Version Changes
Revised. Amendments from Version 1
We have conducted minor changes to the phrasing of the manuscript regarding projections of MSA and the expected increase in MRAD, and corrected the spelling of "Calment". We have also added Supplementary Figure S1 and Supplementary Figure S2 with reference in the text, initially embedded in the supplementary material code, to address the concerns and comments of reviewers.
Abstract
We respond to claims by Dong et al. that human lifespan is limited below 125 years. Using the log-linear increase in mortality rates with age to predict the upper limits of human survival we find, in contrast to Dong et al., that the limit to human lifespan is historically flexible and increasing. This discrepancy can be explained by Dong et al.’s use of data with variable sample sizes, age-biased rounding errors, and log(0) instead of log(1) values in linear regressions. Addressing these issues eliminates the proposed 125-year upper limit to human lifespan.
Keywords: lifespan, human lifespan, contradictory findings, ageing, life history, refutation
Recent findings by Dong et al. 1 suggested fixed upper limits to the human life span. Using the same data, we replicated their analysis to obtain an entirely different result: the upper limit of human life is rapidly increasing.
Dong et al. conclude that the maximum reported age at death (MRAD) is limited to 125 years in humans 1 and that lifespan increases above age 110 are highly unlikely, due to the reduced rate of increase in life expectancy at advanced ages.
We repeated Dong et al.’s 1 analysis using identical data (SI). Replicating these findings requires the inclusion of rounding errors, treating zero-rounded values as log(1) and the incorrect pooling of populations.
The Human Mortality Database ( HMD) data provide both the age-specific probability of survival ( q x) and the survival rates of a hypothetical cohort of 100,000 individuals ( l x). However, l x survival rates are rounded off to the nearest integer value.
The magnitude and frequency of l x rounding errors increases as the probability of survival approaches 1 in 100,000. These rounding errors mask variation in survival rates at advanced ages: over half of l x survival data are rounded to zero above age 90 ( Figure 1b).
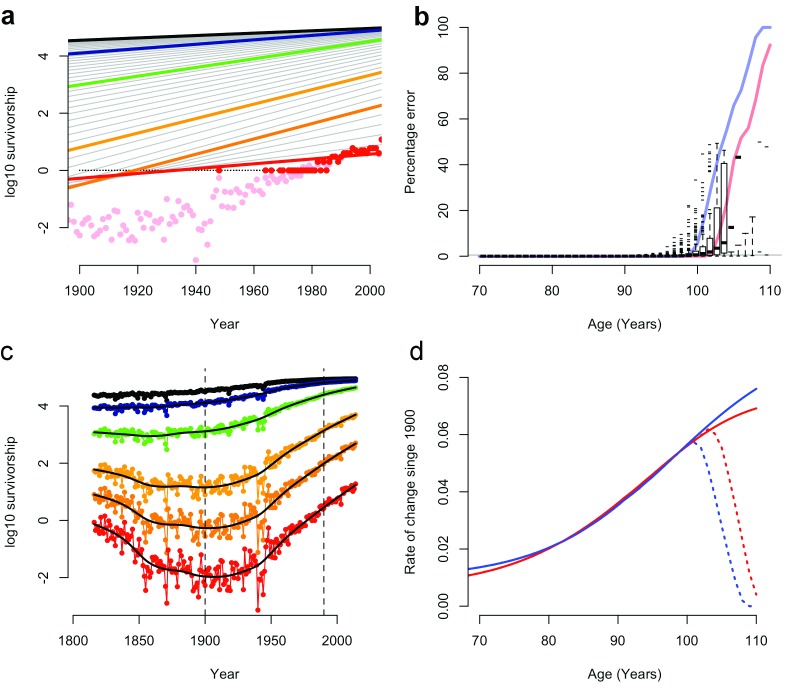
Figure 1. Rate of change in late-life survival for the French population 1816-2014.
( a) Figure modified after Dong et al. Figure 1b, showing rounded survival data (red points), rounded survival data with log(0)=log(1) (black points), the resulting linear regression in Dong et al. (solid red line) and observed survival data (pink points). ( b) Rounding errors in survival data (box-whisker plots; 95% CI) and the proportion of survival data rounded to zero in males (blue line) and females (red line). ( c) Survival data from ( a) with rounding errors removed, showing variation outside the 1900-1990 period (vertical dotted lines). ( d) The rate of change in late-life mortality since 1900 with (dotted lines) and without (solid lines) rounding errors (after Dong et al. Figure 1c).
