Abstract
Lung endothelial cell apoptosis and injury occur throughout all stages of acute lung injury/acute respiratory distress syndrome and impact disease progression. Caspases 1, 4, and 5 are essential for completion of the apoptotic program known as pyroptosis that also involves proinflammatory cytokines. Because gasdermin D (GSDMD) mediates pyroptotic death and is essential for pore formation, we hypothesized that it might direct caspase 1–encapsulated microparticle (MP) release and mediate endothelial cell death. Our present work provides evidence that GSDMD is released by LPS-stimulated THP-1 monocytic cells, where it is packaged into microparticles together with active caspase 1. Furthermore, only MP released from stimulated monocytic cells that contain both cleaved GSDMD and active caspase 1 induce endothelial cell apoptosis. MPs pretreated with caspase 1 inhibitor Y-VAD or pan-caspase inhibitor Z-VAD do not contain cleaved GSDMD. MPs from caspase 1–knockout cells are also deficient in p30 active GSDMD, further confirming that caspase 1 regulates GSDMD function. Although control MPs contained cleaved GSDMD without caspase 1, these fractions were unable to induce cell death, suggesting that encapsulation of both caspase 1 and GSDMD is essential for cell death induction. Release of microparticulate active caspase 1 was abrogated in GSDMD knockout cells, although cytosolic caspase 1 activation was not impaired. Last, higher concentrations of microparticulate GSDMD were detected in the plasma of septic patients with acute respiratory distress syndrome than in that of healthy donors. Taken together, these findings suggest that GSDMD regulates the release of microparticulate active caspase 1 from monocytes essential for induction of cell death and thereby may play a critical role in sepsis-induced endothelial cell injury.
Clinical Relevance
To our knowledge, our novel study is the first to show that microparticulate caspase 1 and gasdermin D may play a critical role in the regulation of sepsis-mediated pulmonary vascular endothelial cell injury. Understanding this regulatory mechanism has therapeutic potential and benefit.
Microparticles are small membrane-coated structures that are released from cells upon activation or during apoptosis (1, 2). In pathological states, such as atherosclerosis, sepsis, acute coronary syndrome, diabetes, or immune disorders, elevated circulating concentrations of microparticles have been detected (3–9). Because microparticles accumulate in areas of disordered blood flow (3–5), we believe that caspase 1–containing microparticles may have pathological consequences to the endothelium. Our previous studies have demonstrated that monocyte-derived microparticles can induce cell death (10–12). In addition to its classical role in IL-1/IL-18 processing (13), caspase 1 has a demonstrated role in microparticle-mediated cell death. However, little is known about the mechanisms of microparticulate caspase 1–induced apoptosis. In this context, gasdermin D (GSDMD) has recently been shown to induce a pyroptotic cell death (14–18). GSDMD is a 487–amino acid cytoplasmic protein that contains an ill-characterized gasdermin domain and lacks any obvious transmembrane segment or signal peptide (19). GSDMD has been shown to be cleaved by inflammatory caspases, and the cleaved p30 amino terminal fragment GSDMD is thought to be involved in the induction of pyroptotic cell death owing to its pore-forming capacity (16–20). In the present study, we show that the p30 form of human GSDMD is released by activated monocytes in microparticles together with the inflammasome protein caspase 1. Importantly, GSDMD is essential for the release of active caspase 1 in microparticles. GSDMD-knockout (GSDMD-KO) cells do not release active caspase 1, despite its continued presence in cell lysates from LPS-activated GSDMD-KO cells. Hence, we propose that active GSDMD regulates the release of active caspase 1–encapsulated microparticles, an event essential for induction of cell death.
Methods
Microparticles (MPs) isolated from stimulated THP-1 cells were analyzed for the presence of cleaved GSDMD together with inflammasome proteins such as active caspase 1. These MPs were also cocultured with human pulmonary vascular endothelial cells (HPMVEC) to test the role of GSDMD in caspase 1–mediated cell death. A detailed description of the methods is provided in the data supplement.
