Abstract
Aspergillus, Penicillium and Talaromyces are diverse, phenotypically polythetic genera encompassing species important to the environment, economy, biotechnology and medicine, causing significant social impacts. Taxonomic studies on these fungi are essential since they could provide invaluable information on their evolutionary relationships and define criteria for species recognition. With the advancement of various biological, biochemical and computational technologies, different approaches have been adopted for the taxonomy of Aspergillus, Penicillium and Talaromyces; for example, from traditional morphotyping, phenotyping to chemotyping (e.g. lipotyping, proteotypingand metabolotyping) and then mitogenotyping and/or phylotyping. Since different taxonomic approaches focus on different sets of characters of the organisms, various classification and identification schemes would result. In view of this, the consolidated species concept, which takes into account different types of characters, is recently accepted for taxonomic purposes and, together with the lately implemented ‘One Fungus – One Name’ policy, is expected to bring a more stable taxonomy for Aspergillus, Penicillium and Talaromyces, which could facilitate their evolutionary studies. The most significant taxonomic change for the three genera was the transfer of Penicillium subgenus Biverticillium to Talaromyces (e.g. the medically important thermally dimorphic ‘P. marneffei’ endemic in Southeast Asia is now named T. marneffei), leaving both Penicillium and Talaromyces as monophyletic genera. Several distantly related Aspergillus-like fungi were also segregated from Aspergillus, making this genus, containing members of both sexual and asexual morphs, monophyletic as well. In the current omics era, application of various state-of-the-art omics technologies is likely to provide comprehensive information on the evolution of Aspergillus, Penicillium and Talaromyces and a stable taxonomy will hopefully be achieved.
Keywords: Aspergillus, Penicillium, Talaromyces, Classification, Evolution, Omics
1. Introduction
Aspergillus, Penicillium and Talaromyces are diverse genera which belong to the Order Eurotiales and contain a large number of species possessing a worldwide distribution and a huge range of ecological habitats. They are ubiquitous and can be found in the air, soil, vegetation and indoor environments [1,2]. Some members are able to grow in extreme environments such as those with high/low temperatures, high salt/sugar concentrations, low acidities or low oxygen levels [3,4]. Species of the three genera are mainly environmental saprobes [3,4] and the primary contribution of these microorganisms to nature is the decomposition of organic materials [1].
Many Aspergillus, Penicillium and Talaromyces species are economically, biotechnologically and medically important with huge social impacts. For example, these species are vital to the food industry and quite a number of them are exploited to produce fermented food such as cheeses (e.g. P. roqueforti), sausages (e.g. P. nalgiovense) and soy sauce (e.g. A. oryzae and A. sojae). These fungi are also important biotechnologically for their strong degradative abilities which have been utilised for the production of enzymes [5,6]. In addition, they are robust producers of a diverse spectrum of secondary metabolites (or extrolites) some of which could be used as drugs and antibiotics or as the lead compounds of potential drug candidates with pharmaceutical or biological activities [7]. On the other hand, many of these species, such as A. chevalieri, A. flavipes, P. citreonigrum and T. macrosporus, are food spoiling decomposers which cause pre- and post-harvest devastation of food crops; and many of these food-spoiling species are also mycotoxin-producers [8]. Even worse, some of them are infectious agents and cause diseases in humans and animals. The most notorious pathogenic species on a global sense is A. fumigatus [9], which is the aetiological agent for the majority of aspergillosis cases [10]. Other commonly encountered pathogenic Aspergillus species include A. flavus, A. nidulans, A. niger and A. terrus. Although Penicillium and Talaromyces species are less commonly associated with human or veterinary infections, the thermally dimorphic fungus T. marneffei, previously known as P. marneffei, is an exception. This notorious fungus is endemic in Southeast Asia and it is able to cause systemic infections particularly in immunocompromised individuals such as HIV-positive patients [11] or patients with impaired cell-mediated immunity [12].
Aspergillus, Penicillium and Talaromyces were traditionally classified according to their morphologies. As technologies capable of characterising biological macromolecules advanced, various approaches focusing on the profiles of different cellular constituents such as lipids, proteins and exometabolites have emerged to supplement the taxonomy of these fungi. The availability of DNA sequencing technology in the past two-to-three decades has generated an enormous amount of DNA sequence data, allowing fungal taxonomy through phylogenetics, including genealogical concordance. The currently accepted consolidated species concept [13], or informally known as the ‘polyphasic taxonomic approach’, has revolutionised fungal taxonomy, and the classification scheme for a vast number of fungi has been revised. In particular, significant changes have been made to reclassify Aspergillus, Penicillium and Talaromyces species in the past seven years. Such revision on the classification of these fungi results in redefined species concepts for Aspergillus, Penicillium and Talaromyces, providing new insights on the evolution of these important filamentous fungi. In this article, the development of various taxonomic approaches as well as species recognition and identification schemes for Aspergillus, Penicillium and Talaromyces is reviewed. These include the traditional morphological/phenotypic approach, the supplementary lipidomic, proteomic and metabolomic approaches, as well as the currently widely used phylogenetic/consolidated approach. The clinical implications of this evolving taxonomy are also discussed.
2. Classification and nomenclature: a brief history and recent development
The name Aspergillus was first introduced by Micheli in 1729 to describe asexual fungi whose conidiophores resembled an aspergillum, a device used to sprinkle holy water [14] (Fig. 1a–f). Later in 1768 von Haller validated the genus [15] and in 1832 Fries sanctioned the generic name [16]. Similarly, the genus Penicillium was erected by Link in 1809 [17] to accommodate asexual fungi which bore penicillum (painter’s brush)-like fruiting bodies (Fig. 1g–l).
Fig. 1.
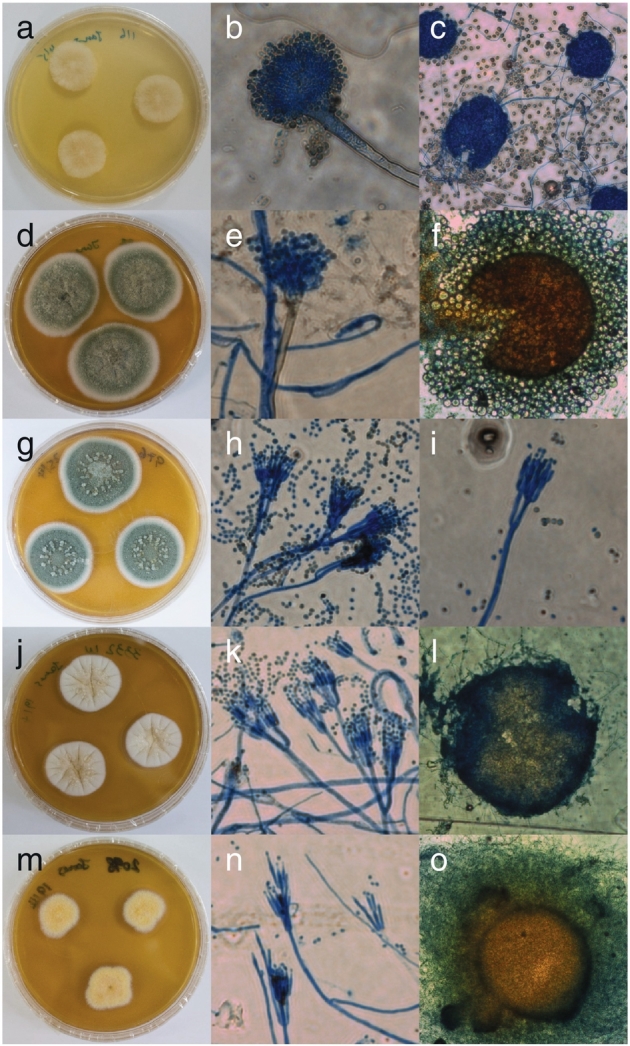
Morphological features of Aspergillus, Penicillium and Talaromyces species. (a) Colony morphology after 7 days of incubation on dichloran 18% glycerol agar, (b) a conidiophore (magnification 400×) and (c) ascomata (Eurotium-like sexual stage, magnification 200×) of A. glaucus NRRL 116T. (d) Colony morphology after 7 days of incubation on malt extract agar (MEA), (e) a conidiophore (magnification 400×) and (f) an ascocarp (Emericella-like sexual stage, magnification 100×) of A. nidulans NRRL 187T. (g) Colony morphology after 7 days of incubation on MEA and (h and i) conidiophores (magnification 400×) of P. expansum NRRL 976T. (j) Colony morphology after 7 days of incubation on MEA, (k) conidiophores (magnification 400×) and (l) an ascocarp (Eupenicillium-like sexual stage, magnification 100×) of P. kewense NRRL 3332T. (m) Colony morphology after 7 days of incubation on MEA, (n) conidiophores (magnification 400×) and (o) an ascocarp (magnification 100×) of T. flavus NRRL 2098T.
Although both Aspergillus and Penicillium were originally described as anamorphic (asexual), some species of the two genera were subsequently found to be ascocarp-forming (Fig. 1c, f and l). For example, the sexual genus Eurotium was first firmly connected to Aspergillus by de Bary in 1854 [18] whereas the ascomycetous genus Eupenicillium has been used to describe Penicillium species capable of producing sclerotoid cleistothecia from as early as 1892 [19]. Since the discovery of the various sexual states of Aspergillus and Penicillium species, it has been controversial as to whether separate sexual generic names should be used to describe species able to produce ascospores. In spite of the fact that several sexual genera had already been established to accommodate the sexual morphs of some Aspergillus and Penicillium species, Thom, Church, Raper and Fennell, in their monographic masterpieces on the taxonomy of these two genera, neglected the use of sexual names. This was because, in their opinions, this would cause unnecessary nomenclatural confusion, especially for strains which were in sexual stages at first and then lost their ascospore-forming ability under laboratory maintenance. In addition, this would also lead to the fragmentation of the large and obviously cohesive Aspergillus/Penicillium groups [[20], [21], [22], [23], [24], [25]]. Nevertheless, in order to abide by the then International Code of Botanical Nomenclature (Stockholm Code), where the first valid names of the ‘perfect states’ (sexual morphs) of fungi took precedence [26], Benjamin assigned Aspergillus species which possess sexual life cycles into the sexual genera Eurotium, Emericella and Sartorya [27]. In addition, he transferred Penicillium species with sexual life cycles to the ascomycetous genus Carpenteles (later synonym of Eupenicillium) [27,28]. During his assignment, Benjamin also established the novel genus Talaromyces to describe Penicillium species which, in their sexual life cycles, possessed soft ascocarps exhibiting indeterminate growth and whose walls were composed of interwoven hyphae [27] (Fig. 1m–o).
As the number of species of the genera Aspergillus, Penicillium and Talaromyces increased, closely related species were grouped into subgroups [[29], [30], [31], [32]]. Such infrageneric classification system underwent vigorous changes since different authors focused on different morphological features when establishing their subgrouping schemes (Table 1). For example, Blochwitz as well as Thom and his co-workers were the first to divide Aspergillus species into seven and 18 subgeneric ‘groups’, respectively, based on their phenotypes [21,24,30,31]. The subgrouping by Thom and associates formed the foundation of Aspergillus subgeneric classification which had been largely followed by other mycologists working on this genus in the last century. However, since these subgeneric ‘groups’ did not possess any nomenclatural status, Gams et al., in 1986, established six subgenera and 18 sections to accommodate these ‘groups’, formalising the subgeneric classification of Aspergillus species [33] (Table 1a). As for Penicillium, Dierckx and Biourge firstly subdivided the genus into the subgenera Aspergilloides (synonym: Monoverticillium) as well as Eupenicillium, which was further separated into sections Biverticillium and Bulliardium (synonym: Asymmetrica) [29,34]. Subsequently, Thom and his co-workers did not follow Dierckx’s and Biourge’s grouping and proposed a new subgeneric classification scheme for Penicillium composed of four main divisions/sections, where species were grouped according to features of their colonies and branching patterns of their conidiophores [20,22]. The system established by Thom and associates for Penicillium was adopted by other mycologists for the next 30 years until Pitt as well as Stolk and Samson in the 1980s proposed two other subgeneric classification schemes based on features of conidiophores, morphology of phialides and growth characteristics, as well as branching patterns of conidiophores and phialide morphology, respectively [35,36] (Table 1b). Similarly, Talaromyces species were also split into four sections based on the structures of their conidial states [32] (Table 1c).
Table 1a.