Dong et al. appear to have used these rounded-off survival data in their models 1 and incorrectly treated log(0) values as log(1) in log-linear regressions ( Figure 1a–d; SI).
These errors have considerable impact. Re-calculating cohort survival from raw data or excluding zero-rounded figures eliminates the proposed decline in old-age survival gains ( Figure 1d; SI).
Likewise, recalculating these data removed their proposed limits to the age of greatest survival gain (SI), which in 15% of cases were the result of the artificial 110-year age limit placed on HMD data 2.
We also found that variation in the probability of death was masked by date censoring 1. Major non-linear shifts in old-age survival occur outside the 1900–1990 period used by Dong et al. ( Figure 1c). Why these data were excluded from this regression, but included elsewhere, is unclear.
Evidence based on observed survival above age 110 appears to support a late-life deceleration in survival gains 1. For the period 1960–2005 Dong et al. present data 1 from 4 of the 15 countries in the International Database on Longevity 3 ( IDL). In their pooled sample of these countries, there is a non-significant (p=0.3) reduction in MRAD between 1995 and 2006 ( Figure 2a).
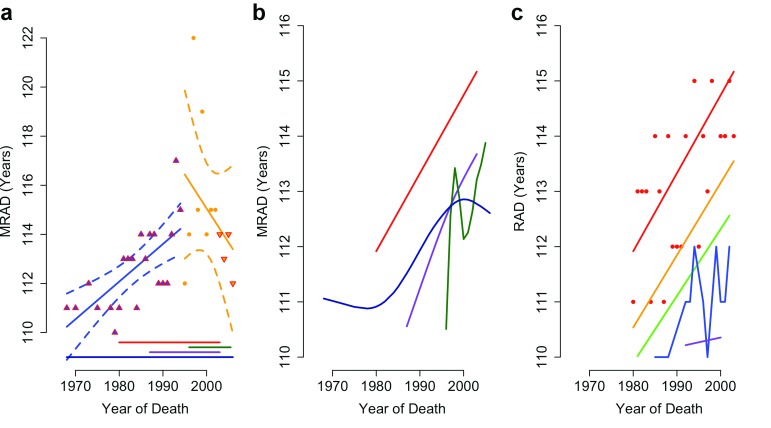
Figure 2. Maximum reported age at death of supercentenarians.
( a) Reproduction of Dong et al. Figure 2a, including 95% CI for increasing (p<0.0001) and falling (p=0.3) maximum recorded age at death (MRAD), showing data biased by the addition and removal (up and down arrows) of populations. ( b) Locally weighted smoothed splines of MRAD in Japan (green), the USA (red), the UK (dark blue) and France (purple). ( c) Locally weighted trends of MRAD in the USA across the oldest 5 reported ages at death (red, orange, green, blue and purple lines show rank 1–5 respectively).
The declining MRAD reported by Dong et al. 1 arises from the use of falling sample sizes. According to the Gerontology Research Group ( GRG), 62% of validated supercentenarians alive in 2007 resided in France and the USA. However, these countries are not surveyed 3 by the IDL after 2003 ( Figure 2a). The proposed post-1995 decline in MRAD results from this dramatic fall in sample size.
Viewed individually, all four countries have an upward trend in the mean reported age at death (RAD; Figure 2b) of supercentenarians (SI) and the top 5 ranked RADs ( Figure 2c). All four countries achieved record lifespans since 1995, as did 80% of the countries in the IDL. Without the pooling of IDL data used by Dong et al. there is no evidence for a plateau in late-life survival gains.
We attempted to reproduce Dong et al.’s supporting analysis of GRG records. The text and Extended Data Figure 6 of Dong et al. do not match annual MRAD records from 1972 as stated 1. However, they do match deaths of the world’s oldest person titleholders from 1955 (GRG Table C, Revision 9) with all deaths in May and June removed (SI).
Actual MRAD data from the GRG support a significant decline in the top-ranked age at death since 1995 (r = -0.47; p = 0.03, MSE = 3.2). However, this trend is not significant if only Jeanne Calment is removed (p = 0.9). Linear models fit to lower-ranked RADs have an order of magnitude better fit, and all indicate an increase in maximum lifespan since 1995 ( Supplementary Figure S1; N= 64; SI).