Results
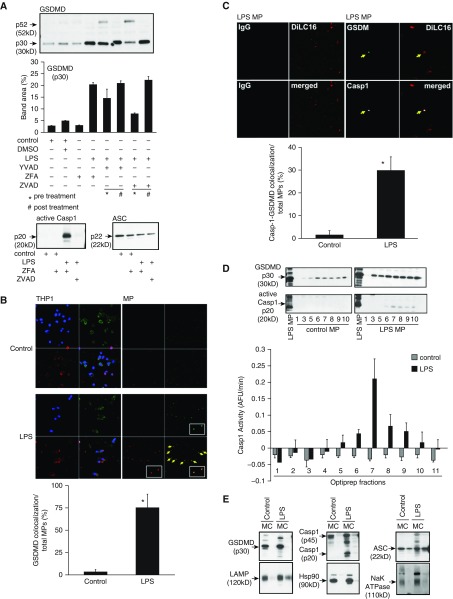
Caspases 1, 4, 5, and 11 can cleave GSDMD to an active fragment that mediates pyroptotic cell death by its induction of cell membrane pores (17, 21–23). Because our recent work established the novel involvement of caspase 1 in microparticle-induced apoptosis, we examined the possible role of GSDMD as a key component of monocyte-derived, caspase-containing microparticles. We detected the cleaved active p30 form of GSDMD in the microparticles released from THP-1 monocytic cells upon stimulation with LPS (1 μg/ml) for 2 hours (Figure 1A). This presence of GSDMD was further confirmed using confocal microscopy. As shown in Figure 1B, THP-1 cells contain endogenous GSDMD; however, upon stimulation with LPS for 2 hours, GSDMD colocalized with released LPS-induced microparticles (LPS MPs). Pretreating THP-1 cells with the caspase 1 inhibitor Y-VAD or the pan-caspase inhibitor Z-VAD reduced the LPS-induced release of p30 GSDMD in MPs (Figure 1A). The release of cleaved GSDMD coincided with the release of active p20 caspase 1 in the MP fractions (Figure 1A, lower left panel). As shown in Figure 1C, GSDMD (green) and caspase 1 (white) were colocalized in released LPS MPs (red). This concurrence of GSDMD and caspase 1 in microparticles was further confirmed using OptiPrep density gradients (AXIS-SHIELD). Active p30 GSDMD and p20/enzymatically active caspase 1 colocalized only in the LPS-generated fractions. Although GSDMD was detected in several fractions of both control and LPS MPs, active GSDMD and p20 enzymatically active caspase 1 were codetected only in fractions 7–9 (Figure 1D). The absence of caspase 1 activity in less dense OptiPrep fractions 1–5 of both control and LPS MPs was not due to lack of MPs, because we detected almost equal numbers of MPs by DilC16 staining of the fractions (see Figure E1 in the data supplement). Of note, MPs also contained the inflammasome protein ASC, critical for caspase 1 activation (Figure 1A).
Figure 1.
Cleaved gasdermin D (GSDMD) colocalizes with active caspase 1 (Casp1) in microparticles (MPs) released from LPS-stimulated THP-1 cells. THP-1 cells were cultured at a concentration of 50 million per milliliter and stimulated with LPS (1 μg/ml) for 2 hours or left untreated. Casp1 inhibitor Y-VAD and pan-caspase inhibitor Z-VAD were used both before and after LPS treatment as indicated. DMSO and Z-FA were used as inhibitor controls. MPs were isolated from each condition and normalized by total protein for analysis. (A) Immunoblots of GSDMD, p20 caspase 1, and ASC from MPs after various treatments. Densitometric scan analysis using ImageJ software (NIH) with immunoblots is representative of four experiments. (B) Cells and MP fractions were analyzed by fluorescence microscopy for colocalization of GSDMD with MPs recovered from control and LPS-treated THP-1 cells. Cells and MPs were stained with DilC16 (lipid stain, red), GSDMD (green), and DAPI (blue). (C) Colocalization of Casp1 and GSDMD in MPs visualized by fluorescence microscopy with DiLC16 (red), GSDMD (green), and Casp1 (white). Quantification of colocalization of GSDMD with MPs was calculated as the percentage of the number of MPs colocalized with GSDMD among the total number of MPs in each field. Quantification of colocalization of Casp1 with GSDMD in MPs was calculated as the percentage of the number of MPs colocalized with Casp1 and GSDMD among the total number of MPs in each field. (D) OptiPrep fractions of control and LPS MPs were analyzed for p30 GSDMD and active p20 Casp1. Casp1 activity in each fraction was determined by fluorimetry of WEHD-afc cleavage. (E) Control and LPS MPs were further fractionated into membrane (M) and MP content (C). Both membrane and content fractions from control and LPS MPs were then analyzed by immunoblotting for p30 GSDMD, Casp1, ASC, LAMP, Hsp90, and Na+/K+-ATPase. *P < 0.05; arrow shows colocalization, inset is 100× magnification. Data are representative of four experiments. AFU = arbitrary fluorescence units.
