Abstract
Aims
The association between progestin-only contraceptive (POC) use and the risk of various cardiometabolic outcomes has rarely been studied. We performed a systematic review and meta-analysis to determine the impact of POC use on cardiometabolic outcomes including venous thromboembolism, myocardial infarction, stroke, hypertension and diabetes.
Methods and results
Nineteen observational studies (seven cohort and 12 case–control) were included in this systematic review. Of those, nine studies reported the risk of venous thromboembolism, six reported the risk of myocardial infarction, six reported the risk of stroke, three reported the risk of hypertension and two studies reported the risk of developing diabetes with POC use. The pooled adjusted relative risks (RRs) for venous thromboembolism, myocardial infarction and stroke for oral POC users versus non-users based on the random effects model were 1.06 (95% confidence interval (CI) 0.70–1.62), 0.98 (95% CI 0.66–1.47) and 1.02 (95% CI 0.72–1.44), respectively. Stratified analysis by route of administration showed that injectable POC with a RR of 2.62 (95% CI 1.74–3.94), but not oral POCs (RR 1.06, 95% CI 0.7–1.62), was associated with an increased risk of venous thromboembolism. A decreased risk of venous thromboembolism in a subgroup of women using an intrauterine levonorgestrel device was observed with a RR of 0.53 (95% CI 0.32–0.89). No effect of POC use on blood pressure was found, but there was an indication for an increased risk of diabetes with injectable POCs, albeit non-significant.
Conclusions
This systematic review and meta-analysis suggests that oral POC use is not associated with an increased risk of developing various cardiometabolic outcomes, whereas injectable POC use might increase the risk of venous thromboembolism.
Keywords: Progestogen, progesterone, progestin-only pill, contraception, stroke, myocardial infarction, venous thromboembolism, hypertension, type 2 diabetes, women, cardiometabolic risk
Introduction
A number of studies have debated the association between combined oral contraceptive use and the risk of cardiometabolic outcomes,1–3 with some studies reporting an increased risk of venous thromboembolism (VTE), stroke and myocardial infarction (MI) for users of combined oral contraceptives (COCs).3,4 COCs can affect lipid profiles, carbohydrate metabolism, haemostatic factors and thrombolysis, and this may be the pathway by which they affect the risk of developing various cardiometabolic outcomes.5–8. It has been postulated that the increased risk of various cardiometabolic outcomes is mainly attributed to the oestrogen content of these contraceptives,9 while the role of exogenous progestins in modulating the oestrogenic effects remains controversial.10 Therefore, over the years the oestrogen content of combined oral contraceptive pills has decreased and new oral contraceptives with progestin-only content have been developed, which are considered to be safer.9 The type of progestin as well as the route of administration are important factors in predicting the risk of various cardiometabolic outcomes. Progestins such as gestodene, norgestimate and desogestrel have been associated with a greater VTE risk than the older progestins (levonorgestrel, lynestrenol, norethisterone).11 Also, studies have reported an elevated risk of VTE with the use of depot medroxyprogesterone (DMPA), which has a relatively higher dose of progestin.12,13 Weight gain is cited as a common side effect and is a major reason for discontinuation of DMPA.14 Previous studies have generally found no association between the use of injectable or implantable progestin-only contraceptives (POCs) and the development of glucose intolerance;15 however, epidemiological evidence has suggested a possible increased risk of diabetes among DMPA users.15 A rise in blood pressure as a side effect of combined oral contraceptives has been theorised to be the critical mechanism for increased cardiovascular risk in women on COCs; however, the evidence on the effect of POCs on blood pressure remains limited.16 To date, there is scarce evidence on how POCs affect the various cardiometabolic outcomes, which might be because of the low occurrence of chronic diseases among women of reproductive age, and therefore low statistical power to estimate the reliable risk due to the usage of POCs.16 Although few reviews have evaluated the role of POCs and the risk of VTE, stroke and MI,11,16–19 these reviews have some limitations. They are focused on specific outcomes (MI or VTE or stroke), include only specific study designs (case–control only), search available literature only within a few databases and are non-quantitative or largely non-systematic in nature. Therefore, an updated and comprehensive quantitative review is important, given the different types of cardiometabolic outcomes that may be affected by POC use by women of child-bearing age.
This systematic review and meta-analysis aims to investigate the impact of POC use on the risk of developing various cardiometabolic outcomes such as MI, stroke, VTE, diabetes and hypertension.
Methods
Data sources and search strategy
The Cochrane Handbook for Systematic Reviews of Interventions and PRISMA and MOOSE guidelines were used to guide the conduct and reporting of this review.20,21 We conducted a literature search of articles from the following electronic databases from the earliest record to 16 January 2017: PubMed, Web of Science and EMBASE. The search strategy was built based on the PICO strategy and followed the recommendations of the Cochrane Review for progestin-only pills.22 The following key words were searched: ‘progesterone only pill’, ‘progesterone’, ‘progestin only’, ‘progestogen only’, ‘cardiovascular disease’, ‘heart disease’, ‘cerebrovascular disease’, ‘stroke’, ‘myocardial infarction’, ‘coronary artery disease’, ‘venous thromboembolism’, ‘diabetes’ and ‘hypertension’. In addition, reference lists of the included studies and relevant reviews, as well as studies that have cited these articles, were searched with Elsevier’s Scopus, the largest abstract and citation database. The detailed master search strategy is shown in Supplementary Figure 1.
