Abstract
Background:
Catheter-related blood stream infections (CRBSI) are an important complication of central venous access devices but are often poorly measured. This article describes the journey of one hospital trust to set up a surveillance process for CRBSI across all specialties of the trust and to reduce CRBSI.
Method:
Using a locally adapted CRBSI criteria and root cause analysis (RCA) for investigation we identified a number of opportunities for a quality improvement programme.
Findings:
Over a 5-year period we saw a significant and sustained reduction in the rate of CRBSI from 5 per 1000 catheter days to 0.23 per 1000 catheter days.
Conclusions:
The surveillance enabled rates of CRBSI to be monitored across the trust and the success of our improvements to be measured.
Keywords: Catheter-related blood stream infections, central line-associated blood stream infections, line surveillance, CRBSI, CLABSI, quality improvement
Introduction
Catheter-related blood stream infections (CRBSI) are a major cause of healthcare-associated bacteraemia, but it is suggested that many of these CRBSI are preventable using evidence-based care (Han et al., 2010; Loveday et al., 2014). CRBSI are costly and associated with a poor prognosis (Tacconelli et al., 2009), causing increased mortality and morbidity in patients (Han et al., 2010; Zingg et al., 2009). Extended length of stay for patients who develop a CRBSI can be in the range of 2–14 days (Tacconelli et al., 2009) with a rate of CRBSI varying between < 1 and > 10 per 1000 catheter days (Han et al., 2010).
Surveillance of central vascular access devices (CVAD)-related infections varies among units (Health Foundation, 2013), with some units not collecting data (Tacconelli et al., 2009). CRBSI and central line-associated blood stream infections (CLABSI) are interchangeably used and can be confusing; CRBSI is a diagnostic tool whereas CLABSI is a surveillance tool and likely to identify a greater number of cases that may not actually be line-related (Fronzo, 2017; O’Grady et al., 2011).
The CRBSI definition is used to diagnose and treat patients who develop an infection with a CVAD in place. It relies on specific laboratory tests to include quantitative culture of the catheter tip or the time differences in growth between catheter and peripheral blood culture specimens. In comparison, the CLABSI definition is used for surveillance purposes and assumes that the presence of a blood stream infection in a patient with a CVAD in the absence of any other identified source will be attributed to the central line (O’Grady et al., 2011).
In 2009, the Matching Michigan campaign was launched in England; this was a national project focused on critical care units to reduce central line infection rates and based on the successful work achieved in Michigan (Pronovost et al., 2006). The campaign focused on the implementation of a bundle with five evidence-based interventions consisting of hand hygiene, full barrier precautions, skin preparation using Chlorhexidine, avoidance of the femoral vein and prompt removal of lines when no longer required using CLABSI as a measure of improvement (Bion et al., 2013).
Background
The Matching Michigan methodology was established in our local critical care units in 2010. In 2011 it was agreed that the same methodology should be extended to all patients with a CVAD in the trust. The trust is a large district general in the north of England with 850 beds including 14 intensive care beds, inpatient oncology and haematology service and a neonatal unit. Patients with CVADs are cared for in many different wards and departments outside of critical care. In addition, the trust has responsibility for community services that care for patients with CVAD in their own home.
The overall aim of the improvement work was to reduce CRBSI in our trust but it was seen as a complex project covering many departments when only the critical care and neonatal units were already collecting surveillance data.
Methodology
First, a CVAD Steering Group was set up to develop the CRBSI surveillance and improvement project. Representatives from all areas involved with insertion and ongoing management of CVAD were invited to join the group; these included critical care, the neonatal unit, and the oncology and haematology unit. Representatives from community nursing, Out-patient Antimicrobial Therapy (OPAT) service and radiology were identified later to join the group. The group was led by a senior nurse, included the lead clinician for CVAD and a medical microbiologist and had a total membership of ten.
With any quality improvement programme it is important from the outset to establish an outcome measure that can be used to demonstrate improvements in practice. For this programme CRBSI was chosen (Gorski et al., 2016). Measurement of CLABSI was already established within the critical care areas, as they were involved with the Matching Michigan programme (Bion et al., 2013); however systems for capturing this data for other areas needed to be established.