Overview of major subgeneric classifications of Aspergillus species.
| Blochwitz [31] | Thom & Church [30], Thom & Raper [21], Raper & Fennell [24] | Gams et al. [33] | Peterson [168] | Peterson et al. [169] | Houbraken & Samson [3] | Houbraken et al. [4] | Jurjević et al. [170], Kocsubé et al. [60], Sklenář et al. [171] |
|---|---|---|---|---|---|---|---|
| Euglobosi | Group A. candidus | Subgenus Aspergillus | Subgenus Aspergillus | Subgenus Aspergillus | Subgenus Aspergillus | Subgenus Aspergillus | fSubgenus Aspergillus |
| Flavi | Group A. cervinus | Section Aspergillus | Section Aspergillus | Section Aspergillus | Section Aspergillus | Section Aspergillus | Section Aspergillus |
| Fulvi | Group A. clavatus | Section Restricti | Section Candidi | Section Restricti | Section Restricti | Section Restricti | Section Restricti |
| Glauci | Group A. cremeus | Subgenus Circumdati | Section Cervini | Subgenus Candidi | Subgenus Circumdati | Subgenus Circumdati | Subgenus Circumdati |
| Nidulantes | Group A. flavipes | Section Candidi | Section Circumdati | Section Candidi | Section Candidi | Section Candidi | Section Candidi |
| Nigroides | Group A. flavus | Section Circumdati | Section Cremei | Subgenus Circumdati | Section Circumdati | Section Circumdati | gSection Circumdati |
| Phaei | Group A. fumigatus | Section Cremei | Section Flavi | Section Circumdati | Section Flavi | Section Flavi | hSection Flavi |
| Group A. glaucus | Section Flavi | Section Flavipedes | Section Cremei | Section Flavipedes | Section Flavipedes | iSection Flavipedes | |
| Group A. nidulans | Section Nigri | Section Nigri | Section Flavi | Section Nigri | Section Nigri | Section Jani | |
| Group A. niger | Section Sparsi | Section Restricti | Section Nigri | Section Terrei | Section Terrei | Section Nigri | |
| Group A. ochraceus | cSection Wentii | Section Terrei | Subgenus Fumigati | Subgenus Fumigati | Subgenus Fumigati | Section Petersonii | |
| aGroup A. ornatus | Subgenus Clavati | Subgenus Fumigati | Section Cervini | Section Cervini | Section Cervini | Section Robusti | |
| Group A. restrictus | Section Clavati | Section Clavati | Section Clavati | Section Clavati | Section Clavati | Section Tanneri | |
| Group A. sparsus | Subgenus Fumigati | Section Fumigati | Section Fumigati | Section Fumigati | Section Fumigati | Section Terrei | |
| Group A. terreus | Section Cervini | Subgenus Nidulantes | aSubgenus Ornati | Subgenus Nidulantes | Subgenus Nidulantes | jSubgenus Cremei | |
| Group A. ustus | Section Fumigati | aSection Ornati | aSection Ornati | Section Aenei | Section Aenei | Subgenus Fumigati | |
| bGroup A. versicolor | aSubgenus Ornati | Section Nidulantes | Subgenus Nidulantes | Section Ochraceorosei | Section Bispori | Section Cervini | |
| cGroup A. wentii | aSection Ornati | Section Sparsi | Section Bispori | Section Nidulantes | Section Cremei | kSection Clavati | |
| Subgenus Nidulantes | Section Ochraceorosei | Section Sparsi | Section Ochraceorosei | lSection Fumigati | |||
| Section Flavipedes | Section Nidulantes | Section Usti | Section Nidulantes | Subgenus Nidulantes | |||
| Section Nidulantes | Section Raperi | Unassigned section | Section Silvati | mSection Aenei | |||
| Section Terrei | Section Silvati | Section Cremei | Section Sparsi | Section Bispori | |||
| Section Usti | Section Sparsi | Section Usti | Section Cavernicolus | ||||
| bSection Versicolores | Section Usti | Section Ochraceorosei | |||||
| Subgenus Terrei | mSection Nidulantes | ||||||
| Section Flavipedes | Section Raperi | ||||||
| Section Terrei | Section Silvati | ||||||
| Subgenus Warcupi | Section Sparsi | ||||||
| dSection Warcupi | mSection Usti | ||||||
| eSection Zonati | Subgenus Polypaecilum |
Merged with section Nidulantes [168]
Merged with section Cremei [172]
Sexual synonym = Eurotium [4]
Sexual synonym = Neopetromyces [4]
Sexual synonym = Petromyces [4]
Sexual synonym = Fennellia [4]
Sexual synonym = Chaetosartorya [4]
Sexual synonym = Dichotomomyces and Neocarpenteles [4]
Sexual synonym = Neosartorya [4]
Sexual synonym = Emericella [4]
Table 1b.
Overview of major subgeneric classifications of Penicillium species.
| Dierckx [29] | Biourge [34] | Thom [20] | Raper et al. [22] | Pitt [35] | Stolk & Samson [36] | Houbraken & Samson [3], Houbraken et al. [173] |
|---|---|---|---|---|---|---|
| Subgenus Aspergilloides | aSubgenus Eupenicillium | Division Asymmetrica | Section Asymmetrica | Subgenus Aspergilloides | Section Aspergilloides | Subgenus Aspergilloides |
| aSubgenus Eupenicillium | bSection Biverticillium | Section Brevi-compacta | bSection Biverticillata-symmetrica | Section Aspergilloides | bSection Biverticillium | Section Aspergilloides |
| Section Bulliardium (=Section Asymmetrica) | Section Fasciculata | Section Monoverticillata | Section Exilicaulis | Section Coremigenum | Section Charlesii | |
| Subgenus Monoverticillium | Section Funiculosa | Section Polyverticillata-symmetrica | bSubgenus Biverticillium | Section Divaricatum | Section Cinnamopurpurea | |
| Section Lanata-divaricata | Section Coremigenum | Section Eladia | Section Citrina | |||
| Section Lanata-typica | Section Simplicium | Section Geosmithia | Section Exilicaulis | |||
| Section Velutina | Subgenus Furcatum | Section Inordinate | Section Fracta | |||
| bDivision Biverticillata-symmetrica | Section Divaricatum | Section Ramosum | Section Gracilenta | |||
| Section Ascogena | Section Furcatum | Section Penicillium | Section Lanata-divaricata | |||
| Section Coremigena | Subgenus Penicillium | Section Torulomyces | Section Ochrosalmonea | |||
| Section Luteo-virida | Section Coronatum | Section Ramigena | ||||
| Section Miscellanea | Section Cylindrosporum | Section Sclerotiora | ||||
| Division Monoverticillata | Section Inordinate | Section Stolkia | ||||
| Section Monoverticillata-stricta | Section Penicillium | Section Torulomyces | ||||
| Section Monoverticillata-Ramigena | Section Thysanophora | |||||
| Division Polyverticillata-symmetrica | Subgenus Penicillium | |||||
| Section Brevicompacta | ||||||
| Section Canescentia | ||||||
| Section Chrysogena | ||||||
| Section Digitata | ||||||
| Section Eladia | ||||||
| Section Fasiculata | ||||||
| Section Osmophila | ||||||
| Section Paradoxa | ||||||
| Section Penicillium | ||||||
| Section Ramosa | ||||||
| Section Robsamsonia | ||||||
| Section Roquefortorum | ||||||
| Section Turbata |
Not referring to the sexual genus Eupenicillium Ludwig
Transferred to genus Talaromyces and excluded from Penicillium
Table 1c.
Overview of major subgeneric classifications of Talaromyces species
| Stolk & Samson [32] | Yaguchi et al. [174] | Yilmaz et al. [63] |
|---|---|---|
| aSection Emersonii | aSection Emersonii | Section Bacillispori |
| Section Purpurea | Section Purpurea | Section Helici |
| Section Talaromyces | Section Talaromyces | Section Islandici |
| bSection Thermophila | bSection Thermophila | Section Purpurei |
| Section Trachyspermus | Section Talaromyces | |
| Section Subinflati | ||
| Section Trachyspermi |
As the species concept for fungi migrates from morphological, physiological, or phenotypic to genetic, phylogenetic (including genealogical concordance) and even consolidated, further changes have been made to the infrageneric classification of Aspergillus, Penicillium and Talaromyces (Table 1). The adoption of the consolidated species concept, with reduced emphasis on morphological properties, in classifying species of these genera resulted in the fact that fungi with aspergillum-shaped conidiophores no longer necessarily are Aspergillus species, while fungi with penicillum-shaped conidiophores no longer necessarily are Penicillium species [37]. One notable change in relation to these genera, also as a result of the recent implementation of the single-naming system(‘One Fungus – One Name’ [1F1N] principle) [[38], [39], [40]], was the transfer of fungi belonging to Penicillium subgenus Biverticillium to the genus Talaromyces [41], whose close chemotaxonomic relationship [42] and phylogenetic connection [[43], [44], [45]] have been recognised since the 1990s, leaving both the genera Penicillium and Talaromyces as monophyletic clades [41] (Fig. 2). Interestingly, during this transfer the species P. aureocephalum (synonym for sexual morph: Lasioderma flavovirens) [46] was also accommodated in the Talaromyces clade. Inclusion of this species, which is also the type and only species of the genus Lasioderma [47], necessitated the renaming of the Talaromyces clade as Lasioderma, since this is an older sexual name with nomenclatural priority [48]. However, such renaming would require many name changes (from Talaromyces species to Lasioderma species) and several species are better scientifically and economically well-known with their Talaromyces names. Also, even though using identical names for botanical/mycological and zoological genera is not forbidden by the Melbourne Code, the name Lasioderma [Ascomycota] is a later homonym to Lasioderma [Arthropoda] currently in use for one of the beetle genera and this might cause confusion to non-taxonomists. Hence, it was proposed to conserve the generic name Talaromyces over Lasioderma (Ascomycota) [49]. Recently, this proposal was approved by both the Nomenclature Committee for Fungi (NCF) [50] and General Committee for Nomenclature [51] of the International Association for Plant Taxonomy, retaining the generic name Talaromyces.
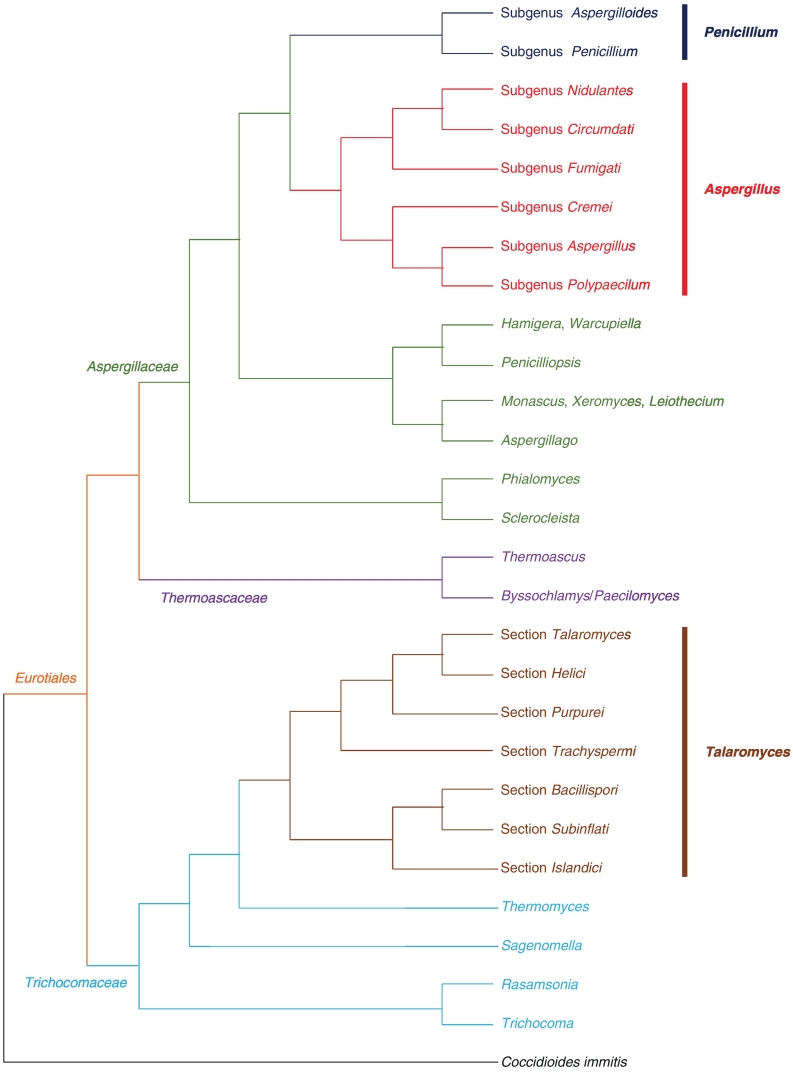
Fig. 2.
Schematic representation of the phylogenetic relationship, as inferred by Houbraken & Samson [3], Yilmaz et al. [63] and Kocsubé et al. [60], amongst members of the order Eurotiales. Aspergillus and Penicillium are sister genera of the family Aspergillaceae whereas Talaromyces is more distantly related to those two genera and belongs to a separate family, Trichocomaceae.
Despite the fact that the taxonomy of Penicillium and Talaromyces seems straight-forward now since both of them clearly represent two separate monophyletic groups [41], the scenario for Aspergillus is much more complicated, involving two opposing generic concepts, namely the wide and narrow Aspergillus concepts. Early work by Benjamin summarised the links between Aspergillus and the sexual genera Emericella, Eurotium and Neosartorya (erroneously as Sartorya by Benjamin which was later found that the original description of Sartorya was based on a contaminant in an A. fumigatus culture receiving radium radiation) [24,27,52]. Following other subsequent changes in Aspergillus classification, seven additional sexual genera, including Chaetosartorya [53], Cristaspora [2], Dichotomomyces [2,54], Fennellia [55], Neocarpenteles [56], Neopetromyces [57] and Petromyces [52], are further connected to Aspergillus. Remarkably, each of these sexual genera only associates with a particular Aspergillus subgenus or section (Table 1a). Subsequent to the adoption of 1F1N, there have been disputes as to whether the generic name Aspergillus should be retained for the large monophyletic clade, although weakly supported (~50–70% bootstrap only) by maximum likelihood analyses [3,4], of classical Aspergillus species (broad/wide Aspergillus concept) [2]; or to adopt sexual names for those well-supported clades containing both pleomorphic species and asexual species with Aspergillus morphologies (narrow Aspergillus concept; i.e. subgenus Aspergillus = Eurotium, subgenus Cremei = Chaetosartorya, subgenus Fumigati = Neosartorya and subgenus Nidulantes = Emericella), leaving the weakly supported (<50% bootstrap) [3] subgenus Circumdati as Aspergillus sensu stricto, even though this group does include several less well-known sexual genera (Fennellia, Neopetromyces and Petromyces) [58]. The latter proposal was advocated based on the fact that the sexual genera Chaetosartorya, Emericella, Eurotium and Neosartorya differ significantly in their morphologies, physiologies, enzymologies, as well as toxicologies [59]. Also, Pitt, Taylor and Göker, proposers of the narrow Aspergillus concept, found in their phylogenetic analyses that classical Aspergillus was paraphyletic, encompassing the monophyletic Penicillium clade. As a result, according to Pitt et al. if the wide Aspergillus concept is to be adopted then Pencillium would also need to be synonymised under Aspergillus to make the whole clade monophyletic [58,59]. On the other hand, the main problem for the narrow Aspergillus concept rests in the retypification by conservation of the genus. This is because under the narrow Aspergillus concept, the type of the genus Aspergillus, A. glaucus of subgenus Aspergillus, would fall in the genus Eurotium instead. Since taxonomic properties of the type and related species determine the circumscription of the genus, if the name Aspergillus is to be applied to subgenus Circumdati, the type of the genus has to be changed to one of the species within this subgenus, for example, A. niger as suggested by Pitt and Taylor because of its more frequent use in literatures and databases [58]. However, in the eyes of the wide Aspergillus concept advocates, such generic retypification is debatable since the new type of choice would depend on the interest of different fields. For instance, A. flavus would be the type of choice for food mycology and mycotoxicology, A. fumigatus for medical mycology, whereas A. nidulans for fungal molecular genetics [2]. Recently, regarding the narrow Aspergillus proposal which considers Aspergillus to be non-monophyletic and recommends to apply the name Aspergillus only to members of the subgenus Circumdati through retypification by conservation while maintaining the sexual names for other supported clades [58,59], Kocsubé et al., supporters of the wide Aspergillus concept, demonstrated in their phylogenetic analyses, based on six and nine genetic markers using both maximum likelihood and Bayesian approaches as well as extrolite profiling, that Aspergillus represents a well-supported monophyletic clade sister to the monophyletic Penicillium clade (Fig. 2) [60], rejecting Pitt et al.’s hypotheses and proposal. They also established the subgenus Polypaecilum to encompass species previously assigned to the genera Phialosimplex and Polypaecilum (Fig. 2), whereas the species A. clavatoflavus and A. zonatus, which are actually phylogenetically distantly related to Aspergillus, were transferred to the novel genera Aspergillago as Aspergillago clavatoflava and Penicilliopsis as Penicilliopsis zonata, respectively [60]. Nevertheless, Pitt and Taylor have submitted a formal proposal to the NCF to retypify Aspergillus with A. niger to redefine the genus to members of subgenus Circumdati only, with sexual names taken up to replace other subgeneric names of Aspergillus [61]. In response to Pitt and Taylor, Samson et al. urged the NCF to reject the conservation proposal based on their arguments that Aspergillus is monophyletic as well as clearly-defined by phenotypic synapomorphies and secondary metabolite chemistry; that the size of the genus Aspergillus is irrelevant; and that conservation with a different generic type (A. niger) would lead to unpredictable name changes and would not result in a more stable nomenclature [62]. Recently, voting was held by the NCF and the proposal by Pitt and Taylor could not obtain a 60% majority for the ‘yes’ vote after two rounds of ballots. Although the ‘no’ vote was also one vote short of reaching 60%, it was in the majority. Since there is no definite recommendation from the NCF, this proposal will be referred to the General Committee on Nomenclature for final decision (Dr Tom May, personal communication).