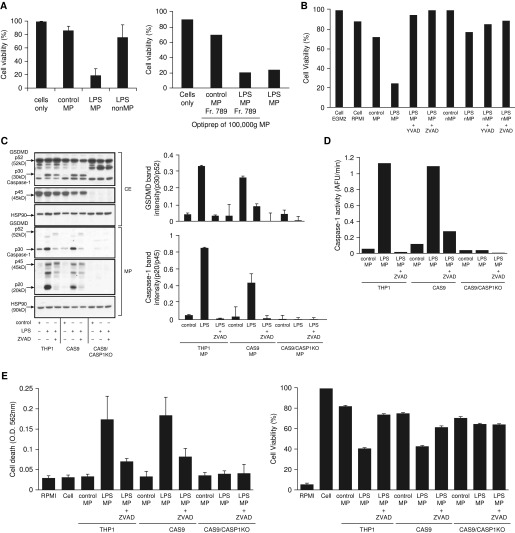
When MPs were disrupted into membrane (M) and content components (C), cleaved GSDMD and active caspase 1 associated with the membrane of the LPS MPs (Figure 1E) because neither was detected in the soluble content of MPs. In contrast, ASC was detectable in the soluble fraction. This finding suggests that GSDMD and active caspase 1 association within the MP membrane may work in concert to induce cell death in target cells. To test this hypothesis, we then asked if GSDMD and caspase 1 association and encapsulation in LPS MPs was essential for its release and cell death induction. First, control MPs and LPS MPs released by THP-1 cells were analyzed for their cytotoxic capacity against HPMVEC. As shown in Figure 2A, only LPS MPs induced cell death, whereas control MPs and LPS non-MP fractions did not. Interestingly, LPS non-MP fractions contained p30 GSDMD (Figure E2), but they did not induce cell death of HPMVEC, suggesting that encapsulation of GSDMD together with active caspase 1 in MPs is essential for cell death. Furthermore, although control MPs sometimes contained p30 GSDMD, possibly owing to activation of GSDMD by a nonclassical pathway, they did not induce cell death, likely because control MPs did not contain active caspase 1. The fact that control MPs did not induce cell death suggests that GSDMD in MPs is essential for caspase 1–encapsulated MP release and thereby caspase 1 for death induction. To further confirm this observation, cell death induction capacity was measured from the density gradient fractions of both control and LPS MPs. As shown in Figure 1D, only fractions 7–9 of LPS MPs contained both p30 GSDMD and p20 caspase 1. Accordingly, only fractions 7–9 from LPS MPs (i.e., containing both GSDMD and active caspase 1) induced cell death, whereas corresponding fractions from control MPs did not. It is noteworthy that control fractions 7–9 did contain p30 GSDMD, as shown in Figure 2A. Other fractions from both control and LPS MPs did not induce cell death.
Figure 2.
