valdivia E., Martínez-Bueno M., Maqueda M. AS-48 bacteriocin: close to perfection. Cell. Mol. Life Sci. 2011;68:2845–2857. doi: 10.1007/s00018-011-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Padrón T., Chiari E., De Souza W. The surface charge of trypanosomatids of the genus Trypanosoma. Mem. Inst. Oswaldo Cruz. 1990;85:215–219. doi: 10.1590/s0074-02761990000200013. [DOI] [PubMed] [Google Scholar]
- Uzcátegui N.L., Carmona-Gutiérrez D., Denninger V., Schoenfeld C., Lang F., Figarella K., Duszenko M. Antiproliferative effect of dihydroxyacetone on Trypanosoma brucei bloodstream forms: cell cycle progression, subcellular alterations, and cell death. Antimicrob. Agents Chemother. 2007;51:3960–3968. doi: 10.1128/AAC.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B., De Muylder G., Nielsen M.J., Pays A., Tebabi P., Dieu M., Raes M., Moestrup S.K., Pays E. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science. 2008;320:677–681. doi: 10.1126/science.1156296. [DOI] [PubMed] [Google Scholar]
- Vreysen M.J.B., Seck M.T., Sall B., Bouyer J. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2013;112(Suppl. 1):S15–S25. doi: 10.1016/j.jip.2012.07.026. Tse Tse Fly Symposium. [DOI] [PubMed] [Google Scholar]
- Welburn S.C., Maudlin I., Simarro P.P. Controlling sleeping sickness – a review. Parasitology. 2009;136:1943–1949. doi: 10.1017/S0031182009006416. [DOI] [PubMed] [Google Scholar]
- Wirtz E., Leal S., Ochatt C., Cross G.M. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999;99:89–101. doi: 10.1016/S0166-6851(99)00002-X. [DOI] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K.K., Wright J.R., Nelson W.J. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wt
WT + 30 min 0.1μM AS48.
Clathrin KD 4h.
Clathrin KD 4h + 30 min 0.1μM AS48.