Collectively, these data indicate an ongoing rebound of upper lifespan limits since 1950, with a progressive increase in observed upper limit of human life. To estimate theoretical limits, we developed a simple approximation of the upper limit of human life.
Mortality rates double with age in human populations ( Figure 3a and b). Log-linear models fit to this rate-increase closely approximate the observed age-specific probability of death 4. These models also provide a simple method of predicting upper limits to human life span that is independent of population size.
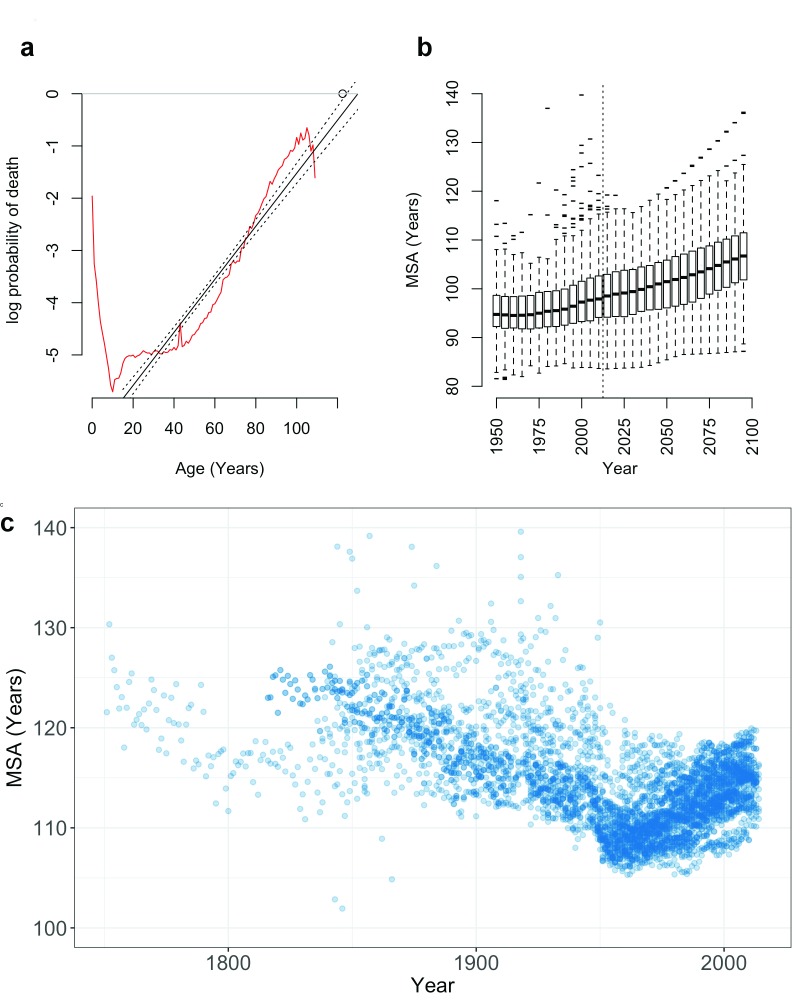
Figure 3. Observed and projected variation in the maximum survivable age (MSA).
( a) In humans, the probability of death q at age x ( q x; red line) increases at an approximately log-linear rate with age (black lines; 95% CI), shown here for the birth cohort of Jeanne Calment (d.122.5 years; circle). Projection of this log-linear increase to log( q) = 0 provides the MSA, the upper limit of human survival, shown here for ( b) observed and projected global populations 5 and ( c) 40 historic HMD populations 1751–2014.
We fit log-linear models to age-specific mortality rates from the HMD data used by Dong et al. 1, and used these models to predict the age at which the probability of death intercepts one. This maximum survivable age (MSA) provides a simple, conservative estimate of the upper limit of human life ( Figure 3c).
Log-linear models closely approximate the observed probability of death in HMD populations for both period and cohort life tables (median R 2 = 0.99; 4501 population-years). The MSA limit is compatible with observed ages at death in the GRG database with 330 out of 331 supercentenarians approaching, but not exceeding, their predicted lifespan limit ( Supplementary Figure S2; SI). These models predict an MSA exceeding 125 years within observed historic periods ( Figure 3b and c; SI).
Furthermore, period HMD data indicate that MSA is steadily increasing from a historic low c.1956 ( Figure 3b and c), tracking the increasing MRAD during the same period. The United Nations 5 global mortality data from 194 nations support this trend, with projected population data from the UN predicting a gradual rise in MRAD and MSA through the year 2100 ( Figure 3b).