3. Species recognition/identification and current advances
Since the establishment of Aspergillus, Penicillium and Talaromyces, species in these genera had been recognised by their morphological features until the dawn of molecular systematics. In particular, morphology of conidial structures, especially their branching patterns as discussed above, has played an important role in species recognition and identification. Other important morphological properties useful for diagnosing a species include cleistothecium and ascus/ascospore (when present) characters [1,2]. Macroscopically, characteristics of the colony, such as texture, growth rate, degree of sporulation, conidial and mycelial colours, as well as production of diffusing pigments, exudates, acids and other secondary metabolites, are also used for species differentiation [1,2,63]. The need for standardisation of culture media and incubation condition for reproducible species identification was recognised as early as Biourge's and Dierckx's time [64]. This is because variations in the immediate cultural environment, such as nutrient availability, temperature, light intensity (including ultraviolet light), water activity, humidity and/or other environmental factors, regardless how subtle these discrepancies are, could change the appearance of the organism since morphology is one of the way in which an organism adapts to and survives in its environment [1]. The effects of these changes in incubation condition have been exemplified by the work by Okura et al. [65,66]. As such, standardised working techniques for morphological characterisation have been recommended for Aspergillus and Penicillium species [1,2]. Although no standard is proposed for Talaromyces, these methods should also be applicable to this genus since by tradition quite a number of Talaromyces species were considered and characterised as Penicillium species.
With the availability of newer techniques, such as gas–liquid chromatography and electrophoresis, for the characterisation of biomolecules in the 20th century, chemotaxonomy has gained popularity in Aspergillus, Penicillium and Talaromyces taxonomy, especially since the 1980s. One of the approaches for chemotaxonomy is zymogram profiling, where species are differentiated based on the polyacrylamide gel-electrophoretic patterns of certain isoenzymes [67]. This technique has been demonstrated to be highly successful in differentiating species of Penicillium subgenus Penicillium, where the isozyme patterns showed a high correlation with morphological species [68,69]. However, when species from other Penicillium subgenera were also included in the analysis it was found that correlation between zymogram grouping and morphological species only existed in some cases [70], rendering the utility of this technique for the identification of Penicillium species questionable. On the other hand, zymogram profiling has also been applied to Aspergillus species and this identification method was found to be practical especially for members of the subgenera Circumdati, Fumigati and Nidulantes [[71], [72], [73]], in spite of the fact that some closely related species, such as the wild type A. flavus and the domesticated counterpart A. oryzae or the wild type A. parasiticus and the domesticated A. sojae, produced very similar isoenzyme patterns and could not be well differentiated [71]. Nonetheless, fingerprinting of isozymes has not been widely employed as a practical identification system since the enzyme profiles for the vast majority of Aspergillus, Penicillium and Talaromyces species remained uncharacterised. Also, there is no consensus as to which isoenzymes should be used for comparison.
Another approach for chemotaxonomy is extrolite profiling. The exometabolome reflects the physiology of an organism in response to its biotic and abiotic environment [74] and profiling of the exometabolome is particularly useful for the chemotaxonomy of Aspergillus, Penicillium and Talaromyces species since these genera are the best known exometabolite producers, having the most diverse spectra of exometabolites amongst 26 different groups of ascomycetes analysed, which represented four different Classes (Dothideomycetes, Eurotiomycetes, Leotiomycetes and Sordariomycetes) [37]. Amongst the various kinds of exometabolites, such as excessive organic acids, extracellular enzymes and accumulated carbohydrates, the one that generally displays more pronounced chemoconsistency and higher species specificity is secondary metabolites [74]. The first insight of the taxonomic value of secondary metabolite profiling was gained when Ciegler et al. attempted to divide P. viridicatum into three subgroups, in which the production of the mycotoxins citrinin, ochratoxin, viomellein and xanthomegnin was characterised as one of the classification criteria [75,76]. However, Ciegler et al.’s method required complicated and tedious pre-treatment of the samples. As a result, their approach was only popularised after the development of simpler techniques which only involve direct spotting of small agar plugs from fungal cultures on thin-layer chromatography plates without the need of any preceding extraction or purification procedures [77,78]. Since then, extrolite data have contributed much to species recognition of Aspergillus, Penicillium and Talaromyces species. For example, using secondary metabolite profiling Frisvad and Filtenborg classified more than 4,000 isolates of terverticillate penicillia into 38 taxa and chemotypes, where infrataxon strains exhibited chemoconsistency in terms of the production of mycotoxins [79]. They also reidentified a large number of misidentified Penicillium strains based on their profiles of secondary metabolites [[79], [80], [81]]. Frisvad and Filtenborg, together with Samson and Stolk, also pioneered the chemotaxonomy of Talaromyces. Again, their analysis demonstrated that the production of secondary metabolites by members of this genus was taxon-specific and they also recognised T. macrosporus and T. luteus as separate species from T. flavus and T. udagawae, respectively, because of their different metabolic profiles [42]. In fact, this chemotaxonomic work offered one of the very first indications of the connection between Talaromyces and Penicillium subgenus Biverticillium [42]. An overview of the extrolite profiles for various Talaromyces species was given in the latest monograph on the genus by Yilmaz et al. [63]. The same also applies to Aspergillus species [[82], [83], [84], [85], [86]]. Notably, different Aspergillus subgenera produce different unique extrolites, as summarised by Frisvad and Larsen [87]. Thus, the production of a certain secondary metabolite by an Aspergillus isolate would serve as a practical hint for identification at the sectional level, whereas the identification of several secondary metabolites of the organism would be an effective tool for species recognition [2]. Currently, (ultra) high-performance liquid chromatography (UHPLC/HPLC) coupled with diode array detection (DAD) and mass spectrometry (MS) is the method of choice for detailed chemotaxonomic characterisation of Aspergillus, Penicillium and Talaromyces [1,2,63,88]. With about 350 accepted species each in Aspergillus [2,60] and Penicillium [1] and more than 100 accepted species in Talaromyces [63,89], qualitative databases equipped with a large volume of verified data on the production of secondary metabolites by various Aspergillus, Penicillium and Talaromyces species is needed for accurate species identification [2]. In view of this, an Aspergillus Secondary Metabolites Database (A2MDB) was established last year [90]. Recently, metabolic fingerprinting has also been demonstrated as a potentially successful tool for differentiating closely related Aspergillus species, without the need of investigating the actual identities of the metabolites. For example, utilising this technique Tam et al. showed that A. nomius and A. tamarii could be distinguished from their morphologically similar sibling A. flavus [91]. In addition, hierarchical cluster analysis by Tsang et al. also showed that except for A. austroafricanus, the metabolic fingerprints of species in the same Aspergillus section clustered together and those of infraspecific strains also formed smaller subclades [92].
Fatty acid profiling is another increasingly used method in diagnosing filamentous fungal species. Although characterisation of fatty acid composition and relative concentration has long been utilised for bacterial and yeast chemotaxonomy [93,94] and there is even a commercial fatty acid methyl ester (FAME)-based bacterial/yeast identification system containing profiles from more than 1,500 different species developed [95], there are only a few studies making use of this technique to characterise the chemotaxonomy of filamentous fungi [96]. This is because filamentous fungi do not produce fatty acids in the quantity and diversity that bacteria do [97] and therefore, traditionally fatty acid profiling had been regarded to have little taxonomic value for filamentous fungi [98]. Blomquist et al. [99] first examined the utility of this technique on the identification of filamentous fungi. They characterised the fatty acid contents of conidia and found that fatty acid profiling, even though performed at different times, could potentially be used to identify Aspergillus and Penicillium species in a reproducible way [99]. In 1996, Stahl and Klug performed a large-scale study to characterise the composition and relative concentration of fatty acids in the mycelia of a number of filamentous fungi from across different phyla [98]. Seven species of Penicillium and one of Aspergillus were included in their study. It was revealed that four fatty acids, namely palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1Δ9[cis]) and linoleic acid (C18:2Δ9,12[cis]), represented more than 95% of the total cellular fatty acid content. These four fatty acids were also common to all the filamentous fungi characterised. In spite of this, discriminant analysis showed that the fatty acid profiles for these species are significantly different. Notably, all the seven Penicillium species characterised were found to possess unique fatty acid profiles [98]. Later in 1998, Da Silva et al. expanded the characterisation to 18 Penicillium species [100]; and they found that different Penicillium subgenera could be readily differentiated by fatty acid profiling. Moreover, in some cases, species of the same subgenus such as Furcatum could be separated based on their fatty acid profiles, which mainly differed in the relative concentration rather than the composition of fatty acids; although difficulties existed for the subgenus Penicillium [100]. The fact that the species differentiation power relied on the variation in fatty acid relative concentration was observed by Mahmoud et al. as well [101]. Fatty acid profiling has also been successfully used to differentiate Aspergillus species [102,103].
A recent chemotaxonomic approach for rapid identification of Aspergillus, Penicillium and Talaromyces is matrix-assisted laser desorption/ionisation–time-of-flight (MALDI–TOF) MS. The technology compares the cellular protein profiles of different organisms to achieve identification at the species level [104]. The advantage of this technique is that the methodology is simple, rapid and inexpensive, requiring a specialised bench-top MALDI–TOF mass spectrometer only. Also, since the majority of proteins analysed by MALDI–TOF MS are constitutively expressed ribosomal proteins, microorganisms can be successfully identified even though varying culture media and incubation conditions are used [104,105]. More importantly, databases consisting of protein mass spectra from over 2,400 microbial species are commercially available [106,107], making the identification of a wide range of microorganisms possible. Given its numerous advantages, MALDI–TOF MS has been gaining popularity for identification of pathogenic microorganisms, including bacteria [108,109], yeasts [[108], [109], [110], [111], [112], [113], [114], [115]] and even filamentous fungi [109,[116], [117], [118]], in clinical microbiology laboratories. The potential of this technology in diagnosing Aspergillus, Penicillium and Talaromyces species has also been evaluated by numerous studies. In general, MALDI–TOF MS is successful in identifying the more commonly found aspergilli/penicillia, such as A. flavus, A. fumigatus, A. nidulans, A. niger, A. sydowii, A. unguis, P. chrysogenum, P. aurantiogriseum and P. purpurogenum, with correct identification rates of ≥78% [[117], [118], [119], [120], [121]]. Yet, for other rare species misidentification is often encountered. Notably, these uncommon species could usually be identified to the sectional level. For example, A. tritici (section Candidi) was misidentified as A. candidus; A. oryzae (section Flavi) as A. flavus; A. fischeri (section Fumigati) as A. fumigatus; A. tubingensis and A. welwitschiae (section Nigri) as A. niger, A. hortai and A. niveus (section Terrei) as A. terreus; as well as A. sydowii (formerly section Versicolores) as A. versicolor [92,122]. A probable reason for this is that the mass spectra for many of these rare species are lacking in the commercial libraries. It should be noted that the Bruker MBT MSP 6903 Library, Bruker MBT Filamentous Fungi Library and Vitek MS V3.0 Knowledge Base only include reference mass spectra for 42, 127 and 82 filamentous fungal species, respectively [106,117,123]. Of these, only up to 22 Aspergillus, 21 Penicillium and 6 Talaromyces, which are still named with their previous Penicillium synonyms, species are included [107,123]. However, the numbers of accepted Aspergillus, Penicillium and Talaromyces species greatly outnumber those included in the MALDI–TOF MS databases, with both Aspergillus and Penicillium having approximately 350 species [1,2,60] and Talaromyces having more than 100 species [63,89]. Despite this, MALDI–TOF MS has still been demonstrated as a potential tool to differentiate members of the three genera by hierarchical cluster analysis of the mass spectra of various species [91,124,125]. As a result, theoretically if more reference mass spectra for different species, especially the rare ones, are generated for inclusion in the databases the species diagnosis power of MALDI–TOF MS would be greatly enhanced and it has already been exemplified by previous studies that the correct identification rates could be improved by the expansion of reference libraries using inhouse generated mass spectra [118,122,125]. To overcome the limitation of small reference data volume of the commercial databases, several organisations have self-established online supplementary databases. For example, the Spectra database (freely available at https://spectra.folkhalsomyndigheten.se/spectra/) by the Public Health Agency of Sweden (Folkhälsomyndigheten) is a platform for MALDI–TOF MS users to deposit and exchange user-generated mass spectra which are curated and continuously updated. Another such complementary database is the MSI Platforme which serves as a webtool for MALDI–TOF MS-based fungal identification. This platform contains more than 11,800 reference mass spectra of more than 900 fungal species, aiming at supplementing the insufficient spectral diversity of the commercial databases so as to improve species identification [126].