During the first phase of screening, two reviewers evaluated the titles and abstracts against the inclusion and exclusion criteria. For each potentially eligible study, two reviewers independently assessed the full text. In cases of disagreement, a decision was made by consensus or, if necessary, a third reviewer was consulted.
Study selection and eligibility criteria
Studies were included if they met all of the following inclusion criteria: (a) used a randomised trial, case–control, cohort (prospective or retrospective), or cross-sectional study design; (b) reported the presence of a treatment arm featuring the use of POCs; (c) reported the use of progestin for the purpose of contraception only; (d) collected data on the incidence of cardiovascular disease (MI, stroke, heart disease, VTE events), diabetes and hypertension; and (e) were based on human data only and reported odds ratio or relative risk (RR) comparing the use of POCs with non-users of contraceptives.
Data extraction
Two reviewers independently extracted data and consensus was reached in the case of any inconsistency with involvement of a third reviewer. A piloted data extraction form was used. This included data on: study size; study design; baseline population; location; age at baseline; duration of follow-up; reported degree of adjustment; type of POC use; type and numbers of outcomes; how outcomes were ascertained; and reported risk ratios. In instances of multiple publications, the most up-to-date information was extracted.
Assessing the risk of bias
Bias within each individual study was evaluated by two independent reviewers using the validated Newcastle–Ottawa scale, a semi-quantitative scale designed to evaluate the quality of non-randomised studies.23 The assessment of study quality was based on the selection criteria of participants, comparability of cases and controls, and exposure and outcome assessment. Studies that received a score of nine stars were judged to be at low risk of bias; studies that scored seven or eight stars were considered at medium risk; those that scored six or less stars were considered at high risk of bias. Detailed information on the assessment of study quality and risk of bias is provided in Supplementary Tables 1 and 2.
Patient involvement
Patients were not involved in our study.
Statistical analysis
We estimated the risk ratio of cardiovascular diseases (VTE, MI and stroke) for users of POCs versus non-users in subgroups according to the route of administration (oral, injectable and intrauterine). Based on previous reports estimating the yearly incidence of those events to about 0.06% per year,24 we considered that cardiovascular events had a low incidence (<10% a year) in women aged less than 50 years taking oral contraceptives. For infrequent events, the RR and odds ratio are considered equivalent measures of RR.25,26 For initial disease risks of 10% or less, even odds ratios of up to eight can reasonably be interpreted as RRs.27 For each study, we used the most adjusted RR with its 95% confidence interval (CI) and we used the inverse variance weighted method to combine RRs to produce a pooled RR using random-effects meta-analysis models, to allow for between-study heterogeneity. We also conducted sensitivity analyses using fixed effects models and we present the results in the forest plots. Furthermore, when a study reported more than one risk estimate, the pooled RR was obtained using a fixed-effects model. A narrative synthesis and construction of descriptive summary tables were performed for those study outcomes that could not be quantitatively pooled. Heterogeneity was quantified using the I2 statistic, classified as low (I2 ≤ 25%), moderate (I2 > 25% and <75%), or high (I2 ≥ 75%).28 In addition, the Q statistic was used to assess the presence of heterogeneity. PQ statistic ≥ 0.05 was considered to indicate no significant heterogeneity among the included studies. Publication bias was assessed through a funnel plot and asymmetry was assessed using the Egger’s test. It was not feasible to perform sensitivity analyses due to the small number of included studies. All tests were two-tailed and P values of 0.05 or less were considered significant. STATA 14 (Stata Corp, College Station, TX, USA) was used for all statistical analyses.
Results
Study identification and selection
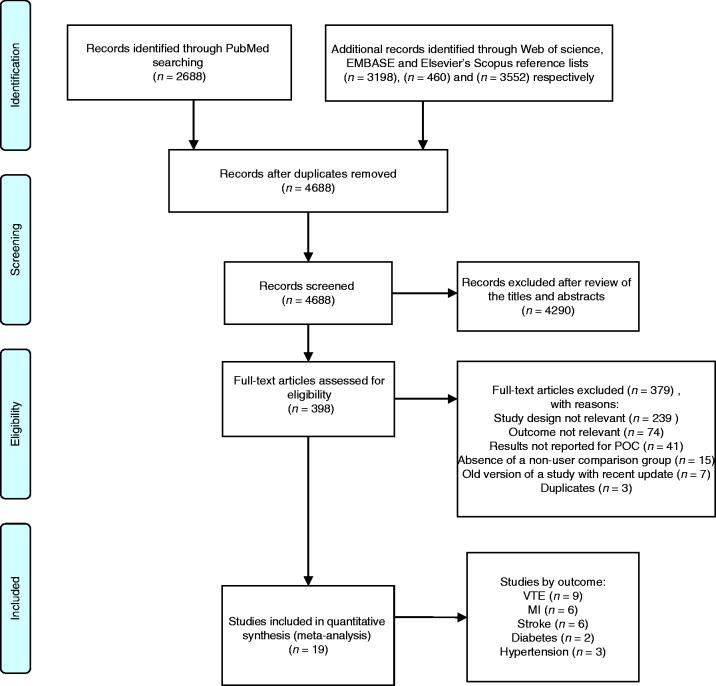
A total of 9898 references were identified: 2688 from PubMed, 3198 from Web of Science, 460 from EMBASE and 3552 from the search in Elsevier’s Scopus (Figure 1). Of these, 5210 duplicates were removed, and 4290 were excluded after review of the titles and abstracts, leaving 398 articles for full-text screening. After full-text assessment 19 articles were included in this review. Of these studies, two were nested case–control studies, 10 case–control studies and seven cohort studies. No randomised clinical trial was found. Nine studies reported the risk of VTE, six studies reported the risk of MI, six reported the risk of stroke, three reported the risk of hypertension and two studies reported the risk of developing diabetes.