This analysis provides an estimate of human lifespan limits that is conservatively low. Log-linear mortality models assume no late-life deceleration in mortality rates 6, which, if present, would increase the upper limits of human lifespan 7. In addition, these models are fit to population rates and cannot provide an estimate of individual variation in the rate of mortality acceleration.
Given historical flexibility in lifespan limits and the possibility of late-life mortality deceleration in humans 8, these models should, however, be treated with caution.
A claim might be made for a general, higher 130-year bound to the human lifespan. However, an even higher limit is possible and should not be ruled out simply because it exceeds observed historical limits.
Methods
Life table data were downloaded from the United Nations 5 (UN) and the Human Mortality Database (HMD) and lifespan records from the International Database on Longevity (IDL) and the Gerontology Research Group (GRG).
Least squared linear models were fit to life table data on the log-transformed age-specific probability of death ( q x), and projected to q x=1 to predict the maximum survivable age in each population ( Figure 1b and c; SI). Maximum lifespan within GRG and IDL data was annually aggregated and fit by locally weighted smoothed splines 9 ( Figure 3b and c).
We reproduced the analysis of Dong et al. in R version 10 3.2.1 (SI), using the code in Supplementary File 1.
An earlier version of this article can be found on bioRxiv (doi: 10.1101/124800).
Data availability
The authors declare that all data are available within the paper and its supplementary material.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 1 approved
Supplementary material
Supplementary File 1: Supplementary information guide. The supplementary information supplied here constitutes two sections of integrated code in the R statistical language:
-
1.
R code required to calculate the maximum survivable age or MSA from both public data and the human mortality data used by Dong et al., and required to reproduce our findings in full.
-
2.
R code required to reproduce Dong et al. 1 with original errors, often including several error-corrected or partially corrected versions.
Both sections are wrapped in the same script, and require several package dependencies and datasets outlined in the annotated code.
Supplementary Figure S2. Relationship between the maximum reported age at death and the theoretical maximum survivable age. Of 331 GRG supercentenarians born into populations with a known lifespan limit, only Jeanne Calment exceeded the theoretical gender-pooled lifespan limit (diagonal line). However, Jeanne Calment did not exceed the theoretical limit of her female cohort (see Figure 3a).
References
- 1. Dong X, Milholland B, Vijg J: Evidence for a limit to human lifespan. Nature. 2016;538(7624):257–259. 10.1038/nature19793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilmoth JR, Andreev K, Jdanov D, et al. : Methods Protocol for the Human Mortality Database.Version 5.0. 83.2007. Reference Source [Google Scholar]
- 3. Maier H, Gampe J, Jeune B, et al. : Supercentenarians. Demogr Res Monogr. 2010;7 10.1007/978-3-642-11520-2 [DOI] [Google Scholar]
- 4. Finch CE: Variations in senescence and longevity include the possibility of negligible senescence. J Gerontol A Biol Sci Med Sci. 1998;53(4):B235–9. 10.1093/gerona/53A.4.B235 [DOI] [PubMed] [Google Scholar]
- 5. United Nations: World Population Prospects: The 2015 Revision. United Nations Econ Soc Aff. 2015;XXXIII:1–66. Reference Source [Google Scholar]
- 6. Rose MR, Drapeau MD, Yazdi PG, et al. : Evolution of late-life mortality in Drosophila melanogaster. Evolution. 2002;56(10):1982–1991. 10.1111/j.0014-3820.2002.tb00124.x [DOI] [PubMed] [Google Scholar]
- 7. Thatcher AR: The long-term pattern of adult mortality and the highest attained age. J R Stat Soc Ser A Stat Soc. 1999;162(Pt 1):5–43. 10.1111/1467-985X.00119 [DOI] [PubMed] [Google Scholar]
- 8. Kannisto V, Lauritsen J, Thatcher AR, et al. : Reductions in Mortality at Advanced Ages: Several Decades of Evidence from 27 Countries. Popul Dev Rev. 1994;20(4):793–810. 10.2307/2137662 [DOI] [Google Scholar]
- 9. Cleveland WS: LOWESS: A program for smoothing scatterplots by robust locally weighted regression. Am Stat. 1981;35(1):54 10.2307/2683591 [DOI] [Google Scholar]
- 10. R Core Development Team: . R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. . 2012 Reference Source [Google Scholar]