With the current adoption of consolidated species recognition where molecular characters play a predominant role, DNA sequencing and phylogenetic analysis have become the gold standard for accurate fungal identification. As in other fungi, early molecular work on Aspergillus, Penicillium and Talaromyces involved the comparison of large and small subunit ribosomal nucleic acid (mitochondrial and/or nuclear) as well as internal transcribed spacer (ITS) sequences [[43], [44], [45],127]. However, subsequent analysis showed that ribosomal genes are too conserved to separate these groups of fungi [128,129]. In addition, although ITS is now accepted as the official DNA barcode for fungi [130], it has also been recognised as an extremely conserved region for Aspergillus, Penicillium and Talaromyces [1,2,63]. Despite the fact that its sequence variability could be used to distinguish species belonging to different sections or series [128], very often it is not useful for the differentiation of species within the same section or series. In view of this and also to better reflect the genealogy of this group of organisms, sequencing of multiple genetic markers, in particular the β-tubulin (benA) and calmodulin (cmdA or CaM) genes, to define species boundaries has been advocated [131]. The exons of these genes are highly conserved and are therefore good locations for primer binding, whereas introns in between the exons act as the major source of sequence variation. As a result, sequences of these genes containing both exons and introns are able to provide variations at different levels for species delimitation [131]. With the majority of Aspergillus, Penicillium and Talaromyces species clearly defined nowadays, sequencing of benA and/or cmdA can be utilised to identify most of these species. In fact, benA and cmdA have been proposed as the secondary identification markers for Penicillium and Aspergillus species, respectively [1,2]. This is because there are universal primers available for these two genes and both of them are easy to amplify. In the case of Aspergillus, although benA could be easily amplified, the presence of paralogous genes (e.g. tubC) in some species which could also be amplified by the universal primers could be confusing and complicate species identification [132,133]. In contrast, although a similar problem has also been noted for cmdA, amplification of a pseudogene only occurred for one Aspergillus strain [134]. Moreover, cmdA is also easy to amplify and its sequence is available for nearly all accepted species. Therefore, cmdA was chosen over benA as secondary identification marker for Aspergillus [2]. On the other hand, as for Penicillium, amplification of benA paralogues has not been reported and since a complete cmdA sequence database is lacking, benA became the secondary identification marker of choice [1]. Although a third option, RNA polymerase II second-largest subunit gene (rpb2), also exists and its lack of introns allows robust and easy alignment for phylogenetic analysis, it was not selected over benA or cmdA because rpb2 is sometimes difficult to amplify and a database with sufficient volume is lacking [1,2]. Nonetheless, when resources are available it is recommended to sequence all the four genetic markers (ITS, benA, cmdA and rpb2) to aid identification, especially when new species are diagnosed [1,2]. Although a recommendation of identification markers has not been put forward for Talaromyces species, they generally follow those for Aspergillus and Penicillium species [63]. In order to achieve accurate identification, sequences from reliable databases should be compared against. Despite the fact that the International Nucleotide Sequence Database Collaboration (INSDC) [135] contains a vast number of sequences, the reliability of the sequence annotation is questionable [136,137]. Notably, ≥10% of the fungal ITS sequences in these databases were found to be misannotated [136]. As such, the Fungal ITS RefSeq Targeted Loci Project has been initiated by the National Center for Biotechnology Information (NCBI) to improve the quality and accuracy of the sequences deposited to INSDC [138,139]. Similarly, the UNITE database was developed to include high-quality type or representative sequences for fungi or fungal species hypothesis with correct or up-to-date taxonomic annotations [140]. The International Society for Human and Animal Mycology (ISHAM) ITS database, specialised in the ITS-based identification of medical fungi, has also been recently established [141] and it contains quite a number of high-quality ITS sequences for Aspergillus, Penicillium and Talaromyces species, which are commonly encountered in the clinical settings. While curated databases for benA, cmdA and rpb2 have not been created, reliable sequences for all the ex-type strains of Aspergillus, Penicillium and Talaromyces accepted species have been listed in the recent monographs on the three genera [1,2,63] or online at http://www.aspergilluspenicillium.org/. In addition to nuclear genes, attempts have also been made to understand the evolution (and thus species recognition) of Aspergillus, Penicillium and Talaromyces by sequencing of mitogenomes [[142], [143], [144], [145]]. Yet, only a handful of mitogenomes are available for these groups of fungi currently and the utility of mitogenomes for species diagnosis awaits further examination.
4. Clinical perspectives
A stable taxonomy is important to the study of Aspergillus, Penicillium and Talaromyces in every aspect including medical mycology. First of all, the nomenclature of pathogenic fungi should be steady over time, without frequent vigorous name changes. The recently implemented 1F1N scheme, where one fungus shall only possess one name, drastically simplified fungal nomenclature. The accepted use of Aspergillus and Penicillium names over their respective ‘sexual names’ is particularly important to the medical community. This is because most clinical fungi are isolated in the asexual forms and these fungi are traditionally named with their asexual names. Use of the ‘sexual names’ would confuse clinicians since they would not be aware of what Eupenicillium, Neosartorya and Emericella are, thus hindering treatment and patient care. This could be exemplified by the recent transfer of P. marneffei to T. marneffei, where the well-known disease name ‘penicilliosis’ also has to be changed to the unfamiliar ‘talaromycosis’. A stable taxonomy also clearly defines species and their identification methods. Therefore, the clinical spectrum of pathogenic species could also be better studied. In particular, rare and new aetiological agents could be revealed (Table 2) [92,[146], [147], [148], [149], [150], [151]]. Accurate identification of the causative pathogen is crucial to epidemiological studies. Correct species diagnosis could also help predict antifungal susceptibility, which varies across different species and this could significantly affect patient treatment, disease management and prognosis. For example, it has been shown that A. tubingensis and A. unguis possessed elevated minimum inhibitory concentrations (MICs) to itraconazole [92]. The fact that triazole agents exhibit various activities against different Aspergillus species has also been demonstrated by other studies [148,149,151]. Also, although triazoles showed moderate activities against Penicillium species, their effectiveness against some Talaromyces species are poor [147].
Table 2.
Novel Aspergillus, Penicillium and Talaromyces species/taxonomic entities described during January, 2013 to December, 2017 sampled from human or non-human vertebrate specimens
| Species | Synonym(s) | Associated human infections or clinical specimensa | Associated non-human vertebrates | Molecular markersb | Year of valid publication | Reference(s) |
|---|---|---|---|---|---|---|
| Aspergillus | ||||||
| A. aurantiopurpureus | Novel species | Kangaroo rat cheek pouch | ITS, benA, cmdA and rpb2 | 2016 | [86] | |
| A. caninus | ≡ Phialosimplex caninus, | Bone marrow aspirate of a dog | rpb2 | 2014 | [2,176,177] | |
| A. capsici | ≡ Scopulariopsis capsica | Fur and skin of hibernating bat | 2014 | [2,178] | ||
| = Leuconeurospora capsica | ||||||
| A. chlamydosporus | ≡ Sagenomella chlamydospore | Disseminated infection in a dog | rpb2 | 2014 | [2,176,179,180] | |
| = Phialosimplex chlamydosporus | ||||||
| A. citrinoterreus | Novel species | Nails, various respiratory specimen, wound and biopsy | benA and cmdA | 2015 | [181] | |
| A. contaminans | Novel species | Fingernail (probably as a contaminant) | ITS, benA, cmdA and rpb2 | 2017 | [182] | |
| A. europaeus | Novel species | Toenail | ITS, benA and cmdA | 2016 | [183] | |
| A. felis | Novel species | Chronic invasive pulmonary aspergillosis and onychomycosis; BAL, oropharyngeal exudate and sputum | Invasive fungal rhinosinusitis in domestic cats and disseminated invasive aspergillosis in a dog | ITS, benA and cmdA | 2013 | [[184], [185], [186], [187]] |
| A. hongkongensis | Novel species | Onychomycosis | ITS, benA, cmdA, rpb2, mcm7 and tsr1 | 2016 | [92] | |
| A. insolitus | ≡ Polypaecilum insolitum | Onychomycosis; ear | cct8, rpb2 and tsr1 | 2014 | [2,188] | |
| A. keratitidis | ≡ Sagenomella keratitidis | Keratitis | ITS and 28S nrDNA | 2017 | [189,190] | |
| A. latilabiatus | Novel species | Sheep dung | ITS, benA, cmdA and rpb2 | 2016 | [86] | |
| A. latus | ≡ Aspergillus nidulans var. latus | Invasive pulmonary aspergillosis | ITS, benA, cmdA and rpb2 | 2016 | [53,86,[191], [192], [193], [194], [195]] | |
| = Aspergillus montenegroi | ||||||
| = Aspergillus sublatus | ||||||
| = Emericella montenegroi | ||||||
| = Emericella nidulans var. lata | ||||||
| = Emericella sublata | ||||||
| A. magnivesiculatus | Novel species | Child carriers | ITS, benA, cmdA and rpb2 | 2017 | [171] | |
| A. mallochii | Novel species | Pack rat dung | benA, cmdA and rpb2 | 2017 | [196] | |
| A. microperforatus | Novel species | Lymph node and toenail | ITS, benA, cmdA and rpb2 | 2017 | [151] | |
| A. pallidofulvus | ≡ Aspergillus sulphureus var. minimus | Invasive pulmonary aspergillosis and disseminated aspergillosis | ITS, benA, cmdA | 2014 | [122,197] | |
| A. parafelis | Novel species | Invasive aspergillosis; oropharyngeal exudate and sputum | Cats | benA, cmdA, rpb2, mcm7 and tsr1 | 2014 | [198,199] |
| A. pragensis | Aspergillus section Candidi | Onychomycosis | ITS, benA and cmdA | 2014 | [200] | |
| A. pseudofelis | Novel species | Invasive aspergillosis; sputum and nail | benA, cmdA, rpb2, mcm7 and tsr1 | 2014 | [198] | |
| A. pseudogracilis | Novel species | Child carrier | ITS, benA, cmdA and rpb2 | 2017 | [171] | |
| A. pseudosclerotiorum | Novel species | BAL, lung and sputum | ITS, benA, cmdA and rpb2 | 2017 | [149] | |
| A. pseudoviridinutans | Novel species | Invasive aspergillosis; mediastinal lymph node | benA, cmdA, rpb2, mcm7 and tsr1 | 2014 | [198] | |
| A. reticulatus | Novel species | Lung biopsy, child carrier | ITS, benA, cmdA and rpb2 | 2017 | [171] | |
| A. sclerotialis | ≡ Sagenomella sclerotialis | Dog | rpb2 | 2014 | [2,176,201] | |
| = Phialosimplex sclerotialis | ||||||
| A. spinulosporus | ≡ Aspergillus nidulans var. echinulatus | Recurrent prosthetic valve endocarditis and invasive pulmonary aspergillosis | ITS, benA, cmdA and rpb2 | 2016 | [2,[202], [203], [204], [205], [206], [207], [208]] | |
| = Aspergillus delacroixii (Samson, Visagie & Houbraken) | ||||||
| = Emericella echinulate | ||||||
| = Emericella nidulans var. echinulata | ||||||
| A. stercorarius | Novel species | Lizard (Uromastix acanthinurus) dung | ITS, benA, cmdA and rpb2 | 2016 | [86] | |
| A. wyomingensis | Novel species | Cat | benA and cmdA | 2014 | [134,209] | |
| Penicillium | ||||||
| P. canis | Novel species | Bone lesion of spayed Rhodesian ridgeback dog with osteomyelitis | ITS, benA and cmdA | 2014 | [210] | |
| P. fimorum | Novel species | Mouse dung | ITS, benA, cmdA and rpb2 | 2016 | [173] | |
| P. paradoxum | ≡ Aspergillus paradoxus | Dung of dog and opossum | ITS, benA, cmdA and rpb2 | 2014 | [1,202,211,212] | |
| = Aspergillus ingratus | ||||||
| = Hemicarpenteles paradoxus | ||||||
| P. robsamsonii | Novel species | Mouse dung | ITS, benA, cmdA and rpb2 | 2016 | [173] | |
| Talaromyces | ||||||
| T. alveolaris | Novel species | BAL | ITS, benA, cmdA and rpb2 | 2017 | [150] | |
| T. atroroseus | Novel species | Mouse dung | ITS, benA and rpb1 | 2013 | [213] | |
| T. columbinus | Novel species | Fungaemia and pulmonary nodule and adjacent rib osteomyelitis | ITS, benA, cmdA, rpb1, rpb2, mcm7 and tsr1 | 2013 | [[214], [215], [216]] | |
| T. georgiensis | Novel species | Animal joint fluid | ITS, benA and rpb2 | 2017 | [150] | |
| T. kabodanensis | Novel species | BAL | ITS, benA, cmdA and rpb2 | 2016 | [150,217] | |
| T. minnesotensis | Novel species | Ear | ITS, benA, cmdA and rpb2 | 2017 | [150] | |
| T. rapidus | Novel species | BAL | ITS, benA, cmdA and rpb2 | 2017 | [150] | |
| T. siglerae | Novel species | Tinea capitis | ITS, benA, cmdA and rpb2 | 2017 | [218] | |
BAL, bronchoalveolar lavage
benA, β-tubulin gene; cct8, chaperonin-containing T-complex protein 1 subunit theta gene; cmdA, calmodulin gene; ITS, internal transcribed spacer; mcm7, mini-chromosome maintenance complex component 7 gene; nrDNA, nuclear ribosomal rRNA gene; rpb1, RNA polymerase II largest subunit gene; rpb2, RNA polymerase II second largest subunit gene; tsr1, ribosome maturation factor for 20S rRNA accumulation gene
5. Summary and outlook
With a consistent taxonomy, understanding on the epidemiology and clinical spectrum of diseases caused by Aspergillus, Penicillium and Talaromyces could be enhanced. This in turn facilitates laboratory diagnosis of these important mycotic pathogens and establishment of patient treatment strategies. The transition from morphological/phenotypic to chemotaxonomic, genetic/phylogenetic, or consolidated species recognition results in the reclassification of these groups of fungi and enables sexual-asexual connection. In the current omics era, advancement in different omics technologies makes characterisation of the complete set of a particular group of characters possible, allowing more thorough analyses and therefore, a more stable taxonomy. For example, comparison of mitogenomes supported the transfer of ‘P. marneffei’ to Talaromyces and demonstrated that Aspergillus and Penicillium are more closely related to each other than to Talaromyces [142,144,145]. The availability of contemporary advanced techniques, such as MALDI–TOF MS as well as UHPLC/HPLC–DAD–MS, significantly improves proteomic and metabolic fingerprinting of fungi, respectively, thus aiding chemotaxonomy. As the cost for second-generation sequencing is getting lower and the emerging third-generation sequencing is becoming more widely accessible, more and more complete/almost complete fungal genomes become available. These genome sequences could advance our knowledge on these fungi, such as T. marneffei [144,[152], [153], [154], [155], [156], [157], [158]], and taxonomy on them could thus be facilitated. With such additional novel data, further reclassification on Aspergillus, Penicillium and Talaromyces is expected. Application of all these state-of-the-art omics technologies is likely to provide comprehensive information on the evolution of the three related genera, and a more stable taxonomy for them will hopefully be achieved. Yet, it should be noted that even though these advanced methodologies are becoming more readily available for the identification and classification of fungi, it is equally important for mycologists to apply standard or best practices when studying fungal taxonomic relationships. In particular, fungal taxonomists should always keep themselves up-to-date with recent trends, tools, standards, recommendations and practices in the field, especially when describing new species [[159], [160], [161], [162], [163]]. When depositing DNA sequence data to public databases, the sequences should be well checked for authenticity as well as reliability [164], and they should be richly annotated as far as possible [165]. Also, multiple genetic markers and proper analytical tools should be used for the inference of phylogenetic relationships [166]. As nowadays taxonomy has entered a deep crisis where descriptive taxonomic studies are not encouraged, it is important for taxonomists to keep the pace for re-growth, to participate actively and to form a good ‘taxonomic culture’ so that the scientific community would value taxonomic work higher [167]. This could also help attract more research funding for more expensive technology or equipment for more detailed taxonomic characterisation. All these efforts could help speed up taxonomic and molecular ecology progress on Aspergillus, Penicillium and Talaromyces significantly.