Figure 1.
Flow diagram of studies included in the review.
Characteristics of included studies
In total, 19 studies were included in this review, including data from 62,088 women of which 11,930 women reported using POCs. The majority of the included studies were conducted in Europe (n = 12) followed by the USA (n = 5). In addition, there were two multi-country studies. The age of participants ranged from 15 years to about 66 years. Fifteen studies reported on POCs administered orally, and five studies by injection, implant or intrauterine device (IUD).
POC use and risk of VTE
POC use and the risk of VTE were reported in nine articles,12,13,29–35 four of which were retrospective case–control studies, two were nested case–control studies and three were cohort studies. The details on study participants can be found in Supplementary Table 3. Eight studies investigated the risk of VTE with oral, two studies with intrauterine and three with injectable POCs. Therefore, we have estimated the fully adjusted (as reported in studies) risk ratio of VTE for POC users versus non-users in each subgroup according to the route of administration (oral, injectable and intrauterine).
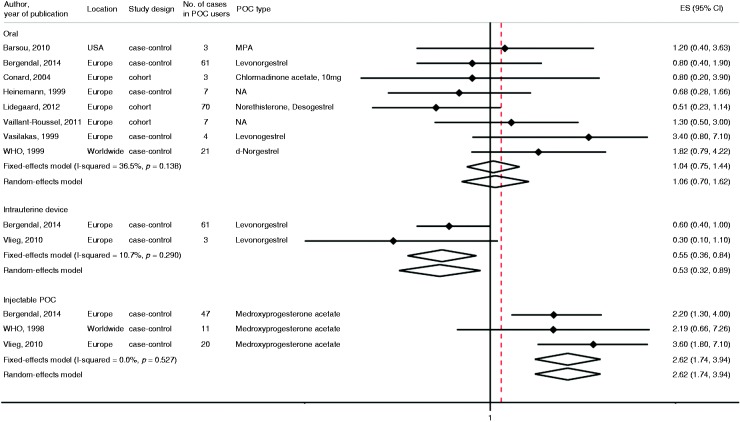
Pooled fully adjusted risk ratios, based on more than 500 women using POCs and 176 VTE events, showed no significant association of oral POC use with the risk of VTE when comparing users with non-users (pooled risk ratio 1.06, 95% CI 0.7–1.62). There was no evidence of high between-study heterogeneity for POC use and the risk of VTE in these studies (I2 = 36.5% and PQ statistic = 0.14). Only three case–control studies reported the risk of VTE with injectable (72 controls, 78 cases) and two studies reported on intrauterine (125 controls and 64 cases) progestin administration. The pooled risk ratio of VTE for users of intrauterine POC formulation (levonorgestrel) was 0.53 (95% CI 0.32–0.89), I2 = 10.7% and PQ statistic = 0.29. On the other hand, the RR of VTE for injectable progestin formulation (DMPA) was 2.62 (1.74–3.94), I2 = 0% and PQ statistic = 0.53 (Figure 2).
Figure 2.
The association between progestin-only contraceptive (POC) use and risk of venous thromboembolism by route of administration. The summary estimates presented were calculated using random effects and fixed effects models. 95% confidence interval (CI) (bars). P comes from Q statistics.
POC use and risk of MI
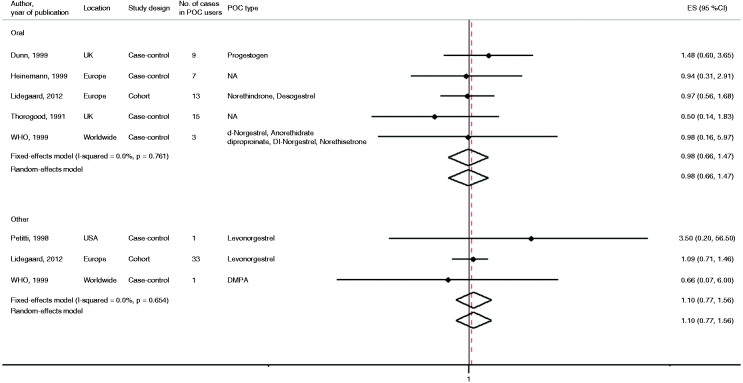
Six studies reported the risk of MI with POC use30,36–40 (Supplementary Table 4). Of those, five were case–control studies and one was a cohort study. Five studies reported RR after oral POC administration, two studies reported RR in women using progestin implants and one study reported RR of MI after injectable and intrauterine POC administration. The adjusted RR of MI for users of POCs versus non-users varied from 0.5 to 3.5, none of the studies reporting a statistically significant association. Pooled results for the fully adjusted models, based on more than 150 women using POCs and 47 MI cases, showed that there was no significant association of MI risk with those who used POCs orally versus those who did not use hormone therapy (pooled risk ratio 0.98, 95% CI 0.66–1.47) (Figure 3). In addition, there was no evidence of between-study heterogeneity for POC use and risk of MI in these studies (I2 = 0% and PQ statistic = 0.72). The pooled RR for MI in the subgroup of women using progestin other than orally was 1.10 (95% CI 0.77–1.56), I2 = 0% and PQ statistic = 0.65.