Microparticulate p30 GSDMD release and human pulmonary microvascular endothelial cell (HPMVEC) death is regulated by caspase 1. (A) MPs were isolated from THP-1 stimulated with LPS (1 μg/ml) for 2 hours or left untreated and then applied to HPMVEC, which were analyzed for cell viability using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Non-MP fractions and OptiPrep fractions 7–9 used in Figure 1 were also analyzed for HPMVEC death. (B) Cell viability using the MTS assay was compared between control MP, LPS MP, and non-MP fractions in the presence or absence of Y-VAD and Z-VAD. (C) Cell extracts from THP-1, CAS9, and CAS9/Casp1-knockout (CAS9/CASP1-KO) cells stimulated with LPS in the presence or absence of Z-VAD or left untreated were analyzed for p30 GSDMD and p45 Casp1. Hsp90 was used as a loading control. MPs were then isolated from the supernatants of these cells and analyzed for GSDMD and Casp1, using Hsp90 as a loading control. Data and densitometric scan of immunoblot are representative of three experiments. (D) Casp1 activity was measured in the above fractions using a WEHD enzymatic assay. (E) Control, LPS, and LPS + Z-VAD MPs from THP-1, CAS9, and CAS9/Casp1-KO cells were then subjected to HPMVEC and analyzed for cell death using trypan blue, and cell viability was analyzed using an MTS assay. Data are representative of three experiments. CE = cell extracts; EGM2 = endothelial cell growth medium 2; O.D. = optical density; RPMI = RPMI1640.
These observations suggest that active GSDMD regulates MP release and requires caspase 1 to be able to induce cell death. To further test this hypothesis, we repeated cell death experiments using control and LPS MPs in the presence or absence of Y-VAD (specific caspase 1 inhibitor) and Z-VAD (pan-caspase inhibitor). As shown in Figure 2B, both Y-VAD and Z-VAD completely abrogated the LPS MP–mediated cell death. The nonmicroparticulate fractions (LPS non-MPs) did not induce cell death, providing a specificity control (Figure 2B). To confirm that GSDMD association with active caspase 1 is critical to induction of cell death, untransformed THP-1 cells, CAS9-expressing THP-1 cells, and CAS9/Casp1-KO THP-1 cells were either left untreated or stimulated with LPS in the presence or absence of Z-VAD. Cell extracts (CE) were first tested for the presence of caspase 1 and GSDMD. No caspase 1 was detected in the CAS9/Casp1-KO cells as compared with both regular THP-1 and CAS9 cells (Figure 2C). Although full-length GSDMD was detected in equal amounts in all three cell types, active p30 GSDMD was higher in the LPS CE and partially suppressed by Z-VAD. No cleaved p30 GSDMD was detected in the CAS9/Casp1-KO CE, suggesting that GSDMD cleavage is regulated by caspase 1 activation.
We then analyzed the MPs generated from these cells. Both active GSDMD and active caspase 1 (p20) were released in LPS MPs from THP-1 and Cas9 cells (Figure 2C, bottom panel). This release of active p30 GSDMD and caspase 1 was significantly inhibited by Z-VAD, although some amount of full-length GSDMD was still released in Z-VAD conditions. That this GSDMD cleavage was related to caspase 1 function was further supported by caspase 1 activity measurements (Figure 2D). Notably, neither full-length nor cleaved GSDMD was easily detected in the microparticles from CAS9/Casp1-KO cells, although full-length p52 GSDMD expression was not impaired in the CE (Figure 2C), suggesting that cleavage of microparticulate GSDMD depends on caspase 1 activation. Subjecting HPMVEC to these microparticles further confirmed the need for the caspase 1–GSDMD association for cell death induction. As expected, LPS MPs containing both active caspase 1 and active GSDMD from THP-1 and CAS9 cells induced significant cell death (Figure 2E). MPs induced by LPS treatment in the presence of Z-VAD did not induce cell death. MPs from CAS9/Casp1-KO cells also did not induce HPMVEC cell death (Figure 2E), further confirming the role of caspase 1 in the regulation of GSDMD activation, release of MPs, and thereby induction of cell death.
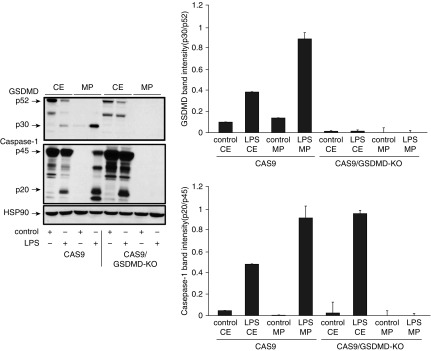
To test active GSDMD’s ability to regulate the release of microparticles containing active caspase 1 from stimulated monocytes, CAS9-expressing and CAS9/GSDMD-KO THP-1 cells were either left untreated or stimulated with LPS (as described in previous experiments), and CE and MP fractions were analyzed for active caspase 1 and GSDMD. As shown in Figure 3, CE from both CAS9- and GSDMD-KO cells detected active p20 caspase 1 upon LPS stimulation, indicating that knocking out GSDMD did not impair LPS-mediated caspase 1 activation. However, when MPs were analyzed, no active caspase 1 was detected in LPS MPs from CAS9/GSDM-KO cells as compared with LPS MPs from Cas9 cells (Figure 3, middle panel), confirming the role of active GSDMD in regulating the release of MPs encapsulating caspase 1.