Acknowledgments
Acknowledgement
This work is supported by the Health and Medical Research Fund (No. HKM-15-M07 [commissioned project]), Food and Health Bureau, Government of the Hong Kong Special Administrative Region, Hong Kong. We are grateful to the Agricultural Research Service (ARS) Culture Collection (NRRL), Department of Agriculture, USA for providing the reference strains A. glaucus NRRL 116T, A. nidulans NRRL 187T, P. kewense NRRL 3332T, P. expansum NRRL 976T and T. flavus NRRL 2098T for free. We also thank Mr Jordan Y. H. Fong, Department of Microbiology, The University of Hong Kong, for his assistance in taking macroscopic photos for these reference fungal strains.
Conflict of interest
Patrick C.Y. Woo has provided scientific advisory/laboratory services for Gilead Sciences, Incorporated; International Health Management Associates, Incorporated; Merck & Corporation, Incorporated and Pfizer, Incorporated. The other authors report no conflicts of interest. The funding sources had no role in study design, data collection, analysis, interpretation or writing of the report. The authors alone are responsible for the content and the writing of the manuscript.
Contributor Information
Susanna K.P. Lau, Email: skplau@hku.hk.
Patrick C.Y. Woo, Email: pcywoo@hku.hk.
References
- 1.Visagie C.M., Houbraken J., Frisvad J.C., Hong S.B., Klaassen C.H.W., Perrone G. Identification and nomenclature of the genus Penicillium. Stud Mycol. 2014;78(Supplement C):343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samson R.A., Visagie C.M., Houbraken J., Hong S.B., Hubka V., Klaassen C.H.W. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173. doi: 10.1016/j.simyco.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houbraken J., Samson R. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol. 2011;70(:1):1–51. doi: 10.3114/sim.2011.70.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houbraken J., de Vries R.P., Samson R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. In: Sariaslani S., Gadd G.M., editors. Academic Press; San Diego: 2014. pp. 199–249. (Adv Appl Microbiol.). [DOI] [PubMed] [Google Scholar]
- 5.Chávez R., Bull P., Eyzaguirre J. The xylanolytic enzyme system from the genus Penicillium. J Biotechnol. 2006;123(4):413–433. doi: 10.1016/j.jbiotec.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Ichishima E. Development of enzyme technology for Aspergillus oryzae, A. sojae, and A. luchuensis, the national microorganisms of Japan. Biosci Biotechnol Biochem. 2016;80(9):1681–1692. doi: 10.1080/09168451.2016.1177445. [DOI] [PubMed] [Google Scholar]
- 7.Frisvad J.C., Smedsgaard J., Larsen TO, Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol. 2004;49:201–241. [Google Scholar]
- 8.Pitt J.I., Hocking A.D. Springer; Boston: 2009. Fungi and Food Spoilage. [Google Scholar]
- 9.Kwon-Chung K.J., Sugui J.A. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013;9(12) doi: 10.1371/journal.ppat.1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latgé J.-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanittanakom N., Cooper C.R., Fisher M.C., Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan J.F.W., Lau S.K.P., Yuen K.-Y., Woo P.C.Y. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5 doi: 10.1038/emi.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quaedvlieg W., Binder M., Groenewald J.Z., Summerell B.A., Carnegie A.J., Burgess T.I. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia. 2014;33:1–40. doi: 10.3767/003158514X681981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micheli P.A. Typis Bernardi Paperinii; Florence: 1729. Nova Plantarum Genera. [Google Scholar]
- 15.von Haller A. Sumptibus Societatis; Bernae: 1768. Historia Stirpium Indigenarum Helvetiae Inchoata. Tomus Secundus. [Google Scholar]
- 16.Fries E.M. Ex Officina Berlingiana; Lundae: 1832. Systema Mycologicum: Sistens Fungorum Ordines, Genera Et Species, Huc Usque Cognitas, Quas Ad Normam Methodi Naturalis Determinavit. [Google Scholar]
- 17.Link H.F. Observationes in ordines plantarum naturales. Ges Naturf Freunde Berlin Mag Neuesten Entdeck Gesammten Naturk. 1809;3(1):3–42. [Google Scholar]
- 18.de Bary A. Ueber die entwickelung und den zusammenhang von Aspergillus glaucus und Eurotium. Bot Zeitung (Berlin) 1854;12(25–27):425–434. 441–451, 465–471. [Google Scholar]
- 19.Ludwig F. von Ferdinand Enke; Stuttgart: 1892. Lehrbuch der Niederen Kryptogamen. [Google Scholar]
- 20.Thom C. Baillière, Tindall and Cox; London: 1930. The Penicillia. [Google Scholar]
- 21.Thom C., Raper K.B. Williams & Wilkins; Baltimore: 1945. A Manual of the Aspergilli. [Google Scholar]
- 22.Raper K.B., Thom C., Fennel D.I. Williams & Wilkins; Baltimore: 1949. A Manual of the Penicillia. [Google Scholar]
- 23.Thom C. The evolution of species concepts in Aspergillus and Penicillium. Ann N Y Acad Sci. 1954;60(1):24–34. doi: 10.1111/j.1749-6632.1954.tb39995.x. [DOI] [PubMed] [Google Scholar]
- 24.Raper K.B., Fennell D.I. Williams & Wilkins; Baltimore: 1965. The Genus Aspergillus. [Google Scholar]
- 25.Raper K.B. Nomenclature in Aspergillus and Penicillium. Mycologia. 1957;49(5):644–662. [Google Scholar]
- 26.Lanjouw J., Baehni C., Merrill E.D., Rickett H.W., Robyns W., Sprague T.A. Chronica Botanica; Waltham: July 1950. International Code of Botanical Nomenclature, Adopted by the Seventh International Botanical Congress, Stockholm; p. 1952. [Google Scholar]
- 27.Benjamin C.R. Ascocarps of Aspergillus and Penicillium. Mycologia. 1955;47(5):669–687. [Google Scholar]
- 28.Stolk A.C., de Scott B. Studies on the genus Eupenicillium Ludwig I. Taxonomy and nomenclature of Penicillia in relation to their sclerotioid ascocarpic states. Persoonia. 1967;4(4):391–405. [Google Scholar]
- 29.Dierckx R.P. Un Essai de revision du genre Penicillium Link. Ann Soc Sci Bruxelles. 1901;25:83–89. [Google Scholar]
- 30.Thom C., Church M. Williams & Wilkins; Baltimore: 1926. The Aspergilli. [Google Scholar]
- 31.Blochwitz A. Die Aspergillaceen. System und Phylogenie. Ann Mycol. 1929;27:185–204. [Google Scholar]
- 32.Stolk A.C., Samson R.A. The genus Talaromyces. Studies on Talaromyces and related genera II. Stud Mycol. 1972;2:1–65. [Google Scholar]
- 33.Gams W., Christensen M., Onions A.H., Pitt J.I., Samson R.A. Infrageneric taxa of Aspergillus. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus Systematics. Springer; Boston: 1986. pp. 55–62. [Google Scholar]
- 34.Biourge P. Les moisissures du groupe Penicillium Link. Cellule. 1923;33:7–331. [Google Scholar]
- 35.Pitt J.I. Academic Press; London: 1979. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. [Google Scholar]
- 36.Stolk A.C., Samson R.A. A new taxonomic scheme for Penicillium anamorphs. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus Systematics. Springer; Boston: 1986. pp. 163–192. [Google Scholar]
- 37.Frisvad J.C. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front Microbiol. 2015;5:773. doi: 10.3389/fmicb.2014.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawksworth D.L., Crous P.W., Redhead S.A., Reynolds D.R., Samson R.A., Seifert K.A. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2(1):105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norvell L.L. Fungal nomenclature. 1. Melbourne approves a new code. Mycotaxon. 2011;116(1):481–490. [Google Scholar]
- 40.Flann C., Turland N., Turland N.J., Monro M. A. Report on botanical nomenclature—Melbourne 2011. XVIII International Botanical Congress, Melbourne: nomenclature section, 18–22 July 2011. PhytoKeys. 2014;41:1–289. doi: 10.3897/phytokeys.41.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samson R.A., Yilmaz N., Houbraken J., Spierenburg H., Seifert K.A., Peterson S.W. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70(1):159–183. doi: 10.3114/sim.2011.70.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frisvad J.C., Filtenborg O., Samson R.A., Stolk A.C. Chemotaxonomy of the genus Talaromyces. Antonie Van Leeuwenhoek. 1990;57(3):179–189. doi: 10.1007/BF00403953. [DOI] [PubMed] [Google Scholar]
- 43.LoBuglio K.F., Taylor J.W. The Fungal Holomorph: Mitotic, Meiotic And Pleomorphic Speciation In Fungal Systematics. CAB International; Wallingford: 1993. Molecular phylogeny of Talaromyces and Penicillium species in subgenus Biverticillium; pp. 115–128. [Google Scholar]
- 44.LoBuglio K.F., Pitt J.I., Taylor J.W. Phylogenetic analysis of two ribosomal DNA regions indicates multiple independent losses of a sexual Talaromyces state among asexual Penicillium species in subgenus Biverticillium. Mycologia. 1993;85(4):592–604. [Google Scholar]
- 45.Berbee M.L., Yoshimura A., Sugiyama J., Taylor J.W. Is Penicillium monophyletic? An evaluation of phylogeny in the family Trichocomaceae from 18S, 5.8S and ITS ribosomal DNA sequence data. Mycologia. 1995;87(2):210–222. [Google Scholar]
- 46.Visagie C.M., Llimona X., Vila J., Louis-Seize G., Seifert K.A. Phylogenetic relationships and the newly discovered sexual state of Talaromyces flavovirens, comb. nov. Mycotaxon. 2013;122(1):399–411. [Google Scholar]
- 47.Montagne J. Cinquième centurie. De plantes cellulaires exotiques nouvelles. Ann Sci Nat Bot. 1845;4:346–367. [Google Scholar]
- 48.McNeill J., Barrie F.R., Buck W.R., Demoulin V., Greuter W., Hawksworth D.L. Koeltz Scientific Books; Königstein: 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. [Google Scholar]
- 49.Seifert K.A., Frisvad J.C., Houbraken J., Llimona X., Peterson S.W., Samson R.A. Proposal to conserve the name Talaromyces over Lasioderma (Ascomycota) Taxon. 2012;61(2):461–462. [Google Scholar]
- 50.May T.W. Report of the nomenclature committee for fungi: 20. Taxon. 2017;66(2):483–495. doi: 10.5598/imafungus.2017.08.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson K.L. Report of the general committee: 18. Taxon. 2017;66(3):742–744. [Google Scholar]
- 52.Malloch D., Cain R.F. The Trichocomataceae: ascomycetes with Aspergillus, Paecilomyces, and Penicillium imperfect states. Can J Bot. 1972;50(12):2613–2628. [Google Scholar]
- 53.Subramanian C.V. The perfect states of Aspergillus. Curr Sci. 1972;41(21):755–761. [Google Scholar]
- 54.Varga J., Due M., Frisvad J.C., Samson R.A. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud Mycol. 2007;59:89–106. doi: 10.3114/sim.2007.59.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiley B.J., Simmons E.G. New species and a new genus of plectomycetes with Aspergillus states. Mycologia. 1973;65(4):934–938. [Google Scholar]
- 56.Udagawa S.-i., Uchiyama S. Neocarpenteles: a new ascomycete genus to accommodate Hemicarpenteles acanthosporus. Mycoscience. 2002;43(1):3–6. [Google Scholar]
- 57.Frisvad J.C., Samson R.A. Neopetromyces gen. nov and an overview of teleomorphs of Aspergillus subgenus Circumdati. Stud Mycol. 2000;45:201–207. [Google Scholar]
- 58.Pitt J.I., Taylor J.W. Aspergillus, its sexual states and the new International Code of Nomenclature. Mycologia. 2014;106(5):1051–1062. doi: 10.3852/14-060. [DOI] [PubMed] [Google Scholar]
- 59.Taylor J.W., Göker M., Pitt J.I. Choosing one name for pleomorphic fungi: The example of Aspergillus versus Eurotium, Neosartorya and Emericella. Taxon. 2016;65(3):593–601. [Google Scholar]
- 60.Kocsubé S., Perrone G., Magistà D., Houbraken J., Varga J., Szigeti G. Aspergillus is monophyletic: evidence from multiple gene phylogenies and extrolites profiles. Stud Mycol. 2016;85(Supplement C):199–213. doi: 10.1016/j.simyco.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitt J.I., Taylor J.W. Proposal to conserve the name Aspergillus (Fungi: Eurotiales: Trichocomaceae) with a conserved type to maintain also the name Eurotium. Taxon. 2016;65(3):631–632. [Google Scholar]
- 62.Samson R.A., Hubka V., Varga J., Houbraken J., Hong S.-B., Klaassen C.H.W. Response to Pitt & Taylor 2016: conservation of Aspergillus with A. niger as the conserved type is unnecessary and potentially disruptive. Taxon. 2017;66(6):1439–1446. [Google Scholar]
- 63.Yilmaz N., Visagie C.M., Houbraken J., Frisvad J.C., Samson R.A. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341. doi: 10.1016/j.simyco.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hennebert G.L. Dierckx’ contribution to the genus Penicillium. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Springer; Boston: 1986. pp. 9–21. [Google Scholar]
- 65.Okuda T. Variation in colony characteristics of Penicillium strains resulting from minor variations in culture conditions. Mycologia. 1994;86(2):259–262. [Google Scholar]