Figure 3.
The association between oral progestin-only contraceptive (POC) use and risk of myocardial infarction by route of administration. The summary estimates presented were calculated using random effects and fixed effects models. 95% confidence interval (CI) (bars). P comes from Q statistics.
POC use and risk of stroke
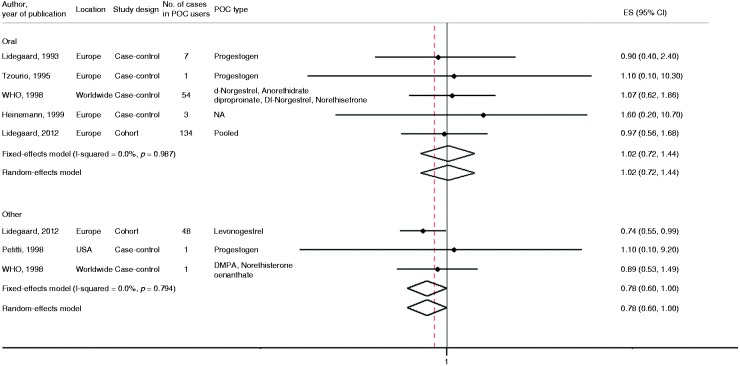
Six studies examined the association between POC use and the risk of stroke30,37,39–42 (Supplementary Table 5). Of those, five were case–control studies and one was a cohort study. The adjusted RR of stroke for users of POCs versus non-users varied from 0.89 to 1.6, none of the studies reporting a statistically significant association. The summary measure from pooled analysis including 350 women using progestin contraceptives orally and 199 stroke events showed no significant evidence to suggest that the use of POCs is associated with the risk of stroke (pooled risk ratio 1.02, 95% CI 0.72–1.44) for the fully adjusted model (Figure 4). There was no evidence of between-study heterogeneity for stroke risk and POP use (I2 = 0% and PQ statistic = 0.99). The pooled RR of stroke in women using POCs other than orally was 0.78 (95% CI 0.6–1), I2 = 0% and PQ statistic = 0.79.
Figure 4.
The association between oral progestin-only contraceptive (POC) use and risk of stroke by route of administration. The summary estimates presented were calculated using random effects and fixed effects models. 95% confidence interval (CI) (bars). P comes from Q statistics.
POC use and risk of hypertension
Only three cohort studies were found to report the impact of POC use with the risk of developing hypertension43–45 (Supplementary Table 6). A study by Spellacy and Birk et al.43 followed 415 predominantly American black women for 2 years and reported that those who used POCs had a significant drop in diastolic blood pressure (P < 0.05). However, most of the women in this study were 4 weeks postpartum, and subsequently using mini pills as a contraceptive method, which might present a bias in case selection. However, two studies44,45 of 119 and 593 participants, respectively, reported that POC use had no significant effect on blood pressure. These studies were limited by small sample size, inadequate adjustment for confounders and lost to follow-up bias.
POC use and risk of diabetes
We found two epidemiological studies that investigated the association between POCs and the risk of developing type 2 diabetes (T2D). A case–control study by Kim et al.46 reported the association of POC use and the risk of developing T2D in a health centre in the USA (Supplementary Table 7). Diabetic cases (n = 284) and non-diabetic controls (n = 570) were matched by age. It was found that users of POCs (DMPA) were at an increased risk of developing diabetes compared to those who used combined pills (oestrogen–progestogen), odds ratio 3.6 (95% CI 1.6–7.9), after adjusting for age and body mass index (BMI). When compared with no history of hormonal contraceptive use, POCs were still associated with the risk of developing diabetes, odds ratio 2.1 (95% CI 1.03–4.2), when adjusted for age, BMI and parity. However, further adjustment for gestational diabetes diagnosed after contraceptives given attenuated and abolished the association, odds ratio 1.6 (95% CI 0.77–3.5). In a cohort study,47 Norplant users (n = 7977) were prospectively compared with age-matched, non-hormonal IUD users (n = 6625) and women who underwent sterilisation (n = 1419). Twelve T2D cases were identified – nine in Norplant initiators (eight current users), two in IUD initiators (three current IUD users) and one in a sterilised woman. The crude incidence rate was higher in current Norplant users compared with controls, but the crude and adjusted rate ratios for Norplant users compared with controls were not significantly different. After adjusting for clinic, age and body weight, the current implant users did not have a significantly higher incidence of T2D compared with the group using IUD or sterilisation, RR 2.4 (95% CI 0.7–8.1).
Study quality and publication bias
Three studies were classified as having a low risk of bias, five as having a medium risk of bias and the rest were classified as having a high risk of bias. We did not find evidence for publication bias from the funnel plots of VTE, MI and stroke, as shown in Supplementary Figure 2.
Discussion
Overall, the available body of literature suggests that the use of oral POCs is not associated with an excess risk of VTE, MI, stroke and hypertension. We found limited evidence that DMPA is associated with an increased risk of VTE, while intrauterine application of levonorgestrel was associated with a decreased risk of VTE. There was, also, an indication for an increased risk of diabetes with injectable POCs, albeit non-significant.