Recognising the differences between CRBSI and CLABSI definitions, neither definition appeared to capture the information for measuring our improvement. The CRBSI definition was too narrow and would miss some potential line infections and CLABSI was too broad and would include infections not related to the CVAD. Initially, the Centers for Disease Control and Prevention (CDC) CRBSI criteria (Association for Professionals in Infection Control and Epidemiology, 2009) was adapted to include cases of blood stream infections (BSI) which the microbiologist and clinical team agreed were probable cases. These included cases where laboratory testing could not provide a definite confirmation of CRBSI and there was no other obvious source for the BSI other than the CVAD. The local criteria were later revised in line with the European Centre for Disease Prevention and Control (2016) guidance. Therefore, CRBSI in this paper refers to our locally developed criteria (Table 1).
Table 1.
CRBSI adapted criteria.
|
Definite CRBSI - criteria require one of the
following: • Bloodstream infection (BSI) occurring 48 h before or after catheter removal AND differential period of CVC culture vs. peripheral blood culture positivity of > 2 h • BSI occurring 48 h before or after catheter removal AND positive culture with the same microorganism from pus from insertion site • BSI semi-quantitative CVC culture > 15 CFU and clinical signs improve within 48 h after catheter removal Probable CRBSI • BSI occurring 48 h before or after catheter removal which does not fulfil the definitions above but there is no alternative source of infection. This needs agreement between microbiologist and the patient’s clinical team BSI defined as: One positive blood culture for a recognised pathogen or patient has at least one of the following signs or symptoms: fever (> 38 °C), chills or hypotension AND two positive blood cultures for a common skin contaminant (from two separate blood samples, usually within 48 h) Skin contaminants = coagulase-negative staphylococci, Micrococcus sp., Propionibacterium acnes, Bacillus sp., Corynebacterium sp. |
Potential CRBSI are reported by the microbiologists following laboratory confirmation of a BSI in all patients with a CVAD. Cases are excluded from the numerator if, after a clinical review, an alternative source of infection is identified. Cases where our trust had no involvement in the care and management of the CVAD, such as some haematology or renal patients, are also excluded although the relevant provider teams are informed.
Although this method relied on the microbiologist reporting the infections, it meant that there was timely reporting. In addition, monthly laboratory reporting was also introduced. This provided a monthly report of cases that met the criteria of positive blood cultures taken from patients with a CVAD; these were all checked to ensure no cases were missed and provides validation of the numerator data. It was evident from this validation process that the reporting by the microbiologists has greatly improved since the start of the data collection in 2011.
To calculate the rate of CRBSI, both numerator data (number of CRBSI) and denominator data (number of line days) needed to be captured. The rate of CRBSI is calculated dividing the numerator data by the denominator data and multiplying by 1000 (Gorski et al., 2016).
At the start of the quality improvement programme, we used the numerator data as the outcome measure recording this as ‘days between infections’ until we established the methods to obtain the denominator data to calculate the CRBSI rate.
Developing a process to collect the number of CVAD days with all the different teams managing the care of CVAD was difficult and relied on manual collection of data in each of the departments. Although we explored some methods of electronic data capture, these were costly as we had no systems already in use at that time in the trust that would be suitable for this type of data collection. Although time-consuming for the wards and departments to collect the numerator data, once they were on board, they started to provide the data.
Establishing the process to collect denominator data for patients in the community with CVAD was much more problematic. Difficulties arose due to patients discharged to community teams from a number of different providers and, therefore, data from community patients are collected but there continues to be some gaps in the denominator data.