- 66.Okuda T., Klich M.A., Seifert K.A., Ando K. Media and incubation effects on morphological characteristics of Penicillium and Aspergillus. In: Samson R.A., Pitt J.I., editors. Integration of modern taxonomic methods for Penicillium and Aspergillus Classification. Harwood Academic Publishers; Amsterdam: 2000. pp. 83–100. [Google Scholar]
- 67.Pitt J.I. Chemotaxonomy of Penicillium and related teleomorphs. Jpn J Med Mycol. 1991;32(Supplement 2):31–38. [Google Scholar]
- 68.Cruickshank R.H., Pitt J.I. The zymogram technique: isoenzyme patterns as an aid in Penicillium classification. Microbiol Sci. 1987;4(1):14–17. [PubMed] [Google Scholar]
- 69.Cruickshank R.H., Pitt J.I. Identification of species in Penicillium subgenus Penicillium by enzyme electrophoresis. Mycologia. 1987;79(4):614–620. [Google Scholar]
- 70.Paterson R.R.M., Bridge P.D., Crosswaite M.J., Hawksworth D.L. A reappraisal of the terverticillate penicillia using biochemical, physiological and morphological features III. An evaluation of pectinase and amylase isoenzymes for species characterization. Microbiology. 1989;135(11):2979–2991. doi: 10.1099/00221287-135-11-2979. [DOI] [PubMed] [Google Scholar]
- 71.Cruickshank R.H., Pitt J.I. Isoenzyme patterns in Aspergillus flavus and closely related species. In: Samson R.A., Pitt J.I., editors. Modern concepts in Penicillium and Aspergillus classification. Springer; Boston: 1990. pp. 259–265. [Google Scholar]
- 72.Varga J., Yu E., Kiss I., Botos B., Kozakiewicz Z. Carbon source utilization and isoenzyme analysis as taxonomic aids for toxigenic Neosartorya species and their relatives. Acta Microbiol Immunol Hung. 1997;44(1):1–11. [PubMed] [Google Scholar]
- 73.Balali G.R., Minaiefar A.A., Sharifnabi B. Pectic zymogram variation and morphological identification of Aspergillus species. Rostaniha. 2010;11(2):163–174. [Google Scholar]
- 74.Frisvad J.C., Larsen TO, de Vries R., Meijer M., Houbraken J., Cabañes F.J. Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins. Stud Mycol. 2007;59:31–37. doi: 10.3114/sim.2007.59.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciegler A., Fennell D.I., Sansing G.A., Detroy R.W., Bennett G.A. Mycotoxin-producing strains of Penicillium viridicatum: classification into subgroups. Appl Microbiol. 1973;26(3):271–278. doi: 10.1128/am.26.3.271-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ciegler A., Lee L.S., Dunn J.J. Production of naphthoquinone mycotoxins and taxonomy of Penicillium viridicatum. Appl Environ Microbiol. 1981;42(3):446–449. doi: 10.1128/aem.42.3.446-449.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Filtenborg O., Frisvad J.C. A simple screening method for toxigenic moulds in pure cultures. Lebenson Wiss Technol. 1980;13(3):128–130. [Google Scholar]
- 78.Filtenborg O., Frisvad J.C., Svendsen J.A. Simple screening method for molds producing intracellular mycotoxins in pure cultures. Appl Environ Microbiol. 1983;45(2):581–585. doi: 10.1128/aem.45.2.581-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frisvad J.C., Filtenborg O. Terverticillate penicillia: chemotaxonomy and mycotoxin production. Mycologia. 1989;81(6):837–861. [Google Scholar]
- 80.Frisvad J.C. Profiles of primary and secondary metabolites of value in classification of Penicillium viridicatum and related species. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Springer; Boston: 1986. pp. 311–325. [Google Scholar]
- 81.Frisvad J.C. The connection between the Penicillia and Aspergilli and mycotoxins with special emphasis on misidentified isolates. Arch Environ Contam Toxicol. 1989;18(3):452–467. doi: 10.1007/BF01062373. [DOI] [PubMed] [Google Scholar]
- 82.Frisvad J.C. Secondary metabolites as an aid to Emericella classification. In: Samson R.A., Pitt J.I., editors. Advances in Penicillium and Aspergillus systematics. Springer; Boston: 1986. pp. 437–444. [Google Scholar]
- 83.Frisvad J.C., Samson R.A. Chemotaxonomy and morphology of Aspergillus fumigatus and related taxa. In: Samson R.A., Pitt J.I., editors. Modern concepts in Penicillium and Aspergillus classification. Springer; Boston: 1990. pp. 201–208. [Google Scholar]
- 84.Samson R.A., Noonim P., Meijer M., Houbraken J., Frisvad J., Varga J. Diagnostic tools to identify black aspergilli. Stud Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samson R.A., Peterson S.W., Frisvad J.C., Varga J. New species in Aspergillus section Terrei. Stud Mycol. 2011;69:39–55. doi: 10.3114/sim.2011.69.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen A.J., Frisvad J.C., Sun B.D., Varga J., Kocsubé S., Dijksterhuis J. Aspergillus section Nidulantes (formerly Emericella): polyphasic taxonomy, chemistry and biology. Stud Mycol. 2016;84(Supplement C):1–118. doi: 10.1016/j.simyco.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frisvad J.C., Larsen TO Chemodiversity in the genus Aspergillus. Appl Microbiol Biotechnol. 2015;99(19):7859–7877. doi: 10.1007/s00253-015-6839-z. [DOI] [PubMed] [Google Scholar]
- 88.Frisvad J.C., Andersen B., Thrane U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res. 2008;112(2):231–240. doi: 10.1016/j.mycres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Chen A.J., Sun B.D., Houbraken J., Frisvad J.C., Yilmaz N., Zhou Y.G. New Talaromyces species from indoor environments in China. Stud Mycol. 2016;84:119–144. doi: 10.1016/j.simyco.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vadlapudi V., Borah N., Yellusani K.R., Gade S., Reddy P., Rajamanikyam M. Aspergillus Secondary Metabolite Database, a resource to understand the secondary metabolome of Aspergillus genus. Sci Rep. 2017;7(1):7325. doi: 10.1038/s41598-017-07436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tam E.W.T., Chen J.H.K., Lau E.C.L., Ngan A.H.Y., Fung K.S.C., Lee K.-C. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: characterization by internal transcribed spacer, β-tubulin, and calmodulin gene sequencing, metabolic fingerprinting, and matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2014;52(4):1153–1160. doi: 10.1128/JCM.03258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsang C.-C., Hui T.W.S., Lee K.-C., Chen J.H.K., Ngan A.H.Y., Tam E.W.T. Genetic diversity of Aspergillus species isolated from onychomycosis and Aspergillus hongkongensis sp. nov., with implications to antifungal susceptibility testing. Diagn Microbiol Infect Dis. 2016;84(2):125–134. doi: 10.1016/j.diagmicrobio.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 93.Vasyurenko Z.P., Frolov A.F. Fatty acid composition of bacteria as a chemotaxonomic criterion. J Hyg Epidemiol Microbiol Immunol. 1986;30(3):287–293. [PubMed] [Google Scholar]
- 94.Welch D.F. Applications of cellular fatty acid analysis. Clin Microbiol Rev. 1991;4(4):422–438. doi: 10.1128/cmr.4.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kunitsky C., Osterhout G., Sasser M. Identification of microorganisms using fatty acid methyl ester (FAME) analysis and the MIDI Sherlock® microbial identification system. In: Miller M.J., editor. Encyclopedia of Rapid Microbiological Methods. Vol. 3. Davis Healthcare International Publishing; River Grove: 2006. pp. 1–17. [Google Scholar]
- 96.Zain M.E., Bahkali A.H., Al-Othman M.R. Developments in using fatty acids in fungal chemotaxonomy. Afr J Microbiol Res. 2013;7(38):4638–4645. [Google Scholar]
- 97.Lechevalier H., Lechevalier M.P. Chemotaxonomic use of lipids—an overview. In: Ratledge C., Wilkinson S.G., editors. Microbial Lipids. Vol. 1. Academic Press; London: 1988. pp. 869–902. [Google Scholar]
- 98.Stahl P.D., Klug M.J. Characterization and differentiation of filamentous fungi based on fatty acid composition. Appl Environ Microbiol. 1996;62(11):4136–4146. doi: 10.1128/aem.62.11.4136-4146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blomquist G., Andersson B., Andersson K., Brondz I. Analysis of fatty acids. A new method for characterization of moulds. J Microbiol Methods. 1992;16(1):59–68. [Google Scholar]
- 100.da Silva T.L., de Sousa E., Pereira P.T., Ferrão A.M., Roseiro J.C. Cellular fatty acid profiles for the differentiation of Penicillium species. FEMS Microbiol Lett. 1998;164(2):303–310. [Google Scholar]
- 101.Mahmoud M.A., Abd-El-Aziz A.R.M., Al-Othman M.R. Molecular and biochemical taxonomic tools for the identification and classification of plant-pathogenic Penicillium species. Biotechnol Biotechnol Equip. 2016;30(6):1090–1096. [Google Scholar]
- 102.Nemec T., Jernejc K., Cimerman A. Sterols and fatty acids of different Aspergillus species. FEMS Microbiol Lett. 1997;149(2):201–205. [Google Scholar]
- 103.Fraga M.E., Santana D.M., Gatti M.J., Direito G.M., Cavaglieri L.R., Rosa C.A. Characterization of Aspergillus species based on fatty acid profiles. Mem Inst Oswaldo Cruz. 2008;103(6):540–544. doi: 10.1590/s0074-02762008000600005. [DOI] [PubMed] [Google Scholar]
- 104.Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valentine N., Wunschel S., Wunschel D., Petersen C., Wahl K. Effect of culture conditions on microorganism identification by matrix-assisted laser desorption ionization mass spectrometry. Appl Environ Microbiol. 2005;71(1):58–64. doi: 10.1128/AEM.71.1.58-64.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruker Corporation . Bruker Corporation; Bremen: 2016. MBT 6903 MSP Library (#1829023). Release Notes. [Google Scholar]
- 107.bioMérieux SA . bioMérieux; Marcy-l’Etoile: 2016. Vitek MS™ V3.0 Knowledge Base. Clinical Use. 161150-556-B. [Google Scholar]
- 108.Deak E., Charlton C.L., Bobenchik A.M., Miller S.A., Pollett S., McHardy I.H. Comparison of the Vitek MS and Bruker Microflex LT MALDI-TOF MS platforms for routine identification of commonly isolated bacteria and yeast in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2015;81(1):27–33. doi: 10.1016/j.diagmicrobio.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Lévesque S., Dufresne P.J., Soualhine H., Domingo M.-C., Bekal S., Lefebvre B. A side by side comparison of Bruker Biotyper and VITEK MS: utility of MALDI-TOF MS technology for microorganism identification in a public health reference laboratory. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mancini N., De Carolis E., Infurnari L., Vella A., Clementi N., Vaccaro L. Comparative evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry systems for identification of yeasts of medical importance. J Clin Microbiol. 2013;51(7):2453–2457. doi: 10.1128/JCM.00841-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pence M.A., McElvania TeKippe E., Wallace M.A., Burnham C.-A.D. Comparison and optimization of two MALDI-TOF MS platforms for the identification of medically relevant yeast species. Eur J Clin Microbiol Infect Dis. 2014;33(10):1703–1712. doi: 10.1007/s10096-014-2115-x. [DOI] [PubMed] [Google Scholar]
- 112.Jamal W.Y., Ahmad S., Khan Z.U., Rotimi V.O. Comparative evaluation of two matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems for the identification of clinically significant yeasts. Int J Infect Dis. 2014;26:167–170. doi: 10.1016/j.ijid.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y., Chan J.F.W., Tsang C.-C., Wang H., Guo D., Pan Y. Clinical characteristics, laboratory identification, and in vitro antifungal susceptibility of Yarrowia (Candida) lipolytica isolates causing fungemia: a multicenter, prospective surveillance study. J Clin Microbiol. 2015;53(11):3639–3645. doi: 10.1128/JCM.01985-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang H., Xiao M., Chen S.C.-A., Kong F., Sun Z.-Y., Liao K. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2012;50(12):3952–3959. doi: 10.1128/JCM.01130-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cassagne C., Normand A.-C., Bonzon L., L'Ollivier C., Gautier M., Jeddi F. Routine identification and mixed species detection in 6,192 clinical yeast isolates. Med Mycol. 2016;54(3):256–265. doi: 10.1093/mmy/myv095. [DOI] [PubMed] [Google Scholar]
- 116.Karabıçak N., Karatuna O., İlkit M., Akyar I. Evaluation of the Bruker matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) system for the identification of clinically important dermatophyte species. Mycopathologia. 2015;180(3):165–171. doi: 10.1007/s11046-015-9898-x. [DOI] [PubMed] [Google Scholar]
- 117.McMullen A.R., Wallace M.A., Pincus D.H., Wilkey K., Burnham C-AD. Evaluation of the Vitek MS matrix-assisted laser desorption ionization–time of flight mass spectrometry system for identification of clinically relevant filamentous fungi. J Clin Microbiol. 2016;54(8):2068–2073. doi: 10.1128/JCM.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sleiman S., Halliday C.L., Chapman B., Brown M., Nitschke J., Lau A.F. Performance of matrix-assisted laser desorption ionization−time of flight mass spectrometry for identification of Aspergillus, Scedosporium, and Fusarium spp. in the Australian clinical setting. J Clin Microbiol. 2016;54(8):2182–2186. doi: 10.1128/JCM.00906-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bille E., Dauphin B., Leto J., Bougnoux M.E., Beretti J.L., Lotz A. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin Microbiol Infect. 2012;18(11):1117–1125. doi: 10.1111/j.1469-0691.2011.03688.x. [DOI] [PubMed] [Google Scholar]