Our findings suggest no effect on VTE risk after oral POCs and a decreased risk of VTE in a subgroup of women using an intrauterine levonorgestrel device with RR 0.53 (95% CI 0.32–0.89). However, the subgroup analysis based on three studies,13,34,40 including 78 VTE events, showed a 2.6-fold increased risk of VTE for injectable progestin users compared to non-users. A study that contributed most to the summary statistic for DMPA and the risk of VTE excluded women in highest risk of VTE (personal history of VTE);12 therefore, it is less likely that the effect observed on injectable progestin is due to confounding by indication. POC intake causes a decrease in sex hormone-binding globulin, which is a marker of venous thrombosis risk, and this effect varies with the dose and type of progestogen used.48,49 Indeed, the plasma concentration of levonorgestrel with IUD ranges between 74 and 166 pg/mL,50 while after an intramuscular injection of 150 mg of DMPA, the peak plasma concentration is 2500–7000 pg/mL and remains greater than 430 pg/mL at 3 months.51,52 Also, progestins may express prothrombotic properties by modulation of protein C resistance,53 by affecting the cellular expression of tissue factor and circulating tissue factor pathway inhibitor.54,55 The third generation progestins (e.g. desogestrel) are suggested to be more prothrombotic than earlier formulations such as levonorgestrel or norethisteron.11 Levonorgestrel does not increase activated protein C resistance, suggesting that this contraceptive does not have a prothrombotic effect.53 While, for instance, in a mouse model of vascular injury, medroxyprogesterone increased thrombin formation and changes in vascular gene expression, resulting in altered plaque matrix either alone and in combination with oestradiol.56
A previous meta-analysis of six case–control studies reported that there was no increase in the MI risk with POC use.17 In our meta-analysis we excluded one of the studies included in previous estimates, as it was investigating the effect of COCs (contained up to 50 µg of oestrogen combined with a fixed dose of progestin),57 and was not a progestin-only pill; however, our results were in line with previous findings. The result was similar according to the route of administration, including implant, injectable and oral POCs. Furthermore, our findings are in line with a previous meta-analysis of six case–control studies18 showing that POC use had no significant effect on the risk of developing stroke. Similarly, a systematic review looking at the association of POC use with high blood pressure also concluded that POC use does not affect diastolic and systolic blood pressures.16 However, all of the included studies were written in the 1970s and 1980s; therefore, they have investigated the first and second generations of POCs, while the information on the third generation of progestins is lacking. Furthermore, an important limitation of these studies is the fact that they investigated POC use in normotensive women, yet future studies should investigate the effects of POCs on blood pressure in women with a history of hypertension.
A case–control study conducted among Navajho women showed that use of injectable POCs significantly increased the risk of developing T2D when adjusted for age, BMI and parity; however, after further adjustment for gestational diabetes diagnosed after contraceptives given, the association was attenuated and not any more significant.46 Women with gestational diabetes are at higher risk of developing T2D, and women who used DMPA were significantly more likely to have a history of gestational diabetes.46 Therefore, it might be that gestational diabetes is on the pathway between POC use and T2D development, which needs further investigation. Nevertheless, a study conducted in breast-feeding Latina women with previous gestational diabetes mellitus demonstrated that oral POCs were associated with an increased risk of diabetes compared with an equal use of low-dose combination oral contraceptives, indicating that if an association between POC use and diabetes exists pathways other than gestational diabetes may be present.58 In this study, however, low-dose progestin and COCs were not associated with risk of diabetes.58 The mechanism linking POC use with a potential increased risk of diabetes is unknown. A possible mechanism might be the adverse effect that POC use has on obesity, which is an important risk factor for diabetes.46 The 2016 Cochrane review investigated the association between POC use and weight changes.59 Although the actual mean weight gain was generally low (<2 kg for most studies) for 6–12 months of follow-up, the effect on weight varied with different formulations and routes of POC administration, being more pronounced with DMPA.14,60–62 Furthermore, using contraception reduces the numbers of pregnancies, which is also considered to be a risk factor for developing diabetes.46 Also, a decrease in sex-hormone-binding globulin is associated with injectable DMPA,63 and low circulating levels of sex hormone-binding globulin are a strong predictor of the risk of T2D in women and men.64 The other possibility is that women taking COCs compared to those receiving DMPA are healthier and have a lower risk of developing T2D.46 Indeed, a systematic review on studies in non-diabetic women based on six studies investigating DMPA use reported an elevation of insulin concentration (compared with baseline before DMPA) at 2–3 hours after the glucose challenge;15 however, most of the studies included in the review did not find any effect of injectable contraceptives on glucose concentrations in lean glucose-tolerant women. Studies that reported increased glucose were performed in subjects who had greater baseline body weight or had a longer duration of POC use.15
Strengths and limitations
Our results are consistent with previously published reviews;11,16–19 however, this is the first systematic review and meta-analysis that looks at the association of POC use with multiple cardiometabolic outcomes such as VTE, MI, stroke, hypertension and diabetes. Nevertheless, there are number of limitations to this review. First, typical with any literature-based review, it is possible that both measured and unmeasured publication bias can limit our overall findings. Although evaluations with the conventional funnel plots and Egger test estimates indicate minimal publication bias, these approaches are limited by a qualitative assessment reliant on visual inspection and the fact that the majority of these assessments were based on a limited number of studies. Thus we cannot exclude the possibility of publication bias coming from the underreporting of negative findings (increased risk of cardiovascular outcomes with POC use). Such a scenario would lead towards null findings and an underestimation of our findings. The studies included in this review were limited by study design and methodology: (a) all studies were observational in nature and thus prone to bias and confounding; (b) they had small numbers of participants using POCs, which explains the wide CIs of some of the studies; and (c) studies did not specify the type and dosage of POCs or the type and dosage of POCs varied considerably. Also, even though the prevalence of intermediate risk factors for cardiovascular disease is low among women of reproductive age, 10% of women aged 18–44 years have high blood pressure, while 15% of women aged 20–45 years have high cholesterol, also 3% of women of reproductive age have T2D.65,66 Although the majority of studies included in our review adjust for potential confounding and intermediate cardiovascular risk factors, they do not adjust for medication use (e.g. statins, antihypertensive medications). Therefore, future studies should take this into account when investigating the risk of diabetes with POC use.