Figure 3.
GSDMD regulates release of microparticulate active caspase 1. CE and MP fractions were isolated from CAS9 and CAS9/GSDM-knockout (CAS9/GSDMD-KO) cells in LPS (1 μg/ml) for 2 hours or were left untreated and analyzed for the presence of cleaved GSDMD and active p20 Casp1. Hsp90 was used as a loading control. Data and densitometric scan of immunoblot are representative of three experiments.
To determine whether caspases 4 and 5 could regulate MP-mediated cell death, MPs from either THP-1– or CAS9/Casp1-KO cells were analyzed for the presence of these proteins. As shown in Figure E3, Casp4, Casp5, and lamin B were not detected in either control MP or LPS MP cells, although these proteins were clearly detected in the cell extracts. This finding suggests that although caspases 4 and 5 are known to be able to cleave GSDMD, these caspases are not involved in MP-mediated cell death. Lamin B blots document separation of MPs from cell nuclei.
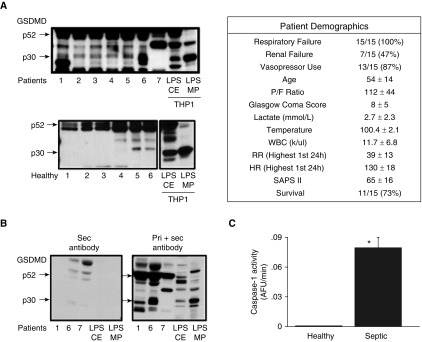
GSDMD has been linked to various diseases, including sepsis (14, 19, 24–27). Researchers in our laboratory previously described the presence of circulating microparticulate active caspase 1 in septic patient plasma and demonstrated a possible correlation of circulating caspase 1 with lymphocyte cell death in septic patients (12). Therefore, we performed a pilot study using plasma from either healthy donors or septic patients with acute respiratory distress syndrome (ARDS). Six plasma samples from healthy donors and seven plasma samples from patients with ARDS were collected during Day 1 of ICU admission and were subjected to microparticle isolation. As shown in Figure 4A, cleaved p30 GSDMD was detected in varying amounts in patients with ARDS. Almost no detectable p30 GSDMD was observed in healthy plasma MPs. MPs were loaded on the basis of protein quantification, which we have found to be representative of MP numbers for analytical purposes (Figure E4). To confirm the specificity of the GSDMD band detected in the patient samples, MPs isolated from three patients and LPS CE and LPS MPs from THP-1 cells (used as markers) were analyzed by either regular immunoblotting (using both primary and secondary antibodies) or with secondary antibody alone. As shown in Figure 4B, both pro-GSDMD (p52) and cleaved p30 GSDMD bands were detected during regular immunoblotting, whereas both bands were completely absent when only secondary antibody was used. This confirmed that the p30 cleaved GSDMD band that was observed only in patient samples and not in healthy donors was not due to detection of human IgG by the secondary antibody. Caspase 1 activity was also measured from these MPs. When compared between healthy donors and patients with ARDS, circulating microparticulate caspase 1 activity was also significantly increased in samples from patients with ARDS as compared with those from healthy donors (P < 0.015) (Figure 4C).
Figure 4.
p30 GSDMD is released in plasma MPs during sepsis. MPs were isolated from plasma of healthy donors (n = 6) and septic patients with acute respiratory distress syndrome on Day 1 of ICU admission (n = 7). (A) Isolated plasma MPs were analyzed for the presence of GSDMD using immunoblotting. LPS CE and LPS MPs from THP-1 cells stimulated with LPS for 2 hours were used as markers. (B) Plasma MPs from patients 1, 6, and 7, as well as LPS CE and MPs, were analyzed by immunoblotting for GSDMD using either secondary antibody alone (left panel) or standard primary and secondary antibodies (right panel). (C) Casp1 activity was measured in healthy donor and patient samples. Casp1 activity was found to be significantly higher (*P < 0.015) in septic patients than in healthy donors. HR = heart rate; P/F = PaO2/FiO2 ratio; RR = respiratory rate; SAPS II = Simplified Acute Physiology Score II; WBC = white blood cell count.