- 120.Ranque S., Normand A.-C., Cassagne C., Murat J.-B., Bourgeois N., Dalle F. MALDI-TOF mass spectrometry identification of filamentous fungi in the clinical laboratory. Mycoses. 2014;57(3):135–140. doi: 10.1111/myc.12115. [DOI] [PubMed] [Google Scholar]
- 121.Park J.H., Shin J.H., Choi M.J., Choi J.U., Park Y.-J., Jang S.J. Evaluation of matrix-assisted laser desorption/ionization time-of-fight mass spectrometry for identification of 345 clinical isolates of Aspergillus species from 11 Korean hospitals: comparison with molecular identification. Diagn Microbiol Infect Dis. 2017;87(1):28–31. doi: 10.1016/j.diagmicrobio.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 122.Masih A., Singh P.K., Kathuria S., Agarwal K., Meis J.F., Chowdhary A. Identification by molecular methods and matrix-assisted laser desorption ionization–time of flight mass spectrometry and antifungal susceptibility profiles of clinically significant rare Aspergillus species in a referral chest hospital in Delhi, India. J Clin Microbiol. 2016;54(9):2354–2364. doi: 10.1128/JCM.00962-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bruker Corporation . Bruker Corporation; Bremen: 2015. MALDI Biotyper Filamentous Fungi Library (1838971) [Google Scholar]
- 124.Hettick J.M., Green B.J., Buskirk A.D., Kashon M.L., Slaven J.E., Janotka E. Discrimination of Penicillium isolates by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Rapid Commun Mass Spectrom. 2008;22(16):2555–2560. doi: 10.1002/rcm.3649. [DOI] [PubMed] [Google Scholar]
- 125.Lau S.K.P., Lam C.S.K., Ngan A.H.Y., Chow W.-N., Wu A.K.L., Tsang D.N.C. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei. BMC Microbiol. 2016;16(1):1–9. doi: 10.1186/s12866-016-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Normand A.C., Becker P., Gabriel F., Cassagne C., Accoceberry I., Gari-Toussaint M. Validation of a new web application for identification of fungi by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2017;55(9):2661–2670. doi: 10.1128/JCM.00263-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Logrieco A., Peterson S.W., Wicklow D.T. Ribosomal RNA comparisons among taxa of the terverticillare Penicillia. In: Samson R.A., Pitt J.I., editors. Modern Concepts in Penicillium and Aspergillus Classification. Plenum Press; New York: 1990. pp. 343–355. [Google Scholar]
- 128.Skouboe P., Taylor J.W., Frisvad J.C., Lauritsen D., Larsen L., Albœk C. Molecular methods for differentiation of closely related Penicillium species. In: Samson R.A., Pitt J.I., editors. Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification. Harwood Academic Publishers; Amsterdam: 2000. [Google Scholar]
- 129.Demİrel R. Comparison of rDNA regions (ITS, LSU, and SSU) of some Aspergillus, Penicillium, and Talaromyces spp. Turk J Bot. 2016;40:576–583. [Google Scholar]
- 130.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A. 2012;109(16):6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Geiser D.M., Harbinski F.M., Taylor J.W. Molecular and analytical tools for characterizing Aspergillus and Penicillium species at the intra- and interspecific levels. In: Samson R.A.P., editor. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Acadmic Publishers; Amsterdam: 2000. pp. 381–394. [Google Scholar]
- 132.Peterson S.W. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia. 2008;100(2):205–226. doi: 10.3852/mycologia.100.2.205. [DOI] [PubMed] [Google Scholar]
- 133.Hubka V., Kolarik M. β-tubulin paralogue tubC is frequently misidentified as the benA gene in Aspergillus section Nigri taxonomy: primer specificity testing and taxonomic consequences. Persoonia. 2012;29:1–10. doi: 10.3767/003158512X658123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nováková A., Hubka V., Dudová Z., Matsuzawa T., Kubátová A., Yaguchi T. New species in Aspergillus section Fumigati from reclamation sites in Wyoming (U.S.A.) and revision of A. viridinutans complex. Fungal Divers. 2014;64(1):253–274. [Google Scholar]
- 135.Cochrane G., Karsch-Mizrachi I., Takagi T., International Nucleotide Sequence Database Collaboration The International Nucleotide Sequence Database Collaboration. Nucleic Acids Res. 2016;44(D1):D48–D50. doi: 10.1093/nar/gkv1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nilsson R.H., Ryberg M., Kristiansson E., Abarenkov K., Larsson K.-H., Kõljalg U. Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS One. 2006;1(1) doi: 10.1371/journal.pone.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bidartondo M.I., Bruns T.D., Blackwell M., Edwards I., Taylor A.F.S., Horton T. Preserving accuracy in GenBank. Science. 2008;319(5870) doi: 10.1126/science.319.5870.1616a. [DOI] [PubMed] [Google Scholar]
- 138.Schoch C.L., Robbertse B., Robert V., Vu D., Cardinali G., Irinyi L. Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. Database. 2014 doi: 10.1093/database/bau061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.National Center for Biotechnology Information Fungal Internal Transcribed Spacer RNA (ITS) RefSeq Targeted Loci Project. http://www.ncbi.nlm.nih.gov/bioproject/PRJNA177353/ Undated; Available from:
- 140.Kõljalg U., Nilsson R.H., Abarenkov K., Tedersoo L., Taylor A.F.S., Bahram M. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2013;22(21):5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- 141.Irinyi L., Serena C., Garcia-Hermoso D., Arabatzis M., Desnos-Ollivier M., Vu D. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database—the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Med Mycol. 2015;53(4):313–337. doi: 10.1093/mmy/myv008. [DOI] [PubMed] [Google Scholar]
- 142.Woo P.C.Y., Zhen H., Cai J.J., Yu J., Lau S.K.P., Wang J. The mitochondrial genome of the thermal dimorphic fungus Penicillium marneffei is more closely related to those of molds than yeasts. FEBS Lett. 2003;555(3):469–477. doi: 10.1016/s0014-5793(03)01307-3. [DOI] [PubMed] [Google Scholar]
- 143.Joardar V., Abrams N.F., Hostetler J., Paukstelis P.J., Pakala S., Pakala S.B. Sequencing of mitochondrial genomes of nine Aspergillus and Penicillium species identifies mobile introns and accessory genes as main sources of genome size variability. BMC Genomics. 2012;13(1):698. doi: 10.1186/1471-2164-13-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tam E.W.T., Tsang C.-C., Lau S.K.P., Woo P.C.Y. Comparative mitogenomic and phylogenetic characterization on the complete mitogenomes of Talaromyces (Penicillium) marneffei. Mitochondrial DNA B Resour. 2016;1(1):941–942. doi: 10.1080/23802359.2016.1261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lau S.K.P., Tsang C.-C., Woo P.C.Y. Talaromyces marneffei genomic, transcriptomic, proteomic and metabolomic studies reveal mechanisms for environmental adaptations and virulence. Toxins. 2017;9(6):192. doi: 10.3390/toxins9060192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hubka V., Kubatova A., Mallatova N., Sedlacek P., Melichar J., Skorepova M. Rare and new etiological agents revealed among 178 clinical Aspergillus strains obtained from Czech patients and characterized by molecular sequencing. Med Mycol. 2012;50(6):601–610. doi: 10.3109/13693786.2012.667578. [DOI] [PubMed] [Google Scholar]
- 147.Guevara-Suarez M., Sutton D.A., Cano-Lira J.F., García D., Martin-Vicente A., Wiederhold N. Identification and antifungal susceptibility of Penicillium-like fungi from clinical samples in the United States. J Clin Microbiol. 2016;54(8):2155–2161. doi: 10.1128/JCM.00960-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siqueira J.P.Z., Sutton D.A., García D., Gené J., Thomson P., Wiederhold N. Species diversity of Aspergillus section Versicolores in clinical samples and antifungal susceptibility. Fungal Biol. 2016;120(11):1458–1467. doi: 10.1016/j.funbio.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 149.Siqueira J.P.Z., Sutton D.A., Gené J., García D., Wiederhold N., Peterson S.W. Multilocus phylogeny and antifungal susceptibility of Aspergillus section Circumdati from clinical samples and description of A. pseudosclerotiorum sp. nov. J Clin Microbiol. 2017;55(3):947–958. doi: 10.1128/JCM.02012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guevara-Suarez M., Sutton D.A., Gené J., García D., Wiederhold N., Guarro J. Four new species of Talaromyces from clinical sources. Mycoses. 2017;60(10):651–662. doi: 10.1111/myc.12640. [DOI] [PubMed] [Google Scholar]
- 151.Siqueira J.P.Z., Sutton D.A., Gené J., García D., Wiederhold N., Guarro J. Species of Aspergillus section Aspergillus from clinical samples in the United States. Med Mycol. 2018;56(5):541–550. doi: 10.1093/mmy/myx085. [DOI] [PubMed] [Google Scholar]
- 152.Woo P.C.Y., Lau S.K.P., Liu B., Cai J.J., Chong K.T.K., Tse H. Draft genome sequence of Penicillium marneffei strain PM1. Eukaryot Cell. 2011;10(12):1740–1741. doi: 10.1128/EC.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Woo P.C.Y., Tam E.W.T., Chong K.T.K., Cai J.J., Tung E.T.K., Ngan A.H.Y. High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei. FEBS J. 2010;277(18):3750–3758. doi: 10.1111/j.1742-4658.2010.07776.x. [DOI] [PubMed] [Google Scholar]
- 154.Woo P.C.Y., Lam C.-W., Tam E.W.T., Leung C.K.F., Wong S.S.Y., Lau S.K.P. First discovery of two polyketide synthase genes for mitorubrinic acid and mitorubrinol yellow pigment biosynthesis and implications in virulence of Penicillium marneffei. PLoS Negl Trop Dis. 2012;6(10) doi: 10.1371/journal.pntd.0001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lau S.K.P., Chow W.-N., Wong A.Y.P., Yeung J.M.Y., Bao J., Zhang N. Identification of microRNA-like RNAs in mycelial and yeast phases of the thermal dimorphic fungus Penicillium marneffei. PLoS Negl Trop Dis. 2013;7(8) doi: 10.1371/journal.pntd.0002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang E., Wang G., Woo P.C.Y., Lau S.K.P., Chow W.-N., Chong K.T.K. Unraveling the molecular basis of temperature-dependent genetic regulation in Penicillium marneffei. Eukaryot Cell. 2013;12(9):1214–1224. doi: 10.1128/EC.00159-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang E., Chow W.-N., Wang G., Woo P.C.Y., Lau S.K.P., Yuen K.-Y. Signature gene expression reveals novel clues to the molecular mechanisms of dimorphic transition in Penicillium marneffei. PLoS Genet. 2014;10(10) doi: 10.1371/journal.pgen.1004662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Woo P.C.Y., Lau S.K.P., Lau C.C.Y., Tung E.T.K., Chong K.T.K., Yang F. Mp1p is a virulence factor in Talaromyces (Penicillium) marneffei. PLoS Negl Trop Dis. 2016;10(8) doi: 10.1371/journal.pntd.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seifert K.A., Rossman A.Y. How to describe a new fungal species. IMA Fungus. 2010;1(2):109–116. doi: 10.5598/imafungus.2010.01.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ariyawansa H.A., Hawksworth D.L., Hyde K.D., Jones E.B.G., Maharachchikumbura S.S.N., Manamgoda D.S. Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers. 2014;69(1):57–91. [Google Scholar]
- 161.Crous P.W., Hawksworth D.L., Wingfield M.J. Identifying and naming plant-pathogenic fungi: past, present, and future. Annu Rev Phytopathol. 2015;53(1):247–267. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- 162.Tedersoo L., Ramirez K.S., Nilsson R.H., Kaljuvee A., Kõljalg U., Abarenkov K. Standardizing metadata and taxonomic identification in metabarcoding studies. GigaScience. 2015;4(1):34. doi: 10.1186/s13742-015-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schoch C.L., Aime M.C., de Beer W., Crous P.W., Hyde K.D., Penev L. Using standard keywords in publications to facilitate updates of new fungal taxonomic names. IMA Fungus. 2018;8(2):70–73. [Google Scholar]
- 164.Nilsson R.H., Tedersoo L., Abarenkov K., Ryberg M., Kristiansson E., Hartmann M. Five simple guidelines for establishing basic authenticity and reliability of newly generated fungal ITS sequences. MycoKeys. 2012;4:37–63. [Google Scholar]
- 165.Tedersoo L., Abarenkov K., Nilsson R.H., Schüssler A., Grelet G.-A., Kohout P. Tidying up international nucleotide sequence databases: ecological, geographical and sequence quality annotation of ITS sequences of mycorrhizal fungi. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hyde K.D., Udayanga D., Manamgoda D.S., Tedersoo L., Larsson E., Abarenkov K. Incorporating molecular data in fungal systematics: a guide for aspiring researchers. Curr Res Environm Appl Mycol. 2013;3(1):1–32. [Google Scholar]