Implications for policy and future research
European guidelines on cardiovascular disease prevention in clinical practice have emphasised the role of the cardiologist in screening for cardiovascular disease risk factors and assessing the baseline cardiovascular risk before advising the type of contraceptives to be used.67 US medical eligibility criteria for contraceptive use advocates the use of POCs for women at high risk of cardiovascular disease,68 which may be a safe recommendation as also supported by our findings of no association between oral POC use and VTE, MI and stroke in women in general. Although the US medical eligibility criteria for contraceptive use68 recognises a previous history of MI and stroke as well as hypertension as contraindications for injectable POC use, a previous history of VTE is not recognised as a contraindication for DMPA. Therefore, based on our findings of an increased risk of developing VTE and present indication of an increased risk of T2D, further investigation is required in order to rule out potential harmful effects of DMPA in these women.
Conclusions
In conclusion, studies included in this meta-analysis suggest that POC use is not associated with an increased risk of developing various cardiometabolic outcomes. However, our findings, based on limited evidence, suggest that an increased risk of VTE might be present for injectable POCs, as well as some indication for an increased diabetes risk. Also, there is some indication that an intrauterine levonorgestrel device might be a safe choice with regard to VTE risk. Nevertheless, this systematic review must be interpreted with caution as the studies included in the review were observational in nature and meta-analyses results were based on studies with a small sample size. Rigorous studies with better quality design and lower risk of bias are needed to determine the true impact of POC use on various cardiometabolic outcomes.
Supplemental Material
Supplemental material for Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis by Marija Glisic, Sara Shahzad, Stergiani Tsoli, Mahmuda Chadni, Eralda Asllanaj, Lyda Z Rojas, Elizabeth Brown, Rajiv Chowdhury, Taulant Muka and Oscar H Franco in European Journal of Preventive Cardiology
Author contribution
TM and OHF contributed to the conception and design, the analysis and interpretation and critically revised the manuscript. MG contributed to the analysis and interpretation, drafted and critically revised the manuscript. SS contributed to the acquisition, analysis and interpretation, drafted and critically revised the manuscript. ST, EB and MC contributed to the acquisition and critically revised the manuscript. EA contributed to the interpretation and critically revised the manuscript. LZR contributed to the analysis and critically revised the manuscript. RC contributed to the interpretation and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Registration: Not registered.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TM and OHF work in ErasmusAGE, a centre for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd.), Metagenics Inc. and AXA. TM reported receiving research support from Metagenics Inc. TM currently works as a pharmaceutical medicine physician at Novo Nordisk, Copenhagen, Denmark. MG and EA have been financially supported by Erasmus Mundus Western Balkans (ERAWEB), a project funded by the European Commission. OHF reported receiving grants or research support from Metagenics Inc. ST is funded by the Economic and Social Research Council. These funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. RC, SS, ST, MC, LZR and EB have nothing to disclose.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Gerstman BB, Piper JM, Tomita DK, et al. Oral contraceptive estrogen dose and the risk of deep venous thromboembolic disease. Am J Epidemiol 1991; 133: 32–37. [DOI] [PubMed] [Google Scholar]
- 2.Khader YS, Rice J, John L, et al. Oral contraceptives use and the risk of myocardial infarction: a meta-analysis. Contraception 2003; 68: 11–17. [DOI] [PubMed] [Google Scholar]
- 3.Mant J, Painter R, Vessey M. Risk of myocardial infarction, angina and stroke in users of oral contraceptives: an updated analysis of a cohort study. Br J Obstet Gynaecol 1998; 105: 890–896. [DOI] [PubMed] [Google Scholar]
- 4.Peragallo R, Urrutia RRC, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol 2013; 122: 380–389. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Willett WC, Colditz GA, et al. A prospective study of past use of oral contraceptive agents and risk of cardiovascular diseases. N Engl J Med 1988; 319: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 6.Lewis MA, Heinemann LA, Spitzer WO, et al. for the Transnational Research Group on Oral Contraceptives and the Health of Young Women. The use of oral contraceptives and the occurrence of acute myocardial infarction in young women. Contraception 1997; 56: 129–140. [DOI] [PubMed] [Google Scholar]
- 7.Petitti DB, Sidney S, Bernstein A, et al. Stroke in users of low-dose oral contraceptives. N Engl J Med 1996; 335: 8–15. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz SM, Siscovick DS, Longstreth WT, Jr, et al. Use of low-dose oral contraceptives and stoke in young women. Ann Intern Med 1997; 127: 596–603. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann LAJ, Dinger JC, Assmann A, et al. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation “pill scare”. Contraception 2010; 81: 401–407. [DOI] [PubMed] [Google Scholar]