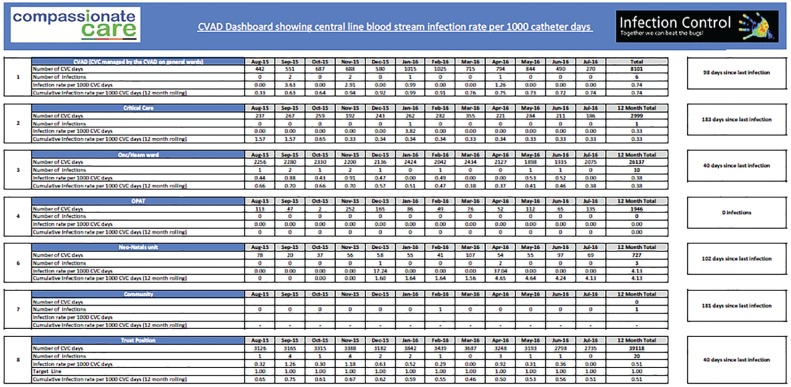
A CVAD dashboard is produced monthly by the health informatics team using the numerator and denominator data to calculate the CRBSI rate per 1000 CVAD days. The dashboard (Figure 1, a more detailed version of Figure 1 can be found online, supplementary material) displays both the CRBSI rate and also the days between infections for the whole trust and separately for each of the individual areas.
Figure 1.
CVAD dashboard.
The focus of the quality improvement work was identified from the root cause analysis. Using a Model for Improvement approach with small tests of change (Plan, Do, Study, Act (PDSA) cycles a number of improvements were achieved as described below (Health Foundation, 2016; Institute for Healthcare Improvement, 2017).
Root cause analysis
Root cause analysis is a formal investigation of an adverse event and one of the methods that can be used to improve patient safety in hospitals (Tamuz and Harrison, 2006). It was important to be able to investigate all cases of CRBSI, both suspected and confirmed, in order to learn and improve.
The clinical teams were encouraged to lead the investigation of all suspected CRBSI by compiling a timeline of relevant events from line insertion to the development of the infection. This timeline was then analysed by the clinical team with support from the infection prevention and control team to look for any gaps in care. If gaps in care were identified, then further work was undertaken to understand why the lapse had occurred.
In addition, to the request for an RCA investigation, these adverse events were reported on the Datix incident management system within the trust. This ensured that the information, investigation and any corrective actions were formally captured. Following the investigation, the key learning was shared with the CVAD Steering Group to focus improvement initiatives.
Improvements in practice
One of the first learning opportunities observed was the lack of standardised care plans and pathways for patients with a CVAD. There was good documentation that had been developed within the critical care units as part of the Matching Michigan project, but this was not used for patients outside of critical care, even if the patient had been transferred from critical care to a ward. There was different documentation used for patients with CVADs in Oncology, Cardiology and general wards.
The CVAD group developed a standardised CVAD pathway for all patients with a CVAD. This included the insertion and ongoing management elements from the Matching Michigan care bundle. A PDSA cycle was used to develop the CVAD pathway, drafting the pathway and testing with one patient and one member of staff. This enabled several changes to be made following feedback from the frontline staff. Testing continued with more patients, modifying the pathway each time, until the frontline staff felt it was ready to be rolled out. This was eventually adapted for use with neonates.
Initially, it was noted that the investigation teams were basing their analysis of events on merely measuring that all the elements of the documentation were completed and not considering wider issues such as the insertion site or tip position of the CVAD. An investigation standard was developed by the CVAD Steering Group to assist the clinical team with their investigation and critical analysis of the relevant events leading to the CRBSI. The standard was based on the evidence available to support best practice as it was acknowledged that not all members of the investigation team had a good knowledge of the whole CVAD policy.
Feedback from investigations to the CVAD Steering Group also identified inconsistencies with training and competency assessments of staff managing CVADs. The critical care team and the oncology team worked closely together to produce a standardised training package with competency assessments. A competency matrix was created to provide assurance of the level of trained and competent staff accessing and managing CVADs.
The learning from the RCA also prompted regular audit of compliance in documentation of the CVAD pathway. This was not only an assurance process but also provided the opportunity to revise and amend the documentation where issues arose; for example, the original pathway did not provide space to document the reasons for dressing change.