- 167.Kotov A.A., Gololobova M.A. Traditional taxonomy: Quo vadis? Integr Zool. 2016;11(6):500–505. doi: 10.1111/1749-4877.12215. [DOI] [PubMed] [Google Scholar]
- 168.Peterson S.W. Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Samson R.A., Pitt J.I., editors. Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification. Harwood Academic Publishers; Amsterdam: 2000. pp. 323–356. [Google Scholar]
- 169.Peterson S.W., Varga J., Frisvad J.C., Samson R.A. Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J., Samson R.A., editors. Aspergillus in the genomic era. Wageningen Academic Publishers; Wageningen: 2008. pp. 33–56. [Google Scholar]
- 170.Jurjević Ž., Kubátová A., Kolařík M., Hubka V. Taxonomy of Aspergillus section Petersonii sect. nov. encompassing indoor and soil-borne species with predominant tropical distribution. Plant Syst Evol. 2015;301(10):2441–2462. [Google Scholar]
- 171.Sklenář F., Jurjević Ž., Zalar P., Frisvad J.C., Visagie C.M., Kolařík M. Phylogeny of xerophilic aspergilli (subgenus Aspergillus) and taxonomic revision of section Restricti. Stud Mycol. 2017:161–236. doi: 10.1016/j.simyco.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Peterson S.W. Phylogenetic analysis of Aspergillus sections Cremei and Wentii, based on ribosomal DNA sequences. Mycol Res. 1995;99(11):1349–1355. [Google Scholar]
- 173.Houbraken J., Wang L., Lee H.B., Frisvad J.C. New sections in Penicillium containing novel species producing patulin, pyripyropens or other bioactive compounds. Persoonia. 2016;36(1):299–314. doi: 10.3767/003158516X692040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yaguchi T., Someya A., S-i Udagawa. A reappraisal of intrageneric classification of Talaromyces based on the ubiquinone systems. Mycoscience. 1996;37(1):55–60. [Google Scholar]
- 175.Houbraken J., Spierenburg H., Frisvad J.C. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek. 2012;101(2):403–421. doi: 10.1007/s10482-011-9647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sigler L., Sutton D.A., Gibas C.F.C., Summerbell R.C., Noel R.K., Iwen P.C. Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichocomaceae. Med Mycol. 2010;48(2):335–345. doi: 10.3109/13693780903225805. [DOI] [PubMed] [Google Scholar]
- 177.Armstrong P.F., Sigler L., Sutton D.A., Grooters A.M., Hitt M. Fungal myelitis caused by Phialosimplex caninus in an immunosuppressed dog. Med Mycol. 2012;50(5):509–512. doi: 10.3109/13693786.2011.633106. [DOI] [PubMed] [Google Scholar]
- 178.Malloch D., Sigler L., Hambleton S., Vanderwolf K.J., Gibas C.F.C., McAlpine D.F. Fungi associated with hibernating bats in New Brunswick caves: the genus Leuconeurospora. Botany. 2016;94(12):1171–1181. [Google Scholar]
- 179.GM E., C J., T P., G I., MdM E., R E. Disseminated mycoses in a dog by Paecilomyces sp. J Vet Med A. 2000;47(4):243–249. doi: 10.1046/j.1439-0442.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 180.Gené J., Blanco J.L., Cano J., García M.E., Guarro J. New filamentous fungus Sagenomella chlamydospora responsible for a disseminated infection in a dog. J Clin Microbiol. 2003;41(4):1722–1725. doi: 10.1128/JCM.41.4.1722-1725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guinea J., Sandoval-Denis M., Escribano P., Peláez T., Guarro J., Bouza E. Aspergillus citrinoterreus, a new species of section terrei isolated from samples of patients with nonhematological predisposing conditions. J Clin Microbiol. 2015;53(2):611–617. doi: 10.1128/JCM.03088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Crous P.W., Wingfield M.J., Burgess T.I., Carnegie A.J., Hardy G.E., Smith D. Fungal planet description sheets: 625–715. Persoonia. 2017;39(1):270–467. doi: 10.3767/persoonia.2017.39.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hubka V., Nováková A., Samson R.A., Houbraken J., Frisvad J.C., Sklenář F. Aspergillus europaeus sp. nov., a widely distributed soil-borne species related to A. wentii (section Cremei) Plant Syst Evol. 2016;302(6):641–650. [Google Scholar]
- 184.Katz M.E., Dougall A.M., Weeks K., Cheetham B.F. Multiple genetically distinct groups revealed among clinical isolates identified as atypical Aspergillus fumigatus. J Clin Microbiol. 2005;43(2):551–555. doi: 10.1128/JCM.43.2.551-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Coelho D., Silva S., Vale-Silva L., Gomes H., Pinto E., Sarmento A. Aspergillus viridinutans: an agent of adult chronic invasive aspergillosis. Med Mycol. 2011;49(7):755–759. doi: 10.3109/13693786.2011.556672. [DOI] [PubMed] [Google Scholar]
- 186.Peláez T., Álvarez-Pérez S., Mellado E., Serrano D., Valerio M., Blanco J.L. Invasive aspergillosis caused by cryptic Aspergillus species: a report of two consecutive episodes in a patient with leukaemia. J Med Microbiol. 2013;62(3):474–478. doi: 10.1099/jmm.0.044867-0. [DOI] [PubMed] [Google Scholar]
- 187.Barrs V.R., van Doorn T.M., Houbraken J., Kidd S.E., Martin P., Pinheiro M.D. Aspergillus felis sp. nov., an emerging agent of invasive aspergillosis in humans, cats, and dogs. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0064871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tanney J.B., Visagie C.M., Yilmaz N., Seifert K.A. Aspergillus subgenus Polypaecilum from the built environment. Stud Mycol. 2017;88:237–267. doi: 10.1016/j.simyco.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hsieh H.-M., Ju Y.-M., Hsueh P.-R., Lin H.-Y., Hu F.-R., Chen W.-L. Fungal keratitis caused by a new filamentous hyphomycete Sagenomella keratitidis. Botanical Stud. 2009;50:331–335. [Google Scholar]
- 190.Martinelli L., Zalar P., Gunde-Cimerman N., Azua-Bustos A., Sterflinger K., Piñar G. Aspergillus atacamensis and A. salisburgensis: two new halophilic species from hypersaline/arid habitats with a phialosimplex-like morphology. Extremophiles. 2017;21(4):755–773. doi: 10.1007/s00792-017-0941-3. [DOI] [PubMed] [Google Scholar]
- 191.Thom C., Raper K.B. The Aspergillus nidulans group. Mycologia. 1939;31(6):653–669. [Google Scholar]
- 192.Horie Y. New or interesting Emericella from herbal drugs. Trans Mycol Soc Jpn. 1979;20:481–491. [Google Scholar]
- 193.Horie Y., Miyaji M., Nishimura K., Franco M.F., KlR Coelho. New and interesting species of Emericella from Brazilian soil. Mycoscience. 1996;37(2):137–144. [Google Scholar]
- 194.Fontbrune F.S., Denis B., Meunier M., Garcia-Hermoso D., Bretagne S., Alanio A. Iterative breakthrough invasive aspergillosis due to TR(34)/L98H azole-resistant Aspergillus fumigatus and Emericella sublata in a single hematopoietic stem cell transplant patient. Transpl Infect Dis. 2014;16(4):687–691. doi: 10.1111/tid.12231. [DOI] [PubMed] [Google Scholar]
- 195.Chrenkova V., Hubka V., Cetkovsky P., Kouba M., Weinbergerova B., Lyskova P. Proven invasive pulmonary aspergillosis in stem cell transplant recipient due to Aspergillus sublatus, a cryptic species of A. nidulans. Mycopathologia. 2018;183(2):423–429. doi: 10.1007/s11046-017-0223-8. [DOI] [PubMed] [Google Scholar]
- 196.Visagie C.M., Yilmaz N., Renaud J.B., Sumarah M.W., Hubka V., Frisvad J.C. A survey of xerophilic Aspergillus from indoor environment, including descriptions of two new section Aspergillus species producing eurotium-like sexual states. MycoKeys. 2017;19:1–30. [Google Scholar]
- 197.Visagie C.M., Varga J., Houbraken J., Meijer M., Kocsubé S., Yilmaz N. Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati) Stud Mycol. 2014;78:1–61. doi: 10.1016/j.simyco.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sugui J.A., Peterson S.W., Figat A., Hansen B., Samson R.A., Mellado E. Genetic relatedness versus biological compatibility between Aspergillus fumigatus and related species. J Clin Microbiol. 2014;52(10):3707–3721. doi: 10.1128/JCM.01704-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chong G.M., Vonk A.G., Meis J.F., Dingemans G.J.H., Houbraken J., Hagen F. Interspecies discrimination of A. fumigatus and siblings A. lentulus and A. felis of the Aspergillus section Fumigati using the AsperGenius® assay. Diagn Microbiol Infect Dis. 2017;87(3):247–252. doi: 10.1016/j.diagmicrobio.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 200.Hubka V., Lyskova P., Frisvad J.C., Peterson S.W., Skorepova M., Kolarik M. Aspergillus pragensis sp. nov. discovered during molecular reidentification of clinical isolates belonging to Aspergillus section Candidi. Med Mycol. 2014;52(6):565–576. doi: 10.1093/mmy/myu022. [DOI] [PubMed] [Google Scholar]
- 201.Gams W. Connected and disconnected chains of phialoconidia and Sagenomella gen. nov. segregated from Acremonium. Persoonia. 1978;10(1):97–112. [Google Scholar]
- 202.Fennell D.I., Raper K.B. New species and varieties of Aspergillus. Mycologia. 1955;47(1):68–89. [Google Scholar]
- 203.Godeas A.M. Micoflora del suelo de la argentina. I algunas formas ascosporicas de la region chaqueña. Mycopathol Mycol Appl. 1972;46(3):189–204. [Google Scholar]
- 204.Horie Y. Ascospores ornamentation and its application to the taxonomic re-evaluation in Emericella. Trans Mycol Soc Jpn. 1980;21:483–493. [Google Scholar]
- 205.Yu J., Mu X., Li R. Invasive pulmonary aspergillosis due to Emericella nidulans var. echinulata, successfully cured by voriconazole and micafungin. J Clin Microbiol. 2013;51(4):1327–1329. doi: 10.1128/JCM.02487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Uhrin G.B., Jensen R.H., Korup E., Grønlund J., Hjort U., Moser C. Recurrent prosthetic valve endocarditis caused by Aspergillus delacroxii (formerly Aspergillus nidulans var. echinulatus) Med Mycol Case Rep. 2015;10:21–23. doi: 10.1016/j.mmcr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ishiwada N., Takeshita K., Yaguchi T., Nagasawa K., Takeuchi N., Hishiki H. The first case of invasive mixed-mold infections due to Emericella nidulans var. echinulata and Rasamsonia piperina in a patient with chronic granulomatous disease. Mycopathologia. 2016;181(3):305–309. doi: 10.1007/s11046-015-9963-5. [DOI] [PubMed] [Google Scholar]
- 208.Hubka V., Nováková A., Peterson S.W., Frisvad J.C., Sklenář F., Matsuzawa T. A reappraisal of Aspergillus section Nidulantes with descriptions of two new sterigmatocystin-producing species. Plant Syst Evol. 2016;302(9):1267–1299. [Google Scholar]
- 209.Barrs V.R., Beatty J.A., Dhand N.K., Talbot J.J., Bell E., Abraham L.A. Computed tomographic features of feline sino-nasal and sino-orbital aspergillosis. Vet J. 2014;201(2):215–222. doi: 10.1016/j.tvjl.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 210.Langlois D.K., Sutton D.A., Swenson C.L., Bailey C.J., Wiederhold N.P., Nelson N.C. Clinical, morphological, and molecular characterization of Penicillium canis sp. nov., isolated from a dog with osteomyelitis. J Clin Microbiol. 2014;52(7):2447–2453. doi: 10.1128/JCM.03602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sarbhoy A.K., Elphick J.J. Hemicarpenteles paradoxus gen. & sp.nov.: the perfect state of Aspergillus paradoxus. Trans Br Mycol Soc. 1968;51(1):155–157. [Google Scholar]
- 212.Yaguchi T., Someya A., Miyadoh S., Udagawa S. Aspergillus ingratus, a new species in Aspergillus section Clavati. Trans Mycol Soc Jpn. 1993;34:305–310. [Google Scholar]
- 213.Frisvad J.C., Yilmaz N., Thrane U., Rasmussen K.B., Houbraken J., Samson R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Horré R., Gilges S., Breig P., Kupfer B., De Hoog G.S., Hoekstra E. Case Report. Fungaemia due to Penicillium piceum, a member of the Penicillium marneffei complex. Mycoses. 2001;44(11-12):502–504. doi: 10.1046/j.1439-0507.2001.00710.x. [DOI] [PubMed] [Google Scholar]
- 215.Santos P.E., Piontelli E., Shea Y.R., Galluzzo M.L., Holland S.M., Zelazko M.E. Penicillium piceum infection: diagnosis and successful treatment in chronic granulomatous disease. Med Mycol. 2006;44(8):749–753. doi: 10.1080/13693780600967089. [DOI] [PubMed] [Google Scholar]
- 216.Peterson S.W., Jurjević Ž. Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces clade 2a. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Crous P.W., Wingfield M.J., Burgess T.I., Hardy G.E., Crane C., Barrett S. Fungal Planet description sheets: 469–557. Persoonia. 2016;37:218–403. doi: 10.3767/003158516X694499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Peterson S.W., Jurjević Ž. New species of Talaromyces isolated from maize, indoor air, and other substrates. Mycologia. 2017;109(4):537–556. doi: 10.1080/00275514.2017.1369339. [DOI] [PubMed] [Google Scholar]