- 10.Tedeschi-Reiner E, Ivekovic R, Novak-Laus K, et al. Endogenous steroid sex hormones and atherosclerosis of retinal arteries in men. Med Sci Monit 2009; 15: CR211–CR216. [PubMed] [Google Scholar]
- 11.Mantha S, Karp R, Raghavan V, et al. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ 2012; 345: e4944–e4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergendal A, Persson I, Odeberg J, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol 2014; 124: 600–609. [DOI] [PubMed] [Google Scholar]
- 13.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol 2010; 30: 2297–2300. [DOI] [PubMed] [Google Scholar]
- 14.Espey E, Steinhart J, Ogburn T, et al. Depo-provera associated with weight gain in Navajo women. Contraception 2000; 62: 55–58. [DOI] [PubMed] [Google Scholar]
- 15.Kahn HS, Curtis KM, Marchbanks PA. Effects of injectable or implantable progestin-only contraceptives on insulin-glucose metabolism and diabetes risk. Diabetes Care 2003; 26: 216–225. [DOI] [PubMed] [Google Scholar]
- 16.Hussain SF. Progestogen-only pills and high blood pressure: is there an association? A literature review. Contraception 2004; 69: 89–97. [DOI] [PubMed] [Google Scholar]
- 17.Chakhtoura Z, Canonico M, Gompel A, et al. Progestogen-only contraceptives and the risk of acute myocardial infarction: a meta-analysis. J Clin Endocrinol Metab 2011; 96: 1169–1174. [DOI] [PubMed] [Google Scholar]
- 18.Chakhtoura Z, Canonico M, Gompel A, et al. Progestogen-only contraceptives and the risk of stroke: a meta-analysis. Stroke 2009; 40: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 19.Tepper NK, Whiteman MK, Marchbanks PA, et al. Progestin-only contraception and thromboembolism: a systematic review. Contraception 2016; 94: 678–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097–e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.handbook.cochrane.org (accessed 18 April 2018).
- 22.Friedrich RM, Lively S, Rubenstein L, et al. The Friedrich–Lively Instrument to Assess the Impact of Schizophrenia on Siblings (FLIISS): Part I – instrument construction. J Nurs Meas 2002; 10: 219–230. [DOI] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 24.Lidegaard O, Lokkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ 2009; 339: b2890–b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano M, Gauvreau K. Principles of biostatistics. 2nd ed. Pacific Grove, Duxbury, 2000.
- 26.Szklo M, Nieto JF. Epidemiology. Beyond the Basics, Sudbury, MA, USA: John and Bartlett, 2004, pp. 110–114. [Google Scholar]
- 27.Talfryn H, Oakley Davies IKC, Tavakoli M. When can odds ratios mislead? BMJ 1988; 316: 989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barsoum MK, Heit JA, Ashrani AA, et al. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case–control study. Thromb Res 2010; 126: 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinemann LA, Assmann A, DoMinh T, et al. Oral progestogen-only contraceptives and cardiovascular risk: results from the Transnational Study on Oral Contraceptives and the Health of Young Women. Eur J Contracept Reprod Health Care 1999; 4: 67–73. [DOI] [PubMed] [Google Scholar]
- 31.Vasilakis C, Jick H, del Mar Melero-Montes M. Risk of idiopathic venous thromboembolism in users of progestagens alone. Lancet 1999; 354: 1610–1611. [DOI] [PubMed] [Google Scholar]
- 32.Conard J, Plu-Bureau G, Bahi N, et al. Progestogen-only contraception in women at high risk of venous thromboembolism. Contraception 2004; 70: 437–441. [DOI] [PubMed] [Google Scholar]
- 33.Vaillant-Roussel H, Ouchchane L, Dauphin C, et al. Risk factors for recurrence of venous thromboembolism associated with the use of oral contraceptives. Contraception 2011; 84: 23–30. [DOI] [PubMed] [Google Scholar]
- 34.Lidegaard O, Nielsen LH, Skovlund CW, et al. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ 2012; 344: e2990–e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lidegaard O, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ 2011; 343: d6423–d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn N, Thorogood M, Faragher B, et al. Oral contraceptives and myocardial infarction: results of the MICA case–control study. BMJ 1999; 318: 1579–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petitti DB, Siscovick DS, Sidney S, et al. Norplant implants and cardiovascular disease. Contraception 1998; 57: 361–362. [DOI] [PubMed] [Google Scholar]
- 38.Thorogood M, Mann J, Vessey MM. Is oral contraceptive use still associated with an increased risk of fatal myocardial infarction? Report of a case–control study. Br J Obstet Gynaecol 1991; 98: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 39.Lidegaard O, Lokkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med 2012; 366: 2257–2266. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives: results of an international, multicenter, case-control study. Contraception 1998; 57: 315–324. [PubMed] [Google Scholar]
- 41.Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case–control study of migraine and risk of ischaemic stroke in young women. BMJ 1995; 310: 830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lidegaard O. Oral contraception and risk of a cerebral thromboembolic attack: results of a case–control study. BMJ 1993; 306: 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spellacy WN, Birk SA. The effect of intrauterine devices, oral contraceptives, estrogens, and progestogens on blood pressure. Am J Obstet Gynecol 1972; 112: 912–919. [DOI] [PubMed] [Google Scholar]