Discussion
Vascular injury is the central component of acute lung injury (ALI)/ARDS and is a hallmark of disease progression. Our in vitro model of HPMVEC monocyte interactions was developed to study the potential role of microparticles as mediators of vascular injury. MPs are known to carry various factors and proteins upon release by activated or apoptotic cells (1–9). For example, monocyte/macrophage-derived microparticles have been shown to transport phosphatidylserine and tissue factor (8, 28–31). We have described that packaging of exogenous caspase 1 into microparticles (11, 12) regulates endothelial cell injury (10). On the basis of the present work, we provide clear evidence that cleaved GSDMD is released into supernatants of LPS-stimulated THP-1 cells and that this exogenous GSDMD is packaged into microparticles together with the inflammasome proteins active caspase 1 and ASC. Although GSDMD was found to be released by control THP-1, GSDMD colocalized with active caspase 1 only under stimulated conditions. This colocalization of GSDMD in MPs was confirmed by immunoblotting as well as fluorescence confocal microscopy.
Recent studies have identified GSDMD protein as critical to pyroptosis (14–17, 19, 20). Several gasdermins are associated with genetic diseases, but their function and activation mechanisms are still unknown. Besides GSDMD, the gasdermin family includes other GSDMs that are insensitive to inflammatory caspases (21). Gasdermin has been shown to associate with the plasma membrane, resulting in its permeabilization and pore formation (22, 23). Current concepts suggest that caspase 1/5 cleaves GSDMD such that the p30 amino terminus now exposes a hydrophobic face that interacts with membrane lipids, forming a pore (17, 19, 22). This pore formation capacity of GSDMD has been shown to regulate cell death by compromising the integrity of the cell membrane. By dividing MPs into membrane and content components, we show that p30 GSDMD is in fact associated with the membrane of the microparticles together with p20 active caspase 1 after LPS challenge. We further demonstrate that this release of microparticulate GSDMD is regulated by caspase 1. Knocking down caspase 1 using the CRISPR/CAS9 system completely abrogated the cleavage and release of p30 GSDMD, as well as affected the release of microparticles in general. This suggests that caspase 1 cleaves GSDMD to its active form, which leads to packaging of p30 GSDMD into microparticles.
The mechanism by which GSDMD triggers pyroptosis is beginning to be understood. As described earlier, GSDMD regulates cell death by forming pores that compromise the integrity of the cell membrane and release of calcium (21–23). In our previous work, we have described the novel role of caspase 1 in splenic B-lymphocyte apoptosis and sepsis survival (12, 13), in smooth muscle cell apoptosis in atherosclerosis (11), and in endothelial cell death (10). The present study provides evidence that the encapsulation of active caspase 1 into MPs is dependent on GSDMD. Thus, a cooperation between caspase 1 and GSDMD drives exogenous caspase 1 release in MPs and thereby induction of cell death by MP-receiving cells. We demonstrate that LPS MPs containing both GSDMD and active caspase 1 induced HPMVEC death, whereas the MPs from control cells or cells treated with caspase inhibitor Z-VAD did not. Although both control MPs and non-MP fractions from control and LPS-stimulated conditions contained GSDMD, neither was able to induce HPMVEC death. This association of GSDMD with MPs is abrogated by inhibition of caspase 1 activity or absence of caspase 1, as seen in the caspase 1–KO model. The absence of GSDMD-containing MPs in caspase 1–KO cells leads us to believe that caspase 1 regulates the packaging of GSDMD in MPs, which in turn may regulate the release of these microparticles. This may be due to the fact that p30 GSDMD activation by caspase 1 is required for the formation and/or release of MPs containing GSDMD. We believe that GSDMD’s pore-forming capacity may be critical to the ability of MPs to target cells. The absence of caspase 1, as seen in CRISPR/CAS9/Casp1 KO, abrogates the necessity of this encapsulation of GSDMD into MPs and thereby its release. This was further confirmed by our observation that knocking down GSDMD using the CRISPR/CAS9 system completely abrogated the release of active caspase 1–containing microparticles, although activation of caspase 1 was not affected in the CE upon stimulation. Further support for the role of GSDMD in assisting caspase 1 in the cell death of targeted cells is the fact that although some amount of p30 GSDMD is detected in control MPs (probably due to autoactivation), control MPs were unable to induce cell death. Thus, we hypothesize that the presence of active caspase 1 together with GSDMD encapsulated in microparticles is essential for apoptosis induction of endothelial cells. This concept is also supported by our observation that although non-MP fractions contain active GSDMD, they do not induce cell death. We suggest that, taken together, our data show that microparticulate caspase 1 induces cell death and that GSDMD assists in the process, possibly by assisting the MPs in release of and/or fusion with cells or by allowing release of p20 caspase 1 from MPs.