Frequent dressing changes were identified from RCA investigations and appeared to be a risk factor in developing CRBSI. Importantly, we needed to understand why some patients were having frequent dressing changes; this was a problem mainly confined to patients in the critical care unit with internal jugular (IJ) lines and high levels of oropharyngeal secretions. The critical care sister led a trial of different dressings to find more adherent dressing suitable for her patients. The placement of IJ lines also prompted discussions among the intensivists to promote subclavian insertion of short-term CVAD as opposed to IJ insertion.
The change of dressings, again using PSDA cycles, allowed each area to test the suitability of the change of dressing for their patient group allowing eventual standardised of dressings across all areas (excluding neonates) using a dressing with a Chlorhexidine island.
The investigation of a number of Methicillin-sensitive Staphylococcus aureus (MSSA) CRBSI gave the CVAD Steering Group the opportunity to review the CVAD policy to include MSSA screening, in addition to Methicillin-resistant Staphylococcus aureus (MRSA) screening of patients before insertion of CVAD and at regular intervals. The use of daily chlorhexidine washes was extended from critical care patients to all inpatients with CVADs apart from neonates.
More recently, RCA investigation of CRBSI has identified a number of ‘non-compliance’ concerns in patients involved with the care of a long-term CVAD both in the community and in hospital. This revealed inconsistencies with the information provided to these patients with little assurance of their understanding of the information provided. A simple care plan was developed by frontline staff and, using a checklist approach of information, was provided to the patient with written confirmation of their understanding of the information given. This also includes observation of practice where this is applicable.
Results
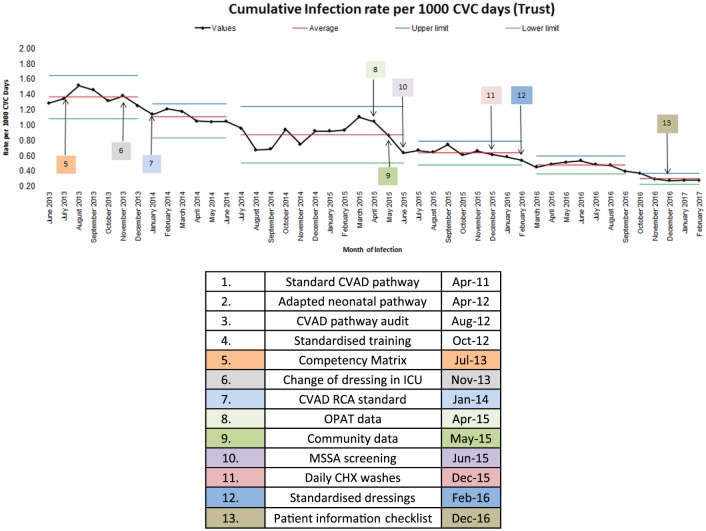
The CRBSI surveillance was able to demonstrate overall improvement with the rate of CRBSI reducing from 5 per 1000 line days in 2011 to 0.23 per 1000 line days in 2017. The data in Figure 2 illustrate the cumulative rate of CRBSI over a rolling 12 months from June 2013 showing the reduction of CRBSI from 1.29 to 0.23 per 1000 line days. Although the data between July 2011 and May 2013 suggested the greatest reduction in CRBSI, because of inconsistencies in reporting of the both the numerator and denominator data, this data has not been displayed.
Figure 2.
Cumulative CRBSI rate.
The various improvement interventions have been annotated on the statistical process control (SPC) chart (Figure 2) comparing the interventions alongside the rate of CRBSI to show the journey of improvement. Step changes on the SPC chart are noted following the change of CVAD dressing in ICU patients (October 2013), the introduction of a written standard for RCA (January 2014), MSSA screening (June 2015) and standardisation of dressings (June 2015).
In addition, an internal ceiling target of 1.5/1000 line days was set in 2013 using the Matching Michigan target of 1.4 as a benchmark but taking into consideration that we were unable to find any CRBSI data that either matched our case definition or our patient population. This target was reduced to 1/1000 line days within 18 months to provide more of an internal focus and challenge.