- 44.Hall WD, Douglas MB, Blumenstein BA, et al. Blood pressure and oral progestational agents. A prospective study of 119 black women. Am J Obstet Gynecol 1980; 136: 344–348. [DOI] [PubMed] [Google Scholar]
- 45.Wilson ES, Cruickshank J, McMaster M, et al. A prospective controlled study of the effect on blood pressure of contraceptive preparations containing different types and dosages of progestogen. Br J Obstet Gynaecol 1984; 91: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 46.Kim C, Seidel KW, Begier EA, et al. Diabetes and depot medroxyprogesterone contraception in Navajo women. Arch Intern Med 2001; 161: 1766–1771. [DOI] [PubMed] [Google Scholar]
- 47.International Collaborative Post-Marketing Surveillance of Norplant. Post-marketing surveillance of Norplant® contraceptive implants: II. Non-reproductive health (1). Contraception 2001; 63: 187–209. [DOI] [PubMed] [Google Scholar]
- 48.van Rooijen M, Silveira A, Hamsten A, et al. Sex hormone-binding globulin – a surrogate marker for the prothrombotic effects of combined oral contraceptives. Am J Obstet Gynecol 2004; 190: 332–337. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JS, Eastell R, Peel NFA. Effects of depot medroxyprogesterone acetate on bone density and bone metabolism before and after peak bone mass: a case–control study. J Clin Endocrinol Metab 2008; 93: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson CG, Lahteenmaki PL, Luukkainen T, et al. Sustained intrauterine release of levonorgestrel over five years. Fertil Steril 1986; 45: 805–807. [DOI] [PubMed] [Google Scholar]
- 51.Nanda K, Amaral E, Hays M, et al. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertil Steril 2008; 90: 965–971. [DOI] [PubMed] [Google Scholar]
- 52.Bogdanov VY, Balasubramanian V, Hathcock J, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med 2003; 9: 458–462. [DOI] [PubMed] [Google Scholar]
- 53.Kemmeren JM, Algra A, Meijers JC, et al. Effect of second- and third-generation oral contraceptives on the protein C system in the absence or presence of the factor V Leiden mutation: a randomized trial. Blood 2004; 103: 927–933. [DOI] [PubMed] [Google Scholar]
- 54.Lockwood CJ, Murk W, Kayisli UA, et al. Progestin and thrombin regulate tissue factor expression in human term decidual cells. J Clin Endocrinol Metab 2009; 94: 2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Vliet HA, Bertina RM, Dahm AE, et al. Different effects of oral contraceptives containing different progestogens on protein S and tissue factor pathway inhibitor. J Thromb Haemost 2008; 6: 346–351. [DOI] [PubMed] [Google Scholar]
- 56.Freudenberger T, Oppermann M, Marzoll A, et al. Differential effects of medroxyprogesterone acetate on thrombosis and atherosclerosis in mice. Br J Pharmacol 2009; 158: 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg L, Palmer JR, Rao RS, et al. Low-dose oral contraceptive use and the risk of myocardial infarction. Arch Intern Med 2001; 161: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 58.Kjos SL, Peters RK, Xiang A, et al. Contraception and the risk of type 2 diabetes mellitus in Latina women with prior gestational diabetes mellitus. JAMA 1998; 280: 533–538. [DOI] [PubMed] [Google Scholar]
- 59.Lopez LM, Ramesh S, Chen M, et al. Progestin-only contraceptives: effects on weight. Cochrane Database Syst Rev 2016; 8: CD008815–CD008815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark MK, DJS, Sowers M, et al. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. Int J Obes 2005; 29: 1252–1258. [DOI] [PubMed] [Google Scholar]
- 61.Pantoja M, Medeiros T, Baccarin MC, et al. Variations in body mass index of users of depotmedroxyprogesterone acetate as a contraceptive. Contraception 2010; 81: 107–111. [DOI] [PubMed] [Google Scholar]
- 62.Modesto W, de Nazaré Silva dos Santos P, Correia VM, et al. Weight variation in users of depot medroxyprogesterone acetate, the levonorgestrel releasing intrauterine system and a copper intrauterine device for up to ten years of use. Eur J Contracept Reprod Health Care 2015; 20: 57–63. [DOI] [PubMed] [Google Scholar]
- 63.Walsh JS, Eastell R, Peel NF. Effects of depot medroxyprogesterone acetate on bone density and bone metabolism before and after peak bone mass: a case–control study. J Clin Endocrinol Metab 2008; 93: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 64.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 2009; 361: 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayes DK, Fan AZ, Smith RA, et al. Trends in selected chronic conditions and behavioral risk factors among women of reproductive age, behavioral risk factor surveillance system, 2001–2009. Prev Chronic Dis 2011; 8: A120–A120. [PMC free article] [PubMed] [Google Scholar]
- 66.Kuklina EV, Yoon PW, Keenan NL. Prevalence of coronary heart disease risk factors and screening for high cholesterol levels among young adults, United States, 1999–2006. Ann Fam Med 2010; 8: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Authors/Task Force Members, Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Atherosclerosis 2016; 252: 207–274. [DOI] [PubMed] [Google Scholar]
- 68.US Medical Eligibility Criteria for Contraceptive Use. 2016. Recommendations and Reports, 2016; 65: 1–104. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Association between progestin-only contraceptive use and cardiometabolic outcomes: A systematic review and meta-analysis by Marija Glisic, Sara Shahzad, Stergiani Tsoli, Mahmuda Chadni, Eralda Asllanaj, Lyda Z Rojas, Elizabeth Brown, Rajiv Chowdhury, Taulant Muka and Oscar H Franco in European Journal of Preventive Cardiology