Much remains to be understood about the relationship between GSDMD and caspase 1 in microparticles. It will be important to determine whether p30 GSDMD creates pores in MPs themselves. If so, do these pores provide a conduit for the transfer of active p20/p10 tetramers to MP-targeted cells? Also, why are MPs not induced to rupture in the presence of p30 GSDMD? Possibly, MPs are somehow protected from GSDMD pore-mediated rupture owing to constraints on membrane stress as a result of the small radius of MPs or because of unique aspects of the MP cytoskeleton. Furthermore, a critical role of caspase 1 in membrane reconstitution has been shown previously, which may be important in the replenishment of MP membranes (32). Clearly, the present findings support the need for more specific questions about the GSDMD-caspase 1 relationship in MPs.
GSDMD has been described to be involved in immune regulation in various inflammatory disease models, including sepsis (19). When septic patients were analyzed for the presence of circulating microparticulate GSDMD, significantly higher concentrations of p30 GSDMD and active caspase 1 were detected in MPs isolated from plasma of septic patients with ARDS than those in healthy control subjects. Our previous work had described elevated concentrations of microparticulate active caspase 1 in septic patients (12). That GSDMD and active caspase 1 concentrations are also significantly higher in plasma MPs from septic patients further supports our hypothesis that GSDMD regulates release of circulatory microparticulate caspase 1 and thereby cell death.
We conducted a pilot study with only seven patient samples. Researchers at our laboratory are currently working on actively recruiting patient samples as well as generating sensitive alternative detection techniques to screen patient samples from limited sample volumes. Further investigation of that aspect of GSDMD kinetics is needed. Much remains to be understood about the role of GSDM in sepsis and the pathobiology of the critical role of caspase 1 and pyroptosis in ALI- or sepsis- induced endothelial dysfunction. Notably, caspase 1–deficient mice have been reported to have a prolonged inflammatory response, suggesting more pronounced or longer inflammation (33). However, in our previous work, we have shown that although systemic Escherichia coli infections are relatively benign in caspase 1–KO animals (13), there was a relationship between pyroptotic death of cells (albeit in the spleen) and survival. Researchers at our laboratory are actively working on experiments involving in vivo studies to evaluate the pathogenic effect of microparticle-encapsulated GSDMD in models of sepsis (e.g., systemic or pulmonary LPS). However, there are many limitations to performing such studies using MPs. The generation and purification of sufficient numbers of mouse-specific MPs is a technical challenge that we are currently attempting to surmount.
In summary, the present work provides evidence that cleaved GSDMD is released from cells packaged in microparticles together with active caspase 1 and other inflammasome proteins such as ASC. This cleavage of p30 GSDMD is likely essential for the formation of caspase 1–encapsulated MPs that are capable of inducing pyroptosis of affected cells.
Conclusions
Although much is known about the pathogenesis of lung cell injury and death in ALI/ARDS, knowledge gaps remain. Our present work provides evidence that GSDMD is packaged in microparticles together with active caspase 1 and released by LPS-stimulated THP-1. This association of GSDMD and active caspase 1 can induce HPMVEC death. We believe that GSDMD encapsulation in microparticles together with caspase 1 may be essential for effective release of caspase 1–encapsulated MPs from monocytes to vascular cells, thereby regulating caspase 1–mediated endothelial cell death. Significantly higher concentrations of microparticulate GSDMD were detected in septic patients with ARDS. We report that, taken together, our data show that microparticle-encapsulated GSDMD plays a critical role in caspase 1–mediated endothelial cell injury.