Discussion
The results of this project have shown an overall reduction in CRBSI which compares favourably with documented levels of between < 1 to > 10 per 1000 catheter days (Hans et al., 2010). However, the authors acknowledge that it took almost two years to refine the process for collecting both the numerator and denominator.
There are some important limitations to this work that have been acknowledged. First, the process for collecting the denominator data has not been validated and relies on the various teams to collect the data. It is envisaged that we will eventually be able to validate the denominator data with our new electronic patient record system. The second important limitation is the difficulty of comparing rates of CRBSI with both other organisations or published literature because of differences in patient case-mix. Most of the literature looks at specific patient groups—such as critical care, neonatal or oncology patients—whereas we included all of these, as well as all patients with CVAD from community, general surgery, cardiology and general medical services.
Establishing the CVAD Steering Group with frontline staff who were able to lead change in their areas was an important factor in the success of this improvement project. Members of the Steering Group were able to review the learning from the RCA investigations providing understanding of the local problems that are important factors in quality improvement (Health Foundation, 2016) and moreover empowering them to make changes.
The standardisation of the CVAD pathway for insertion and management combined with audit of compliance along with the introduction of standard training and competency would appear to have made the greatest impact on our journey to reduce CRBSI. We observed, what we considered a high rate of CRBSI in July 2011, fall markedly in the first 12 months, although we were unable to reliably demonstrate this with our data. On reflection, the development of the standard CVAD pathway at the beginning of the project was one of the most difficult areas for improvement. The pathway included both the insertion and ongoing care of the CVAD and was developed predominantly by nurses. The anaesthetic doctors inserted the majority of the CVADs and were resistant to what they perceived as a ‘nursing document’. The key to the success of any quality improvement is engagement (Health Foundation, 2016) and we had failed to involve the medical staff in this important change. Involvement in the RCA process provided the opportunity to be included in further changes.
Measuring the improvement was difficult to establish at the start of the project and as a result we are unable to provide data from the start of our quality improvement journey. It was difficult for the frontline teams to see the need to collect the denominator data (line days) or for the microbiologists to remember to flag the potential CRBSI to the Infection Prevention and Control Team. As the project progressed, the importance of measurement and evaluation were acknowledged by those involved. While the process and measurement we have established is not suitable for benchmarking, it has provided us with one of the tools for improvement (Institute for Healthcare Improvement, 2017).
Adaption of the CRBSI locally provided us with consistency in measurement but allowed engagement of staff with what clinicians considered meaningful data. Use of the CLABSI definition (O’Grady et al., 2011) would have resulted, in our opinion, in clinicians disengaging with the data and the improvement work due to the broad criteria. Allowing clinicians to be involved with deciding whether to include potential BSI to be considered in the numerator enabled ownership of the data.
Celebrating each of the successes on this journey has been an important part of the leadership of the project. Displaying each of the team’s data as ‘days between’ CRBSI provides a positive measure and had been successful in motivating and energising the teams it was therefore continued, even after we had established the denominator data.
Arguably, the lack of national targets for CRBSI has allowed this project to be owned by frontline staff and not dictated by senior managers. There is evidence that involvement of frontline staff to make changes rather than a top-down approach provides more sustainable improvement (Health Foundation, 2016). We consider that this engagement has been the greatest benefit to our project. Providing staff with the knowledge, skills and experience of quality improvement and an established surveillance process has allowed continuous improvement. Interestingly, without the mandate for national surveillance of CRBSI, it appears that many trusts do not invest in the time to collect such data.
Conclusions
During the last five years, we have been successful in establishing robust processes to identify definite and probable cases of CRBSI within our trust. Investigation of these in real time has led to improvement in all aspects of CVAD care which has manifested as a sustained reduction in the number of CRBSI cases across all relevant areas.
We are able to monitor the rate of CRBSI for each department with patients and can accurately assess problems and identify trends at an early stage to implement effective change as required. However, the locally developed criteria for CRBSI makes it difficult to benchmark our data with other hospitals, particularly as our rate this includes patients with CVAD in critical care, neonatal unit, oncology and haematology units, general wards, community and OPAT.
Setting up the measurement for CRBSI was difficult, but failure to measure and investigate CRBSI would not have allowed us to identify the problems and, most importantly, to improve practice and provide safer care for our patients with CVADs.
Supplemental Material
Supplemental material, JIP767759_Suppl_Mat for Establishing catheter-related bloodstream infection surveillance to drive improvement by Carole Hallam, Tim Jackson, Anu Rajgopal and Belinda Russell in Journal of Infection Prevention
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
References
- Association for Professionals in Infection Control and Epidemiology. (2009) Guide to the Elimination of Catheter-Related Bloodstream Infections. Washington, DC: APIC; Available at: http://www.apic.org/Resource_/EliminationGuideForm/259c0594–17b0–459d-b395-fb143321414a/File/APIC-CRBSI-Elimination-Guide.pdf (last accessed 29th January 2017). [Google Scholar]
- Bion J, Richardson A, Hibbert P, Beer J, Abrusci T, McCutcheon M, Cassidy J, Eddleston J, Gunning K, Bellingan Patten M, Harrison D. (2013) ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Quality & Safety 22: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. (2016) Point prevalence survey of healthcare- associated infections and antimicrobial use in European acute care hospitals – protocol version 5.3. Solna: ECDC; Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5–3.pdf (last accessed 11th November 2017). [Google Scholar]
- Fronzo C. (2017) Approaches for standardising best practice to reduce CRBSI and CLBSI. British Journal of Nursing 26: 19 S32–35. [DOI] [PubMed] [Google Scholar]
- Gorski L, Hadaway L, Hagle M, McGoldrick, Orr M, Doellman D. (2016) Infusion therapy standards of practice. Journal of Infusion Nursing 39 (1S): S1–S159. [DOI] [PubMed] [Google Scholar]
- Han Z, Liang S, Marschall J. (2010) Current Strategies for the prevention and management of central line-associated bloodstream infections. Infection and Drug Resistance 3: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Foundation. (2013) Lining up: How is harm measured? Lessons from an ethnographic research study of interventions to reduce central line infections. London: Health Foundation; Available at: http://www.health.org.uk/sites/health/files/LiningUpHowIsHarmMeasured.pdf (last accessed 29th January 2017). [Google Scholar]
- Health Foundation. (2016) Quality improvement made simple. London: Health Foundation. [Google Scholar]
- Institute for Healthcare Improvement. (2017) The model for improvement. Boston, MA: IHI; Available at: http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx (last accessed 29th January 2017). [Google Scholar]
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, Browne J, Prieto J, Wilcox M. (2014) epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals. Journal of Hospital Infection 86(Suppl. 1): S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady N, Alexander M, Burns L, Dellinger E, Garland J, Heard S, Lipsett P, Masur H, Mermel L, Pearson M, Raad I, Randolph A, Saint S, Rupp M. and the Healthcare Infection Control Practices Advisory Committee. (2011) Guidelines for the prevention of intravascular catheter-related infections. Clinical Infectious Diseases 52: e162–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. (2006). An intervention to decrease catheter-related bloodstream infections in the ICU. New England Journal of Medicine 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- Tacconelli E, Smith G, Hieke K, Lafuma A, Bastide P. (2009) Epidemiology, medical outcomes and costs of catheter-related blood stream infections in intensive care units of four European countries: literature- and registry-based estimates. Journal of Hospital Infection 72: 97–103. [DOI] [PubMed] [Google Scholar]
- Tamuz M, Harrison M. (2006) Improving patient safety in hospitals: contributions of high-reliability theory and normal accident theory. Health Services Research 41: 1654–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg W, Sax H, Inan C, Cartier V, Diby M, Clergue F, Pitter D, Walder B. (2009) Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. Journal of Hospital Infection 73: 41–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JIP767759_Suppl_Mat for Establishing catheter-related bloodstream infection surveillance to drive improvement by Carole Hallam, Tim Jackson, Anu Rajgopal and Belinda Russell in Journal of Infection Prevention