Footnotes
Supported by National Institutes of Health (NIH) grants R01HL24325 (A.S.) and R01GM108928 (A.S. and M.D.W.).
Author Contributions: A.S.: conception of hypothesis, design and acquisition of data, analysis and interpretation, and drafting manuscript for intellectual content; M.D.W.: involved in analysis and interpretation as well as drafting of the manuscript; S.M.: design and acquisition of data; M.E.: acquisition of patient samples; F.H.: fluorescence microscopy; and M.A.G., P.J.B., and S.L.M.: generation of CRISP/CAS9 cells.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0393OC on January 24, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hugel B, Martínez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 2.Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–690. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 3.Martínez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288:H1004–H1009. doi: 10.1152/ajpheart.00842.2004. [DOI] [PubMed] [Google Scholar]
- 4.Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 5.Martin S, Tesse A, Hugel B, Martínez MC, Morel O, Freyssinet JM, et al. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–1659. doi: 10.1161/01.CIR.0000124065.31211.6E. [DOI] [PubMed] [Google Scholar]
- 6.Distler JH, Huber LC, Hueber AJ, Reich CF, III, Gay S, Distler O, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–741. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 7.Huber LC, Jüngel A, Distler JH, Moritz F, Gay RE, Michel BA, et al. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007;12:363–374. doi: 10.1007/s10495-006-0622-7. [DOI] [PubMed] [Google Scholar]
- 8.McKechnie NM, King BCR, Fletcher E, Braun G. Fas-ligand is stored in secretory lysosomes of ocular barrier epithelia and released with microvesicles. Exp Eye Res. 2006;83:304–314. doi: 10.1016/j.exer.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe J, Marathe GK, Neilsen PO, Weyrich AS, Harrison KA, Murphy RC, et al. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J Biol Chem. 2003;278:33161–33168. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]
- 10.Mitra S, Wewers MD, Sarkar A. Mononuclear phagocyte-derived microparticulate caspase-1 induces pulmonary vascular endothelial cell injury. PLoS One. 2015;10:e0145607. doi: 10.1371/journal.pone.0145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4:e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Exline MC, Justiniano S, Hollyfield JL, Berhe F, Besecker BY, Das S, et al. Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis. PLoS One. 2014;9:e90968. doi: 10.1371/journal.pone.0090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, et al. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1β and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Gao W, Shao F. Pyroptosis: gasdermin mediated programmed necrotic cell death. Trends Biochem Sci. 2016;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Man SM, Kanneganti TD. Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res. 2015;25:1183–1184. doi: 10.1038/cr.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 19.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, He WT, Hu L, Li J, Fang Y, Wang X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HY, Lin PH, Wu SH, Yang LT. Inducible expression of gasdermin A3 in the epidermis causes epidermal hyperplasia and skin inflammation. Exp Dermatol. 2015;24:897–899. doi: 10.1111/exd.12797. [DOI] [PubMed] [Google Scholar]
- 25.Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89:618–629. doi: 10.1016/j.ygeno.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11:718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 27.Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J Immunol. 2016;197:1353–1367. doi: 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carta S, Tassi S, Semino C, Fossati G, Mascagni P, Dinarello CA, et al. Histone deacetylase inhibitors prevent exocytosis of interleukin-1β-containing secretory lysosomes: role of microtubules. Blood. 2006;108:1618–1626. doi: 10.1182/blood-2006-03-014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 30.Wewers MD. IL-1β: an endosomal exit. Proc Natl Acad Sci USA. 2004;101:10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 32.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Rowe SJ, Allen L, Ridger VC, Hellewell PG, Whyte MK. Caspase-1-deficient mice have delayed neutrophil apoptosis and a prolonged inflammatory response to lipopolysaccharide-induced acute lung injury. J Immunol. 2002;169:6401–6407. doi: 10.4049/jimmunol.169.11.6401. [DOI] [PubMed] [Google Scholar]